Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
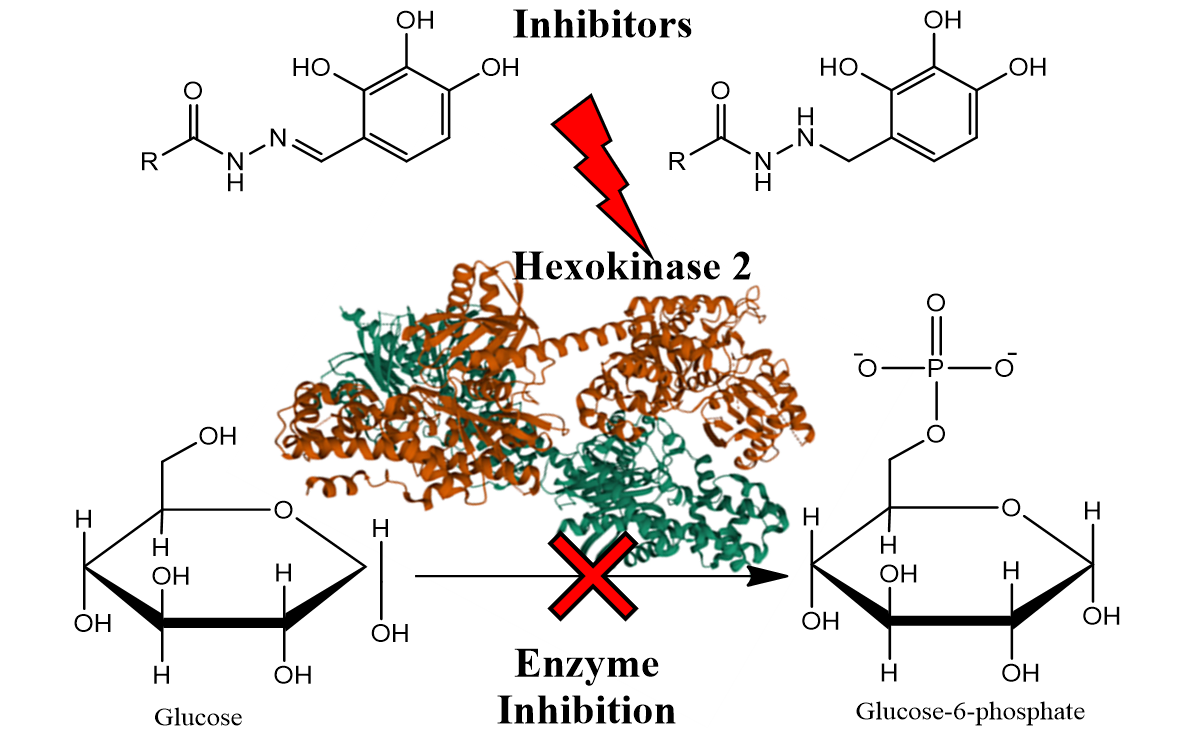
Keywords:
1. Introduction
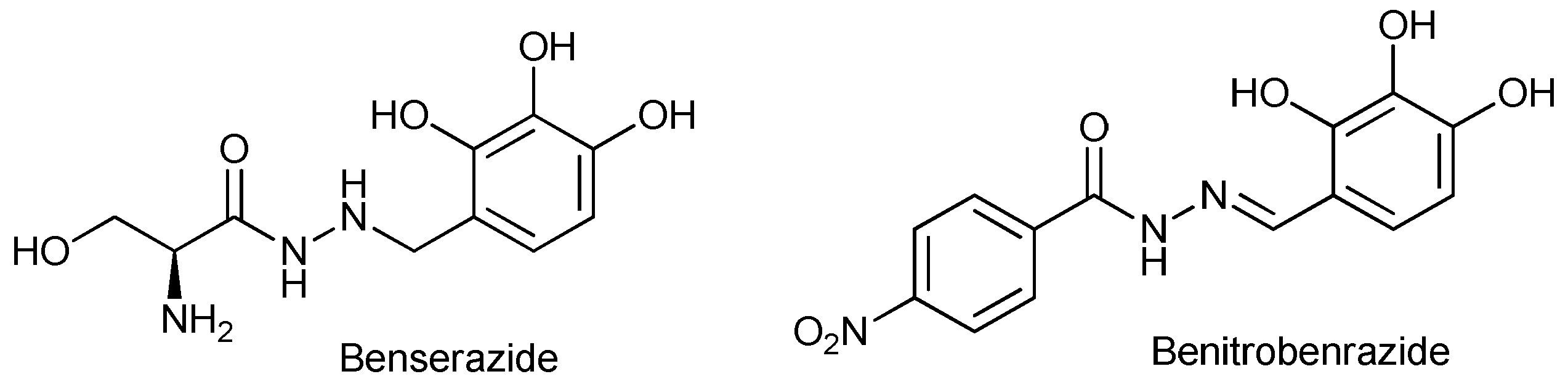
2. Results and Discussion
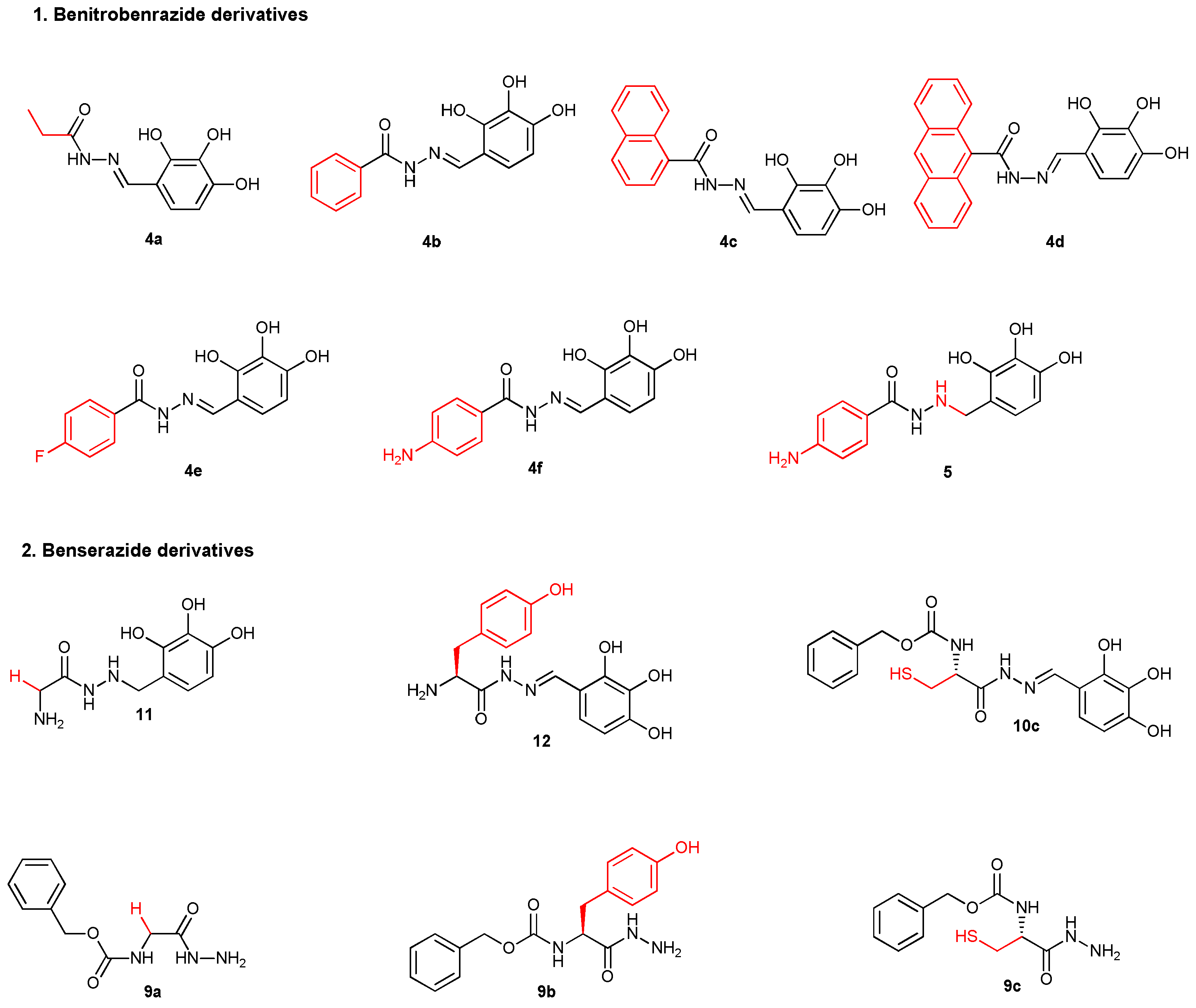
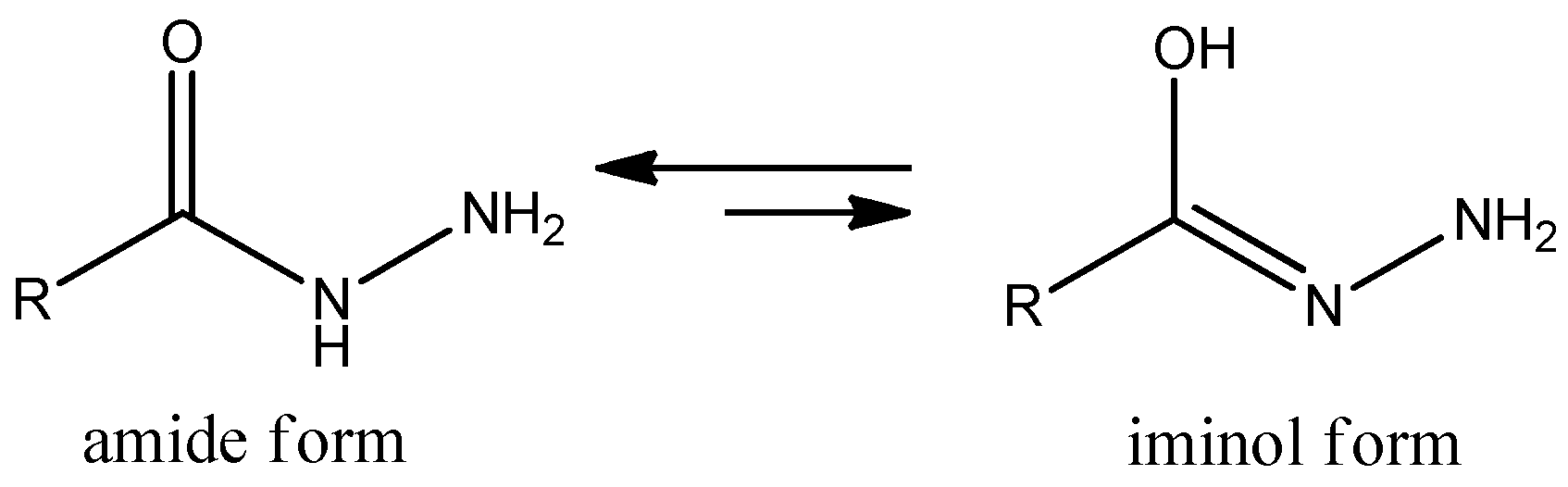
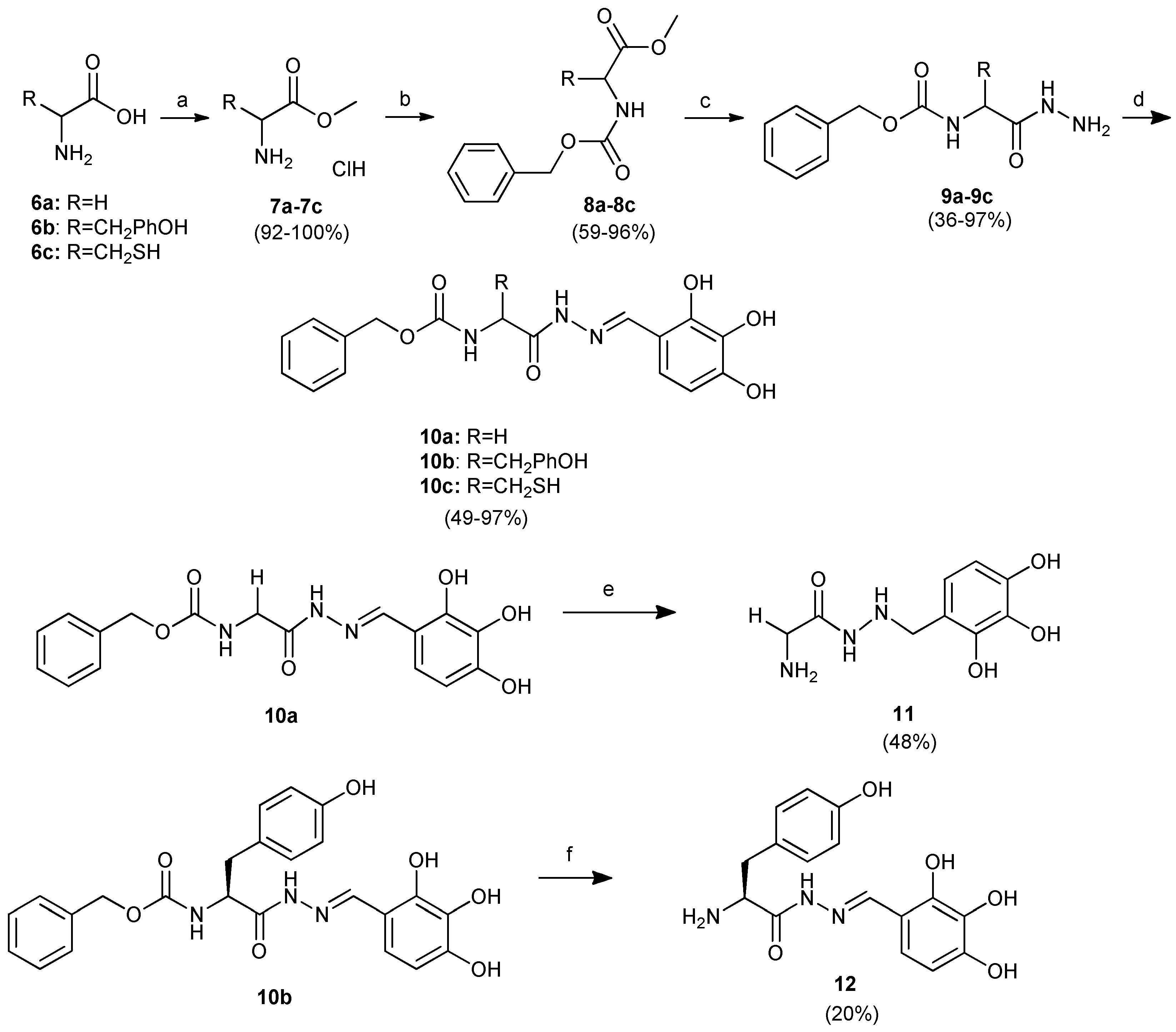
2.1. Chemistry of benitrobenrazide and benserazide derivatives
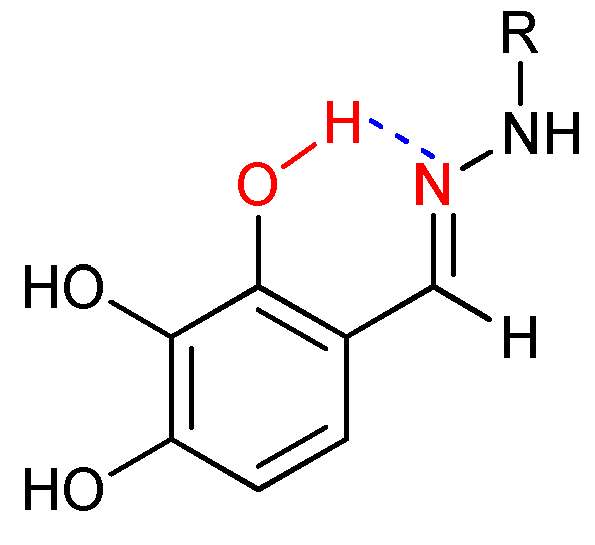

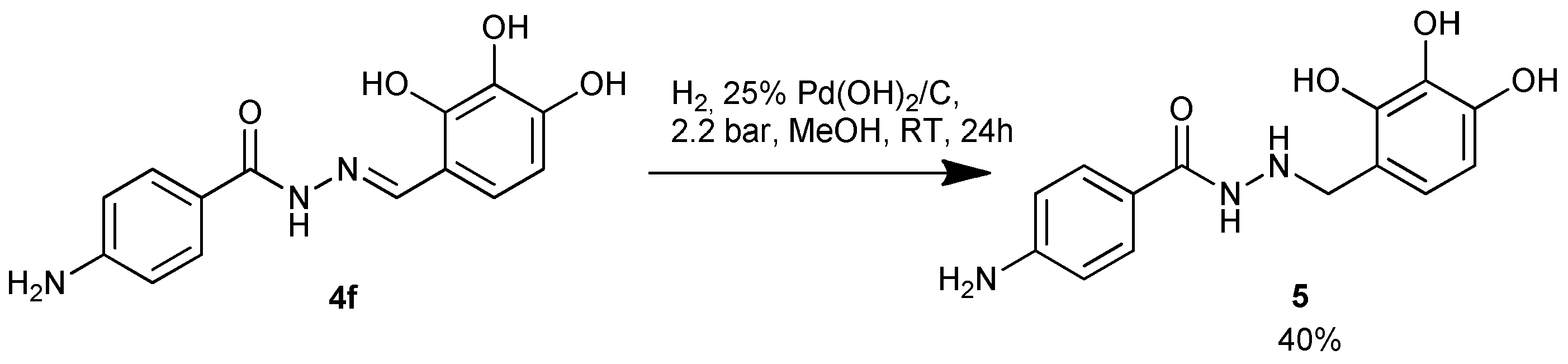
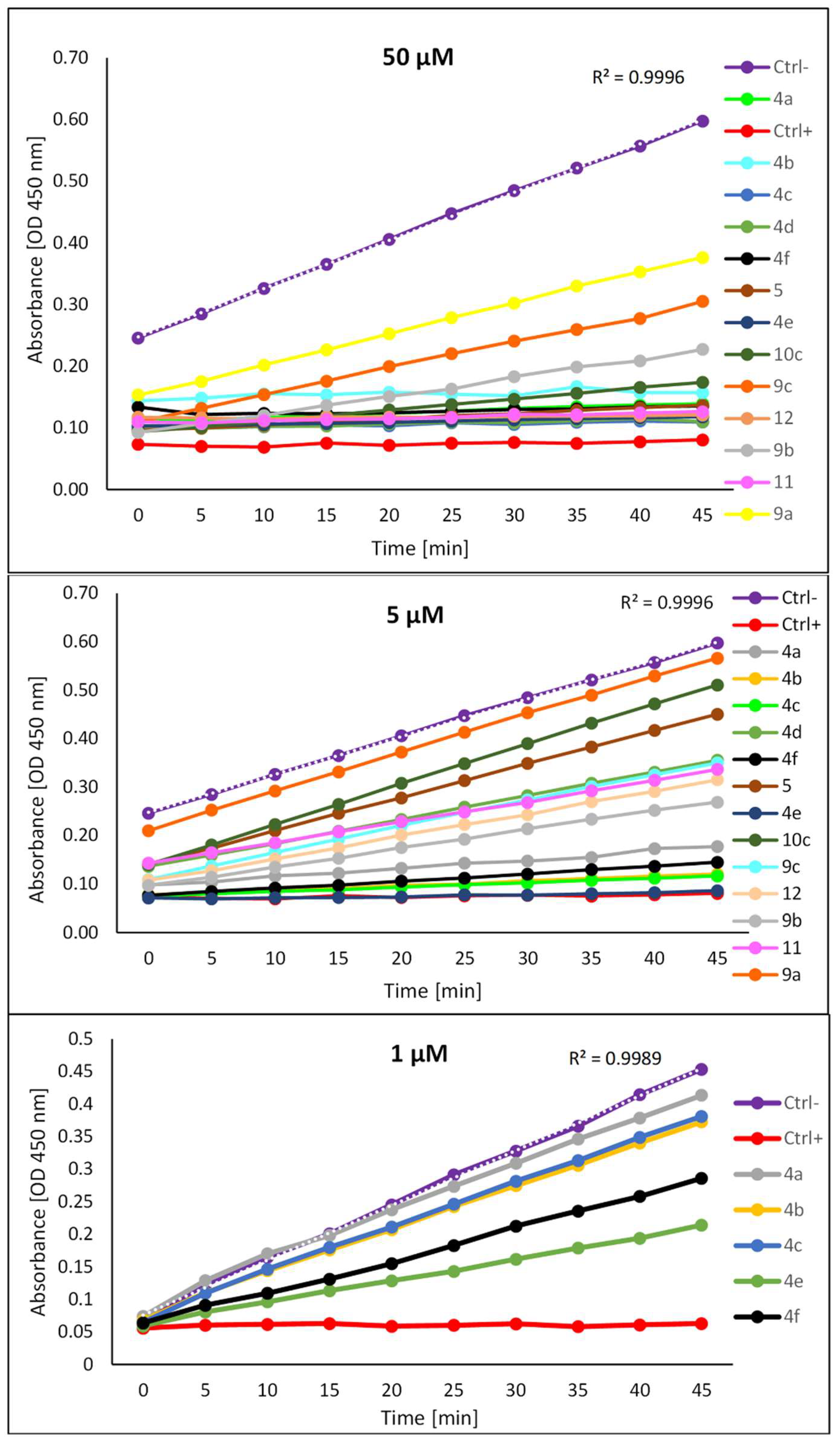
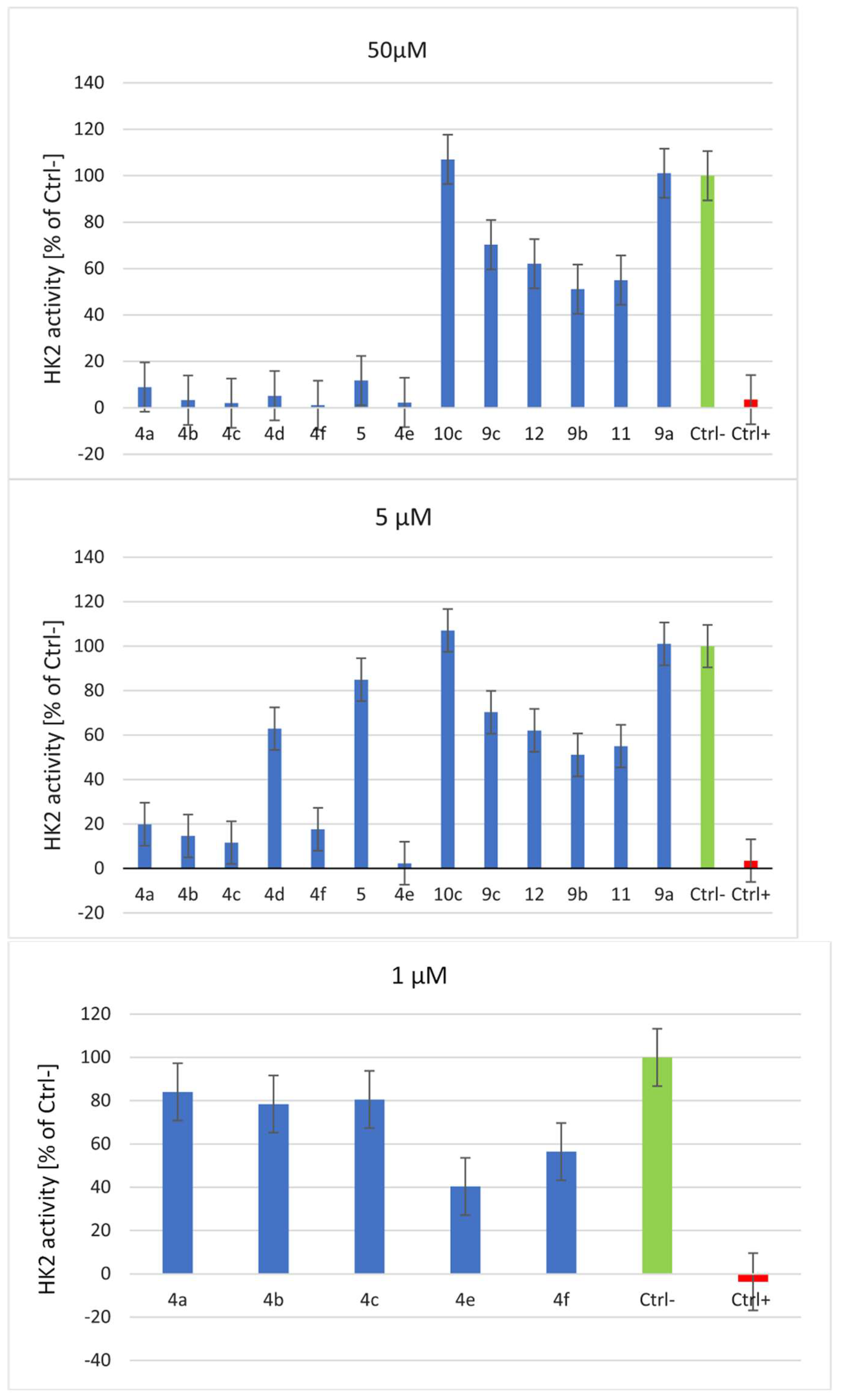
2.2. Inhibitory effect of benserazide and benitrobenrazide derivatives on HK2 enzymatic activity.
4. Materials and Methods
3.1. Chemistry
3.1.1. General procedure for the synthesis of esters 2e-f
3.1.2. General procedure for the synthesis of hydrazides 3a-3f.
3.1.3. General procedure for the synthesis of hydrazones 4a-4f and hydrazide 5
3.1.4. General procedure for the synthesis of amino acid methyl ester hydrochloride 7a-7c
3.1.5. General procedure for the synthesis of N-benzyloxycarbonyl-L-amino acid methyl esters 8a-8c
3.1.6. General procedure for the synthesis of N-benzyloxycarbonyl-L-amino acid hydrazides 9a-9c
3.1.7. General procedure for the synthesis of hydrazones of N-benzyloxycarbonyl-amino acids 10a-10c.
3.1.8. General procedure for the synthesis of substituted amino acid hydrazide 11 and substituted amino acid hydrazone 12.
3.2. Computational methods
3.3. Hexokinase activity assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raczuk E.; Dmochowska B.; Samaszko-Fiertek J.; Madaj J. Different Schiff Bases-Structure, Importance and Classification. Molecules, 2022, 27, 787. [CrossRef]
- Uddin N.; Faisal R.; Saqib A.; Tirmizi S. A.; Ahmad I.; Zaid S.; Zubair M.; Diaconescu P. L.; Tahir M. N.; Iqbal J.; Haider A. Synthesis, characterization, and anticancer activity of Schiff bases. J. Biomol. Struct. Dyn. 2020, 38, 3246–3259. [CrossRef]
- Kajal A.; Bala S.; Kamboj S.; Sharma N.; Saini V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 1–14. [CrossRef]
- Mounika K.; Anupama B.; Pragathi J.; Gyanakumari C. Synthesis, characterization and biological activity of a Schiff base derived from 3-ethoxy salicylaldehyde and 2-amino benzoic acid and its transition metal complexes. J. Scient. Res. 2010, 2 (3), 513–524. [CrossRef]
- Kargar H.; Fallah-Mehrjardi M.; Behjatmanesh-Ardakani R.; Tahir M. N.; Ashfaqc M.; Munawar K. S. Synthesis, crystal structure determination, Hirshfeld surface analysis, spectral characterization, theoretical and computational studies of titanium(IV) Schiff base complex, J. Coord. Chem. 2021, 74 (16). 2682–2700. [CrossRef]
- Awantu A. F.; Fongang Y. S. F.; Ayimele G. A.; Nantia E. A.; Fokou P. V. T.; Boyom F. F.; Ngwang C. K.; Bruno N. Lenta B. N.; Ngouela, S. A. Novel Hydralazine Schiff Base Derivatives and Their Antimicrobial, Antioxidant and Antiplasmodial Propertie Int. J. Org. Chem. 2020, 10 (1), 1-16. [CrossRef]
- Taha M.; Ismail N. H.; Imran S.; Anouar H.; Selvaraj M.; Jamil W.; Ali M.; Kashif S. M.; Rahim F.; Khan K. M.; Adenan M. I. Synthesis and molecular modelling studies of phenyl linked oxadiazole-phenylhydrazone hybrids as potent antileishmanial agents. Eur. J. Med. Chem. 2017, 126, 1021-1033. [CrossRef]
- Li L-Y.; Peng J-D.; Zhou W.; Qiao H.; Deng X.; Li, Z-H.; Li J-D.; Fu Y-D.; Li S.; Sun K.; Liu H-M.; Zhao W. Potent hydrazone derivatives targeting esophageal cancer cells. Eur. J. Med. Chem. 2018, 148, 359-371. [CrossRef]
- Küçükgüzel S. G.; Mazi A.; Sahin F. Öztürk, S.; Stables S. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [CrossRef]
- Khan K. M.; Rasheed M.; et al. Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogues. Bioorg. Med. Chem. 2003, 11, 1381–1387. [CrossRef]
- Durcik M.; Tammela P.; et al. Synthesis and Evaluation of N-Phenylpyrrolamides as DNA Gyrase B Inhibitors. Chem. Med. Chem. 2018, 13, 186–198. [CrossRef]
- Kareem H. S.; Ariffin A.; Nordin N.; Heidelberg T.; Azlina Abdul-Aziz A.; Kong K. W.; Yehye W.A. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3,4,5-trimethoxy benzyl moiety. Eur. J. Med. Chem. 2015, 103, 497-505. [CrossRef]
- Liu Y.; Li M.; Zhang Y.; Wu C.; Yang K.; Gao S.; Zheng M.; , Li X.; Li H.; Chen L. Structure based discovery of novel hexokinase 2 inhibitors. Bioorg. Chem. 2020, 96, 103609. [CrossRef]
- Wei L.; Mengzhu Z.; Shuangping W.; Gao S.; Yang M.; Li Z.; Min Q.; Sun W.; Chen L.; Xiang G.; Li H. Benserazide, a dopa decarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017, 36(58), 1–12. [CrossRef]
- Zheng M.; Wu C.; Yang K.; Yang Y.; Liu Y.; Gao S.; Wang Q.; Li Ch.; Chen L.; Li H. Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol. Res. 2021, 164, 105367. [CrossRef]
- Pelicano H.; DS Martin D. S.; Xu R-H.; Huang P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633-4646. [CrossRef]
- Hay N. Aerobic glycolysis Oxidative phosphorylation Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Publ. Gr. 2016, 16, 635. [CrossRef]
- Heiden M. G. V.; Cantley L. C.; Thompson C. B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science, 2009, 324, no. 5930, 1029. [CrossRef]
- Semenza G.L. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51-56. [CrossRef]
- Wu J.; Hu L.; Wu F.; Zou J.; He T. Poor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysis. Oncotarget, 2017, 8, 32332. [CrossRef]
- Ciscato F.; Ferrone L.; Masgras I.; Laquatra C.; Rasola A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int. J. Mol. Sci. 2021, 22, 4716. [CrossRef]
- Counihan J. L.; Grossman E. A.; Nomura D. K. Cancer Metabolism: Current Understanding and Therapies. Chem. Rev. 2018, 118, 6893-6923. [CrossRef]
- Wilson J. E. Isozymes of mammalian hexokinase: structure, subcellular localization, and metabolic function. J. Exp. Biol. 2003, 206, 2049-2057. [CrossRef]
- Garcia S. N.; Guedes R. C.; Marques M. M. Unlocking the Potential of HK2 in Cancer Metabolism and Therapeutics. Curr. Med. Chem. 2019, 26, 7285-7322. [CrossRef]
- Tsai J.H.; Wilson J. E. Functional organization of mammalian hexokinases: characterization of the rat type III isozyme and its chimeric forms, constructed with the N- and C-terminal halves of the type I and type II isozymes. Arch. Biochem. Biophys. 1997, 338, 183-192. [CrossRef]
- Zhang F. Angelova A.; Garamus V. M.; Angelov B.; Tu, S.; Kong L.; Zhang X.; Li N.; Zou A. Mitochondrial Voltage-Dependent Anion Channel 1−Hexokinase-II Complex-Targeted Strategy for Melanoma Inhibition Using Designed Multiblock Peptide Amphiphiles. ACS Appl. Mater. Interfaces 2021, 13, 35281-35293. [CrossRef]
- Lin H.; Zeng J.; Xie R.; Schulz M. J.; Tedesco R.; Qu J.; Erhard K. F.; Mack J. F.; Raha K.; Rendina A. R.; Szewczuk L. M.; Kratz P. M.; Jurewicz A. J.; Cecconie T.; Martens S.; McDevitt P. J.; Martin J. D.; Chen S. B.; Jiang Y.; Nickels L.; Schwartz B. J.; Smallwood A.; Zhao B.; Campobasso N.; Qian Y.; Briand J.; Rominger C. M.; Oleykowski C.; Hardwicke M. N.; Luengo J. I. Discovery of a Novel 2,6-Disubstituted Glucosamine Series of Potent and Selective Hexokinase 2 Inhibitors. ACS Med. Chem. Lett. 2016, 7, 217-222. [CrossRef]
- Tanbin, S.; Fuad F. A. A.; Hamid A. A. A. Virtual Screening for Potential Inhibitors of Human Hexokinase II for the Development of Anti-Dengue Therapeutics. Bio. Tech. 2021, 10, 1-28. [CrossRef]
- Juszczak K.; Kubicka A.; Kitel R.; Zawadzki S.; Marczak A.; Dzido G.; Walczak K.; Łabieniec-Watała M.; Matczak K.; Tomczyk M. D. Hexokinase 2 Inhibition and Biological Effects of BNBZ and Its Derivatives: The Influence of the Number and Arrangement of Hydroxyl Groups. Int. J. Mol. Sci. 2022, 23, 2616. [CrossRef]
- Song Y-J.; Zeng H-B.; Peng A-H.; Ma J-H.; Lu D-D.; Li X.; Zhang H-Y.; Xie W-D. Strepantibins A-C: Hexokinase II Inhibitors from a Mud Dauber Wasp Associated Streptomyces sp. J. Nat. Prod. 2019, 82, 1114–1119. [CrossRef]
- Agnihotri S.; Mansouri S.; Burrell K.; et al. Ketoconazole and Posaconazole Selectively Target HK2-expressing Glioblastoma Cells. Clin. Cancer Res. 2019, 25, 844-855. [CrossRef]
- Salani B.; Del Rio A.; Marini C.; Sambuceti G.; Cordera R.; Maggi D. Metformin, cancer, and glucose metabolism. Endocrine-Related Cancer 2014, 21, 461-471. [CrossRef]
- Nepali K.; Lee H. Y.; Liou J. P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851-2893. [CrossRef]
- Nishiwaki N. A Walk through Recent Nitro Chemistry Advances. Molecules, 2020, 25, 3680. [CrossRef]
- Wang Q.; Jianlin Han J.; Sorochinsky A.; Landa A.; Soloshonok V. A. The Latest FDA-Approved Pharmaceuticals ContainingFragments of Tailor-Made Amino Acids and Fluorine. Pharmaceuticals 2022, 15, 999. [CrossRef]
- Al-Harthy T.; Zoghaib W.; Abdel-Jalil R. Importance of Fluorine in Benzazole Compounds. Molecules 2020, 25, 4677. [CrossRef]
- Hosangadi B. D.; Dave. R. H. An efficient general method for esterification of aromatic carboxylic acids. Tetrah. Lett. 1996, 37 (35), 6375-6378. [CrossRef]
- Li X.; Zhao Z.; Li G.; Shi P.; Design and Synthesis of Novel Molecular Tweezer Anion Receptors based on Diphenic Acid Carbonyl Thiosemicarbazide. J. Chem. Res, 2010, 410-413. [CrossRef]
- Rohane S. H.; Chauhan A. J.; Fuloria N. K.; Fuloria S. Synthesis and invitro antimycobacterial potential of novel hydrazones of eugenol. Arab. J. Chem. 2020, 13, 4495-4504. [CrossRef]
- Gloaguen E.; Brenner V.; Alauddin M.; Tardivel B.; Mons M.; Zehnacker-Rentien A.; Declerck V.; Aitkenet D. J. al. Direct spectroscopic evidence of hyperconjugation unveils the conformational landscape of hydrazides. Angew. Chem. Int. Ed. Engl. 2014, 53, 13756–13759. [CrossRef]
- Özen A. S.; De Proft F.; Aviyente V.; Geerlings P. Interpretation of hydrogen bonding in the weak and strong regions using conceptual DFT descriptors. J. Phys. Chem. A. 2006, 110, 5860-5868. [CrossRef]
- Green T. W.; Wuts P. G. M. Protective Groups in Organic Synthesis. Wiley-Interscience, NY 1999, 531-537, 736-739.
- Kozioł A.; Lendzion-Paluch A.; Manikowski A. A fast and effective hydrogenation process of protected pentasaccharide: A key step in the synthesis of fondaparinux sodium. Org. Process Res. Dev. 2013, 17, 869-875. [CrossRef]
- Bartholomew C. H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212(1-2), 17-60. [CrossRef]
- Guan Y.; Wang J.; Sun J. A method for determination of hexokinase activity by RP-HPLC. J. Nat. Sci. 2011, 16, 535-540. [CrossRef]
- Rodrigues D. A.; et al. Design, Synthesis, and Pharmacological Evaluation of First-in-Class Multitarget N-Acylhydrazone Derivatives as Selective HDAC6/8 and PI3Kα Inhibitors. ChemMedChem, 2020, 15, 539–551. [CrossRef]
- Saha A.; Kumar R.; Kumar R.; Devakumar C. Development and assessment of green synthesis of hydrazides. Indian J Chem. 2010, 49B, 526-531.
- Younis A.; Awad G. E. A.Utilization of Ultrasonic as an Approach of Green Chemistry for Synthesis of Hydrazones and Bishydrazones as Potential Antimicrobial Agents. Egypt.J.Chem. 2020, 63(2), 599-610. [CrossRef]
- de Fátima S. Barreto A.; dos Santos V. A.; Andrade C. K. Z. Synthesis of acylhydrazino-peptomers, a new class of peptidomimetics, by consecutive Ugi and hydrazino-Ugi reactions. Beilstein J. Org. Chem. 2016, 12, 2865-2872. [CrossRef]
- Schröder E.; Gibian H. Über Peptidsynthesen, XII. Synthese von Glukagon-Teilsequenzen. Liebigs Ann. Chem. 1962, 656 (1), 190-204. [CrossRef]
- Eugen Schnabel. E. Nebenreaktionen bei der Synthese von Peptiden nach dem Azidverfahren von Curtius. Liebigs Ann. Chem. 1962, 659 (1), 168-184. [CrossRef]
- Bartholini G.; Hegedues B. Hoffmann La Roche. Ein Hydrazid und dessen Saeureadditionssalze. CPC C07C243/34 (EP); C07C251/72 (EP); DE1941261, 1970, A1 [Chem.Abstr., vol. 81, # 91225].

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




