1. Introduction
By 2050 the human population is projected to have increased to more than 9 billion; meanwhile, energy demand is expected to have nearly doubled [
1]. The continued excessive consumption of non-renewable energy from fossil fuels continues to raise serious environmental concerns [
2]. Furthermore, population growth will generate more organic waste, which will also cause environmental pollution. Anaerobic digestion (AD), being able to utilize a range of substrates, is an effective way to treat organic waste and produce renewable energy. Besides, anaerobic digestion is also an essential part of circular agriculture. The safety of biogas slurry and digestate is closely related to the performance of the reactor
AD occurs as a result of the activities of bacteria and archaea and involves the following three stages: (i) hydrolysis of macromolecules (polysaccharides, fats, and proteins) and subsequent production of monosaccharides, fatty acids, amino acids, and CO
2; (ii) conversion of monosaccharides, fatty acids and amino acids to volatile fatty acids (VFAs) and H
2; and (iii) conversion of VFAs, CO
2 and H
2 to methane [
3]. Any measure that affects these three stages may change the performance of the reactor. In recent years, AD still faces some enormous challenges. For example, the low hydrolysis rate of lignocellulosic agricultural wastes, ammonia inhibition in livestock and poultry manure, lack of nutrition in some digestion system [
4,
5,
6]. To solve those problems and achieve high performance in AD, conventional strategies that have been developed including pretreatment, co-digestion, and recirculation. Pretreatment technology is mainly applied to substrates that are difficult to directly decompose by microorganism such as crop straw, microalgae, and activated sludge. Co-digestion is the most useful promotion strategy, and its practical effect has been widely confirmed. Recirculation has unique advantages in areas where water is scarce. The functional consequences of these traditional strategies on promoting anaerobic digestion have been widely confirmed.
Recently, the understanding of the AD process has improved immensely and additional strategies and technologies are being developed. For example, in the past AD was considered to require a strict anaerobic environment. However, recent research has demonstrated that supplying a little amount of oxygen can promote the AD process [
7]. Previously, electron transports were only supposed to use H
2 as a shuttle by syntrophic partners [
8]. Nevertheless, it has recently been found that electron transfer can occur directly between different microorganisms, and such direct interspecies electron transport (DIET) can be enhanced by conductive materials [
9]. Those emerging promotion strategies mainly include additive and microaeration. There are various types of additives, such as conductive materials, functional microorganisms and enzymes, and trace elements. The microaeration strategy is mainly used in substrate fermentation, which is difficult to hydrolyze.
Although a lot of experiments have proved the actual effect of traditional and emerging strategies on digestion performance, a systematic summary of their digestion-promoting mechanisms is still lacking. The objective of this review is to offer a comprehensive overview of the main strategies to promote the AD process as reported by the latest research progress (Last 20 years). Critical think and summary about those promotion strategies were executed considering their mechanisms, reactor performance, and availability. Finally, conclusions and the outlook for future research into AD were given.
2. Strategies to promote AD process
AD produces biogas as renewable energy and is environmentally favorable in terms of low greenhouse gas and odor emissions. While, AD still faces many problems which limit the development of biogas projects such as low hydrolysis efficiency for lignocellulosic biomass, instability for FW (Food waste), and ammonia inhibition for livestock manure [
10,
11,
12,
13,
14,
15]. In order to improve the efficiency of AD, several strategies have been developed. In fact, research interest on strategies to promote anaerobic digestion has increased in recent 5 years (
Table 1). As shown in
Table 1, more than half of the research paper in anaerobic digestion field was on this topic of strategies in 2019. In this section, those strategies are discussed considering their mechanism, availability, and actual effect on reactor performance.
2.1. Pretreatment
Pretreatment includes physical, chemical and biological pretreatment. After those pretreatment processes, the digestion substrate will get higher availability. The effects of physical, biological, and chemical pretreatments on methane yields from different substrates are shown in
Table 1. The actual results of each pre-treatment method rely on different mechanisms.
Table 2 and
Table 3 clearly shown the mechanisms, advantages and disadvantages, and tangible effects on digestion performance for different pretreatment methods. In general, each pretreatment method has its limitations. Therefore, synergistic pretreatment results will happen when use a combined pretreatment method.
2.1.1. Biological pretreatment
Biological pretreatment is a safe and environmentally-friendly method and has some unique advantages such as low energy consumption and environment friendly [
4,
16]. Biological pretreatment includes mainly fungal, microbial consortium, and enzyme pretreatment. Fungal pretreatment (mainly white-rot fungi) is the most common biological pretreatment method.
2.1.1.1. Microbial pretreatment
Fungi have the ability to secrete cellulases, hemicellulases, and ligninase, which have the function of decomposing lignocellulosic biomass (mainly crop straw, energy crops and lignocellulose fraction of municipal solid waste (MSW)) [
17,
18,
19]. The role of fungi in lignocellulosic biomass pretreatment mainly includes modifying lignin structure (the ratio of guaiacyl/sinapyl), decreasing cellulose crystalline, increasing substrate porosity and changing hemicellulose structure (the ratio of xylose/arabinose) [
20].
Fungi comprise white-rot fungi (WRF), brown-rot fungi (BRF), and soft-rot fungi (SRF). Degradation mechanisms of SRF are still not clear [
20]. BRF is able to degrade cellulose and hemicelluloses and modify little lignin. Compared to SRF and BRF, WRF is better at delignification. To degrade lignocellulose, WRF uses hydrolases and a ligninolytic system which includes lignin peroxidase, manganese peroxidase and laccase [
20]. The unique enzymatic system gives fungi the ability to degrade lignin (mainly phenolic structures) into CO
2 [
21]. Recently, some lab-scale studies have confirmed the effectiveness of fungi in pretreating lignocellulose. Zhao et al. [
19] reported that methane yields of unsterilized branches increased from 20 L/kg VS to 40 L/kg VS after WRF pretreating. Thus, it demonstrated that fungal pretreatment could increase digestibility. Consistently, Mustafa et al. [
22] reported that methane production increased linearly with lignin degradation of WRF pretreated rice straw. However, the pure culture of fungi was applied hardly to pretreatment in the large scale-biogas project due to the limited degradation activity and ability. In general, the pure culture fungi only degraded the simple structure substrates such as artificial xylan and pure cellulose [
16].
To overcome the disadvantage of pure culture fungi. The microbial consortium was developed. The microbial consortium also is used to the pretreatment of lignocellulosic biomass [
16,
23]. The method of restrictive culture was usually be used to screen ideal microbial consortium. if the targeted microorganism is cellulolytic bacteria, cellulose material can be as the substrate for continuous subculture. The function of those microbial consortiums in pretreatment has been reported in the lab-scale level. Yuan et al. [
16] reported that a thermophilic microbial consortium (including
Clostridium straminisolvens CSK1,
Clostridium sp. FG4b,
Pseudoxanthomonas sp. train M1-3,
Brevibacilus sp. M1-5, and
Bordetella sp. M1-6) was used to pretreat lignocellulose in MSW (including office paper, newspaper, cardboard and mixture) and found that methane production was more than doubled after this pretreatment. Similarly, Zhong et al. [
23] reported that methane yield increased by 75.6% after microbial consortium pretreatment. Interestingly, the efficacy of microbial consortium pretreatment is often dependent on the duration of the pretreatment. For example, Yuan et al. [
16] reported that there was no obvious increase in methane yield when the pretreatment time exceeded 10 d because the microorganism consumed lots of organic matter. This phenomenon has also been found in another study [
17]. The main drawback of microbial pretreatment is the loss of organic carbon due to microbial growth. Therefore, the culture time is the most crucial thing which needs to optimize to reduce the loss of organic matter and obtain the optimal pretreatment results.
To obtain better pretreatment results, it’s a good choice to combined microbial pretreatment with physical pretreatment or chemical pretreatment. In general, microbial pretreatment has good results in mainly lab-scale research due to manageable conditions. But it still needs to explore further in large scale-project. Anyway, microbial pretreatment has considerable potential for further application.
2.1.1.2. Enzyme pretreatment
Since the condition of microbial pretreatment was challenging to control, some researchers attempted to screen out and utilize enzymes for the pretreatment. According to the substrate characteristics, the substrates often using the enzyme pretreatment method were divided into two categories: (i) Agricultural waste (mainly lignocellulosic biomass); (ii) Sludge (mainly waste activated sludge). For other substrates with high natural hydrolysis rates such as FW, OFMSW (Organic Fraction of Municipal Solid Waste), they were not suitable for using enzyme pretreatment due to limited results [
24]. For lignocellulosic biomass, enzymes pretreatment obtained better results than the conventional microbial pretreatments [
17]. Laccases and peroxidases were often used to degrade lignin and showed the actual pretreatment effect on lignocellulosic biomass. Schroyen et al. [
25] reported that methane yield increase by 17% when applied laccase to pretreat corn stover. Consistently, Frigon et al. [
26] obtained an obvious increase in methane production by 29% and 41%, respectively when used lignin and manganese peroxidases to pretreat switchgrass. However, Schroyen et al. [
27] reported that the higher the lignin concentration of lignocellulosic biomass, the lower the pretreatment efficiency when used laccases and versatile peroxidases to pretreat lignocellulosic biomass. In fact, a large release of phenolic compounds from lignin degradation strongly inhibited anaerobic digestion. This indicated that the choice of the substrate was very significant when focused on lignin degradation to increase methane yield [
24,
27]. In order to degrade carbohydrates, cellulases, hemicellulases, amylases, and pectinases were widely tested on agricultural waste and got good results considering methane yield [
24]. Even so, for a given substrate, it needs to be careful about the choice of enzymes, incubation time, temperature and pH; otherwise, negative results will appear [
28]. In fact, the high cost of existing commercial enzymes limits their application in the AD of lignocellulosic biomass. Recently, the technology of solid-state fermentation producing enzyme was proposed in order to obtain cheap enzyme to pretreat lignocellulosic biomass [
12,
29]. Due to its advantages of low cost (mainly culture substrate is organic wastes), environmental friendliness, and good pretreatment effect, solid-state fermentation producing enzyme has the potential to be applied in large scale projects.
Sludge is a by-product of wastewater treatment plants (WWTP) and is another substrate which often used enzyme pretreatment. Sludge can be divided into primary sludge and waste activated sludge (WAS). WAS consists of flocs which is rich in microbial biomass and EPS, which are mostly carbohydrates and proteins [
24]. To our knowledge, mainly carbohydrases and proteases were used to sludge pretreatment due to its composition. Yu et al. [
30] reported that the biogas yield increased by 23% after using amylases and proteases pretreatment at 37 °C. Consistently, Yin et al. [
31] reported that hydrolysis rate and methane yield increased by three times and 50%, respectively when applied a fungal mash (mainly rich in carbohydrases) to pretreat WAS. Unfortunately, the activity lifetime of enzymes in sludge was a very short time (generally less than 24 h) because of enzyme degradation by endogenous proteases and inhibition by unknown compounds in sludge [
24,
32]. Therefore, it needs a high cost to get desired effects due to the short lifetime of the enzyme. Recently, garbage enzymes were used to pretreat WAS and it successfully improved solubilization and biodegradability of WAS in AD [
33,
34,
35]. Garbage enzymes are by definition crude enzyme solution which is made of fruit wastes digestion. The advantages of garbage enzymes are low production cost, environment friendly and pleasant pretreat results. Therefore, garbage enzyme pretreatment is a potential pretreatment method to sludge in a large scale-biogas project.
2.1.2. Chemical pretreatment
Chemical pretreatments include alkali, acids, thermo-chemical, and electrical-chemical pretreatment methods and so on [
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47]. Similar to microbial pretreatment, chemical pretreatment is commonly used to treat lignocellulosic biomass because of its effectiveness in breaking ester bonds between polysaccharides and lignin [
45]. Alkali treatment is the most frequently used method in chemical pretreatment due to its effect on reducing cellulose crystallinity, removing lignin and increasing surface area and porosity, which makes the substrate easier to be digested.
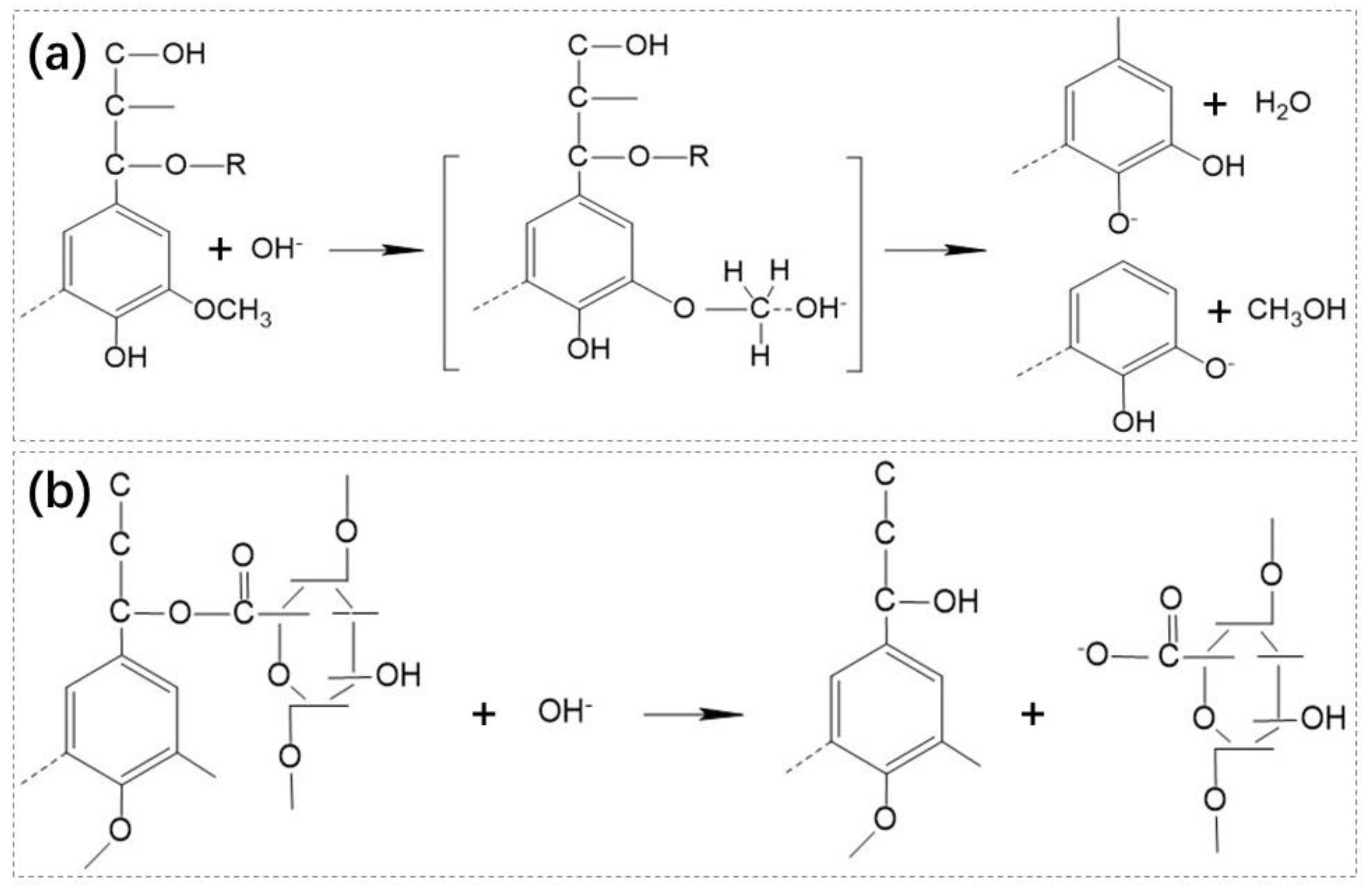
Figure 1a and
Figure 1b show the mechanism of the reaction between lignin, lignin complex and OH
-, respectively. The OH
- can partially resolve and separate the lignin and hemicellulose by breaking and weakening the ester ether bond between lignin and polysaccharides, and the hydrogen bond between hemicellulose and cellulose, respectively [
48]. In general, CaO and NaOH have commonly used alkalis and are highly effective for pretreatment. Solé-Bundó et al. [
36] reported that the methane yields increased by 11.99% after pretreatment using 10% CaO. Likewise, Mancini et al. [
40] showed that alkaline pretreatment led to a 155% enhancement in methane yields from wheat straw. In addition to CaO and NaOH, Yao et al. [
43] suggested that urea also had a function of loosening the constituent cell wall polymers in wheat straw lignocellulose. Although the dissolution of lignin makes lignocellulose more susceptible to be digested, the reaction system will be inhibited when the lignin content is too high in the digestion system. Koyama et al. [
42] reported that the step of hydrolysis was susceptible to be inhibited by dissolved lignin during methanogenesis and acidogenesis. Hydrolysis efficiency decreased by 25% at a 1.0 g L
-1 dissolved lignin concentration [
42]. Acid can also obviously change the biodegradability of lignocellulosic biomass by dissolving the hemicellulose. The commonly used acids are H
2SO
4, HCl, H
2O
2, and CH
3COOH. In general, it can’t get better results when use low concentration of acids than alkali to pretreat lignocellulosic biomass because of the limited effect of low concentration acids on the lignocellulosic structure. For acid pretreatment, the impact of inorganic acids is better than organic acids due to the mild chemical properties of organic acids. Song et al. [
49] compared the effects of H
2SO
4, H
2O
2, HCl, and CH
3COOH on pretreating rice straw and found that the biogas yields were in the order of 3% H
2O
2 > 2% H
2SO
4 > 2% HCl > 4% CH
3COOH pretreatment. In general, low concentration of organic acid pretreatment can’t get good results considering biogas production and high level of organic acid pretreatment will lead to a large amount of dry matter lost which is detrimental to anaerobic digestion [
48,
50].
Thermo-chemical and electrical-chemical pretreatments are also efficient methods. Passos et al. [
44] reported that the methane potential of dairy cow manure increased by 23.6% after pretreatment, with 10% of NaOH at 100 °C. Solé-Bundó et al. [
36] also found that thermo-chemical pretreatment increased the methane yield of microalgae by 15% compared to the untreated control. Electrical-chemical pretreatment was also often used to pretreat waste activated sludge (WAS). Electrolysis in conjunction with alkali treatment was able to disrupt the microbial cells surrounded in WAS gels and release the biopolymers (protein and polysaccharide) which enhanced breakdown/solubilization of sludge floc [
46,
47]. However, the technical-economic analysis showed that thermo-chemical pretreatment had no advantage over no pretreatment [
44].
It should base on the properties of the substrate when we selected chemical pretreatment methods. In the selection of chemical reagents, attempts should be made to use reagents that are beneficial to subsequent processing such as KOH which can be used as fertilizer. Although chemical pretreatment is a very effective method to enhance AD efficiency, its disadvantages are also obvious such as secondary pollution and high economic input. Therefore, chemical pretreatment should not be preferred.
2.1.3. Physical pretreatment
Physical pretreatment methods include milling, radiation (microwave and ultrasound) and thermal pretreatments [
51,
52,
53,
54,
55,
56,
57,
58,
59].
Milling pretreatment can decrease the digestion time due to its function of reducing substrate particle size, the crystallinity of lignocellulose substrate and increasing surface area which makes it easier to be digested [
48]. It is usually used prior to other pretreatment methods to obtain a synergistic pretreatment effect. Mustafa et al. [
22] reported that combinations of milling pretreatment and fungal pretreatment improved methane production by 165%. In general, this method is not economically feasible due to high energy consumption to get the desired particle size [
60]. At the lab-scale level, the desired particle size is a few millimeters that need highly energy input and is sometimes not recommended for a large-scale project [
61]. In addition, the energy consumption also depends largely on the type of material. Mönch-Tegeder et al. [
62] reported that energy consumption for milling pretreatment was negligible considering the 26.5% increase in methane production in a large-scale biogas plant when the particle size of horse manure was 3 mm [
62]. Thus, the availability of milling pretreatment mainly needs to consider the type and milling degree of the digestion substrate [
20].
Microwave pretreatment is often used to treat sludge and microalgae [
52,
53,
54]. Sludge is composed mainly of microbial cells within an extra-cellular polymeric floc matrix. Microwave pretreatment can disrupt the cell walls of microbial cells and then release the intracellular organics which are easy to be digested [
54]. For microalgae, although microwave pretreatment is not capable of inducing cell wall lysis, microwave pretreated cells are more susceptible to microbial attack which leads to the enhancement of their biodegradability [
53]. Ultrasound pretreatment is also commonly used due to its ability to enhance the solubilization of organic matter [
51]. Liu et al. [
51] reported that the maximum VFA yield and the highest percentage of H
2 in the biogas increased by 65.3% and 59.1% respectively under optimal sonication conditions. However, the installation cost of microwave and ultrasound is too high in the sizeable scale-biogas project. Thus, radiation only can as an auxiliary process together with other pretreatment methods.
Thermal pretreatment is one of the physical pretreatments in which the lignocellulosic biomass is subjected to heating at a specific pressure and temperature. The temperature range is 50-240 °C for this purpose [
58]. Marsolek et al. [
57] found that the biogas yield of algae during AD increased by 35.8% after being pretreated at a temperature of 90 °C for 12 h. Passos et al. [
59] compared microwave, ultrasound and thermal pretreatment in microalgae and found that solubilization of organic matter was improved in all three cases. Furthermore, the methane yield increased by 72% after thermal pretreatment and 21% after microwave pretreatments, whereas no significant increase was found as a result of ultrasound pretreatment. The broad range of differences in the properties of substrates is reflected in different results of the different physical pretreatments.
Physical pretreatment, especially milling, is necessarily combined with other pretreatment methods. When using physical pretreatment, the energy input and output efficiency should be considered first to determine its potential application value.
2.2. Co-digestion
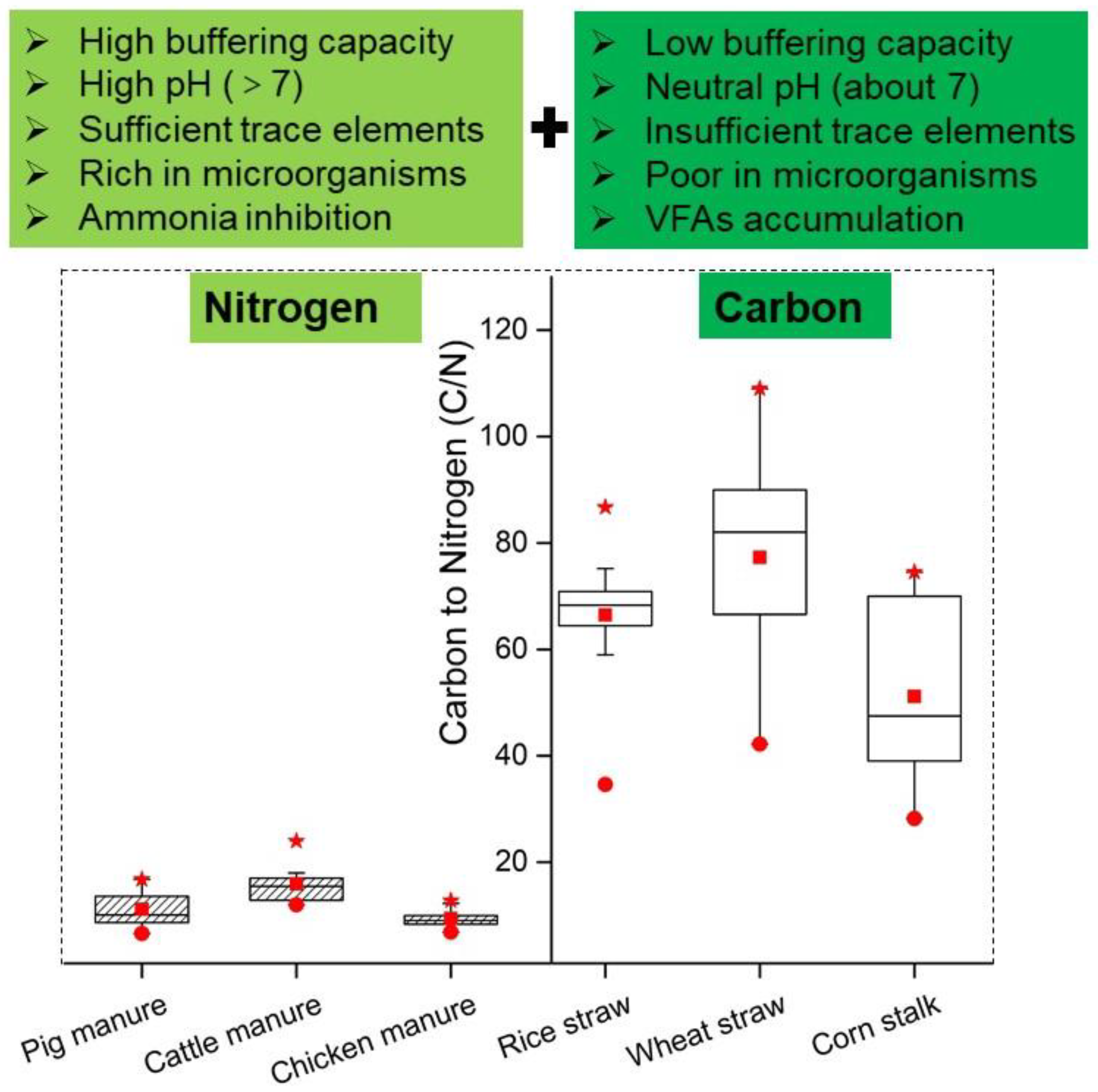
Co-digestion is the most commonly used strategy to enhance AD performance. Compared with mono-digestion, co-digestion utilizes multiple substrates to avoid a C/N imbalance, low buffering capacity, and the lack of trace elements and those are common obstacles in mono-digestion.
2.2.1. C/N ratio: the most important parameter in co-digestion
The most common substrates for co-digestion are livestock manure and crop straws. As shown in
Figure 3, the C/N ratio of lignocellulosic biomass is often greater than 60 which leads to instability of the system due to low buffer capacity and a high accumulation of VFAs. Conversely, the C/N ratio of livestock manure is often less than 20 which leads to ammonia inhibition and low biogas production efficiency [
14,
15]. Therefore, the co-digestion of livestock manure and lignocellulosic biomass is an effective method to improve the overall decomposition efficiency [
63]. In general, the optimal ratio of co-digestion between lignocellulosic biomass and livestock manure was in the range of 1:2 to 2:1 (Based on TS) [
63,
64,
65]. Although co-digestion is an effective method to improve digestion efficiency, this method is not always effective because substrates may be seasonable or site-specific, especially for livestock manure and lignocellulosic biomass [
66]. In recent years, the digestion substrates gradually diversified such as food industry wastewater, FW, energy crops, microalgae, vegetable waste, dairy wastewater, slaughtering waste and OFMSW. Co-digestion using more than two materials is becoming a trend. The C/N should be an important research indicator when the co-digestion of various materials is explored. Although some studies have confirmed that the digestion efficiency reached the highest when the C/N ratio was around 25, inconsistent results have also been reported. For example, Zheng et al. [
63] reported that co-digestion of cow manure and switchgrass (typical lignocellulosic biomass) the optimal C/N ratio was 29.4. However, Latha et al. [
67] shown that methane yield reached a maximum when C/N ratio was 12.9 for the Co-digestion of FW and sludge. The obvious difference of optimal C/N may be due to the large differences in the characteristics (mainly biodegradability, trace elements, etc.) of the raw materials [
68]. Therefore, further research about the C/N ratio should be done considering specific substrate properties.
2.2.2. Mechanism of Co-digestion to promote digestion efficiency
Co-digestion technology promotes nutritional balance and increase the buffering capacity of the digestion system. These effects are ultimately reflected in the microbial community structure and the absolute number of microorganisms. Zhang et al. [
69] reported that co-digestion of horse manure and FW led to significantly differing dominant bacteria (
Aminobacterium and
Proteiniphilum) and methanogenic archaea (
Methanobacterium and
Methanosarcina) comparing to dominant bacteria and archaea of mono-digestion of FW. Zhang et al. [
70] shown that the relative abundances of hydrogenotrophic methanogenesis, acetoclastic methanogenesis, and bacteria in co-digestion of blackwater with kitchen organic waste were higher than those in mono-digestion of blackwater. Consistently, Mu et al. [
71] also found that the absolute abundance of bacteria and archaea on co-digestion of sewage sludge and FW were higher than those in mono-digestion of FW. In general, the methanogenic archaea are more sensitive to the change of digestion condition than bacteria. Therefore, when the digestion system is under stress (mainly high concentration VFAs and ammonia), the differences of methanogenic archaea are more evident than those of bacteria. For example, Capson-Tojo et al. [
72] reported that the acetoclastic methanogenesis pathway changed to the hydrogenotrophic methanogenesis pathway when ammonia concentration increased gradually. Methanogenic archaea include acetoclastic methanogenesis archaea, mixotrophic methanogenesis archaea, and hydrogenotrophic archaea. Specifically, the dominant methanogenic archaea will change from
Methanosaeta (strict cetoclastic methanogenesis archaea) to
Methanosarcina (mixotrophic methanogenesis archaea) and eventually change to
Methanoculleus,
Methanobacterium or
Methanobrevibacter (hydrogenotrophic archaea) [
72]. In general, the acetoclastic methanogenesis pathway is considered as the main pathway in co-digestion [
73]. However, the methanogenesis pathway will change when the digestion system is under stress conditions. Co-digestion provides a suitable environment for digestion which makes the community structure of methanogenic archaea more efficient than mono-digestion. Therefore, co-digestion should be used as the preferred strategy to promote the efficiency of anaerobic digestion when the type and amount of raw materials are enough.
2.3. Recirculation
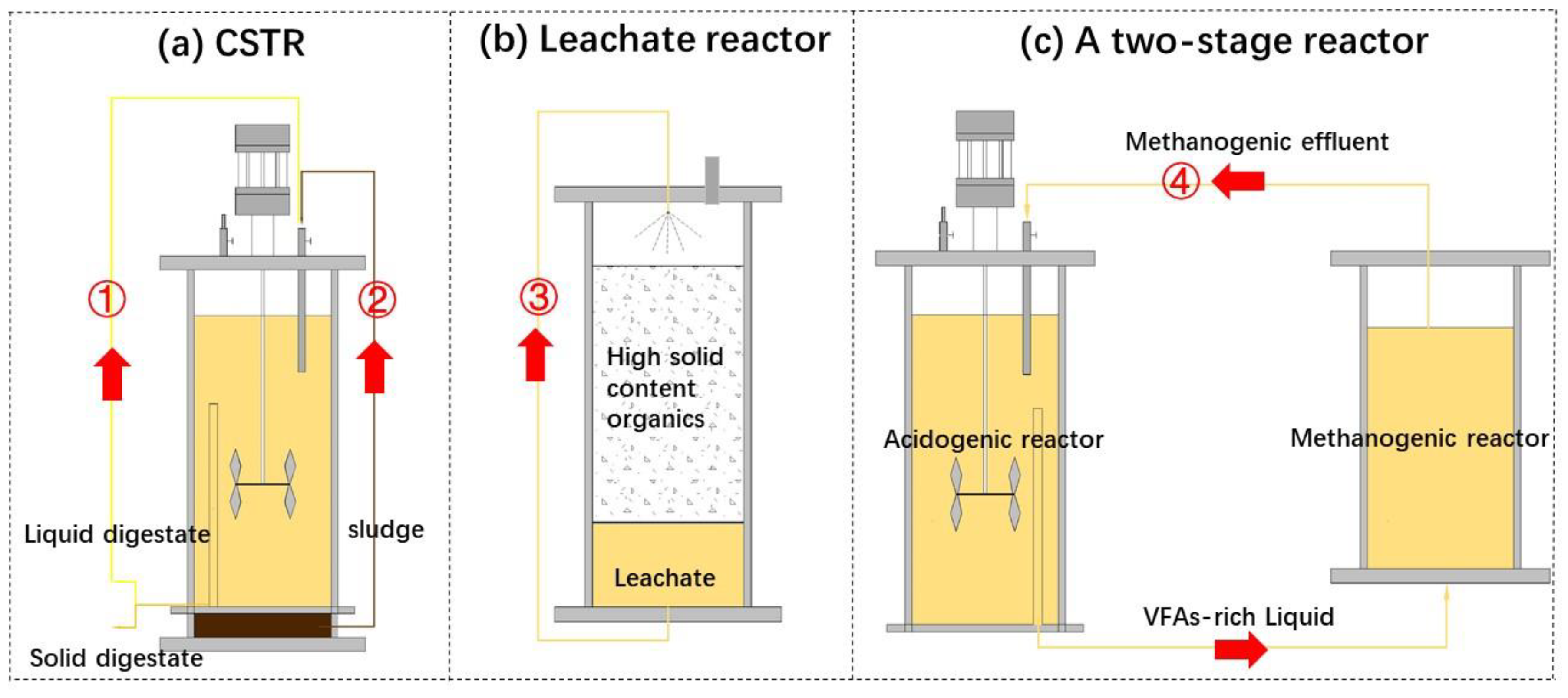
Recirculation is the process of recirculating digestates, sludge residue, and leachate to the reactor. As shown in
Figure 2, there are four common recirculation types including liquid digestates recirculation, sludge recirculation, leachate recirculation, and methanogenic effluent recirculation. Recirculation can reduce the discharge of digestate, improve the buffering capacity and alkalinity of the system, increase the stability of the digestion system, change the dominant microorganism and methanogenic pathways, increase mass transfer [
74,
75,
76,
77,
78,
79,
80]. These effects ultimately lead to an increase of hydrolysis rate, acidification efficiency and methane yield [
76,
79,
80].
Table 4 shows the effect of different recirculation types on the digestion system considering different substrates, reactor types, recirculation types, and recirculation rates. Wu et al. [
76] found that digestate recirculation had a positive effect on methane yield, OLR, and systematic hydrolysis rates. Recirculation also increased the system alkalinity and maintained an ideal pH for methanogens. Pezzolla et al. [
77] reported that recirculation prevented the accumulation of VFAs, which led to the digestion system more stable. However, this contrasted with studies by Qian et al. [
80] who reported that rapid accumulation of VFAs and a high VFA concentration peak occurred in a short time. These contrasting findings may be due to the differences in recirculation frequencies and rates. Qian et al. [
80] also reported that a higher recirculation frequency obviously contributed to hydrolysis and acidogenesis, and mass transfer. Conversely, Ni et al. [
79] demonstrated that methane yield decreased, which was caused by a considerable increase in viscosity from 30 to 1000 mPas and a decrease in mass transfer under excessive recirculation conditions. In addition, excessive recirculation may cause accumulation of heavy metals (mainly Mn, Pb, Zn, and Cu) and ammonia in the digestate [
76,
79], thereby disrupting the metabolic balance between methanogens and bacteria.
Recirculation can also lead to changes in metabolic pathways and the structure of the microbial community. For example, stable carbon isotope analysis indicates that the hydrogenotrophic methanogenesis pathway becomes dominant with digestate recirculation, while acetoclastic methanogen pathways dominate in the absence of digestate recirculation [
79]. Zamanzadeh et al. [
78] reported that recirculation could change dominated bacterium from
Chloroflexi to
Firmicutes in mesophilic FW AD. Actually, the effect of recirculation on microorganisms and dominated methanogenesis pathways ultimately originates from the changes of the substances in the digestion system such as nutrient enrichment, increased buffer substances, and increased microorganisms.
In summary, the effect of recirculation on AD can be both advantageous and disadvantageous. The extent and frequency of proper recirculation must be controlled to achieve good results. Different substrates often require different recirculation strategies (mainly recirculation types, recirculation ratio, and recirculation frequency), and those are particularly important to anaerobic of FW and livestock manure which are prone to ammonia accumulation.
2.4. Microaeration
Microaeration is also often described as micro oxygenation, limited aeration, and a microaerobic condition. Microaeration means supplying small volumes of air or oxygen (typically ranging from 0.005 to 5 L O
2/L
reactor/d) into the anaerobic reactor [
81]. Traditionally, oxygen or air should be avoided due to its adverse effects on the growth and metabolism of methanogens which are obligate anaerobes [
7]. However, in recent years, scientists have discovered that microaeration could overcome significant problems such as low hydrolysis rates, toxic of high concentration hydrogen sulfide, and instability at high OLRs [
13,
82,
83,
84,
85].
2.4.1. Digestion performance under microaerobic condition
Table 5 clearly shows that the different effects can be achieved when the reactor under different microaerobic conditions. The microaerobic status depends on the O
2 utilization rate which is related to inoculum (source and adaptation to substrate), substrate (type and organic loading rate), the microaerobic method (air/O
2, single/continuous, injection phase) and the O
2 transfer rate which is related to reactor configuration (one/two-staged, batch/CSTR) [
7].
Microaeration is directly related to the growth and metabolism of microorganisms. In general, the bacteria are responsible for 80% of the total DNA considering bacteria and archaea in the digestion system. Bacteria are mainly involved in the hydrolysis and acidification stages of anaerobic digestion.
Firmicutes,
Bacteroidetes,
Proteobacteria, and
Actinobacteria are the dominant bacterial phyla.
Bacteroidetes and
Firmicutes phyla are accountable for the hydrolysis of hemicellulose, cellulose, and other polysaccharides, while the primary function of
Proteobacteria is to utilize glucose and VFAs [
7]. Previous studies have proven that the hydrolysis step was the rate-limiting step in the strict anaerobic AD of high solids organic substrates and lignocellulosic biomass [
11,
86]. Microaeration is conducive to form diverse hydrolytic and fermentative bacterial communities with high activity [
87]. For example, Fu et al. [
87] reported that a high percentage of the
Firmicutes phylum appeared under microaerobic conditions which was linked with high hydrolytic rates [
87]. An abundant hydrolytic bacteria community can produce high level of extracellular hydrolytic enzymes (e.g., amylases, cellulases, and proteases) and thereby enhance the hydrolysis of proteins, carbohydrates, and other complex organic substrates [
88]. For example, after micro aeration treatment, the Firmicutes population of organic wastewater and FW increased from 58 to 72% [
88]. Consistently, the activity of
Clostridia and
Bacilli classes (belongs to the phylum
Firmicutes) increases under microaerobic conditions which leads to a 3-fold increase in butyric and acetic acid concentrations and then consequently causes high methane yield [
89].
Previous studies have shown that 30% of the protein released during AD belongs to archaea which comprise less than 4% of the microbial population [
90]. Under microaerobic conditions, enhancement in hydrolysis and acidogenesis leads to more substrates for methanogens thereby results in higher methanogenic activity [
87]. Microaeration also directly affects the methane-producing step by changing the main methanogenic pathway. The shift between hydrogenotrophic and acetoclastic pathways is highly dependents on the balance of symbiotic acetic acid oxidizing bacteria (transforming acetate to CO
2 and H
2) and homoacetogenic bacteria (reducing CO
2 to acetate) [
91]. Under the microaerobic condition, the traditional syntrophic collaboration between symbiotic acetic acid oxidizing bacteria and hydrogenotrophic methanogens can be substituted by the synergistic collaboration between facultative bacteria (generating CO
2 and H
2) and hydrogenotrophic methanogens (consuming H
2 and CO
2) [
92]. Therefore, under the microaerobic condition, the changes of VFAs, CO
2 and H
2 concentration can affect the archaeal communities and then change the dominant methanogenic pathway.
In addition to the above functions, the microaerobic condition can also protect the reaction system from H
2S by regulating the activity of sulfide/sulfur-oxidizing bacteria (SOB) and sulfate-reducing bacteria (SRB). SRB converts sulfate to hydrogen sulfide (H
2S) which is highly toxic and corrosive due to the dissolved sulfide (mainly H
2S+HS
−) in the liquid phase. Dissolved sulfide (H
2S+HS
-) is an inhibitor for methanogens and is corrosive to the reactor equipment. Furthermore, it is vital to consider the competition for substrates (acetate, H
2, and other intermediates) between methanogens and SRB. SRB has a higher substrate affinity for acetate and H
2 and has a higher growth rate than methanogens which leads to high H
2S content and low methane yield [
7,
93]. SOB is responsible for the conversion of sulfide (HS
−) to elemental sulfur (S
o), thiosulfate (S
2O
32−) and sulfate (SO
42−). Both SOB and SRB exist in the AD system of sulfate-rich substrates, where SOB converts sulfide to S
o and/or SO
42− and SRB SO
42−to sulfide. As shown in
Table 5, H
2S can be effectively removed when the anaerobic condition is controlled to create a microaerobic condition [
83,
94,
95].
In recent years, microaerobic technology is researched and developed. But the knowledge is still limited. The oxygen or air supply needs to be optimized for different purposes. For example, increasing hydrolysis efficiency requires higher oxygen concentrations than hydrogen sulfide removal. Under different digestion process conditions such as substrate, reactor types, OLR and stir, the effect of microaerobic conditions on the digestion system should be investigated more extensively. It is a very promising technology and is expected to be applied in large-scale biogas projects, especially substrates that are difficult to hydrolyze.
2.4.2. Co-existence and synergistic interaction mechanisms between bacteria and archaea in microaerobic condition
In microaerobic conditions, facultative microorganism utilizes and partly reduced oxygen, producing reactive oxygen species (ROS) (i.e., •OH, H
2O
2, and O
2-). These ROS are greatly oxidative and can damage protein and DNA, the cell membrane of microorganisms [
7]. Aerobic and facultative bacteria can generate anti-oxidative enzymes (AOE) to neutralize these ROS and this allows them to grow well in an aerobic environment. Recent studies showed that several strict anaerobic microorganisms (Mainly methanogenic archaea) can also generate AOE to adapt to the oxidative environment under the microaerobic environment [
96]. Such adaptive responses of strictly anaerobic microorganisms make itself has a high tolerance to the microaerobic environment.
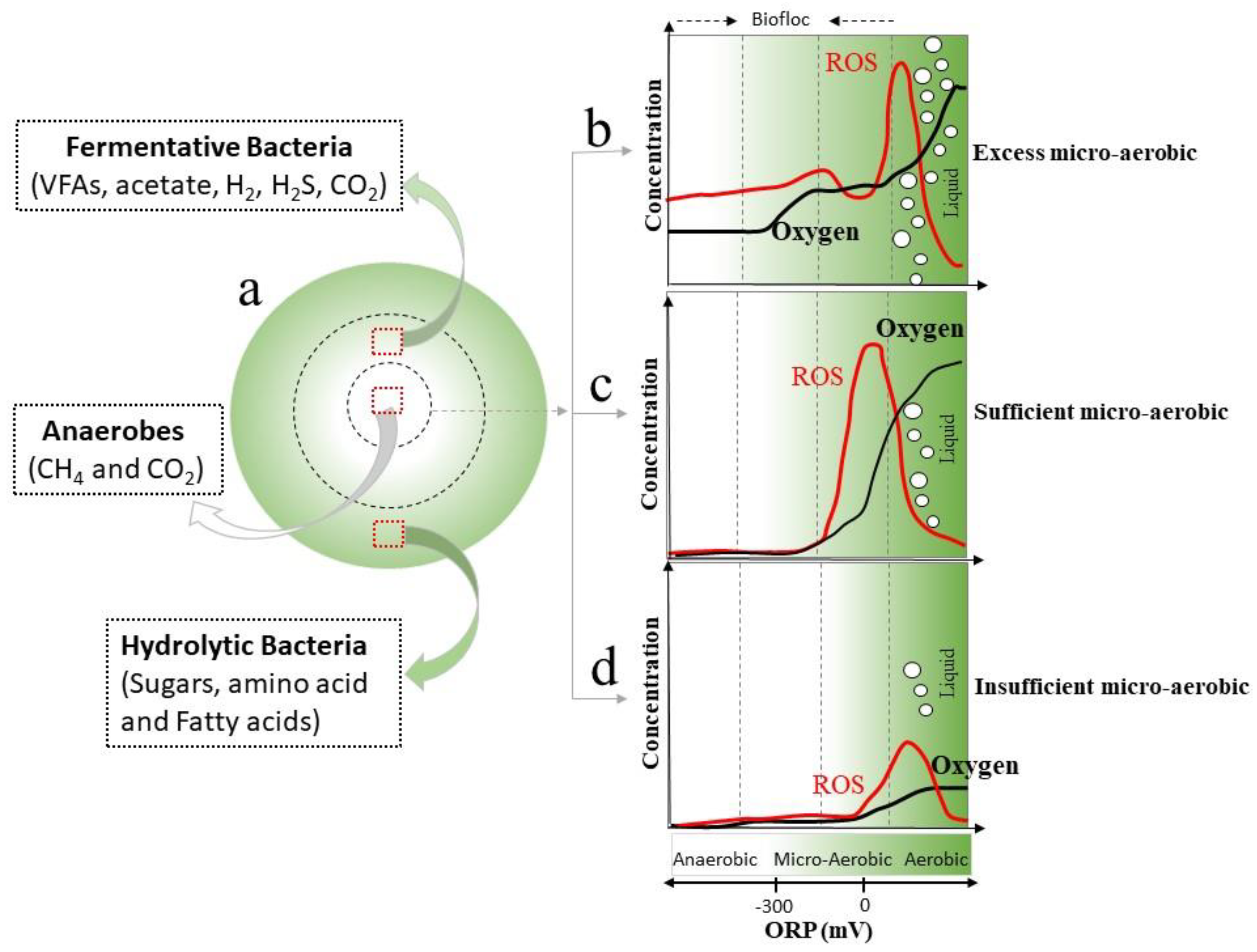
In the AD system, strictly anaerobic archaea and facultative bacteria tend to form bioflocs with facultative bacteria at the outer layer and strictly anaerobic archaea at the inner core (
Figure 4). Hydrolytic and fermentative bacteria (Facultative bacteria) with high AOE activity can scavenge ROS to protect the anaerobes which are strict anaerobic microorganisms and every susceptible to oxygen environment. The concentrations of ROS and Oxygen will decrease when they go through the bioflocs layer in that way protecting anaerobes located at the inner core of the bioflocs from oxidative material [
97]. As shown in
Figure 4, the synergetic relationship between facultative bacteria and anaerobic archaea also exists in the substrate flow of the aggregated bioflocs (from outer to inner section). In the bioflocs, hydrolytic bacteria can break down complex organics into diverse intermediates which work as substrates for fermentative bacteria and then for methanogenic archaea. Because of the synergetic relationship, the remaining oxygen (represented by ORP) decreases to a tolerable level for methanogenic archaea (
Figure 4c). Therefore, the facultative bacteria in the outer layer possibly act as a physical and biological barrier against oxygen thereby protecting strict anaerobic microorganism, and allowing strict anaerobic microorganism to function effectively under a microaerobic environment. Also, previous research showed that the phospholipid membrane and DNA of strict anaerobic microorganism might be damaged due to the high concentration of ROS when the amount of aeration exceeds the oxygen utilization rate and anti-oxidation abilities of facultative bacteria [
89]. Furthermore, insufficient oxygen dosing may also cause a negative effect on AD performance because it may change the delicate balance between facultative bacteria and anaerobic archaea [
98]. As shown in fig 4d, under insufficient oxygen concentration environment, anaerobic archaea will not be damaged by ROS and oxidizing substances. But the efficiency of facultative bacteria will decrease, which undoubtedly affects the growth and metabolism of anaerobic archaea.
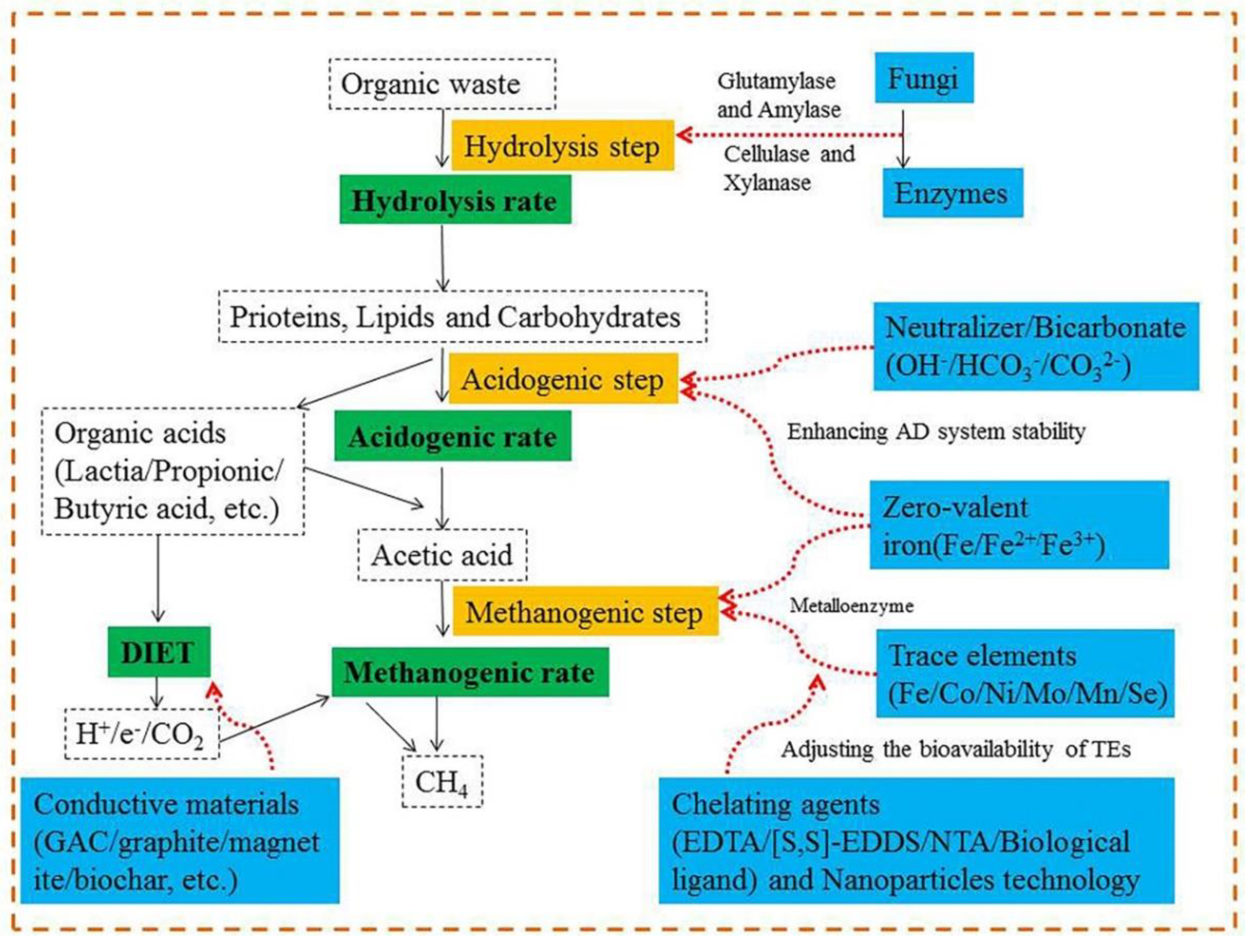
2.5. Additives
Trace elements, biochar and biological additives (bioaugmentation and enzymes) are the main additives applied in AD. The prominent feature of the additives is that small quantities can obtain desired results. As shown in
Figure 5, different types of additives have different mechanisms for stimulating AD.
2.5.1. Conductive material
Conductive materials include carbon-based conductive materials (activated carbon (AC), biochar, carbon cloth, carbon nanotubes, graphene, and graphite) and non-carbon-based conductive materials (magnetite, hematite, and stainless steel) [
9]. In recent years, conductive materials have been used for the digestion system, especially for lab-scale research.
2.5.1.1. The mechanism of conductive materials to promote anaerobic digestion
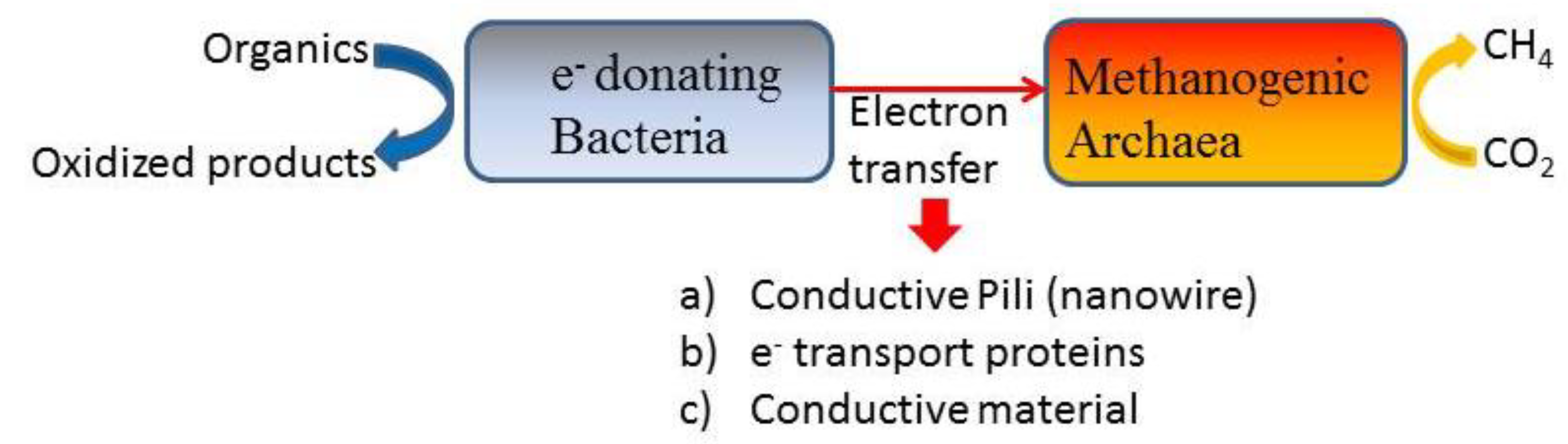
Electron transfer through interspecies hydrogen transfer (IHT) between acetogens and methanogens using H
2 as a shuttle was understood to be the main way that methane was produced by hydrogenotrophic methanogens [
8]. However, researchers have recently discovered that direct interspecies electron transport (DIET) is also a route for methane generation [
99]. DIET is an electron transfer mechanism between microbes that avoids the use of mediating diffusive electron transporters. Three types of DIET mechanisms have been identified: membrane-bound electron transport proteins, conductive pili, and conductive materials (shown in
Figure 6) [
9,
99]. Electronic transfer through membrane-bound electron transport proteins (such as cytochromes) and conductive pili are inherent mechanisms in microbial species and challenging to regulate. In a recent study, researchers have found that conductive materials can also provide a carrier for DIET [
8,
100,
101,
102,
103,
104,
105,
106,
107,
108]. For carbon-based conductive materials, Lin et al. [
100] reported that the addition of 1.0 g/L graphene led to an enhancement of 25.0% in methane yield, and the ethanol degradation constant was improved by 29.1%. Microbial analyses revealed that bacteria
Geobacter and
Pseudomonas along with archaea
Methanobacterium and
Methanospirillum participate in DIET. Park et al. [
9] found that the AC supplementation increased the methane yield and its production rate by 31% and 72% compared to control treatments along with an enhancement of the CO
2 reduction pathway according to metagenomics analysis. Dang et al. [
104] found that enrichment of
Sporanaerobacter and
Methanosarcina (typical hydrogenotrophic methanogens) occurred on the carbon cloth surface and determined that
Sporanaerobacter can participate in direct interspecies electron transfer with
Methanosarcina. The phenomenon of
Methanosarcina enrichment was also found in other studies [
106]. In addition to carbon-based conductive materials, non-carbon-based conductive materials also play an active role in DIET. Wang et al. [
8] reported that magnetite clearly reduced the accumulation of VFAs and accelerated the methane yield by 26.6% along with an improvement in acetate-dependent methanogenesis in the AD of high-solids sewage sludge. In addition, the expression of both cytochromes and pili was reduced which indicated that magnetite could substitute their roles for effective electron transfer between acetogens and methanogens. Similarly, Zhao et al. [
101] reported that ferrosoferric oxide as a conductive material could significantly increase the digestion performance of WAS. In this study, DIET was enhanced between
Syntrophomonas and
Methanosaeta indicating that the aceticlastic methanogenesis pathway was enhanced.
There are challenges associated with the enhancing of DIET using conductive materials. For example, DIET is impossible to participate in the direct decomposition of complex organics. Therefore, it is advantageous to combine enhancing DIET with other strategies that can improve the degradation of the complex organics. In addition, inexpensive and efficient conductive materials and applied styles should be developed.
2.5.2. Bioaugmentation and enzymes
In this section, bioaugmentation and enzymes refer to the treatment of the digestion process, not pretreatment. In general, bioaugmentation means that apply functional microorganisms to the reactor and get better reactor performance. For example, adding functional microorganisms to accelerate decomposition and transformation of organic macromolecules and VFAs or resist some toxic substances (mainly organic acids and ammonia nitrogen) [
109]. In recent years, extensive researches have been conducted to investigate the actual effects of bioaugmentation on reactor performance, especially at the laboratory scale. The addition of hydrolytic microbes may be an alternative way to enhance hydrolysis rate and extent. For example, the biogas yield during AD of fiber-rich swine manure was considerably improved after a lignocellulolytic microbial consortium was used. The cellulose and hemicellulose digestion efficiencies were improved significantly from 15% to 30–62% and 23% to 31–75%, respectively [
110]. Weiss et al. [
111] reported that methane yield increased by 53% when added hemi-cellulolytic bacteria to AD reactor. Similarly, Martin-Ryals et al. [
112] shown that adding cellulolytic microorganisms achieved a significant increase in soluble chemical oxygen demand (sCOD) production (+25%) and methane yield (+15%). The addition of pure bacterial cultures with high hydrolytic abilities has also been assessed. Lü et al. [
113] shown that the inoculation of
Coprothermobacter proteolyticus enhanced the hydrolysis of the constituent polysaccharides and proteins. In addition to bacterial consortia, the consortia of methanogens are also effective. Li et al. [
114,
115] reported that adding methanogens consortium could restructure the methanogen community by increasing the populations of
Methanothrix (acetoclastic methanogens) and
Methanolinea (hydrogenotrophic methanogens), which accelerates the VFAs degradation and then increases the methane yield.
Bioaugmentation is an important method to relieve or eliminate ammonia inhibition. In general, hydrogenotrophic methanogens and syntrophic acetate oxidizing bacteria (SAOB) are the most common bioaugmentation additives used to relieve ammonia inhibition [
116,
117,
118,
119,
120]. Tian et al. [
117] and Fotidis et al. [
118] found that the methane production increased by about 30% after adding hydrogenotrophic
Methanoculleus bourgensis at high ammonia concentrations (i.e., 5 and 11g NH
4+-N L
-1) [
117,
118]. Moreover, Tian et al. [
119] also shown that another hydrogenotrophic methanogen
Methanoculleus thermophilus could create thermodynamically favorable environments for the process of acetate oxidation and then stimulate the growth of SAOB
Thermacetogenium phaeum. Yang et al. [
120] explored the effect of seven pure strains of bacteria and archaea (including SAOB, facultative aceticlastic methanogen, obligate aceticlastic methanogen, and hydrogenotrophic methanogen) on digestion performance and found that together SAOB
Syntrophaceticu schinkii and hydrogenotrophic methanogen
Methanobrevibacter smithii was the optimal choice considering the methane yield (71.1% higher than that in control).
In addition to microorganisms, enzymes can also be directly added into AD systems. For example, inoculating lipases (0.33 % v/v) in AD of sewage sludge considerably improved the methane yield from 365 to 452 mL CH
4 g VS
-1 [
121]. Sometimes the effect of the enzyme is better than bioaugmentation. This may be due to the following reasons: (i) enzymes can remain active under a wide range of environmental conditions (e.g., pH, salinity, and temperature) even though these conditions quickly change; (ii) enzymes can work in the presence of microbes, inhibitors of microbial metabolism, and predators, avoiding the adverse effects on microorganism; and (iii) enzymes have higher solubility and mobility and more effective at direct interaction with the substrates than microorganisms [
109].
In general, bioaugmentation and enzymes are very effective strategies to improve the efficiency of AD. Microbial consortia tend to be more effective than single strains. However, bioaugmentation and enzymes must be added at above certain threshold levels to achieve the desired effect. The cost of bioaugmentation and enzymes is an obstacle to its application in large-scale biogas projects.
2.5.3. Trace elements
2.5.3.1. Relationship between trace elements (TEs) and anaerobic digestion
Metal elements can be classified based on their concentration in the cells into major elements (K, Mg, Ca), and trace elements (Mn, Fe, Co, Cu, Mo, Ni, Se, and W) [
122]. Metal concentration in the cells ranges from 10
−7 to 10
−3 M for the major elements, and from 10
−6 to 10
−15 M for trace elements (TEs) [
123].
The mechanism of trace elements in digestion system includes: (i) Trace elements change the metabolic environment to a more favorable one such as decreasing oxidative–reductive potential (ORP), increasing buffer capacity and changing sludge properties; and (ii) its role as a cofactor of several key enzymatic activities [
109,
124]. AD is a complex biochemical reaction involving a considerable number of metalloenzymes. TEs are involved in the formation of metalloenzymes and play an important role in microbial metabolism [
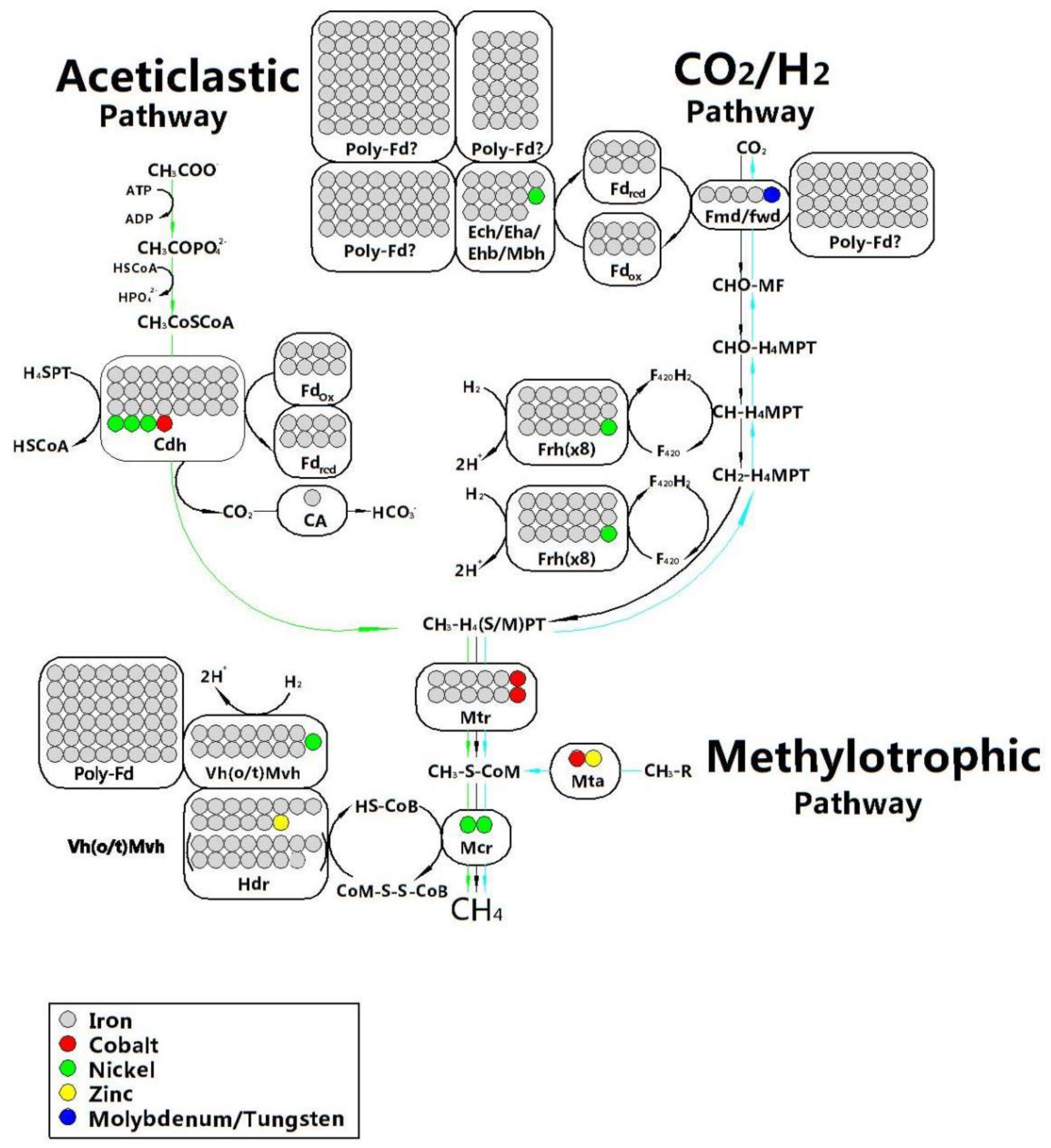
109]. As shown in
Figure 7, whether it is the aceticlastic pathway or the CO
2/H
2 pathway, there are a large number of trace elements involved in the composition of important metalloenzymes needed to form methane especially the formation of intermediates methyl-tetrahydrosarcinapterin (CH
3-H
4SPT) or methyl-tetrahydromethanopterin (CH
3-H
4MPT). TEs have a function of generating the active site, the structure itself and cofactor in the metalloenzyme. For example, to initiate CO
2/H
2 pathway, two isoenzymes of formylmethabofuran dehydrogenase demand at least one binding element of Mo or W [
125]. In the aceticlastic pathway, the first metalloenzyme is carbon monoxide dehydrogenase/acetyl-coenzyme A synthase (Cdh) complex enzyme, which the structure of its subunit CdhA contains Fe and Ni [
126]. In addition, the cofactor 5-hydroxybenzimidazolylcobamide (factor III, with Co) and the crystal structure of methyl coenzyme M reductase (Mcr) of CH
3- H
4M(S)PT-coenzyme M methyltransferase (Mtr) has been revealed with two Ni-containing cofactors F
430 as the active sites [
127]. As shown in
Figure 8, the trace element requirement trend in methanogenesis is Fe >> Ni > Co > Zn = Mo/W without taking consideration of the substrate medium [
128]. In fact, Cai et al. [
129] found that the demand trend for trace elements didn’t follow to this law. Therefore, although those methane production pathways have been understood well, further research is also required to assess the possible utilization mechanism for trace elements required rules considering some specific factors (such as ammonia concentration, pH of digestion system and bioavailability of TEs) which may have an impact on the methanogenesis pathway.
Figure 7 clearly shows that Fe, Co, Ni, Zn, W, and Mo have a direct relationship to related enzymes in the methanogenesis stage. In addition to these directly trace elements, some studies have proven that Se and Mn which have no direct relationship to the metalloenzymes also had positive effects on the digestion system. The further mechanism of this phenomenon needs to be revealed [
6,
14,
129,
130].
2.5.3.2. The requirements for trace elements in the digestion system
Trace elements are broadly used as additives in AD to relieve the accumulation of VFAs and increase methane yield [
130,
131]. As shown in
Figure 5, the main role of TEs is to promote the conversion of VFAs, H
2, and CO
2 to methane. In previous studies, the optimal demand for TEs has been studied and summarized [
2,
109,
122,
130,
132]. The concentration of TEs required in AD differs significantly and depends on the substrate type, digestion mode (mono or co-digestion) and operating temperature. Cai et al. [
124] reported that the concentration of TEs presents in livestock manure is usually higher than that exists in FW and in lignocellulosic biomass. Therefore, the digestion system of kitchen waste and lignocellulosic biomass is more prone to the lack of TEs than the AD of livestock manure [
2,
133]. The substrates with low concentrations of TEs can be co-digested with livestock manure to prevent the deficiency in TEs. Operating temperature is also an important factor affecting the demand for TEs. Takashima et al. [
134] reported that the required concentrations of TEs in thermophilic glucose digestion were significantly higher than those required for mesophilic digestion, which implies an increase in TEs requirements at thermophilic temperatures.
2.5.3.3. The bioavailability of trace element and the possibility to regulate
Based on the definition of ISO 11074 (2005), bioavailability is the degree to which elements are available for interaction in biological systems [
135,
136]. The concept of TEs bioavailability in AD has been poorly understood in the past [
122]. Although TEs are available in the digestion system, the stimulating effects of adding TEs still can be obtained which indicates that part of those TEs may be present in the non-bioavailable form [
137]. Therefore, it is vital to understand how TEs’ speciation affects their bioavailability to reduce the number of trace elements supplemented and maximize methanogenic activity. The pH and anions (S
2-, CO
32- and HPO
42-) have a capacity of forming or dissolving precipitates of TEs. Therefore, they are the two most important factors affecting the bioavailability of TEs [
124,
138]. For example, Marcato et al. [
135] reported Zn gradually dissolved from the slurry into solution when the pH decreased below about 6.0 and dissolved Zn accounted for 40% of the total Zn at pH 2.7. When sulfur-rich materials were used as AD substrates, a large amount of S
2- would be produced. The solubility constants for complexes between most of TEs and S
2- are low and the formation of those complexes is likely to reduce the bioavailability of TEs [
139]. Similarly, the concentration of CO
32- and HPO
42- can also affect the bioavailability of trace elements by forming complexes with TEs.
In order to regulate the bioavailability of TEs, chelating agents (Nitrilotriacetic acid (NTA), Ethylenediamine-N,N’-disuccinic acid ([S, S]-EDDS), Ethylenediaminetetraacetic acid (EDTA), soluble microbial products (SMPs), extracellular polymeric substances (EPS) and Yeast extract) have often been used to AD [
122]. Thanh et al. [
140] reported that the Fe-EDDS complex was effective in controlling the change of sulfide levels in the submerged anaerobic membrane bioreactor and then increased methane yields by 9.46%. Zhang et al. [
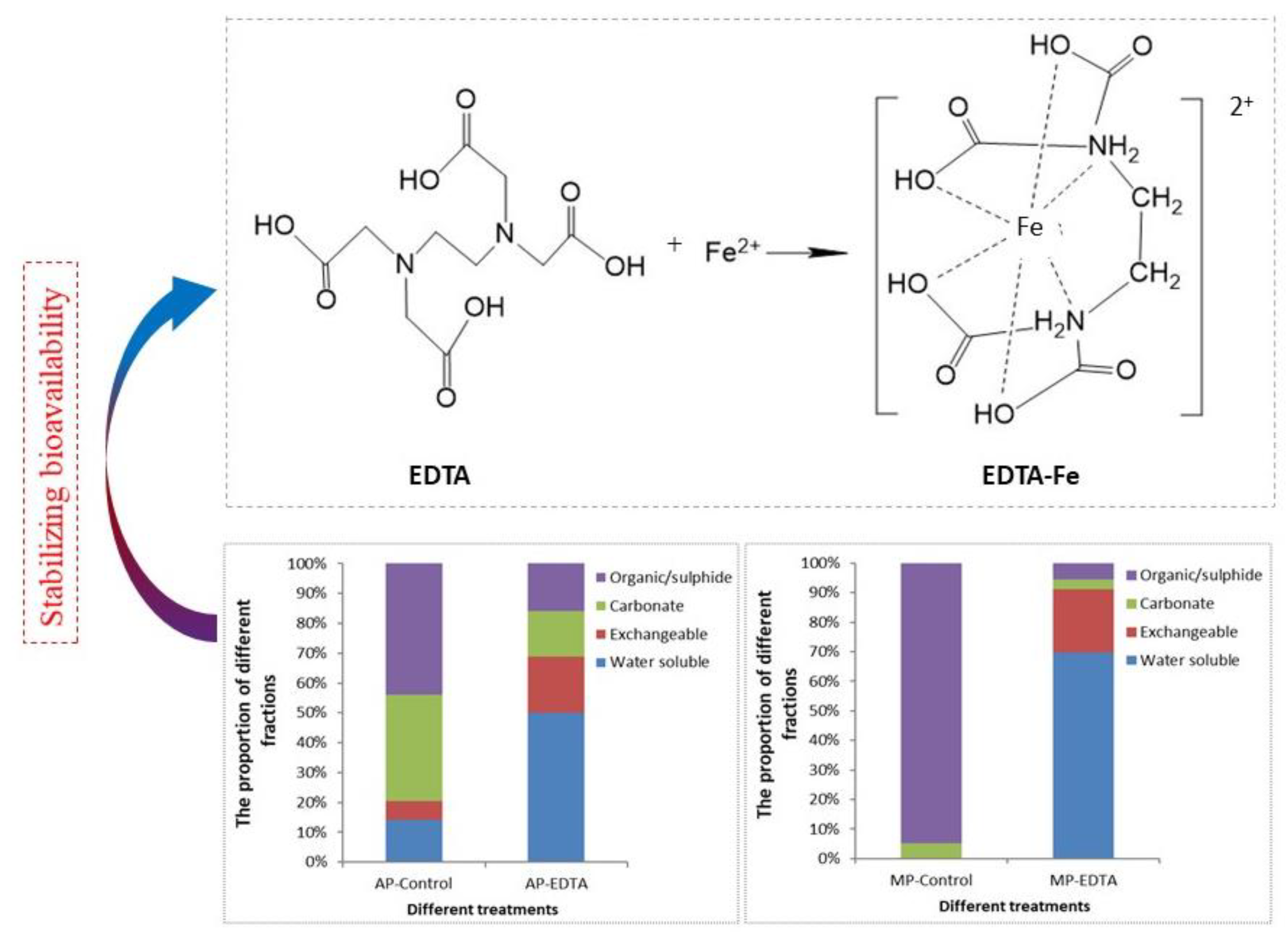
141] found that the addition of EDDS improved the bioavailability of TEs for microbial uptake and then amplified the stimulatory effects of TEs on the AD of FW. The optimum supplementation dose of TEs could be reduced by 50% when EDDS was added at a concentration of 20 mg/L. Similarly, Vintiloiu et al. [
142] showed that if TEs are complexed with EDTA before their supplementation, the concentration needed could be reduced by up to 75% compared to the dose required of non-complexed TEs. Cai et al. [
143] explored the effect of EDTA and Fe on acidogenic phase (MP) and methanogenic phase (MP) and found that EDTA could effectively improve the bioavailability of Fe by increasing the ratio of water-soluble fraction Fe and exchangeable fraction Fe in both the AP and the MP (As shown in
Figure 8). EDTA improves the bioavailability of TEs based on the chelation effect. Other kinds of ligands such as EDDS, NTA, SMPs and EPS also have a similar impact on TEs bioavailability due to chelation, binding or adsorption [
143]. Yeast extract, SMPs, and EPS are organic complexes produced by microorganisms and they are difficult to obtain in large quantities at low cost, so such ligands aren’t suitable to be applied in practice.
Although the supplementation of trace elements plays a positive role in AD, excessive addition will lead to toxic effects and the influence of ligands on the environment should also be taken seriously [
144,
145].
2.5.4. Biochar
Biochar is a material produced from biomass pyrolysis under a low oxygen concentration and high-temperature condition [
146]. As shown in
Figure 9, several inherent characteristics of biochar endue its ability to enhance AD performance such as rich in functional groups, a large surface area, porous and conductivity. These characteristics give biochar the functions of adsorbing toxic substances, enhancing DIET process and immobilizing microorganisms. Different sorption mechanisms exist because of the co-existed non-carbonized and carbonized fractions in the biochar surface. The adsorption process is facilitated by hydrogen bonding, ion exchange, electrostatic attraction and hydrophobic effect [
147].
Figure 9a and
Figure 9b shows the mechanism of adsorption for inorganic and organic pollutants, respectively. As fig 9a shown, there are four adsorption routes for inorganic pollutants including ion exchange, anionic metal attraction, cationic metal attraction, and precipitation. The existence of charged functional groups and metal ions that can be exchanged in the biochar surface provides the possibility of anionic, cationic metal attraction and ion exchange, respectively. As fig 9b shown, electrostatic, polar organic and Non-polar organic attraction are main adsorptions for organic pollutant adsorption. Electrostatic attraction is possible because of potential metal exchange with alkali metals (such as K
+ and Na
+) which is available on the biochar surface [
147]. Biochar surfaces are normally negatively charged because of the dissociation of oxygen-containing functional groups. This enables the electrostatic attraction of positively charged organic pollutants [
147,
148,
149]. However, Ahmad et al. 2014 reported that biochar became little polar because of the loss of the oxygen and hydrogen-containing functional groups when the pyrolytic temperature exceeds 450 °C [
149]. This affects the adsorption effect of the biochar on polar organic pollutants. Besides, hydrophobic sites that existed in biochar surface can attract non-polar organic pollutants.
In recently published work, it suggested that biochar could work as an electron carrier to favor DIET, thereby accelerating the production of methane [
150]. The mechanism of DIET was shown in
Figure 6 and
Figure 9d. The inhibitive effects of ammonia on AD can be relieved by its adsorption onto biochar, which was augmented by the large biochar surface area and functional groups [
147,
151]. In addition, as
Figure 9c shown, the abundant pores in biochar provide a microenvironment to immobilize microbes resulting in an improvement in AD performance [
151,
152]. The alkaline nature of biochar facilitates the reaction of CO
2 with H
2S increasing in the methane yield [
153]. Wang et al. [
150] reported that biochar addition could reduce the concentration of VFAs, which reflected the buffering capacity of biochar in AD. Furthermore, the addition of 20 g/L biochar to wastewater AD systems could reduce the environmental risk of antibiotic resistance genes by adsorbing antibiotics [
146].
Although biochar has a good effect on AD, its application in large-scale biogas projects remains limited because it can be used only once - it is difficult to reuse. This results in a high cost.
3. Conclusions and recommendations for future research
3.1. Conclusions
Given the advantages and disadvantages of pretreatment, combined biological pretreatment with physical pretreatment or chemical pretreatment is the most promising pretreatment model due to complementary advantages. Co-digestion and recirculation have multiple positive effects on balancing water, substance, and microorganism. With the diversification of digestion feedstock, co-digestion should be applied first when the type and amount of raw materials are sufficient due to its less negative impact on environment. Microaerobic technology can achieve a delicate balance between bacteria and methanogens and its advantages are obvious, especially when the digestion substrate is difficult to hydrolyze (mainly lignocellulosic mass). Additives technology can be as a complementary strategy of other strategies. Cheap, reusable and effective additives are still scarce which limits their application in large-scale biogas projects. To obtain sustainable development, the potential impact of those strategies on the environment and agriculture must be considered.
3.2. Recommendations for future research
A large amount of literature has reported the effects of different strategies on the anaerobic digestion system, and sometimes many excellent results have been obtained. Nevertheless, it still needs further research to accelerate the application of these strategies in large-scale project.
Both pretreatment and additive strategies involve additional substance or energy, therefore more reasonable models should be developed considering practicality, economy, and sustainability of the entire biogas industry chain. Some of pretreatment and additive strategies still stay at the laboratory level, such as microbial or enzyme additives, microbial pretreatment. The initial investment and additional operating costs can’t be ignored when those strategies were considered to be applied in large scale project. Therefore, larger-scale experiments should be done to evaluate their economy and practicality in further research. The characteristics and functions of biochar obtained under different conditions such as hydrolysis temperature and raw material should be further explored to achieve the ideal effect considering different purposes such as adsorption and high specific surface area. The bioavailability of TEs has not been taken seriously, and the change law of TEs bioavailability under various digestion conditions still needs to be further explored. The value of adding biochar and TEs on utilization of biogas digestate also need evaluated further.
Co-digestion and recirculation are very practical strategies. The most important point of these two strategies is promoting the balance of substances environment in the reactor. Both of strategies should adapt to local conditions and consider the match of substrates.
The substrates of anaerobic digestion are gradually diversifying. Besides traditional digestion substrate (mainly livestock manure), lignocellulosic biomass (crop straw, microalgae and energy grass) also attracted broad interest of researchers. The hydrolysis stage of lignocellulosic biomass is limited-speed step in anaerobic. Therefore, microaerobic technology should be applied first when digestion substrates are difficult to hydrolyze. In order to obtain optimal condition for hydrolytic fermentation bacteria and methanogenic archaea, two-stage digestion mode has obvious advantage and should be studied further, especially for microaerobic control under different operating conditions (substrate, reactor types, temperature etc.).
Author Contributions
Conceptualization, X.L and Z.W.; methodology, Y.H. and Y.W.; software, S.W. and Z.Z.; validation, Y.C. and S.W.; formal analysis, Y.C and S.W; investigation, Z.W. Y.H. and Y.W.; resources, S.W.; data curation, Z.Z.; writing - original draft preparation, X.L. and Y.H.; writing - review and editing, Z.W. and Y.C.; visualization, J.X.; supervision, H.Y.; project administration, S.W.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (52200178; 32300092), National Engineering Research Center of Solid-state Brewing (GFGS-2023000501; 2023HX02), Henan Province Science and Technology Research (222102310142), the Key Research Project of the Higher Education Institutions of Henan Province, China (22A530010), and the Key Program for Collaborative and Innovation of Nanyang, Henan Province, China (21XTCX21001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Interested parties can request data from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
WRF, white-rot fungi; BRF, brown-rot fungi; SRF, soft-rot fungi; AC, activated carbon; AD, anaerobic digestion; C/N, carbon-to-nitrogen; COD, chemical oxygen demand; CSTR, continuously-stirred tank reactor; DIET, direct interspecies electron transfer; EDTA, ethylenediaminetetraacetic acid, EPS extracellular polymeric substances; IHT, interspecies hydrogen transfer; ISR, inoculum substrate ratio; OLR, organic loading rate; ORP, oxidation-reduction potential; sCOD, soluble chemical oxygen demand; SMPs, soluble microbial products; SOB, sulfide/sulfur-oxidizing bacteria; SRB, sulfate-reducing bacteria; [S, S]-EDDS, ethylenediamine-N,N’-disuccinic acid; TEs, trace elements; TS, total solids; UASB, up-flow anaerobic sludge bed reactor; VFA, volatile fatty acid; VS, volatile solids; FW, Food waste; WAS, Waste activated sludge; WWTP, Wastewater treatment plants; AOE, anti-oxidative enzymes; OFMSW, Organic Fraction of Municipal Solid Waste; MSW, municipal solid waste; SAOB, syntrophic acetate oxidizing bacteria. CA, carbonic anhydrase; Ech/Eha/Ehb/Mbh, energy-converting hydrogenase; Cdh, carbon monoxide dehydrogenase/acetyl-CoA synthase; Fd, ferredoxin; Frh, F420-reducing hydrogenase; Fmd/Fwd, Mo/W formylmethanofuran dehydrogenase; Hdr, heterodisulfide reductase; Hmd, Ni-free Fe hydrogenase; Mta, methanol-coenzyme M methyltransferase; Mcr, methyl coenzyme M reductase; Vh(o/t)/Mvh, Ni-Fe hydrogenase; Mtr, CH3-H4M(S)PT-coenzyme M methyltransferase.
References
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Improving the sustainability of organic waste management practices in the food-energy-water nexus: A comparative review of anaerobic digestion and composting. Renew Sustain Energy Rev. 2018, 89, 151–167. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour Technol. 2018b, 250, 163–170. [Google Scholar] [CrossRef]
- Wang, ZY.; Li, X.; Siddiqui, MA.; Liu, H.; Zhou, T.; Zheng, L.; Huang, SY.; Gao, L.; Lin, C.S.K.; Wang, Q.L. Effect of humic substances on the anaerobic digestion of secondary sludge in wastewater treatment plants: a review. Environ Chem Lett. 2023, 21, 3023–3040. [Google Scholar] [CrossRef]
- Neshat, SA.; Mohammadi, M.; Najafpour, GD.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew Sustain Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Liu, H. Enhanced methane production and syntrophic connection between microorganisms during semi-continuous anaerobic digestion of chicken manure by adding biochar. J Clean Prod. 2019, 240, 118178. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Çalli, B. Long-term influence of trace element deficiency on anaerobic monodigestion of chicken manure. J Environ Manage. 2018a, 223, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Khanal, SK. A little breath of fresh air into an anaerobic system: How Microaeration facilitates anaerobic digestion process. Biotechnol Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite Triggering Enhanced Direct Interspecies Electron Transfer: A Scavenger for the Blockage of Electron Transfer in Anaerobic digestion of High-Solids Sewage Sludge. Environ Sci Technol. 2018a, 52, 7160–7169. [Google Scholar] [CrossRef]
- Park, JH.; Kang, HJ.; Park, KH.; Park, HD. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour Technol. 2018a, 254, 300–311. [Google Scholar] [CrossRef]
- Hashemi, B.; Sarker, S.; Lamb, J.J.; Lien, K.M. Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J Clean Prod. 2021, 288, 125447. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Yuan, X.; Zhu, W.; Wang, X.; Cheng, X.; Cui, Z. The effect of mixing intensity on the performance and microbial dynamics of a single vertical reactor integrating acidogenic and methanogenic phases in lignocellulosic biomass digestion. Bioresour Technol. 2017a, 238, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.C.; Liu, H.B.; Zhang, H.D.; Xu, S.Y.; Lichtfouse, E.; Yun, Y.B. Anaerobic digestion and recycling of kitchen waste: a review. Environ Chem Lett. 2022, 20, 1745–1762. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, AI.; Ai, P.; Zhou, ZB.; Meng, FA.; Rooney, DW. Bioenergy production from chicken manure: a review. Environ Chem Lett. 2023, 21, 2707–2727. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Sürmeli, RO.; Çalli, B. Anaerobic digestion of chicken manure by a leach-bed process coupled with side-stream membrane ammonia separation. Bioresour Technol. 2018, 258, 41–47. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, B.; Ma, X.; Zhu, W.; Wang, X.; Cheng, X.; Cui, Z. Enhancing the anaerobic digestion of lignocellulose of municipal solid waste using a microbial pretreatment method. Bioresour Technol. 2014, 154, 1–9. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, K.; Zhang, Y.; Zheng, Z.; Cai, Y.; Wen, B.; Cui, Z.; Wang, X. Improving the methane yield of maize straw: Focus on the effects of pretreatment with fungi and their secreted enzymes combined with sodium hydroxide. Bioresour Technol. 2018a, 250, 204–213. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of feed/inoculum ratios and waste cooking oil content on the mesophilic anaerobic digestion of food waste. Waste Manag. 2018a, 73, 156–164. [Google Scholar] [CrossRef]
- Zhang, H.W.; Yao, Y.Q.; Deng, J.; Zhang, J.L.; Qiu, Y.J.; Li, G.F.; Liu, J. Hydrogen production via anaerobic digestion of coal modified by white-rot fungi and its application benefits analysis. Renew Sust Energ Rev. 2022, 157, 112091. [Google Scholar] [CrossRef]
- Rouches, E.; Herpoël-Gimbert, I.; Steyer, JP.; Carrere, H. Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: A review. Renew Sustain Energy Rev. 2016, 59, 179–198. [Google Scholar] [CrossRef]
- Wang, Z.W.; Deuss, P.J. The isolation of lignin with native-like structure. Biotechnol Adv. 2023, 68, 108230. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.P.; Basaglia, M.; Casella, S.; Favaro, L. Rice waste streams as a promising source of biofuels: feedstocks, biotechnologies and future perspectives. Renew Sust Energ Rev. 2022, 167, 112673. [Google Scholar] [CrossRef]
- Brémond, U.; Buyer, R.D.; Steyer, J.P.; Bernet, N.; Carrere, H. Biological pretreatments of biomass for improving biogas production: an overview from lab scale to full-scale. Renew Sustain Energy Rev. 2018, 90, 583–604. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Van, Hulle, S.W.H.; Raes, K. Impact of enzymatic pretreatment on corn stover degradation and biogas production. Bioresour Technol. 2014, 173, 59–66. [Google Scholar] [CrossRef]
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pretreatments on the methane yield from the anaerobic digestion of switchgrass. Biomass-Bioenergy. 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.H.; Raes, K. Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef]
- Leca, E.; Zennaro, B.; Hamelin, J.; Carrère, H.; Sambusiti, C. Use of additives to improve collective biogas plant performances: A comprehensive review. Biotechnol. 2023, 65, 108129. [Google Scholar] [CrossRef]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic waste valorisation strategy through enzyme and biogas production. Bioresour Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, G.; Li, J.; Zhao, Z.; Kang, X. Effect of endogenous hydrolytic enzymes pretreatment on the anaerobic digestion of sludge. Bioresour Technol. 2013, 146, 758–761. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, YJ.; Meng, SJ.; Kiran, EU.; Liu, Y. Enzymatic pretreatment of activated sludge, food waste and their mixture for enhanced bioenergy recovery and waste volume reduction via anaerobic digestion. Appl Energy. 2016, 179, 1131–1137. [Google Scholar] [CrossRef]
- Odnell, A.; Recktenwald, M.; Stensén, K.; Jonsson, BH.; Karlsson, M. Activity, lifetime and effect of hydrolytic enzymes for enhanced biogas production from sludge anaerobic digestion. Water Res. 2016, 103, 462–471. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Enhanced production of biohydrogen from dairy waste activated sludge pretreated using multi hydrolytic garbage enzyme complex and ultrasound-optimization. Energ Convers Manage. 2018, 164, 277–287. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Solubilization of waste activated sludge using a garbage enzyme produced from different pre- consumer organic waste. RSC Adv. 2015, 5, 51421–51427. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Study on optimization of process parameters for enhancing the multi-hydrolytic enzyme activity in garbage enzyme produced from preconsumer organic waste. Bioresour Technol. 2017, 226, 200–210. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Carrère, H.; Garfí, M.; Ferrer, I. Enhancement of microalgae anaerobic digestion by thermo-alkaline pretreatment with lime (CaO). Algal Res. 2017a, 24, 199–206. [Google Scholar] [CrossRef]
- Dong, C.; Chen, J.; Guan, R.; Li, X.; Xin, Y. Dual-frequency ultrasound combined with alkali pretreatment of corn stalk for enhanced biogas production. Renew Energy. 2018, 127, 444–451. [Google Scholar] [CrossRef]
- Chacana, J.; Alizadeh, S.; Labelle, M.; Laporte, A.; Hawari, J.; Barbeau, B.; Comeau, Y. Effect of ozonation on anaerobic digestion sludge activity and viability. Chemosphere. 2017, 176, 405–411. [Google Scholar] [CrossRef]
- Kang, X.; Sun, Y.; Li, L.; Kong, X.; Yuan, Z. Improving methane production from anaerobic digestion of Pennisetum Hybrid by alkaline pretreatment. Bioresour Technol. 2018, 255, 205–212. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew Energy. 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Zhang, L.; Laloo, A.; Duan, H.; Batstone, DJ.; Yuan, Z. Free nitrous acid pre-treatment of waste activated sludge enhances volatile solids destruction and improves sludge dewaterability in continuous anaerobic digestion. Water Res. 2018, 130, 13–19. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem Eng J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Yao, Y.; Bergeron, AD.; Davaritouchaee, M. Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew Energy. 2018, 115, 139–148. [Google Scholar] [CrossRef]
- Passos, F.; Ortega, V.; Donoso-Bravo, A. Thermochemical pretreatment and anaerobic digestion of dairy cow manure: Experimental and economic evaluation. Bioresour Technol. 2017, 227, 239–246. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Eskicioglu, C.; Garfí, M.; Carrère, H.; Ferrer, I. Anaerobic co-digestion of microalgal biomass and wheat straw with and without thermo-alkaline pretreatment. Bioresour Technol. 2017b, 237, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zan, FX.; Hao, TW.; Khanal, SK.; Chen, GH. Sewage sludge digestion beyond biogas: Electrochemical pretreatment for biochemicals. Water Res. 2022, 208, 117839. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Li, Y.; Zhao, Y. Combined electrical-alkali pretreatment to increase the anaerobic hydrolysis rate of waste activated sludge during anaerobic digestion. Appl Energ. 2014, 128, 93–102. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew Sustain Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Song, Z.L.; Liu, X.F.; Ya, Z.Y.; Yuan, Y.X.; Liao, Y.Z. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS One. 2014, 9, e93801. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Cao, J.; Chang, Z.Z.; Ye, X.M.; Du, J. Effect of oganic acids pretreatment on physico-chemical property and biogas production of wheat straw. Acta Energiae Solaris Sin. 2015, 36, 2559–2564. [Google Scholar]
- Liu, J.; Yang, M.; Zhang, J.; Zheng, J.; Xu, H.; Wang, Y.; Wei, Y. A comprehensive insight into the effects of microwave-H2O2 pretreatment on concentrated sewage sludge anaerobic digestion based on semi-continuous operation. Bioresour Technol. 2018, 256, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Siles, JA.; Martín, MA.; Chica, AF.; Estevez-Pastor, FS.; Toro-Baptista, E. Effect of microwave pretreatment on semi-continuous anaerobic digestion of sewage sludge. Renew Energy. 2018, 115, 917–925. [Google Scholar] [CrossRef]
- Bin Kabir, S.; Khalekuzzaman, M.; Hossain, N.; Jamal, M.; Alam, MA.; Abomohra, A. Progress in biohythane production from microalgae-wastewater sludge co-digestion: An integrated biorefinery approach. Biotechnol. 2022, 57, 107933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, B.; Yao, FB.; He, L.; Chen, F.; Ma, YH.; Shu, XY.; Hou, KJ.; Wang, DB.; Li, XM. Biogas production from anaerobic co-digestion of waste activated sludge: co-substrates and influencing parameters. Rev Environ Sci Biotechnol. 2019, 18, 771–793. [Google Scholar] [CrossRef]
- Cesaro, A.; Velten, S.; Belgiorno, V.; Kuchta, K. Enhanced anaerobic digestion by ultrasonic pretreatment of organic residues for energy production. J Clean Prod. 2014, 74, 119–124. [Google Scholar] [CrossRef]
- Chen, H.; Xia, A.; Zhu, X.; Huang, Y.; Zhu, XQ.; Liao, Q. Hydrothermal hydrolysis of algal biomass for biofuels production: A review. Bioresource Technology. 2022, 344, 126213. [Google Scholar] [CrossRef]
- Rajput, AA.; Sheikh, Z.; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J Environ Manage. 2018, 221, 45–52. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem Eng J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Maurya, DP.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. Biotech. 2015, 5, 597–609. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, L.L.; Fan, Y.M.; Wang, Z.G. The case-dependent lignin role in lignocellulose nanofibers preparation and functional application-A review. Green Energy Environ. 2023, 8, 1553–1566. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H. Enhancement of methane production with horse manure supplement and pretreatment in a full-scale biogas process. Energy. 2014, 73, 523–530. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl Energ. 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Zou, H.; Chen, Y.; Shi, J.; Zhao, T.; Yu, Q.; Yu, S.; Shi, D.; Chai, H.; Gu, L.; He, Q.; Ai, H. Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester. Bioresour Technol. 2018, 268, 371–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, F.; Yu, J.; Cai, Y.; Luo, X.; Cui, Z.; Hu, Y.; Wang, X. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour Technol. 2018b, 269, 143–152. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Sun, Y.; Yuan, Z.; Lv, P.; Kang, X.; Zhang, Y.; Yang, G. Influence of the Feedstock Ratio and Organic Loading Rate on the Co-digestion Performance of Pennisetum hybrid and Cow Manure. Energ Fuel. 2018b, 32, 5171–5180. [Google Scholar] [CrossRef]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J Clean Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Sole-Bundo, M.; Passos, F.; Romero-Guiza, M.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew Sustain Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Lee, J.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Three-stage anaerobic codigestion of food waste and horse manure. Sci Rep. 2017, 7, 1269. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, B.; Zhang, Q.; Florentino, A.; Xu, R.; Zhang, Y.; Liu, Y. Co-digestion of blackwater with kitchen organic waste: Effects of mixing ratios and insights into microbial community. J Clean Prod. 2019, 236, 117703. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, JP. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew Sustain Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Kurade, M.B.; Saha, S.; Salama, E.S.; Patil, S.M.; Govindwar, S.P.; Jeon, B.H. Acetoclastic methanogenesis led by Methanosarcina in anaerobic codigestion of fats, oil and grease for enhanced production of methane. Bioresour Technol. 2019, 272, 351–359. [Google Scholar] [CrossRef]
- Rajendran, K.; Mahapatra, D.; Venkatraman, AV.; Muthuswamy, S.; Pugazhendhi, A . Advancing anaerobic digestion through two-stage processes: Current developments and future trends. Renew Sust Energ Rev. 2020, 123, 109746. [Google Scholar] [CrossRef]
- Gottardo, M.; Micolucci, F.; Bolzonella, D.; Uellendahl, H.; Pavan, P. Pilot scale fermentation coupled with anaerobic digestion of food waste - Effect of dynamic digestate recirculation. Renew Energy. 2017, 114, 455–463. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef]
- Pezzolla, D.; Maria, FD.; Zadra, C.; Massaccesi, L.; Sordi, A.; Gigliotti, G. Optimization of solid-state anaerobic digestion through the percolate recirculation. Biomass Bioenerg. 2017, 96, 112–118. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, LH.; Svensson, K.; Linjordet, R.; Horn, SJ. Anaerobic digestion of food waste-Effect of recirculation and temperature on performance and microbiology. Water Res. 2016, 96, 246–254. [Google Scholar] [CrossRef]
- Ni, P.; Lyu, T.; Sun, H.; Dong, R.; Wu, S. Liquid digestate recycled utilization in anaerobic digestion of pig manure: Effect on methane production, system stability and heavy metal mobilization. Energy. 2017, 141, 1695–1704. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Li, R.; Nelles, M.; Stinner, W.; Li, Y. Effects of Percolate Recirculation on Dry Anaerobic Co-digestion of Organic Fraction of Municipal Solid Waste and Corn Straw. Energ Fuel. 2017, 31, 12183–12191. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: a review. Rev Environ Sci Biotechnol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Sasidhar, KB.; Somasundaram, M.; Ekambaram, P.; Arumugam, SK.; Nataraj, G.; Murugan, MA. A critical review on the effects of pneumatic mixing in anaerobic digestion process. J Clean Prod. 2022, 378, 134513. [Google Scholar] [CrossRef]
- Krayzelova, LP.; Mampaey, KE.; Vannecke, TPW.; Bartacek, J.; Jenicek, P.; Volcke, EIP. Model-based optimization of Microaeration for biogas desulfurization in UASB reactor. Chem Eng J. 2017, 125, 171–179. [Google Scholar] [CrossRef]
- Ramos, I.; Fdz-Polanco, M. The potential of oxygen to improve the stability of anaerobic reactors during unbalanced conditions: results from a pilot-scale digester treating sewage sludge. Bioresour Technol. 2013, 140, 80–85. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei, Y.; Zhang, Q.; Zhang, J.; Lu, T.; Pei, Y. Enhancement of surfactant biodegradation with an anaerobic membrane bioreactor by introducing microaeration. Chemosphere. 2018, 208, 343–351. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, SK.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: current status and future perspectives. Bioresour Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Fu, S.; Wang, F.; Shi, X.; Guo, R. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem Eng J. 2016, 287, 523–528. [Google Scholar] [CrossRef]
- Wang, ZY.; Li, X.; Liu, H.; Zhou, T.; Qin, ZH.; Mou, JH.; Sun, J; Huang, SY.; Chaves, AV.; Gao, L. Bioproduction and applications of short-chain fatty acids from secondary sludge anaerobic fermentation: A critical review. Renew Sust Energ Rev. 2023, 183, 113502. [Google Scholar] [CrossRef]
- Xu, S.; Selvam, A.; Wong, JWC. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014, 34, 363–369. [Google Scholar] [CrossRef]
- Hanreich, A.; Schimpf, U.; Zakrzewski, M.; Schlüter, A.; Benndorf, D.; Heyer, R.; Rapp, E.; Pühler, A.; Reichl, U.; Klocke, M. Metagenome and metaproteome analyses of microbial communities in mesophilic biogas-producing anaerobic batch fermentations indicate concerted plant carbohydrate degradation. Syst Appl Microbiol. 2013, 36, 330–338. [Google Scholar] [CrossRef]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: a review. Biotechnol Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Díaz, I.; Donoso-Bravo, A.; Fdz-Polanco, M. Effect of microaerobic conditions on the degradation kinetics of cellulose. Bioresour Technol. 2011, 102, 10139–10142. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jenicek, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef]
- Horne, AJ.; Lessner, DJ. Assessment of the oxidant tolerance of Methanosarcina acetivorans. FEMS Microbiol Lett. 2013, 343, 13–19. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Zhu, M.; Lü, F.; Hao, LP.; He, PJ.; Shao, LM. Regulating the hydrolysis of organic wastes by micro-aeration and effluent recirculation. Waste Manag. 2009, 29, 2042–2050. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Yu, Q.; Zhang, Y. Ferroferric oxide triggered possible direct interspecies electron transfer between Syntrophomonas and Methanosaeta to enhance waste activated sludge anaerobic digestion. Bioresour Technol. 2018c, 250, 79–85. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Murphy, J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem Eng J. 2018b, 350, 681–691. [Google Scholar] [CrossRef]
- Park, J.H.; Seong, H.J.; Sul, W.J.; Jin, H.K.; Park, H.D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour Technol. 2018b, 259, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, DE.; Zhao, Z.; Woodard, TL.; Zhang, Y.; Sun, D.; Wang, L.; Nevin, KP.; Lovley, D. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017b, 115, 266–277. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Quan, X.; Zhao, H. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour Technol. 2016, 200, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron–graphite electrode. Chem Eng J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Gao, X.; Chen, H.; Xu, X.; Zhu, L. Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour Technol. 2018b, 268, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, MS.; Vila, J.; Mata-Alvarez, J.; Chimenos, JM.; Astals, S. The role of additives on anaerobic digestion: A review. Renew Sustain Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Tuesorn, S.; Wongwilaiwalin, S.; Champreda, V.; Leethochawalit, M.; Nopharatana, A.; Techkarnjanaruk, S.; Chaiprasert, P. Enhancement of biogas production from swine manure by a lignocellulolytic microbial consortium. Bioresour Technol. 2013, 144, 579–586. [Google Scholar] [CrossRef]
- Weiss, S.; Tauber, M.; Somitsch, W.; Meincke, R.; Müller, H.; Berg, G.; Guebitz, GM. Enhancement of biogas production by addition of hemicellulolytic bacteria immobilised on activated zeolite. Water Res. 2010, 44, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ryals, A.; Schideman, L.; Li, P.; Wilkinson, H.; Wagner, R. Improving anaerobic digestion of a cellulosic waste via routine bioaugmentation with cellulolytic microorganisms. Bioresour Technol. 2015, 189, 62–70. [Google Scholar] [CrossRef]
- Lü, F.; Li, T.; Wang, T.; Shao, L.; He, P. Improvement of sludge digestate biodegradability by thermophilic bioaugmentation. Appl Microbiol Biotechnol. 2014, 98, 969–977. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sun, Y.; Yuan, Z. Bioaugmentation strategy for enhancing anaerobic digestion of high C/N ratio feedstock with methanogenic enrichment culture. Bioresour Technol. 2018c, 261, 188–195. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Li, L.; Sun, Y. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manage. 2018d, 2018, 744–751. [Google Scholar] [CrossRef]
- Fotidis, IA.; Karakashev, D.; Angelidaki, I. Bioaugmentation with an acetate-oxidising consortium as a tool to tackle ammonia inhibition of anaerobic digestion. Bioresour Technol. 2013, 146, 57–62. [Google Scholar] [CrossRef]
- Tian, H.; Mancini, E.; Treu, L.; Angelidaki, I.; Fotidis, IA. Bioaugmentation strategy for overcoming ammonia inhibition during biomethanation of a protein-rich substrate. Chemosphere. 2019a, 231, 415–422. [Google Scholar] [CrossRef]
- Fotidis, IA.; Wang, H.; Fiedel, NR.; Luo, G.; Karakashev, DB.; Angelidaki, I. Bioaugmentation as a solution to increase methane production from an ammoniarich substrate. Environ Sci Technol. 2014, 48, 7669–7676. [Google Scholar] [CrossRef]
- Tian, H.; Yan, M.; Treu, L.; Angelidaki, I.; Fotidis, IA. Hydrogenotrophic methanogens are the key for a successful bioaugmentation to alleviate ammonia inhibition in thermophilic anaerobic digesters. Bioresour Technol. 2019b, 293, 122070. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Liu, C.; Zhang, R.; Liu, G. Mitigation of ammonia inhibition through bioaugmentation with different microorganisms during anaerobic digestion: Selection of strains and reactor performance evaluation. Water Res. 2019, 155, 214–224. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Fdz-Polanco, M. Anaerobic co-digestion of sewage sludge and grease trap: assessment of enzyme addition. Process Biochem. 2013, 48, 936–940. [Google Scholar] [CrossRef]
- Thanh, PM.; Ketheesan, B.; Zhou, Y.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. The distribution of elements in cells. Coord Chem Rev. 2000, 200-202, 247–248. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, X.; Cui, Z. A critical review on trace elements and bioavailability in anaerobic digestion system. Journal of China Agricultural University. 2017b, 22, 1–11. (In Chinese) [Google Scholar]
- Hochheimer, A.; Hedderich, R.; Thauer, RK. The formylmethanofuran dehydrogenase isoenzymes in Methanobacterium wolfei and Methanobacterium thermoautotrophicum: induction of the molybdenum isoenzyme by molybdate and constitutive synthesis of the tungsten isoenzyme. Arch Microbiol. 1998, 170, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Harrop, TC.; Mascharak, PK. Structural and spectroscopic models of the Acluster of acetyl coenzyme a synthase/carbon monoxide dehydrogenase: nature’s Monsanto acetic acid catalyst. Coord Chem Rev. 2005, 249, 3007–3024. [Google Scholar] [CrossRef]
- Ferry, JG. The chemical biology of methanogenesis. Planet Space Sci. 2010, 58, 1775–1783. [Google Scholar] [CrossRef]
- Glass, JB.; Orphan, VJ. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front Microbiol. 2012, 3, 1–20. [Google Scholar] [CrossRef]
- Cai, Y.; Hua, B.; Gao, L.; Hu, Y.; Yuan, X.; Cui, Z.; Zhu, W.; Wang, X. Effects of adding trace elements on rice straw anaerobic mono-digestion: Focus on changes in microbial communities using high-throughput sequencing. Bioresour Technol. 2017a, 239, 454–463. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Zhao, Y.; Zhang, Y.; Guo, S.; Cui, Z.; Wang, X. Effects of molybdenum, selenium and manganese supplementation on the performance of anaerobic digestion and the characteristics of bacterial community in acidogenic stage. Bioresour Technol. 2018a, 266, 166–175. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Mahdy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe2+ and Ni2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Choong, YY.; Norli, I.; Abdullah, AZ.; Yhaya, MF. Impacts of trace element supplementation on the performance of AD process: A critical review. Bioresour Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Shimada, K.; Speece, RE. Minimum Requirements for trace metals (iron, nickel, cobalt, and zinc) in thermophilic and mesophilic methane fermentation from glucose. Water Environ Res. 2011, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Marcato, C.; Pinelli, E.; Cecchi, M.; Winterton, P.; Guiresse, M. Bioavailability of Cu and Zn in raw and anaerobically digested pig slurry. Ecotoxicol Environ Saf. 2009, 72, 1538–1544. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Zhao, Y.; Zhao, X.; Zheng, Z.; Wen, B.; Cui, Z.; Wang, X. A new perspective of using sequential extraction: To predict the deficiency of trace elements during anaerobic digestion. Water Res. 2018c, 140, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zandvoort, MH.; van Hullebusch, ED.; Fermoso, FG.; Lens, P.N.L. Trace metals in anaerobic granular sludge reactors: bioavailability and dosing strategies. Eng Life Sci. 2006, 6, 293–301. [Google Scholar] [CrossRef]
- Frunzo, L.; Fermoso, FG.; Luongo, V.; Mattei, MR.; Esposito, G. ADM1-based mechanistic model for the role of trace elements in anaerobic digestion processes. J Environ Manage. 2019, 241, 587–602. [Google Scholar] [CrossRef]
- Gustavsson, J.; Yekta, SS.; Sundberg, C.; Karlsson, A.; Ejlertsson, J.; Skyllberg, U.; Svensson, BH. Bioavailability of cobalt and nickel during anaerobic digestion of sulfur-rich stillage for biogas formation. Appl Energ. 2013, 112, 473–477. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Stuckey, D.C.; Zhou, Y. Dosing of Ethylenediamine-N,N’-disuccinic acid (EDDS) to improve the bioavailability of Fe2+ in the presence of sulfide in a submerged anaerobic membrane bioreactor. Chem Eng J. 2017, 330, 175–182. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Boxriker, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T.; Mathies, E.; Ramhold, D. Effect of ethylenediaminetetraacetic acid (EDTA) on the bioavailability of trace elements during anaerobic digestion. Chem Eng J. 2013, 223, 436–441. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, K.; Zheng, Z.; Zhang, Y.; Guo, S.; Zhao, X.; Cui, Z.; Wang, X. Effects of adding EDTA and Fe2+ on the performance of reactor and microbial community structure in two simulated phases of anaerobic digestion. Bioresour Technol. 2019, 275, 183–191. [Google Scholar] [CrossRef]
- Chen, J.; Steele, T.W.J.; Stuckey, D.C. Stimulation and inhibition of anaerobic digestion by nickel and cobalt: a rapid assessment using the resazurin reduction assay. Environ Sci Technol. 2016, 50, 11154–11163. [Google Scholar] [CrossRef]
- Serrano, A.; Pinto-Ibieta, F.; Bra, AFgaM.; Jeison, D.; Borja, R.; Fermoso, F.G. Risks of using EDTA as an agent for trace metals dosing in anaerobic digestion of olive mill solid waste. Environ Technol. 2017, 38, 3137–3144. [Google Scholar] [CrossRef]
- Sun, W.; Gu, J.; Wang, X.; Qian, X.; Tuo, X. Impacts of biochar on the environmental risk of antibiotic resistance genes and mobile genetic elements during AD of cattle farm wastewater. Bioresour Technol. 2018, 256, 342–349. [Google Scholar] [CrossRef]
- Masebinu, SO.; Akinlabi, ET.; Muzenda, E.; Aboyade, AO. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew Sustain Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Qambrani, NA.; Rahman, MM.; Won, S.; Shim, S.; Ra, C. Biochar properties and ecofriendly applications for climate change mitigation, waste management, and wastewater treatment: a review. Renew Sustain Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, AU.; Lim, JE.; Zhang, M.; Bolan, N.; Mohan, D.; et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef]
- Fagbohungbe, M.; Herbert, B.; Hurst, L.; Li, H.; Usmani, S.; Semple, K. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, JL.; Ignacio-de Leon, PAA.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J Clean Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.; Zou, L.; Qian, G.; Xu, Z. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J Clean Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies. 2018, 11, 107. [Google Scholar] [CrossRef]
- Guan, R.; Li, X.; Wachemo, AC.; Yuan, H.; Liu, Y.; Zou, D. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci Total Environ. 2018, 637–638, 9–17. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Sergent, M.; Raouche, S.; Carrere, H. Influence of white-rot fungus Polyporus brumalis BRFM 985 culture conditions on the pretreatment efficiency for anaerobic digestion of wheat straw. Biomass Bioenerg. 2018, 110, 75–79. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Wachemo, AC.; Li, X. Effects of liquid fraction of digestate recirculation on system performance and microbial community structure during serial anaerobic digestion of completely stirred tank reactors for corn stover. Energy. 2018e, 160, 309–317. [Google Scholar] [CrossRef]
- Luo, L.; Wong, J.W.C. Enhanced food waste degradation in integrated two-phase anaerobic digestion: Effect of leachate recirculation ratio. Bioresour Technol. 2019, 291, 121813. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, SJ.; Shahzad, H.M.A. Impact of sludge recirculation ratios on the performance of anaerobic membrane bioreactor for wastewater treatment. Bioresour Technol. 2019, 288, 121473. [Google Scholar] [CrossRef]
- Duarte, M.S.; Silva, S.A.; Salvador, A.F.; Cavaleiro, A.J.; Stams, A.J.M.; Alves, M.M.; Pereira, M.A. Insight into the Role of Facultative Bacteria Stimulated by Microaeration in Continuous Bioreactors Converting LCFA to Methane. Environ Sci Technol. 2018, 52, 6497–6507. [Google Scholar] [CrossRef] [PubMed]
- Jenicek, P.; Celis, CA.; Krayzelova, L.; Anferova, N.; Pokorna, D. Improving products of anaerobic sludge digestion by microaeration. Water Sci Technol. 2014, 69, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Diak, J.; Örmeci, B.; Kennedy, KJ. Effect of micro-aeration on anaerobic digestion of primary sludge under septic tank conditions. Bioprocess Biosyst Eng. 2013, 36, 417–424. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).