Submitted:
04 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
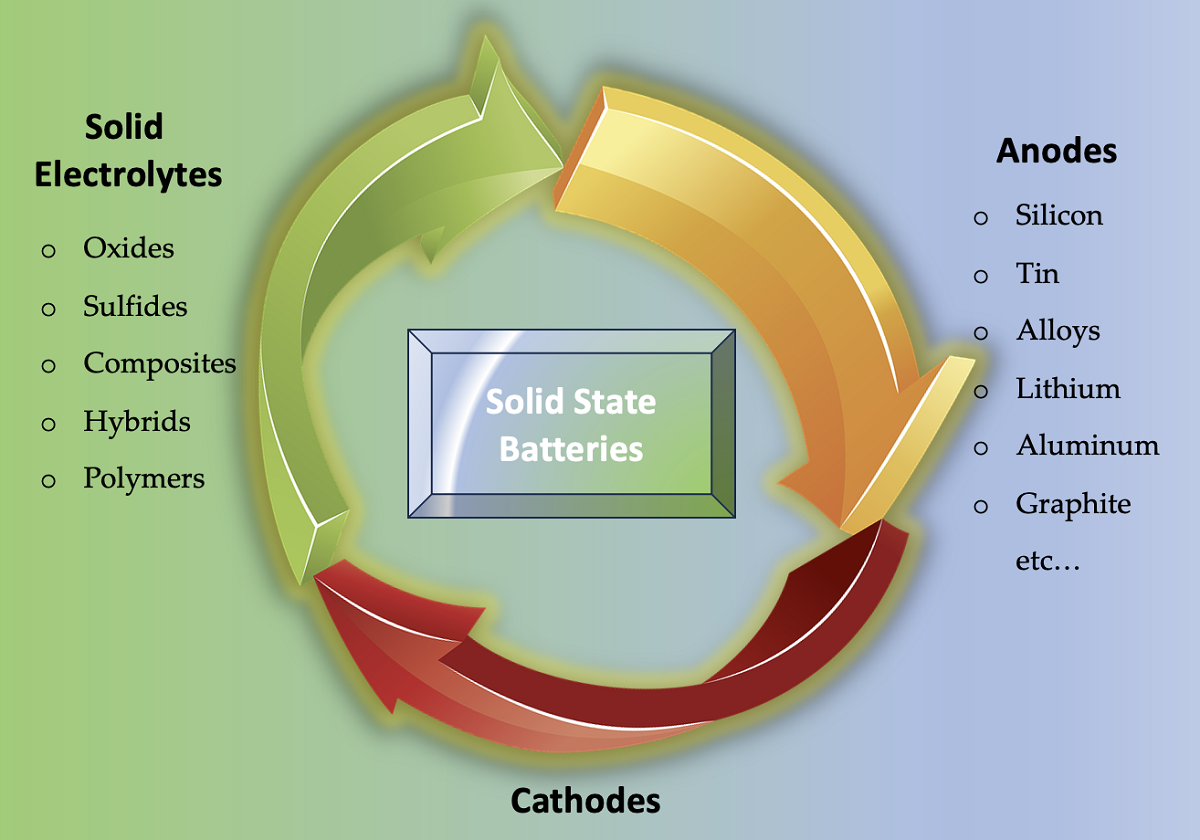
Keywords:
1. Introduction
1.1. Contextualizing the Shift to Solid-State Energy Storage
1.2. The Constraints of Liquid Electrolyte Lithium-Ion Batteries
1.3. Advancements and Concepts of Solid-State Batteries (SSBs)
1.4. Advantages Relative to Conventional Battery Technologies
1.5. Technological Hurdles in the Adoption of Solid-State Batteries
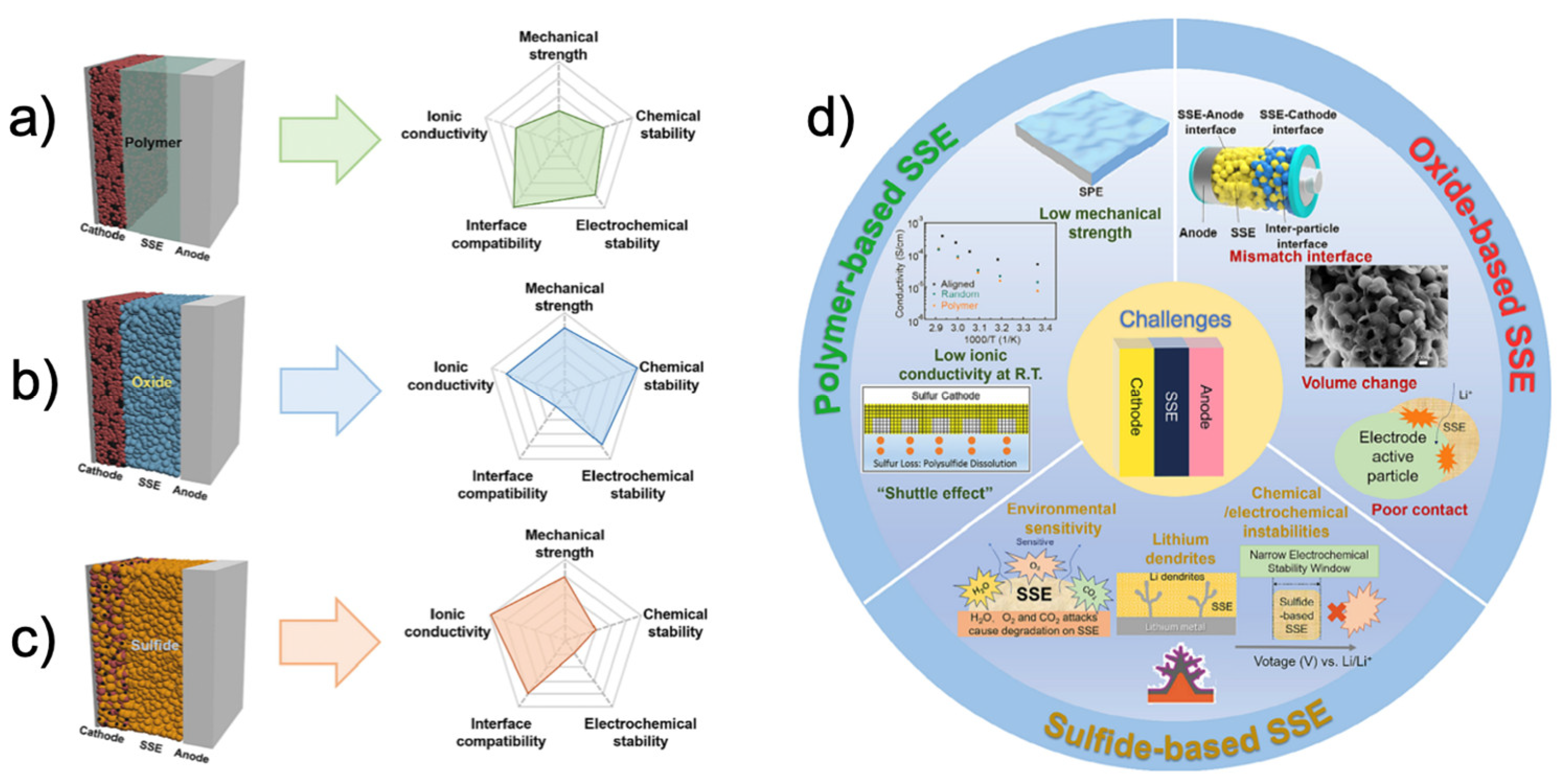
2. Solid Electrolytes: The Heart of Solid-State Batteries
 |
- o Oxide Electrolytes: LIPON, NASICON and Garnet Type
- o Sulfide Electrolytes: LPS and Argyrodites
- o Polymer Electrolytes
- o Composite Electrolytes
- o Hybrid Solid-Liquid Electrolytes
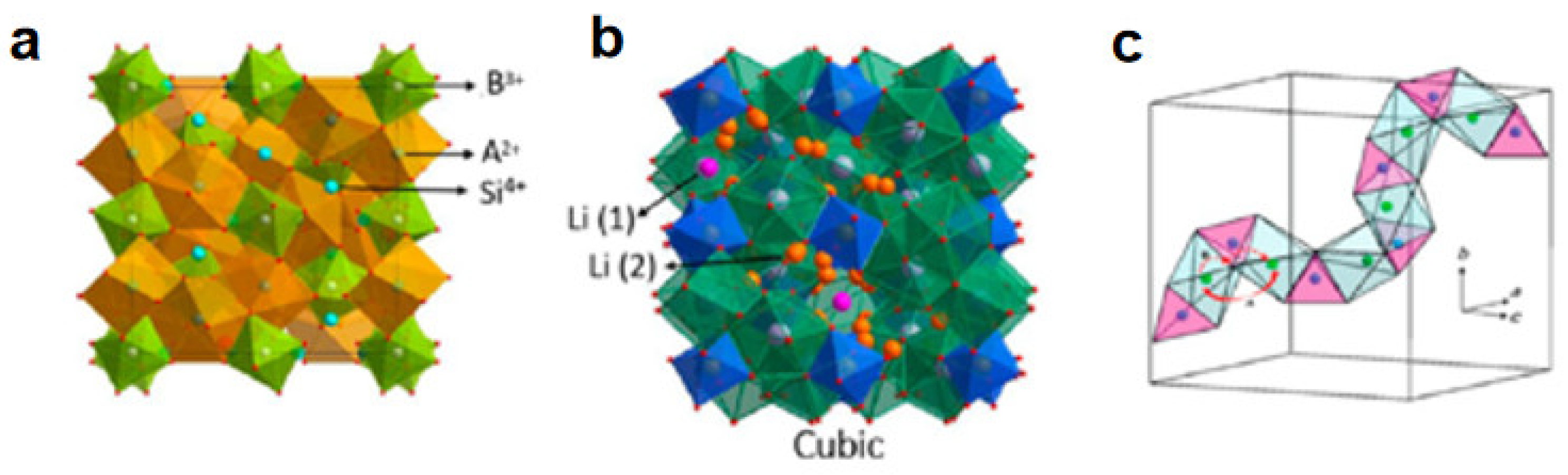
2.1. Oxide Electrolytes
2.1.1. LIPON
2.1.2. NASICON
2.1.3. Garnet Type
2.2. Sulfide Electrolytes
2.2.1. LPS
2.2.2. Argyrodites
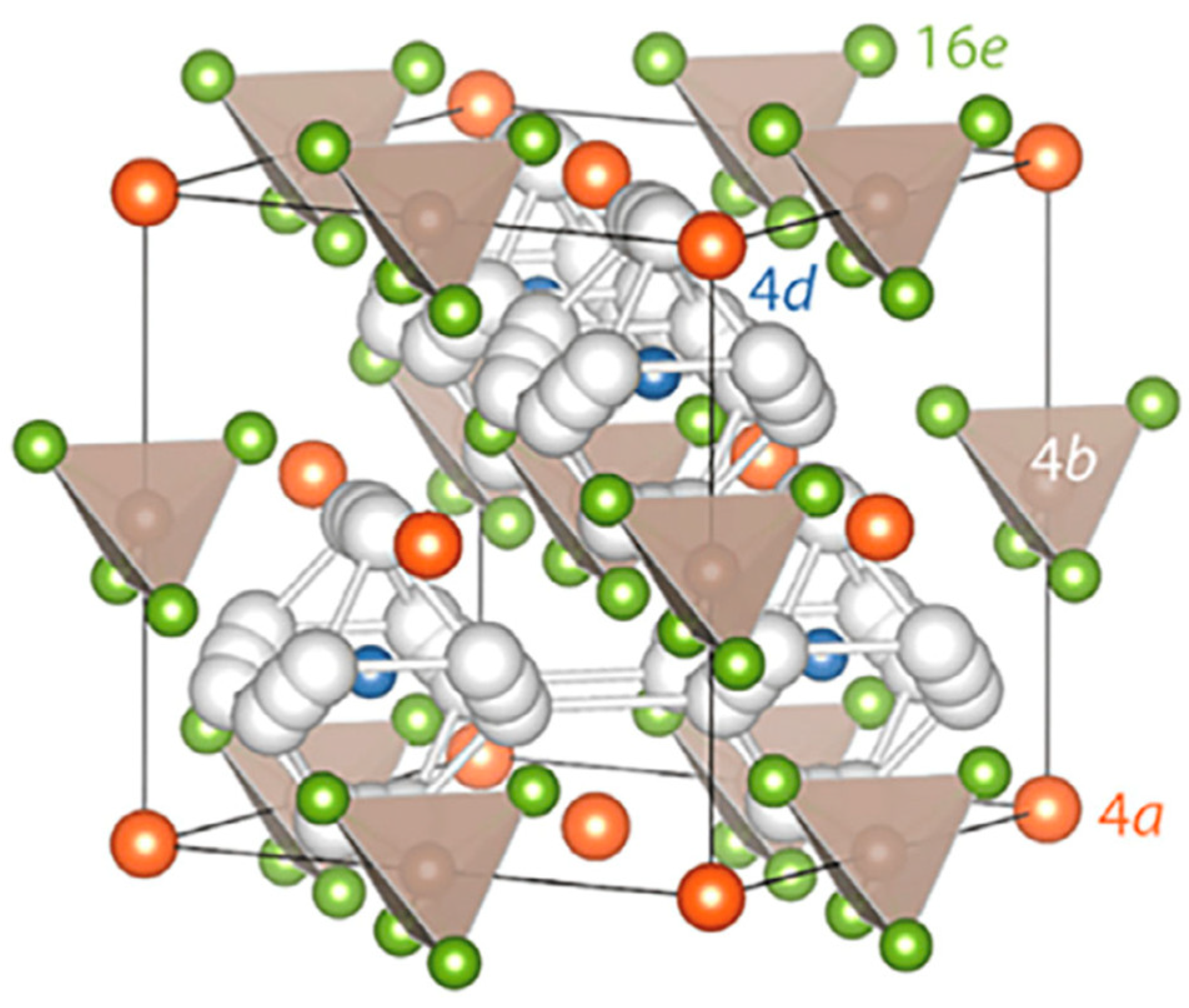
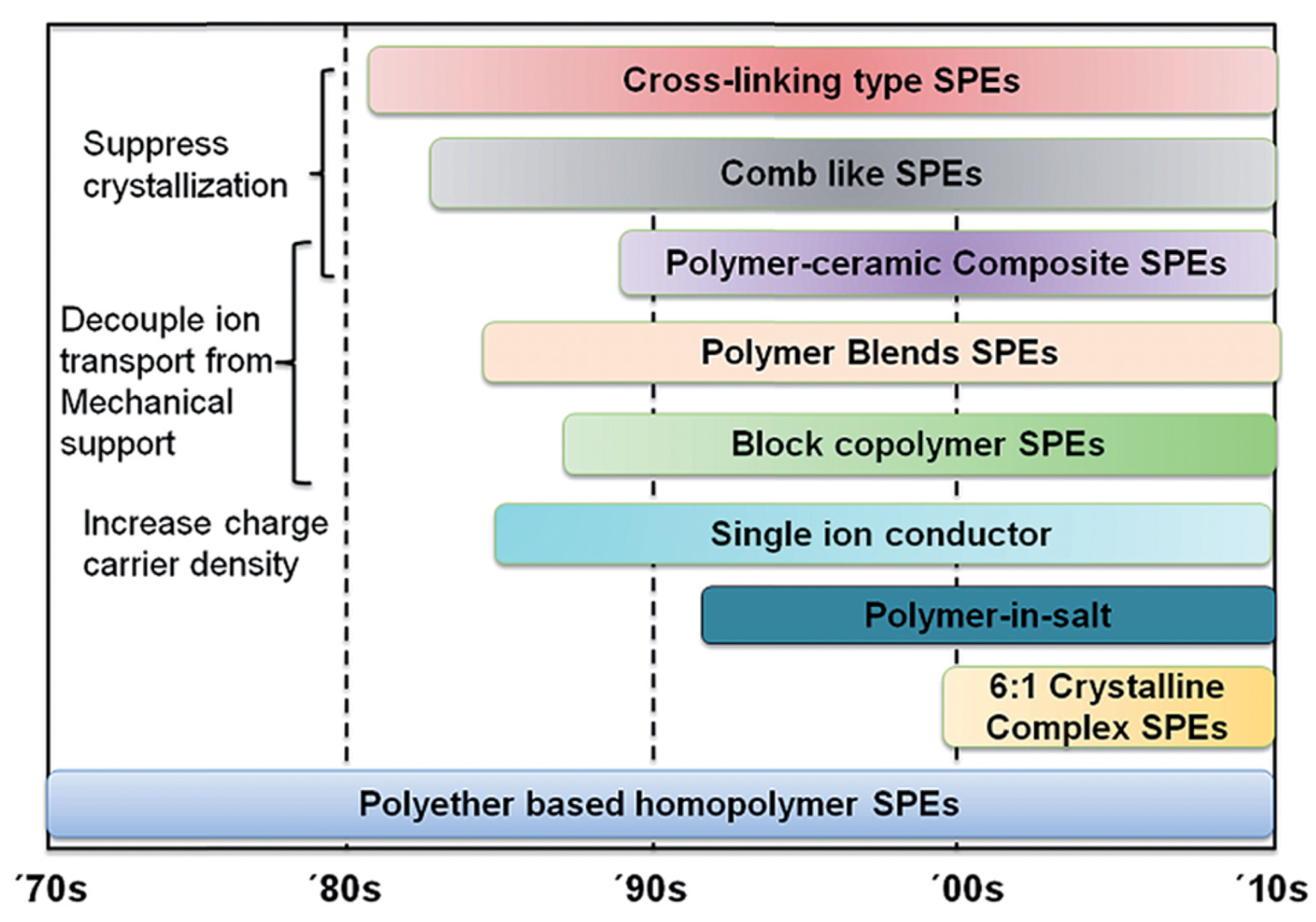
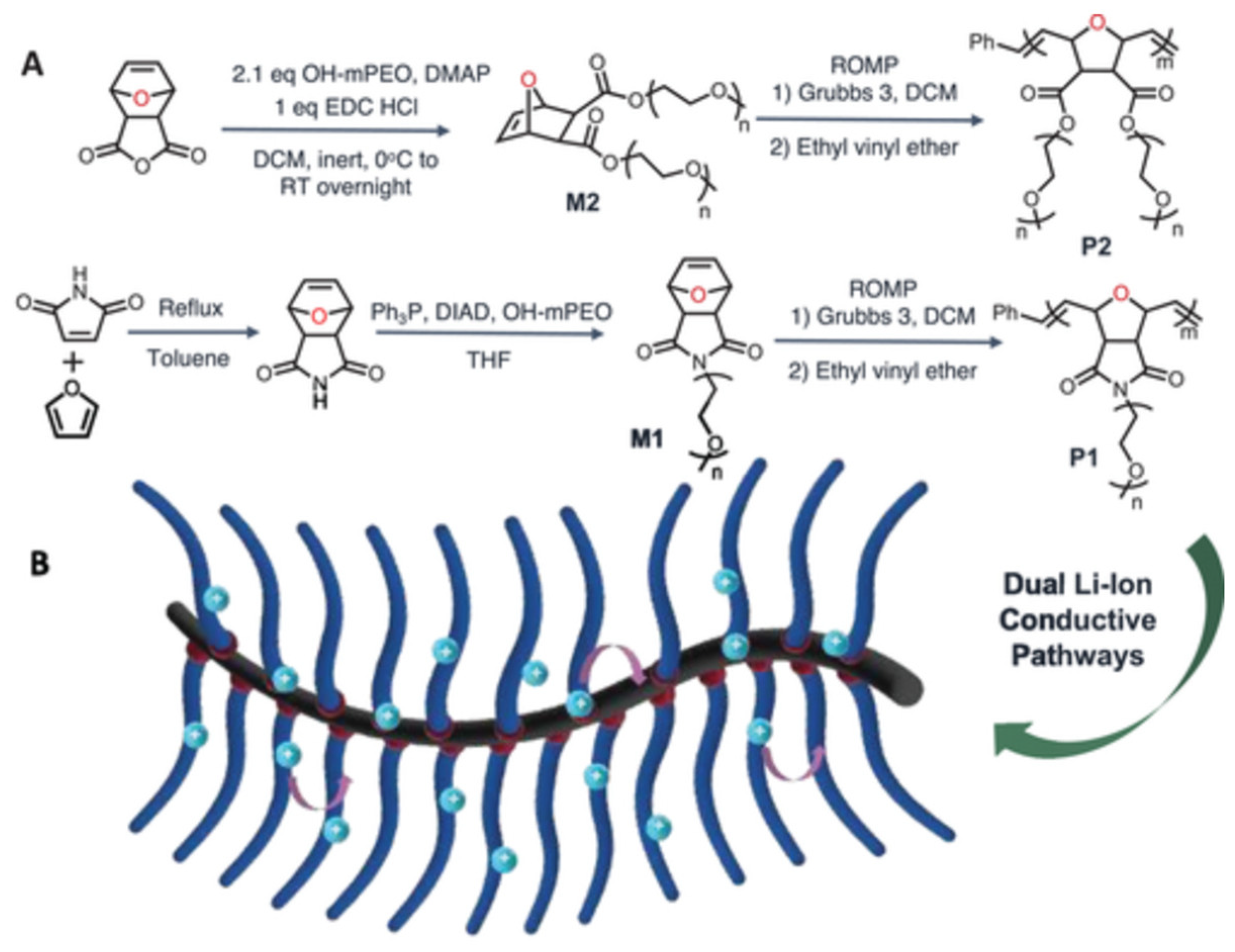
2.3. Polymer Electrolytes
2.4. Composite Electrolytes
2.5. Hybrid Solid Electrolyte-Liquid Electrolyte
2.6. Progress, Challenges and Prospects in Solid Electrolytes
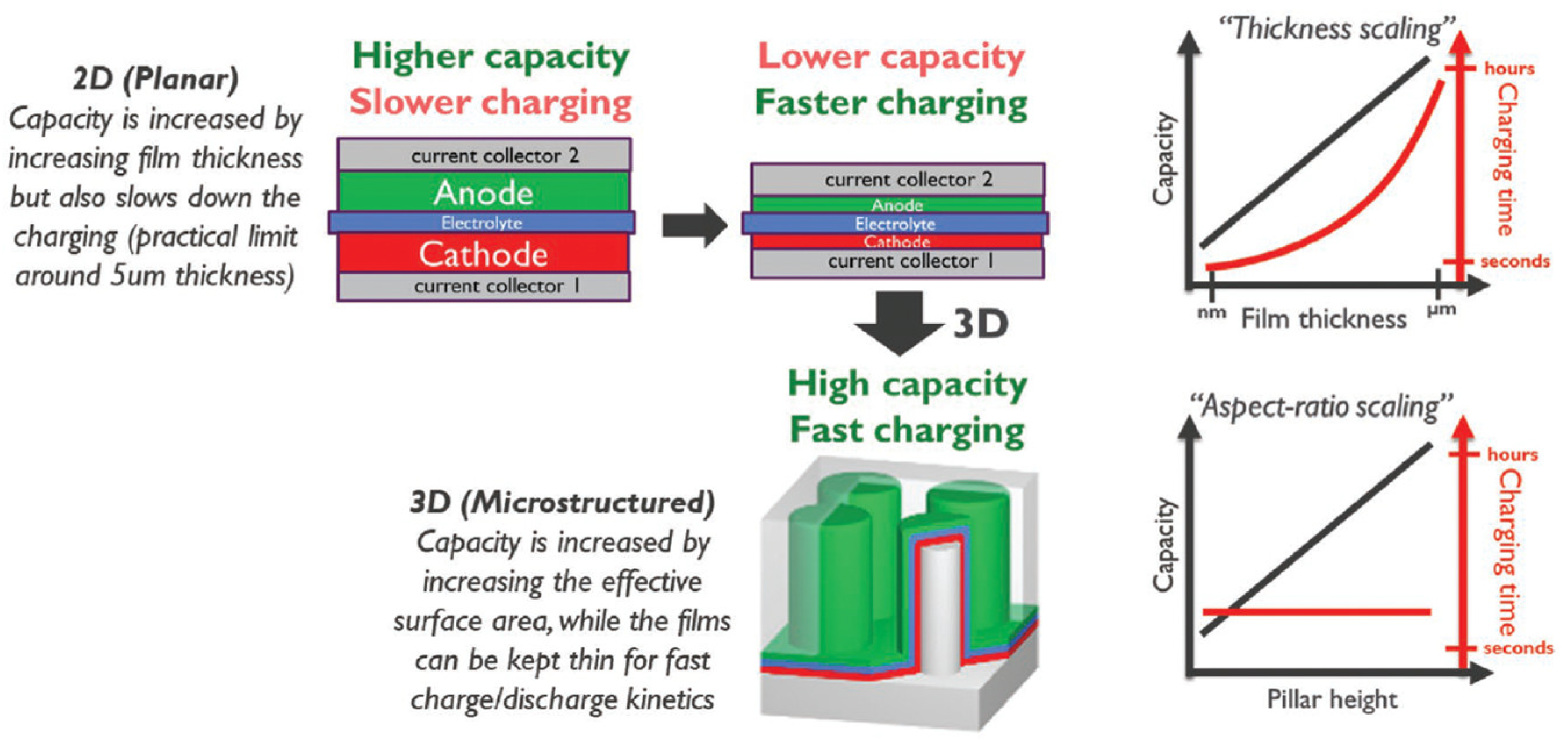
3. Anode Innovations in Solid-State Batteries
3.1. Importance of Anode Material in Solid-State Batteries (SSBs)
3.2. Anode Material Selection for SSBs
3.3. Overcoming Anode Challenges
3.3.1. Prevention of Dendritic Lithium Formation
3.3.2. Enhancement of Anode/Electrolyte Contact
3.3.3. Augmentation of Anode Life Cycle and Efficiency
3.4. Anode Enhancement Techniques
3.4.1. Surface Modification and Coating
3.4.2. Nanoengineering for Improved Performance
3.4.3. Formation of Protective Layers
4. The Convergence of Solid Electrolytes and Anodes
4.1. Designing for Synergy between Anodes and Solid Electrolytes
4.2. Analytical and Experimental Insights into Solid Electrolyte-Anode Parings
4.3. Computational Approaches in Predicting and Enhancing Performance
5. Conclusions and Forward Look
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Acronyms
| AEF | Area enhancement factor |
| AF-ASSB | Anode-free all-solid-state battery |
| AFB | Anode-free battery |
| ASSB | Anode/solid-state electrolyte interface |
| DLP | Digital light processing |
| DME | Dimethoxyethane |
| EV | Electric vehicle |
| FEC | Fluoroethylene carbonate |
| GCPE | Gradient composite polymer solid electrolyte |
| INPC | Inorganic nanoparticle/polymer combination |
| INFPC | Inorganic nanofiber/polymer structure |
| ISE | Inorganic solid electrolyte |
| LAGP | Li₁₊ₓAlₓGe₂₋ₓ(PO₄)₃ |
| LATP | Li1+xAxTi2-x(PO₄)₃ (where ‘A’ represents Al, Cr, Ga, Fe, In, La, Sc, or Y) |
| LE-LIB | Liquid electrolyte lithium-ion battery |
| LGPS | Li10GeP2S12 |
| LiFSI | Lithium bis(fluorosulfonyl)imide |
| LiPON | Lithium phosphorus oxynitride |
| LiTFSI | LiN(CF3SO2)2 |
| LLTO | Li0.33La0.557TiO3 |
| LLZO | Li7La3Zr2O12 |
| LLZTO | Li6.4La3Zr1.4Ta0.6O12 |
| LPS | Glass-ceramic lithium thiophosphate |
| NAS | Na3SbS4 |
| NASICON | Sodium super-ionic conductor |
| NPS | Na3PS4 |
| NZSP | Na3Zr2Si2PO12 |
| PAN | Polyacrylonitrile |
| PE | Polymer solid electrolyte |
| PEO | Polyethylene oxide |
| PVDF | Polyvinylidene fluoride |
| SEI | Solid electrolyte interphase |
| SLEIs | Solid-liquid electrolyte interfaces |
| SLA | Stereolithography |
| SSE | Solid-state electrolyte |
| SSLB | Solid-state lithium battery |
| SSBs | Solid-state batteries |
| TFBs | Planar thin-film battery |
| VOC | Volatile organic compound |
References
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J.; et al. Challenges for and Pathways toward Li-Metal-Based All-Solid-State Batteries. ACS Energy Lett. 2021, 1399–1404. [Google Scholar] [CrossRef]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-State Batteries: The Critical Role of Mechanics. Science 2023, 381, eabg5998. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent Advances in All-Solid-State Rechargeable Lithium Batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. Challenges in Speeding up Solid-State Battery Development. Nat Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Wu, C.; Lou, J.; Zhang, J.; Chen, Z.; Kakar, A.; Emley, B.; Ai, Q.; Guo, H.; Liang, Y.; Lou, J.; et al. Current Status and Future Directions of All-Solid-State Batteries with Lithium Metal Anodes, Sulfide Electrolytes, and Layered Transition Metal Oxide Cathodes. Nano Energy 2021, 87, 106081. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Al Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of Energy Storage Services, Applications, Limitations, and Benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; He, Z.; Li, Y.; Mao, J.; Dai, K.; Yan, C.; Zheng, J. An Advance Review of Solid-State Battery: Challenges, Progress and Prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Shuai, H.; Luo, Z.; Wang, B.; Fang, S.; Zou, G.; Hou, H.; Peng, H.; Ji, X. Recent Advances of Composite Electrolytes for Solid-State Li Batteries. J. Energy Chem. 2022, 67, 524–548. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Wu, Y.; Li, H.; Yang, J.; Liao, Y.; Qu, R.; Zhang, Z. Liquid Electrolytes for Low-Temperature Lithium Batteries: Main Limitations, Current Advances, and Future Perspectives. Energy Storage Mater. 2023, 56, 642–663. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Kuterbekov, K.; Kabyshev, A.; Kubenova, M.M.; Bekmyrza, K.Z.; Azad, A.K. Emerging and Recycling of Li-Ion Batteries to Aid in Energy Storage, A Review. Recycling 2023, 8, 48. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Kong, L.; Xing, Y.; Pecht, M.G. In-Situ Observations of Lithium Dendrite Growth. IEEE Access 2018, 6, 8387–8393. [Google Scholar] [CrossRef]
- Hou, W.; Lu, Y.; Ou, Y.; Zhou, P.; Yan, S.; He, X.; Geng, X.; Liu, K. Recent Advances in Electrolytes for High-Voltage Cathodes of Lithium-Ion Batteries. Trans. Tianjin Univ. 2023, 29, 120–135. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of High-Energy Li-Ion Batteries Comprising Silicon-Containing Anodes and Insertion-Type Cathodes. Nat Commun 2021, 12, 5459. [Google Scholar] [CrossRef]
- Zachmann, N.; Petranikova, M.; Ebin, B. Electrolyte Recovery from Spent Lithium-Ion Batteries Using a Low Temperature Thermal Treatment Process. J. Ind. Eng. Chem. 2023, 118, 351–361. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The Environmental Impact of Li-Ion Batteries and the Role of Key Parameters—A Review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; He, Y.-B.; Kang, F.; Chen, L.; Li, H.; Yang, Q.-H. Solid-State Lithium Batteries: Safety and Prospects. eScience 2022, 2, 138–163. [Google Scholar] [CrossRef]
- Mauger; Julien; Paolella; Armand; Zaghib Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing Solid-State Electrolytes for Safe, Energy-Dense Batteries. Nat Rev Mater 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Chen, X.C.; Cobb, C.L.; Dasgupta, N.P.; Dixit, M.B.; Marbella, L.E.; McDowell, M.T.; Mukherjee, P.P.; Verma, A.; Viswanathan, V.; et al. Challenges in Lithium Metal Anodes for Solid-State Batteries. ACS Energy Lett. 2020, 5, 922–934. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Bai, Z.; Fan, L.-Z. Enhancing Interfacial Stability in Solid-State Lithium Batteries with Polymer/Garnet Solid Electrolyte and Composite Cathode Framework. J. Energy Chem. 2021, 52, 210–217. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Wu, C.; Gao, J.; Xu, H.; Li, Y.; Guo, X.; Li, H.; Zhou, W. A Multilayer Ceramic Electrolyte for All-Solid-State Li Batteries. Angew Chem Int Ed 2021, 60, 3781–3790. [Google Scholar] [CrossRef]
- Kundu, S.; Ein-Eli, Y. A Review on Design Considerations in Polymer and Polymer Composite Solid-State Electrolytes for Solid Li Batteries. J. Power Sources 2023, 553, 232267. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and Manufacturing of next Generation Lithium-Based All Solid-State Batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Bubulinca, C.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. Batteries 2023, 9, 157. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, B.; Li, S.; Yu, Y.; Li, X.; Chang, Z.; Wu, H.; Hu, Y.; Huang, K.; Liu, L.; et al. Life Cycle Environmental Impact Assessment for Battery-Powered Electric Vehicles at the Global and Regional Levels. Sci Rep 2023, 13, 7952. [Google Scholar] [CrossRef]
- Solid State Batteries Volume 1: Emerging Materials and Applications; Gupta, R. K., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, 2022; Volume 1413, ISBN 978-0-8412-9768-5. [Google Scholar]
- Miao, X.; Guan, S.; Ma, C.; Li, L.; Nan, C. Role of Interfaces in Solid-State Batteries. Adv. Mater. 2023, 2206402. [Google Scholar] [CrossRef]
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J.; et al. Challenges for and Pathways toward Li-Metal-Based All-Solid-State Batteries. ACS Energy Lett. 2021, 1399–1404. [Google Scholar] [CrossRef]
- Jetybayeva, A.; Uzakbaiuly, B.; Mukanova, A.; Nurpeissova, A.; Bakenov, Z. Solid-State Nanobatteries. In ACS Symposium Series; Gupta, R.K., Ed.; American Chemical Society: Washington, DC, 2022; Volume 1414, pp. 201–248. ISBN 978-0-8412-9766-1. [Google Scholar]
- Shalaby, M.S.; Alziyadi, M.O.; Gamal, H.; Hamdy, S. Solid-State Lithium-Ion Battery: The Key Components Enhance the Performance and Efficiency of Anode, Cathode, and Solid Electrolytes. J. Alloys Compd. 2023, 969, 172318. [Google Scholar] [CrossRef]
- Waidha, A.I.; Salihovic, A.; Jacob, M.; Vanita, V.; Aktekin, B.; Brix, K.; Wissel, K.; Kautenburger, R.; Janek, J.; Ensinger, W.; et al. Recycling of All-Solid-State Li-ion Batteries: A Case Study of the Separation of Individual Components Within a System Composed of LTO, LLZTO and NMC**. ChemSusChem 2023, 16, e202202361. [Google Scholar] [CrossRef]
- Lu, P.; Xia, Y.; Huang, Y.; Li, Z.; Wu, Y.; Wang, X.; Sun, G.; Shi, S.; Sha, Z.; Chen, L.; et al. Wide-Temperature, Long-Cycling, and High-Loading Pyrite All-Solid-State Batteries Enabled by Argyrodite Thioarsenate Superionic Conductor. Adv Funct Mater. 2023, 33, 2211211. [Google Scholar] [CrossRef]
- Pacios, R.; Villaverde, A.; Martínez-Ibañez, M.; Casas-Cabanas, M.; Aguesse, F.; Kvasha, A. Roadmap for Competitive Production of Solid-State Batteries: How to Convert a Promise into Reality. Adv. Energy Mater. 2023, 13, 2301018. [Google Scholar] [CrossRef]
- Pandey, G.P.; Brown, J.E.; Li, J. Architectural Design for Flexible Solid-State Batteries. In ACS Symposium Series; Gupta, R.K., Ed.; American Chemical Society: Washington, DC, 2022; Volume 1414, pp. 289–309. ISBN 978-0-8412-9766-1. [Google Scholar]
- Hantanasirisakul, K.; Sawangphruk, M. Sustainable Reuse and Recycling of Spent Li-Ion Batteries from Electric Vehicles: Chemical, Environmental, and Economical Perspectives. Glob. Chall. 2023, 7, 2200212. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.-H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding Interface Stability in Solid-State Batteries. Nat Rev Mater 2019, 5, 105–126. [Google Scholar] [CrossRef]
- Singer, C.; Schnell, J.; Reinhart, G. Scalable Processing Routes for the Production of All-Solid-State Batteries—Modeling Interdependencies of Product and Process. Energy Tech 2021, 9, 2000665. [Google Scholar] [CrossRef]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring Inorganic–Polymer Composites for the Mass Production of Solid-State Batteries. Nat Rev Mater 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Richter, F.H. Viscoelastic Glass Electrolytes. Nat Energy 2023, 8, 1182–1183. [Google Scholar] [CrossRef]
- Raj, V.; Venturi, V.; Kankanallu, V.R.; Kuiri, B.; Viswanathan, V.; Aetukuri, N.P.B. Direct Correlation between Void Formation and Lithium Dendrite Growth in Solid-State Electrolytes with Interlayers. Nat. Mater. 2022, 21, 1050–1056. [Google Scholar] [CrossRef]
- Widyantara, R.D.; Zulaikah, S.; Juangsa, F.B.; Budiman, B.A.; Aziz, M. Review on Battery Packing Design Strategies for Superior Thermal Management in Electric Vehicles. Batteries 2022, 8, 287. [Google Scholar] [CrossRef]
- Quérel, E.; Williams, N.J.; Seymour, I.D.; Skinner, S.J.; Aguadero, A. Operando Characterization and Theoretical Modeling of Metal|Electrolyte Interphase Growth Kinetics in Solid-State Batteries. Part I: Experiments. Chem. Mater. 2023, 35, 853–862. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Wu, X.; Feng, X.; Wu, Q.; Sun, Y.; Xiang, H.; Wang, J. Solid-State Electrolytes for Solid-State Lithium-Sulfur Batteries: Comparisons, Advances and Prospects. J. Energy Chem. 2022, 73, 370–386. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on Solid Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable Organic Electrolytes for High-Safety Lithium-Ion Batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Shannon, R.D.; Taylor, B.E.; English, A.D.; Berzins, T. New Li Solid Electrolytes. Electrochim. Acta 1977, 22, 783–796. [Google Scholar] [CrossRef]
- Torres, V.M.; Kalnaus, S.; Martin, S.W.; Duggan, C.; Westover, A.S. Structure-mechanical Properties Correlation in Bulk LiPON Glass Produced by Nitridation of Metaphosphate Melts. J Am Ceram Soc. 2023, 106, 6565–6576. [Google Scholar] [CrossRef]
- De Souza, J.E.; Rojas De Souza, S.; Gebhardt, R.; Kmiec, S.; Whale, A.; Warthen Martin, S. LiPON and NaPON Glasses: A Study of the Ammonolysis of Lithium and Sodium Metaphosphate Melts. Int J Appl Glass Sci 2020, 11, 78–86. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid Electrolyte: The Key for High-Voltage Lithium Batteries. Adv. Energy Mater. 2015, 5, 1401408. [Google Scholar] [CrossRef]
- Koo, M.; Park, K.-I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable Inorganic Thin-Film Battery for Fully Flexible Electronic Systems. Nano Lett. 2012, 12, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Kalnaus, S.; Westover, A.S.; Kornbluth, M.; Herbert, E.; Dudney, N.J. Resistance to Fracture in the Glassy Solid Electrolyte Lipon. J. Mater. Res. 2021, 36, 787–796. [Google Scholar] [CrossRef]
- Kim, Y.; Dudney, N.J.; Chi, M.; Martha, S.K.; Nanda, J.; Veith, G.M.; Liang, C. A Perspective on Coatings to Stabilize High-Voltage Cathodes: LiMn 1.5 Ni 0.5 O 4 with Sub-Nanometer Lipon Cycled with LiPF 6 Electrolyte. J. Electrochem. Soc. 2013, 160, A3113–A3125. [Google Scholar] [CrossRef]
- Martha, S.K.; Nanda, J.; Kim, Y.; Unocic, R.R.; Pannala, S.; Dudney, N.J. Solid Electrolyte Coated High Voltage Layered–Layered Lithium-Rich Composite Cathode: Li1.2Mn0.525Ni0.175Co0.1O2. J. Mater. Chem. A 2013, 1, 5587. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Wu, Z.; Zhu, Y.; Yang, Y.; Chen, J.; Jing, M.; Li, F.; Banks, C.E. First Exploration of Na-Ion Migration Pathways in the NASICON Structure Na3V2(PO4)3. J. Mater. Chem. A 2014, 2, 5358. [Google Scholar] [CrossRef]
- Passerini, S.; Bresser, Dominic; Moretti, Arianna; Varzi, Alberto Batteries : Present and Future Energy Storage Challenges; Wiley-VCH.; ISBN 978-3-527-82731-2.
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. Ionic Conductivity of Solid Electrolytes Based on Lithium Titanium Phosphate. J. Electrochem. Soc. 1990, 137, 1023–1027. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High Ionic Conductivity in Lithium Lanthanum Titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Tolganbek, N.; Serikkazyyeva, A.; Kalybekkyzy, S.; Sarsembina, M.; Kanamura, K.; Bakenov, Z.; Mentbayeva, A. Interface Modification of NASICON-Type Li-Ion Conducting Ceramic Electrolytes: A Critical Evaluation. Mater. Adv. 2022, 3, 3055–3069. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y. Perovskite-type Li-ion Solid Electrolytes: A Review. J Mater Sci: Mater Electron 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Turney, D.E.; Yadav, G.G.; Gallaway, J.W.; Kolhekar, S.; Huang, J.; D’Ambrose, M.J.; Banerjee, S. Aqueous Mn-Zn and Ni-Zn Batteries for Sustainable Energy Storage. In Energy-Sustainable Advanced Materials; Alston, M., Lambert, T.N., Eds.; Springer International Publishing: Cham, 2021; pp. 1–26. ISBN 978-3-030-57491-8. [Google Scholar]
- Tolganbek, N.; Yerkinbekova, Y.; Khairullin, A.; Bakenov, Z.; Kanamura, K.; Mentbayeva, A. Enhancing Purity and Ionic Conductivity of NASICON-Typed Li1.3Al0.3Ti1.7(PO4)3 Solid Electrolyte. Ceram. Int. 2021, 47, 18188–18195. [Google Scholar] [CrossRef]
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Li, Y.; Liu, S.; Xie, K. New Class of LAGP-Based Solid Polymer Composite Electrolyte for Efficient and Safe Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 41837–41844. [Google Scholar] [CrossRef]
- Hamao, N.; Yamaguchi, Y.; Hamamoto, K. Densification of a NASICON-Type LATP Electrolyte Sheet by a Cold-Sintering Process. Materials 2021, 14, 4737. [Google Scholar] [CrossRef]
- Grady, Z.M.; Tsuji, K.; Ndayishimiye, A.; Hwan-Seo, J.; Randall, C.A. Densification of a Solid-State NASICON Sodium-Ion Electrolyte Below 400 °C by Cold Sintering with a Fused Hydroxide Solvent. ACS Appl. Energy Mater. 2020, 3, 4356–4366. [Google Scholar] [CrossRef]
- Zhao, E.; Ma, F.; Guo, Y.; Jin, Y. Stable LATP/LAGP Double-Layer Solid Electrolyte Prepared via a Simple Dry-Pressing Method for Solid State Lithium Ion Batteries. RSC Adv. 2016, 6, 92579–92585. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic Solid Li Ion Conductors: An Overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Chen, R.; Nolan, A.M.; Lu, J.; Wang, J.; Yu, X.; Mo, Y.; Chen, L.; Huang, X.; Li, H. The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium. Joule 2020, 4, 812–821. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li 5 La 3 M 2 O 12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Abouali, S.; Yim, C.-H.; Merati, A.; Abu-Lebdeh, Y.; Thangadurai, V. Garnet-Based Solid-State Li Batteries: From Materials Design to Battery Architecture. ACS Energy Lett. 2021, 6, 1920–1941. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li 7 La 3 Zr 2 O 12. Angew Chem Int Ed 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Baral, A.K.; Narayanan, S.; Ramezanipour, F.; Thangadurai, V. Evaluation of Fundamental Transport Properties of Li-Excess Garnet-Type Li5+2xLa3Ta2−xYxO12 (x = 0.25, 0.5 and 0.75) Electrolytes Using AC Impedance and Dielectric Spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 11356. [Google Scholar] [CrossRef]
- Xie, H.; Alonso, J.A.; Li, Y.; Fernández-Díaz, M.T.; Goodenough, J.B. Lithium Distribution in Aluminum-Free Cubic Li 7 La 3 Zr 2 O 12. Chem. Mater. 2011, 23, 3587–3589. [Google Scholar] [CrossRef]
- Geiger, C.A.; Alekseev, E.; Lazic, B.; Fisch, M.; Armbruster, T.; Langner, R.; Fechtelkord, M.; Kim, N.; Pettke, T.; Weppner, W. Crystal Chemistry and Stability of “Li 7 La 3 Zr 2 O 12 ” Garnet: A Fast Lithium-Ion Conductor. Inorg. Chem. 2011, 50, 1089–1097. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Al-Doped Li7La3Zr2O12 Synthesized by a Polymerized Complex Method. J. Power Sources 2011, 196, 8683–8687. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Li 6 ALa 2 Ta 2 O 12 (A = Sr, Ba): Novel Garnet-Like Oxides for Fast Lithium Ion Conduction. Adv Funct Mater. 2005, 15, 107–112. [Google Scholar] [CrossRef]
- Rettenwander, D.; Wagner, R.; Reyer, A.; Bonta, M.; Cheng, L.; Doeff, M.M.; Limbeck, A.; Wilkening, M.; Amthauer, G. Interface Instability of Fe-Stabilized Li 7 La 3 Zr 2 O 12 versus Li Metal. J. Phys. Chem. C 2018, 122, 3780–3785. [Google Scholar] [CrossRef]
- Kotobuki, M.; Munakata, H.; Kanamura, K.; Sato, Y.; Yoshida, T. Compatibility of Li[Sub 7]La[Sub 3]Zr[Sub 2]O[Sub 12] Solid Electrolyte to All-Solid-State Battery Using Li Metal Anode. J. Electrochem. Soc. 2010, 157, A1076. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152, A396. [Google Scholar] [CrossRef]
- Ni, J.E.; Case, E.D.; Sakamoto, J.S.; Rangasamy, E.; Wolfenstine, J.B. Room Temperature Elastic Moduli and Vickers Hardness of Hot-Pressed LLZO Cubic Garnet. J Mater Sci 2012, 47, 7978–7985. [Google Scholar] [CrossRef]
- Shen, F.; Dixit, M.B.; Xiao, X.; Hatzell, K.B. Effect of Pore Connectivity on Li Dendrite Propagation within LLZO Electrolytes Observed with Synchrotron X-Ray Tomography. ACS Energy Lett. 2018, 3, 1056–1061. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Roddatis, V.; Chandran, C.V.; Ma, Q.; Uhlenbruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li 7 La 3 Zr 2 O 12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. [Google Scholar] [CrossRef]
- Cheng, E.J.; Sharafi, A.; Sakamoto, J. Intergranular Li Metal Propagation through Polycrystalline Li6.25Al0.25La3Zr2O12 Ceramic Electrolyte. Electrochim. Acta 2017, 223, 85–91. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Q.; Ning, T.; Liu, T.; Luo, Y.; He, X.; Luo, Z.; Lu, A. Critical Challenges and Progress of Solid Garnet Electrolytes for All-Solid-State Batteries. Mater. Today Chem. 2020, 18, 100368. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Muramatsu, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Structural Change of Li2S–P2S5 Sulfide Solid Electrolytes in the Atmosphere. Solid State Ion. 2011, 182, 116–119. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Hayashi, A. Sulfide Glass-Ceramic Electrolytes for All-Solid-State Lithium and Sodium Batteries. Int J Appl Glass Sci 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A Lithium Superionic Conductor. Nat. Mater 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A Sulphide Lithium Super Ion Conductor Is Superior to Liquid Ion Conductors for Use in Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Dietrich, C.; Weber, D.A.; Sedlmaier, S.J.; Indris, S.; Culver, S.P.; Walter, D.; Janek, J.; Zeier, W.G. Lithium Ion Conductivity in Li 2 S–P 2 S 5 Glasses—Building Units and Local Structure Evolution during the Crystallization of Superionic Conductors Li 3 PS 4, Li 7 P 3 S 11 and Li 4 P 2 S 7. J. Mater. Chem. A 2017, 5, 18111–18119. [Google Scholar] [CrossRef]
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. High Lithium Ion Conducting Glass-Ceramics in the System Li2S–P2S5. Solid State Ion. 2006, 177, 2721–2725. [Google Scholar] [CrossRef]
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide Solid Electrolytes for All-Solid-State Lithium Batteries: Structure, Conductivity, Stability and Application. Energy Storage Mater. 2018, 14, 58–74. [Google Scholar] [CrossRef]
- Jung, Y.S.; Oh, D.Y.; Nam, Y.J.; Park, K.H. Issues and Challenges for Bulk-Type All-Solid-State Rechargeable Lithium Batteries Using Sulfide Solid Electrolytes. Isr. J. Chem. 2015, 55, 472–485. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Hayashi, A. Superionic Glasses and Glass–Ceramics in the Li2S–P2S5 System for All-Solid-State Lithium Secondary Batteries. Solid State Ion. 2012, 225, 342–345. [Google Scholar] [CrossRef]
- Ohno, S.; Banik, A.; Dewald, G.F.; Kraft, M.A.; Krauskopf, T.; Minafra, N.; Till, P.; Weiss, M.; Zeier, W.G. Materials Design of Ionic Conductors for Solid State Batteries. Prog. Energy 2020, 2, 022001. [Google Scholar] [CrossRef]
- Deiseroth, H.; Kong, S.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li 6 PS 5 X: A Class of Crystalline Li-Rich Solids with an Unusually High Li + Mobility. Angew Chem Int Ed 2008, 47, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Park, K.-H.; Sun, X.; Lalère, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-Engineered Design of Argyrodite Li 6 PS 5 X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Lett. 2019, 4, 265–270. [Google Scholar] [CrossRef]
- Bai, X.; Duan, Y.; Zhuang, W.; Yang, R.; Wang, J. Research Progress in Li-Argyrodite-Based Solid-State Electrolytes. J. Mater. Chem. A 2020, 8, 25663–25686. [Google Scholar] [CrossRef]
- Zulfiqar, Z.; Zulfiqar, S.; Abbas, Q.; Mirzaeian, M.; Raza, R. Potential Electrolytes for Solid State Batteries and Its Electrochemical Analysis—A Review. Energy Storage 2023, est2.506. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M.F. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent Advances in Solid Polymer Electrolytes for Lithium Batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Iojoiu, C.; Paillard, E. Solid-State Batteries with Polymer Electrolytes; Bard, A.J., Wiley, 2007.
- Manuel Stephan, A.; Nahm, K.S. Review on Composite Polymer Electrolytes for Lithium Batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Benrabah, D.; Baril, D.; Sanchez, J.-Y.; Armand, M.; Gard, G.G. Comparative Electrochemical Study of New Poly(Oxyethylene)–Li Salt Complexes. J. Chem. Soc., Faraday Trans. 1993, 89, 355–359. [Google Scholar] [CrossRef]
- Fang, Z.; Ma, Q.; Liu, P.; Ma, J.; Hu, Y.-S.; Zhou, Z.; Li, H.; Huang, X.; Chen, L. Novel Concentrated Li[(FSO 2 )(n-C 4 F 9 SO 2 )N]-Based Ether Electrolyte for Superior Stability of Metallic Lithium Anode. ACS Appl. Mater. Interfaces 2017, 9, 4282–4289. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Filler, R.; Mandal, B.K. Synthesis and Properties of a New Class of Fluorine-Containing Dilithium Salts for Lithium-Ion Batteries. Solid State Ion. 2010, 180, 1640–1645. [Google Scholar] [CrossRef]
- Ganapatibhotla, L.V.N.R.; Maranas, J.K. Interplay of Surface Chemistry and Ion Content in Nanoparticle-Filled Solid Polymer Electrolytes. Macromolecules 2014, 47, 3625–3634. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Passerini, S. PEO-Carbon Composite Lithium Polymer Electrolyte. Electrochim. Acta 2000, 45, 2139–2145. [Google Scholar] [CrossRef]
- Scrosati, B. Progress in Lithium Polymer Battery R&D. J. Power Sources 2001, 100, 93–100. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew Chem Int Ed 2016, 55, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Smith, D.M.; Pan, Q.; Wang, S.; Li, C.Y. Anisotropic Ion Transport in Nanostructured Solid Polymer Electrolytes. RSC Adv. 2015, 5, 48793–48810. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Li, Y.; Liu, R.; Yang, H. Accelerated Ion Transportation in Liquid Crystalline Polymer Networks for Superior Solid-State Lithium Metal Batteries. Chem. Eng. J. 2023, 476, 146658. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, 2101051. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Wu, X.; Zhao, Y.; Liu, T.; Yin, R.; Ahn, J.H.; Walker, L.M.; Whitacre, J.F.; Matyjaszewski, K. Highly Conductive Polyoxanorbornene-Based Polymer Electrolyte for Lithium-Metal Batteries. Adv. Sci. 2023, 10, 2302932. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid Halide Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer Electrolytes for Lithium Polymer Batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Grundish, N.S.; Goodenough, J.B.; Khani, H. Designing Composite Polymer Electrolytes for All-Solid-State Lithium Batteries. Curr. Opin. Electrochem. 2021, 30, 100828. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Mater. Sci. Eng. R: Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Pitawala, H.M.J.C.; Dissanayake, M.A.K.L.; Seneviratne, V.A.; Mellander, B.-E.; Albinson, I. Effect of Plasticizers (EC or PC) on the Ionic Conductivity and Thermal Properties of the (PEO)9LiTf: Al2O3 Nanocomposite Polymer Electrolyte System. J Solid State Electrochem 2008, 12, 783–789. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, D.; Li, M.; Chen, Z. Nanohybrid Electrolytes for High-Energy Lithium-Ion Batteries: Recent Advances and Future Challenges. Nanotechnology 2019, 30, 302002. [Google Scholar] [CrossRef]
- Hallinan, D.T.; Villaluenga, I.; Balsara, N.P. Polymer and Composite Electrolytes. MRS Bull. 2018, 43, 759–767. [Google Scholar] [CrossRef]
- Wang, W.; Yi, E.; Fici, A.J.; Laine, R.M.; Kieffer, J. Lithium Ion Conducting Poly(Ethylene Oxide)-Based Solid Electrolytes Containing Active or Passive Ceramic Nanoparticles. J. Phys. Chem. C 2017, 121, 2563–2573. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef]
- Fu, K. (Kelvin); Gong, Y.; Dai, J.; Gong, A.; Han, X.; Yao, Y.; Wang, C.; Wang, Y.; Chen, Y.; Yan, C.; et al. Flexible, Solid-State, Ion-Conducting Membrane with 3D Garnet Nanofiber Networks for Lithium Batteries. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 7094–7099. [Google Scholar] [CrossRef]
- Vargas-Barbosa, N.M.; Roling, B. Dynamic Ion Correlations in Solid and Liquid Electrolytes: How Do They Affect Charge and Mass Transport? ChemElectroChem 2020, 7, 367–385. [Google Scholar] [CrossRef]
- Park, K.; Yu, B.-C.; Jung, J.-W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical Nature of the Cathode Interface for a Solid-State Lithium-Ion Battery: Interface between LiCoO 2 and Garnet-Li 7 La 3 Zr 2 O 12. Chem. Mater. 2016, 28, 8051–8059. [Google Scholar] [CrossRef]
- Schwietert, T.K.; Arszelewska, V.A.; Wang, C.; Yu, C.; Vasileiadis, A.; De Klerk, N.J.J.; Hageman, J.; Hupfer, T.; Kerkamm, I.; Xu, Y.; et al. Clarifying the Relationship between Redox Activity and Electrochemical Stability in Solid Electrolytes. Nat. Mater. 2020, 19, 428–435. [Google Scholar] [CrossRef]
- Woolley, H.M.; Vargas-Barbosa, N.M. Hybrid Solid Electrolyte-Liquid Electrolyte Systems for (Almost) Solid-State Batteries: Why, How, and Where To? J. Mater. Chem. A 2023, 11, 1083–1097. [Google Scholar] [CrossRef]
- Vivek, J.P.; Meddings, N.; Garcia-Araez, N. Negating the Interfacial Resistance between Solid and Liquid Electrolytes for Next-Generation Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kazyak, E.; Dasgupta, N.P.; Sakamoto, J. Electrochemical and Surface Chemistry Analysis of Lithium Lanthanum Zirconium Tantalum Oxide (LLZTO)/Liquid Electrolyte (LE) Interfaces. J. Power Sources 2020, 474, 228598. [Google Scholar] [CrossRef]
- Hatz, A.-K.; Calaminus, R.; Feijoo, J.; Treber, F.; Blahusch, J.; Lenz, T.; Reichel, M.; Karaghiosoff, K.; Vargas-Barbosa, N.M.; Lotsch, B.V. Chemical Stability and Ionic Conductivity of LGPS-Type Solid Electrolyte Tetra-Li 7 SiPS 8 after Solvent Treatment. ACS Appl. Energy Mater. 2021, 4, 9932–9943. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L.M. Processing Thin but Robust Electrolytes for Solid-State Batteries. Nat Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D Thin-Film Li-Ion Batteries. Adv Mater. Inter 2019, 6, 1900805. [Google Scholar] [CrossRef]
- Sahal, M.; Molloy, J.; Narayanan, V.R.; Ladani, L.; Lu, X.; Rolston, N. Robust and Manufacturable Lithium Lanthanum Titanate-Based Solid-State Electrolyte Thin Films Deposited in Open Air. ACS Omega 2023, 8, 28651–28662. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Yuan, X.; Jin, H.; Zhao, Y. NaBr-Assisted Sintering of Na 3 Zr 2 Si 2 PO 12 Ceramic Electrolyte Stabilizes a Rechargeable Solid-State Sodium Metal Battery. ACS Appl. Mater. Interfaces 2023, 15, 49321–49328. [Google Scholar] [CrossRef]
- Lin, C.; Ihrig, M.; Kung, K.; Chen, H.; Scheld, W.S.; Ye, R.; Finsterbusch, M.; Guillon, O.; Lin, S. Low-Temperature Sintering of Li0.33La0.55TiO3 Electrolyte for All-Solid-State Li Batteries. J. Eur. Ceram. Soc. 2023, 43, 7543–7552. [Google Scholar] [CrossRef]
- Ramos, E.; Browar, A.; Roehling, J.; Ye, J. CO 2 Laser Sintering of Garnet-Type Solid-State Electrolytes. ACS Energy Lett. 2022, 7, 3392–3400. [Google Scholar] [CrossRef]
- Chen, A.; Qu, C.; Shi, Y.; Shi, F. Manufacturing Strategies for Solid Electrolyte in Batteries. Front. Energy Res. 2020, 8, 571440. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single Lithium-Ion Conducting Solid Polymer Electrolytes: Advances and Perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A Review of Lithium and Non-Lithium Based Solid State Batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Chen, A.-N.; Wu, J.-M.; Liu, K.; Chen, J.-Y.; Xiao, H.; Chen, P.; Li, C.-H.; Shi, Y.-S. High-Performance Ceramic Parts with Complex Shape Prepared by Selective Laser Sintering: A Review. Adv. Appl. Ceram. 2018, 117, 100–117. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, M.; Yang, X.; Sun, R.; Zhang, J.; Sun, X. Emerging Application of 3D-Printing Techniques in Lithium Batteries: From Liquid to Solid. Mater. Today 2022, 59, 161–181. [Google Scholar] [CrossRef]
- Chen, C.; Zuo, Y.; Ye, W.; Li, X.; Deng, Z.; Ong, S.P. A Critical Review of Machine Learning of Energy Materials. Adv. Energy Mater. 2020, 10, 1903242. [Google Scholar] [CrossRef]
- Xu, Z.; Xia, Y. Progress, Challenges and Perspectives of Computational Studies on Glassy Superionic Conductors for Solid-State Batteries. J. Mater. Chem. A 2022, 10, 11854–11880. [Google Scholar] [CrossRef]
- Garcia-Mendez, R.; Smith, J.G.; Neuefeind, J.C.; Siegel, D.J.; Sakamoto, J. Correlating Macro and Atomic Structure with Elastic Properties and Ionic Transport of Glassy Li 2 S-P 2 S 5 (LPS) Solid Electrolyte for Solid-State Li Metal Batteries. Adv. Energy Mater. 2020, 10, 2000335. [Google Scholar] [CrossRef]
- Sadowski, M.; Albe, K. Computational Study of Crystalline and Glassy Lithium Thiophosphates: Structure, Thermodynamic Stability and Transport Properties. J. Power Sources 2020, 478, 229041. [Google Scholar] [CrossRef]
- Smith, J.G.; Siegel, D.J. Low-Temperature Paddlewheel Effect in Glassy Solid Electrolytes. Nat Commun 2020, 11, 1483. [Google Scholar] [CrossRef] [PubMed]
- Cangaz, S.; Hippauf, F.; Reuter, F.S.; Doerfler, S.; Abendroth, T.; Althues, H.; Kaskel, S. Enabling High-Energy Solid-State Batteries with Stable Anode Interphase by the Use of Columnar Silicon Anodes. Adv. Energy Mater. 2020, 10, 2001320. [Google Scholar] [CrossRef]
- Xi, L.; Zhang, D.; Xu, X.; Wu, Y.; Li, F.; Yao, S.; Zhu, M.; Liu, J. Interface Engineering of All-Solid-State Batteries Based on Inorganic Solid Electrolytes. ChemSusChem 2023, 16, e202202158. [Google Scholar] [CrossRef]
- Aktekin, B.; Riegger, L.M.; Otto, S.-K.; Fuchs, T.; Henss, A.; Janek, J. SEI Growth on Lithium Metal Anodes in Solid-State Batteries Quantified with Coulometric Titration Time Analysis. Nat Commun 2023, 14, 6946. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Y.; Wan, S.; Ma, W.; Rong, J.; Xiao, Y.; Hou, G.; Chen, S. Recent Advances in Designing Solid-State Electrolytes to Reduce the Working Temperature of Lithium Batteries. Mater. Chem. Front. 2023, 7, 6061–6084. [Google Scholar] [CrossRef]
- Huang, Y.; Shao, B.; Wang, Y.; Han, F. Solid-State Silicon Anode with Extremely High Initial Coulombic Efficiency. Energy Environ. Sci. 2023, 16, 1569–1580. [Google Scholar] [CrossRef]
- Li, H.; Yamaguchi, T.; Matsumoto, S.; Hoshikawa, H.; Kumagai, T.; Okamoto, N.L.; Ichitsubo, T. Circumventing Huge Volume Strain in Alloy Anodes of Lithium Batteries. Nat Commun 2020, 11, 1584. [Google Scholar] [CrossRef]
- Cui, Y. Silicon Anodes. Nat Energy 2021, 6, 995–996. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Huang, F. A Comparative Overview of Carbon Anodes for Nonaqueous Alkali Metal-Ion Batteries. J. Mater. Chem. A 2021, 9, 27140–27169. [Google Scholar] [CrossRef]
- Yan, W.; Mu, Z.; Wang, Z.; Huang, Y.; Wu, D.; Lu, P.; Lu, J.; Xu, J.; Wu, Y.; Ma, T.; et al. Hard-Carbon-Stabilized Li–Si Anodes for High-Performance All-Solid-State Li-Ion Batteries. Nat Energy 2023, 8, 800–813. [Google Scholar] [CrossRef]
- Li, W.; Tchelepi, H.A.; Tartakovsky, D.M. Screening of Electrolyte-Anode Buffers to Suppress Lithium Dendrite Growth in All-Solid-State Batteries. J. Electrochem. Soc. 2023, 170, 050510. [Google Scholar] [CrossRef]
- Singh, D.K.; Fuchs, T.; Krempaszky, C.; Mogwitz, B.; Burkhardt, S.; Richter, F.H.; Janek, J. Overcoming Anode Instability in Solid-State Batteries through Control of the Lithium Metal Microstructure. Adv Funct Mater. 2023, 33, 2211067. [Google Scholar] [CrossRef]
- Cronau, M.; Szabo, M.; Renz, D.; Duchardt, M.; Pescara, L.P.; Roling, B. Deposition-Type Lithium Metal All-Solid-State Batteries: About the Importance of Stack-Pressure Control and the Benefits of Hot Pressing during Initial Cycling. Adv Mater. Inter 2023, 10, 2202475. [Google Scholar] [CrossRef]
- Deng, C.; Chen, N.; Hou, C.; Liu, H.; Zhou, Z.; Chen, R. Enhancing Interfacial Contact in Solid-State Batteries with a Gradient Composite Solid Electrolyte. Small 2021, 17, 2006578. [Google Scholar] [CrossRef] [PubMed]
- Deysher, G.; Chen, Y.-T.; Sayahpour, B.; Lin, S.W.-H.; Ham, S.-Y.; Ridley, P.; Cronk, A.; Wu, E.A.; Tan, D.H.S.; Doux, J.-M.; et al. Evaluating Electrolyte–Anode Interface Stability in Sodium All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2022, 14, 47706–47715. [Google Scholar] [CrossRef]
- Cao, D.; Ji, T.; Wei, Z.; Liang, W.; Bai, R.; Burch, K.S.; Geiwitz, M.; Zhu, H. Enhancing Lithium Stripping Efficiency in Anode-Free Solid-State Batteries through Self-Regulated Internal Pressure. Nano Lett. 2023, 23, 9392–9398. [Google Scholar] [CrossRef]
- Wu, B.; Chen, C.; Danilov, D.L.; Chen, Z.; Jiang, M.; Eichel, R.; Notten, P.H.L. Dual Additives for Stabilizing Li Deposition and SEI Formation in Anode-Free Li-Metal Batteries. Energy Amp; Env. Mater. 2023, e12642. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Yang, J.; Wang, J.; Yao, L.; Yao, X.; Cui, P.; Xu, X. Interface Re-Engineering of Li 10 GeP 2 S 12 Electrolyte and Lithium Anode for All-Solid-State Lithium Batteries with Ultralong Cycle Life. ACS Appl. Mater. Interfaces 2018, 10, 2556–2565. [Google Scholar] [CrossRef]
- Humana, R.M.; Ortiz, M.G.; Thomas, J.E.; Real, S.G.; Sedlarikova, M.; Vondrak, J.; Visintin, A. Characterization of Anodes for Lithium-Ion Batteries. J Solid State Electrochem 2016, 20, 1053–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Nguyen, M.; Cho, J.; Katyal, N.; Vishnugopi, B.S.; Hao, H.; Fang, R.; Wu, N.; Liu, P.; et al. Stable Anode-Free All-Solid-State Lithium Battery through Tuned Metal Wetting on the Copper Current Collector. Adv. Mater. 2023, 35, 2206762. [Google Scholar] [CrossRef]
- Garcia-Calvo, O.; Gutiérrez-Pardo, A.; Combarro, I.; Orue, A.; Lopez-Aranguren, P.; Urdampilleta, I.; Kvasha, A. Selection and Surface Modifications of Current Collectors for Anode-Free Polymer-Based Solid-State Batteries. Front. Chem. 2022, 10, 934365. [Google Scholar] [CrossRef]
- Xia, H.; Wang, D.; Wang, Y.; Fu, Z. Study on Stable Lithiophilic Ag Modification Layer on Copper Current Collector for High Coulombic-Efficiency Lithium Metal Anode. J. Electrochem. Soc. 2023, 170, 060546. [Google Scholar] [CrossRef]
- Fuchs, T.; Haslam, C.G.; Moy, A.C.; Lerch, C.; Krauskopf, T.; Sakamoto, J.; Richter, F.H.; Janek, J. Increasing the Pressure-Free Stripping Capacity of the Lithium Metal Anode in Solid-State-Batteries by Carbon Nanotubes. Adv. Energy Mater. 2022, 12, 2201125. [Google Scholar] [CrossRef]
- Li, M.M.; Tripathi, S.; Polikarpov, E.; Canfield, N.L.; Han, K.S.; Weller, J.M.; Buck, E.C.; Engelhard, M.H.; Reed, D.M.; Sprenkle, V.L.; et al. Interfacial Engineering with a Nanoparticle-Decorated Porous Carbon Structure on Β″-Alumina Solid-State Electrolytes for Molten Sodium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 25534–25544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, J.; Zheng, B.; Chen, Z.; Su, Y.; Zhang, M.; Xie, C.; Su, M.; Yang, Y. Constructing a High-Energy and Durable Single-Crystal NCM811 Cathode for All-Solid-State Batteries by a Surface Engineering Strategy. ACS Appl. Mater. Interfaces 2021, 13, 41669–41679. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, X.; Yang, L.; Song, D.; Wu, Y.; Ohsaka, T.; Matsumoto, F.; Wu, J. In Situ Ion-Conducting Protective Layer Strategy to Stable Lithium Metal Anode for All-Solid-State Sulfide-Based Lithium Metal Batteries. Adv Mater. Inter 2021, 8, 2001698. [Google Scholar] [CrossRef]
- Fan, Z.; Ding, B.; Li, Z.; Hu, B.; Xu, C.; Xu, C.; Dou, H.; Zhang, X. Long-Cycling All-Solid-State Batteries Achieved by 2D Interface between Prelithiated Aluminum Foil Anode and Sulfide Electrolyte. Small 2022, 18, 2204037. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, C.; Wu, P.; Yuan, H.; Feng, W.; Liu, Z.; Lu, Y.; Sun, S.; Fu, Z.; Hu, J.; et al. Anode-Free Solid-State Lithium Batteries: A Review. Adv. Energy Mater. 2022, 12, 2201044. [Google Scholar] [CrossRef]
- Miao, X.; Wang, H.; Sun, R.; Wang, C.; Zhang, Z.; Li, Z.; Yin, L. Interface Engineering of Inorganic Solid-State Electrolytes for High-Performance Lithium Metal Batteries. Energy Environ. Sci. 2020, 13, 3780–3822. [Google Scholar] [CrossRef]
- Wu, E.A.; Kompella, C.S.; Zhu, Z.; Lee, J.Z.; Lee, S.C.; Chu, I.-H.; Nguyen, H.; Ong, S.P.; Banerjee, A.; Meng, Y.S. New Insights into the Interphase between the Na Metal Anode and Sulfide Solid-State Electrolytes: A Joint Experimental and Computational Study. ACS Appl. Mater. Interfaces 2018, 10, 10076–10086. [Google Scholar] [CrossRef]
- Shinde, S.S.; Wagh, N.K.; Kim, S.; Lee, J. Li, Na, K, Mg, Zn, Al, and Ca Anode Interface Chemistries Developed by Solid-State Electrolytes. Adv. Sci. 2023, 10, 2304235. [Google Scholar] [CrossRef]
- Gao, X.; Xing, Z.; Wang, M.; Nie, C.; Shang, Z.; Bai, Z.; Dou, S.X.; Wang, N. Comprehensive Insights into Solid-State Electrolytes and Electrode-Electrolyte Interfaces in All-Solid-State Sodium-Ion Batteries. Energy Storage Mater. 2023, 60, 102821. [Google Scholar] [CrossRef]
- Li, W.; Lutz, D.M.; Wang, L.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Peering into Batteries: Electrochemical Insight Through In Situ and Operando Methods over Multiple Length Scales. Joule 2021, 5, 77–88. [Google Scholar] [CrossRef]
- Yang, K.; Liu, D.; Qian, Z.; Jiang, D.; Wang, R. Computational Auxiliary for the Progress of Sodium-Ion Solid-State Electrolytes. ACS Nano 2021, 15, 17232–17246. [Google Scholar] [CrossRef] [PubMed]
- Dutra, A.C.C.; Dawson, J.A. Computational Design of Antiperovskite Solid Electrolytes. J. Phys. Chem. C 2023, 127, 18256–18270. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Shao, H.; Gao, B.; Zhu, Z.; Zhu, Y.; Rao, Y.; Xiang, Y. matExplorer: Visual Exploration on Predicting Ionic Conductivity for Solid-State Electrolytes. IEEE Trans. Visual. Comput. Graph. 2022, 28, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on Modeling of the Anode Solid Electrolyte Interphase (SEI) for Lithium-Ion Batteries. npj Comput Mater 2018, 4, 15. [Google Scholar] [CrossRef]
- Xiao, Y.; Miara, L.J.; Wang, Y.; Ceder, G. Computational Screening of Cathode Coatings for Solid-State Batteries. Joule 2019, 3, 1252–1275. [Google Scholar] [CrossRef]
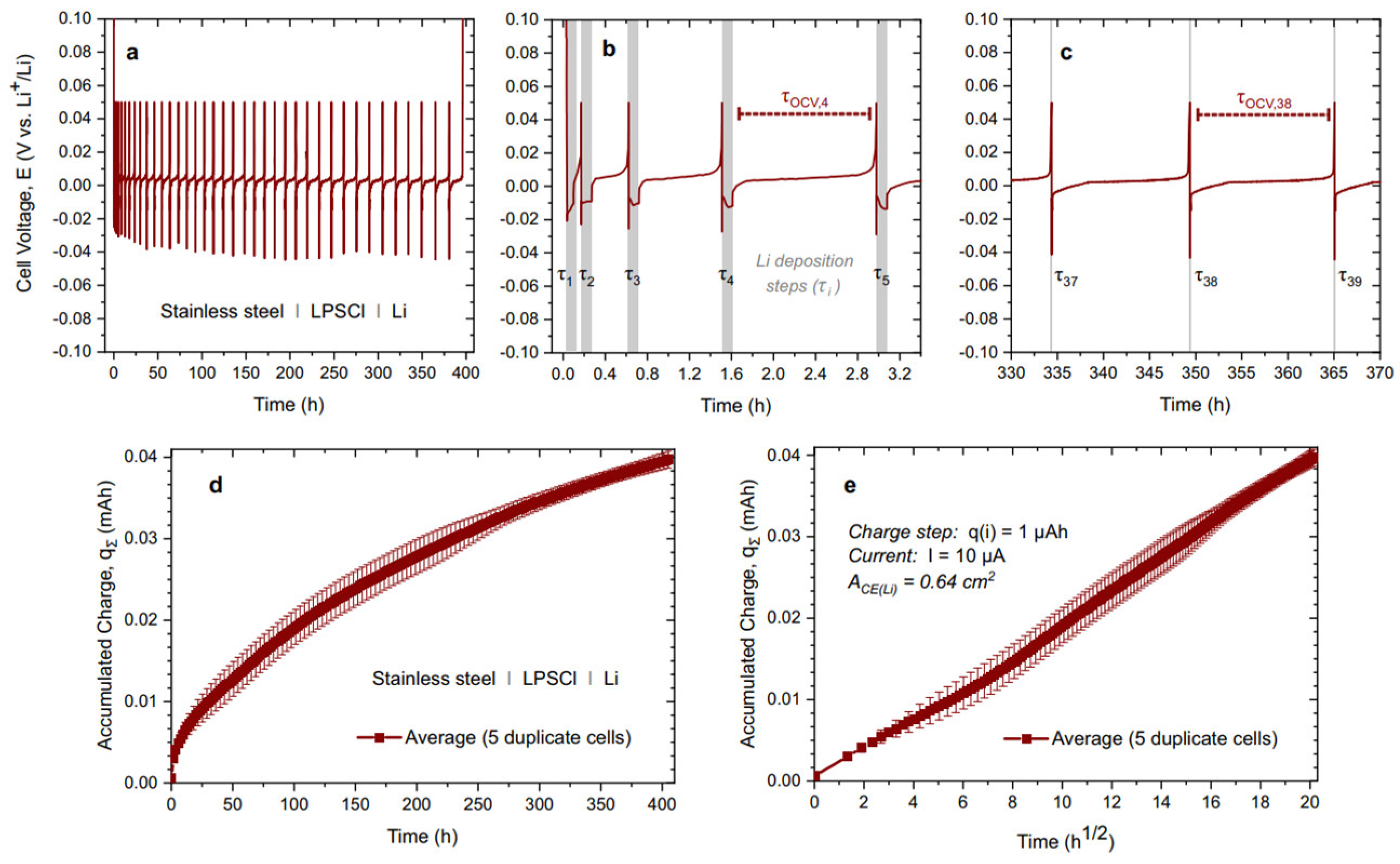
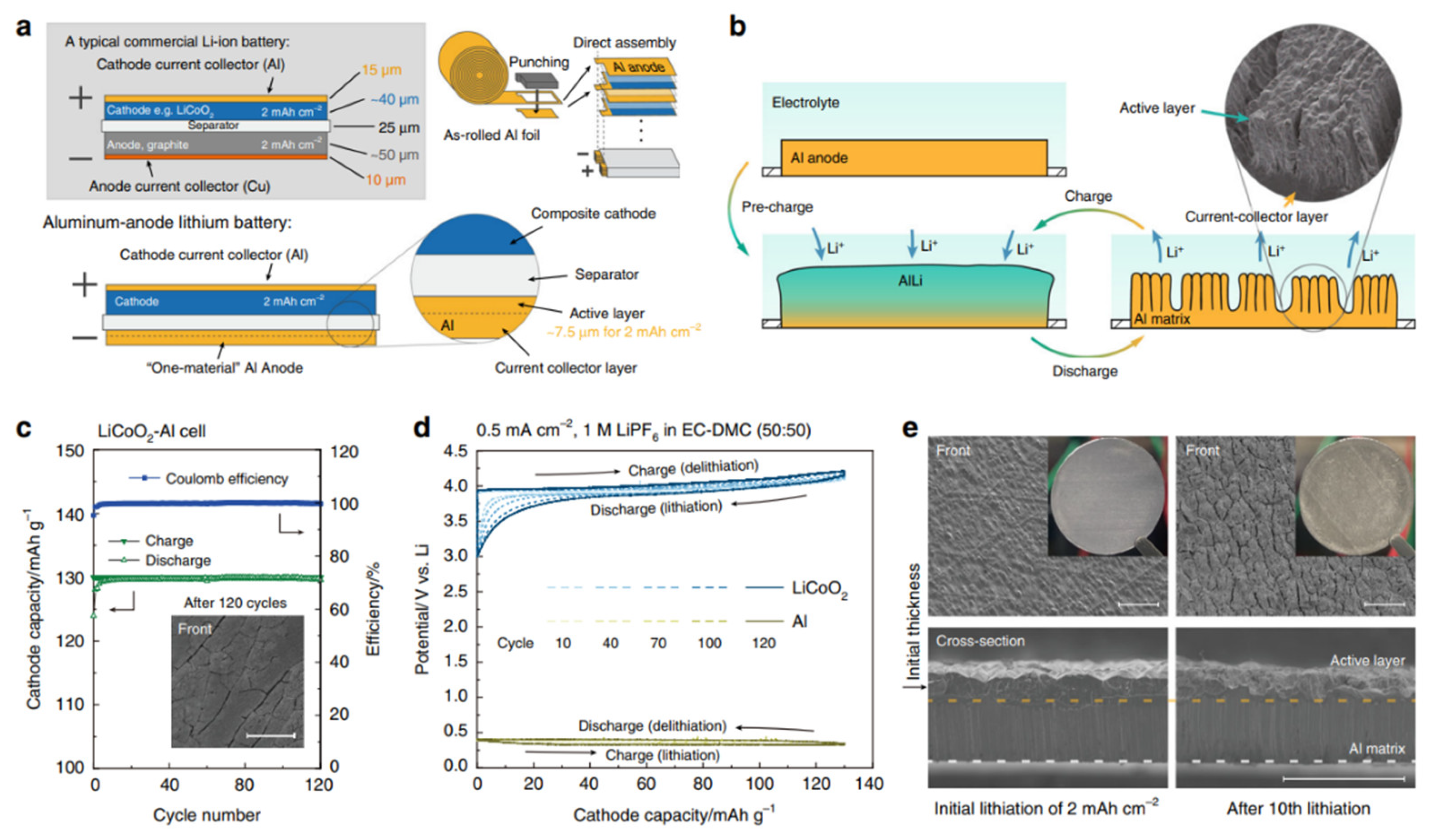
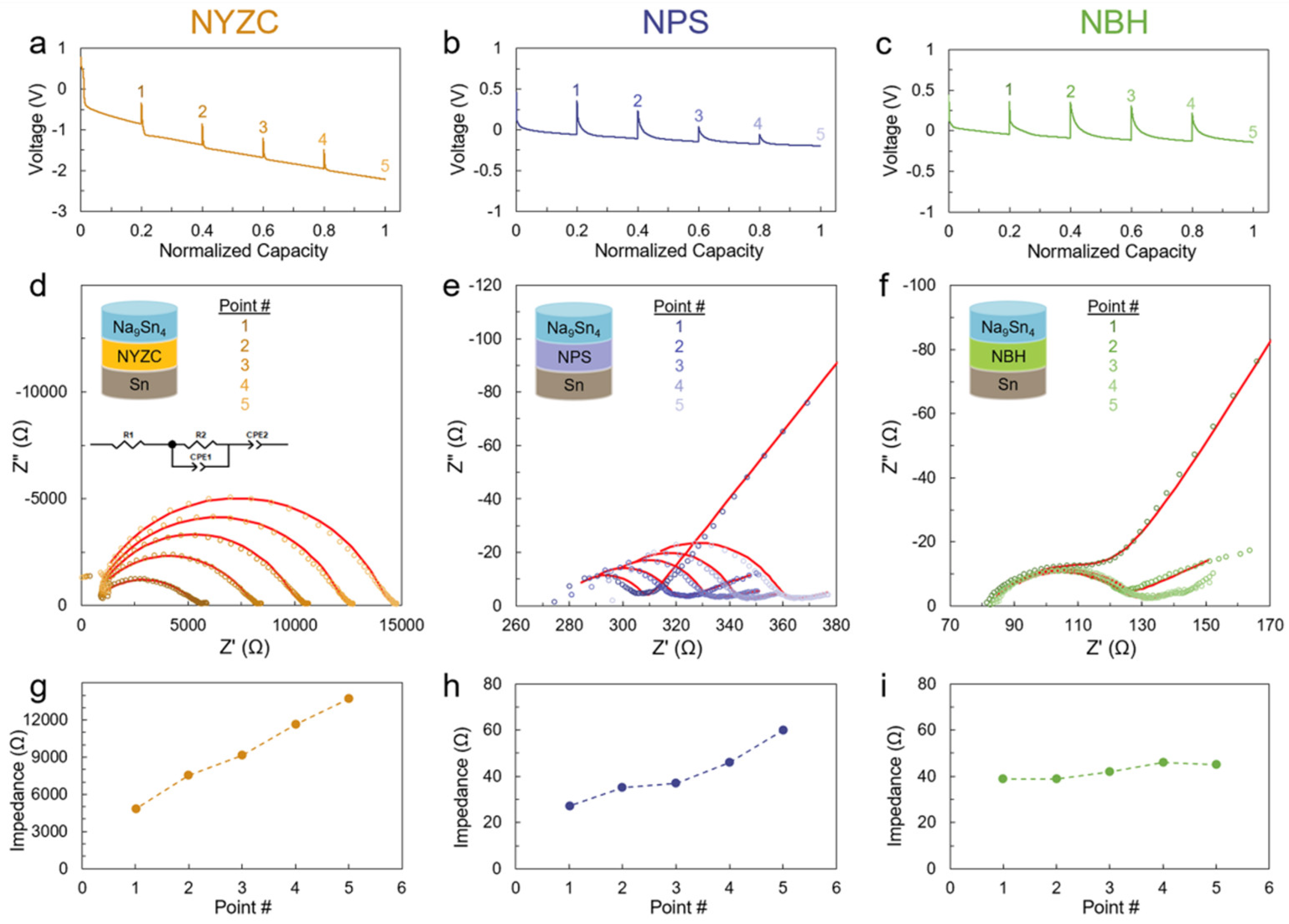
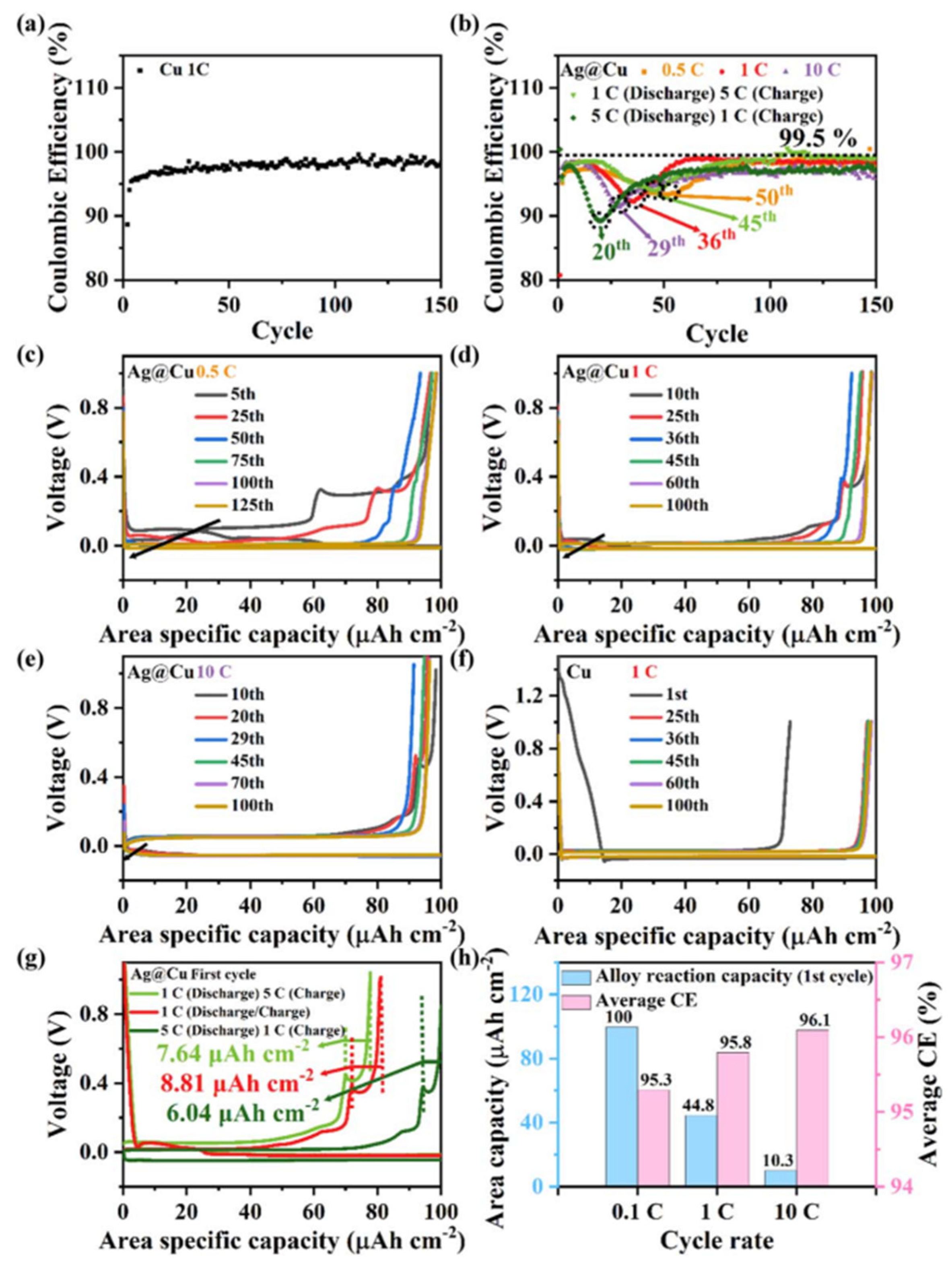
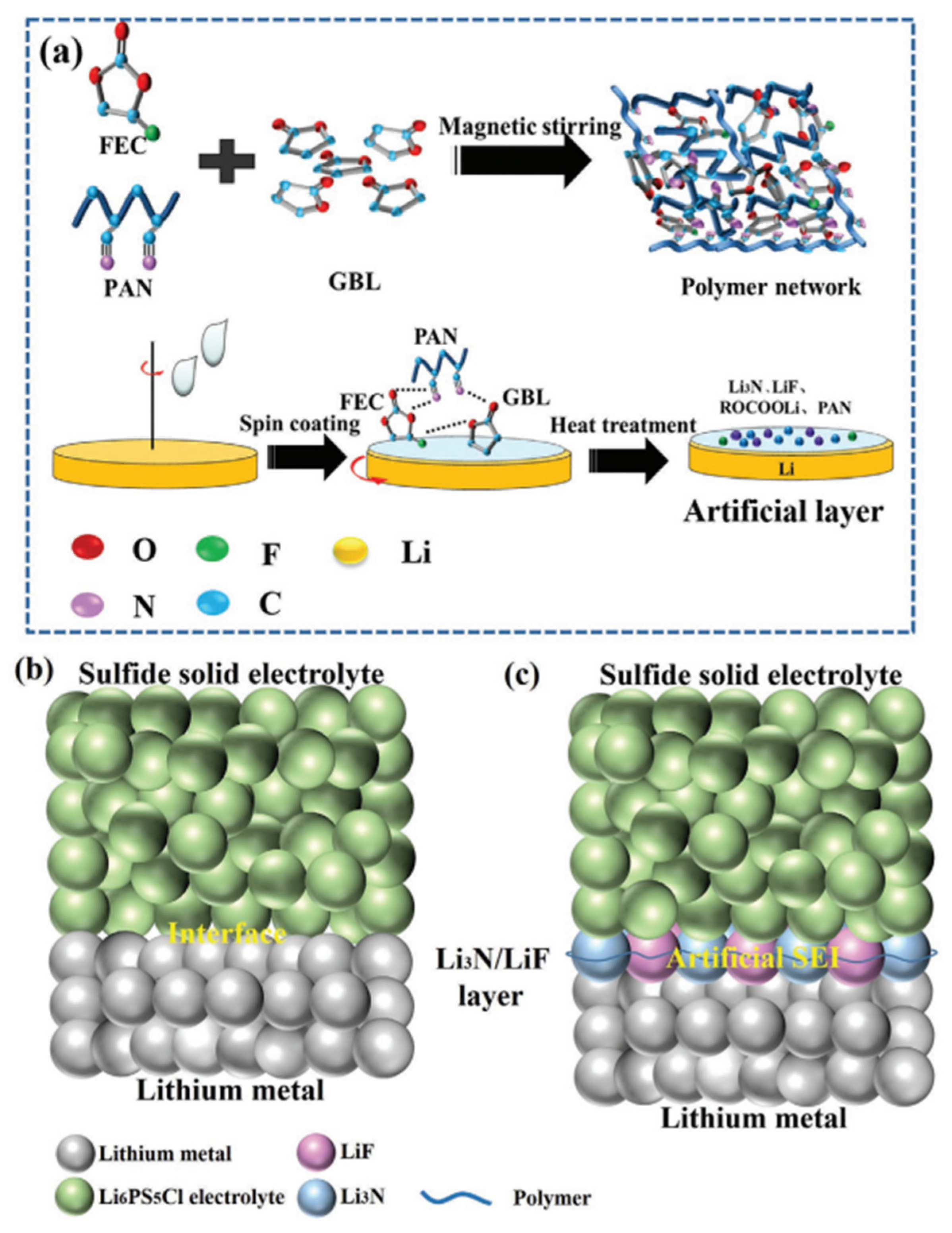
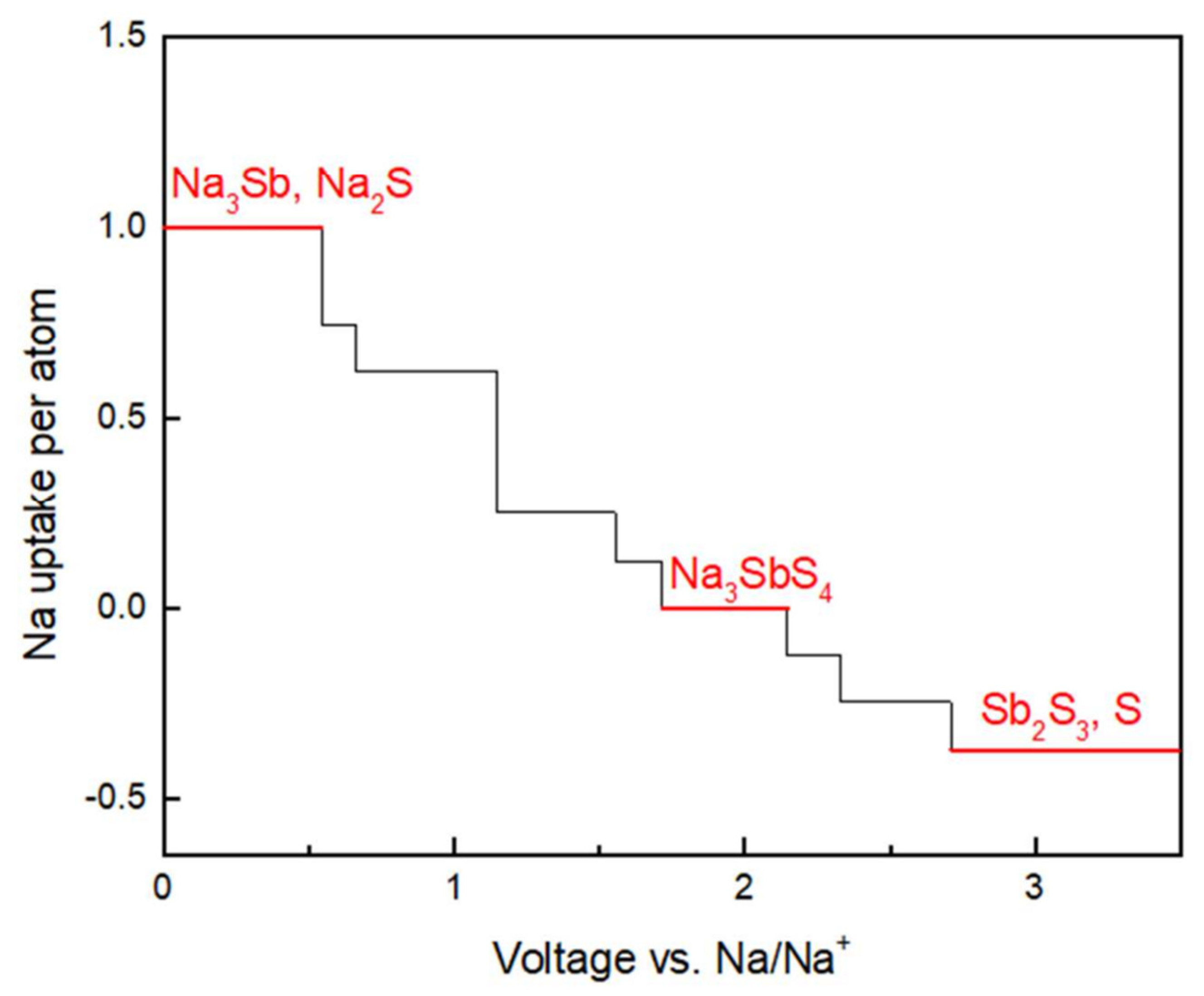
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





