1. Introduction
Breast cancer is the most common malignant tumor in women with an incidence of around 25 % and is responsible for 15 % of all cancer related deaths in women [
1]. Whereas the mortality rates of mammary carcinoma are declining due to improved therapeutic options and early detection screening, the incidence is continuously increasing [
2,
3]. Although non-surgical therapy options including radiotherapy and chemotherapy are available, tumor resection remains the crucial mainstay [
4,
5] and for later stages of breast cancer mastectomy is often inevitable [
6]. This procedure is accompanied with a high degree of psychological strain for the patients leading to a strong desire for breast reconstruction [
7]. Until a few decades ago implant-based reconstruction was the gold standard for breast reconstruction [
8,
9,
10,
11]. However, using the patients’ own tissue in a more regenerative approach has increasingly gained interest in the last years.
Lipofilling or autologous fat grafting (AFT) is one of the most common procedures in plastic surgery. Adipose tissue can be harvested with minimally invasive methods and is rich in adipose tissue-derived stem cells (ADSCs). The immunomodulatory, angiogenic, and proliferation-supporting properties of ADSCs makes adipose tissue an ideal material for regenerative medicine [
12,
13,
14]. Due to complication with silicone implants AFT has also gained increasing interest as therapy after breast tumor resection and mastectomies in the last years [
8]. In contrast to the implant-based reconstruction, the autologous reconstruction prevents the risk of implant failure or foreign body reactions. Additionally, autologous reconstruction leads to a higher patient satisfaction and postoperative quality of life [
7]. When reconstruction with autologous tissue alone is not possible, for example in very lean patients, the additional usage of adipose tissue in the implant-based reconstruction has been shown to improve the aesthetic outcome [
15,
16]. However, there are some concerns regarding the oncological safety of autologous fat grafting. ADSCs may be capable of supporting neoangiogenesis and cell proliferation through paracrine signaling and therefore, ADSCs may contribute to tumor relapse [
17]. On the other hand, antitumoral properties of both ADSCs and extracellular vesicles (EVs) from ADSCs have been reported [
18,
19].
The influence of cells on each other both between the same cells and different cell types often relies on paracrine signaling by secreted particles and molecules [
20]. EVs are defined as naturally secreted particles with a lipid bilayer that are not self-replicatory [
21]. Several subtypes can be identified that differ in terms of particle size as well as the composition of lipids, proteins and nucleic acids [
22]. The most important subtypes are microvesicles and exosomes. Microvesicles are between 100 nm and 1000 nm in diameter and as ectosomes emerge from the outward budding and fission of the plasma membrane. Exosomes (between 30 nm and 100 nm in diameter) are initially formed by endocytosis as endosomes. After combining with a multivesicular body the endosomes are released as exosomes by exocytosis. Exosomes carry the tetraspanin CD63 on the surface. Exosomes have been shown to influence cellular behaviour and might mediate the adaptive immune responses to tumors [
23].
Here, we evaluated the effect of exosomes isolated from adipose tissue-derived stem cells on the viability and gene expression of breast tumor cell line MCF-7 in comparison to the conditioned medium (CM).
2. Results
2.1. Exosome detection
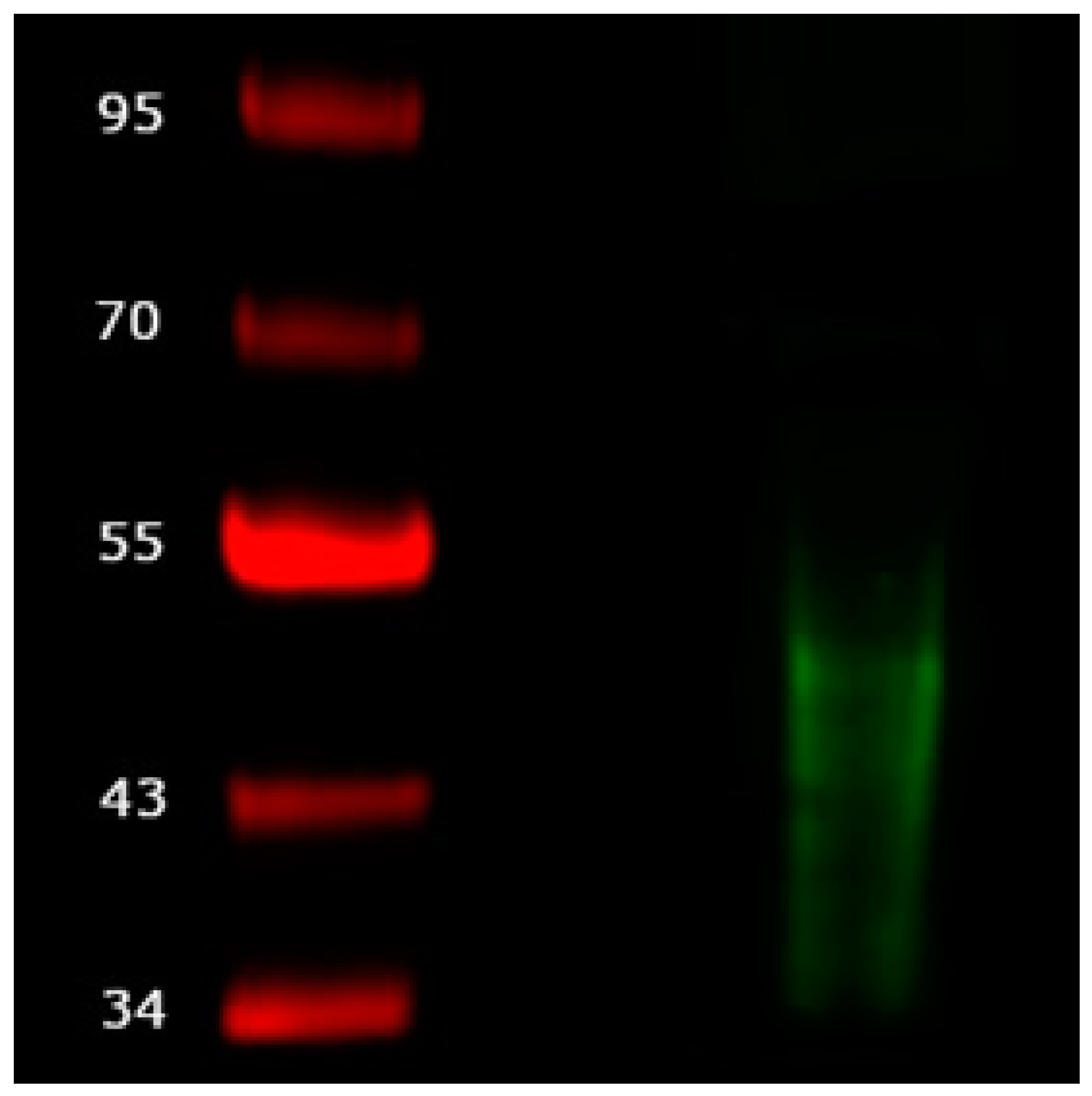
Isolated exosomes were evaluated using western blot. After electrophoretic separation and blotting a signal from the mouse anti-human CD63 antibody can be detected between 30 kDa and 50 kDa. No signal is seen for the conditioned medium (
Figure 1).
2.2. Cell viability and cytotoxicity
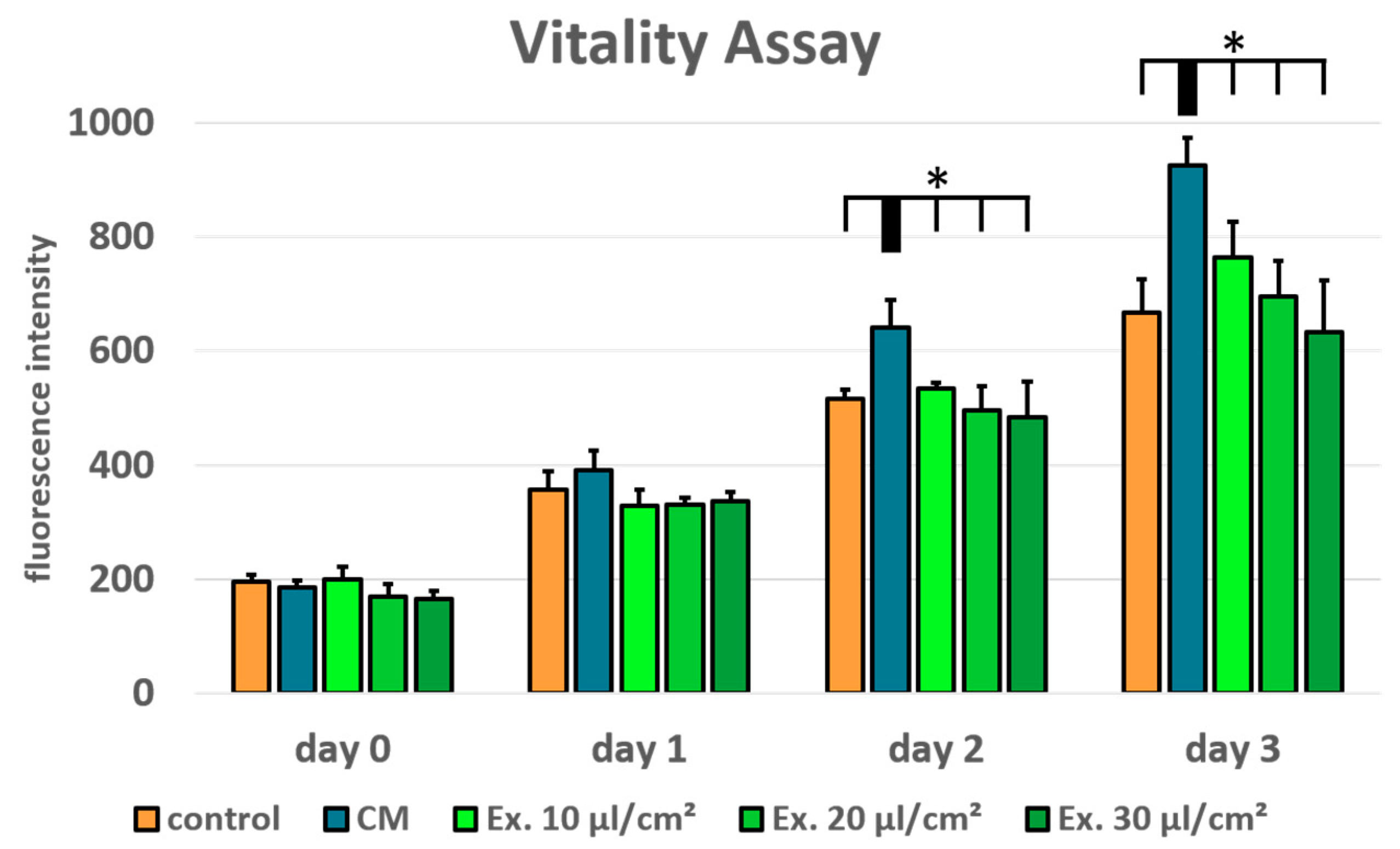
Cell viability was tested using a resazurin assay for MCF-7 treated with conditioned medium and medium supplemented with exosomes in the concentrations 10 µl/cm
2, 20 µl/cm
2, and 30 µl/cm
2. Normal growth medium served as control. In comparison to the conditioned medium, the ES medium shows a concentration dependent negative effect on MCF-7 cell viability. However, in the tested concentrations no significant negative effect in comparison to the control medium can be seen (
Figure 2). ADSC-conditioned medium has a positive effect on MCF-7 cell viability. In comparison to the conditioned medium the ES medium shows a significantly reduced cell viability in all tested concentrations on day 2 and day 3.
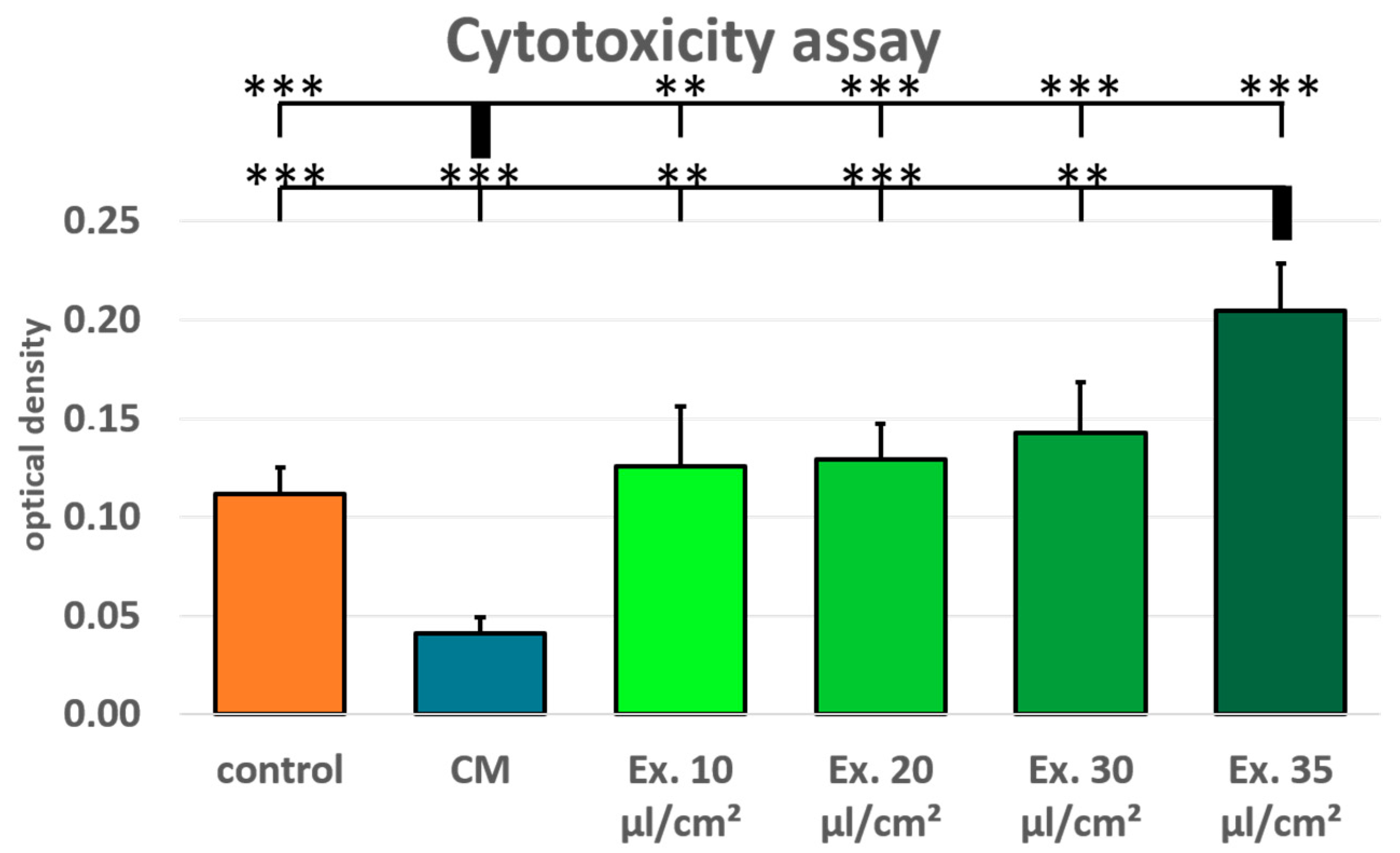
Additionally, cytotoxicity was tested using a LDH assay for MCF-7 treated with conditioned medium and medium supplemented with exosomes in the concentrations 10 µl/cm
2, 20 µl/cm
2, 30 µl/cm
2, and 35µl/cm
2. Normal growth medium served as control. The MCF-7 cells treated with ES medium show an increased LDH release after 24 hours, both in comparison with the conditioned medium and the control medium. LDH release in the conditioned medium was lower than in the control medium, whereas LDH release was higher in all exosome concentrations than in the control medium (
Figure 3). However, only the 35 µl/cm
2 exosome concentration sample was significantly higher than the control medium.
2.3. Gene expression
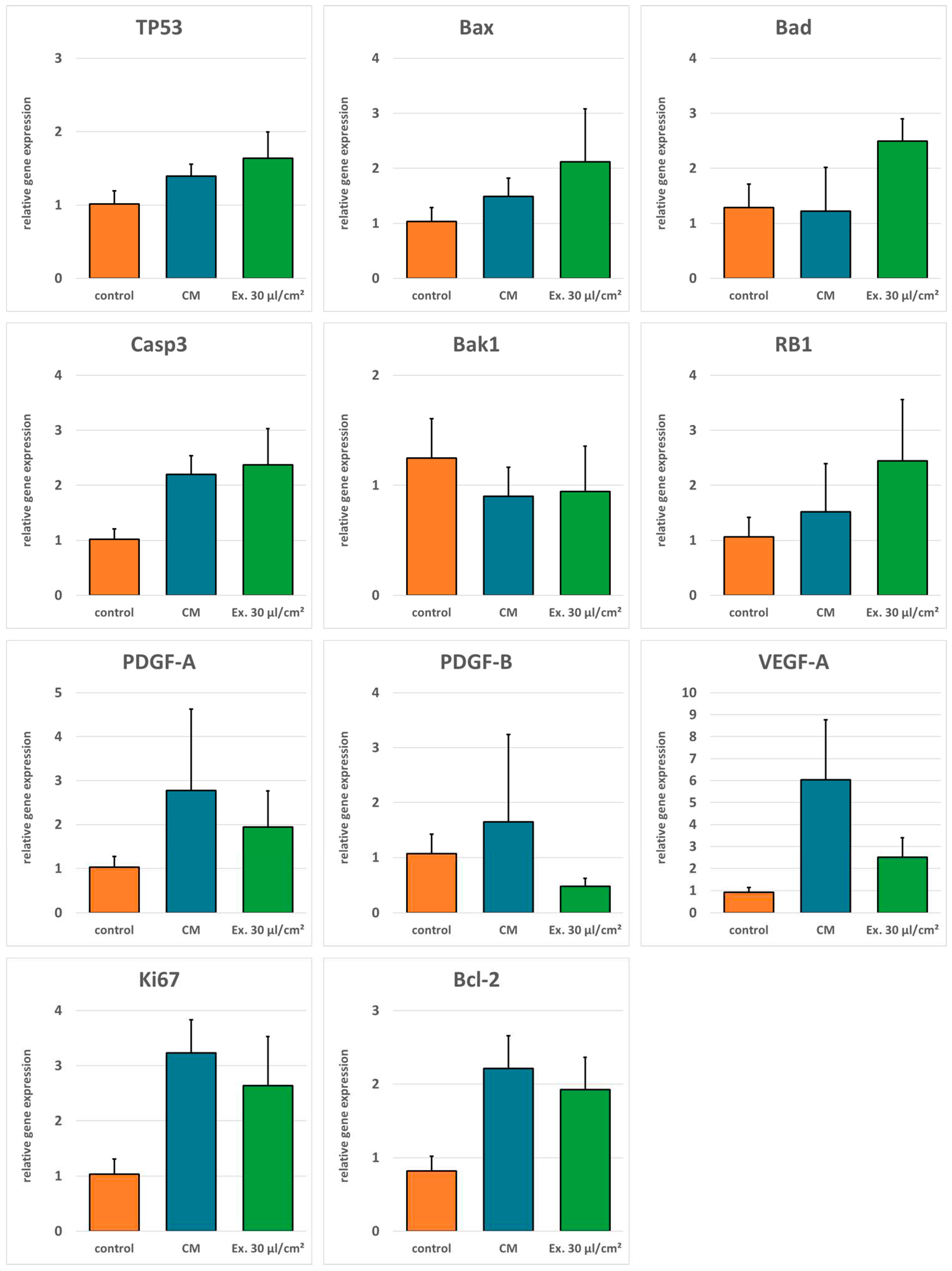
Real time-RT-PCR was done to evaluate gene regulation after exosome treatment. Expression of pro-apoptotic genes TP53, Bax, Bad, Casp3, Bak1, and RB1 was generally higher in cells treated with the exosome medium compared to both the cells treated with the conditioned medium and the control cells. Only pro-apoptotic gene Bak1 was downregulated in exosome treated cells compared to the control. For anti-apoptotic gene Bcl-2, angiogenesis promoting genes VEGF-A, PDGF-A, and PDGF-B, and the proliferation marker Ki67 expression was higher in cells treated with the conditioned medium compared to the exosome medium. However, except for PDGF-B, expression of these genes was not downregulated in exosome treated cells in comparison to the control cells (
Figure 4).
3. Discussion
Lipofilling is commonly used in plastic surgery for the treatment of soft tissue deficits [
24,
25,
26]. Adipose tissue is rich in mesenchymal stem cells which possess a multipotent differentiation potential and have immunomodulatory, angiogenetic and proliferation supporting properties, making it an ideal biomaterial for regenerative medicine [
27,
12,
13,
28,
29]. However, these properties might also exert a prooncogenic effect on residual cells after tumor resection. Therefore, AFT is viewed critically for breast reconstruction after mastectomy.[
30,
31]. On the other hand, anti-tumor properties of mesenchymal stem cells have been reported. Especially exosomes as part of the adaptive immune response to tumors could exert antitumoral properties [
19,
23].
The gold standard for exosome isolation is ultracentrifugation [
32]. Lacking the necessary equipment, we have utilized a method based on magnetic beads. Exosomes were successfully isolated from ADSC-conditioned cell culture medium as confirmed with immunodetection (
Figure 1). CD63 is a marker for exosomes although it has also been detected on the surface of other vesicles [
33,
34]. However, when separated electrophoretically, exosomes show a size range between 30 kDa and 60 kDa [
35,
36], matching the size of the particles detected with our western blot.
Conditioned medium is rich in cytokines and growth factors. It has been shown that ADSC-conditioned medium can increase proliferation in a mammary carcinoma cell line [
37]. This is in accordance with our findings. Compared to control medium, ADCS conditioned medium supported MCF-7 cell proliferation significantly (
Figure 2). Additionally, ADCS conditioned medium causes a reduced LDH release in MCF-7 cells, which suggests protective properties of ADSC from cell death (
Figure 3). For the cytotoxicity assay, ES medium had the adverse effect. LDH release was higher for all tested exosome concentrations than in the control medium. However, only for the highest concentration these differences were statistically significant. In the viability assay, the effects of the ES medium were unambiguou. For all concentrations the cell viability in ES treated cells was lower than in the cells treated with CM, but only for the highest concentration cell viability was lower compared to the control medium, and this difference was statistically not significant. However, the shown viability decrease is concentration dependent, and it is likely that with higher exosome concentrations a more distinct impact on MCF-7 cell viability would have been observed. This is in accordance with the observation that the only significant LDH release increase was seen with the 35 µl/cm
2 concentration which was not tested in the viability assay. Preparation of exosome isolate is material- and time-consuming. The LDH assay showed significant results after 24 hours. Therefore, fewer samples were needed which allowed for the testing of higher exosome concentrations. For the viability assay, new ES medium was needed after every measurement, restricting the examinable concentrations. However, for following studies a higher concentration should be used for the viability assays.
There are several properties a cell needs to acquire to become a tumor cell. Among these hallmarks of cancer are the ability to evade apoptosis as well as sustained angiogenesis [
38,
39]. Therefore, it is important whether ADSCs support angiogenesis and apoptosis resistance in residual tumor cells at the recipient site after AFT. ADSC CM enhances the gene expression of genes for PDGF and VEGF, which are important for neovascularization, of the proliferation marker Ki67, and the antiapoptotic gene Bcl-2 both in comparison to control medium and ES medium. Antiapoptotic genes on the other hand are downregulated in comparison to the ES medium. In contrast, MCF-7 cells treated with ES medium showed increased gene expression for pro-apoptotic genes compared to both cells treated with control medium and cells treated with ADSC CM. This is accordance with other studies where a pro-apoptotic effect of exosomes isolated from mesenchymal stem cells was shown [
40]. In our study, antiapoptotic genes, proliferation marker and angiogenesis-related genes are downregulated in comparison to cells treated with ADSC CM. However, compared with the control medium these markers were upregulated, too for the tested concentration. The strong increase in LDH release observed for the highest exosome concentration supports the hypothesis that a higher exosome concentration could cause a further downregulation of these genes, resulting in increased apoptosis and decreased proliferation and angiogenesis. However, it is also possible that a more distinct gene regulation is unnecessary to explain the pronounced effect observed for the LDH assay. An important factor for the initiation of apoptosis is the ratio between Bcl-2 and Bad. If Bcl-2 is predominant, apoptosis is inhibited; if Bad is predominant apoptosis is induced [
41]. This mechanism might explain why small changes in the relative gene expression of these genes after CM or ES medium incubation can cause a profound difference in apoptosis induction observed with the LDH assay.
The growth factors and cytokines found in ADSC CM support cell proliferation, cell survival, and angiogenesis. Therefore, concerns about the oncological safety of AFT after tumor resection are justified. However, in our own studies we didn’t find any evidence that autologous fat gafting increases the risk for cancer recurrence [
42]. Here, we confirm these properties for the ADSC secretome. However, in most cases of lipofilling these are desired properties to improve the graft take rate. Actually, efforts are being made to improve transplant survival through enhanced angiogenesis and cell survival. In order to reach this goal lipoaspirate is for example enriched with stem cells or supplemented with platelet rich plasma [
43,
44]. Nonetheless a risk for tumor recurrence from the ADSC secretome cannot be dismissed. Exosomes isolated from ADSCs however seem to have a concentration-dependent opposite effect in regard to apoptosis, proliferation, and angiogenesis. Maybe the intentional enrichment of lipoaspirate should be enriched with exosomes prior to injection for tissue augmentation should be considered specifically for post-oncologic patients, just like adipose tissue lipoaspirate is currently augmented with growth factors or growth factor producing stem cells for non-oncologic indications.
One limitation of our study is the exosome isolation method without ultracentrifugation. In future studies the exosome concentration that caused a significant increase in LDH increase or even higher concentrations should be tested for their effect on cell viability and gene expression.
4. Materials and Methods
4.1. Cell Culture
ADSCs were isolated as previously described XXX. Briefly, lipoaspirate from patients that underwent liposuction was enzymatically digested with a 0.05 % collagenase solution (Collagenase from C. histolyticum, Sigma Aldrich) at 37 °C for 60 minutes, filtered through a 100-µm-filter and centrifuged at 500 rcf for 5 minutes. The supernatant was discarded and the cell pellet was washed with PBS (Pan Biotech) and seeded into cell culture flasks with αMEM (Pan Biotech) supplemented with 10 % fetal calf serum (FCS) and 1 % of a penicillin/streptomycin solution (growth medium). When reaching subconfluency cells were detached using a trypsin/EDTA solution and seeded in a density of 5000 cells per cm2. ADSCs in passage 3 were used for all experiments. MCF-7 cell line was purchased from CLS Cell Lines Service (Eppelheim, Germany) and cultured in the same growth medium. Seeding density was 30,000 cells per cm2.
4.2. Medium harvesting, exosome isolation and exosome detection
ADSCs were seeded into cell culture flasks. Upon reaching subconfluency the medium was changed to a serum-free medium. The medium was harvested 24 hours later. For exosome isolation the harvested medium underwent serial centrifugation (300 rcf for 10 minutes, 2000 rcf for 20 minutes and 4500 rcf for 45 minutes) to remove cell debris. The supernatant was transferred to a new centrifugation tube after every centrifugation step and filtered through a 0.2 µm filter after the last centrifugation step. Subsequently, the exosomes were preconcentrated using Centricon Plus-70 Centrifugal Filter Units (Merck Millipore) with a Molecular Weight Cut Off of 100 kDa. In this way 75 ml medium collected from 5 T175 cell culture flasks with a combined growth area of 875 cm2 were concentrated to a volume of ca. 350 µl. For the final exosome isolation from the medium concentrate the Pan Human Exosome Isolation Kit (Miltenyi Biotec) based on magnetic beads-coupled antibodies was used according to the manufacturers’ instructions. The final isolation volume was 100 µl. For the experiments, normal growth medium was supplemented with different concentrations of exosome isolate. For standardization, the volume of exosome isolate per treated cell growth area in µl/cm2 was used as unit.
ADSC-conditioned medium (CM) was also harvested after 24-hour incubation in serum free medium and was supplemented with 10 % FCS prior to the experiments.
Isolated exosomes were detected using western blot. For SDS-PAGE the Mini-PROTEAN® Tetra Cell electrophoresis chamber (BioRad, Hercules, CA, USA) and the PageRuler Prestained NIR Protein Ladder (Thermo Fisher Scientific) were used. After electrophoresis, separated vesicles were blotted onto a nitrocellulose membrane (Amersham Protran 0.2 NC, Sigma-Aldrich) using the Mini Trans-Blot® Cell wet blotting system (BioRad). Membranes were incubated with primary antibody (mouse anti-human CD63 antibody (Thermo Scientific)) overnight at 4 °C, and the next day with infrared-labeled secondary antibody IRDye 680CW goat anti-mouse (Li-Cor Biosciences, Lincoln, NE, USA)) for one hour at room temperature. Visualization was performed using the Odyssey® Infrared Imaging System.
4.3. Viability and cytotoxicity assays
MCF-7 cells were seeded into 96-well plates (4.800 Zellen/cm2) and incubated with different concentrations of exosome isolate, with conditioned medium and normal growth medium as control. Viability was evaluated using a resazurin assay on 4 consecutive days where day 0 refers to the measurement prior to treatment start. Cells were incubated with normal growth medium supplemented with 0.07 µM resazurin (Sigma Aldrich) for two hours. Metabolic conversion of resazurin into the fluorescent resorufin was detected using a multiwell plate reader (excitation: 530 nm, emission: 590 nm, Varioskan, Thermo Scientific). Cytotoxicity was evaluated using the LDH Assay (abcam) according to themanufacturers’ instructions.
4.4. RNA isolation and RT-PCR
MCF-7 cells were seeded into 24 well plates (50,000 cells per well) in triplicates and allowed to adhere. Afterwards, the medium was changed to control medium (normal growth medium), conditioned medium (CM) or exosome supplemented (ES) medium (30 µl/cm
2). RNA was harvested after 24 hours using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Isolated RNA was reversely transcribed using the QuantiTect Reverse Transcription Kit (Qiagen). For real-time RT-PCR the DyNAmo HS SYBR Green qPCR Kit (Life Technologies) with the Eco™ Real-Time PCR System (Illumina, San Diego, CA, USA) was used. Primers were designed using Primer3 and were purchased from Eurofins MWG Operon. Primer sequences are listed in
Table 1. The relative gene expressions were calculated using the ΔΔC
t method XVX181, and normalized to the control using glycerinaldehyd-3-phosphat-dehydrogenase (GAPDH) as the housekeeping gene. The gene expression was evaluated for genes related to angiogenesis and apoptosis.
Author Contributions
“Conceptualization, O.F. and L.P.; methodology, S.V.; software, S.V.; validation, O.F., A.E. and S.K.; formal analysis, A.E.; investigation, O.F., S.V., and A.E.; resources, S.K. and L.P..; data curation, S.V.; writing—original draft preparation, S.V.; writing—review and editing, O.F. and S.K.; visualization, S.V.; supervision, L.P.; project administration, L.P.; funding acquisition, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University Hospital Regensburg (08/117).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciuba, A.; Wnuk, K.; Nitsch-Osuch, A.; Kulpa, M. Health Care Accessibility and Breast Cancer Mortality in Europe. Int J Environ Res Public Health 2022, 19, 13605. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Francisci, S.; Capocaccia, R.; Verdecchia, A.; Allemani, C.; Berrino, F. Time Trends of Breast Cancer Survival in Europe in Relation to Incidence and Mortality. Int J Cancer 2006, 119, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Canelo-Aybar, C.; Ferreira, D.S.; Ballesteros, M.; Posso, M.; Montero, N.; Solà, I.; Saz-Parkinson, Z.; Lerda, D.; Rossi, P.G.; Duffy, S.W.; et al. Benefits and Harms of Breast Cancer Mammography Screening for Women at Average Risk of Breast Cancer: A Systematic Review for the European Commission Initiative on Breast Cancer. J Med Screen 2021, 28, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, M.M.; Jean, J.; Graham, R.; Chatterjee, A. Surgical Trends in Breast Cancer: A Rise in Novel Operative Treatment Options over a 12 Year Analysis. Breast Cancer Res Treat 2019, 173, 267–274. [Google Scholar] [CrossRef]
- Zhu, H.; Doğan, B.E. American Joint Committee on Cancer’s Staging System for Breast Cancer, Eighth Edition: Summary for Clinicians. Eur J Breast Health 2021, 17, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J Pers Med 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.A. da C.; Bailão-Junior, A.; de Oliveira-Junior, I. Does Breast Oncoplastic Surgery Improve Quality of Life? Front Oncol 2022, 12, 1099125. [CrossRef] [PubMed]
- Djohan, R.; Gage, E.; Bernard, S. Breast Reconstruction Options Following Mastectomy. Cleve Clin J Med 2008, 75 Suppl 1, S17–23. [Google Scholar] [CrossRef]
- Atisha, D.; Alderman, A.K.; Lowery, J.C.; Kuhn, L.E.; Davis, J.; Wilkins, E.G. Prospective Analysis of Long-Term Psychosocial Outcomes in Breast Reconstruction: Two-Year Postoperative Results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg 2008, 247, 1019–1028. [Google Scholar] [CrossRef]
- Krastev, T.K.; Schop, S.J.; Hommes, J.; Piatkowski, A.A.; Heuts, E.M.; van der Hulst, R.R.W.J. Meta-Analysis of the Oncological Safety of Autologous Fat Transfer after Breast Cancer. Br J Surg 2018, 105, 1082–1097. [Google Scholar] [CrossRef]
- Wederfoort, J.L.M.; Kleeven, A.; Hommes, J.E.; Van Kuijk, S.M.J.; van der Hulst, R.R.W.J.; Piatkowski, A.; M. D for The Breast trial investigators Aesthetic Evaluation of Breast Reconstruction with Autologous Fat Transfer vs. Implants. Aesthetic Plast Surg 2023, 47, 593–604. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Biol Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.Y.; Mirzabeigi, M.N.; Vonderhaar, R.J.; Bucky, L.P. Utilizing Large Volume Fat Grafting in Breast Reconstruction after Nipple Sparing Mastectomies. Gland Surg 2018, 7, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.; Schmauss, D.; Brucato, D.; Harder, Y. Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind? J Clin Med 2022, 11, 3079. [Google Scholar] [CrossRef] [PubMed]
- Piccotti, F.; Rybinska, I.; Scoccia, E.; Morasso, C.; Ricciardi, A.; Signati, L.; Triulzi, T.; Corsi, F.; Truffi, M. Lipofilling in Breast Oncological Surgery: A Safe Opportunity or Risk for Cancer Recurrence? Int J Mol Sci 2021, 22, 3737. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor microRNA to Breast Cancer Cells. Mol Ther 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Rezaie, Z.; Ardeshirylajimi, A.; Ashkezari, M.D.; Seifati, S.M. Antitumoral Potential of Microvesicles Extracted from Human Adipose-Derived Mesenchymal Stem Cells on Human Breast Cancer Cells. J Cancer Res Ther 2019, 15, 1114–1119. [Google Scholar] [CrossRef]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy. ” Biomolecules 2021, 11, 878. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alonso, M.L.; García-Posadas, L.; Diebold, Y. Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos. Stem Cell Rev Rep 2022, 18, 854–901. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Zhang, Z.-R.; Schluesener, H.J.; Xu, S.-Q. Role of Exosomes in Immune Regulation. J Cell Mol Med 2006, 10, 364–375. [Google Scholar] [CrossRef]
- Rohrich, R.J. The American Society of Plastic Surgeons’ Procedural Statistics: What They Really Mean. Plast Reconstr Surg 2003, 112, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Gir, P.; Brown, S.A.; Oni, G.; Kashefi, N.; Mojallal, A.; Rohrich, R.J. Fat Grafting: Evidence-Based Review on Autologous Fat Harvesting, Processing, Reinjection, and Storage. Plast Reconstr Surg 2012, 130, 249–258. [Google Scholar] [CrossRef]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, Applications, and Outcomes of Autologous Fat Grafting. Ann Med Surg (Lond) 2017, 20, 49–60. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic Review of Patient Factors Affecting Adipose Stem Cell Viability and Function: Implications for Regenerative Therapy. Stem Cell Res Ther 2017, 8, 45. [Google Scholar] [CrossRef]
- Zou, J.; Wang, W.; Kratz, K.; Xu, X.; Nie, Y.; Ma, N.; Lendlein, A. Evaluation of Human Mesenchymal Stem Cell Senescence, Differentiation and Secretion Behavior Cultured on Polycarbonate Cell Culture Inserts. Clin Hemorheol Microcirc 2018, 70, 573–583. [Google Scholar] [CrossRef]
- Krastev, T.K.; Jonasse, Y.; Kon, M. Oncological Safety of Autologous Lipoaspirate Grafting in Breast Cancer Patients: A Systematic Review. Ann Surg Oncol 2013, 20, 111–119. [Google Scholar] [CrossRef]
- Cohen, S.; Sekigami, Y.; Schwartz, T.; Losken, A.; Margenthaler, J.; Chatterjee, A. Lipofilling after Breast Conserving Surgery: A Comprehensive Literature Review Investigating Its Oncologic Safety. Gland Surg 2019, 8, 569–580. [Google Scholar] [CrossRef]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of Extracellular Vesicles with Combined Enrichment Methods. J Chromatogr B Analyt Technol Biomed Life Sci 2021, 1169, 122604. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA Profiles in Subpopulations of Extracellular Vesicles: Apoptotic Bodies, Microvesicles and Exosomes. J Extracell Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Cheng, T.-S.; Liao, H.-J.; Chuang, M.-H.; Chen, H.-T.; Chen, C.-H.; Zhang, K.-L.; Chang, C.-H.; Lin, P.-C.; Huang, C.-Y.F. Mesenchymal Stem Cell Secreted-Extracellular Vesicles Are Involved in Chondrocyte Production and Reduce Adipogenesis during Stem Cell Differentiation. Tissue Eng Regen Med 2022, 19, 1295–1310. [Google Scholar] [CrossRef]
- Oksvold, M.P.; Neurauter, A.; Pedersen, K.W. Magnetic Bead-Based Isolation of Exosomes. Methods Mol Biol 2015, 1218, 465–481. [Google Scholar] [CrossRef]
- Kowal, E.J.K.; Ter-Ovanesyan, D.; Regev, A.; Church, G.M. Extracellular Vesicle Isolation and Analysis by Western Blotting. Methods Mol Biol 2017, 1660, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, S.; Gencarelli, J.; Gareau, A.J.; Levatte, T.; Dugandzic B Sc, A.; Johnston, B.; Bezuhly, M. Promotion of Primary Murine Breast Cancer Growth and Metastasis by Adipose-Derived Stem Cells Is Reduced in the Presence of Autologous Fat Graft. Plast Reconstr Surg 2019, 143, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Takahara, K.; Ii, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem Cells Dev 2016, 25, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-L.; Zhang, X.; Zhang, J.-Y.; Hou, L.; Tian, R.-H. The Mechanisms on Apoptosis by Inhibiting VEGF Expression in Human Breast Cancer Cells. Int Immunopharmacol 2009, 9, 389–395. [Google Scholar] [CrossRef]
- Kempa, S.; Brix, E.; Heine, N.; Hösl, V.; Strauss, C.; Eigenberger, A.; Brébant, V.; Seitz, S.; Prantl, L. Autologous Fat Grafting for Breast Reconstruction after Breast Cancer: A 12-Year Experience. Arch Gynecol Obstet 2022, 305, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Toyserkani, N.M.; Quaade, M.L.; Sørensen, J.A. Cell-Assisted Lipotransfer: A Systematic Review of Its Efficacy. Aesthetic Plast Surg 2016, 40, 309–318. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; van Boxtel, J.; Harmsen, M.C.; Stevens, H.P. The Development of Facial Lipofilling from a Historical Point of View. Facial Plast Surg 2019, 35, 358–367. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).