1. Introduction
The concept of biochemical recurrence (BCR) in prostate cancer (PC) and the relevance it has in clinical practice is unique if compared with in other neoplasms. BCR represents, in most cases, the first form of failure after primary treatment with curative intent. It is certainly a concept better defined after radical prostatectomy (RP) but still present even after radiotherapy (RT), it develops in a significant percentage of cases subjected to such treatments and defines their limits in terms of radicality.
In the case of BCR, the patient is informed that the primary treatment has not achieved radicality and will probably never achieve it, however he is an asymptomatic patient in full well-being. The isolated progressive increase in total prostatic specific antigen (PSA) in these patients describes the stage of the disease with completely negative imaging and clinical findings. It is important to remember that these patients are in a condition of complete well-being and that in a non-negligible percentage of cases, they will remain in this condition for a period of more than 5 years, without developing clinical or radiological progression. In these cases, active surveillance without treatment may represent the best choice, often thwarted by a “PSA syndrome” with close checks and initiation of therapies against PSA rather than against PC. On the other hand, a percentage of these BCRs shortly develops clinical and radiological progression with the appearance of distant metastases. The survival and quality of life of a patient with non-metastatic PC is certainly better than the metastatic PC and the prevention of the development of a metastatic phase, even if hormone sensitive, is certainly a significant target for these subjects. Consequently, patients with BCR represent an extremely heterogeneous PC category and one of the most complex phases to manage.
The first unmet need is to correctly stratify patients between BCR at low and high risk of early progression to a metastatic disease, identifying the factors useful for this definition. The second need is to free low-risk BCR patients from unnecessary and often harmful treatments as well as from overly burdensome follow-ups. The third target is to prolong as much as possible through effective treatments, the phase of simple BCR in the high-risk group, preventing and slowing down the progression to the metastatic stage.
BCR represents one of the fields of PC where precision medicine and a tailored management based on predictive parameters not limited to PSA is necessary. On the contrary, to date the management of BCR remains extremely heterogeneous in different centers, often submitted to an overtreatment with different strategies.
The concept of anticipation and intensification of therapy which in recent years has overwhelmed PC starting from the later phases up to globally affecting metastatic hormone sensitive patients, will soon also involve patients with BCR after primary treatment. This aspect makes it even more urgent to correctly stratify and define patients with BCR at low and high risk of early progression to the metastatic phase so as to precisely identify when an anticipation and intensification of treatment may be considered appropriate.
2. Definition and incidence of biochemical recurrence after primary treatments
2.1. Definition and incidence
RP and RT for PC generally provide pleasing disease control and favorable long-term survival. However, between 27% and 53% of all patients undergoing RP or RT develop a PSA recurrence [
1]. Approximately 30% (20-40%) of patients following RP and 30-50% of males treated with RT will experience BP within 10 years post-therapy [
2]. For several years, PSA has been used for detection of recurrent and progressive disease after primary treatments. According to the European Association of Urology (EAU) guidelines, a progressive and confirmed serum PSA level > 0.2 ng/mL after RP is considered a biochemical recurrence [
3]. The use of ultrasensitive PSA assays for routine post-RP follow-up is still debatable. Males who have a PSA nadir of less than 0.01 ng/mL have a high (96%) chance of not having a relapse within two years. Furthermore, clinical features such as ISUP grade and surgical margin status combined with a post-RP PSA levels > 0.01 ng/mL are able to predict a BCR and be helpful in determining follow-up intervals [
4]. A PSA increase > 2 ng/mL higher than the PSA nadir value, regardless of the serum concentration of the nadir is the RTOG-ASTRO Phoenix Consensus Conference definition of PSA failure following primary RT [
5]. The frequent association of androgen deprivation therapy (ADT) with RT in intermediate and high-risk PC can make the definition and identification of BP more complex.
2.2. How to define low and high risk BCR
Over time, it was possible to realize that PSA relapse after primary treatments has different meanings accordingly to clinicopathological features, as Gleason score, PSA doubling time (PSA-DT), clinical stage and surgical margins status.
Table 1.
Which parameters can contribute to distinguish BCR after primary treatments at low and high risk for early clinical progression. BCR= Biochemical recurrence. HRR= homologous recombinant repair. PV= pathogenetic variant.
Table 1.
Which parameters can contribute to distinguish BCR after primary treatments at low and high risk for early clinical progression. BCR= Biochemical recurrence. HRR= homologous recombinant repair. PV= pathogenetic variant.
| Parameter |
Low Risk BCR |
High Risk BCR |
| T stage |
T2 |
T3 |
| ISUP grading |
1-2 |
3-5 |
| Surgical margins |
negative |
positive |
| Time to primary treatment |
>12 months |
≤12 months |
| Pretreatment PSA value |
< 10 ng/ml |
≥ 10 ng/ml |
| PSA DT |
≥ 10 months |
< 10 months |
| HRR PV |
BRCA2 - |
BRCA2 + |
EAU guidelines recommend the first PSA test three months after surgery, but PSA levels should be undetectable within four weeks and early testing at 4–8 weeks after surgery could be useful for determining patient prognosis [
6]. After 4-8 weeks from RP, 5-20% of PC patients shows detectable or persistent PSA > 0.1 ng/ml [
7]. It may result from persistent local disease, pre-existing metastases or residual benign prostate tissue.
Different definitions of PSA kinetics (PSA-DT and PSA velocity) have been used to stratify patients with BCR into low or high risk for early clinical or metastatic progression [
8]. In non-metastatic castration resistant PC (nmCRPC), PSA-DT has been associated with metastasis-free survival and overall survival (OS) results and is used to identify high-risk nmCRPC patients who benefit from intensified therapy (PSA-DT threshold < 10 months) [
9].
According to the results of a systematic review and meta-analysis of Van den Broeck et al., patients with BCR after primary treatments have a higher risk of developing distant metastases, prostatic cancer specific and overall mortality (OM) [
10]. Nevertheless, the effect size of BP as a risk factor for mortality is extremely variable [
11]: after primary RP its impact ranges from HR 1.03 (95% CI: 1.004–1.06) to HR 2.32 (95% CI: 1.45–3.71) [
12] whereas after primary RT, OS rates are approximately 20% lower at 10-year follow-up even in men with minimal co-morbidity [
13]. In this systematic review, different clinical and pathologic variables (e.g., T-category, PSA, ISUP grade) and PSA kinetics (PSA-DT and interval to PSA failure), significantly predict the probability of future metastases, PC-specific mortality, and OM [
10]. Recently, Falagario et al., on 16,311 PC cases, evaluated the association of BP after RP or RT with prostate cancer specific mortality (PCSM) [
14]. Following RP, the 15-year cumulative incidence of BCR was 16% (95% CI, 15%-18%) in low-risk PC, 30% (95% CI, 27%-32%) in intermediate risk group, and 46% (95% CI, 42%-51%) in high-risk PC. Following RT, the 15-year cumulative incidences of BCR were 18% (95% CI, 15%-21%) in low-risk PC, 24% (95% CI, 21%-26%) in intermediate-risk group, and 36% (95% CI, 33%-39%) in high-risk PC. The 10-year cumulative incidence of prostate cancer specific mortality (PCSM) after RP and RT was 4% (95% CI, 2%-6%) and 24% (95% CI, 19%-29%) in low-risk BCR (Gleason score <8 and PSADT >12 mo) and 9% (95% CI, 5%-13%) and 46% (95% CI, 40%-51% in high-risk BCR (Gleason score ≥8 or PSADT ≤12 mo), respectively. In patients with BCR after RP the following outcomes were found to be significantly correlated with PCSM and OM: high pathologic ISUP grade, short interval to biochemical failure, short PSA-DT, positive surgical margins, high pT category.
The corresponding results for patients with BCR following RT as predictors of PCSM and OS were short interval to biochemical failure; distant metastatic recurrence: high biopsy ISUP grade, high cT category, short interval to biochemical failure, high initial (pre-treatment) PSA.
Homologous recombinant repair (HRR) pathway of DNA damage repair (DDR) genes family and in particular BRCA1-2 pathogenetic variants (PV) are now used to tailor therapy with poly-ADP-ribose polymerase (PARP) inhibitors in metastatic CRPC. An early determination of somatic HRR PVs in non-metastatic PC could have a prognostic impact on the oncological outcomes of these patients and could help to better define high and low risk cases for a metastatic clinical progression. Castro et al. evaluated the prognostic value of BRCA PV in terms of risk for metastatic progression and cancer specific survival after RP or RT for localized PC [
15]. At 3,5,10-year interval from primary treatments, 97%,94% and 84% respectively of BRCA- and 90%,72% and 50% respectively of BRCA+ cases were free of metastasis (p<0.001). The cancer specific survival rates were significantly better in the BRCA- cohort (99%,97% and 85% respectively at 3,5,10 years) than in the BRCA+ (96%,76% and 61%, respectively; p<0.001). Also at multivariate analysis the risk of metastasis-free survival (MFS) was significantly lower in BRCA+ cases (HR 2.36; CI 1.38-4.03; p=0.002).
3. Imaging for biochemical recurrence
In the event of BCR key question remains whether the PSA rise is reflective of metastatic disease or is a consequence of a locally confined recurrence. With the development of new metastasis-directed therapies (MDTs) or the possibility of local treatments even for metastatic patients with a low disease burden, a correct identification and use of imaging is essential for a tailored treatment planning [
16].
A recent systematic review by De Visschere et al. evaluated the role of the existing imaging techniques in early recurrent PC [
17]. A total of 98 studies evaluating transrectal ultrasonography (TRUS), computed tomography (CT), bone scintigraphy (BS), multiparametric magnetic resonance imaging (mpMRI), whole-body MRI (wbMRI), and positron emission tomography (PET)-CT/MRI using different tracers were included. TRUS is rarely used in this setting and the few studies evaluating its efficacy present conflicting results. Despite that, color/power Doppler, contrast enhanced TRUS or multiparametric ultrasound are emerging as adjuvant techniques to increase the sensitivity and specificity of grayscale TRUS [
18]. Authors found three studies reporting the detection rates of CT in the setting of early PC recurrence. Sensitivity and specificity were very low both for local recurrence and distant metastasis detection meaning that morphological and size criteria are not enough especially for lymph nodes or for low metastatic burden. Despite the non-unanimous results presented by De Visschere et al., mpMRI shows good accuracy when it comes to local recurrence detection, even with low PSA levels. With PSA levels ≤5.0 ng/mL, positivity rates ranged from 66.7% to 86.7% and sensitivity was around 90%, but the detection rate was good even in subgroups with lower PSA threshold. Panebianco et al. obtained a sensitivity of 94–100% and a specificity of 92–97% in 116 patients with recurrent PSA 1.4–2.9 ng/ml, while in a study comparing mpMRI with 18F choline PET-CT for local recurrence, the former showed sensitivity of 92.0%, specificity of 75.0%, and a positivity rate of 82.1% with PSA levels of 0.8–1.4 ng/mL [
19,
20]. BS has been widely used for the detection of bone metastases in PC patients. De Visschere et al. found three studies reporting detection rates of BS in the early recurrence setting. The detection rate ranged from 10% in cases with a recurrent PSA <1.0 ng/ml to 20% when a level of <4.0 ng/mL was considered [
17]. The 18F FDG-PET-CT has not been widely used in the setting of PC, mainly because of the low glucose metabolism of PC cells and the accumulation of the tracer in the urinary system [
21]. A 2020 retrospective analysis by Michaud et al. evaluated the role of 11C-choline PET/CT in detecting BCR PC in 282 cases [
22], scoring the level of suspicion in 0, negative; 1, equivocal; 2, positive by 2 readers. A score of 2 was found in 28%, 46%, 62%, and 81%, respectively, for patients with PSA < 0.5 ng/mL, 0.5–0.99 ng/mL, 1.0–1.99 ng/mL, and ≥ 2.0 ng/mL. 18F (fluoro)choline is another widely studied PET tracer for PC. For local recurrence, De Visschere et al. found detection rates ranging from 16.7% to 76.0% in patients with BCR after primary treatment and a PSA level <1 ng/ml, lower than that demonstrated by mpMRI [
17]. Regarding bone metastasis, 18F choline PET-CT appears to have better detection rates when compared with BS, mainly because of the early bone infiltration detection, before the occurrence of osteoblastic reaction. To date, the most promising data are those generated with radiotracers targeting the PSMA, which is overexpressed in most PC cells. A 2022 systematic review by Mazrani et al. included 20 prospective studies evaluating the role of 68Ga and 18F PSMA PET/CT and PET/MRI in 2110 PC cases with BCR. Pooled PSMA PET positivity was 66.6% and the only factor significantly associated with PSMA PET positivity was PSA level at the time of the analysis [
23].
After RP, 68Ga PSMA-11 PET/CT demonstrates good detection rates even at very low PSA levels. De Visschere et al. reported positivity rates ranging from 11.0% to 65.0% with a PSA serum level <0.5 ng/mL [
17]. The largest available series was published by Afshar-Oromieh et al. reporting a 43.3% detection rate in 226 patients with PSA ≤0.2 ng/mL after RP [
24]. In the setting of a BCR after primary RT, 68Ga PSMA PET/CT accuracy differs according to the PSA level at the time of the exam. Einspieler et al. reported a 90.7% detection rate for 68Ga PSMA PET/CT in 118 patients with BCR after primary RT and a median PSA of 6.4 ng/mL; the detection rates were, respectively, 81.8%, 95.3% and 96.8% for PSA values of 2 to <5, 5 to <10, and ≥10 ng/mL [
25]. The detection rate of 68Ga PSMA PET/CT can vary depending on the metastatic site. In a systematic review by Eissa et al., detection rate reached 74.5%, 89.7%, 61% and 14.3% for prostate recurrence, lymph nodes, bone and visceral metastasis, respectively [
26]. Specifically for lymph node recurrence, conventional imaging has limited value with a pool sensitivity and specificity of 42% and 82% for CT and 39% and 82% for MRI [
27]. Regarding 68Ga PSMA PET/CT, two studies by Rauscher et al. and Jilg et al. reported positive predictive values of 94.6% and 99.1% and negative predictive values of 87.8% and 92.3% for lymph node recurrence detection, respectively [
28,
29].
In summary, the ability of currently available imaging modalities in the detection of both local and metastatic recurrence in the setting of BP after primary treatments is still highly dependent on PSA levels. The strength of recommendation of current EAU guidelines in this setting is still weak. In the event of a PSA-only recurrence after RP, PSMA PET/CT seems to be the imaging modality with the highest sensitivity at low PSA levels (< 0.5 ng/mL) [
30]. Results of PSMA PET/CT may help distinguish patients with local recurrences in the prostatic fossa from those with low volume distant metastases. After RT, the high sensitivity of mpMRI may help in the detection of local recurrences, in case of a local salvage treatment indication [
31]. Nonetheless, a systemic staging is still needed before treatment initiation in the event of local recurrence, because of the high morbidity of post-RT salvage therapies. Choline-, fluciclovine- or PSMA-PET/CT can all be used in these patients but again the highest sensitivity of PSMA PET/CT is preferred, in order to more accurately rule-out any distant metastasis.
4. How biochemical progression is treated today
4.1. BCR after RP
There is still limited evidence and controversy regarding the correct time and treatment of patients with PSA-only recurrence after RP. Salvage radiotherapy and ADT are the treatment modalities accepted by current guidelines, while other options, such as salvage pelvic lymph node dissection (sPLND) in the event of nodal recurrence are still waiting for validation [
32].
Salvage RT (SRT) has been shown to decrease the onset of distant metastasis and improve PC specific mortality, providing a possibility of cure in patients with an increased PSA after RP. In post-RP patients with PSA levels over 0.1–0.2 ng/mL, the RAVES and RADICAL trials demonstrated 5-year biochemical recurrence (BCR)-free survival rates of 88% for SRT [
33,
34]. A ten-years older study by Boorjian et al. evaluating the impact of SRT on disease progression and survival showed that SRT decreased the risk of local recurrence (HR 0.13, 95% CI 0.06–0.28, p <0.0001) and delayed hormonal therapy (HR 0.81, 95% CI 0.71–0.93, p = 0.003) and systemic progression (HR 0.24, 95% CI 0.13–0.45, p <0.0001), when compared to non-SRT [
35]. Pre-SRT PSA has proved to be one of the main prognostic factors for treatment response. In a 2012 study by Siegmann et al. patients with a PSA of < 0.28 ng/ml before SRT had a higher two-year failure-free survival and no evidence of disease (bNED) than those with a pre-SRT PSA level of > 0.28 ng/ml (78% vs 61% respectively) [
36]. Two systematic reviews confirmed the role of PSA level in predicting the 5-year BCR-free survival after SRT [
37,
38]. In the analyzed studies, the probability of achieving an undetectable PSA was >60% when treatment was commenced with a PSA <0,5ng/dl and In addition to PSA, ISUP grade, margin status, and pT stage seem to have an impact on metastasis-free and over-all survival [
39].
Basing on current guidelines, it is still uncertain whether the addition of ADT to SRT in patients with PSA-only recurrence can improve OS and progression-free survival (PFS) and treatment modalities and duration are not yet standardized. Molecular imaging advancements are now revealing that greater pre-SRT PSA levels are associated with an increased risk of extra-pelvic metastatic illness. In this scenario, it is reasonable to believe that patients with a higher pre-SRT PSA may have an increased benefit of ADT for occult metastasis. Data from the RTOG 9601 suggest a cancer specific survival (CSS) and OS benefit of the addition of 2 years of Bicalutamide to early-SRT [
40]. In a secondary analysis of this multicenter, double-blind, placebo-controlled randomized clinical trial, ADT was associated with an OS benefit in patients with a PSA greater than 1.5 ng/ml (25% 12-year absolute benefit; HR 0,45; 95% CI 0.25.0.81), but not in those with a PSA lever of 1,5 ng/ml or less. Moreover, in patients receiving early SRT (PSA<0.6 ng/ml), there was no improvement in OS but an increased OM risk (HR 1.94; 95% CI, 1.17-3.20), and a higher incidence of late grade 3 to 5 cardiac and neurologic side effects, when adding ADT [
41]. A systematic review of the benefit of combining ADT with SRT proposed a risk stratification of patients, in order to individualize treatment, based on pre-SRT PSA (0.5, 0.6-1, > 1 ng/mL), margin status, and ISUP grade [
42]. Regarding the duration of ADT, data from a recent multi-centric study suggested a significant effect of long-term ADT for patients with two or more adverse features (pT stage ≥pT3b, pathologic Gleason ≥8, and PSA level at SRT >0.5 ng/ml). Short-term ADT, on the other hand, was sufficient for patients with a single risk factor, whilst those without any risk factors did not benefit significantly from simultaneous ADT [
43].
The oncological role and safety of sPLND in the era of modern imaging was evaluated by a recent systematic review by Ploussard et al. The reference imaging technique was PSMA or choline PET/CT and mean follow-up was 29.4 months The 2- and 5-year biochemical PFS rates ranged from 23% to 64% and from 6% to 31%, respectively and 5-year OS was 84% [
44]. However, despite interest in the role and safety of sPLND in nodal recurrence, high-level evidence is still lacking.
4.2. BCR after RT
4.2.1. Local treatments
Treatment options for BP after RT include re-irradiation, local procedures and a ‘wait and see approach’. The choice should be based on available strategies and EAU risk groups. Salvage re-irradiation options include stereotactic ablative body radiotherapy (CyberKnife
® or linac-based treatment) and salvage brachytherapy [either High Dose Rate (HDR) or Low Dose Rate (LDR)]. Stereotactic ablative body radiotherapy (SBRT) should be offered to patients with a good IPSS-score and a biopsy proven local recurrence. For the linac-based approach, a prospective single-center study by Bergamin et al. showed a 2-year BCR-free rate of 80% with a total dose (TD) of 36-38 Gy delivered in fractions of 6-6.2 Gy [
45]. For the Cyberknife treatment, two large retrospective series of 50 and 100 patients with a TD of 34 and 36 Gy demonstrated, respectively, a 5-year BCR-free survival rate of 60% and a 3-year BCR-free survival rate of 55% [
46,
47]. In a systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer (MASTER), SBRT showed a rate of severe genitourinary (GU) toxicity and gastrointestinal (GI) toxicity of 4.2% and 0.0%, respectively, lower than any other local salvage approach [
48]. Regarding brachytherapy, in the MASTER trial, the 2-year and 5-year BCR-free survival rates for HDR and LDR were, respectively, 77% and 60%, and 81% and 56%. With rates of severe GU toxicity of 8% for HDR and 8.1% of LDR and rates of severe GI toxicity of 0% for HDR and 1.5% for LDR, brachytherapy proved to be safer than salvage RP or salvage HIFU, but not safer than salvage SBRT.
Compared to primary surgery, salvage RP after RT is associated with a higher risk of anastomotic stricture (47% vs. 5.8%), urinary retention (25.3% vs. 3.5%) and rectal injury (9.2 vs. 0.6%), as well as a higher probability of poor long-term functional outcomes such as urinary incontinence and erectile dysfunction [
49]. In a recent multicenter retrospective study of 414 salvage RP patients with a median follow-up of 36 (IQR 20.4–60.5) months, 59.8% (n = 229) of men did not experience BCR, 9.4% (n = 39) had disease persistence after salvage RP and 30.9% (n = 115) had BCR (median time of BCR being 12 (IQR 5.75–30) months from surgery) [
50]. In an older systematic review, Chade et al. demonstrated a similar BCR-free survival (47-82%) and a 10-year BCR-free survival and OS of 28-53% and 54-89% respectively [
51]. Currently, no data supports salvage RP in patients with a negative biopsy. Ideal candidates for this salvage surgical treatment are patients with a positive pre-treatment biopsy, T1-T2 stage before RT, no evidence of distant metastasis or lymph-node involvement, PSA <10 ng/ml and a life expectancy >10 years.
Alternatives to SRT and salvage RP are salvage cryoablation of the prostate (SCAP) and salvage high intensity focused ultrasound (S-HIFU). A retrospective study comprising 418 patients with BCR and local recurrent disease after EBRT treated with S-HIFU, showed a 5-year BCR-free survival of 49% [
52]. Two similar studies with a shorter follow-up demonstrated comparable results in terms of BCR-free rate [
53,
54]. Urine incontinence, urine retention, rectourethral fistula, and erective dysfunction are the most common side effects associated with S-HIFU and the MASTER trial adjusted pooled analysis for severe GU toxicity was 22.66% (95% CI: 16.98–28.85%) [
48]. At the state of the art, due to the lack of high-certainty data, one should only offer S-HIFU in a clinical trial setting. Regarding SCAP, one of the largest series currently available included 898 patients with a BCR after primary RT. The 5-year BCR-free probability was 71.3% [
55]. Less optimistic results were proposed by the MASTER systematic review, with an adjusted pooled analysis for 5-year BCR-free survival of 50.25% for a total of 32 studies and 5513 patients [
48].
4.2.2. Active observation
Despite the indication for salvage treatments, a ‘wait and see‘ strategy remains an option for the EAU BCR ‘Low-Risk’ group. For instance, studies report that only approximately 30% of patients with BCR after primary surgery will eventually develop clinical recurrence, with only 16.4% dying from PC [
10]. An observational study of 407 patients experiencing BCR following RP used PSA kinetics and DT pattern to evaluate the need for adjuvant treatment (ADT or SRT) or active observation (AO). The Risk assessment was performed using the PSA- DT (>12 vs. <12 months), and PC cases with rapidly decreasing DTs were assigned to treatment [
56]. The PCSM in the treatment group (TG) was 10.7% whereas in the AO group was 0% (p < 0.001). An initial PSA-DT >12 months was present in 73.6% of the AO group versus 22.6% of the TG (p < 0.001). In the AO group an increasing PSA-DT trend was seen in 71.5% whereas in the treatment group in only 32.7% of cases (p < 0.001. Using this selection, 33% of patients with BCR were managed conservatively without compromising their life-expectancy. The key benefit of AO is to avoid overtreatment, with a positive impact on quality of life and reduced side effects, in addition to the monetary savings of avoided ADT, RT and their side effects management.
4.3. Systemic treatment
Conflicting results were published regarding the clinical effectiveness of early ADT in the setting of BCR after curative therapy of primary PC without overt clinical disease. The TOAD trial is a multicenter, phase 3 trial including 261 patients with PSA relapse after primary treatment (group 1) and 32 with non-curable disease (group 2) randomized on a 1:1 ratio to delayed ADT (delayed therapy arm) or immediate ADT (immediate therapy arm) [
57]. Median follow-up was 5 years. Specifically for group 1, 26 men (19%) assigned to the delayed therapy arm and 14 men (11%) assigned to the immediate therapy arm died. The estimated 5-year OS rates were 78.2% (95% CI 67·2–85·8) in the delayed therapy arm and 84.3% (73·9–90·8) in the immediate therapy arm. The TOAD study was the only randomized clinical trial (RCT) to report a favorable but limited effect of early ADT in a subsequent systematic review of this topic, while the majority of the other studies reported no differences in terms of OS between early and delayed ADT. No data were found on the effectiveness of different types of ADT [
58]. Due to the lack of clear effectiveness and the considerable adverse effects, early ADT should only be considered in patients with the highest risk of disease progression (short PSA-DT and high initial ISUP grade) and a life expectancy >10 years.
Intermittent androgen deprivation (IAD) for PSA elevation after primary treatment (RP or RT) was proposed to improve quality of life and delay hormone resistance, without negatively impacting life expectancy. A 2012 RCT by Crook et al. randomly assigned 1386 patients with BCR in a 1:1 ratio to either intermittent or continuous therapy [
59]. The median follow-up was 6.9 years. The median OS was 8.8 years and 9.1 years in the IAD group and in the continuous-therapy group, respectively. The hazard ratio (HR) for death with IAD versus continuous therapy was 1.02 (95% CI, 0.86 to 1.21). At baseline, there were no meaningful differences in quality-of-life scores between the 2 groups. At 5 years, IAD was associated with significantly better scores for hot flashes (P<0.001), desire for sexual activity (P<0.001), and urinary symptoms (P = 0.006). An important limitation of this RCT is the lack of any stratifying criteria such as PSA-DT or initial risk factors. In another RCT of 77 patients there was no difference in terms of disease progression and quality-of-life between IAD and CAD after a median follow-up of 48 months [
60].
Table 2.
How to define and treat BCR after RP or RT at low and high risk for early progression. BCR= biochemical recurrence. RP= radical prostatectomy. RT= radiotherapy. SRT= salvage radiotherapy. AO= active observation. ADT= androgen deprivation therapy. ARSI= androgen receptor signaling inhibitor. HIFU= high intensity focalized ultrasound.
Table 2.
How to define and treat BCR after RP or RT at low and high risk for early progression. BCR= biochemical recurrence. RP= radical prostatectomy. RT= radiotherapy. SRT= salvage radiotherapy. AO= active observation. ADT= androgen deprivation therapy. ARSI= androgen receptor signaling inhibitor. HIFU= high intensity focalized ultrasound.
| |
BCR after RP |
BCR after RT |
| |
Low risk |
High risk |
Low risk |
High risk |
| How to define |
PSA-DT ≥ 10 ng/ml |
PSA-DT <10 months |
PSA-DT ≥ 10 ng/ml |
PSA-DT < 10 months |
| Which imaging to exclude metastases |
PSMA PET/CT |
PSMA PET/CT |
PSMA PET/CT |
PSMA PET/CT |
| How to treat today |
- AO
- SRT |
- SRT
- ADT |
- AO
- Stereotactic RT
- Cryoablation
- HIFU |
ADT |
| How will be treated in a near future |
- AO
- SRT |
ARSI+ ADT |
- AO
- Stereotactic RT
- Cryoablation
- HIFU |
ARSI + ADT |
5. How systemic treatment for BCR can condition the development of a non-metastatic CRPC
An indiscriminate use of ADT in patients with only BCR, can produce an aberration of the natural history of PC, with the development of castration resistance (CR) that precedes that of metastases. CRPC is defined as the association of castrate serum testosterone <50 ng/dL or 1.7 nmol/L and either biochemical progression (three consecutive rises in PSA levels, resulting in two 50% increases over the nadir, and a PSA >2 ng/mL), or radiological progression (>2 bone metastases identified by bone scan, or a visceral metastasis defined by RECIST criteria) [
61,
62].The annual incidence of nmCRPC in the United States has been estimated at roughly 60,000 cases in 2020, with a 16% annual OM [
63]. Prior to 2018, no treatment approach for non-metastatic patients with a PSA rise during ADT (nmCRPC) had demonstrated an OS benefit [
64].
For asymptomatic nmCRPC patients, the Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence (RADAR) group guidelines recommended PSA testing every 3 months and evaluation by conventional imaging at PSA levels of 2 ng/mL, 5 ng/mL, and then every time the PSA level doubles [
65]. In a 2019 study, PSMA-PET detection rate for pelvic disease and distant metastasis was evaluated on 200 high-risk nmCRPC patients. PSMA-PET detected any disease in nearly all patients (196 of 200) and M1 disease in 55% of patients previously diagnosed with nmCRPC [
66]. Although PSMA-PET is widely used in clinical practice also for initial staging or for BCR, current guidelines do not endorse its indication beyond these settings. It is important to note that, despite the high probability of micrometastatic disease in high-risk nmCRPC defined by conventional imaging, this did not preclude a survival benefit in the three studies evaluating novel treatment options for nmCRPC defined by the conventional imaging [
67,
68,
69].
Few studies have analyzed risk factors for the development of CR in BCR non-metastatic patients. A recent study by Salciccia et al. retrospectively evaluated in 170 PC cases with BCR after primary treatments, the impact of continuous ADT (CAD) and intermittent ADT (IAD) on the development of CRPC in both metastatic and non-metastatic settings (PET/CT was used as imaging). At univariate analysis the use of a continuous administration of ADT significantly increases the HR for CRPC-M0 progression (HR 3.48; 95%CI 1.66-7.29; P = 0.01) when compared to the IAD administration, either in cases treated with RP or RT and the result remains significant also at multivariate analysis (RR 2.34, 95%CI 1.52-5.33; P = 0.03) [
70].
Similarly to BCR PC group, nmCRPC is an heterogeneous condition, ranging from indolent disease to aggressive forms that rapidly progress to symptomatic metastases, with significant differences in terms of OS and CSS [
71]. In a study on 201 patients with nmCRPC, at 2 years of follow up, only 33% of patients developed radiologically evident metastases (on conventional imaging) and median bone metastasis-free survival (BMFS) was 30 months. Baseline PSA level greater than 10 ng/mL and PSA velocity independently predicted shorter time to first bone metastasis, OS, and metastasis-free survival [
9]. A more recent phase 3 randomized trial of denosumab in nmCRPC suggested that a PSA-DT of ≤10 months predicted a shorter OS and BMFS [
72]. Of the above-mentioned parameters, PSADT is considered the most relevant predictive factor of disease progression, supporting its use in the selection of high-risk patients [
73].
Delaying the onset of metastasis and thus increasing OS are the main treatment goals for nmCRPC. Prior to 2019, NCCN guidelines recommended observation for patients with PSA-DT >10 months given the low risk of progression and, for those with PSA-DT ≤10 months, enrolling them in a clinical trial [
74]. The first two new generation androgen receptor signaling inhibitors (ARSI) to be approved for nmCRPC were Apalutamide and Enzalutamide in 2018. The following year, FDA and EMA also approved the use of Darolutamide in this setting. PROSPER, SPARTAN and ARAMIS trials are all phase 3, randomized, double-blind, placebo-controlled study on high-risk nmCRPC (defined as PSA-DT ≤10 months and PSA level ≥2 ng/mL) using as primary end-point metastasis-free survival (MFS) [
67,
68,
69]. The definition of non-metastatic disease was obtained using the conventional imaging and all the three ARSI demonstrated a significant MFS benefit when compared to the ADT arm. No direct comparison of the three aforementioned agents has been published, indirect comparisons from three meta-analyses suggest a similar effectiveness, whereas darolutamide seems to have better tolerability [
75,
76,
77].
6. Differences between real world and clinical trials in the management of biochemical recurrence
Real-world data (RWD) are data gathered outside of RCTs that reflect actual clinical practice. While RCTs provide the highest degree of evidence for demonstrating the efficacy of medical therapies, studies using RWD provide further data concerning the effectiveness, long-term results, and safety of those interventions in real-world situations [
78]. The significance of RWD in in-depth investigation of PC etiology, diagnosis, and treatment efficacy is expanding as a result of the establishment of high-quality clinical national and worldwide registers. However, real world studies to verify the results obtained in RCTs, continue to remain little used, particularly in the setting of BCR PC. A recent 2021 review demonstrated the value of a high-quality national register, the National Prostate Cancer Register (NPCR) of Sweden, in generating high-quality evidence in different PC settings, such as etiology, treatment and adverse events management [
79].
After RP, literature describes a >30% risk of developing BCR. A recent American retrospective cohort study used RWD extrapolated from nationally representative Optum© Electronic Medical Records (EMR) from 2010 to 2021 [
80]. The study included a total of 15198 patients and the median follow-up was 3.9 years. RT after primary treatment was initiated in 14.3% of patients, either adjuvant or salvage. BCR occurred in 19.8% of patients and among patients with BCR, 6.3% had a PSA-DT ≤ 10 months, 17.1% developed metastasis, 13.8% developed CRPC, and 9.7% died. At multivariate analysis, authors found that a PSA ≥ 20 ng/mL, Charlson Comorbidity Index (CCI) >0, age ≥ 65 and african-american race were the most relevant baseline risk factors for BCR.
Regarding the staging of patients with a PSA rise after primary treatment, a 2023 study by Burgard et al. evaluated the real-world efficacy of PSMA-PET in detecting tumor localization after early (PSA ≤0.2 ng/mL) BCR [
81]. Authors conducted a retrospective analysis in 115 men with BCR after RP for intermediate or high-risk PCs at very low PSA level (≤0.2 ng/mL) and a PSA-DT ≤12 months. The primary endpoint was the per-patient visual detection rate (positive scans/total scans). Secondary endpoints included the detection rate by lesion type, PSA concentration, PSA-DT, and Gleason score. 25.2% of patients had lesions suspicious for PC recurrence at PSMA-PET/CT with a total of 44 lesions, including 11 putative local recurrences, 22 putative lymph node metastases, and 11 putative bone metastases. When comparing these data with available literature, the detection rate seems to be significantly lower than that demonstrated by the largest available series by Afshar-Oromieh et al. (43.3% detection rate in 226 RP patients with PSA ≤0.2 ng/mL) [
24]. By contrast, these RWD results are in line with those demonstrated in a 2020 meta-analysis (33% detection rate in 197 RP patients) [
82] and even better than those reported by Meredith et al. in 2016 [
83].
In 2021, data from a nationwide US survey on treatment modalities for nmPC were published. Physicians included were board-certified urologists and oncologists with at least five years of clinical practice and at least 30 PC patients treated monthly [
84]. At final analysis, 4415 patients with nmPC were stratified by stage. After 5 years from initial therapy, relapse occurred in 22.1%, 31.4%, 43.6% and 60.4% in stage I, II, III and IV (M0) patients, respectively. Stage I and II patients were most likely to experience a biochemical progression only, while metastatic recurrence was considerably more frequent among stage IV(M0) patients (41.2%). Regardless of the initial treatment, more than half of the recurrent patients received systemic therapy with or without RT, only roughly 10% of patients with a recurrence were addressed to AS and following a relapse after RP, 49% and 17.6% of patients underwent only systemic therapy or systemic therapy + RT, respectively.
In 2023 Tilki et al. used multivariable Cox regression analysis on a multinational database of 25,551 patients with pT2-4N0M0 or NXM0 PC after RP to evaluate the existence of a prespecified PSA level associated with an increased all-cause mortality (ACM) at the beginning of SRT [
85]. ACM was evaluated at a starting PSA of 0.10 ng/ml and then every 0.05 ng/ml increase up to 0.5 ng/ml. 1,556 (6.09%) cases were submitted to SRT at a low PSA level ≤0.25 ng/mL, whereas 1,677 (6.56%) when the PSA level was >0.25 ng/mL. 39,65% of cases with PSA > 0.25 ng/ml was submitted to ADT as compared to 27.44% cases with PSA ≤0.25 ng/mL. At a median follow-up of 6 years, patients submitted to SRT at a PSA level >0.25 ng/mL had a significantly higher ACM-risk (AHR, 1.49; 95% CI, 1.11 to 2.00; P = 0.008) compared with those submitted to SRT at a PSA ≤0.25 mg/mL.
Tilki et al. showed no significant differences in ACM-risk between adjuvant RT and salvage SRT at PSA ≤0.25 ng/mL in patients with 1 high-risk factor (Gleason score 8-10 or pT3/4). Similarly, the Radiotherapy and Androgen Deprivation after Local Surgery-RT randomized trial, demonstrated no superiority of adjuvant to early (PSA < 0.25 ng/ml) salvage post-RP SRT in terms of disease-free survival [
34].
7. The concept of anticipation and incrementation in the treatment of biochemical recurrence
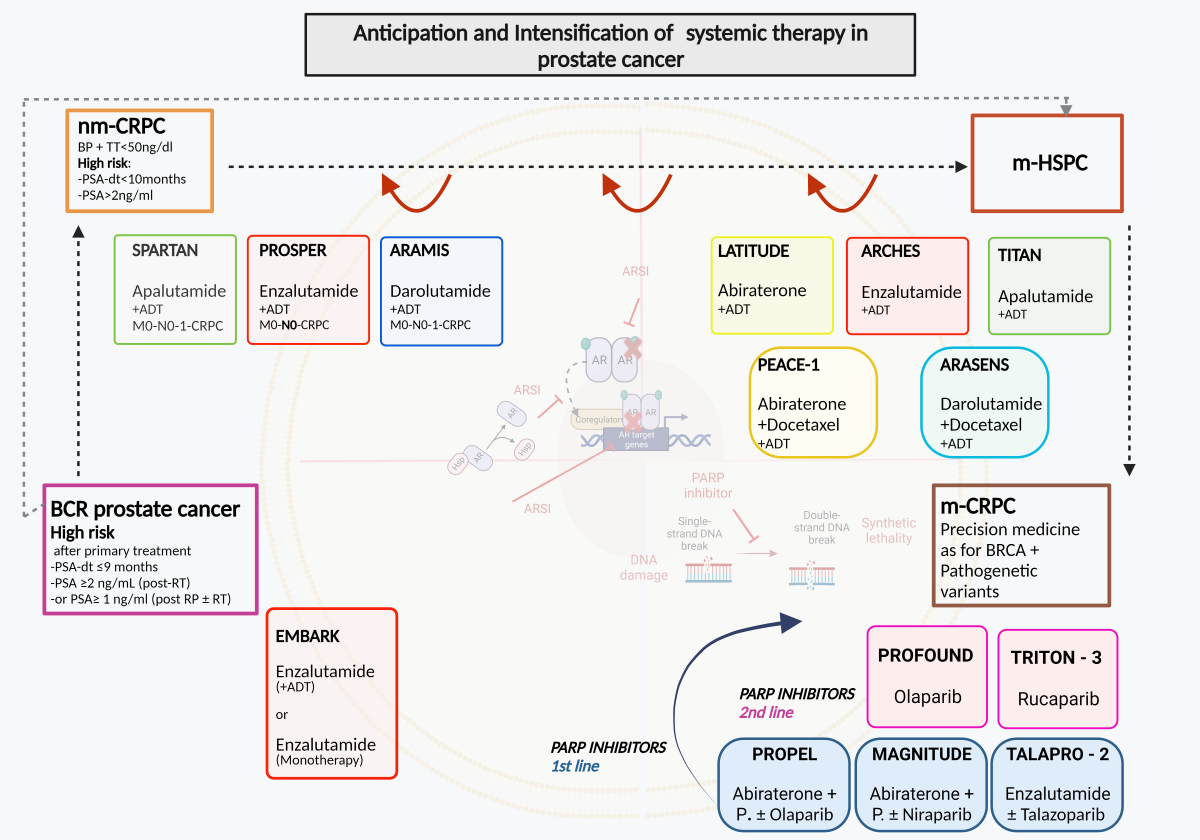
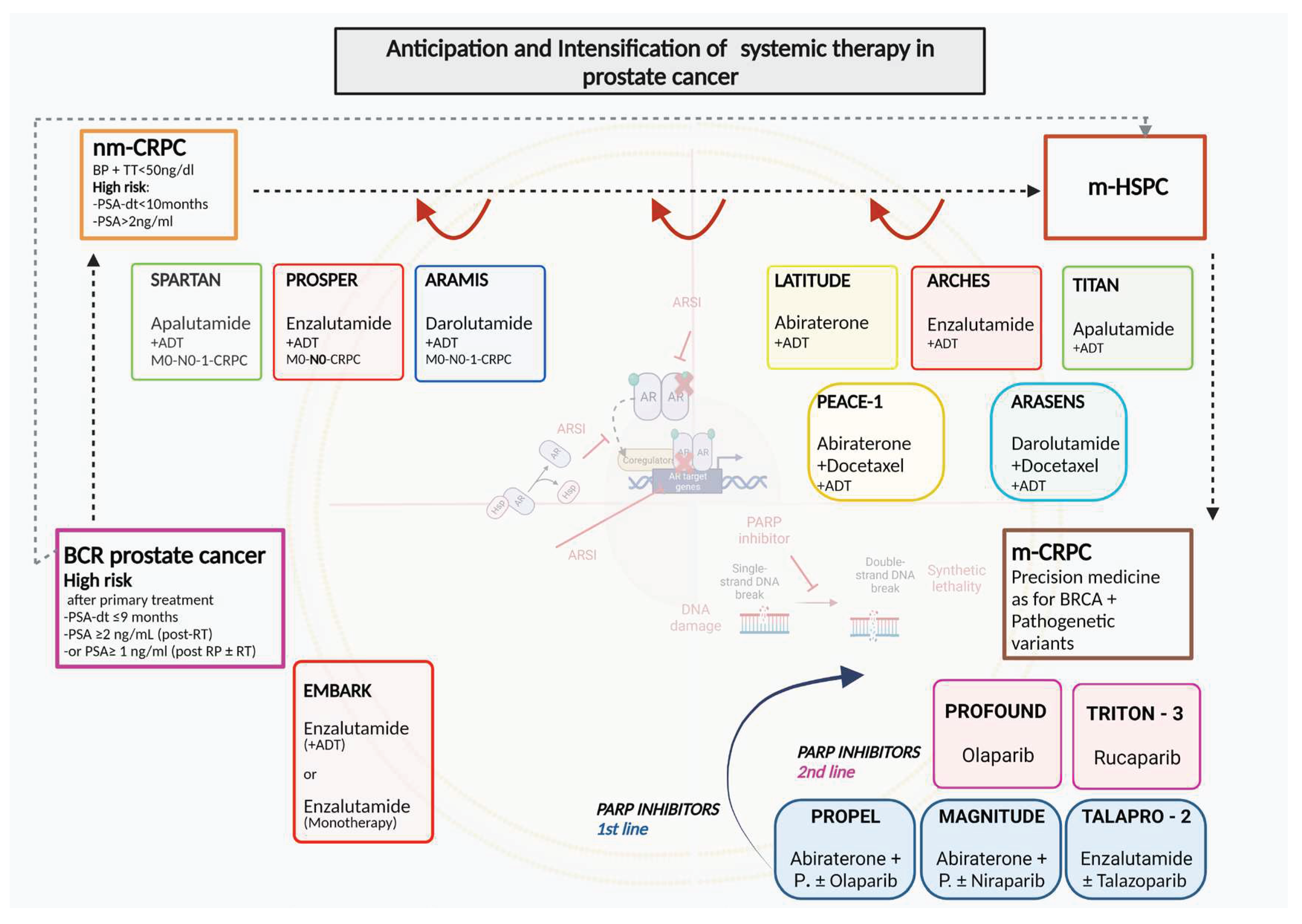
ARSI therapies in PC have led to indications increasingly aimed at a concept of anticipation and intensification of therapy starting from the CRPC phase and then completely involving the metastatic HSPC phase. Recently this concept has been proposed in even earlier phases of the disease as neoadjuvant to primary treatments and in case of BCR after primary therapies.
Figure 1.
Anticipation and intensification of systemic therapy in prostate cancer. ADT: androgen deprivation therapy; AR: androgen receptor; ARSI: androgen receptor signal inhibitors; BCR: biochemical recurrence; BP: Biochemical progression; HSP: heat shock protein; m-CRPC: metastatic Castration resistant prostate cancer; m-HSPC: metastatic Hormone sensitive prostate cancer; nm-CRPC: non-metastatic castration resistant prostate cancer; P.: prednisone or prednisolone; PARP: Poly (ADP-ribose) polymerase.
Figure 1.
Anticipation and intensification of systemic therapy in prostate cancer. ADT: androgen deprivation therapy; AR: androgen receptor; ARSI: androgen receptor signal inhibitors; BCR: biochemical recurrence; BP: Biochemical progression; HSP: heat shock protein; m-CRPC: metastatic Castration resistant prostate cancer; m-HSPC: metastatic Hormone sensitive prostate cancer; nm-CRPC: non-metastatic castration resistant prostate cancer; P.: prednisone or prednisolone; PARP: Poly (ADP-ribose) polymerase.
7.1. EMBARK study
The EMBARK study is probably one of the most innovative studies to date in analyzing the anticipated role of ARSI in PC patients with BCR after primary treatment [
86]. The study uses the concept of PSA-DT to select only nonmetastatic PC with high risk BCR.
EMBARK is a randomized, phase III study focused on nmHSPC patients with a PSADT of ≤9 months, and screening PSA of ≥1 ng/mL post RP (+/- RT) or ≥2 ng/mL above nadir post-RT, and serum testosterone ≥150 ng/dL. The PSA value used to start therapy after RT corresponds to that defined as BCR, on the contrary in patients undergoing RP it expects a value higher than the normally used cutoff of 0.2 ng/ml. Men are randomized 1:1:1 to enzalutamide 160 mg/d plus leuprolide (LHRH analogue) or placebo plus leuprolide in double-blind arms or enzalutamide monotherapy (open label). EMBARK represents the first study where a treatment with ARSI that replaces and is not only associated with ADT is proposed. In this direction it has the courage to analyze whether the inclusion of ARSI in such an early phase of the disease such as BCR can determine the non-used of the classic ADT. Patients have treatment suspended on week 37 if PSA levels are <0.2 ng/mL and reinstated if levels increase to ≥2.0 ng/mL (RP) or ≥5.0 ng/mL (RT). Patients with a PSA ≥0.2 ng/mL stay on the treatment until the requirements to stop it are satisfied. This interruption of therapy in the event of a reduction in PSA levels to undetectable values suggests the use of a non-continuous treatment but does not represent a true intermittent strategy like IAD. The primary endpoint is metastasis free survival (MFS) comparing enzalutamide plus LHRHa vs placebo plus LHRHa. Key secondary endpoints are MFS in the enzalutamide monotherapy group in comparison with the other two arms, time to PSA progression, time to first use of new antineoplastic therapy, and overall survival. Progression-free survival on first subsequent therapy (PFS2) is an exploratory endpoint. The study confirms that the maintenance of a non-metastatic disease stage must be considered the primary endpoint in this disease phase with only BCR, similarly to what is considered in trials on patients with nmCRPC. 1068 randomized patients were followed for a median of 60.7 months. The 5-year MFS was 87.3% (95% confidence interval [CI], 83.0 to 90.6) in the combination group, 71.4% (95% CI, 65.7 to 76.3) in the leuprolide-alone group, and 80.0% (95% CI, 75.0 to 84.1) in the enzalutamide monotherapy group. Either the combination enzalutamide plus leuprolide or enazalutamide monotherapy was superior to leuprolide alone (HR 0.42; 95% CI, 0.30 to 0.61; P<0.001 and HR 0.63; 95% CI, 0.46 to 0.87; P=0.005, respectively). Compared to leuprolide alone, patients treated with enzalutamide plus leuprolide demonstrated a significant reduction in the risk of PSA progression, longer time to the first use of new antineoplastic therapy and above all a longer OS (HR 0.59; CI 038-0.91; p<0.02).Compared with leuprolide alone, patients treated with enzalutamide alone demonstrated a significant reduction in the risk of PSA progression, longer time to the first use of new antineoplastic therapy but no significant advantage in terms of OS (HR 0.78; CI 0.52-1.17; p=0.23). These results were maintained regardless to the type of primary treatment used (RP or RT).
7.2. Ongoing trials
Aggarwal et al. during the 2022 ESMO annual meeting presented the PRESTO trial, a randomized phase III, open-label trial in patients with BCR PC and PSA-DT ≤ 9 months, M0 at conventional imaging (no PET-CT imaging was used) [
87]. The primary end-point was BCR-free survival and secondary end-points included safety, quality of life, MFS and castration resistance. Patients (stratified in PSA -DT < 3 vs 3-9 months) were randomly treated with a 52-week course of ADT, ADT + apalutamide, or ADT + apalutamide + abiraterone acetate plus prednisone. The enrolment started in March 2017, including a period in March 2020 where COVID-19 pandemic led to a mitigation plan with flexible LHRH analog dosing schedules. 504 patients were randomly assigned to ADT alone (n = 167), ADT + apalutamide (n = 168) or ADT + apalutamide + abiraterone acetate plus prednisone (n = 169). At a median follow-up of 21.5 months, both combination arms significantly prolonged BCR-free survival when compared to the ADT alone group. In particular, median BCR-free survival was 24.9 months for ADT + apalutamide vs 20.3 months for ADT (HR 0.52 (95% CI 0.35–0.77)) and 26.0 months for ADT + apalutamide + abiraterone acetate plus prednisone vs 20.0 months for ADT (HR 0.48 (95% CI 0.32–0.71)). Hypertension was the most common grade ≥ 2 adverse event (19.4%, 23.4%, 30.4% in ADT, ADT + apalutamide and ADT + apalutamide + abiraterone acetate plus prednisone arms, respectively). Treatment was concluded for adverse events in only 1.8% cases across all treatment arms.
ARASTEP is an ongoing, randomized, double-blind, phase 3 study evaluating the use of darolutamide compared to placebo in patients with high-risk BCR PC. Patients (estimated enrollment, n=750) will be randomized to darolutamide 600 mg twice daily (BID) or placebo BID, both in combination with ADT for a maximum duration of 24 months, or until disease progression and severe toxicity [
88].
ARAMON is an ongoing, open-label, phase 2 study evaluating the use of darolutamide and enzalutamide as monotherapy in the treatment of patients with PC experiencing BCR following definitive treatment for localized disease [
89]. During the lead-in phase of the study, 25 patients will receive darolutamide 600 mg BID. A testosterone assessment will be conducted at 12 weeks. The randomized phase of the study will only proceed if, during the lead-in phase, testosterone level criteria will be respected. The randomized phase will enroll approximately 40 patients randomized 1:1 to darolutamide 600 mg BID or enzalutamide 160 mg once daily. In either phase, patients will receive treatment for 52 weeks, or until unacceptable toxicity, PSA progression or death.
8. Conclusion
The introduction of new therapeutic strategies, in particular with ARSI, has led to a rush towards the concept of anticipation and intensification of therapy in PC. The advantage of this concept is represented by the possibility to block the disease at an earlier stage when the patient’s quality of life is better preserved as well as having a positive impact on overall survival. The major risk is represented by overtreatment in a non-negligible percentage of patients as well as the development of resistance which can reduce therapeutic options in the subsequent phases.
The introduction of the concept of anticipation and intensification as therapeutic strategy for mHSPC and nmCRPC phases has been associated with a stratification of patients into risk categories. In nmCRPC patients, intensification of therapy with ARSI is now recommended only in high-risk subjects based on a PSA-DT < 10 months. On the contrary in mHSPC, the current guidelines recommend the intensification of ADT therapy with ARSI or chemotherapy in all patients while stratifying based on the volume of disease according to the Charteed criteria (low and high volume) or Latitude risk classes (low and high risk). These stratifications do not represent real tailored indications for patients according to a precision medicine concept. The possibility of using a genetic characterization with the PVs of HRRs to precisely identify when to anticipate the use of PARP inhibitors in metastatic CRPC could represent a real example of precision medicine.
EMBARK study represents the first significant evidence in support of the use of an anticipation and intensification of therapy with ARSI in the BCR phase after primary treatments. In this phase of the disease the primary advantage of this concept is represented by the delay in the evolution of a metastatic phase (MFS), even with the same OS, maintaining patients in an asymptomatic condition with good quality of life. The greatest risk is that of overtreatment in subjects who could remain non-metastatic for several years even without therapies.
The present review would underline the following aspects to correctly realize the concept of anticipation and intensification of therapy in BCR PC:
BCR phase is extremely heterogeneous and at least a stratification in low and high- risk cases for early progression in metastatic disease is necessary.
At now PSA-DT represents the best parameter to define low and high risk BCR PC, however other factors (T stage, ISUP grading) can be considered through risk calculators or nomograms.
Precision medicine is strongly suggested to define a tailored management of patients with BCR and the anticipation in the use of HRR PVs in non-metastatic PC could help to achieve it
Before defining management, it is necessary to exclude the presence of low volume metastasis associated with PSA progression using new generation imaging preferably with PSMA PET/CT
Low risk BCR cases should be actively observed without early systemic therapies.
Early treatment of low risk BCR with continuous ADT can produce disadvantages such as an aberration in the natural history of PC with the development of castration resistance before the appearance of metastases (nmCRPC).
Patients with high risk BCR benefit from early systemic therapy. The primary end point must be metastasis-free survival, even with the same OS.
The EMBARK study is able to impact on the management of high risk BCR, by introducing the concept of anticipation and intensification through the use of ARSI and ADT combination therapy.
In high risk (PSA-DT ≤ 9 months) BCR cases, the combination of enzalutamide with leuprolide significantly improves MFS when compared to leuprolide alone, maintaining the quality of life unchanged in an asymptomatic phase of the disease.
The possibility of using ARSI alone in this early disease setting is suggested by the EMBARK study (arm with enzalutamide alone) with less evidence than the intensification of the combination therapy.
Continued use versus discontinuation upon reaching undetectable PSA levels of enzalutamide plus leuprolide intensified therapy needs to be better defined with further analysis.
Real world analysis must verify the significant results obtained in the context of a phase 3 study.
Author Contributions
All authors actively contribute in the organization and writing of the review.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Giraud, N.; Benziane-Ouaritini, N.; Schick, U.; Beauval, J.-B.; Chaddad, A.; Niazi, T.; et al. Post-Operative Radiotherapy in Prostate Cancer: Is It Time for a Belt and Braces Approach? Front Oncol. 2021, 11, 781040. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer. In: Uroweb—European Association of Urology [Internet]. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 28 December 2023).
- Patrikidou, A.; Zilli, T.; Baciarello, G.; Terisse, S.; Hamilou, Z.; Fizazi, K. Should androgen deprivation therapy and other systemic treatments be used in men with prostate cancer and a rising PSA post-local treatments? Ther Adv Med Oncol. 2021, 13, 17588359211051870. [Google Scholar] [CrossRef] [PubMed]
- Roach, M., 3rd; Hanks, G.; Thames HJr Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Urabe, F.; Sasaki, H.; Kimura, T.; Miki, K.; Egawa, S. Prognostic Significance of Prostate-Specific Antigen Persistence after Radical Prostatectomy: A Systematic Review and Meta-Analysis. Cancers. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Fossati, N.; Wiegel, T.; D’Amico, A.; Hofman, M.S.; Gillessen, S.; et al. Management of Persistently Elevated Prostate-specific Antigen After Radical Prostatectomy: A Systematic Review of the Literature. Eur Urol Oncol. 2021, 4, 150–169. [Google Scholar] [CrossRef]
- Campbell, S.R.; Tom, M.C.; Agrawal, S.; Efstathiou, J.A.; Michalski, J.M.; Abramowitz, M.C.; et al. Integrating Prostate-specific Antigen Kinetics into Contemporary Predictive Nomograms of Salvage Radiotherapy After Radical Prostatectomy. Eur Urol Oncol. 2022, 5, 304–313. [Google Scholar] [CrossRef]
- Smith, M.R.; Kabbinavar, F.; Saad, F.; Hussain, A.; Gittelman, M.C.; Bilhartz, D.L.; et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005, 23, 2918–2925. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur Urol. 2019, 75, 967–987. [Google Scholar] [CrossRef]
- Jackson, W.C.; Suresh, K.; Tumati, V.; Allen, S.G.; Dess, R.T.; Salami, S.S.; et al. Intermediate Endpoints After Postprostatectomy Radiotherapy: 5-Year Distant Metastasis to Predict Overall Survival. Eur Urol. 2018, 74, 413–419. [Google Scholar] [CrossRef]
- Trock, B.J.; Walsh, P.C. Impact of postoperative prostate-specific antigen disease recurrence and the use of salvage therapy on the risk of death. Cancer. 2011, 656, author reply 656. [Google Scholar] [CrossRef]
- Royce, T.J.; Chen, M.-H.; Wu, J.; Loffredo, M.; Renshaw, A.A.; Kantoff, P.W.; et al. Surrogate End Points for All-Cause Mortality in Men With Localized Unfavorable-Risk Prostate Cancer Treated With Radiation Therapy vs Radiation Therapy Plus Androgen Deprivation Therapy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2017, 3, 652–658. [Google Scholar] [CrossRef]
- Falagario, U.G.; Abbadi, A.; Remmers, S.; Björnebo, L.; Bogdanovic, D.; Martini, A.; et al. Biochemical Recurrence and Risk of Mortality Following Radiotherapy or Radical Prostatectomy. JAMA Netw Open. 2023, 6, e2332900. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Goh, C.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Dadaev, T.; et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol. 2015, 68, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Giannarini, G.; Fossati, N.; Gandaglia, G.; Cucchiara, V.; Ficarra, V.; Mirone, V.; et al. Will Image-guided Metastasis-directed Therapy Change the Treatment Paradigm of Oligorecurrent Prostate Cancer? Eur Urol. 2018, 74, 131–133. [Google Scholar] [CrossRef] [PubMed]
- De Visschere, P.J.L.; Standaert, C.; Fütterer, J.J.; Villeirs, G.M.; Panebianco, V.; Walz, J.; et al. A Systematic Review on the Role of Imaging in Early Recurrent Prostate Cancer. Eur Urol Oncol. 2019, 2, 47–76. [Google Scholar] [CrossRef]
- Rouvière, O.; Vitry, T.; Lyonnet, D. Imaging of prostate cancer local recurrences: Why and how? Eur Radiol. 2010, 20, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Musio, D.; Forte, V.; Gentile, V.; et al. Prostate cancer recurrence after radical prostatectomy: The role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol. 2013, 23, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Sciarra, A.; Lisi, D.; Galati, F.; Buonocore, V.; Catalano, C.; et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP). Eur J Radiol. 2012, 81, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Zhu, Y.; Shi, Y.; Xu, L.; Huang, G.; et al. The Added Value of F-FDG PET/CT Compared with Ga-PSMA PET/CT in Patients with Castration-Resistant Prostate Cancer. J Nucl Med. 2022, 63, 69–75. [Google Scholar] [CrossRef]
- Michaud, L.; Touijer, K.A.; Mauguen, A.; Zelefsky, M.J.; Morris, M.J.; Lyashschenko, S.K.; et al. C-Choline PET/CT in Recurrent Prostate Cancer: Retrospective Analysis in a Large, U.S. Patient Series. J Nucl Med. 2020, 61, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Mazrani, W.; Cook, G.J.R.; Bomanji, J. Role of 68Ga and 18F PSMA PET/CT and PET/MRI in biochemical recurrence of prostate cancer: A systematic review of prospective studies. Nucl Med Commun. 2022, 43, 631–637. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; da Cunha, M.L.; Wagner, J.; Haberkorn, U.; Debus, N.; Weber, W.; et al. Performance of [Ga]Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur J Nucl Med Mol Imaging. 2021, 48, 2925–2934. [Google Scholar] [CrossRef]
- Einspieler, I.; Rauscher, I.; Düwel, C.; Krönke, M.; Rischpler, C.; Habl, G.; et al. Detection Efficacy of Hybrid Ga-PSMA Ligand PET/CT in Prostate Cancer Patients with Biochemical Recurrence After Primary Radiation Therapy Defined by Phoenix Criteria. J Nucl Med. 2017, 58, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.; Elsherbiny, A.; Coelho, R.F.; Rassweiler, J.; Davis, J.W.; Porpiglia, F.; et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol Nefrol. 2018, 70, 462–478. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Beer, A.J.; Graner, F.-P.; Haller, B.; Weirich, G.; et al. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J Nucl Med. 2016, 57, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Jilg, C.A.; Drendel, V.; Rischke, H.C.; Beck, T.; Vach, W.; Schaal, K.; et al. Diagnostic Accuracy of Ga-68-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer. Theranostics. 2017, 7, 1770–1780. [Google Scholar] [CrossRef]
- Prostate Cancer. In: Uroweb—European Association of Urology [Internet]. Available online: https://uroweb.org/guidelines/prostate-cancer/chapter/treatment (accessed on 28 December 2023).
- Dinis Fernandes, C.; van Houdt, P.J.; Heijmink, S.W.T.P.J.; Walraven, I.; Keesman, R.; Smolic, M.; et al. Quantitative 3T multiparametric MRI of benign and malignant prostatic tissue in patients with and without local recurrent prostate cancer after external-beam radiation therapy. J Magn Reson Imaging. 2019, 50, 269–278. [Google Scholar] [CrossRef]
- McCormick, B.Z.; Mahmoud, A.M.; Williams, S.B.; Davis, J.W. Biochemical recurrence after radical prostatectomy: Current status of its use as a treatment endpoint and early management strategies. Indian J Urol. 2019, 35, 6–17. [Google Scholar] [CrossRef]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet. 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Karnes, R.J.; Crispen, P.L.; Rangel, L.J.; Bergstralh, E.J.; Blute, M.L. Radiation therapy after radical prostatectomy: Impact on metastasis and survival. J Urol. 2009, 182, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, A.; Bottke, D.; Faehndrich, J.; Brachert, M.; Lohm, G.; Miller, K.; et al. Salvage radiotherapy after prostatectomy—what is the best time to treat? Radiother Oncol. 2012, 103, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Ohri, N.; Dicker, A.P.; Trabulsi, E.J.; Showalter, T.N. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer. 2012, 48, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Bolla, M.; Briganti, A.; Carroll, P.; Cozzarini, C.; Joniau, S.; et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014, 65, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, E.; Matheu, R.; Muní, M.; Sureda, J.; García-Sorroche, M.; Ribal, M.J.; et al. The Effect of Adverse Surgical Margins on the Risk of Biochemical Recurrence after Robotic-Assisted Radical Prostatectomy. Biomedicines. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med. 2017, 376, 417–428. [Google Scholar] [CrossRef]
- Dess, R.T.; Sun, Y.; Jackson, W.C.; Jairath, N.K.; Kishan, A.U.; Wallington, D.G.; et al. Association of Presalvage Radiotherapy PSA Levels After Prostatectomy With Outcomes of Long-term Antiandrogen Therapy in Men With Prostate Cancer. JAMA Oncol. 2020, 6, 735–743. [Google Scholar] [CrossRef]
- Spratt, D.E.; Dess, R.T.; Zumsteg, Z.S.; Lin, D.W.; Tran, P.T.; Morgan, T.M.; et al. A Systematic Review and Framework for the Use of Hormone Therapy with Salvage Radiation Therapy for Recurrent Prostate Cancer. Eur Urol. 2018, 73, 156–165. [Google Scholar] [CrossRef]
- Fossati, N.; Robesti, D.; Karnes, R.J.; Soligo, M.; Boorjian, S.A.; Bossi, A.; et al. Assessing the Role and Optimal Duration of Hormonal Treatment in Association with Salvage Radiation Therapy After Radical Prostatectomy: Results from a Multi-Institutional Study. Eur Urol. 2019, 76, 443–449. [Google Scholar] [CrossRef]
- Ploussard, G.; Gandaglia, G.; Borgmann, H.; de Visschere, P.; Heidegger, I.; Kretschmer, A.; et al. Salvage Lymph Node Dissection for Nodal Recurrent Prostate Cancer: A Systematic Review. Eur Urol. 2019, 76, 493–504. [Google Scholar] [CrossRef]
- Bergamin, S.; Eade, T.; Kneebone, A.; Booth, J.; Hsiao, E.; Schembri, G.P.; et al. Interim Results of a Prospective Prostate-Specific Membrane Antigen-Directed Focal Stereotactic Reirradiation Trial for Locally Recurrent Prostate Cancer. Int J Radiat Oncol Biol Phys. 2020, 108, 1172–1178. [Google Scholar] [CrossRef]
- Fuller, D.; Wurzer, J.; Shirazi, R.; Bridge, S.; Law, J.; Crabtree, T.; et al. Retreatment for Local Recurrence of Prostatic Carcinoma After Prior Therapeutic Irradiation: Efficacy and Toxicity of HDR-Like, S.B.R.T. Int J Radiat Oncol Biol Phys. 2020, 106, 291–299. [Google Scholar] [CrossRef]
- Pasquier, D.; Martinage, G.; Janoray, G.; Rojas, D.P.; Zerini, D.; Goupy, F.; et al. Salvage Stereotactic Body Radiation Therapy for Local Prostate Cancer Recurrence After Radiation Therapy: ARetrospective Multicenter Study of the, G.E.T.U.G. Int J Radiat Oncol Biol Phys. 2019, 105, 727–734. [Google Scholar] [CrossRef]
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur Urol. 2021, 80, 280–292. [Google Scholar] [CrossRef]
- Gontero, P.; Marra, G.; Alessio, P.; Filippini, C.; Oderda, M.; Munoz, F.; et al. Salvage Radical Prostatectomy for Recurrent Prostate Cancer: Morbidity and Functional Outcomes from a Large Multicenter Series of Open versus Robotic Approaches. J Urol. 2019, 202, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Karnes, R.J.; Calleris, G.; Oderda, M.; Alessio, P.; Palazzetti, A.; et al. Oncological outcomes of salvage radical prostatectomy for recurrent prostate cancer in the contemporary era: A multicenter retrospective study. Urol Oncol. 2021, 39, 296.e21–296.e29. [Google Scholar] [CrossRef] [PubMed]
- Chade, D.C.; Shariat, S.F.; Cronin, A.M.; Savage, C.J.; Karnes, R.J.; Blute, M.L.; et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: A multi-institutional collaboration. Eur Urol. 2011, 60, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, S.; Blana, A.; Murat, F.J.; Pasticier, G.; Brown, S.C.W.; Conti, G.N.; et al. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int. 2017, 119, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Kanthabalan, A.; Peters, M.; Van Vulpen, M.; McCartan, N.; Hindley, R.G.; Emara, A.; et al. Focal salvage high-intensity focused ultrasound in radiorecurrent prostate cancer. BJU Int. 2017, 120, 246–256. [Google Scholar] [CrossRef]
- Jones, T.A.; Chin, J.; Mcleod, D.; Barkin, J.; Pantuck, A.; Marks, L.S. High Intensity Focused Ultrasound for Radiorecurrent Prostate Cancer: A North American Clinical Trial. J Urol. 2018, 199, 133–139. [Google Scholar] [CrossRef]
- Ginsburg, K.B.; Elshafei, A.; Yu, C.; Jones, J.S.; Cher, M.L. Avoidance of androgen deprivation therapy in radiorecurrent prostate cancer as a clinically meaningful endpoint for salvage cryoablation. Prostate. 2017, 77, 1446–1450. [Google Scholar] [CrossRef]
- Huang, E.; Huynh, L.M.; Tran, J.; Gordon, A.M.; Chandhoke, R.; Morales, B.; et al. Active Observation of Biochemical Recurrence without Treatment following Radical Prostatectomy: Long-Term Analysis of Outcomes. Cancers. 2022, 14. [Google Scholar] [CrossRef]
- Duchesne, G.M.; Woo, H.H.; Bassett, J.K.; Bowe, S.J.; D’Este, C.; Frydenberg, M.; et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016, 17, 727–737. [Google Scholar] [CrossRef]
- van den Bergh, R.C.N.; van Casteren, N.J.; van den Broeck, T.; Fordyce, E.R.; Gietzmann, W.K.M.; Stewart, F.; et al. Role of Hormonal Treatment in Prostate Cancer Patients with Nonmetastatic Disease Recurrence After Local Curative Treatment: A Systematic Review. Eur Urol. 2016, 69, 802–820. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; O’Callaghan, C.J.; Duncan, G.; Dearnaley, D.P.; Higano, C.S.; Horwitz, E.M.; et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012, 367, 895–903. [Google Scholar] [CrossRef]
- Casas, F.; Henríquez, I.; Bejar, A.; Maldonado, X.; Alvarez, A.; González-Sansegundo, C.; et al. Intermittent versus continuous androgen deprivation therapy to biochemical recurrence after external beam radiotherapy: A phase 3 GICOR study. Clin Transl Oncol. 2017, 19, 373–378. [Google Scholar] [CrossRef]
- Morote, J.; Aguilar, A.; Planas, J.; Trilla, E. Definition of Castrate Resistant Prostate Cancer: New Insights. Biomedicines. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Solo, K.; Valant, J.; Todd, M.B.; Mehra, M. Prevalence of Prostate Cancer Clinical States and Mortality in the United States: Estimates Using a Dynamic Progression Model. PLoS ONE. 2015, 10, e0139440. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Brandt, M.P.; Heck, M.M. Treatment of non-metastatic castration resistant prostate cancer in 2020, What is the best? Urol Oncol. 2020, 38, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Stone, N.N.; Yu, E.Y.; Koo, P.J.; Freedland, S.J.; Slovin, S.F.; et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014, 83, 664–669. [Google Scholar] [CrossRef]
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.S.; Calais, J.; Czernin, J.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Salciccia, S.; Frisenda, M.; Tufano, A.; Di Pierro, G.; Bevilacqua, G.; Rosati, D.; et al. Intermittent Versus Continuous Androgen Deprivation Therapy for Biochemical Progression After Primary Therapy in Hormone-Sensitive Nonmetastatic Prostate Cancer: Comparative Analysis in Terms of CRPC-M0 Progression. Clin Genitourin Cancer. 2023. [Google Scholar] [CrossRef]
- Berruti, A.; Bracarda, S.; Caffo, O.; Cortesi, E.; D’Angelillo, R.; Del Re, M.; et al. nmCRPC, a look in the continuous care of prostate cancer patients: State of art and future perspectives. Cancer Treat Rev. 2023, 115, 102525. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Oudard, S.; Shore, N.; Fizazi, K.; Sieber, P.; et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: Exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013, 31, 3800–3806. [Google Scholar] [CrossRef]
- Dong, L.; Su, Y.; Zhu, Y.; Markowski, M.C.; Xin, M.; Gorin, M.A.; et al. The European Association of Urology Biochemical Recurrence Risk Groups Predict Findings on PSMA PET in Patients with Biochemically Recurrent Prostate Cancer After Radical Prostatectomy. J Nucl Med. 2022, 63, 248–252. [Google Scholar] [CrossRef]
- Gupta, R.; Sheng, I.Y.; Barata, P.C.; Garcia, J.A. Non-metastatic castration-resistant prostate cancer: Current status and future directions. Expert Rev Anticancer Ther. 2020, 20, 513–522. [Google Scholar] [CrossRef]
- Kumar, J.; Jazayeri, S.B.; Gautam, S.; Norez, D.; Alam, M.U.; Tanneru, K.; et al. Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: A systematic review and network meta-analysis. Urol Oncol. 2020, 38, 826–834. [Google Scholar] [CrossRef]
- Chowdhury, S.; Oudard, S.; Uemura, H.; Joniau, S.; Pilon, D.; Ladouceur, M.; et al. Matching-Adjusted Indirect Comparison of the Efficacy of Apalutamide and Enzalutamide with ADT in the Treatment of Non-Metastatic Castration-Resistant Prostate Cancer. Adv Ther. 2020, 37, 501–511. [Google Scholar] [CrossRef]
- Mori, K.; Mostafaei, H.; Pradere, B.; Motlagh, R.S.; Quhal, F.; Laukhtina, E.; et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: A systematic review and network meta-analysis. Int J Clin Oncol. 2020, 25, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Averitt, A.J.; Weng, C.; Ryan, P.; Perotte, A. Translating evidence into practice: Eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med. 2020, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, K.; Garmo, H.; Franck Lissbrant, I.; Stattin, P. The Value of Real-World Data in Understanding Prostate Cancer Risk and Improving Clinical Care: Examples from Swedish Registries. Cancers. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; McKay, R.R.; Spratt, D.E.; Constantinovici, N.; Chen, G.; Ortiz, J.A.; et al. Biochemical recurrence (BCR) and outcomes in patients (pts) with prostate cancer (PC) following radical prostatectomy (RP). J Clin Oncol. 2023, 41, e17112. [Google Scholar] [CrossRef]
- Burgard, C.; Hoffmann, M.A.; Frei, M.; Buchholz, H.-G.; Khreish, F.; Marlowe, R.J.; et al. Detection Efficacy of Ga-PSMA-11 PET/CT in Biochemical Recurrence of Prostate Cancer with Very Low PSA Levels: A 7-Year, Two-Center “Real-World” Experience. Cancers. 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol. 2020, 77, 403–417. [Google Scholar] [PubMed]
- Meredith, G.; Wong, D.; Yaxley, J.; Coughlin, G.; Thompson, L.; Kua, B.; et al. The use of Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int. 2016, 118 Suppl 3, 49–55. [Google Scholar] [CrossRef]
- de Sá Moreira, E.; Robinson, D.; Hawthorne, S.; Zhao, L.; Hanson, M.; Kanas, G.; et al. Patterns of Care and Outcomes for Non-Metastatic Prostate Cancer in the United States: Results of the CancerMPact Survey 2018. Cancer Manag Res. 2021, 13, 9127–9137. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Chen, M.-H.; Wu, J.; Huland, H.; Graefen, M.; Mohamad, O.; et al. Prostate-Specific Antigen Level at the Time of Salvage Therapy After Radical Prostatectomy for Prostate Cancer and the Risk of Death. J Clin Oncol. 2023, 41, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- LBA02-09 EMBARK: A Phase 3 Randomized Study of Enzalutamide or Placebo Plus Leuprolide Acetate and Enzalutamide Monotherapy in High-risk Biochemically Recurrent Prostate Cancer. J Urol. 2023, 210, 224–226. [CrossRef] [PubMed]
- Aggarwal, R.R.; Heller, G.; Hillman, D.W.; Xiao, H.; Picus, J.; Taplin, M.-E.; et al. Baseline characteristics associated with PSA progression-free survival in patients (pts) with high-risk biochemically relapsed prostate cancer: Results from the phase 3 PRESTO study (AFT-19). J Clin Oncol. 2023, 41, 208–208. [Google Scholar] [CrossRef]
- CTG Labs—NCBI. Available online: https://clinicaltrials.gov/study/NCT05794906 (accessed on 28 December 2023).
- CTG Labs—NCBI. Available online: https://clinicaltrials.gov/study/NCT05526248 (accessed on 28 December 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).