1. Introduction
Interstitial lung disease (ILD) is a frequent complication in systemic sclerosis (SSc) patients and despite the recent advances in the treatment it is still the major cause of death of these patients [
1,
2]. ILD usually may be established during the first 4 years of the SSc being frequently subclinical [
3,
4]. Thus, particular attention in detecting early this complication is a crucial need in the clinical setting, because it may lead to a poor prognosis and quality of life. So, an accurate strategy is desirable to detect ILD in its early (or pre-clinical) stages.
Beyond the clinical history, currently, to assess the presence of SSc-ILD there are different tools including respiratory functional tests (RFT) and imaging methods such as X-ray and high-resolution computer tomography (HRCT).
As previously mentioned, the clinical manifestations might be ambiguous or absent in the initial stages of the SSc-ILD [
5]. Moreover, the RFT could shows unspecific or normal findings despite an established ILD [
6,
7]. In this context, imaging can play a key role in detecting the earliest SSc-ILD.
In past, chest radiography was the most frequently imaging method adopted to assess ILD, but currently its application has dramatically reduced due to the low sensitivity in the initial stages of ILD.
HRCT is the most common imaging technique used in the assessment of ILD. It is the gold standard imaging method reference for the diagnosis/prognosis, the quantification of severity, pattern analysis and therapy monitoring of ILD [
8,
9]. Furthermore, it has shown ability to detect both early pulmonary changes and subclinical lung involvement [
8]. Despite these qualities, its routine use is very limited due to high costs, scarce availability in non-tertiary health centres, long waiting times in some realities, and ionizing radiation. This latest aspect is of relevance since recently the issue of radiation exposure has been raised [
10,
11]. In this context pulmonary ultrasound (US) has emerged as a potential tool to for the assessment of ILD in patients with SSc [
12,
13,
14,
15,
16]. In fact, in the last years pulmonary US has been demonstrated to be reliable, feasible and valid for the assessment of SSc-ILD [
17,
18,
19].
Despite the growing body of evidence supporting its utility in ILD, there are no solid data regarding its potential role in both detecting ILD in subclinical stages and monitoring the evolution of ILD in these SSc patients. Taking into account of this gap of knowledge, we decide to investigate the validity of pulmonary US in detecting subclinical ILD in SSc and to determine its potential role in monitoring the ILD progression.
2. Material and Methods
2.1. Patients
We included consecutive patients with diagnosis of SSc according the accepted international criteria [
20], attending the outpatient and inpatient clinics of the centres involved in the study.
The inclusion criteria included: age > 18 years, non-smokers and patients with diagnosis of SSc without respiratory symptoms including dyspnoea or cough. Patients with a previous diagnosis of ILD, pulmonary diseases such as chronic obstructive pulmonary disease and pulmonary oedema were exclude mainly to avoid overlap of lung findings.
2.2. Study design
All patients underwent a complete clinical evaluation by an expert rheumatologist in order to confirm the absence of respiratory symptoms. Particular attention was paid to the detection of fine basilar dry inspiratory crackles or “velcro sound” at the lung auscultation. Rodnan skin score (RSS) and Borg Dyspnoea scale (Borg score) were also additionally performed in all patients.
Chest X-ray and RFT were performed the same day in all patients. Successively, pulmonary US was performed by a rheumatologist expert in lung examination (with more than 12 years of experience in US) who was blinded to clinical and laboratory assessment.
To determine the concurrent validity, HRCT was performed within the 7 days after pulmonary US assessment by an operator who was blinded to clinical, RFT, and pulmonary US findings. Finally, serologic tests (anti-centromere, anti-Scl70) were obtained from all patients.
In order to evaluate the inter-observer reliability, a second rheumatologist with 1 year of US experience, who received previously a 1-month dedicated and intensive training in lung US, has scanned half of patients. A healthy group, sex and age matched were included as control group.
The patients were followed every 12 weeks for 48 weeks performing pulmonary US, RFT and Borg scale at each visit.
2.3. US assessment
US examinations were assessed using a GE Versana Premier machine provided by a 5-13 MHz linear transducer. The patient position for the US examination and the scanning technique were those previously described [
16]. Shortly, for the anterior chest, the 2
nd lung intercostal spaces (LIS) along the para-sternal lines, the 4th LIS along the mid-clavear, the anterior axillary and the mild-axillary lines were assessed. For the posterior chest, the 8th LIS along the paravertebral, the sub-scapular and the posterior axillary lines were assessed.
2.4. US interpretation
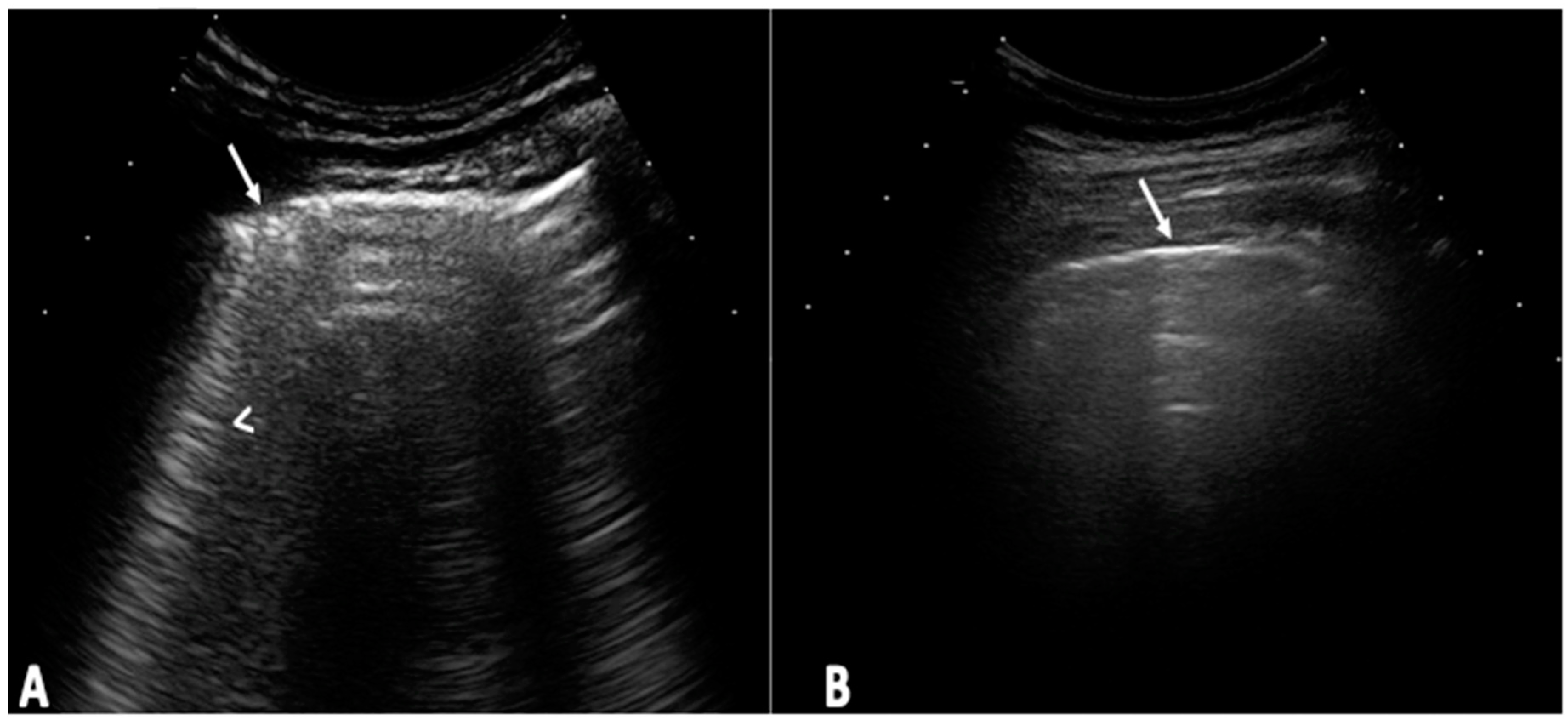
The US elementary lesions evaluated were the US B-lines (
Figure 1) [
21,
22]. The US B-lines total sum of all LIS was recorded and classified according to the following semiquantitative US scale [0=normal (≤5 B-lines); 1=slight (≥6 and ≤15 B-lines); 2=moderate, (≤16 and ≥30 B-lines); 3=severe (≥30 B-lines)] [
16].
2.5. HRCT assessment
HRCT examination was performed using a CT 64 E light Speed VCT power scanner with a rotation tube with a scanning time of 0.65 seconds. Pulmonary involvement was evaluated by lung segments according to the Warrick score [
23]. The severity of disease was obtained by adding single point values. The extension of the pulmonary involvement was obtained by counting the number of bronchopulmonary segments involved for each abnormality: one to three segments scored as 1; four to nine segments scored as 2; more than nine segments scored as 3. The total HRCT score of severity and extension were calculated between the range from 0 to 30 as described previously [
18]. The following semi-quantitative scoring was adopted to correlate accurately the US with HRCT findings: [0 = normal (0 points); 1 = mild (< 8 points); 2 = moderate (from 8 to 15 points) and 3 = marked (> 15 points)].
2.6. Statistical analysis
Statistical analysis was performed using Stata v13.0 (StataCorp.,TX, USA). Standard descriptive results were expressed both as means ± standard deviations (SDs) and medians. Categorical data were expressed as proportions. For the multivariate analysis (to determine associations between variables and US findings) a binary logistic regression was conducted with chi-square interpretation and momio reason. Spearman rank correlation coefficient (rho coefficient) was used for the correlation between the US and HRCT.
Accuracy, including sensitivity, specificity and predictive values of X-ray, pulmonary US, pulmonary auscultation and RFT were measured by the area under the ROC curve.
ROC curves were created by plotting the true-positive proportion versus the false-positive proportion (sensitivity versus specificity respectively). The area under the ROC curve (AUC) was employed to quantify the discriminative accuracy of US. AUC from 0.50 to 0.70 represent poor accuracy, those from 0.70 and 0.90 are useful for some purposes whereas higher values represent high accuracy.
To detect differences between US, RFT and Borg score in the longitudinal assessment Cochran's Q test was used. Inter-observer reliability of US findings a weighted kappa statistic was adopted [
24]. The feasibility was calculated according the time spent during each US examination by the independent samples t –test (p values less than 0.005 was considered statistically significant).
3. Results
One hundred and fifty-four SSc patients were recruited to be included in the study. From those 22 patients were excluded due to the presence of at least one-exclusion criteria. The study was conducted definitively on 133 SSc patients and 133 healthy sex- and age-matched controls.
3.1. Baseline assessment
A total of 1.862 LIS were globally scanned. Demographic and clinical data of the study population are reported in the
Table 1.
A total of 79 out of 133 patients (59.4%) showed US signs of ILD with respect to the healthy controls (4.8%) (p=0.0001).
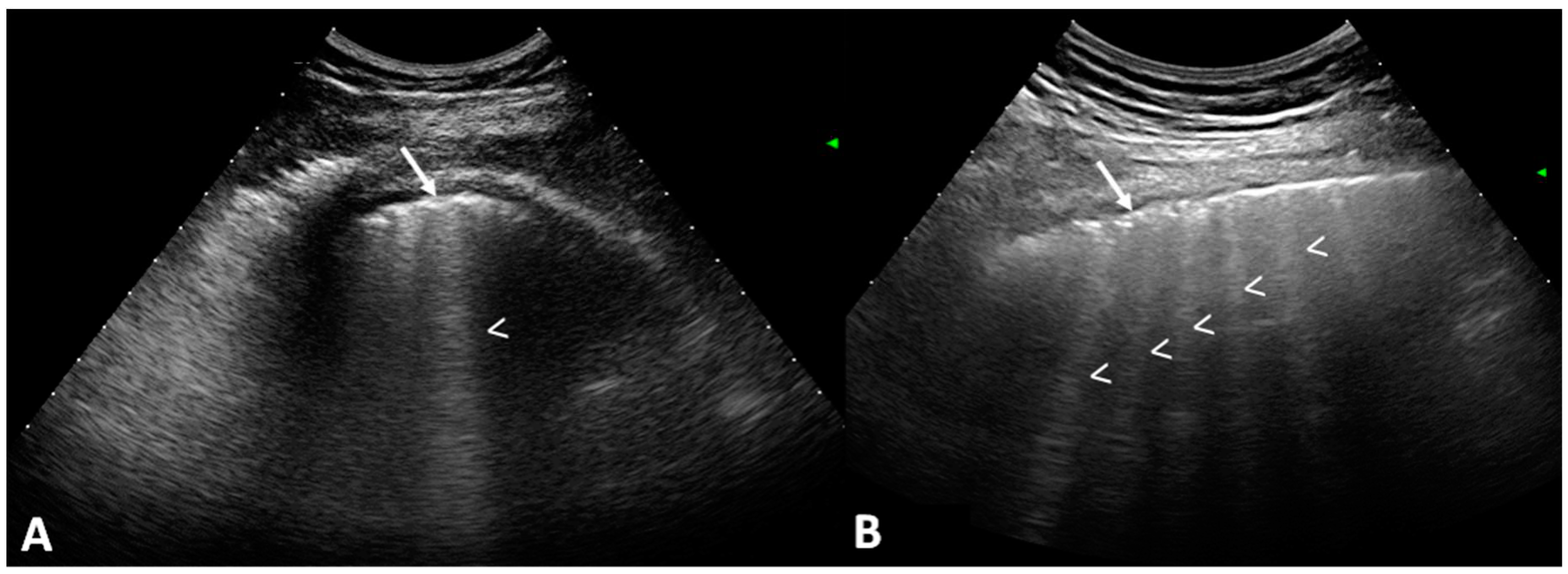
Figure 2, shows US B-lines of SSc patients.
RSS and anti-centromere antibodies were the variables that showed association with pulmonary US-ILD (p=0.003 and p=0.005 respectively) whereas no association was found with gender, age, disease duration, chest radiography and RFT findings (
Table 2).
US B-lines showed a positive correlation with HRCT Warrick score (rho=0.802; p = 0.0001). A 90.2% of concordance between the two imaging methods was additionally showed in the overall population, with a sensitivity and specificity of US with respect HRCT of 91.2% and 88.6% respectively. The 13 discordant cases were related to false positives at US (6 cases) and false negatives in 7 cases.
Sensitivity and specificity for the chest X-ray, pulmonary auscultation and RFT in the assessment of ILD were 2.5% and 98.1%, 8.7% and 98.1%, 27.5% and 77.3% respectively. The
Table 3 shows more details regarding the sensitivity, specificity, predictive value and area under the ROC (AUC-ROC) curve. The AUC-ROC analysis confirmed the analytical relationship between the pulmonary US B-lines and the presence of ILD at HRCT.
The global kappa values for the inter-observer reliability of pulmonary US semi-quantitative score showed a good agreement between the two investigators (overall kappa = 0.72).
The mean time spent to perform the pulmonary US assessment was 8.6 minutes (± SD 1.4, range 6 to 12 minutes).
3.2. Longitudinal assessment
All 133 SSc patients started the follow-up; however, 12 patients did not complete the 1-year of follow-up (4 patients abandoned the study within the 3 months, 5 patients within the 6 months, 1 patient within the 9 months and 2 patients within the 12 months). Finally, 121 SSc patients completed the 1-year follow-up.
Thirty out of 79 patients (37.97%) that showed US signs of ILD (B-lines) at baseline assessment, demonstrated a progression in severity of ILD according the US semiquantitative score. Supplementary file 1, shows the progression in terms of number of B-lines from the baseline assessment to the 12-months follow-up.
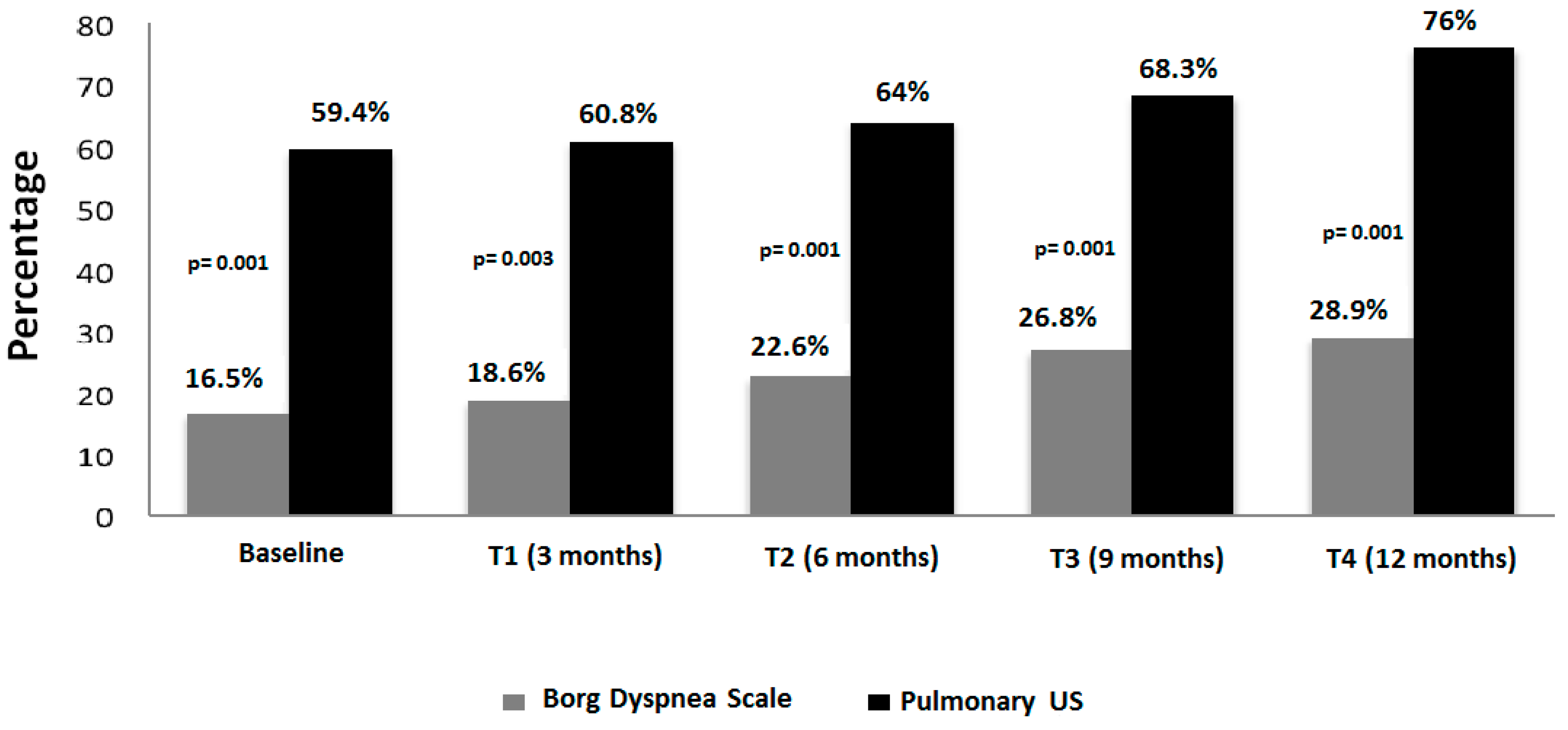
Only nine of those 30 patients (30%) developed respiratory symptoms during the follow-up. The elapsed time in which progression of ILD or clinical conditions was documented, was around 6 and 9 months of follow-up (supplementary file 2). The progression of both US and Borg dyspnoea scale changes are illustrated in the
Figure 3.
4. Discussion
ILD is a leading cause of mortality in patients with SSc [
1,
2,
3,
4,
5] So, its early diagnosis is mandatory and represents one of the primary goals in order to improve the prognosis of the disease.
Although HRCT is and will be, the current gold-standard imaging method for the assessment of ILD in SSc patients, including diagnosis and prognosis purposes [
25,
26], pulmonary US is providing interesting and solid data regarding its potential to assess SSc-ILD [
27,
28,
29,
30]. These researches have provided important information regarding the correlation between pulmonary US and HRCT findings in SSc patients with established ILD [
16,
31,
32,
33]. Interesting data regarding the good performance of US in terms of reliability and feasibility of SSc-ILD were also recently documented [
15,
33,
34,
35,
36,
37]. These data open up an interesting research opportunity to consolidate the utility of pulmonary US as biomarker of SSc-ILD.
Despite the emerging literature, there are no studies aimed to explore the diagnostic value of pulmonary US in the subclinical (or pre-clinical) stages of ILD and its potential role in monitoring ILD progression in SSc patients.
Our results showed a high prevalence of subclinical ILD detected by pulmonary US (59.4%) in SSc patients in spite Borg score and RFT resulting in normal or without significant restrictive abnormalities (
Table 1). We can interpreted and confirm that ILD may be asymptomatic during several years before the symptoms appear [
25,
26]. However, it is not surprising since in autopsy studies, previously performed, 90% of patients showed ILD by HRCT [
38,
39] whereas, surprisingly, only 40–55% showed changes in RFT [
40]. Our results confirm also the good sensitivity and specificity of pulmonary US in assessing ILD with respect to the HRCT, whereas radiographs, pulmonary auscultation and RFT showed a poor sensitivity and specificity for ILD in pre-clinical stages.
Interesting data have also emerged from the follow-up of patients. Thirty (55%) out of 79 SSc patients that showed US signs of ILD at baseline showed a progression in their US score of ILD. However, only 9 of those patients developed dyspnoea. Although this number is very low, some considerations can be formulated: a) pulmonary US can detect progression of the ILD in spite the clinical scale for measure dyspnoea remain unchanged, b) there is no a relationship between the changes in severity of ILD by the number of B-lines and the start of symptoms. Several patients changed the ILD status from mild to moderate, but not all developed respiratory symptoms (supplementary file 2).
From a further analysis of our results additional considerations could be made:
Firstly, the terms subclinical and/or pre-clinical could resulting still confusing in the routine nomenclature of the clinicians to describe interstitial lung abnormalities (ILA). However, it is important underline that a recent expert position paper recommended that incidentally detected ILA in SSc patients, should be classified as “preclinical or subclinical ILD” due to the presence of a risk factor for progressive disease [
41].
Second, chest radiograph confirms its scarce utility for the assessment of early phases of ILD as well as pulmonary auscultation and RFT may fail in detecting early ILD in early. Third, US offers many advantages for ILD assessment. It is a widely available tool, inexpensive, and largely accepted by the patient. Moreover, portable machines can be sufficient for a detailed ILD assessment as demonstrated previously [
36] Fourth, pulmonary US cannot replace the meaningful information obtained with the HRCT for the final diagnosis of ILD or for a complete evaluation of lung involvement; on the contrary, US could be considered as a complementary tool to implement in the very early stages of the SSc (as a screening tool) in order to identify patients with the potential risk of developing SSc-ILD or to move on HRCT to determine the phenotype and prognosis evolution. This, in light that to date, there is no evidence to recommend screening for early or subclinical SSc-ILD with HRCT [
42].
The ability of US to identify early signs of ILD may position its routine use essential in the management of patients who require serial examinations. Moreover, minimize radiation exposure is important in SSc because of the observed puzzling relation between SSc and breast cancer which usually appears on average 20 years after SSc onset [
43,
44,
45].
According our opinion and experience, in a near future pulmonary US might take part of the algorithm of ILD diagnosis together other examinations such as RFT and HRCT.
We are aware that our study presents potential limitations. First, the low number of enrolled patients, which does not permit an accurate evaluation in terms of sensitivity and specificity that could more strongly support these data. Second, additional DLco, that is the most important functional test demonstrating better correlation with the extent of lung fibrosis were not performed [
25]. It was omitted mainly due to the fact that in our institution requires additional cost in charge of the patients. Third, HRCT assessment was not performed during the longitudinal phase of study that limits a more solid support to the usefulness of pulmonary US in monitoring the progression of ILD. HRCT was also not performed by the costs and ethics aspects since it is difficult to justify a sequential HRCT assessment of SSc patients in absence of respiratory symptoms. Despite of this, we used RFT and Borg scale during the follow-up, which are tests largely used in real life to identify initial signs of ILD in SSc patients. Fourth, the 12-months of longitudinal follow-up could be a limited time to support the role of US in monitoring the progression of ILD. However, although a minimal quote of patients developed respiratory symptoms (dyspnoea), most of them (55%) showed an ILD progression by US during the 12 months, in absence of symptoms. This aspect is of great interest in order to reflect on the clinical and US disparity, which seems independently of the evolution time. Ongoing follow-up of our cohort of patients will elucidate better this aspect. Fifth, the additional causes of dyspnoea of those patients that developed it during the follow-up have not accurately investigated, in order to determine if the symptoms were closely related to the new-onset ILD. Sixth, only B-lines were considered as a US elementary lesion of ILD. Recently has been proposed the pleural irregularity as a new US lesion of ILD [
46]. However, its clinical implication is not still consolidated. Finally, a comparison with a control group with other diseases involving the pulmonary interstitium that would have added important information was not considered. However, this was addressed in previous papers, which showed data similar to our findings [
16,
36,
37,
38,
39].
5. Conclusions
US is a valid, reliable and feasible tool to detect very early (pre-clinical or subclinical stages) the ILD and to follow its progression of patients with SSc. Despite a great deal of work remains to be done (construct validity, reliability and sensitivity to change studies in order to validate lung US as outcome measurement instrument in ILD-SSc), our preliminary results can represent a milestone towards a possible implementation, in a nearly future, of pulmonary US as an innocuous screening tool for a very early diagnosis of ILD in SSc and for monitoring its progression.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
MG participated in the design of the study, the acquisition and interpretation of data, the drafting of the manuscript and gave final approval of the version of the paper to be published. DCC performed the statistical analysis, made substantial contributions to the manuscript preparation and were involved in revising the manuscript for important intellectual content. CB participated in the study conception, made substantial contributions to the manuscript preparation and were involved in revising the manuscript for important intellectual content. EZV made substantial contributions to the manuscript preparation and were involved in revising the manuscript for important intellectual content. All authors read and approved the final version of manuscript.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Center of Excellence in Rheumatic and Musculoskeletal Diseases (04/21).
Informed Consent Statement
The local Ethics Committee gave approval for the study and informed consent was obtained from all patients.
Conflicts of Interests
The authors would like to make the following statements with regard to their conflicts of interest/financial disclosures: MG has attended advisory board meetings, scientific consultancies and has obtained speaking fees for: AbbVie, Novartis, UCB, Esaote SpA, Janssen, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Sanofi Aventis. CB, EZV and DCC declare that they have no competing interests.
References
- Cappelli S.; Bellando Randone S.; Camiciottoli G.; De Paulis A.; Guiducci S.; Matucci-Cerinic M. Interstitial lung disease in systemic sclerosis: where do we stand? Eur. Respir. Rev. 2015;24:411-9. [CrossRef]
- Adler S.; Huscher D.; Siegert E.; Allanore Y.; Czirják L.; DelGaldo F.; Denton CP.; Distler O.; Frerix M.; Matucci-Cerinic M.; et al. Systemic sclerosis associated interstitial lung disease -individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group. Arthritis Res. Ther. 2018;30;20:17.
- Salaffi F.; Carotti M.; Baldelli S.; Bichi Secchi E.; Manganelli P.; Subiaco S.; Salvolini L. Subclinical interstitial lung involvement in rheumatic diseases. Correlation of high resolution computerized tomography and functional and cytologic findings. Radiol Med. 1999;97:33-41.
- Manganelli P.; Salaffi F.; Pesci A. Clinical and subclinical alveolitis in connective tissue diseases assessed by bronchoalveolar lavage. Semin. Arthritis Rheum. 1997;26:740-54. [CrossRef]
- D Roofeh D.; Brown KK.; Kazerooni EA.; Tashkin D.; Assassi S.; Martinez F.; Wells AU.; Raghu G.; Denton CP.; Chung L.; et al. Systemic sclerosis associated interstitial lung disease: a conceptual framework for subclinical, clinical and progressive disease. Rheumatology (Oxford). 2023;62:1877-1886. [CrossRef]
- Le Gouellec N.; Duhamel A.; Perez T.; Hachulla AL.; Sobanski V.; Faivre JB.; Morell-Dubois S.; Lambert M.; Hatron PY.; Hachulla E.; et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS One 2017;12:e0181692. [CrossRef]
- Showalter K.; Hoffmann A.; Rouleau G.; Aaby D.; Lee J.; Richardson C.; Dematte J.; Agrawal R.; Chang RW.; Hinchcliff M. Performance of Forced Vital Capacity and Lung Diffusion Cutpoints for Associated Radiographic Interstitial Lung Disease in Systemic Sclerosis. J. Rheumatol. 2018;45:1572-1576. [CrossRef]
- Molberg Ø.; Hoffmann-Vold AM. Interstitial lung disease in systemic sclerosis: progress in screening and early diagnosis. Curr. Opin. Rheumatol. 2016;28:613-18. [CrossRef]
- Picano E.; Semelka R.; Ravenel J.; Matucci-Cerinic M. Rheumatological diseases and cancer: the hidden variable of radiation exposure. Ann Rheum Dis 2014; 73: 2065-8. [CrossRef]
- Frauenfelder T.; Winklehner A.; Nguyen TD.; Dobrotav R.; Baumueller S.; Maurer B.; Distler O. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Ann. Rheum. Dis. 2014; 73: 2069-73. [CrossRef]
- Bernstein EJ.; Khanna D.; Lederer DJ. Screening High Resolution Computed Tomography of the Chest to Detect Interstitial Lung Disease in Systemic Sclerosis: A Global Survey of Rheumatologists. Arthritis Rheumatol 2018;70:971-2. [CrossRef]
- Moazedi-Fuerst FC.; Kielhauser S.; Brickmann K.; Tripolt N.; Meilinger M.; Lufti A.; Lufti A. Sonographic assessment of interstitial lung disease in patients with rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Clin. Exp. Rheumatol 2015;33(4 Suppl 91):S87-91.
- Gargani L.; Doveri M.; D'Errico L.; Frassi F.; Bazzichi ML.; Delle Sedie A.; Scali MC.; Monti S.; Mondillo S.; Bombardieri S.; et al. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009;48:1382-7. [CrossRef]
- Sperandeo M.; De Cata A.; Molinaro F.; Trovato FM.; Catalano D.; Simeone A.; Varriale A.; Martines GF.; Trovato G. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand. J. Rheumatol. 2015; 44:389-98. [CrossRef]
- Tardella M.; Gutierrez M.; Salaffi F.; Carotti M.; Ariani A.; Bertolazzi C.; Filippucci E.; Grassi. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: correlation with high-resolution computed tomography. J. Rheumatol. 2012; 39:1641-7. [CrossRef]
- Gutierrez M.; Salaffi F.; Carotti M.; Tardella M.; Pineda C.; Bertolazzi C.; Bichisecchi E.; Filippucci E.; Grassi W. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders--preliminary results. Arthritis Res. Ther. 2011;13:R134. [CrossRef]
- Hughes M.; Bruni C.; Cuomo G.; Delle Sedie A.; Gargani L.; Gutierrez M.; Lepri G.; Ruaro B.; Santiago T.; Suliman Y.; et al. The role of ultrasound in systemic sclerosis: On the cutting edge to foster clinical and research advancement. J. Scleroderma Relat. Disord. 2021;6:123-132. [CrossRef]
- Reyes-Long S.; Gutierrez M.; Clavijo-Cornejo D.; Alfaro-Rodríguez A.; González-Sámano K.; Cortes-Altamirano JL.; Muñoz-Louis R.; Cruz-Arenas E.; Camargo K.; Gonzalez F.; et al. Subclinical interstitial lung disease in patients with systemic sclerosis. A pilot study on the role of ultrasound. Reumatol. Clin. (Engl Ed) 2021;17:144-149. [CrossRef]
- Gutierrez M.; Soto-Fajardo C.; Pineda C.; Alfaro-Rodriguez A.; Terslev L.; Bruyn GA.; Iagnocco A.; Bertolazzi C.; D'Agostino MA.; Delle Sedie A. Ultrasound in the Assessment of Interstitial Lung Disease in Systemic Sclerosis: A Systematic Literature Review by the OMERACT Ultrasound Group. J. Rheumatol. 2020;47:991-1000. [CrossRef]
- Van den Hoogen F.; Khanna D.; Fransen J.; Johnson SR.; Baron M.; Tyndall A.; Matucci-Cerinic M.; Naden RP.; Medsger TA Jr.; Carreira PE.; et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2013;72:1747-55. [CrossRef]
- Lichtenstein D.; Mezière G.; Biderman P, .; Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999; 25:383-8. [CrossRef]
- Warnecke K.; Galanski M.; Peters E.; Hansen J. Pneumothorax: evaluation by ultrasound Preliminary results. J. Thorac. Imaging 1987;2:76-8.
- Warrick JH.; Bhalla M.; Schabel SI.; Siver RM. High resolution computed tomography in early scleroderma lung disease. J. Rheumatol. 1991;18:1520-8.
- 24 Landis JR.; Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977, 33:159-74. [CrossRef]
- Salaffi F.; Carotti M.; Di Donato E.; Di Carlo M.; Ceccarelli L.; Giuseppetti G. Computer-Aided Tomographic Analysis of Interstitial Lung Disease (ILD) in Patients with Systemic Sclerosis (SSc). Correlation with Pulmonary Physiologic Tests and Patient-Centred Measures of Perceived Dyspnea and Functional Disability. PLoS One 2016;11:e0149240. [CrossRef]
- Salaffi F.; Carotti M.; Baldelli S.; Bichi Secchi E.; Manganelli P.; Subiaco S.; Salvolini L. Subclinical interstitial lung involvement in rheumatic diseases. Correlation of high resolution computerized tomography and functional and cytologic findings. Radiol. Med. 1999;97:33-41.
- Mohammad Reza Beigi D.; Pellegrino G.; Loconte M.; Landini N.; Mattone M.; Paone G.; Truglia S.; Di Ciommo FR.; Bisconti I.; Cadar M.; et al. Lung ultrasound compared to computed tomography detection and automated quantification of systemic sclerosis-associated interstitial lung disease: preliminary study. Rheumatology (Oxford). 2023:kead324. [CrossRef]
- Gomes Guerra M.; Machado Pinto T.; Águeda A.; Rodrigues J.; Marona J.; Violante A.; Oliveira M. The Role of Lung Ultrasound in Systemic Sclerosis: A Systematic Review. J. Clin. Rheumatol. 2023;29:e32-e39. [CrossRef]
- Bruni C.; Mattolini L.; Tofani L.; Gargani L.; Landini N.; Roma N.; Lepri G.; Orlandi M.; Guiducci S.; Bellando-Randone S.; et al. Lung Ultrasound B-Lines in the Evaluation of the Extent of Interstitial Lung Disease in Systemic Sclerosis. Diagnostics (Basel). 2022;12:1696. doi: 10.3390/diagnostics12071696.
- Gargani L.; Bruni C.; Romei C.; Frumento P.; Moreo A.; Agoston G.; Guiducci S.; Bellando-Randone S.; Lepri G.; Belloli L.; et al. Prognostic Value of Lung Ultrasound B-Lines in Systemic Sclerosis. Chest. 2020;158:1515-1525). [CrossRef]
- Aghdashi M.; Broofeh B.; Mohammadi A. Diagnostic performances of high resolution trans-thoracic lung ultrasonography in pulmonary alveoli-interstitial involvement of rheumatoid lung disease. Int. J. Clin. Exp. Med. 2013; 6:562-6.
- Hassan RI, Lubertino LI, Barth MA, Quaglia MF, Montoya SF, Kerzberg E, Binda MDC.;et al. Lung Ultrasound as a Screening Method for Interstitial Lung Disease in Patients With Systemic Sclerosis. J. Clin. Rheumatol. 2019;25:304-307. [CrossRef]
- Moazedi-Fuerst FC.; Zechner PM.; Tripolt NJ.; Kielhauser SM.; Brickmann K.; Scheidl S.; Lutfi A.; Graninger WG. Pulmonary echography in systemic sclerosis. Clin. Rheumatol. 2012; 31:1621-5. [CrossRef]
- Gigante A.; Rossi Fanelli F.; Lucci S.; Barilaro G.; Quarta S.; Barbano B.; Giovannetti A.; Amoroso A.; Rosato E. Lung ultrasound in systemic sclerosis: correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern Emerg Med 2016;11:213–17. [CrossRef]
- Mohammadi A.; Oshnoei S.; Ghasemi-Rad M. Comparison of a new, modified lung ultrasonography technique with high-resolution CT in the diagnosis of the alveolo-interstitial syndrome of systemic scleroderma. Med. Ultrason. 2014;16:27–31. [CrossRef]
- Delle Sedie A.; Doveri M.; Frassi F.; Gargani L.; D'Errico G.; Pepe P.; Bazzichi L.; Riente L.; Caramella D.; Bombardieri S. Ultrasound lung comets in systemic sclerosis: a useful tool to detect lung interstitial fibrosis. Clin Exp Rheumatol 2010;28:S54.
- Barskova T.; Gargani L.; Guiducci S.; Randone SB.; Bruni C.; Carnesecchi G.; Conforti ML.; Porta F.; Pignone A.; Caramella D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013; 72:390-5. [CrossRef]
- Rubio-Rivas M.; Royo C.; Simeón CP.; Corbella X.; Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin. Arthritis. Rheum. 2014; 44: 208-19. [CrossRef]
- Mouthon L.; Berezné A.; Brauner M.; Kambouchner M.; Guillevin L.; Valeyre D. Interstitial lung disease in systemic sclerosis. Rev. Mal. Respir. 2007; 24: 1035-46.
- Steen VD.; Conte C.; Owens GR.; Medsger TA. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283-9.
- Hatabu H.; Hunninghake GM.; Richeldi L.; Brown KK.; Wells AU.; Remy-Jardin M.; Verschakelen J.; Nicholson AG.; Beasley MB.; Christiani DC.; et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir. Med. 2020;8:726-37. [CrossRef]
- Volkmann ER.; Sparks JA.; Hoffmann-Vold AM.; Doyle TJ.; Emery P.; Dieudé P. Preclinical/subclinical rheumatoid arthritis-associated interstitial lung disease: misleading terms with potentially deleterious consequences. Lancet Rheumatol. 2023;5:e116-e118.
- European Commission. Radiation protection 118: referral guidelines for imaging. http://europa.eu.int/comm/environment/radprot/118/rp-118-en.pdf (accessed 18 April 2010).
- Picano E.; Matucci-Cerinic M. Unnecessary radiation exposure from medical imaging in the rheumatology patient. Rheumatology 2011;50:1537–9. [CrossRef]
- Lu TY.; Hill CL.; Pontifex EK.; Roberts-Thompsom PJ. Breast cancer and systemic sclerosis: a clinical description of 21 patients in a population-based cohort study. Rheumatol. Int. 2008;28:895–9. [CrossRef]
- Pinal-Fernandez I.; Pallisa-Nuñez E.; Selva-O’Callaghan A.; Castella-Fierro E.; Simeon-Aznar CP.; Fonollosa-Pla V.; Vilardell-Tarres M. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin. Exp. Rheumatol. 2015;33:136-41.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).