1. Introduction
The components interaction in
R-
T-
X intermetallic systems (
R = rare earth metal;
T = transition metal;
X = other metal) results in many ternary compounds with recurrent stoichiometries in different concentration ranges. These compounds have been widely studied both for their applicative and fundamental properties [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10], and they represent an ever growing database for studying structural relationships aiming simplified descriptions and rational generalizations.
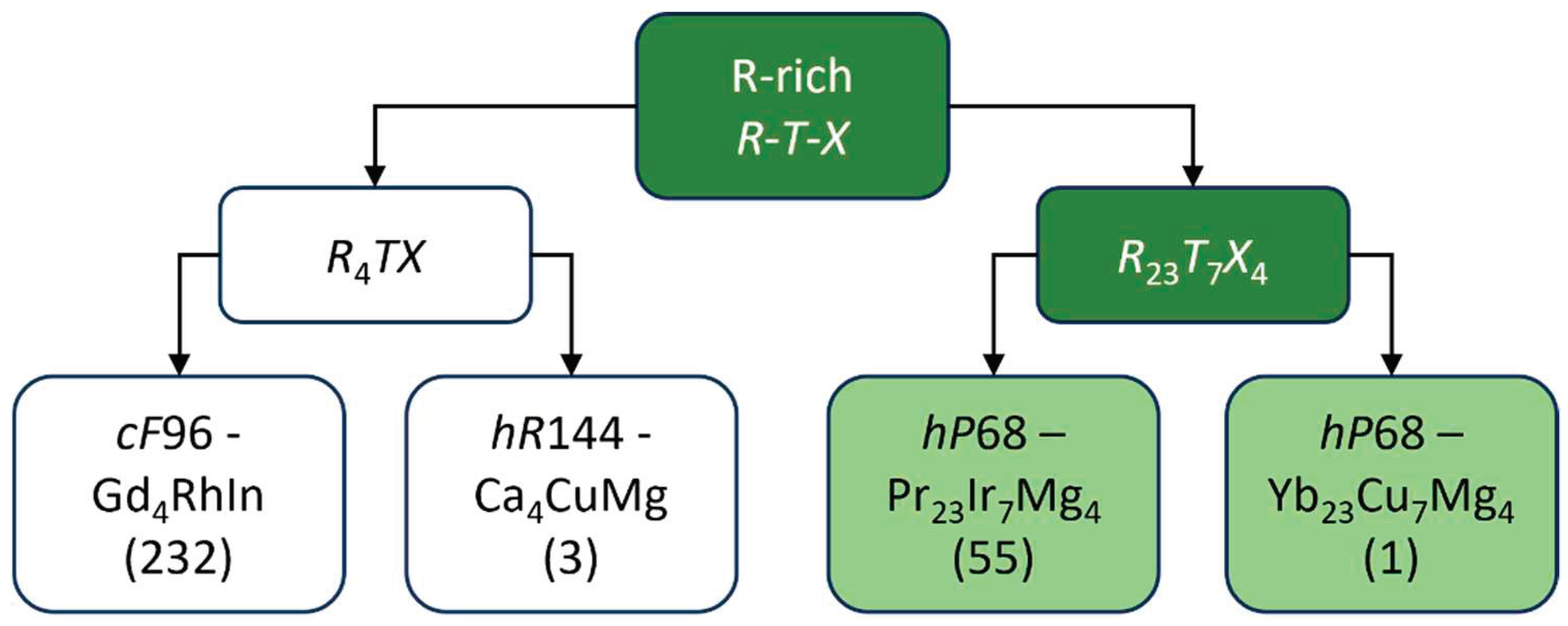
Families of rare earth-rich
R-
T-
X representatives are highly populated, especially for
R4TX and
R23T7X4 stoichiometries, each characterized by two different crystal structures (see
Figure 1).
In the framework of a recent investigation on the
R4TX family, the new {Ca,Eu,Yb}
4CuMg compounds (
hR144) were discovered and structurally elucidated in terms of intergrowth concept [
11].
As a logical evolution of this research, our attention focused on the
R23T7X4 family, mostly showing the
hP68-Pr
23Ir
7Mg
4 structure; an overview of chemical compositions of the known so far members is presented in
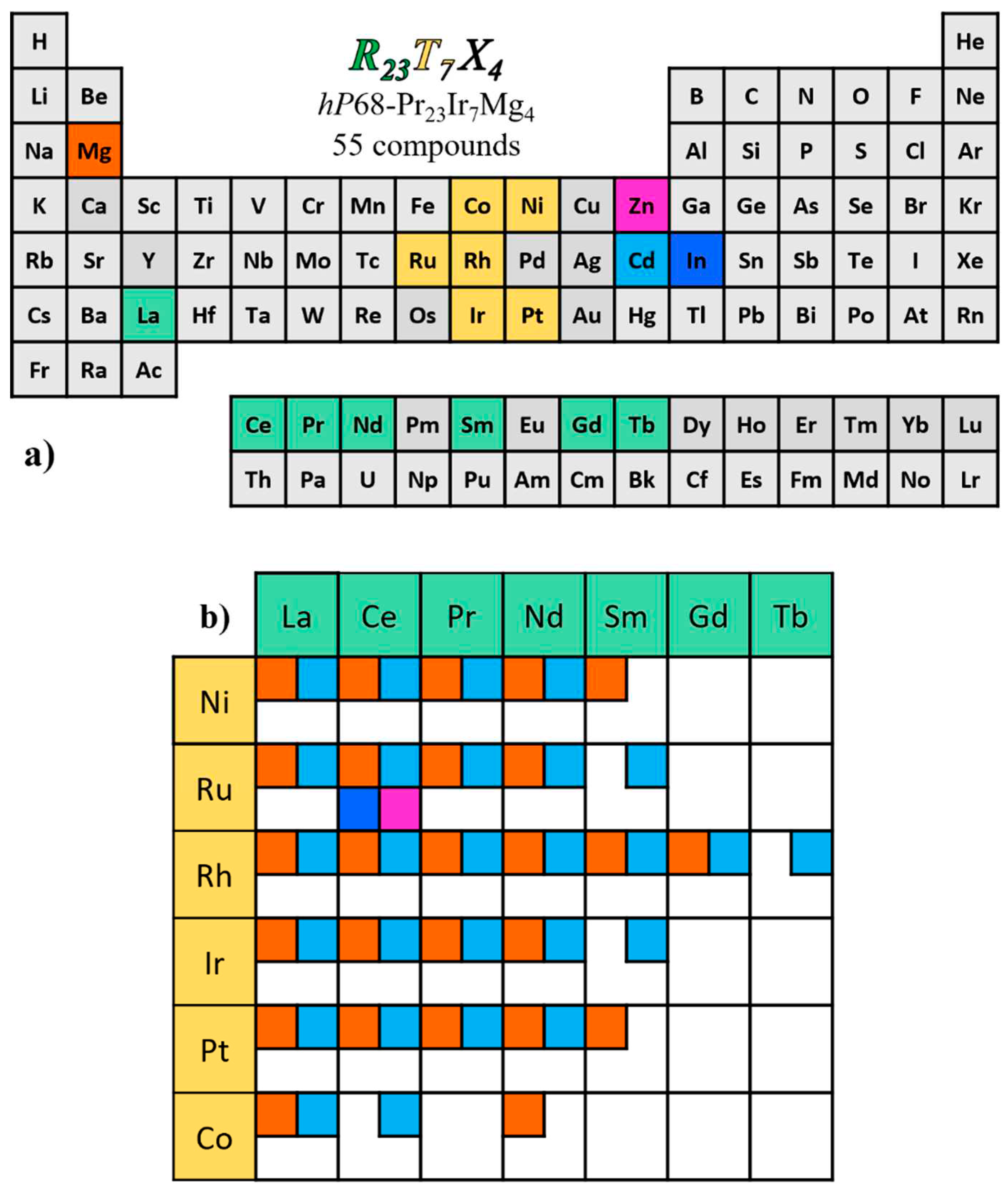
Figure 2. This periodic table map shows that the components of these phases are light trivalent rare earth metals (
R with Z < 65), late transition elements from 8 to 10 group (
T) and Mg, Zn, Cd or In (
X). As derives from the expanded table in Figure2b, the Mg and Cd containing groups are the most abundant, Zn and In having only one representative each, in combination with Ce and Ru.
The
hP68-Yb
23Cu
7Mg
4 structure is only represented by the prototype [
12], thus it was decided to perform some explorative syntheses on divalent
R analogues (
R = Ca, Eu) with the aim to enrich this family and looking for meaningful structural and chemical generalization criteria.
2. Experimental Section
Samples of nominal composition Ca67.6Cu20.6Mg11.8 (total mass = 0.5 g) and Eu66.7Cu20.3Mg13 (total mass = 0.8 g) were prepared from pure (>99.9 mass %) components. The starting metals were weighted in stoichiometric amounts and placed in tantalum crucibles, arc-sealing their cap to prevent Mg evaporation. These operations were done in a glove box filled with Ar, to minimize side reactions with oxygen and water. The Ta crucibles were put in a quartz glass tube sealed under an inert atmosphere, then placed in a resistance furnace where the following thermal cycle was applied: 20 °C → (5 °C/min) → 850 °C (10 min) → (-0.1 °C/min) → 400 °C (5 min) → (-0.2 °C/min) → 100 °C (furnace switched off).
For microscopic characterization, some pieces of each sample were selected and embedded in a conductive phenolic resin, polymerized in a hot mounting press machine Opal 410 (ATM GmbH, Germany). Surfaces were smoothed by SiC abrasive papers with no lubricant and polished with the aid of diamond pastes with particle size decreasing from 6 to 1 μm, using petroleum ether as lubricant. An automatic polishing machine Saphir 520 (ATM GmbH, Germany) was applied for this purpose.
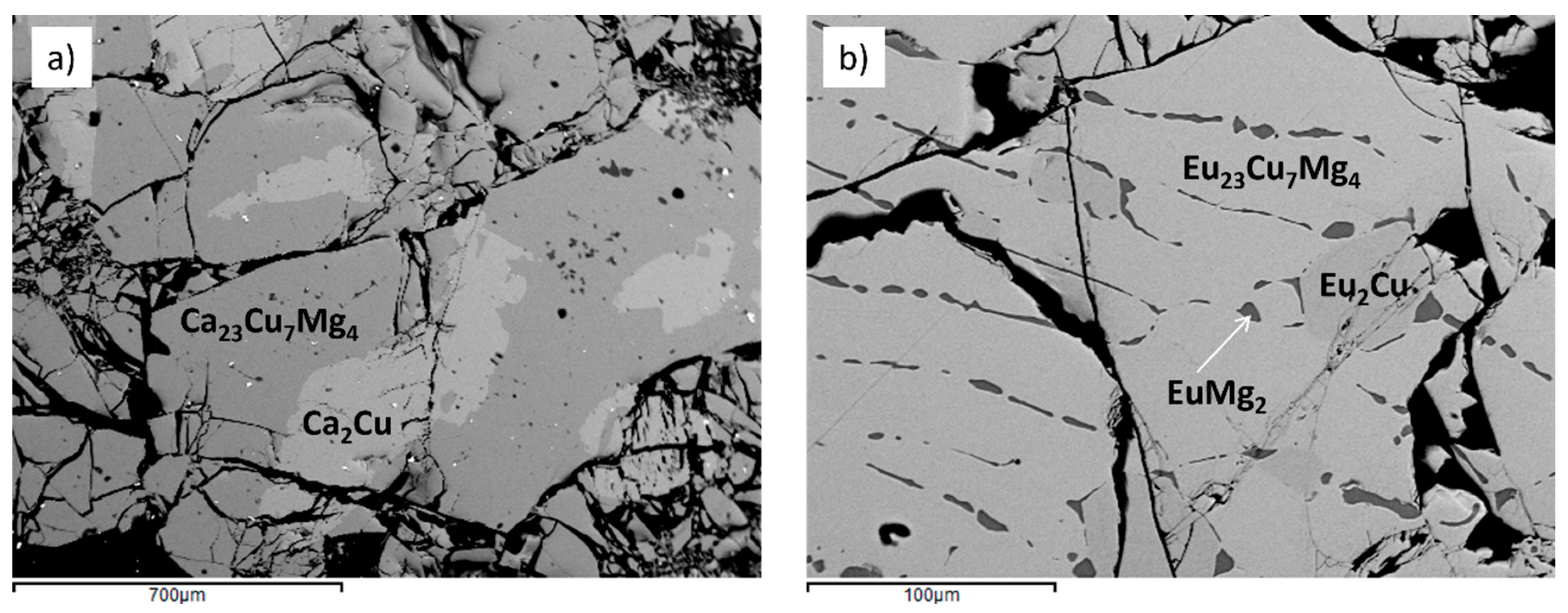
Microstructure observation together with qualitative and quantitative analyses were conducted on a Zeiss Evo 40 Scanning Electron Microscope (SEM) equipped with a Dispersive X-ray Spectroscopy (EDXS) system (INCA X-ACT) managed by the INCA Energy software (Oxford Instruments, Analytical Ltd., Bucks, U.K.). Both samples showed a good yield of the compound of interest, of average composition ~ 67 at.%
R, 20 at.% Cu, 13 at.% Mg (see
Figure 3), and were therefore subjected to X-ray diffraction studies. X-ray Powder Diffraction (XRPD) patterns were recorded on a Philips X'Pert MPD diffractometer (Cu K
α radiation, step mode of scanning) and indexed by Powder Cell [
13] software.
Good quality single crystals were selected with the help of a light microscope from mechanically crushed alloys covered with mineral oil. Crystals, embedded in an excess of grease to prevent oxidation, were then glued to pins and remained stable for several days.
The X-ray diffraction data were collected on a three-circle Bruker D8 QUEST diffractometer equipped by a PHOTON III 14 photon counting detector, using the graphite monochromatized Mo Kα radiation. Data collection strategies, consisting of both ω- and ϕ-scans, were decided using the APEX4 software [
14] to obtain good data completeness, redundancy, and resolution limit. Data were collected over the reciprocal space up to ~ 31° in θ (resolution of
ca. 0.7 Å) with exposures of 30-40 s per frame. The software SAINT [
15] and XPREP [
16] were used for data reduction. Lorentz, polarization, and absorption effects were corrected by SADABS [
17]; the crystal structure was solved and refined with the aid of SHELXTL [
18].
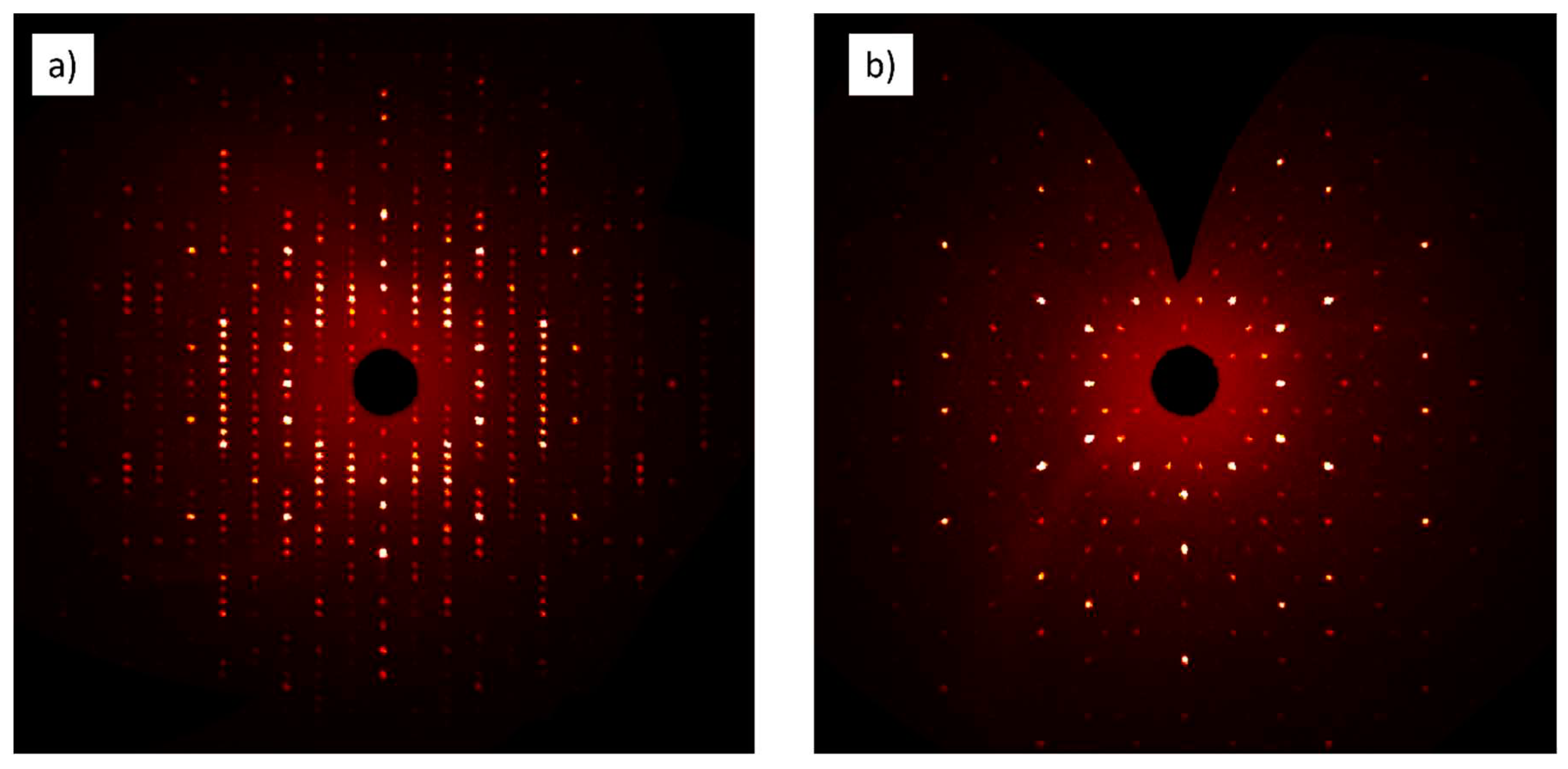
Both crystals possess hexagonal symmetry, and their diffraction patterns show systematic absences due to the presence of a
c-type glide plane. Reconstructed intensity profiles of selected zones are shown in
Figure 4 for the Eu-compound diffraction pattern.
The best structural model was found using the intrinsic phasing method in the
P6
3/
mmc space group (N. 194), corresponding to the
hP68-Yb
23Cu
7Mg
4 prototype. The unit cell, containing 2 formula units of
R23Cu
7Mg
4 composition, accounts for 68 atoms, distributed among 5 Wyckoff sites of
R, 2 sites of Cu and 2 of Mg. In the case of Ca
23Cu
7Mg
4, the first refinement resulted in somewhat high isotropic displacement parameters for Mg atoms in the 2
a position. A Mg/Ca statistical mixture was refined for this site, resulting in 0.93/0.07 ratio and significantly improving the structural model. For the Eu representative, no need of statistical mixture was evidenced, and the structural model turns out to be perfectly stoichiometric. The final anisotropic full-matrix least-squares refinements converged to good residuals for both compounds. Details of data collection and structure refinement are summarized in
Table 1 together with selected crystal data; standardized atomic coordinates, site occupancy factors and equivalent displacement parameters are listed in
Table 1. The corresponding CIF files, available as supplementary material, were deposited at the Cambridge Database.
3. Results and Discussion
The studied {Ca,Eu}
23Cu
7Mg
4 compounds, together with the Yb-containing prototype, form a small sub-family of
R23T7X4 with crystal structure (
hP68-Yb
23Cu
7Mg
4, Wyckoff sequence
k4h2fca, see
Table 2) different from all the others (
hP68-Pr
23Ir
7Mg
4, Wyckoff sequence
c10b2a2), however having in common with them the hexagonal symmetry and the number of atoms in the unit cell.
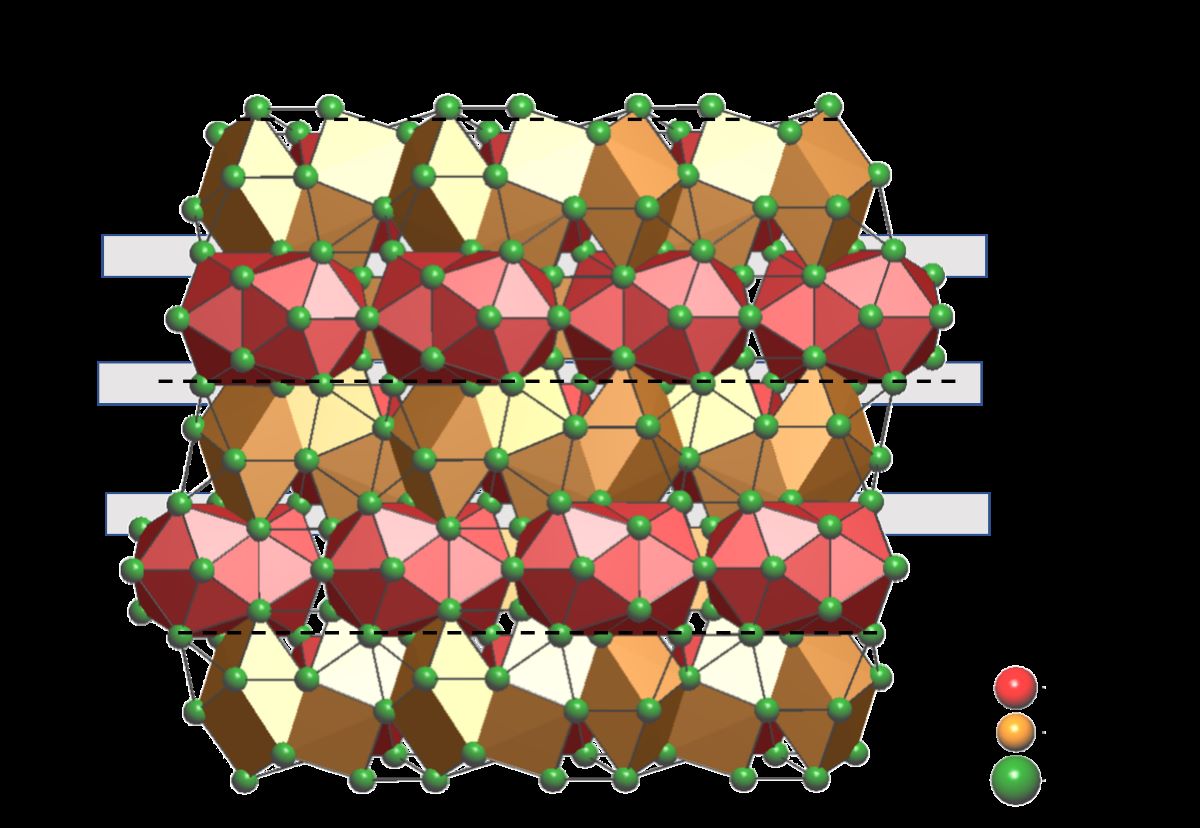
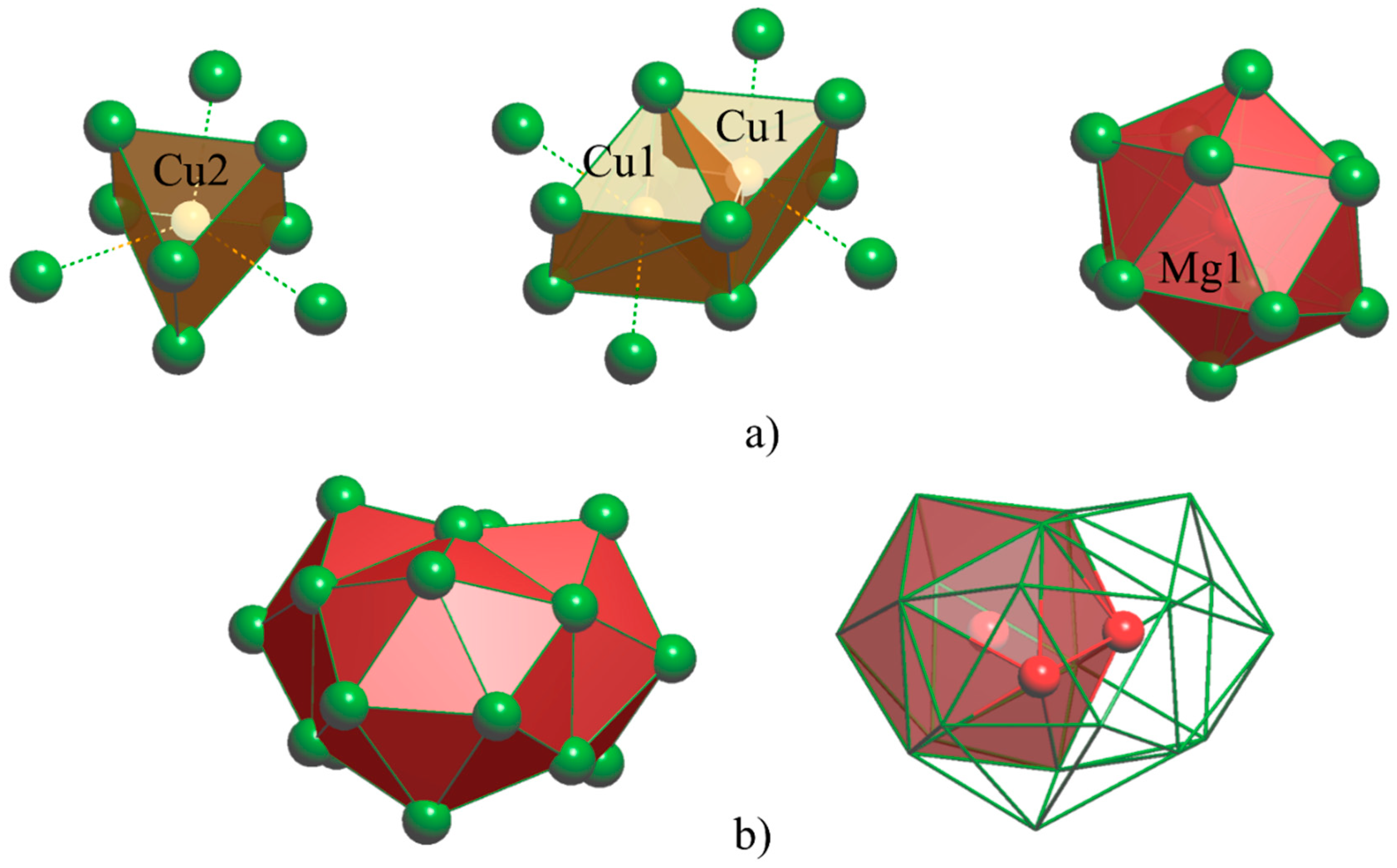
A first glance to the crystal structures of interest in terms of interatomic distances shows that the
R–Mg (3.52 ÷ 3.74 Å for Ca, 3.69 ÷ 3.90 Å for Eu), Cu–Cu (2.49 for Ca, 2.56 for Eu) and Mg–Mg (3.19 for Ca, 3.26 for Eu) contacts are compatible with the metallic radii sums [
19], instead Mg and Cu are well apart and do not interact. Also, the first coordination spheres of the different species are geometrically similar to those in other
R-
T-
X compounds rich in
R. These polyhedra are: capped Cu-centered trigonal prisms, either isolated (Cu@
R(6+3)) or sharing a rectangular face (Cu@Cu
R(6+2)), isolated Mg-centered icosahedra (Mg@
R12) and polyicosahedral Mg3@
R20 core-shell clusters (see
Figure 5).
The same coordination polyhedra were observed in
R4CuMg (
R=Ca, Eu, Yb) compounds [
11], with a difference in the Mg-centered polyicosahedral units: in the 23:7:4 these are formed by three fused icosahedra, instead in the 4:1:1 six fused icosahedra form core-shell clusters of Mg7@
R32 composition. Recently, we proposed an elegant description of the
R4CuMg structure in terms of linear intergrowth of slabs of
R9.5CuMg
3.5 and
R13Cu
6Mg composition [
11]. Considering the cited similarities, an analogous description was attempted with success also for
R23Cu
7Mg
4 with divalent
R.
In fact, the structure of title compounds can be interpreted as a stacking of slabs from the same parent structures, that have been extensively described in [
11]: the hexagonal
hP28-
kh2ca (SG 194) adopted by many
R9TX4 and
R10TX3 compounds and the rhombohedral
hR60-
b6a2 (SG 160) only adopted by Lu
13Ni
6In [
20]. Slabs of each parent type are alternatively stacked along the
c-direction, fulfilling the crystal space with no gaps neither need of “gluing” atoms (see
Figure 6a). Slabs, possessing the same
p3
m1 layer symmetry, are joined by a common corrugated layer composed exclusively by
R atoms, showing two types of nodes represented by (3
6)
1;(3
2434)
6 Schläfli notation (see
Figure 6b).
As a consequence of this, the composition of slabs from the hexagonal parent type is R10CuMg3, instead the composition of other slabs is exactly R13Cu6Mg. Therefore, the 23:7:4 unit cell content can be easily described by properly considering the composition and number of stacked slabs: 2×R10CuMg3 + 2×R13Cu6Mg = 2×R23Cu7Mg4.
It should be noted that the
P6
3/
mmc space group of title compounds is the only centrosymmetric one among the three hexagonal space groups compatible with the
p3
m1 layer symmetry of the stacked slabs [
11]. Lattice parameters of representatives with the same slabs stacked along
c should be related to those of the parent structures, with
a and
b being similar or integer multiples and
c correlated to the total number of slabs in the unit cell: this is true for 23:7:4 and 4:1:1 compounds, having
a =
b ≈ 10 Å and
c ≈ 24 Å and 51 Å, respectively.
The compositions of the two families of divalent
R-rich compounds can be plotted on a Gibbs triangle highlighting the relation with compositions of parent types (see
Figure 7) and helping to develop the structural/compositional generalization idea: in fact, hypothetic new compounds with similar intergrowth architectures should show stoichiometries laying along the dotted tie-lines.
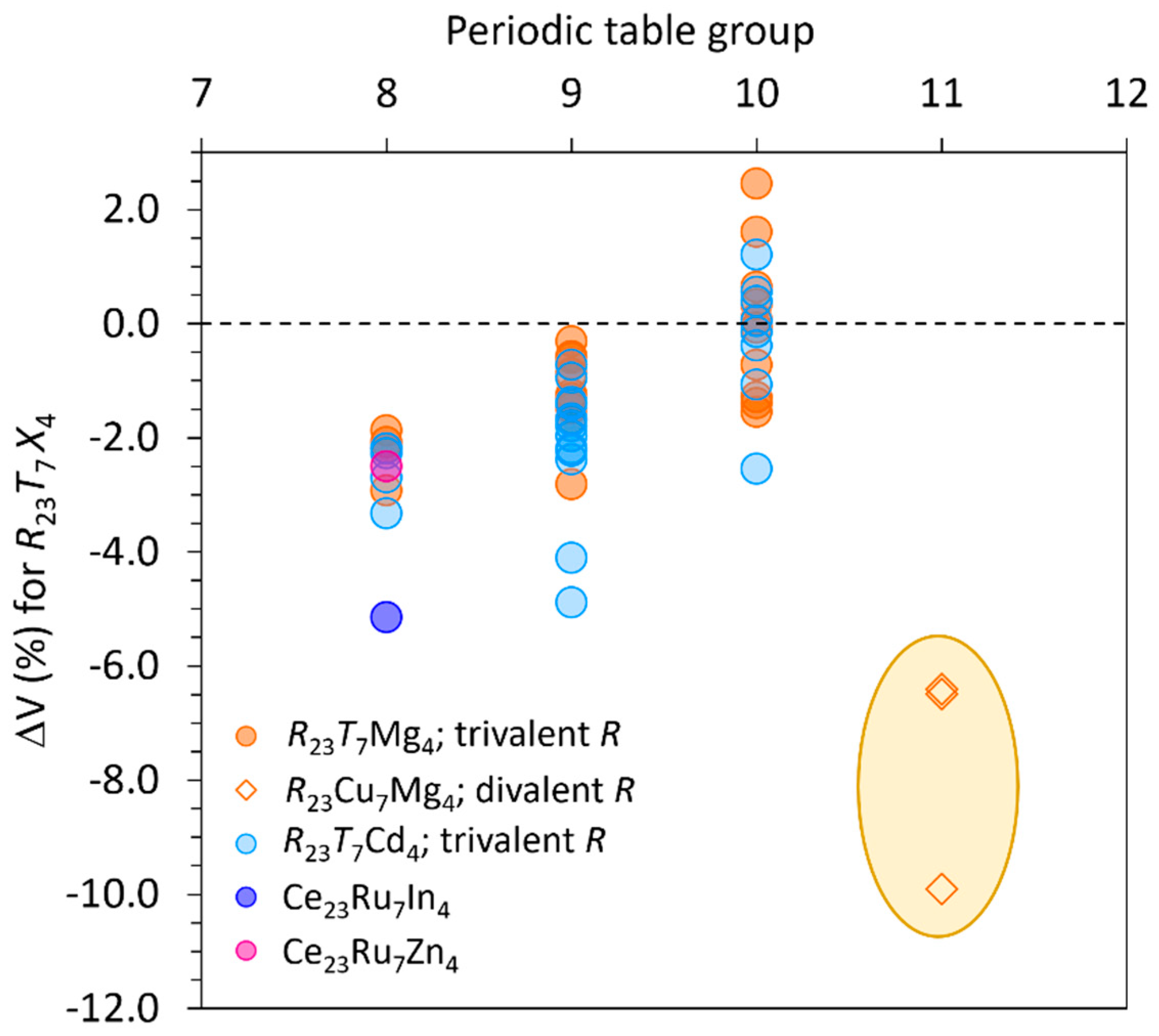
At this point, it is interesting to compare the two structural sub-families of
R23T7X4 intermetallics in terms of their component nature. A similar analysis was applied to
R4TMg using the volume contraction as a criterion. This is defined as
, where
, (
Ni = number of
i-type atoms in the unit cell,
Vi = atomic volume of the
i-type species taken from [
21]) and
Vmeas is the experimentally determined volume [
22]. Values of Δ
Vf (%) for
R23T7X4 are plotted in
Figure 8, as a function of the
T group and of the
R trivalent/divalent nature.
A major part of ΔVf (%) are negative, except for compounds with T = Pt, for which values lay in the range between 0 and +2.4%. No separation is observed as a function of X nature.
Instead, the title compounds, the only representatives known for divalent R, form a clearly clustered group, showing the most prominent volume contractions, extending down to -10% and indicating strong chemical interactions.
It is worth to note that both
R and
T nature are determinant for the formation and structure of these
R-rich compounds; for example, the existence of
R23T7Mg
4 has been excluded for several combinations of trivalent
R with {Cu, Ag, Au}[
4,
23,
24,
25]. On the other hand, for
T belonging to the 10
th group no representatives were found with divalent
R so far: considering the similar trend observed for the structurally related 4:1:1, these combinations are indeed worth to be investigated.
4. Conclusions
In this work, the crystal structures of the {Ca,Eu}23Cu7Mg4 compounds were solved by single crystal X-ray measurements, being the second and third representatives of the Yb23Cu7Mg4 prototype, and forming a small structural sub-family of 23:7:4 with a divalent R constituent. These few compounds are characterized by pronounced volume contractions if compared with the highly populated R23T7X4 family with trivalent R having Pr23Ir7Mg4-type structure. The distribution of members of both groups discovered so far as a function of the nature of components suggests combinations for new exploratory syntheses aiming to enrich the Yb23Cu7Mg4-type representatives, for example R23{Ag,Au}7Mg4, R 23{Ni,Pd,Pt}7Mg4 and R23Cu7{Zn,Cd,Al,In}4 with R= Ca, Eu, Yb.
The crystal structure of the title compounds was interpreted in terms of linear intergrowth of slabs R10CuMg3 (parent type: hP28-kh2ca, SG 194) and R13Cu6Mg (parent type: hR60-b6a2, SG 160), alternating along c-axis in the 1:1 ratio. This description brings together {Ca,Eu,Yb}23Cu7Mg4 and {Ca,Eu,Yb}4CuMg compounds, the latter being formed by the same type of slabs in a 2:1 ratio. An evolution of this idea pushes towards the recognition/discovery of new structural families based on different intergrowths of the same slabs. To this purpose, the following conditions should be fulfilled:
- (1)
Composition restraint – stoichiometries laying along the lines joining the end-members
- (2)
Symmetry restraint – rhombohedral, hexagonal and cubic space groups including the
p3
m1 among possible s
d linear orbits [
11,
26]
- (3)
Metric restraint – for hexagonal and rhombohedral representatives a=b≈10÷11 Å or their multiples
The results of structural/chemical analysis illustrated here constitute a further step towards a planned wider generalization aimed to a simple and chemically significant representation in terms of few common building blocks of complex R-rich ternary intermetallics.