Submitted:
04 January 2024
Posted:
05 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Surgery and Postoperative care:
2.2. Patient selection:
2.3. Outcomes:
2.4. Inflammatory biomarkers and the NUn score:
2.5. Statistical analysis:
3. Results
3.1. Demographics of the study cohort:
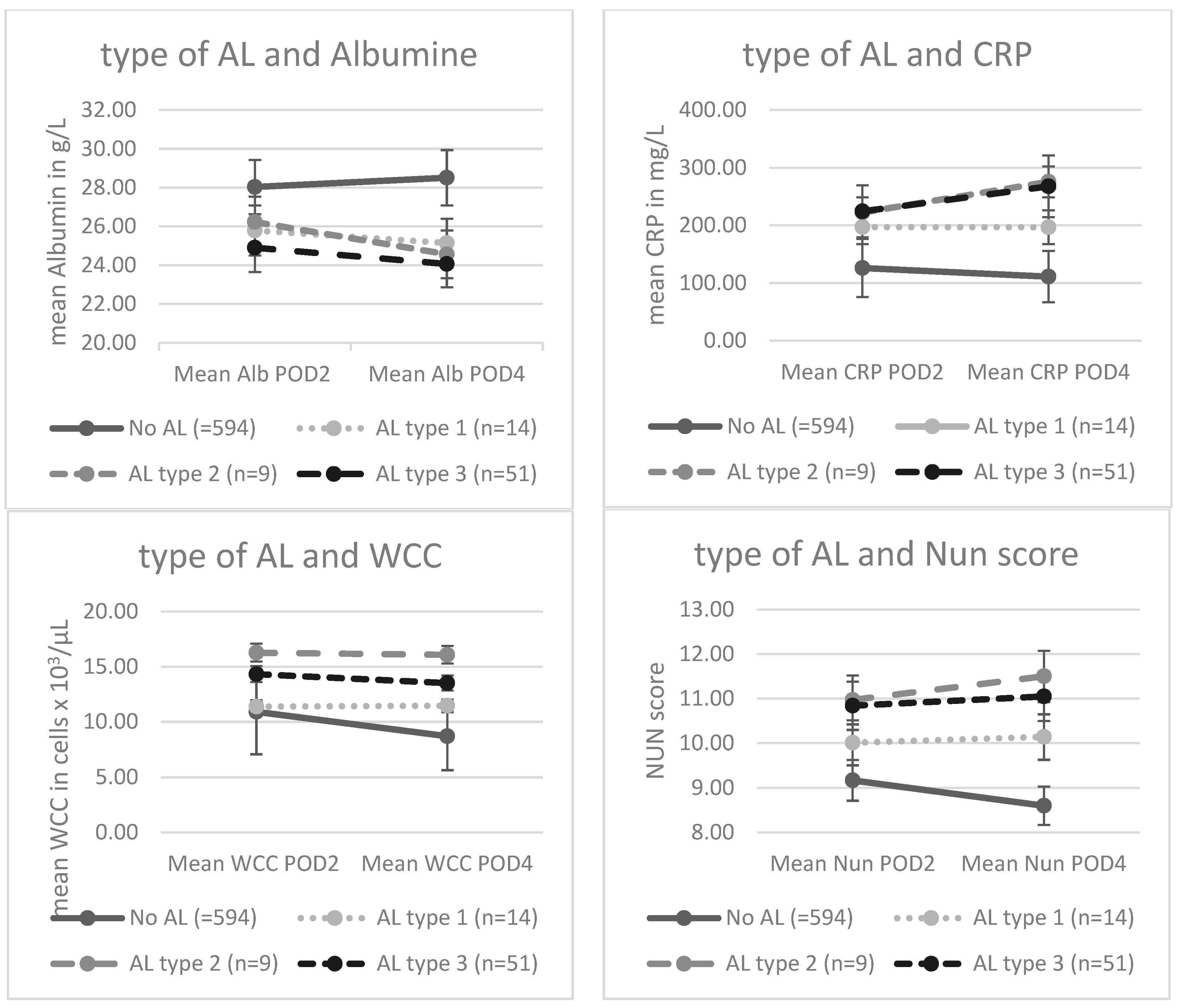
3.2. Mean levels of inflammatory biomarkers and severity of the AL:
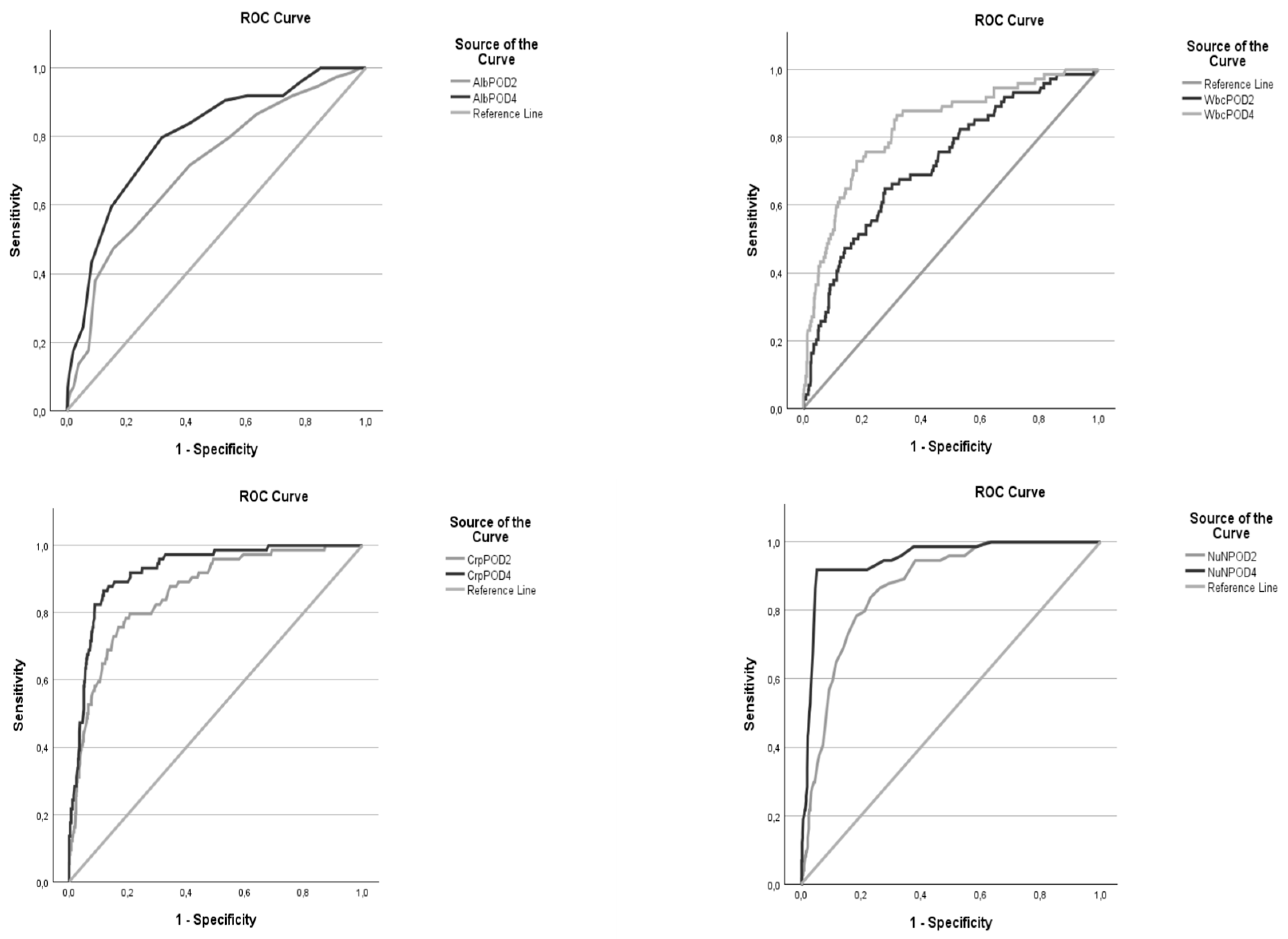
3.3. Optimal cut-off and predictive accuracy of Albumin:
3.4. Optimal cut-off and predictive accuracy of CRP:
3.5. Optimal cut-off and predictive accuracy of WCC:
3.6. Optimal cut-off and predictive accuracy of the NUn score:
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, et al CROSS Group. Preoperative chemo radiotherapy for esophageal or junctional cancer. N Engl J Med. 2012 May 31;366(22):2074-84. [CrossRef] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, et al. CROSS study group. Neoadjuvante chemoradiotherapy plus surgery versus surgery alone for esophageal or junctional cancer (CROSS): long term results of a randomized controlled trial. Lancet Oncol. 2015 Sep;16(9):1090-1098. doi: 10.1016/S1470-2045(15)00040-6. Epub 2015 Aug 5. [CrossRef] [PubMed]
- Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A, Smyth EC and clinicalguidelines@esmo.org., ESMO Guidelines Committee. Electronic address:. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Oct;33(10):992-1004. [CrossRef] [PubMed]
- Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D and Committee., ESMO Guidelines. Oesophageal cancer: ESMO CLinical proactice guidelines for diagnosis, treatment and follow up. Ann Oncol. 2016 Sep;27(suppl 5):v50-v57. [CrossRef] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G, Davies A, D'Journo XB, Gisbertz SS, Griffin SM, Hardwick R, Hoelscher A, Hofstetter W, Jobe B, Kitagawa Y, Law S, Mariette C, Maynard N, Morse CR, Nafteux P, Pera M, Pramesh CS, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg. 2019 Feb;269(2):291-298. [CrossRef] [PubMed]
- Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Schmidt HM, Gisbertz SS, Moons J, et al. Ann Surg. 2017;266:814–821.
- Collaborative., Oesophago-Gastric Anastomosis Study Group on behalf of the West Midlands Research. Comparison of short-term outcomes from the International Oesophago-Gastric Anastomosis Audit (OGAA), the Esophagectomy Complications Consensus Group (ECCG), and the Dutch Upper Gastrointestinal Cancer Audit (DUCA). BJS Open. 2021 May 7;5(3):zrab010. Erratum in: BJS Open. 2022 Jan 6;6(1):. [CrossRef] [PubMed]
- Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A, Friel CM, LaPar DJ, Stukenborg GJ, Jones RS. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg. 2015 Feb;220(2):195-206. Epub 2014 Nov 8. [CrossRef] [PubMed]
- Kassis E, Kosinski A, Ross P Jr, Koppens K, Donahue J, Daniel V. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013 Dec;96(6):1919-26. Epub 2013 Sep 24. [CrossRef] [PubMed]
- Ubels S, Verstegen M, Klarenbeek B, Bouwense S, van Berge Henegouwen M, Daams F, van Det MJ, Griffiths EA, Haveman JW, Heisterkamp J, Koshy R, Nieuwenhuijzen G, Polat F, Siersema PD, Singh P, Wijnhoven B, Hannink G, et al. TENTACLE—Esophagus Collaborative. Severity of oEsophageal Anastomotic Leak in patients after oesophagectomy: the SEAL score. Br J Surg. 2022 Aug 16;109(9):864-871. [CrossRef] [PubMed]
- Aiolfi A, Asti E, Rausa E, Bonavina G, Bonitta G, Bonavina L. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: Systematic review and Bayesian meta-analysis. PLoS One. 2018 Dec 17;13(12):e0209272. [CrossRef] [PubMed]
- Noble F, Curtis N, Harris S, Kelly JJ, Bailey IS, Byrne JP, Underwood TJ and (SC-OG)., South Coast Cancer Collaboration–Oesophago-Gastric. Risk assessment using a novel score to predict anastomotic leak and major complications after oesophageal resection. J Gastrointest Surg. 2012 Jun;16(6):1083-95. [CrossRef] [PubMed]
- Findlay JM, Tilson RC, Harikrishnan A, Sgromo B, Marshall RE, Maynard ND, Gillies RS, Middleton MR. Attempted validation of the NUn score and inflammatory markers as predictors of esophageal anastomotic leak and major complications. Dis Esophagus. 2015 Oct;28(7):626-33. [CrossRef] [PubMed]
- Bundred J, Hollis AC, Hodson J, Hallissey MT, Whiting JL, Griffiths EA. Validation of the NUn score as a predictor of anastomotic leak and major complications after Esophagectomy. Dis Esophagus. 2020 Jan 16;33(1):doz041. [CrossRef] [PubMed]
- Paireder M, Jomrich G, Asari R, Kristo I, Gleiss A, Preusser M, Schoppmann SF. External validation of the NUn score for predicting anastomotic leakage after oesophageal resection. Sci Rep. 2017 Aug 29;7(1):9725. [CrossRef] [PubMed]
- Liesenfeld LF, Sauer P, Diener MK, Hinz U, Schmidt T, Müller-Stich BP, Hackert T, Büchler MW, Schaible A. Prognostic value of inflammatory markers for detecting anastomotic leakage after esophageal resection. BMC Surg. 2020 Dec 9;20(1):324. [CrossRef] [PubMed]
- Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, DʼJourno XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SY, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJ. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015 Aug;262(2):286-94. [CrossRef] [PubMed]
- Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Annals of Surgery 240(2):p 205-213, August 2004. [CrossRef]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP and Initiative., STROBE. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014 Dec;12(12):1495-9. [CrossRef] [PubMed]
- Collaborative., Oesophago-Gastric Anastomosis Study Group on behalf of the West Midlands Research. Rates of Anastomotic Complications and Their Management Following Esophagectomy: Results of the Oesophago-Gastric Anastomosis Audit (OGAA). Ann Surg. 2022 Feb 1;275(2):e382-e39. [CrossRef] [PubMed]
- Aiolfi A, Griffiths EA, Sozzi A, Manara M, Bonitta G, Bonavina L, Bona D. Effect of Anastomotic Leak on Long-Term Survival After Esophagectomy: Multivariate Meta-analysis and Restricted Mean Survival Times Examination. . Ann Surg Oncol. 2023 Sep;30(9):5564-5572. [CrossRef] [PubMed]
- Markar S, Gronnier C, Duhamel A, Mabrut JY, Bail JP, Carrere N, Lefevre JH, Brigand C, Vaillant JC, Adham M, Msika S, Demartines N, Nakadi IE, Meunier B, Collet D, Mariette C, FREGAT Working Group, FRENCH, and AFC. The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg. 2015;262(6):972–80.
- Fransen LFC, Berkelmans GHK, Asti E, van Berge Henegouwen MI, Berlth F, Bonavina L, Brown A, Bruns C, van Daele E, Gisbertz SS, Grimminger PP, Gutschow CA, Hannink G, Hölscher AH, Kauppi J, Lagarde SM, Mercer S, Moons J, et al. EsoBenchmark Collaborative. The effect of postoperative complications after minimally invasive esophagectomy on long-term survival: an international multicenter cohort study. Ann Surg. 2021;274(6):e1129-37.
- Kamarajah SK, Navidi M, Wahed S, Immanuel A, Hayes N, Griffin SM, Phillips AW. Anastomotic Leak Does Not Impact on Long-Term Outcomes in Esophageal Cancer Patients. Ann Surg Oncol. 2020 Jul;27(7):2414-2424. [CrossRef] [PubMed]
- Gao C, Xu G, Wang C, Wang D. Evaluation of preoperative risk factors and postoperative indicators for anastomotic leak of minimally invasive McKeown esophagectomy: a single-center retrospective analysis. J Cardiothorac Surg. 2019 Feb 28;14(1):46. [CrossRef] [PubMed]
- Shao CY, Liu KC, Li CL, Cong ZZ, Hu LW, Luo J, Diao YF, Xu Y, Ji SG, Qiang Y, Shen Y. C-reactive protein to albumin ratio is a key indicator in a predictive model for anastomosis leakage after esophagectomy: Application of classification and regression tree analysis. Thorac Cancer. 2019 Apr;10(4):728-737. [CrossRef] [PubMed]
- Lindenmann J, Fink-Neuboeck N, Porubsky C, Fediuk M, Anegg U, Kornprat P, Smolle M, Maier A, Smolle J, Smolle-Juettner FM. A nomogram illustrating the probability of anastomotic leakage following cervical esophagogastrostomy. Surg Endosc. 2021 Nov;35(11):6123-6131. [CrossRef] [PubMed]
- Zhang C, Li XK, Hu LW, Zheng C, Cong ZZ, Xu Y, Luo J, Wang GM, Gu WF, Xie K, Luo C, Shen Y. Predictive value of postoperative C-reactive protein-to-albumin ratio in anastomotic leakage after esophagectomy. J Cardiothorac Surg. 2021 May 17;16(1):133. [CrossRef] [PubMed]
- Labgaa I, Mantziari S, Genety M, Elliott JA, Kamiya S, Kalff MC, Winiker M, Pasquier J, Allemann P, Messier M, van Berge Henegouwen MI, Nilsson M, Reynolds JV, Piessen G, Hübner M, Demartines N, Schäfer M. Early postoperative decrease of albumin is an independent predictor of major complications after oncological esophagectomy: A multicenter study. J Surg Oncol. 2021 Feb;123(2):462-469. [CrossRef] [PubMed]
- Asti E, Bonitta G, Melloni M, Tornese S, Milito P, Sironi A, Costa E, Bonavina L. Utility of C-reactive protein as predictive biomarker of anastomotic leak after minimally invasive esophagectomy. Langenbecks Arch Surg. 2018 Mar;403(2):235-244 . [CrossRef] [PubMed]
- Tsujimoto H, Ono S, Takahata R, Hiraki S, Yaguchi Y, Kumano I, Matsumoto Y, Yoshida K, Aiko S, Ichikura T, Yamamoto J, Hase K. Systemic inflammatory response syndrome as a predictor of anastomotic leakage after esophagectomy. Surg Today. 2012 Jan;42(2):141-6. [CrossRef] [PubMed]
- Hoeboer SH, Groeneveld AB, Engels N, van Genderen M, Wijnhoven BP, van Bommel J. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg. 2015 Apr;19(4):613-24. [CrossRef] [PubMed]
- Gordon AC, Cross AJ, Foo EW, Roberts RH. C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg. 2018 Mar;88(3):223-227. [CrossRef] [PubMed]
- Park JK, Kim JJ, Moon SW. C-reactive protein for the early prediction of anastomotic leak after esophagectomy in both neoadjuvant and non-neoadjuvant therapy case: a propensity score matching analysis. J Thorac Dis. 2017 Oct;9(10):3693-3702. [CrossRef] [PubMed]
- Giulini L, Dubecz A, Solymosi N, Tank J, Renz M, Thumfart L, Stein HJ. Prognostic Value of Chest-Tube Amylase Versus C-Reactive Protein as Screening Tool for Detection of Early Anastomotic Leaks After Ivor Lewis Esophagectomy. J Laparoendosc Adv Surg Tech A. 2019 Feb;29(2):192-197. [CrossRef] [PubMed]
- McAnena P, Neary C, Doyle C, Kerin MJ, McAnena OJ, Collins C. Serial CRP levels following oesophagectomy: a marker for anastomotic dehiscence. Ir J Med Sci. 2020 Feb;189(1):277-282. [CrossRef] [PubMed]
- Prochazka V, Marek F, Kunovsky L, Svaton R, Farkasova M, Potrusil M, Moravcik P, Kala Z. C-reactive protein as predictor of anastomotic complications after minimally invasive oesophagectomy. J Minim Access Surg. 2019 Jan-Mar;15(1):46-50. [CrossRef] [PubMed]
- Dutta S, Fullarton GM, Forshaw MJ, Horgan PG, McMillan DC. Persistent elevation of C-reactive protein following esophagogastric cancer resection as a predictor of postoperative surgical site infectious complications. World J Surg. 2011 May;35(5):1017-25. [CrossRef] [PubMed]
- Ji L, Wang T, Tian L, Gao M. The early diagnostic value of C-reactive protein for anastomotic leakage post radical gastrectomy for esophagogastric junction carcinoma: A retrospective study of 97 patients. Int J Surg. 2016 Mar;27:182-186. [CrossRef] [PubMed]
- Babic B, Tagkalos E, Gockel I, Corvinus F, Hadzijusufovic E, Hoppe-Lotichius M, Lang H, van der Sluis PC, Grimminger PP. C-reactive Protein Levels After Esophagectomy Are Associated With Increased Surgical Trauma and Complications. Ann Thorac Surg. 2020 May;109(5):1574-1583. [CrossRef] [PubMed]
- Neary C, McAnena P, McAnena O, Kerin M, Collins C. C-Reactive Protein-Lymphocyte Ratio Identifies Patients at Low Risk for Major Morbidity after Oesophagogastric Resection for Cancer. Dig Surg. 2020;37(6):515-523. [CrossRef] [PubMed]
- Miki Y, Toyokawa T, Kubo N, Tamura T, Sakurai K, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M. C-Reactive Protein Indicates Early Stage of Postoperative Infectious Complications in Patients Following Minimally Invasive Esophagectomy. World J Surg. 2017 Mar;41(3):796-803. [CrossRef] [PubMed]
- Stuart SK, Kuypers TJL, Martijnse IS, Heisterkamp J, Matthijsen RA. C-reactive protein and drain amylase: their utility in ruling out anastomotic leakage after minimally invasive Ivor-Lewis esophagectomy. . Scand J Gastroenterol. 2023 May;58(5):448-452. [CrossRef] [PubMed]
- Rat P, Piessen G, Vanderbeken M, Chebaro A, Facy O, Rat P, Boisson C, Ortega-Deballon P. C-reactive protein identifies patients at low risk of anastomotic leak after esophagectomy. Langenbecks Arch Surg. 2022 Dec;407(8):3377-3386. [CrossRef] [PubMed]
- Hagens ERC, Feenstra ML, Lam WC, Eshuis WJ, Lameris W, van Berge Henegouwen MI, Gisbertz SS. C-Reactive Protein as a Negative Predictive Marker for Anastomotic Leakage After Minimally Invasive Esophageal Surgery. World J Surg. 2023 Aug;47(8):1995-2002. [CrossRef] [PubMed]
- Baker EH, Hill JS, Reames MK, Symanowski J, Hurley SC, Salo JC. Drain amylase aids detection of anastomotic leak after esophagectomy. J Gastrointest Oncol. 2016 Apr;7(2):181-8. [CrossRef] [PubMed]
|
All patients (n= 668) |
no AL (n= 594) |
AL (n= 74) |
P value | ||
|---|---|---|---|---|---|
| Age, (y) | Mean ± SD | 64,0 ± 12,2 | 64,8 ± 10,2 | 65,6 ± 8,9 | 0,508 |
| BMI (kg/m2) | Mean ± SD | 25,3 ± 4,6 | 25,2 ± 4,5 | 25,9 ± 4,9 | 0,252 |
| ASA score, n (%) | 1 | 27 (4,0%) | 24 (4,1%) | 3 (4,1%) | 0,036 |
| 2 | 286 (42,8%) | 261 (43,9%) | 25 (33,7%) | ||
| 3 | 335 (50,1%) | 292 (49,2%) | 43 (58,1%) | ||
| 4 | 4 (0,6%) | 2 (0,3%) | 2 (2,7%) | ||
| Gender, n (%) | Male | 527 (78,9%) | 468 (78,8%) | 59 (79,7%) | 0,851 |
| Female | 141 (21,1%) | 126 (21,2%) | 15 (20,3%) | ||
| Comorbidities, n (%) | Kidney disease | 21 (3,1%) | 19 (3,2%) | 2 (2,7%) | 0,818 |
| Cardiovascular disease | 257 (38,5%) | 226 (38,0%) | 31 (41,9%) | 0,522 | |
| Pulmonary disease | 161 (24,1%) | 140 (23,6%) | 21 (28,4%) | 0,362 | |
| Diabetes | 88 (13,2%) | 75 (12,6%) | 13 (17,6%) | 0,236 | |
| Smoking | 230 (34,4%) | 200 (33,7%) | 30 (40,5%) | 0,241 | |
| Corticosteroids | 20 (3,0%) | 16 (2,7%) | 4 (5,4%) | 0,197 | |
| Tumor Location, n (%) | proximal | 17 (2,5%) | 12 (2,0%) | 5 (6,8%) | 0,094 |
| mid | 121 (18,1%) | 110 (18,5%) | 11 (14,9%) | ||
| distal | 402 (60,2%) | 357 (60,1%) | 45 (60,8%) | ||
| GEJ | 128 (19,2%) | 115 (19,4%) | 13 (17,6%) | ||
| Neoadjuvant therapy, n (%) | none | 179 (26,8%) | 158 (26,6%) | 21 (28,4%) | 0,932 |
| Chemotherapy | 97 (14,5%) | 87 (14,6%) | 10 (13,5%) | ||
| Radiochemotherapy | 392 (58,7%) | 349 (58,8%) | 43 (58,1%) | ||
| Histology, n (%) | Adeno Ca | 451 (67,5%) | 402 (67,7%) | 49 (66,2%) | 0,719 |
| Squamous cell Ca | 200 (29,9%) | 176 (29,6%) | 24 (32,4%) | ||
| Other | 17 (2,5%) | 16 (2,7%) | 1 (1,4%) | ||
| cT-stage, n (%)* | Tx | 8 (1,2%) | 7 (1,2%) | 1 (1,4%) | 0,641 |
| T1 | 56 (8,4%) | 49 (8,2%) | 7 (9,5%) | ||
| T2 | 136 (20,4%) | 118 (19,9%) | 18 (24,3%) | ||
| T3 | 455 (68,1%) | 407 (68,5%) | 48 (64,9%) | ||
| T4 | 13 (1,9%) | 13 (2,2%) | 0 (0,0%) | ||
| cN-stage, n (%)* | N0 | 227 (34,0%) | 203 (34,2%) | 24 (32,4%) | 0,898 |
| N1 | 308 (46,1%) | 276 (46,5%) | 32 (43,2%) | ||
| N2 | 112 (16,8%) | 97 (16,3%) | 15 (20,3%) | ||
| N3 | 13 (1,9%) | 11 (1,9%) | 2 (2,7%) | ||
| cM-stage, n (%)* | M0 | 625 (93,6%) | 556 (93,6%) | 69 (93,2%) | 0,989 |
| M1 | 35 (5,2%) | 31 (5,2%) | 4 (5,4%) | ||
| Procedure, n (%) | IL | 586 (87,7%) | 522 (87,9%) | 64 (86,5%) | 0,010 |
| McK | 25 (3,7%) | 18 (3,0%) | 7 (9,5%) | ||
| THE | 57 (8,5%) | 54 (9,1%) | 3 (4,1%) | ||
| Approach, n (%) | Open | 327 (49,0%) | 299 (50,3%) | 28 (37,8%) | 0,008 |
| Hybride | 139 (20,8%) | 127 (21,4%) | 12 (16,2%) | ||
| MIE | 202 (30,2%) | 168 (28,3%) | 34 (45,9%) | ||
| type of surgery, n (%) | Elective | 608 (91,0%) | 545 (91,8%) | 63 (85,1%) | <0,001 |
| Emergency | 5 (0,7%) | 1 (0,2%) | 4 (5,4%) | ||
| Salvage | 55 (8,2%) | 48 (8,1%) | 7 (9,5%) |
| No AL (=594) | AL type 1 (n=14) | AL type 2 (n=9) | AL type 3 (n=51) | p value | |
|---|---|---|---|---|---|
| Alb POD2 (mean ± SD) | 28,0 (± 3,7) | 25,8 (± 2,6) | 26,2 (± 4,4) | 24,9 (± 4,1) | <,001 |
| Alb POD4 (mean ± SD) | 28,5 (± 3,9) | 25,1 (± 3,4) | 24,6 (± 4,0) | 24,1 (± 3,2) | <,001 |
| CRP POD2 (mean ± SD) | 125,9 (± 55,4) | 197,0 (± 89,8) | 222,3 (± 73,2) | 224,4 (± 67,9) | <,001 |
| CRP POD4 (mean ± SD) | 111,1 (± 62,8) | 196,8 (± 84,9) | 275,4 (± 86,4) | 267,6 (± 74,0) | <,001 |
| WCC POD2 (mean ± SD) | 10,9 (± 5,2) | 11,4 (± 3,6) | 16,3 (± 6,5) | 14,3 (± 4,2) | <,001 |
| WCC POD4 (mean ± SD) | 8,7 (± 2,9) | 11,46 (± 3,7) | 16,1 (± 5,7) | 13,5 (± 4,5) | <,001 |
| Nun POD2 (mean ± SD) | 9,2 (± 1,2) | 10,0 (± 0,8) | 10,9 (± 1,4) | 10,8 (± 1,0) | <,001 |
| Nun POD4 (mean ± SD) | 8,6 (± 1,0) | 10,1 (± 1,2) | 11,5 (± 0,8) | 11,1 (± 0,9) | <,001 |
| variable | AUC | 95% CI | p value | Cut-off | sens | spec | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|---|---|
| Alb POD2 | 0,710 | 0,646 - 0,774 | < 0,001 | 24,5 | 47,30% | 84,40% | 27,42% | 92,78% | 3,032 | 0,624 |
| POD4 | 0,799 | 0,746 - 0,853 | < 0,001 | 26,5 | 79,70% | 68,20% | 23,79% | 96,42% | 2,506 | 0,298 |
| CRP POD2 | 0,859 | 0,816 - 0,903 | < 0,001 | 165,5 | 79,70% | 79,30% | 32,42% | 96,90% | 3,850 | 0,256 |
| POD4 | 0,924 | 0,896 - 0,953 | < 0,001 | 181,5 | 86,50% | 88,20% | 47,73% | 98,13% | 7,330 | 0,153 |
| WCC POD2 | 0,724 | 0,662 - 0,786 | < 0,001 | 12,255 | 64,90% | 72,30% | 22,59% | 94,30% | 2,343 | 0,486 |
| POD4 | 0,829 | 0,777 - 0,880 | < 0,001 | 10,885 | 73,00% | 82,00% | 33,57% | 96,06% | 4,056 | 0,329 |
| Nun POD2 | 0,869 | 0,833 - 0,905 | < 0,001 | 9,75 | 83,80% | 76,80% | 31,03% | 97,44% | 3,612 | 0,211 |
| POD4 | 0,948 | 0,923 - 0,972 | < 0,001 | 10,05 | 91,90% | 94,70% | 68,36% | 98,95% | 17,340 | 0,086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





