1. Introduction
Neoplastic proliferation of plasmacytoid dendritic cells (pDCs) is known to give rise to BPDCN, as wells as to the clonal expansion of mature pDCs [
1]. First identified as a unique category in 1994, BPDCN is a rare and aggressive hematologic cancer originating from the malignant transformation of the non-activated precursors of pDC. This malignancy represents less than 1% of all hematologic tumor cases [
2,
3]. Its primary impact is observed in older adults, commonly surfacing in the sixth decade of life. Significantly, there is a higher prevalence among males, and individuals of Caucasian descent are slightly more affected than those of other demographics. Beyond its epidemiological profile, BPDCN exhibits a set of unique clinical, morphological, and immunophenotypic features, setting it apart from other hematopoietic neoplasms [
4]. The nomenclature evolved in 2016 when the World Health Organization (WHO) classified BPDCN as a distinct malignancy within myeloid neoplasms and acute leukemia [
5,
6]. While the specific developmental pathway of pDC malignancies remains a subject of debate, prevailing evidence supports that malignant proliferation of pDC precursors is specifically involved in the pathogenesis of BPDCN [
3,
7,
8]. To comprehend this, it is essential to delve into the classification of DCs and their functional subtypes.
2. Dendritc Cells Classification
Dendritic cells (DCs), originating from the bone marrow, are a diverse group found in the circulation, lymphoid organs, and various tissues. Modern classification recognizes two principal functional subtypes: conventional DCs (cDCs) and pDCs [
9,
10]. In the bloodstream, there are cDCs that express CD11c, including cDC2 CD1c
+ and cDC1 CD141
+ cells. Plasmacytoid dendritic cells, characterized by CD123
+ and CD303
+ markers, are also present but do not express CD11c. Although pDCs make up a minor fraction of the dendritic cell population, they predominantly reside in blood and lymphoid tissues Top of Form[
9,
10,
11]. These cells are renowned for their robust antiviral defense capabilities, primarily through the secretion of type I interferon (IFN). Despite their antiviral function, pDCs also play a role in immune tolerance, as evidenced by their ability to stimulate regulatory T cells (Tregs) and T cells producing interleukin (IL)-10, indicating a potentially dualistic role in immune response regulation [
12,
13,
14].
3. Clinical Manifestations
The importance of accurately identifying and understanding BPDCN is emphasized to enhance clinical management and treatment strategies for this specific subset of hematologic malignancies. BPDCN patients typically initially show skin involvement, which can extend to affect the bone marrow (BM), peripheral blood (PB), and lymph nodes (LN). Extra-cutaneous manifestations often appear at diagnosis, particularly in regional LNs, progressing to involve PB and BM as the disease advances [
2,
15]. This malignancy is characterized by rapid progression, frequent relapses, and poor overall survival (OS), highlighting the need for effective treatment strategies [
16].
Historically, diagnosing and treating BPDCN has been challenging due to its rarity and the lack of standardized approaches. It predominantly affects the elderly, with a noticeable male prevalence, and most patients experience cytopenias, lymphadenopathy, and/or splenomegaly [
2]. Although a leukemic presentation is infrequent, occurring in less than 1% of acute leukemia cases, only 10% of those initially presenting with isolated skin lesions progress rapidly to leukemia [
2,
17,
18]. Interestingly, a minority of cases present with leukemia but without skin involvement [
15]. Advances in immunophenotyping and molecular profiling have uncovered potential therapeutic targets.
4. Diagnosis of BPDCN
The accurate identification and classification of BPDCN require a combination of clinical expertise and precise diagnostic laboratory tests due to its clinical and biological heterogeneity. Immunophenotypic criteria, using either flow cytometry (FC) or immunohistochemistry (IHC), are the primary methods for identifying this neoplasm [
19].
Flow cytometry is considered a crucial diagnostic tool for BPDCN due to its objectivity and quantitative precision. It has the ability to detect small populations of cells with abnormal phenotypes among normal leukocytes, which is particularly important for accurately diagnosing BPDCN. FC outperforms IHC in diagnosing BPDCN because it can simultaneously detect multiple antigens, including those that are not routinely examined by IHC. This heightened sensitivity is essential in overcoming the challenges associated with accurately diagnosing BPDCN. However, diagnosing BPDCN is challenging due to its immunophenotypic heterogeneity, which often leads to overlapping features with other hematologic neoplasms such as NK/T cell leukemia/lymphoma, cutaneous T cell lymphoma, and acute myelomonocytic leukemia expressing CD4 or CD56 [
20]. Moreover, the initial assumption that BPDCN derived from NK cells based on the absence of common lineage markers and the expression of CD4 and CD56 has been reconsidered. Further studies have revealed the intricate nature of BPDCN and its shared characteristics with pDCs. These include the absence of T cell receptor and immunoglobulin heavy chain gene rearrangements, as well as the production of IFN-alfa (IFN-α) and Th2 polarization following Interleukin-3 (IL-3) induction [
21,
22,
23,
24].
In conclusion, successfully addressing the diagnostic challenges posed by BPDCN requires a deep understanding of its immunophenotypic complexities, the strategic use of advanced diagnostic methods like flow cytometry, and staying informed about evolving insights into its cellular origins and molecular characteristics
5. Immunophenotype of BPDCN
The diagnosis of BPDCN hinges on discerning specific immunophenotypic features within the neoplastic cells [
25]. Typically, BPDCN cells coexpress CD4 and CD56, in addition to CD34, CD36, and pDC-associated antigens like CD123 and CD303. Rare instances may involve aberrant expression of CD5, CD7, CD33, TdT, and CD79a. The presence of detrimental lineage-specific antigens (Lin-) has also been observed [
20,
24,
26]
.
A recent study has categorized BPDCN into three maturation-associated subgroups based on the expression of CD34 and CD117 in pDCs. In stage 1, CD34 is expressed in some immature blasts; in stage 2, blast cells partially express CD117, while CD34 is negative; in stage 3, both CD34 and CD117 are negative in blast cells. These distinctive maturation profiles contribute to the diverse clinical presentations and laboratory characteristics of BPDCN.
While the origin and development of pDC malignancies remain debated, contemporary evidence leans towards considering BPDCN cells as the malignant counterparts of precursor pDCs [
3,
7].
6. Molecular and Genetic Mutations
Molecular and genetic analyses play a crucial role in the detailed characterization of BPDCN by offering valuable diagnostic and prognostic insights. Cytogenetic techniques, such as karyotyping and fluorescence in situ hybridization (FISH), have been instrumental in identifying frequent chromosomal anomalies associated with BPDCN. Notable abnormalities include deletions or rearrangements of chromosomes 5q, 12p, and 13q, and mutations in the TP53 gene. Further refinement in detection methods comes from next-generation sequencing (NGS), which sheds light on specific genetic mutations. Alterations in genes like Tet methyl cytosine dioxygenase (TET2), ASXL transcriptional regulator 1 (ASXL1), and DNA methyltransferase 3 alpha (DNMT3A) are commonly reported in BPDCN [24-28]. Moreover, chromosomal changes commonly linked to BPDCN include anomalies in regions 5q, 6q, monosomy 9, 12p, 13q, and 15q [
18,
26,
27,
28].
7. Treatment of BPDCN
7.1. Treatment Strategies for BPDCN
The recognition of BPDCN as a distinct disease entity is a recent development, and due to its rarity, the establishment of standardized treatment protocols is yet to be achieved. Traditionally, conventional chemotherapy has been the primary method of treating BPDCN.
7.2. Conventional Chemotherapy
Chemotherapy regimens originally designed for treating acute leukemia have shown varying degrees of success in inducing sustained clinical responses in BPDCN patients. Regimens that combine anthracycline-based induction therapies with high-dose cytarabine have produced encouraging remission rates. In clinical settings, programs such as HyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) have been employed [
2,
29]. The determination of the most effective treatment plan, including the regimen and length of therapy, remains contentious, largely due to the scarcity of comprehensive clinical trials and variability in patient profiles.
7.3. Stem Cell Transplantation
In selected BPDCN patients, allogeneic stem cell transplantation (allo-SCT) offers a potentially curative approach and is considered the gold-standard consolidation therapy in patients who attain remission and are fit for the procedure. This, however, often excludes the majority of patients over the age of 65 or those with compromised clinical conditions and comorbidities [
5,
30,
31]. Evidence indicates that conducting allo-SCT during the first complete remission (CR1) or after securing a durable remission through induction chemotherapy can enhance OS rates and decrease relapse risks [
4,
29]. Nevertheless, refining patient selection criteria, deciding on the optimal timing for transplantation, and determining effective conditioning regimens are all active fields of investigation.
8. CD123 Molecule
CD123 has emerged as a promising therapeutic target for patients with BPDCN [
32]. This cell surface molecule is expressed on pDCs, basophils, monocytes, and eosinophils, with a noted overexpression in a variety of hematological malignancies [
33]. A pivotal discovery in the understanding of BPDCN was the identification of CD123 overexpression in nearly all cases of the disease [
34,
35,
36]. High levels of CD123 expression were detected in up to 100% of the samples analyzed through flow cytometry, not just in BPDCN but also in Acute Myeloid Leukemia (AML), B-cell Acute Lymphoblastic Leukemia (B-ALL), Hairy Cell Leukemia (HCL), Myelodysplastic Syndromes (MDS), and chronic eosinophilic leukemia [
37,
38,
39,
40]. As the alpha subunit of the interleukin-3 receptor (IL-3Ra), CD123 plays a fundamental role in the maturation of hematopoietic cells [
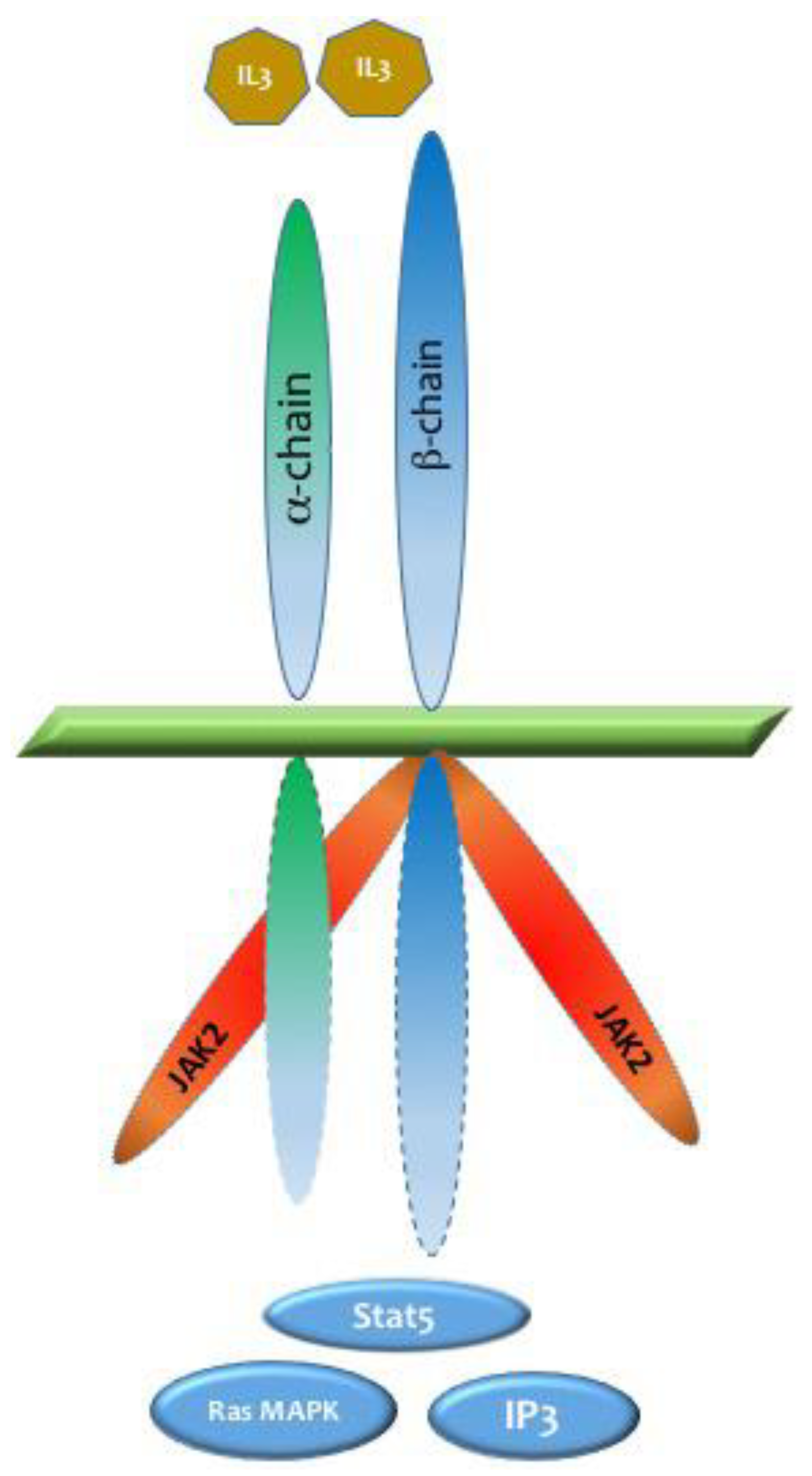
41]. The binding of IL-3 to IL-3Ra activates the beta chain, forming a heterodimeric IL-3 receptor that transmits vital signals leading to the activation of multiple pathways, including Janus kinase/signal transducers and activators of transcription (JAK/STAT), Ras-mitogen-activated protein kinase (Ras/MAPK), and phosphoinositide 3-kinase (PI3K) (
Figure 1). These pathways are particularly important for anti-apoptotic and cell-proliferative signaling [
41].
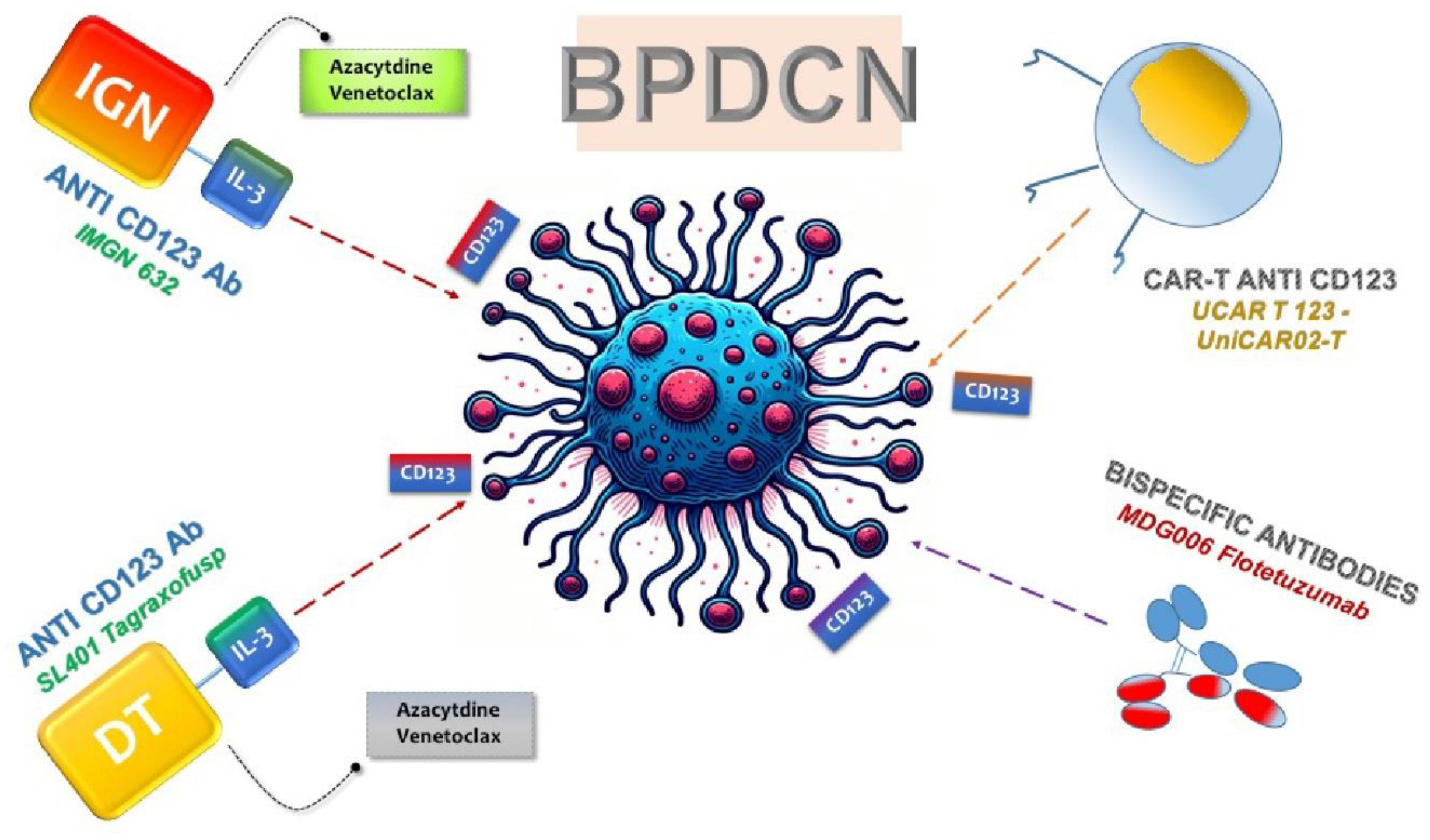
The pronounced overexpression of CD123 on neoplastic cells has paved the way for the design of targeted therapeutic agents aimed at this molecule, such as monoclonal antibodies, bispecific T cell antibodies (BsAbs), and cellular therapies like chimeric antigen receptor (CAR) T cells (
Figure 2). These therapies have progressed to early phase clinical trials for BPDCN, utilizing the specificity for CD123 to selectively target tumor cells [
42,
43,
44].
8.1. ANTI-CD123 Tag
The preliminary pilot studies on a CD123-targeted agent have unveiled a pioneering recombinant protein medication. This cutting-edge therapeutic, known as Diphtheria toxin (DT)-IL3, incorporates a modified DT combined with recombinant human IL-3 [
45] (
Figure 2). Initially employed in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), including a subset with BPDCN [
46], DT-IL3 underwent further examination in a pilot study by Frankel et al. Specifically focusing on BPDCN patients, the targeted therapy was subsequently renamed SL-401 (Tagraxofusp, Stemline Therapeutics, New York, NY, USA) [
43].
In a phase I–II clinical trial involving 11 patients with relapsed/refractory BPDCN, Tagraxofusp demonstrated significant efficacy. Treatment-naïve patients exhibited major responses in 90% of cases, with 72% of these responses culminating in CR. Commonly reported adverse events included thrombocytopenia, hypoalbuminemia, and elevated liver function tests [
47].
This promising outcome led to a prospective multi-institutional phase II clinical study, encompassing 47 subjects with untreated or relapsed BPDCN. Among the 29 previously untreated patients, a remarkable 90% overall response rate was observed, with 45% of them qualifying for hematopoietic stem cell transplantation (HSCT). The 2-year OS rate reached 52%. Among the fifteen patients who had received prior treatment, the response rate reached 67%, with a median OS time of 8.5 months [
48].
Tagraxofusp significant advancements in BPDCN treatment were evident in both in vitro and in vivo models, displaying potent antitumor activity at minimal concentrations [
48,
49]. Consequently, it received approval from the United States Federal Drug Administration for patients aged 2 and older with previously untreated or relapsed/refractory BPDCN, followed by approval in the European Union for adults as a first-line treatment in January 2021 [
48,
49].
While Tagraxofusp has proven efficacious in reducing disease burden, it does come with a notable side effect profile, most frequently encountered in the first cycle of therapy. Notable side effects include hepatotoxicity (88%), thrombocytopenia (49%), and capillary leak syndrome (CLS) (19%) [
48,
50]. Despite facing numerous obstacles, the effectiveness of CD123-targeted therapy in eliciting a clinical response establishes its role as a crucial element in the management of BPDCN for patients who are undergoing initial treatment as well as those with refractory disease
8.2. Resistance to Tagraxofusp - Combination therapy
Resistance in cancer cells doesn't stem from CD123 loss but rather from their ability to resist diphtheria toxin, facilitated by DNA methylation-induced silencing of genes in the diphthamide synthesis pathway [
51,
52,
53,
54]. Azacytidine has proven effective in reversing diphthamide silencing, thus addressing Tag resistance [
55] (
Figure 2). Additionally, post-Tag treatment, surviving cancer cells exhibit heightened expression of the anti-apoptotic protein BCL2. Combating these adaptations and boosting sensitivity involves the strategic use of the BCL2 inhibitor Venetoclax alongside conventional chemotherapy [
56] (
Figure 2).
To address these complexities, ongoing clinical trials (NCT03113643) are exploring Tag in combination with Azacitidine and Venetoclax for patients with BPDCN, acute myeloid leukemia (AML), and myelodysplastic syndrome (MDS). Emerging data suggest a synergistic effect, leading to enhanced cell death. Azacytidine impact on DNA methylation is crucial, reactivating Tag sensitivity in cells that manage to escape, often by down regulating the diphthamide pathway [
56].
Ongoing and completed clinical trials are illustrated in
Table 1.
Table 1.
|
Study ID
|
Therapeutic Strategy
|
Condition/Disease
|
Phase
|
Status
|
| NCT03113643 |
Tagraxofusp
plus azacitidine ± venetoclax |
AML, MDS, BPDCN |
I |
Recruiting |
| NCT04216524 |
Tagraxofusp
plus azacitidine and chemotherapy |
BPDCN |
II |
Recruiting |
| NCT03386513 |
IMGN632 |
AML, ALL, BPDCN, MPN |
I/II |
Active, not recruiting |
| NCT04086264 |
IMGN632 alone
or plus azacitidine ± venetoclax |
CD123-Positive AML |
I/II |
Recruiting |
| NCT03203369 |
Chimeric Antigen Receptor T cells UCART 123
|
BPDCN |
I |
Terminated |
| NCT04109482 |
Chimeric Antigen Receptor T cells MB-102
|
BPDCN |
I/II |
Terminated |
| NCT02159495 |
Chimeric Antigen Receptor T cells CD123+ CAR T cells
|
AML, BPDCN |
I |
Active, not recruiting |
| NCT04230265 |
Chimeric Antigen Receptor T cells UniCAR02-T + TM123
|
AML, BPDCN |
I |
Recruiting |
| NCT04681105 |
Bispecific antibodies Flotetuzumab
|
AML, BPDCN |
I |
Active, not recruiting |
8.3. IMGN632
IMGN632 stands as a noteworthy advancement in cancer therapeutics, being a humanized IgG1 monoclonal antibody meticulously engineered for CD123, coupled with a pioneering DNA-alkylating indolinobenzodiazepine pseudodimer (IGN) (
Figure 2). This antibody-drug conjugate selectively binds to CD123-expressing cells, undergoing internalization and releasing FGN849, a potent DNA alkylating agent capable of inducing cell lysis and apoptosis (
Figure 2). Notably, IMGN632 exhibits robust efficacy against the BPDCN cell line CAL1 and demonstrates significant activity in vivo within patient-derived xenograft (PDX) models of BPDCN [
57,
58]. Further supporting its targeted action, studies reveal that decreased CD123 expression in normal hematopoietic stem and progenitor cells correlates with reduced sensitivity to IMGN632 [
57,
58].
In a recent phase I/II clinical study (NCT03386513), IMGN632 was evaluated as a single agent in patients with relapsed/refractory AML, BPDCN, or other CD123 hematologic malignancies. Among the 23 patients with relapsed/refractory BPDCN, administered at a dose level of 0.045 mg/kg via intravenous infusion once every three weeks, 30% demonstrated an objective response with a composite CR rate of 22% (2 CR, 2 clinical CR [CRc], 1 CRi, and 2 Partial Response). Impressively, the duration of response for the CR/CRc patients ranged between 3 and 9 months, even without undergoing stem cell transplantation. IMGN632 showcased a favorable safety profile, with no grade 3 or higher adverse events reported in more than one patient. The most common adverse events included nausea, peripheral edema, and infusion-related reactions. Notably, in contrast to tagraxofusp, no instances of CLS were observed [
59].
In light of this promising preliminary evidence, IMGN632 received breakthrough therapy designation (BTD) from the US Food and Drug Administration in 2020, specifically for the treatment of BPDCN. This designation bestows priority review status upon the agent and its manufacturer for future evaluation by the regulatory agency, marking a significant step forward in the pursuit of effective therapies for BPDCN.
8.4. Combination therapy
IMGN632 is undergoing further investigation in a Phase 1b/2 study for patients with AML, where it is being assessed in combination with azacitidine, venetoclax, or a combination of both (NCT04086264). The outcomes of this study, encompassing both safety and efficacy data, may potentially pave the way for exploring IMGN632 combinations in the context of BPDCN in the future.
Ongoing and completed clinical trials are illustrated in
Table 1.
9. Anti-CD123 Chimeric Antigen Receptors (CAR)-T in BPDCN
Autologous T cells engineered to express chimeric antigen receptors (CAR) represent a promising avenue for the treatment of diverse tumors, eliciting a robust T cell immune response that specifically targets and eliminates cancerous cells [
60,
61,
62]. Notably, CAR T cell immunotherapy targeting the pan-B-cell antigen CD19 has demonstrated significant success, particularly in achieving high remission rates among patients with acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL), leading to accelerated FDA approvals in 2017 [
63,
64]. In the quest for effective T cell-based immunotherapy, CD123 emerges as an attractive target. Preclinical studies have shown that anti-CD123 CAR-T therapy holds substantial promise in the treatment of BPDCN [
65]. To mitigate potential adverse effects, such as Cytokine Release Syndrome (CRS) resulting from a robust immune response, these trials have incorporated safety switches in the form of various CAR designs [
66,
67].
Specifically, T cells from BPDCN patients transduced with CD28/4-1BB CD123 CAR have demonstrated efficacy in vitro, successfully eliminating autologous BPDCN blasts, and reducing BPDCN blast burden in vivo, without causing significant on-target/off-tumor toxicity effects [
68]. A recent development in the field is the emergence of TCRαβ-negative allogeneic CAR T cells, often referred to as "universal" CAR T cells (
Figure 2). Notably, UCAR T 123, composed of allogeneic T cells from healthy donors expressing anti-CD123 CAR and edited using Transcription Activator-Like Effector Nuclease (TALEN), presents a promising candidate for treating relapsed/refractory acute myeloid leukemia (AML) and BPDCN (
Figure 2) [
67].
UCART123 cell therapy, as a salvage method for BPDCN patients unable to harvest normal T cells for CAR T generation, has demonstrated success in xenograft mouse models with primary patient-derived BPDCN. However, challenges may arise due to the potential loss of CD123 antigen, detected in some BPDCN cases. In summary, these results provide a preclinical proof-of-principle that allogeneic UCART123 cells have potent anti-BPDCN activity [
67].
Clinical trials, such as NCT03203369 and NCT04109482, are actively assessing the efficacy of anti-CD123 CAR T cells for BPDCN treatment, although submission of results is still pending, according to
ClinicalTrials.gov.
In addition to UCAR T 123, several other CAR-T products are currently under investigation in clinical trials. Noteworthy among them are MB-102 (NCT04109482 and NCT02159495) and UniCAR02-T (NCT04230265), both targeted for relapsed or refractory CD123 hematologic neoplasms and BPDCN patients [
65,
69,
70] (
Figure 2). Results from two phase clinical studies (NCT04109482 and NCT02159495) using MB-102 have shown initial responses in four out of seven patients, including one with BPDCN. Meanwhile, a phase I study (NCT04230265) employing UniCAR02-T in patients with relapsed AML, ALL, and BPDCN is currently recruiting, and results are awaited. Notably, UniCAR02-T was built-in combining UniCAR T cells with a specific targeting of a CD123 recombinant antibody derivative molecule (TM123), making it active against its target only in the presence of TM123 [
69]
In conclusion, these developments underscore the significant potential of CAR-T therapies, particularly those targeting CD123, in the treatment of hematologic neoplasms, although ongoing clinical trials will provide critical insights into their real-world effectiveness.
Table 1 shows ongoing and completed clinical trials.
10. Anti-CD123 Bispecific antibodies
Bispecific antibodies (BsAbs) have emerged as a groundbreaking therapeutic strategy aimed at concurrently targeting specific antigens on both tumor and immune cells, such as CD3. This innovative class of protein drugs is designed to recruit immune cells to the vicinity of cancer cells, thereby triggering a more targeted and potent immune response. Notably, Blinatumomab, a BsAbs therapy engaging CD19 on leukemia cells and CD3 on T cells, has demonstrated success in treating B-cell acute lymphoblastic leukemia (B-ALL) patients [
71].
Expanding the scope of BsAbs therapy, researchers are exploring CD123 as a target to redirect the natural immune response towards malignant BPDCN cells (
Figure 2). Flotetuzumab (MDG006), a dual affinity retargeting antibody (DART), has shown promise in redirecting T CD3
+ lymphocytes against AML cells, displaying potent anti-leukemic activity both in vitro and in vivo [
68]. A phase I/II study in relapsed/refractory AML patients revealed a noteworthy CR/CR with partial hematologic recovery (CRh) rate of 26.7% and a median OS of 10.2 months [
68]. Ongoing research, including a basket trial evaluating Flotetuzumab in CD123-positive malignancies, is eagerly awaited (NCT04681105).
XmAb14045 represents another potent BsAb targeting both CD123 and CD3, designed for intermittent administration due to its extended serum half-life. In a phase I clinical trial (NCT02730312), XmAb14045 exhibited evidence of anti-leukemic activity, with promising clinical outcomes, including a 23% CR or CR with incomplete hematologic recovery (CR/CRi) at the two highest dose levels [
72,
73]. However, current data are limited to 104 AML and ALL individuals, necessitating further exploration.
A recent addition to the BsAb landscape is APVO436, a novel anti-CD123 x anti-CD3 BsAb. Preclinical data demonstrate its efficacy both in vitro and in vivo, showcasing superior T-cell activation, proliferation, and effective CD123
+ cell depletion compared to MGD006 (Flotetuzumab). Remarkably, APVO436 exhibits enhanced safety with lower T-cell cytokine release. In subcutaneous tumor models, it inhibits tumor growth, indicating T-cell migration and engagement at the tumor site when human T cells are intravenously implanted. These findings provide a strong rationale for further investigating APVO436 as a potential treatment for AML and other hematological malignancies, including BPDCN [
74,
75,
76]. The unique combination of efficacy and safety profile positions APVO436 as a promising candidate for advancing therapeutic options in these challenging diseases. Future clinical trials will be crucial in validating and expanding upon these encouraging preclinical results, ultimately paving the way for its potential inclusion in the arsenal against hematological malignancies.
Table 1 illustrates ongoing and completed clinical trials.
11. Conclusions
BPDCN represents a very rare and aggressive hematologic malignancy, arising from precursors of pDC, behaving like acute myeloid leukemia with high-risk clinical features [
3].
Although there has been significant progress in research specifically focused on BPDCN, the development of better treatments remains an urgent priority. In recent times, there has been a notable shift in both clinical and research endeavors towards advancing CD123-targeted immunotherapies for myeloid malignancies, including BPDCN. CD123 stands out as a promising target due to its abnormal expression on BPDCN and AML blasts, distinguishing them from normal hematopoietic stem cells and myeloid progenitors. The success of Tagraxofusp highlights the effectiveness of CD123 targeting in drug development for BPDCN. Such strategies, especially when directed at common antigens like CD123, could broaden the impact of new treatments across various cancers. The continued development of immunotherapies that target CD123 for hematologic malignancies points to additional progress in this field.
The successful clinical translation of SL-401 and CD123CAR T cells has fueled active development in other CD123-targeted immunotherapies, including BsAb, for treating BPDCN and various CD123-related hematologic malignancies. As we await mature results from ongoing studies, it's crucial to consider emergent observations that could impact the efficacy of CD123-targeted immunotherapies to enhance antineoplastic responses and elevate the survival rates for patients dealing with BPDCN and related CD123 neoplasms.
Author Contributions
S.Z. and D.G contributed to the writing, reviewing and editing of the review. All authors approved the final version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Xiao, W.; Chan, A.; Waarts, M.R.; Mishra, T.; Liu, Y.; Cai, S.F.; Yao, J.; Gao, Q.; Bowman, R.L.; Koche, R.P.; et al. Plasmacytoid dendritic cell expansion defines a distinct subset of RUNX1-mutated acute myeloid leukemia. Blood 2021, 137, 1377–1391. [Google Scholar] [CrossRef]
- Pagano, L.; Valentini, C.G.; Pulsoni, A.; Fisogni, S.; Carluccio, P.; Mannelli, F.; Lunghi, M.; Pica, G.; Onida, F.; Cattaneo, C.; et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 2013, 98, 239–246. [Google Scholar] [CrossRef]
- Galati, D.; Corazzelli, G.; De Filippi, R.; Pinto, A. Dendritic cells in hematological malignancies. Crit Rev Oncol Hematol 2016, 108, 86–96. [Google Scholar] [CrossRef]
- Pemmaraju, N. Blastic plasmacytoid dendritic cell neoplasm. Clin Adv Hematol Oncol 2016, 14, 220–222. [Google Scholar]
- Aoki, T.; Suzuki, R.; Kuwatsuka, Y.; Kako, S.; Fujimoto, K.; Taguchi, J.; Kondo, T.; Ohata, K.; Ito, T.; Kamoda, Y.; et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood 2015, 125, 3559–3562. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Liu, K.; Nussenzweig, M.C. Development and homeostasis of dendritic cells. Eur J Immunol 2010, 40, 2099–2102. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.; Park, S.H.; Jo, J.C. Plasmacytoid dendritic cell neoplasms. Blood Res 2023, 58, 90–95. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74-80. [Google Scholar] [CrossRef]
- Mildner, A.; Jung, S. Development and function of dendritic cell subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef]
- Diebold, S.S. Activation of dendritic cells by toll-like receptors and C-type lectins. Handbook of experimental pharmacology 2009, 3–30. [Google Scholar] [CrossRef]
- Moseman, E.A.; Liang, X.; Dawson, A.J.; Panoskaltsis-Mortari, A.; Krieg, A.M.; Liu, Y.J.; Blazar, B.R.; Chen, W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol 2004, 173, 4433–4442. [Google Scholar] [CrossRef]
- Haniffa, M.; Collin, M.; Ginhoux, F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv Immunol 2013, 120, 1–49. [Google Scholar] [CrossRef]
- Dai, H.; Thomson, A.W.; Rogers, N.M. Dendritic Cells as Sensors, Mediators, and Regulators of Ischemic Injury. Front Immunol 2019, 10, 2418. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med 2014, 138, 564–569. [Google Scholar] [CrossRef]
- Pagano, L.; Valentini, C.G.; Grammatico, S.; Pulsoni, A. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol 2016, 174, 188–202. [Google Scholar] [CrossRef]
- Jacob, M.C.; Chaperot, L.; Mossuz, P.; Feuillard, J.; Valensi, F.; Leroux, D.; Bene, M.C.; Bensa, J.C.; Briere, F.; Plumas, J. CD4+ CD56+ lineage negative malignancies: a new entity developed from malignant early plasmacytoid dendritic cells. Haematologica 2003, 88, 941–955. [Google Scholar]
- Riaz, W.; Zhang, L.; Horna, P.; Sokol, L. Blastic plasmacytoid dendritic cell neoplasm: update on molecular biology, diagnosis, and therapy. Cancer Control 2014, 21, 279–289. [Google Scholar] [CrossRef]
- Dietrich, S.; Andrulis, M.; Hegenbart, U.; Schmitt, T.; Bellos, F.; Martens, U.M.; Meissner, J.; Kramer, A.; Ho, A.D.; Dreger, P. Blastic plasmacytoid dendritic cell neoplasia (BPDC) in elderly patients: results of a treatment algorithm employing allogeneic stem cell transplantation with moderately reduced conditioning intensity. Biol Blood Marrow Transplant 2011, 17, 1250–1254. [Google Scholar] [CrossRef]
- Deotare, U.; Yee, K.W.; Le, L.W.; Porwit, A.; Tierens, A.; Musani, R.; Barth, D.; Torlakovic, E.; Schimmer, A.; Schuh, A.C.; et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: 10-Color flow cytometry diagnosis and HyperCVAD therapy. Am J Hematol 2016, 91, 283–286. [Google Scholar] [CrossRef]
- Garnache-Ottou, F.; Feuillard, J.; Saas, P. Plasmacytoid dendritic cell leukaemia/lymphoma: towards a well defined entity? Br J Haematol 2007, 136, 539–548. [Google Scholar] [CrossRef]
- Martin-Martin, L.; Lopez, A.; Vidriales, B.; Caballero, M.D.; Rodrigues, A.S.; Ferreira, S.I.; Lima, M.; Almeida, S.; Valverde, B.; Martinez, P.; et al. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget 2015, 6, 19204–19216. [Google Scholar] [CrossRef]
- Reimer, P.; Rudiger, T.; Kraemer, D.; Kunzmann, V.; Weissinger, F.; Zettl, A.; Konrad Muller-Hermelink, H.; Wilhelm, M. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant 2003, 32, 637–646. [Google Scholar] [CrossRef]
- Garnache-Ottou, F.; Feuillard, J.; Ferrand, C.; Biichle, S.; Trimoreau, F.; Seilles, E.; Salaun, V.; Garand, R.; Lepelley, P.; Maynadie, M.; et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol 2009, 145, 624–636. [Google Scholar] [CrossRef]
- Wang, W.; Khoury, J.D.; Miranda, R.N.; Jorgensen, J.L.; Xu, J.; Loghavi, S.; Li, S.; Pemmaraju, N.; Nguyen, T.; Medeiros, L.J.; et al. Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a 10-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm. Haematologica 2021, 106, 1047–1055. [Google Scholar] [CrossRef]
- Garnache-Ottou, F.; Vidal, C.; Biichle, S.; Renosi, F.; Poret, E.; Pagadoy, M.; Desmarets, M.; Roggy, A.; Seilles, E.; Soret, L.; et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv 2019, 3, 4238–4251. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, M.R.; Pileri, A.; Derenzini, E.; Melle, F.; Motta, G.; Fiori, S.; Calleri, A.; Pimpinelli, N.; Tabanelli, V.; Pileri, S. Blastic Plasmacytoid Dendritic Cell Neoplasm: State of the Art and Prospects. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, J.; Yang, M.; Wang, L.; Jin, J. New perspectives in genetics and targeted therapy for blastic plasmacytoid dendritic cell neoplasm. Crit Rev Oncol Hematol 2020, 149, 102928. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Kantarjian, H.; Sweet, K.; Wang, E.; Senapati, J.; Wilson, N.R.; Konopleva, M.; Frankel, A.E.; Gupta, V.; Mesa, R.; et al. North American Blastic Plasmacytoid Dendritic Cell Neoplasm Consortium: position on standards of care and areas of need. Blood 2023, 141, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.C.; Janssen, J.J.; Boers, J.E.; Kluin, P.M.; Verdonck, L.F. Allogeneic stem-cell transplantation for blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol 2012, 30, e102–103. [Google Scholar] [CrossRef]
- Roos-Weil, D.; Dietrich, S.; Boumendil, A.; Polge, E.; Bron, D.; Carreras, E.; Iriondo Atienza, A.; Arcese, W.; Beelen, D.W.; Cornelissen, J.J.; et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood 2013, 121, 440–446. [Google Scholar] [CrossRef]
- Mezzanzanica, D.; Canevari, S.; Mazzoni, A.; Figini, M.; Colnaghi, M.I.; Waks, T.; Schindler, D.G.; Eshhar, Z. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther 1998, 5, 401–407. [Google Scholar]
- Testa, U.; Pelosi, E.; Frankel, A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res 2014, 2, 4. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Han, L.; Qiu, P.; Zeng, Z.; Jorgensen, J.L.; Mak, D.H.; Burks, J.K.; Schober, W.; McQueen, T.J.; Cortes, J.; Tanner, S.D.; et al. Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells. Cytometry A 2015, 87, 346–356. [Google Scholar] [CrossRef]
- Jordan, C.T.; Upchurch, D.; Szilvassy, S.J.; Guzman, M.L.; Howard, D.S.; Pettigrew, A.L.; Meyerrose, T.; Rossi, R.; Grimes, B.; Rizzieri, D.A.; et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000, 14, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.M.; Sugita, M.; Ewing-Crystal, N.; Lam, L.; Galetto, R.; Gouble, A.; Smith, J.; Hassane, D.C.; Roboz, G.J.; Guzman, M.L. Selection and characterization of antibody clones are critical for accurate flow cytometry-based monitoring of CD123 in acute myeloid leukemia. Leuk Lymphoma 2018, 59, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kato, S.; Kohno, K.; Satou, A.; Eladl, A.E.; Asano, N.; Kono, M.; Kato, Y.; Taniwaki, M.; Akiyama, M.; et al. Clinicopathological analysis of 46 cases with CD4(+) and/or CD56(+) immature haematolymphoid malignancy: reappraisal of blastic plasmacytoid dendritic cell and related neoplasms. Histopathology 2017, 71, 972–984. [Google Scholar] [CrossRef]
- An, H.J.; Yoon, D.H.; Kim, S.; Shin, S.J.; Huh, J.; Lee, K.H.; Suh, C. Blastic plasmacytoid dendritic cell neoplasm: a single-center experience. Ann Hematol 2013, 92, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tsagarakis, N.J.; Kentrou, N.A.; Papadimitriou, K.A.; Pagoni, M.; Kokkini, G.; Papadaki, H.; Pappa, V.; Marinakis, T.; Anagnostopoulos, N.I.; Vadikolia, C.; et al. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res 2010, 34, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.E.; Dhagat, U.; Hercus, T.R.; Nero, T.L.; Grimbaldeston, M.A.; Bonder, C.S.; Lopez, A.F.; Parker, M.W. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 2012, 250, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Eidenschink Brodersen, L.; Alonzo, T.A.; Wang, J.; Pardo, L.; Sung, L.; Cooper, T.M.; Kolb, E.A.; Aplenc, R.; Tasian, S.K.; et al. CD123 Expression Is Associated With High-Risk Disease Characteristics in Childhood Acute Myeloid Leukemia: A Report From the Children's Oncology Group. J Clin Oncol 2022, 40, 252–261. [Google Scholar] [CrossRef]
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Pemmaraju, N.; Medeiros, B.C.; Carraway, H.E.; Frankfurt, O.; Forman, S.J.; Yang, X.A.; Konopleva, M.; et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014, 124, 385–392. [Google Scholar] [CrossRef]
- Pelosi, E.; Castelli, G.; Testa, U. CD123 a Therapeutic Target for Acute Myeloid Leukemia and Blastic Plasmocytoid Dendritic Neoplasm. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J.; Gomez, S.M.; Segesman, K.D.; Hunkapiller, T.; Laug, W.E.; Hood, L. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: implications for T cell receptor complex formation and activation. Cell 1990, 60, 929–939. [Google Scholar] [CrossRef]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Alfayez, M.; Konopleva, M.; Pemmaraju, N. Role of tagraxofusp in treating blastic plasmacytoid dendritic cell neoplasm (BPDCN). Expert Opin Biol Ther 2020, 20, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N Engl J Med 2019, 380, 1628–1637. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Konopleva, M. Approval of tagraxofusp-erzs for blastic plasmacytoid dendritic cell neoplasm. Blood Adv 2020, 4, 4020–4027. [Google Scholar] [CrossRef]
- Diaz Acedo, R.; Dominguez Munoz, M.A.; Navajas Laguna, C.; Morales Camacho, R.; Simon Pilo, I.; Calama Ruiz-Mateos, V.P.; Yebenes Ramirez, M.; Vahi Sanchez de Medina, M.; Artacho Criado, S.; Rodriguez Perez, A.; et al. Tagraxofusp as first-line treatment for blastic plasmacytoid dendritic cell neoplasm. Leuk Lymphoma 2022, 63, 1762–1764. [Google Scholar] [CrossRef]
- Stephansky, J.; Togami, K.; Ghandi, M.; Montero, J.; vonEgypt, N.; Lindsay, R.; Brooks, C.; Aster, J.C.; Johannessen, C.; Lane, A.A. Resistance to SL-401 in AML and BPDCN Is Associated with Loss of the Diphthamide Synthesis Pathway Enzyme DPH1 and Is Reversible By Azacitidine. Blood 2017, 130. [Google Scholar] [CrossRef]
- Montero, J.; Stephansky, J.; Cai, T.Y.; Griffin, G.K.; Cabal-Hierro, L.; Togami, K.; Hogdal, L.J.; Galinsky, I.; Morgan, E.A.; Aster, J.C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discovery 2017, 7, 156–164. [Google Scholar] [CrossRef]
- Togami, K.; Pastika, T.; Stephansky, J.; Ghandi, M.; Christie, A.L.; Jones, K.L.; Johnson, C.A.; Lindsay, R.W.; Brooks, C.L.; Letai, A.; et al. DNA methyltransferase inhibition overcomes diphthamide pathway deficiencies underlying CD123-targeted treatment resistance. J Clin Invest 2019, 129, 5005–5019. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Abu-Salah, A.; Salous, T.; Nassiri, M. Relapse of tagraxofusp treated blastic plasmacytoid dendritic cell neoplasm with loss of CD123 expression. J Hematop 2022, 15, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Thomassen, K.; Kurch, L.; Opitz, S.; Franke, G.N.; Bach, E.; Platzbecker, U.; Kayser, S. Combination of Tagraxofusp and Azacitidine Is an Effective Option for Relapsed Blastic Plasmacytoid Dendritic Cell Neoplasm After Allogeneic Hematopoietic Stem-Cell Transplantation. Clin Lymphoma Myeloma Leuk 2021, 21, e579–e582. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.A.; Stein, A.S.; Garcia, J.S.; Garzon, J.L.; Galinsky, I.; Luskin, M.R.; Stone, R.M.; Winer, E.S.; Leonard, R.; Mughal, T.I.; et al. Safety and Efficacy of Combining Tagraxofusp (SL-401) with Azacitidine or Azacitidine and Venetoclax in a Phase 1b Study for CD123 Positive AML, MDS, or BPDCN. Blood 2021, 138, 2346–2346. [Google Scholar] [CrossRef]
- Angelova, E.; Audette, C.; Kovtun, Y.; Daver, N.; Wang, S.A.; Pierce, S.; Konoplev, S.N.; Khogeer, H.; Jorgensen, J.L.; Konopleva, M.; et al. CD123 expression patterns and selective targeting with a CD123-targeted antibody-drug conjugate (IMGN632) in acute lymphoblastic leukemia. Haematologica 2019, 104, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Jones, G.E.; Adams, S.; Harvey, L.; Audette, C.A.; Wilhelm, A.; Bai, C.; Rui, L.; Laleau, R.; Liu, F.; et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv 2018, 2, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.G.; Montesinos, P.; DeAngelo, D.J.; Wang, E.S.; Papadantonakis, N.; Deconinck, E.; Erba, H.P.; Pemmaraju, N.; Lane, A.A.; Rizzieri, D.A.; et al. Clinical Profile of IMGN632, a Novel CD123-Targeting Antibody-Drug Conjugate (ADC), in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) or Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN). Blood 2019, 134, 734–734. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.A.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Slaney, C.Y.; von Scheidt, B.; Davenport, A.J.; Beavis, P.A.; Westwood, J.A.; Mardiana, S.; Tscharke, D.C.; Ellis, S.; Prince, H.M.; Trapani, J.A.; et al. Dual-specific Chimeric Antigen Receptor T Cells and an Indirect Vaccine Eradicate a Variety of Large Solid Tumors in an Immunocompetent, Self-antigen Setting. Clin Cancer Res 2017, 23, 2478–2490. [Google Scholar] [CrossRef] [PubMed]
- Till, B.G.; Jensen, M.C.; Wang, J.; Chen, E.Y.; Wood, B.L.; Greisman, H.A.; Qian, X.; James, S.E.; Raubitschek, A.; Forman, S.J.; et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008, 112, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, E.; Shahani, S.A.; Shah, N.N. Updates on CAR T-cell therapy in B-cell malignancies. Immunol Rev 2019, 290, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.I.; Pont, M.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 2018, 131, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Mardiros, A.; Dos Santos, C.; McDonald, T.; Brown, C.E.; Wang, X.; Budde, L.E.; Hoffman, L.; Aguilar, B.; Chang, W.C.; Bretzlaff, W.; et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 2013, 122, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- Bole-Richard, E.; Pemmaraju, N.; Cael, B.; Daguindau, E.; Lane, A.A. CD123 and More: How to Target the Cell Surface of Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Gouble, A.; Black, K.L.; Skwarska, A.; Naqvi, A.S.; Taylor, D.; Zhao, M.; Yuan, Q.; Sugita, M.; Zhang, Q.; et al. Targeting CD123 in blastic plasmacytoid dendritic cell neoplasm using allogeneic anti-CD123 CAR T cells. Nat Commun 2022, 13, 2228. [Google Scholar] [CrossRef]
- Bole-Richard, E.; Fredon, M.; Biichle, S.; Anna, F.; Certoux, J.M.; Renosi, F.; Tse, F.; Molimard, C.; Valmary-Degano, S.; Jenvrin, A.; et al. CD28/4-1BB CD123 CAR T cells in blastic plasmacytoid dendritic cell neoplasm. Leukemia 2020, 34, 3228–3241. [Google Scholar] [CrossRef]
- Loff, S.; Dietrich, J.; Meyer, J.E.; Riewaldt, J.; Spehr, J.; von Bonin, M.; Grunder, C.; Swayampakula, M.; Franke, K.; Feldmann, A.; et al. Rapidly Switchable Universal CAR-T Cells for Treatment of CD123-Positive Leukemia. Mol Ther Oncolytics 2020, 17, 408–420. [Google Scholar] [CrossRef]
- Xue, T.; Budde, L.E. Immunotherapies Targeting CD123 for Blastic Plasmacytoid Dendritic Cell Neoplasm. Hematol Oncol Clin North Am 2020, 34, 575–587. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Ravandi, F.; Bashey, A.; Foran, J.M.; Stock, W.; Mawad, R.; Blum, W.; Saville, M.W.; Johnson, C.M.; Vanasse, K.G.J.; Ly, T.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of XmAb14045, a CD123 x CD3 T Cell-Engaging Bispecific Antibody: Initial Results of a Phase 1 Study. Blood 2018, 132. [Google Scholar] [CrossRef]
- Ravandi, F.; Bashey, A.; Stock, W.; Foran, J.M.; Mawad, R.; Egan, D.; Blum, W.; Yang, A.; Pastore, A.; Johnson, C.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 x CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood 2020, 136. [Google Scholar] [CrossRef]
- Comeau, M.R.; Gottschalk, R.; Daugherty, M.; Sewell, T.; Misher, L.; Jeannette, B.; Johnson, S.; Parr, L.; Kumer, J.; Jablonski, D.; et al. APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity with limited cytokine release, is well tolerated in repeat dose toxicology studies in cynomolgus macaques. Cancer Research 2019, 79. [Google Scholar] [CrossRef]
- Comeau, M.R.; Miller, R.E.; Bannink, J.; Johnson, S.; Bader, R.; Gottschalk, R.; Misher, L.; Mitchell, D.; Parr, L.; DeFrancesco, M.; et al. Characterization of APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity, in preclinical models of AML and nonhuman primates. Mol Cancer Ther 2018, 17. [Google Scholar] [CrossRef]
- Comeau, M.R.; Miller, R.E.; Bader, R.; Gottschalk, R.; Daugherty, M.; Sewell, T.; Misher, L.; Parr, L.; DeFrancesco, M.; Bienvenue, D.; et al. APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR® molecule for redirected T-cell cytotoxicity, induces potent T-cell activation, proliferation and cytotoxicity with limited cytokine release. Cancer Research 2018, 78. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).