Submitted:
04 January 2024
Posted:
05 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods:
2.2.1. Evaluating the food risk assessment scheme in three egg sorting/packing units with regards to the implemented food safety initiatives
2.2.2. Elaboration of PRPs
2.2.3. Elaboration of the HACCP Plan
2.2.3. Analyses
3. Results and discussion
3.1. Materials
3.2. Evaluating the risk factors associated with eggs at three sorting and packing stations that have implemented distinct food safety protocols.
3.2. Assessment and Implementation of the PRPs
3.3. Implementation of HACCP plan
Preliminary steps to enable hazard analysis (Step 1-6) include:
Food safety teams
Specifications and intended purpose of the product
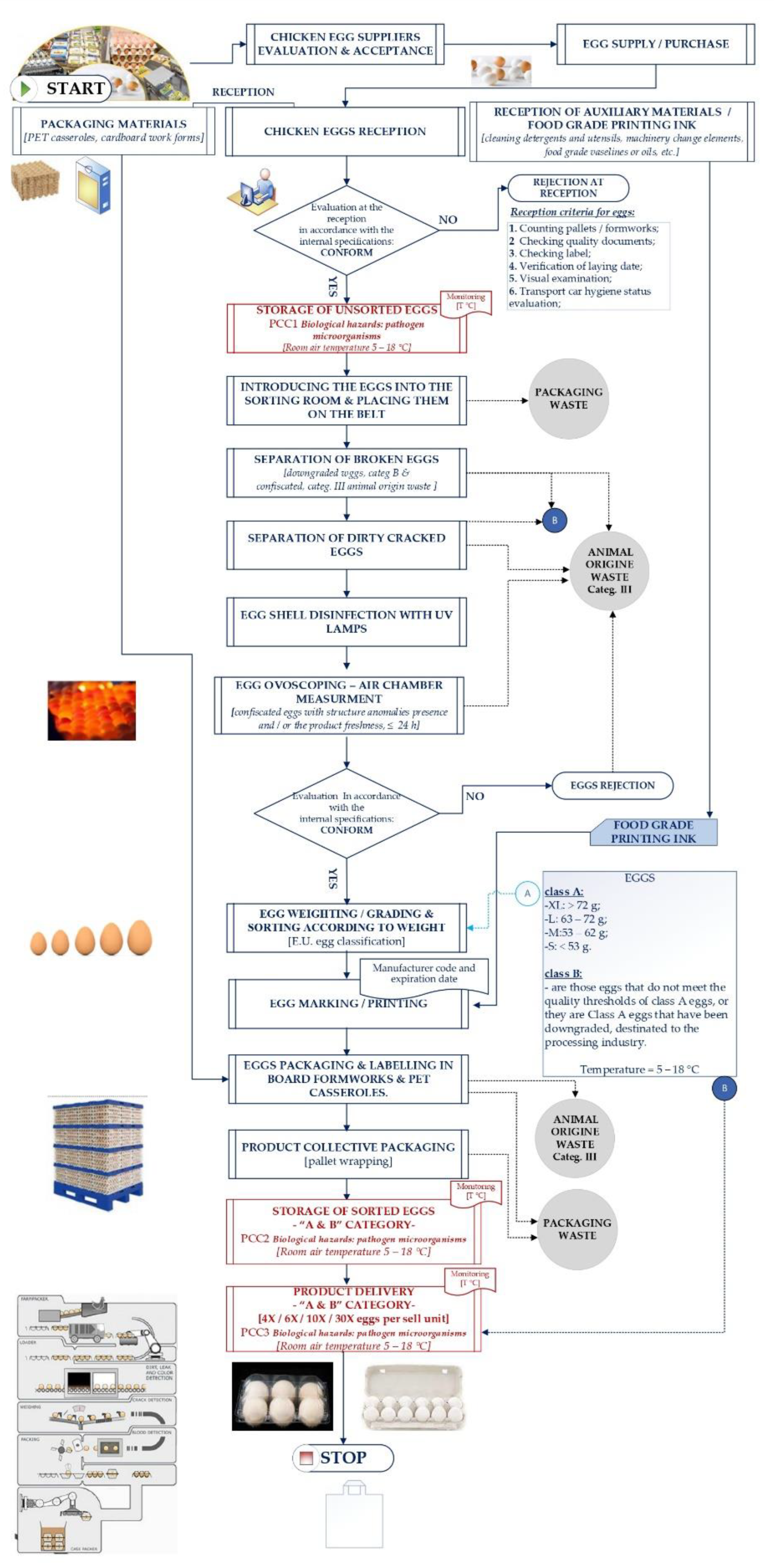
Flow diagram
The concepts of the HACCP plan (Steps 7-12)
Assessing risks and establishing permissible thresholds
3.4. Findings and discourse on the analysis
3.4.1. Evaluation of quality parameters
3.4.2. Evaluation of veterinary drugs
3.4.3. The egg quality and safety characteristics
3.4.4. The contamination of the eggs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magdelaine: P., Marché de l’œuf et des ovoproduits:. from https://www.itavi.asso.fr/ (accessed 2023-12-12), 21 July 2021.
- Nys, Y., et al., Qualités des oeufs de consommation. 2018.
- Rusu, M., et al., TRANSFER OF HEAVY METALS IN SOIL IN-PLUM CULTIVATION: A FIELD STUDY IN ADAMACHI IASI, ROMANIA. Journal of Applied Life Sciences and Environment, 2023. 56: p. 59-74. [CrossRef]
- (BIOHAZ)., E.P.o.B.H., Scientific Opinion on the Public Health Risks of Table Eggs Due to Deterioration and Development of Pathogens: Public Health Risks of Table Eggs Due to Deterioration and Development of Pathogens. EFSA 2014, 12 (7), 3782. [CrossRef]
- Hazards, E.Panel o.B., et al., Salmonella control in poultry flocks and its public health impact. 2019. 17(2): p. e05596. [CrossRef]
- (ORAR), O.f.R.A.R., Advice on the Risks in the Egg Supply Chain Netherlands Food and Consumer Product Safety Authority. Ministry of Agriculture Nature and Food Quality;, 2017.
- Allata, S., A. Valero, and L. Benhadja, Implementation of traceability and food safety systems (HACCP) under the ISO 22000:2005 standard in North Africa: The case study of an ice cream company in Algeria. Food Control, 2017. 79: p. 239-253. [CrossRef]
- Kamboj, S., et al., Food safety and hygiene: A review. International Journal of Chemical Studies, 2020. 8: p. 358-368. [CrossRef]
- Manley, D., 3 - Quality management systems and hazard analysis critical control point (HACCP) in biscuit manufacture, in Manley’s Technology of Biscuits, Crackers and Cookies (Fourth Edition), D. Manley, Editor. 2011, Woodhead Publishing. p. 23-28. [CrossRef]
- Ammar, D.e., et al., Implementation of the Hazard Analysis Critical Control Point (haccp) System for Processed Cheese Production Line. 2017. [CrossRef]
- Nada, S., et al., Implication of food safety measures on microbiological quality of raw and pasteurized milk. Food Control, 2012. 25(2): p. 728-731. [CrossRef]
- Chen, H., et al., Establishment the critical control point methodologies of seven major food processes in the catering industry to meet the core concepts of ISO 22000:2018 based on the Taiwanese experience. 2019. 39(6): p. e12691. [CrossRef]
- Panghal, A., et al., Role of Food Safety Management Systems in safe food production: A review. 2018. 38(4): p. e12464. [CrossRef]
- ISO22000:2018, ISO 22000-Food Safety Management Systems Requirements for Any Organization in the Food Chain; ISO. Geneva, Switzerland.
- Chhikara, N., et al., Importance of Traceability in Food Supply Chain for Brand Protection and Food Safety Systems Implementation. Annals of biology, 2018. 34: p. 111-118. [CrossRef]
- Panghal, A., et al., Role of Food Safety Management Systems in safe food production: A review. Journal of Food Safety, 2018. 38: p. e12464. [CrossRef]
- WHO., F.a., CODEX – 60 years of standards. Codex Alimentarius Commission. Rome. 2023.
- Surareungchai, S., et al., Comparison of Risk Assessment Schemes in GHPs and HACCP, FSMA Preventive Controls for Human Food, ISO 22000, and GFSI Recognized Standards with Risk Scoring Guidance in General Use with Fresh Produce. 2022. 8(2): p. 181. [CrossRef]
- Cusato, S., et al., Food safety systems in a small dairy factory: implementation, major challenges, and assessment of systems’ performances. Foodborne Pathog Dis, 2013. 10(1): p. 6-12. [CrossRef]
- Mureşan, C.C., et al., Food Safety System (HACCP) as Quality Checkpoints in a Spin-Off Small-Scale Yogurt Processing Plant. 2020. 12(22): p. 9472. [CrossRef]
- V6, F.F.S.M.S.C., FSSC 22000 Food Safety Management System Certification V6. 2023.
- v8, I.F., IFS FOOD. IFS FOOD, 2023.
- Adesiyun, A., et al., Prevalence of Antimicrobial Residues in Table Eggs in Trinidad. Journal of Food Protection, 2005. 68(7): p. 1501-1505. [CrossRef]
- Szkoda, J. and J. Zmudzki, Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bulletin of the Veterinary Institute in Pulawy, 2005. 49: p. 89-92.
- ten Dam, G., et al., The performance of atmospheric pressure gas chromatography–tandem mass spectrometry compared to gas chromatography–high resolution mass spectrometry for the analysis of polychlorinated dioxins and polychlorinated biphenyls in food and feed samples. Journal of Chromatography A, 2016. 1477: p. 76-90. [CrossRef]
- Theurillat, X., et al., An LC-MS/MS method for the quantitative determination of 57 per- and polyfluoroalkyl substances at ng/kg levels in different food matrices. Food Additives & Contaminants: Part A, 2023. 40(7): p. 862-877. [CrossRef]
- Wang, P.C., et al., Determination of cyromazine and melamine in chicken eggs using quick, easy, cheap, effective, rugged and safe (QuEChERS) extraction coupled with liquid chromatography-tandem mass spectrometry. Anal Chim Acta, 2012. 752: p. 78-86. [CrossRef]
- Charalampous, A.C., K.S. Liapis, and E.D. Bempelou, Fipronil in eggs. Is LC-MS/MS the only option? A comparison study of LC-MS/MS and GC-ECD for the analysis of fipronil. Journal of Chromatography B, 2019. 1129: p. 121785. [CrossRef]
- Owusu-Apenten, R. and E. Vieira, Food Safety Management, GMP & HACCP, in Elementary Food Science, R. Owusu-Apenten and E.R. Vieira, Editors. 2023, Springer International Publishing: Cham. p. 217-236. [CrossRef]
- 1441/2007, C.R.E., 1441/2007 OF THE COMMISSION of December 5, 2007 amending Regulation (EC) no. 2073/2005 regarding microbiological criteria for food products.
- 2023/915, C.R.E., 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006.
- 96/23/EC, C.D., COMMISSION DECISION of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results.
- 37/2010, C.R.E.N., No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (Text with EEA relevance).
- 396/2005, R.E.N., NO 396/2005 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC.
- 2023/710, C.R.E., Commission Regulation (EU) 2023/710 of 30 March 2023 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bromopropylate, chloridazon, fenpropimorph, imazaquin and tralkoxydim in or on certain products (Text with EEA relevance.
- 2023/1049, C.R.E., Commission Regulation (EU) 2023/1049 of 30 May 2023 amending Annexes II and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fish oil, pendimethalin, sheep fat and spirotetramat in or on certain products (Text with EEA relevance).
- 2023/1042, C.R.E., Commission Regulation (EU) 2023/1042 of 26 May 2023 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for folpet in or on certain products (Text with EEA relevance).
- 2016/52, C.R.E., 2016/52 of 15 January 2016 laying down maximum permitted levels of radioactive contamination of food and feed following a nuclear accident or any other case of radiological emergency, and repealing Regulation (Euratom) No 3954/87 and Commission Regulations (Euratom) No 944/89 and (Euratom) No 770/90.
- Service, F.S.a.I., Egg Products Hazards and Controls Guide. United States Department of Agriculture 2020.
- Lee, J.C., et al., Implementation of Food Safety Management Systems along with Other Management Tools (HAZOP, FMEA, Ishikawa, Pareto). The Case Study of Listeria monocytogenes and Correlation with Microbiological Criteria. Foods, 2021. 10(9). [CrossRef]
- 1998, O.n.d.d., ORDIN nr. 976 din 16 decembrie 1998 Romanian Ministry of Health 1998.
- COUNCIL, R.E.N.O.T.E.P.A.O.T. and o.O. 2004, REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 October 2004. THE EUROPEAN PARLIAMENT AND OF THE COUNCIL, 2004.
- 2023/2006, C.R.E.N. and o.D. 2006, COMMISSION REGULATION (EC) No 2023/2006 of 22 December 2006. Official Journal of the European Union, 2006.
- Motarjemi, Y. and B.R. Warren, Chapter 36 - Hazard Analysis and Critical Control Point System (HACCP), in Food Safety Management (Second Edition), V. Andersen, H. Lelieveld, and Y. Motarjemi, Editors. 2023, Academic Press: San Diego. p. 799-818.
- van Asselt, E.D., et al., Risk-based monitoring of chemical substances in food: Prioritization by decision trees. Food Control, 2018. 93: p. 112-120. [CrossRef]
- Cardoso, M.J., et al., Salmonella in eggs: From shopping to consumption—A review providing an evidence-based analysis of risk factors. 2021. 20(3): p. 2716-2741. [CrossRef]
- Charles Robert Stilz, S.C., Katie Garman, and John R. Dunn, Salmonella Enteritidis Outbreaks Associated with Egg-Producing Farms Not Regulated by Food and Drug Administration’s Egg Safety Rule. 2022. 19(8): p. 529-534. [CrossRef]
- Upadhyaya, I., et al., Chapter 19 - Natural Approaches for Improving Postharvest Safety of Egg and Egg Products, in Producing Safe Eggs, S.C. Ricke and R.K. Gast, Editors. 2017, Academic Press: San Diego. p. 391-420. [CrossRef]
- 589/2008, C.R.E.N., No 589/2008 of 23 June 2008 laying down detailed rules for implementing Council Regulation (EC) No 1234/2007 as regards marketing standards for eggs.
| Item | Requirements | ||
|---|---|---|---|
| 2 GFSI schemes | Station C: GPFH [GHPs HACCP, v. 2023] |
||
| Station A: IFS Food v8, April 2023 | Station B: FSSC 22000 v6, April 2023 | ||
| Contaminants |
|
|
|
| Cleaning and disinfection |
|
|
|
| Product description |
|
|
|
| Process description |
|
|
|
| Operational control |
|
|
|
| Operational monitoring |
|
|
|
| Corrective actions in case of process failure |
|
|
|
| Validation |
|
|
|
| Verification |
|
|
|
| Records |
|
|
|
| Hazards |
|
|
|
| Hazard sources |
|
|
|
| Occurrence in absence of control |
|
|
|
| Severity in absence of control |
|
|
|
| Significant hazard |
|
|
|
| Control measure |
|
|
|
| Control limit |
|
|
|
| Limit control definition |
|
|
|
| Monitoring |
|
|
|
| Correction |
|
|
|
| Corrective action |
|
|
|
| Validation |
|
|
|
| Verification |
|
|
|
| Test reports |
|
|
|
| Records keeping |
|
|
|
| Recall |
|
|
|
| Input raw material and auxiliary risk assessment |
|
|
|
| Fraud assessment |
|
|
|
| Threat assessment / Food defense |
|
|
|
| Supplier control |
|
|
|
| Incoming inspection |
|
|
|
| Quality control |
|
|
|
| Incident management |
|
|
|
| Specification | Description | Mentions |
|---|---|---|
| Product name | Eggs – category A | |
| Technical quality conditions | The eggs come from hens farms that are sanitary and veterinary authorized for consumption. Hens for consumption eggs are raised in batteries or on the ground in compliance with the legal requirements regarding the welfare of the consumption egg hens. |
|
| Qualitative characteristics | Shell and cuticle: clean, intact, normal; Air chamber: the height does not exceed 6 mm, immovable; however, for eggs marketed with the mention "extra", it must not exceed 4 mm; Yolk: visible in the beam of light only as a shadow, without a precise outline; when the egg is turned, the yolk is slightly mobile and returns to the central position; Albumen: clear, translucent; Foreign bodies: no foreign bodies; Foreign odor: no foreign odor. |
Tolerances for category A quality defects: At the packing center, just before shipping - 5% of the eggs have quality defects; In the other stages of marketing - 7% of the eggs have quality defects; For eggs with the mention "extra", no tolerance for the height of the air chamber is allowed during the inspection carried out during packaging; The percentages are doubled when the controlled lot contains less than 180 eggs. |
| Classification of eggs according to weight | XL - very large - weight greater than or equal to 73 g; L - large - weight less than 73 g and greater than or equal to 63 g, M - medium - weight less than 63 g and greater than or equal to 53 g; S - small - weight less than 53 g |
Tolerances for egg weight A batch can contain no more than 10% of eggs from the weight categories close to the one marked on the package, but no more than 5% from the weight category immediately below. When eggs of different sizes are packed in the same package, the minimum net weight of these eggs is indicated in grams, and the mention "eggs of different sizes" is applied on the outside of the package. Category A eggs are neither washed nor cleaned, neither before nor after classification. Eggs should not be washed or cleaned, as this can cause damage to the shell, which due to its antimicrobial characteristics represents an effective barrier against bacterial contamination. |
| Physical - chemical characteristics | The protein content of the albumen: 11 – 12 % pH albumen: 7.8 – 9.3 The protein content of the yolk: 16 – 17 % pH yolk: 5.6 – 7 |
|
| Microbiological conditions | Salmonella (Spp/25 g): absent | According Reg. 1441 / 2007 [30] |
| Maximum contaminant limits | Sum of dioxin – max 2.5 pg/g fat Sum of dioxins and dioxin-like PCBs – max 5.0 pg/g fat |
According Reg. 915 / 2023 [31] |
| Residues of medicine | ≤ 200 - Chlortetracyclin, Oxytetracycline, Tetracycline, Tylosin; ≤ 150 - Erythromycin; ≤ 400 - Neomycin; ≤ 1000 - Tiamulin |
According DC 657/2002/EC [32]; Reg. 37 / 2010 [33] |
| Residues of pesticides | absent | According Reg. 396 / 2005 [34]; Reg. 710 / 2023 [35]; Reg. 1049 / 2023 [36]; Reg. 1042 / 2023 [37] |
| Radioactive contamination | absent | According Reg. 52 / 2016 [38] |
| Melamine | max 2.5 mg/kg | According Reg. 915 / 2023 [31] |
| Rules for checking quality | Checking the quality of the eggs is carried out according to the "Monitoring and measuring" procedure. Each batch is examined with an ovoscope before marking and packaging. | The verification of the microbiological and physico-chemical conditions is done by collecting samples, according to the self-control program and analyzing them in authorized laboratories with which the unit collaborates. |
| Marking and packaging | Eggs are packed in formwork, they are palletized and wrapped. Eggs are marked in an automated system with the code of the farm of origin and the expiration date. Sale of eggs in bulk: information are communicated visibly and perfectly legibly, information regarding: quality category; weight category; the way of raising chickens; manufacturer code; explanation of the meaning of the manufacturer’s code; minimum validity date. The bands and labels for category A eggs will be white, and the indications will be printed in black. |
Marking of packages containing category A eggs: - the packages containing category A eggs have written on the outside, easily visible and perfectly legible; - packaging center code; the meaning of the code is explained on the outside or inside the packaging; letters and numbers of at least 2 mm; - Quality category category A or by the letter A accompanied or not by the mention "fresh"; - Weight category; a 12 mm circle around the mark for the weight class, consisting of letters at least 2 mm high; - Storage conditions "keeping eggs in the refrigerator after purchase"; - Method of raising chickens: "eggs raised in batteries"; - Minimum validity date: it must be a maximum of 28 days calculated from the laying date; letters and numbers of at least 2 mm including the day and month; for packaging "to be consumed, preferably, before..."; for the egg, the date of minimum durability followed by the date, the day, expressed in numbers from 1 to 31 and the month expressed in letters from 1 to 12 or 4 letters from the alphabet; - The "extra" mentions can only be used on packages containing category A eggs until the 9th day after laying; the laying date and the 9-day period must be written; - The way of feeding the chickens can also be indicated. Tolerances regarding the marking of packaging and eggs A tolerance of 20% is allowed for eggs bearing illegible markings during batch and packaging control. |
| Storage, transport, documentations | 5 – 18oC, in clean spaces, free of pests. Eggs should not be refrigerated in spaces with a temperature ˂5 oC. | Eggs are transported with properly equipped, authorized and well-sanitized means of transport. During transport, the cold chain must be maintained. Eggs are delivered according to the "Product release" procedure. Documents: The transport of eggs is accompanied by the following documents: shipping notice, declaration of conformity |
| Terms of validity | 28 days from the date of laying. | |
| Intended use | Chicken eggs are widely used in many types of food, both sweet and salty, including baked ones. Eggs can be scrambled, fried, boiled, soft-boiled and pickled. They can also be eaten raw, although this is not recommended for people who may be particularly sensitive to salmonellosis. | The average weekly consumption of eggs should be reduced to 4 pieces. Eggs are part of the group of potentially allergenic foods, they can cause allergies. |
| The step of the technological process |
Identify potential hazards introduced, controlled, or improved at this step | Does this potential hazard need to be addressed in the HACCP plan? Yes/No |
Justify your decision | Hazard assessment | What measure(s) can be applied to prevent or eliminate the hazard or reduce in to an acceptable level? | |||
|---|---|---|---|---|---|---|---|---|
| S | P | HR | ||||||
| General, for all steps | B | Human diseases such as the SARS-CoV-2 virus or different zoonosis | Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Chemical residues of substances used inside the facility | No | It can lead to comsumer health impact | 2 | 1 | 2 | ||
| P | Foregn bodies from company infrastructure | No | The presence of these hazards have low impact; in general lead to damages of the egg, which will not be delivered to the consumer | 2 | 1 | 2 | ||
| Egg supplier election | B | Presence of Salmonella spp. and Campylobacter jejuni for the supplied eggs | Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Pesticide residues, mycotoxins, heavy metals, drugs, hormones, dioxines, radioactivity, allergens (other than eggs protein). | Yes | It can lead to comsumer health impact | 3 | 1 | 3 | ||
| P | Presence of insects, rodent droplets, plastic, glass. | No | The presence of these hazards have low impact; in general lead to damages of the egg, which will not be delivered to the consumer | 2 | 1 | 2 | ||
| Fraud | No | 97% of the eggs come from own farms; exception → station A with 5 external suppliers, and the matrix does not lead itself to fraud | 2 | 1 | 2 | |||
| Egg supply |
B | Development of pathogenic microorganisms due to improper transport temperature (Salmonella spp. and Campylobacter jejuni). | Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Chemical residues of substances used to sanitize means of transport. | Yes | It can lead to comsumer health impact | 2 | 1 | 2 | ||
| P | Contamination with foreign bodies during transport: minerals, insects parts, rodents, dust | No | The presence of these hazards has a low impact; in general, they lead to damage to the egg, which cannot be processed further or delivered to the consumer. | 2 | 1 | 2 | ||
| Reception | B | Development of pathogenic microorganisms due to farm conditions and / or improper transport: Salmonella spp., Campylobacter jejuni; |
Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Pesticide residues, mycotoxins, heavy metals, melamine, drugs, hormones, dioxins, radioactivity, allergens (eggs protein), chemical residues of substances used to sanitize farms and means of transport; | Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
|
| P | Presence of minerals, insects, rodents, rodent drops, plastic, glass, metals, etc. Contamination with foreign bodies during transport: |
No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| - | Food fraud | No | 97% of the eggs come from own farms; exception → station A with 5 external suppliers, and the matrix does not lead itself to fraud | 2 | 1 | 2 |
|
|
| - | Food Defence | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Reception of packaging [cardboard packaging, paper rolls, PET casserols, PP bags], labels, and food-grade ink | B | Presence of Total viable count (TVC) and coliforms | Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Components that can migrate into the product (global migration, heavy metals), toxic substances in the marking ink | Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
|
| P | Presence of metals, glass, dust, insects, rodents traces | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Storage of unsorted eggs | B | Proliferation of pathogenic microorganisms in favorable temperature conditions results in the formation of condensation on the eggshell. Contamination from the storage space Salmonella spp, Campylobacter jejuni, TVC, Moulds) |
Yes | It can result an unsuitable product or possibly have a health repercussion leading to various illnesses. | 3 | 1 | 3 |
|
| C | Residues from pest control activities and/or cleaning chemicals | No | The presence of this hazards can cause illness and injury to the consumer | 2 | 1 | 2 |
|
|
| P | Cracked egg. Contamination with foreign bodies during storage and internal manipulation from the storage: glas, parts of insects, hard plastic, dust |
No | The presence of these hazards has a low impact; in general, they lead to damage to the egg, which cannot be processed further or delivered to the consumer. | 2 | 1 | 2 |
|
|
| Storage of packaging | B | Contamination from the storage space (TVC, Moulds) | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
| C | Residues from pest control activities and / or clenning chemicals | No | The presence of this hazards can cause illness and injury to the consumer | 2 | 1 | 2 |
|
|
| P | Presence of glass, insects, rodents, dust |
No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Introducing eggs for sorting | B | Contamination from personnel, MOBA work line or working space (TVC, Moulds, Staphylococcus haemolyticus, Staphylococcus coagulase positive, Enterobacteriacea) |
No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
| C | Residues from pest control activities and / or clenning chemicals | No | The presence of this hazards can cause illness and injury to the consumer | 2 | 1 | 2 |
|
|
| P | Presence of glass, metal, insects, rodents or rodents traces | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Separation and removal of confiscated, dirty and cracked eggs | B | Contamination from machinery, and personnel; Contamination due to breaking eggs |
No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
| C | Contamination with oils used to lubricate equipment. Contamination with residues and substances used for sanitation and pest control activities |
No | the presence of residues of oils used for greasing or washing substances cannot cause serious illness | 2 | 1 | 2 |
|
|
| P | Presence of glass, metal, parts from other eggs | No | It can lead to comsumer health impact | 1 | 1 | 2 |
|
|
| Disinfection with UV lamp | B | Inefficient disinfection [for Total viable count of germs (TVC), coliform bacteria] |
No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
| C | - | - | - | - | - | - | - | |
| P | Presence of: glass, hard plastic, metal | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Egg ovoscopy and air chamber measurement | B | Inappropriate removal of eggs with dirty shell, broken | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
| C | Contamination with residues and substances used for sanitation and pest control activities or oils used to lubricate equipment. | No | the presence of residues of oils used for greasing or washing substances cannot cause serious illness | 2 | 1 | 2 |
|
|
| P | Presence of metals, glass, plastic | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Weighing eggs and sorting according to weight | B | Development of pathogenic bacteria in favorable temperature conditions. The formation of condensation on the egg shell. Contamination from machinery or working area. |
Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Residues of chemical substances used for sanitation of MOBA equipments; lubricants | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| P | - | - | - | - | - | - | - | |
| Marking eggs/Printing | B | - | - | - | - | - | - | - |
| C | Heavy metals in the substances used for marking/ printing [Pb, Cd, As, Hg) | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| P | Presence of glass, metal, insects, rodents | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Egg packaging in formwork and labeling | B | The development of pathogenic bacteria in favorable temperature conditions. The formation of condensation on the surface of the eggs. Contamination from packaging materials. |
Yes | It can lead to comsumer health impact | 3 | 1 | 3 |
|
| C | Chemical components that can migrate from the packaging to the product. Residues from substances used for sanitation and pest control activities. |
No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| P | Presence of glass, metal, insects. | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Storage of sorted eggs, category A and B | B | Development of pathogenic bacteria, due to improper storage conditions (Salmonella spp., Campylobacter jejun, Aerobic TVC, Moulds, Coliforms) | Yes | it can lead to obtaining an inappropriate product or even to a health impact causing different diseases. | 3 | 1 | 3 |
|
| C | Residues of chemicals from cleaning operations and / or pest control activities | Yes | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| P | Presence of glass, plastic, insects, rodents | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Product delivery A and B category | B | Development of pathogenic bacteria as a result of non-compliance with storage temperatures or the formation of condensation on the surface of the eggshell (Salmonella spp., Campylobacter jejuni, Aerobic TVC, Moulds, Coliforms ) | Yes | It can lead to obtaining an inappropriate product or even to a health impact causing different diseases. | 3 | 1 | 3 |
|
| C | Residues of chemicals from cleaning operations, fuel residue, or other residue from products transported | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| P | Presence of impurities: metal, plastic protection lamps, and stitches from windows | No | It can lead to comsumer health impact | 2 | 1 | 2 |
|
|
| Process step | Significant hazard | Q11 | Q22 | Q33 | Q44 | CCP/ CP YES / NO |
|---|---|---|---|---|---|---|
| Egg supplier election | B [f.e.: Salmonella spp. and Campylobacter jejuni]: the supplied eggs | Yes | No | No | - | CP 1 |
| C [f.e.: pesticide residues, mycotoxins, heavy metals, drugs, hormones, dioxines, radioactivity, allergens (other than eggs protein).]: eggs can be contaminated from the farm; | Yes | No | No | - | ||
| Egg supply | B [f.e.: Salmonella spp. and Campylobacter jejuni]: eggs can be contaminated from improper transport temperature; | Yes | No | No | - | CP2 |
| Reception | B [f.e.: Salmonella spp, Campylobacter jejuni, TVC, Moulds]: egg supplier election | Yes | No | No | - | CP 3 |
| C [f.e. Pesticide residues, mycotoxins, heavy metals, melamine, drugs, hormones, dioxins, radioactivity, allergens (eggs protein), chemical residues of substances used to sanitize farms and means of transport]: egg supplier election | Yes | No | No | - | ||
| Reception of packaging materials, labels and ink | B [f.e. TVC, coliforms]: contamination from the manufacturer or transport; | Yes | No | No | - | CP 4 |
| C [chemicals residue, overall migration limit (OML) for plastic packaging > 60mg/kg food, or 10 mg/dm2 of the contact material]: contamination from the manufacture; | Yes | No | No | - | ||
| Storage of unsorted eggs | B [f.e.: Salmonella spp]: contamination due to the improper temperature [limits → 5 – 18 °C]; | Yes | No | Yes | No | CCP - 1 |
| Weighing eggs and sorting according to weight | B [f.e.: Salmonella spp., Campylobacter jejuni, TVC, Coliforms]: contamination of eggs due to improper temperatures, condensation or equipment; | Yes | No | No | - | CP - 5 |
| Egg packaging in formwork and labeling | B [f.e.: Salmonella spp., Campylobacter jejun, TVC, Coliforms]: contamination of eggs due to improper temperatures, condensation or equipment; | Yes | No | No | - | CP - 6 |
| Storage of sorted eggs, category A și B | B [f.e.: Salmonella spp., Campylobacter jejun, Aerobic TVC, Moulds, Coliforms]: contamination due to the improper temperature [limits → 5 – 18 °C]; | Yes | No | Yes | No | CCP - 2 |
| Product delivery A and B category | B [f.e.: Salmonella spp., Campylobacter jejun, Aerobic TVC, Moulds, Coliforms]: contamination due to the improper temperature [limits → 5 – 18 °C]; | Yes | No | Yes | No | CCP - 3 |
| CCPs | Significant hazard (s) | CCP parame- ter |
Value pro-grammed and validated | Critical limits | Monitoring procedure | Correction and Corrective action | Records | |||
|---|---|---|---|---|---|---|---|---|---|---|
| What? | How? | When -frequency? | Who? | |||||||
|
Storage of unsorted eggs CCP – 1 Storage of sorted eggs, category A and B CCP - 2 |
Biological hazard: Proliferation of pathogenic microorganisms in favorable temperature conditions, formation of condensation on the eggshell; Contamination from the storage space. |
Temp. in the storage room | 5 – 18 oC | > 18oC for more than 3 hours | Air temp. | Reading and recording storage space temperature Checking/ validation of the internal system with the ethalon thermometer [standard measuring and monitoring devices]. |
Continue through electronic systems and physical by the stockkeeper 2 x/ day from Monday to Sunday. | Monitoring: the stockkeeper and the person responsible for security during the weekend; Verification: Quality Assurance Manager and Production Responsible; Corective action: Production Responsible and/or Administrator; |
Correction If the temperature is near the critical limit (> 15 °C), immediate notification of the technical department and production responsible is done. During storage, a free space is ensured between the formwork / boxes, sufficient for the circulation of cold air. If the defect cannot be fixed and there is a danger that the temperature of the egg warehouse [at reception or at delivery] will exceed the value of 18 °C, the eggs should be urgently inserted for sorting if possible or transferred to another space with a corresponding temperature of 5–18 °C [case of CCP1], and / or delivered urgently or transferred to another space with a corresponding temperature of 5–18 °C [case of CCP2]. If the temperature of the air warehouse has reached > 18 °C for more than 3 hours, the product lots are identified as potentially unsafe and treated according to the procedure "Control of non-compliant products [quarantified, externally tested reports for Salmonella and sensoy parameters, and the decision of the Food Safety Team]. Sorting, packaging, and commercialization within a maximum of 3 days of eggs that have been stored at a temperature < 5 °C. Moldy, rotten, cloudy, or even opaque eggs, without separation between white and yolk, or those with dark spots on the inner side of the shell, produced by various molds or bacteria, are confiscated and destined for denature. Corrective action Maintaining the annual verifications of the cooling system according with the internal schedule for preventive measures. If the electricity supply stops, the electric group will be automatically turned on to ensure the appropriate conditions. Establishment and application of equipment maintenance program Establishing and following the specific training of the employees (on food safety and on technical part). |
Online system database and temperature sheet |
|
Product delivery CCP - 3 |
Biological hazard: Development of pathogenic bacteria as a result of non-compliance with storage temperatures, or the formation of condensation on the surface of the eggshell; contamination from the means of transport. |
Temp. during product delivery [inside the truck] | 5 – 18oC | > 18oC for more than 3 hours | Air temp. | Reading and recording the temperature inside the truck at product loading; Automatic system, checking the thermodiagrame before unloading the product |
Continue through electronic systems. Visualisation every 2 hours during transport [inside the driver cabine] |
Monitoring: the driver; Verification: Logistic Responsible Corective action: Logistic Responsible and / or Administrator; |
Correction The product is not loaded in the truck until the temperature of the truck-transported room is max. 10 oC. In case of failure of the system, the truck will be changed (maximum 3 hours) or will be redirected nearest the closest refrigerated warehouse [due to our networking partners and collaborations]. Corrective action Revision in time on all trucks and on all refrigerated systems. between If the temperature are not in the range 5 – 18oC Compliance with GMP, GHP measures and staff training. Respecting the product legal parameteres and compliance with product technical parameters; Corect sanitation of the transport trucks after easch delivery [thawing process and sanitation]; |
Thermodiagrame picture at delivery |
| No. crt. | Field of verification / item | Frequency | Responsible for verification |
|---|---|---|---|
| 1. | Verification of compliance with the procedure for selecting suppliers; | Annual or at introduction of a new supplier in the system | Purchase Responsible |
| 2. | Checking the quality and safety of eggs: - quality parameters (pH, sensory) → once every 3 months; - safety parameters: veterinary residue, mycotoxins, PCB, heavy metals, drugs, hormones, dioxins, melamine, radioactivity, allergens (other than egg protein), chemical residues of substances used to sanitize the farms (e.g., fipronil) → annual. - Sallmonela spp., Campylobacter jejuni: monthly |
Annual, biannual and / or monthly | HACCP team leader |
| 3. | Checking the conformity of transport at reception (daily or each reception) and at delivery (each delivery); | Daily or each reception / each transport | Stockkeeper Logistic responsible |
| 4. | Checking the temperature and hygiene conditions from storage warehouses and transport, until sale; | Daily / as long the product is kept into the storage or transported | Logistic Responsible Production Responsible Stockkeeper Driver |
| 5. | Potable water supply check | Annual | Hygiene Responsible |
| 6. | Verification of compliance with the stages of the technological flow | Monthly | Technological engineer |
| 7. | Verification of compliance with equipment maintenance | Annual, biannual and / or monthly | Maintenance manager |
| 8. | Verification of calibration of measuring and control device | Annual or when it is necessary. | Maintenance responsible |
| 9. | Checking the hygiene of production protective equipment, spaces, annexes, and social groups | Internal (weekly) External (1x/ 3 months) |
Hygiene Responsible HACCP Team leader |
| 10. | Checking the control of the health of the staff | Biannual | Production Responsible |
| 11. | Checking the hygiene of the work equipment | Internal (weekly) External (1x/ 3 months) |
Hygiene Responsible HACCP Team leader |
| 12. | Checking efficiency for waste disposal | Monthly | HACCP team leader |
| 13. | Verification of compliance with the pest control procedure | Monthly | Hygiene Responsible |
| 14. | Verification of CCP records; deviations from critical limits; execution of corrections and / or corrective actions | Daily | HACCP team leader |
| 15. | Checking CP records | Daily | Production responsible HACCP team leader |
| 15. | Checking the efficiency of employees training | Once every three months | HR Manager Production Responsible HACCP team leader |
| 16. | Checking the quality control and safety of the finished eggs | Internal (daily) External (monthly) |
Production Responsible HACCP team leader |
| 17. | Checking the registration activity | Monthly | HACCP team secretary |
| 18. | Checking the registration and settlement mode of complaints, trend analysis conclusions | Monthly | HACCP team leader |
| 19. | Checking team biovigilance | Annual | TACCP team |
| 20. | Checking the fraud vulnerability | Annual | VACCP team |
| Eggs quality parameters | Station A | Station B | Station C | Referince description [Reg. E.U. 589 / 2008] [49] |
|
|---|---|---|---|---|---|
| (a) shell and cuticle: | irregular shape (%) | 0.31 | 0.26 | 0.16 | normal shape, clean and undamaged; |
| dirty (%) | 0.81 | 1.15 | 1.98 | ||
| damaged (%) | 1.49 | 1.53 | 1.27 | ||
| (b) air space: | height > 6 mm (%) | 0.37 | 0.44 | 0.28 | height not exceeding 6mm, stationary; however, for eggs to bemarketed as ‘extra’, it may not exceed 4mm; |
| for extra eggs: height ≤ 4 mm (%) | nd | nd | nd | ||
| (c) yolk: | abnormalities presence at yolk | 0.16 | < 0.1 | < 0.1 | referince values: visible on candling as a shadow only, without clearly discernible outline, slightly mobile upon turning the egg, and returning to a central position; |
| (d) white: | unclear, nontranslucent | < 0.1 | clear, translucent; | ||
| development | nd | nd | nd | imperceptible development; | |
| (f) foreign matter | presence | nd | nd | nd | not permissible |
| (g) foreign smell | presence | nd | presence 2 cases / rejection at reception | nd | not permissible |
| Veterinary drugs | Station A | Station B | Station C | Reference values [Reg. E.U.37 / 2010. max. μg/kg] [33] |
Observations |
|---|---|---|---|---|---|
| X±sx | X±sx | X±sx | |||
| Chlortetracyclin | nd | nd | nd | ≤ 200 | waiting period 6 days |
| Erythromycin | nd | nd | nd | ≤ 150 | waiting period 4 days |
| Neomycin | na | na | na | ≤ 500 | for this type of antibiotic no waiting period is required |
| Oxytetracycline | nd | nd | nd | ≤ 200 | waiting period 4 days |
| Tetracycline | nd | nd | nd | ≤ 200 | waiting period 4 days |
| Tiamulin | na | na | na | ≤ 1000 | for this type of antibiotic no waiting period is required |
| Tylosin | nd | nd | nd | ≤ 200 | waiting period 4 days |
| Parameter | Station A | Station B | Station C | Reference values |
|---|---|---|---|---|
| X±sx | X±sx | X±sx | ||
| RH | 76.8±1.33 | 73.2±2.13 | 79.1±1.02 | 70-80% [4] |
| pH yolk | 6.3±2.14 | 6.2±1.46 | 6.4±1.62 | 6 [4] |
| pH white | 7.8±1.09 | 7.1±2.03 | 7.2±2.59 | 7.6 [4] |
| Temparature | 5.6±2.53 | 5.1±1.36 | 7.2±3.02 | 5 - 18 °C [49] |
| Parameter | Station A | Station B | Station C | Reference values |
|---|---|---|---|---|
| X±sx | X±sx | X±sx | ||
| Sum of dioxins (pg WHO- PCDD/F- TEQ/g) | 1.7±.03 | 1.2±0.21 | 1.6±0.02 | 2,5 pg/g fat [31] |
| Sum of dioxins and dioxin-like PCBs (pg WHO-PCDD/ F-PCB-TEQ/g) | 2.6±0.34 | 3.2±1.29 | 3.4±1.25 | 5,0 pg/g fat [31] |
| Sum of non dioxin-like PCBs (ng/g) | 28±1.32 | 27.1±2.38 | 30.2±2.61 | 40 ng/g fat [31] |
| Sum of PFOS, PFOA, PFNA and PFHxS (Perfluoroalkyl and polyfluoroalkyl substances) | 0.9±0.34 | 0.2±0.21 | 0.3±0.07 | 1.7 [31] |
| Melamine | 1.9±0.05 | 2.1±1.36 | 1.9±1.01 | 2.5 mg/kg [31] |
| Fipronil (sum Fipronil + Fipronil sulfone) | < LOD | <LOD | <LOD | LOD = 0.005 mg/kg [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





