1. Introduction
Obesity has been on the rise worldwide, especially in recent decades, and the rate of obesity has continued to increase at an alarming rate [
1]. Furthermore, the global increase in obesity is associated with an increased incidence of significant health risk factors and conditions, including insulin resistance, type 2 diabetes, non-alcoholic fatty liver disease, atherosclerosis, and specific cancers [
2]. Obesity is influenced by the processes of lipogenesis and lipolysis [
3,
4].
Specifically, middle age is the period when immunity begins to decrease, and the effects of unhealthy weight and other bad health habits accumulate [
5]. Obesity in middle age is more sensitive to arteriosclerosis than obesity in youth [
6]. In particular, in the case of middle-aged women who have reached menopause, abdominal and hepatic fat accumulation, and waist circumference increase due to changes in hormonal and metabolic profiles. In addition, middle-aged women with these metabolic disturbances have an increased risk of cardiovascular disease and acute coronary artery disease due to excessive production of very low-density lipoprotein (VLDL) and even have a poorer prognosis than men [
7]. However, numerous studies have focused on short-term dietary for several weeks, but not many papers have examined the effects until middle age.
Autumn olive (
Elaeagnus umbellata Thunb.) is one of the wild spinies branched shrubs and a plant in the family Elaeagnaceae that is native to Asia [
8,
9]. Autumn olive berries (AOB) mature between September and November to an edible dark red color and are characterized by sweet, sour, and juicy [
8,
10]. AOB is known to contain a lot of catechin, lutein, phytoene, phytofluene, β-cryptoxanthin, β-carotene, and α-cryptoxanthin [
11,
12]. AOB has effects on obesity, type 2 diabetes, and oxidative stress [
13,
14].
In this experiment, we aim to improve obesity using this approach. First, we investigated the effect of autumn olive berries on lipid accumulation in nematodes fed a high-glucose diet by middle age. Second, we evaluated the effect of autumn olive berries on menopausal symptoms (reduced behavior, decreased fertility, ROS accumulation) in middle age. Last, we studied whether the autumn olive berries effect is affected by the intervention of lipid metabolism (lipogenesis and lipolysis) related genetic factors.
2. Results
2.1. Confirmation of the antioxidant activity and polyphenol contents of FAOB and SAOB
The total phenol contents (TPC) of freeze-dried AOB (FAOB) and spray-dried AOB (SAOB) were 0.258±0.01 mg GAE/100g and 0.063±0.02 mg GAE/100g, respectively (
Table 1). The DPPH scavenging capacity of FAOB and SAOB were 3842.23±294.91 mg AA/100g and 3151.78±395.08 mg AA/100g, respectively (
Table 1).
Table 1.
TPC and DPPH values of freeze-dried AOB (FAOB) and spray-dried AOB (SAOB).
Table 1.
TPC and DPPH values of freeze-dried AOB (FAOB) and spray-dried AOB (SAOB).
| Samples |
TPC (mg GAE/100 g) |
DPPH (mg AA/100 g) |
| FAOB |
0.26 0.01 |
3842.23 294.91 |
| SAOB |
0.06 0.02 |
3151.78 395.08 |
|
p-value* |
0.05 |
0.03 |
2.2. Catechin identification
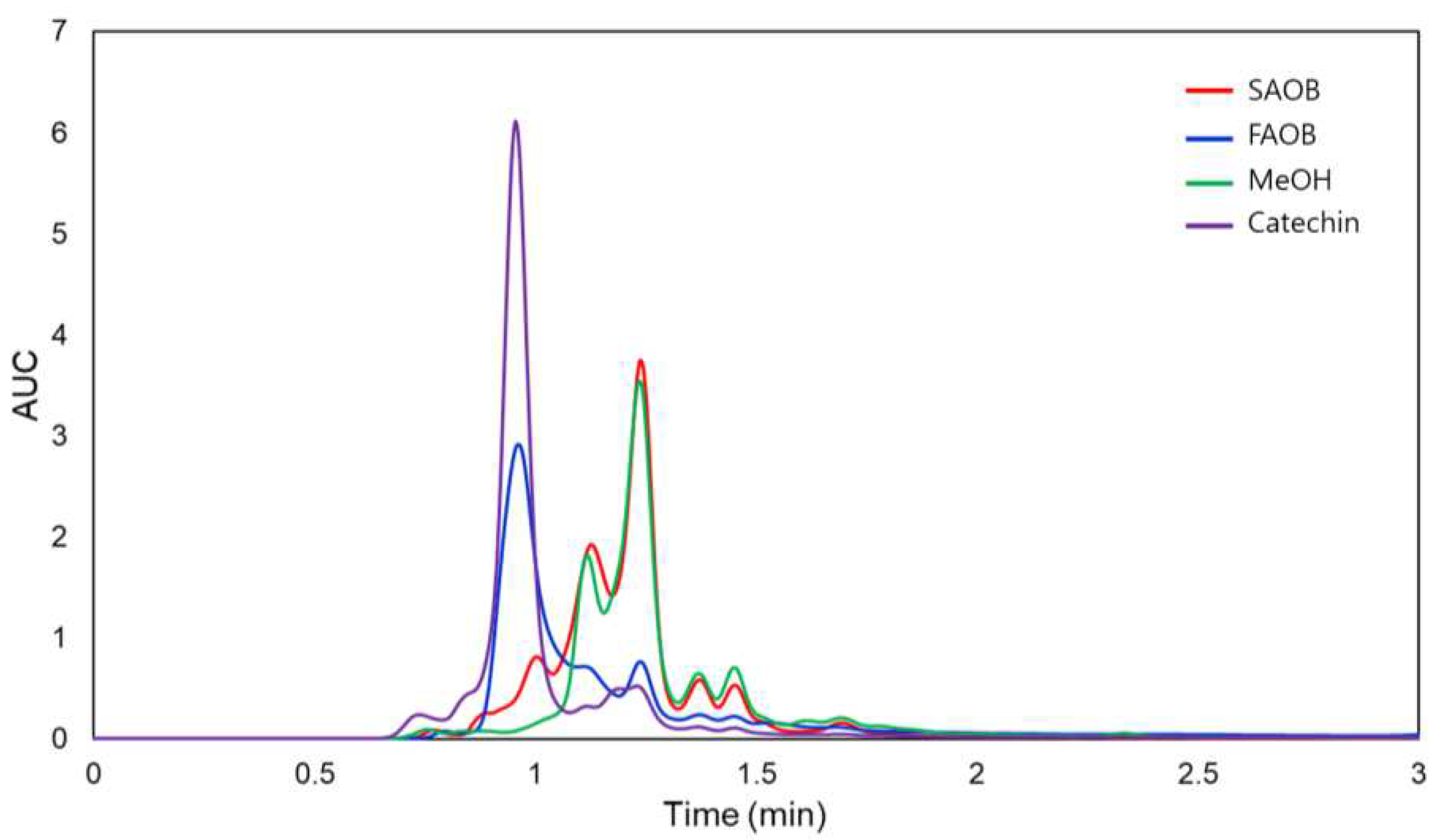
HPLC chromatograms of FAOB and SAOB are shown in
Figure 1. The results show that catechin was the key compound of FAOB. Interestingly, the catechin peak appeared high in FAOB and small in SAOB. SAOB almost coincided with the chromatogram of methanol used as the mobile phase. Quantification of catechin in FAOB and SAOB showed that they contained 10.93±0.93 and 0.14±0.01 μg/g, respectively (
Table 2). There were statistically significant differences in the catechin content of FAOB and SAOB (p-value=0.002), suggesting that differences in the physiological activities of FAOB and SAOB might be attributed to the catechin content. Based on the previously indicated polyphenol and catechin contents and antioxidant capacity results, we considered that FAOB has more potential and thus decided to use FAOB for further experiments.
2.3. Safety of AOB in C. elegans
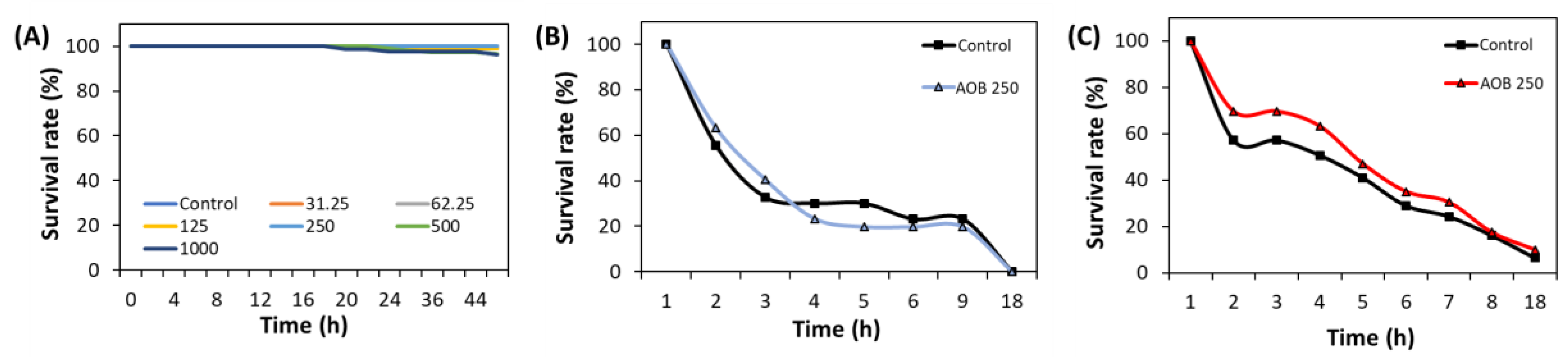
To determine the safe concentrations of AOB, the acute toxicity test was conducted with different concentrations (31.25-1000 μg/mL) of AOB. As a result, any toxicity was not observed at the concentration range from 31.25 μg/mL to 250 μg/mL. However, worm survival decreased in the concentration range of 500-1000 μg/mL of AOB. Consequently, safe concentrations (62.5, 125, and 250 μg/mL) of AOB were used in subsequent experiments (
Figure 2A). Also, FAOB did not affect the survival of nematodes even under oxidative and heat stress conditions (
Figure 2B and 2C).
2.4. AOB inhibits lipid accumulation in C. elegans
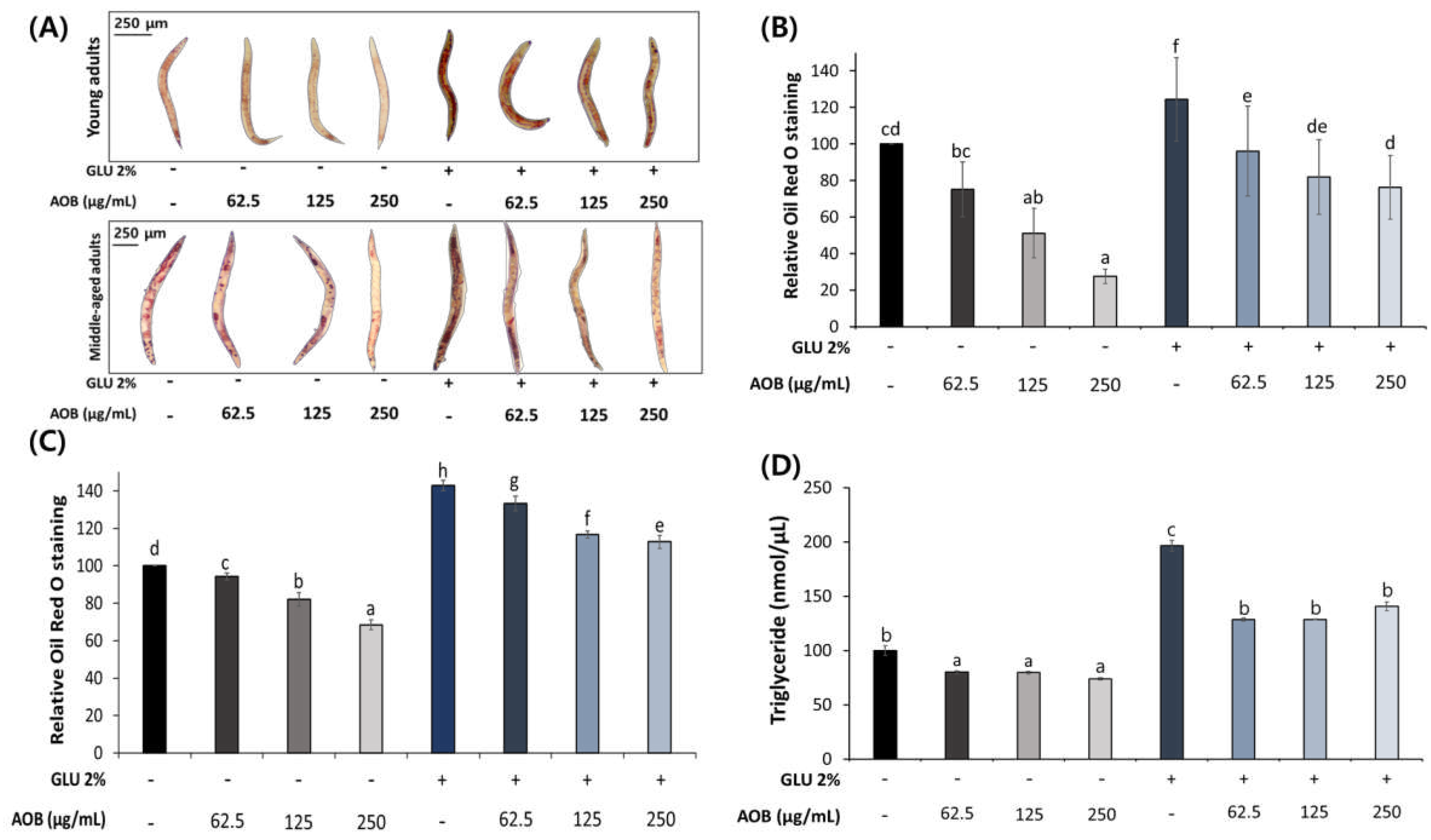
We examined the effect of AOB on fat accumulation in young and middle-aged adult worms. AOB showed a concentration-dependent decrease in total fat accumulation compared to the control under normal and 2% GLU diet conditions in young adult worms (
Figure 3A).
The inhibitory effect of AOB on total fat accumulation was also confirmed in middle-aged worms (
Figure 3B). Also, the TG content in the AOB-treated group was reduced compared to the control under normal and 2% GLU diet conditions (
Figure 3C).
2.5. AOB reduces ROS accumulation in middle-aged C. elegans
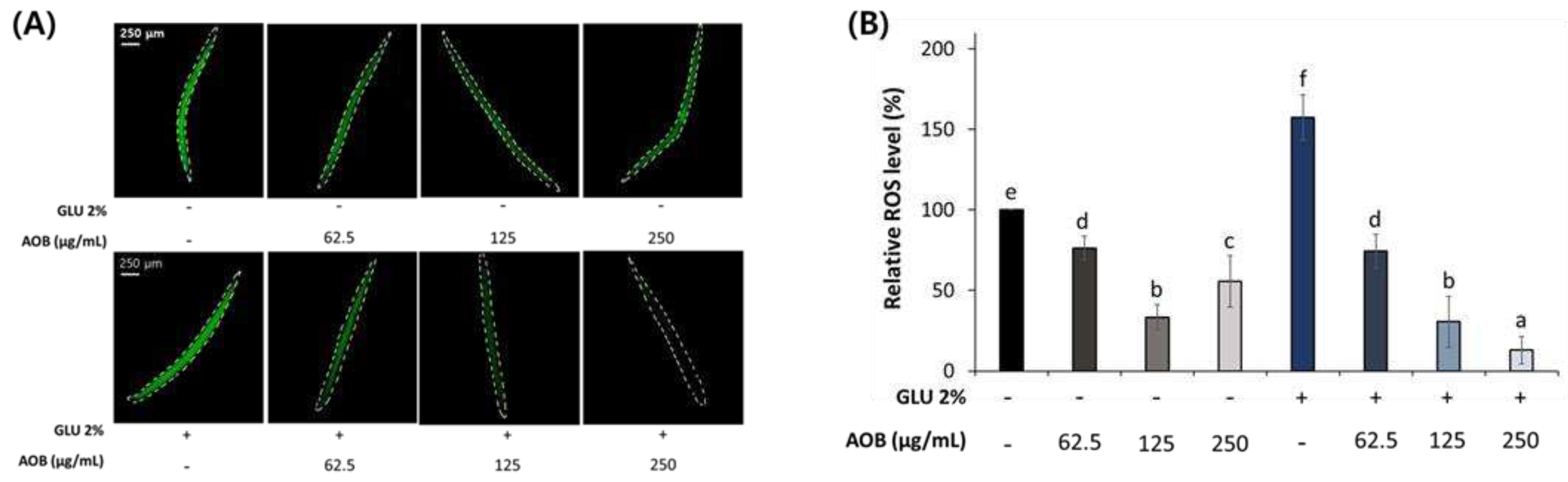
ROS is a metabolite that causes aging, and it has been reported in previous studies that a high-GLU diet causes ROS accumulation [
15]. AOB exhibited a reduction effect on ROS production in both young and middle-aged worms (
Figure 4).
2.6. AOB exhibits an aging-delaying effect in C. elegans
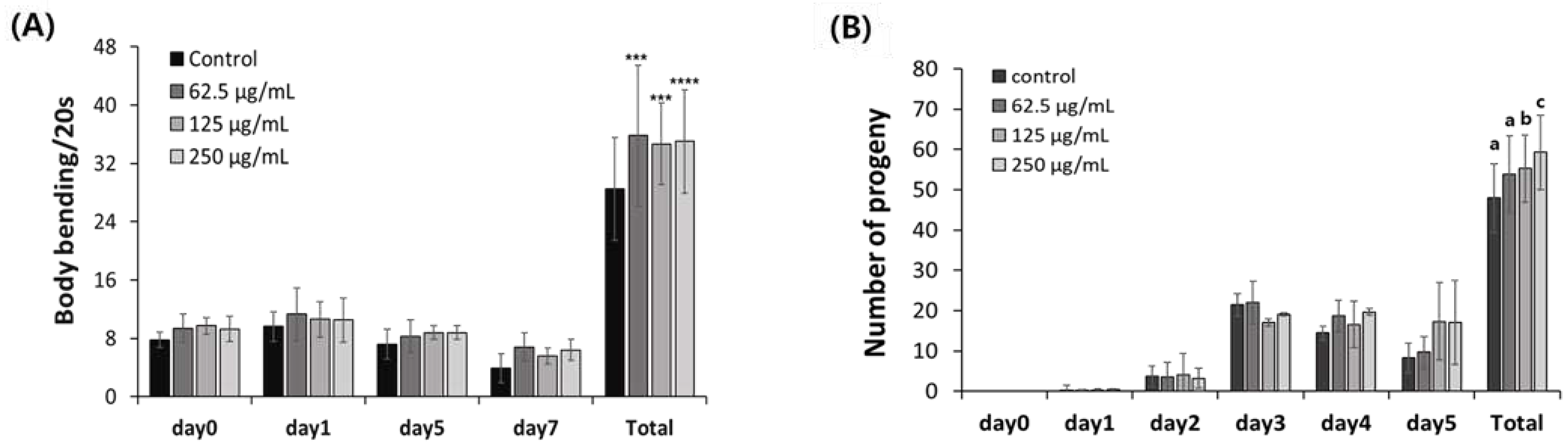
In aging studies using nematodes, behavioral changes, and offspring production ability are identified as biomarkers [
16]. In our results, AOB increased behavior and fertility that were reduced by aging (
Figure 5).
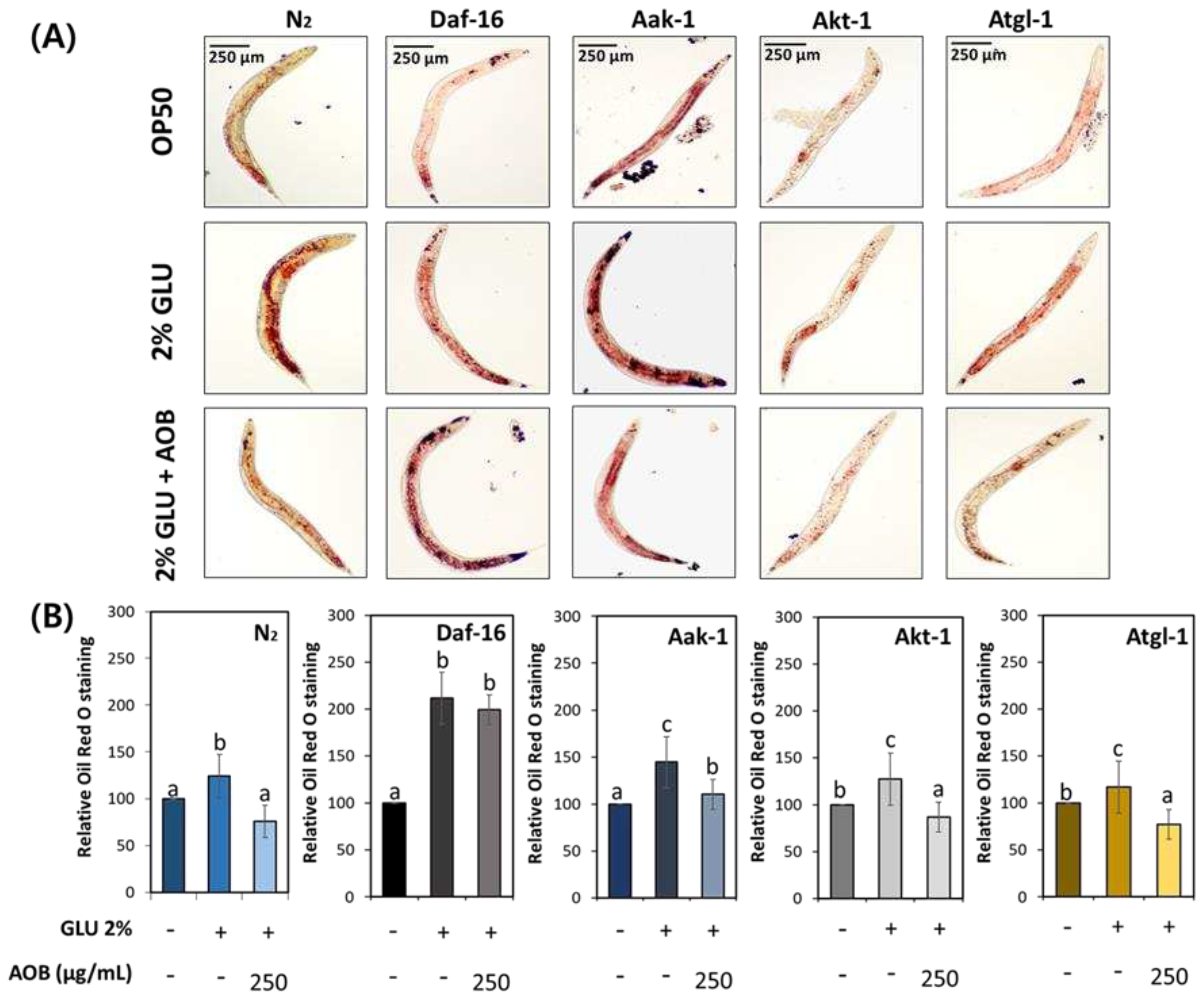
2.7. AOB regulates lipid accumulation by daf-16 under 2% GLU-diet condition
To investigate whether AOB regulates lipid metabolism in middle-aged worms under GLU-feeding conditions, the total fat contents were analyzed using N2, daf-16, aak-1, akt-1, and atgl-1 knockdown worms. Although body fat reduction was found in the AOB-treated group in wild-type N2 worms, such an effect of AOB was not seen in daf-16 worms (
Figure 6). Other worm strains had statistically reduced body fat by AOB treatment, similar to N2. This means that the inhibitory effect of AOB on body fat accumulation by GLU diet depends on daf-16.
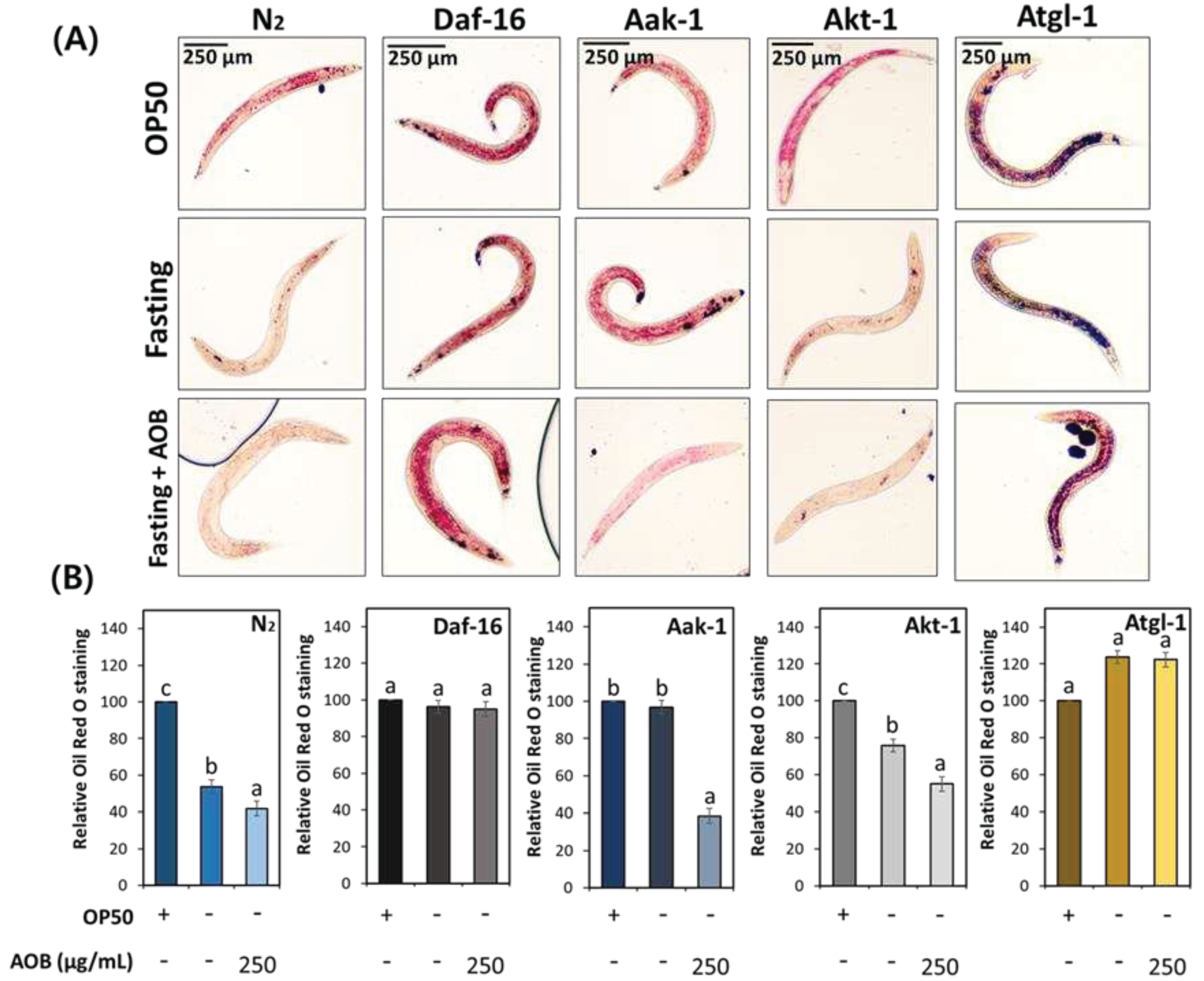
2.8. AOB is involved lipolysis signaling pathway under fasting condition
To investigate whether AOB involved lipolysis in middle-aged worms during fasting, the total fat contents were analyzed using N2, daf-16, aak-1, akt-1, and atgl-1 knockdown worms. Body fat reduction was found in the AOB treatment group compared to the control in the wild-type N2 worm under fasting condition (
Figure 7). This effect of AOB has not been seen in daf-16 and atgl-1 worms (
Figure 7). In other worm strains, body fat was statistically reduced by AOB treatment similar to N2. Daf-16 and atgl-1 are factors involved in lipolysis signaling, and our results indicate that AOB depends on daf-16 and atgl-1 to intervene in lipolysis.
3. Discussion
Aging and lipid metabolism disorders show close associations [
17]. In particular, increased lipotoxicity under specific conditions, such as aging, may contribute to a variety of age-related diseases, including cardiovascular disease, cancer, arthritis, type 2 diabetes, and Alzheimer's disease [
18,
19,
20,
21,
22]. Recent studies have described the mechanisms of changes in lipolysis during aging [
23,
24]. However, although they can explain system-level changes in lipid metabolism, how these changes influence aging and aging-related diseases has not been verified [
17]. Our results showed that during obesity induction with a high-GLU diet, middle-aged and young worms accumulated 20% and 40% lipid, respectively (
Figure 3). It has been verified that more lipids accumulate with age when given the same high-GLU diet over the same period.
There is an opinion that obesity, especially in middle age, can be caused not only by changes in the metabolic system, but also by a decrease in physical activity [
25]. In the present study, day 7 worms were observed to have a decline by more than 50% in physical activity compared to day 1 worms (
Figure 5A). This result is consistent with the view that there is a decline in behavior in middle age that may lead to obesity. However, further research is required as we have not confirmed whether the metabolic system at the molecular level has changed.
Lipid metabolism is regulated by lipid synthesis (lipogenesis) and lipid degradation (lipolysis and beta-oxidation) [
3,
4]. These processes are intertwined at the molecular level and require a logical understanding to gain insight. Crucial in lipid metabolism is the working of daf-16/FOXO and atgl-1/ATGL [
26,
27,
28]. In particular, FOXO is a key transcription factor in lipid metabolism that controls the balance of lipogenesis and lipolysis by downregulating ATGL and is involved in lipid accumulation in mammals [
28]. However, there are only a few reports on the interaction between daf-16/FOXO and atgl-1/ATGL in
C. elegans.
We prepared worms under high-GLU diet and fasting conditions to clearly define the role of daf-16 and atgl-1 concerning the AOB effect. As a result of AOB administration, it was confirmed that the effect seen in wild-type disappeared in daf-16 knockdown worms under both GLU feeding and fasting conditions (
Figure 6 and
Figure 7). This indicates that AOB is dependent on daf-16 related to lipogenesis and lipolysis. In addition, the effect of AOB seen in N2 was lost in atgl-1 knockdown worms under fasting conditions, indicating that AOB cooperates with atgl-1 under certain lipolytic conditions such as fasting. Previous studies have shown that daf-16 and atgl-1 can be regulated by akt-1/AKT and aak-1/AMPK [
29,
30,
31,
32]. However, our results confirmed that the AOB effect is dependent on daf-16 and atgl-1 but is not related to akt-1 and aak-1 (
Figure 6 and
Figure 7). In other words, this suggests that AOB acts directly on daf-16 or atgl-1 rather than daf-16 and atgl-1 being involved by regulating the upstream factors akt-1 or aak-1. Although daf-16 is upstream of atgl-1, further gene expression analysis is needed to clarify their upper- and lower-relationship for the effects of AOB.
AOB is a red fruit that is reported to contain high levels of bioactive compounds such as carotenoids, organic acids, cinnamic acids, benzoic acids, flavonols, anthocyanins, tannins, and catechins [
11,
12,
33]. We focused on phenolic compounds among the bioactive substances of AOB, and as a result of HPLC analysis, catechin was detected as the main substance of AOB (
Figure 1,
Table 2). Catechins have been widely recognized as the main functional components in tea and wine including catechin, epicatechin, epigallocatechin (EGC), epicatechin-3-gallate (ECG), epigallocatechin-3-gallate (EGCG) and gallocatechin gallate (GCG) [
34]. Catechins exhibit anti-cancer, anti-obesity, anti-diabetic, anti-cardiovascular, anti-infection, liver-protective, and nerve-protective properties [
35]. In particular, catechin has antioxidant activity that scavenges ROS and at the same time can positively regulate lipid metabolism [
35,
36]
. Therefore, it is believed that AOB containing catechin has the potential to effectively prevent obesity and related chronic degenerative diseases.
4. Materials and Methods
4.1. Preparation of AOB extracts
AOB samples used in the present study were provided by NutriAdvisor (Seong-Nam, Gyeonggi-do, Korea) and used. AOB was cultivated in Hapcheon, Gyeongnam, Korea. The AOB was freeze- or spray-dried and then extracted with distilled water (1:10 w/v) under reflux at 80℃. After that, the extracts were evaporated to remove the solvent, and made into powder. The powder samples were stored at -80℃, and then diluted fresh to an appropriate concentration right before the experiment.
4.2. Reagents
All reagents used in our studies were HPLC or molecular biology grade. Reagents, unless otherwise specified, were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
4.3. HPLC Analysis
To profile phenolic compounds in AOB, Information on equipment, column, and analysis methods required for HPLC operation was presented in
Table 1. The catechin used was D-catechin (CAS no. 154-23-4), which was dissolved in distilled water immediately before analysis.
Table 1.
HPLC analysis condition.
Table 1.
HPLC analysis condition.
| HPLC (Agilent 1100) |
|---|
| Column |
ZORBAX Eclipse Plus C18 (4.6x100mm, 3.5 µm) |
| Mobile phase |
Isocratic, methanol (Duksan, Ansan-si, Gyeonggi-do, Korea) |
| Flow rate |
mL/min |
| Inject volume |
20 μL |
| Detector |
Diode array detector (DAD) |
| Wavelength |
250 nm |
4.4. Total Phenol Contents (TPC) of AOB
The total phenolic contents (TPC) in the AOB were determined using the Folin-Ciocalteu (FC) method. Simply, 700 μL of the sample (mixed with FC in a 1:1 ratio) was combined with 700 μL of sodium carbonate and left in the dark for 1 hour. The absorbance reading was performed at 720 nm. The total phenolic contents of the sample were calculated using the equation derived from the gallic acid standard curve. The results were expressed as gallic acid equivalents (GAE) per gram.
4.5. DPPH radical scavenging capacity
In a simple procedure, a sample of the same volume was mixed with a DPPH solution (0.2 mM) and left at room temperature for 30 m. The reaction mixture was then measured at 517 nm using a microplate reader (SYNERGY HTX, Biotek, CA, USA).
4.6. Worm study
4.6.1. Worm culture
To conduct in vivo experiments, we use C. elegans strain N2 (wild-type) and its derivative mutant strains; daf-16 (tm5030), atgl-1 (tm12352), and aak-1 (tm1944) and akt-1 (tm399) were obtained from the National BioResource Project (NBRP) of Japan.
All these strains were maintained on nematode growth medium (NGM) plates that were spread with E. coli OP50 and maintained at a temperature of 20°C throughout the entire duration of the experiment. Age synchronization of nematodes was achieved by separating the eggs from gravid adults using a solution comprising 6% sodium hypochlorite (Yuhanclorox, Seoul, Korea) and 5 M NaOH.
4.6.2. Acute toxicity
Synchronized L4 was washed twice with M9 buffer and suspended in M9 buffer containing cholesterol. Subsequently, 1 milliliter of this suspension was transferred to each well of a 24-well plate (20-30 worms per well) and combined with 10 μL of various concentrations of FAOB and SAOB. The plates were then incubated at 20°C for 24 hours. The results of acute toxicity were quantified as percent survival after counting alive worms.
4.6.3. Determination of stress resistance
In the context of thermal and oxidative stress analysis, 50 synchronized N2 L1 larvae were prepared as follows: OP50 and AOB were combined, and the mixture was seeded on NGM plates. The worm plate was first maintained at 20 °C for 60 hours to assess the impact of thermal stress. Subsequently, it was incubated at 35 °C for 18 hours. To investigate the effect on oxidative stress, pretreated worms were transferred to a 24-well plate containing a 100 μM juglone (5-hydroxy-1,4-naphthoquinone) solution and incubated at 20°C.
4.6.4. Oil red O staining
The oil red O assay was modified by Cho et al [
16]. L1 worms were exposed to different concentrations of extracts for 7 days and then incubated with 100 μM H2DCF-DA in the dark for 3 h. Subsequently, the nematodes were fixed on microscope slides using NAN3 (2%). These slides were observed using a Nikon ECLIPS Ci fluorescence microscope (Nikon, Seoul, Korea). The fluorescence intensities were examined by Image J software. Ten worms per group were chosen for quantification.
4.6.5. Triglyceride quantification assay
The adult worms that were in culture were suspended in 500 μL of M9 buffer containing a 0.05% Tween-20 solution. They were then homogenized on ice for 5 minutes using a glass homogenizer (ALLSHENG, regional, national) to collect the pellet, which was subsequently centrifuged at 1000 x g for 5 minutes. The obtained supernatant was analyzed for TG content. TG content was measured by absorbance at 570 nm using a TG kit (Biomax, Gyeonggi-do, Korea).
4.6.6. Reproduction and pumping rates
To count the number of progenies, worms were transferred to fresh NGM plates daily throughout the reproductive period, and eggs were left on plates to hatch. The offspring of each worm were counted when they reached the L2 or L3 stage. The test was performed three times.
To evaluate behavior activity, we counted the number of pharyngeal pumps on 0, 1, 5, and 7 days. Specifically, 10 nematodes were randomly selected for each concentration and age point and the pumping frequency was determined three times for 20 sec. This test was conducted three times.
4.6.7. Determination of ROS level
L1 worms were maintained in different concentrations of extracts for 7 days and then incubated with 100 μM H2DCF-DA in the dark for 3 hours. Subsequently, the nematodes were fixed on microscope slides using NAN3 (2%). These slides were observed using a Nikon ECLIPS Ci fluorescence microscope. The fluorescence intensities were examined by Image J software. An average of 10 worms per group was chosen for quantification.
4.7. Statistical analysis
Results are presented as the means ± standard deviations (SD) of three independent replicates. The significance of intergroup differences was determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test. SPSS 27.0 was used for all statistical analyses except lifespan.
P-values<0.05 were considered to be significant. The survival results were analyzed with the Kaplan-Meier method using the OASIS application (
https://sbi.postech.ac.kr/oasis/).
P-values of survival differences were determined with the log-rank test. Statistical analyses were performed by Student’s t test (unpaired, two tailed) with at least three replicates, unless otherwise indicated. Statistical analyses were performed in SPSS 27.0.
5. Conclusions
This study investigated the relationship between obesity and aging in midlife and examined the positive effects of AOB. The bio-activity of plants can vary depending on the drying method [
37], and FAOB showed more effective radical scavenging ability compared to SAOB, which is believed to be because FAOB contained more polyphenols, including catechin. In the
C. elegans study, treatment with AOB significantly reduced lipid accumulation in both the young and the middle-aged groups. In addition, AOB inhibited ROS accumulation in middle-aged worms under 2% GLU condition. Interestingly, AOB attenuated aging symptoms, including increasing the movement and reproductive capacity of aged worms. To understand the inhibitory effect of AOB on lipid accumulation at the molecular level, we performed further investigations on the daf-16, aak-1, akt-1, and atgl-1 genes, which are related to lipid metabolism. The lipid accumulation reduction effect of AOB was nullified under high-GLU condition in the daf-16 knockdown mutant. The AOB effect under fasting conditions was also nullified in the daf-16 and atgl-1 knockdown mutants. This suggests that these two factors are involved in the in vivo anti-obesity activity of AOB in middle-aged worms, and further suggests their potential for anti-aging benefits. In summary, AOB acts on lipogenesis and lipolysis by regulating daf-16 and atgl-1 in middle-aged worms and alleviates aging symptoms. These results suggest that AOB is a valuable material with the potential to delay aging by modulating lipid metabolism.
Author Contributions
Conceptualization, M.J.; methodology, Y.K. and S.N.; software, Y.K. and S.N.; validation, Y.K.; formal analysis, J.L.; writing—original draft preparation, Y.K.; writing—review and editing, M.J.; visualization, Y.K.; supervision, M.J.; project administration, M.J.; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by project for Collabo R&D between Industry, Academy, and Research Institute funded Korea Ministry of SMEs and Startups in 2023 (Project No. G21002258981).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, I.J.; Yin, L.; Jensen-Urstad, A.P.; Funai, K.; Coleman, T.; Baird, J.H.; El Ramahi, M.K.; Razani, B.; Song, H.; Fu-Hsu, F. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metab. 2012, 16, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Catecholamine-induced lipolysis in obesity. Int. J. Obes. 1999, 23, S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Ory, M.G.; Anderson, L.A.; Friedman, D.B.; Pulczinski, J.C.; Eugene, N.; Satariano, W.A. Cancer prevention among adults aged 45–64 years: Setting the stage. Am. J. Prev. Med. 2014, 46, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Arvandi, M.; Pasha, E.; Haley, A.; Stanforth, P.; Tanaka, H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 495–502. [Google Scholar] [CrossRef]
- Hodson, L.; Banerjee, R.; Rial, B.; Arlt, W.; Adiels, M.; Boren, J.; Marinou, K.; Fisher, C.; Mostad, I.L.; Stratton, I.M. Menopausal status and abdominal obesity are significant determinants of hepatic lipid metabolism in women. J. Am. Heart Assoc. 2015, 4, e002258. [Google Scholar] [CrossRef]
- Pei, R.; Yu, M.; Bruno, R.; Bolling, B.W. Phenolic and tocopherol content of autumn olive (Elaeagnus umbellate) berries. J. Funct. Foods 2015, 16, 305–314. [Google Scholar] [CrossRef]
- Ahmad, S.D.; Sabir, M.S.; Juma, M.; Asad, H.S. Morphological and biochemical variations in Elaeagnus umbellata Thunb. from mountains of Pakistan. Acta Bot. Croat. 2005, 64, 121–128. [Google Scholar]
- Ishaq, S.; Rathore, H.A.; Sabir, S.M.; Maroof, M.S. Antioxidant properties of Elaeagnus umbellata berry solvent extracts against lipid peroxidation in mice brain and liver tissues. Food Sci. Biotechnol. 2015, 24, 673–679. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Hassan, A.M.; Koca, I. Optimization of drying temperature for the assessment of functional and physical characteristics of autumn olive berries. J. Food Process. Preserv. 2021, 45, e15658. [Google Scholar] [CrossRef]
- Fordham, I.M.; Clevidence, B.A.; Wiley, E.R.; Zimmerman, R.H. Fruit of autumn olive: A rich source of lycopene. HortScience 2001, 36, 1136–1137. [Google Scholar] [CrossRef]
- Nazir, N.; Zahoor, M.; Nisar, M.; Khan, I.; Karim, N.; Abdel-Halim, H.; Ali, A. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: Pharmacological and computational approach. BMC Complement. Altern. Med. 2018, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Baek, H.-J.; Han, D.-W.; Yun, J.-A. Autumn olive (Elaeagnus umbellata Thunb.) berry reduces fasting and postprandial glucose levels in mice. Nutr. Res. Pract. 2019, 13, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.-b.; Cho, M.; Choe, S.; Jang, M. Indian almond (Terminalia catappa linn.) leaf extract extends lifespan by improving lipid metabolism and antioxidant activity dependent on AMPK signaling pathway in Caenorhabditis elegans under high-glucose-diet conditions. Antioxidants 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Kim, Y.; You, S.; Hwang, D.Y.; Jang, M. Chlorogenic acid of Cirsium japonicum resists oxidative stress caused by aging and prolongs healthspan via SKN-1/Nrf2 and DAF-16/FOXO in Caenorhabditis elegans. Metabolites 2023, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W. Advances in understanding of the role of lipid metabolism in aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Morishita, R. The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front. Aging Neurosci. 2015, 7, 199. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, C.; Tan, Z.; Li, N.; Tang, M.; Yang, L.; Cao, Y. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McGrath, C.M.; Young, S.P. Lipid and metabolic changes in rheumatoid arthritis. Curr. Rheumatol. Rep. 2015, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mennes, E.; Dungan, C.M.; Frendo-Cumbo, S.; Williamson, D.L.; Wright, D.C. Aging-associated reductions in lipolytic and mitochondrial proteins in mouse adipose tissue are not rescued by metformin treatment. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2014, 69, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.D.; Sander, J.; Spadaro, O.; Lee, A.; Nguyen, K.Y.; Wing, A.; Goldberg, E.L.; Youm, Y.-H.; Brown, C.W.; Elsworth, J. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 2017, 550, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Szlejf, C.; Parra-Rodríguez, L.; Rosas-Carrasco, O. Osteosarcopenic obesity: Prevalence and relation with frailty and physical performance in middle-aged and older women. J. Am. Med. Dir. Assoc. 2017, 18, 733–e731. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.; Van Den Heuvel, A.; Birnbaum, M. The role of FoxO in the regulation of metabolism. Oncogene 2008, 27, 2320–2336. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Xie, H.; Schweiger, M. Of mice and men: The physiological role of adipose triglyceride lipase (ATGL). Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 880–899. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Kandror, K.V. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J. Biol. Chem. 2009, 284, 13296–13300. [Google Scholar] [CrossRef]
- Han, Y.; Hu, Z.; Cui, A.; Liu, Z.; Ma, F.; Xue, Y.; Liu, Y.; Zhang, F.; Zhao, Z.; Yu, Y. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat. Commun. 2019, 10, 623. [Google Scholar] [CrossRef]
- Jang, M.; Choi, S.I. Schisandrin C isolated from Schisandra chinensis fruits inhibits lipid accumulation by regulating adipogenesis and lipolysis through AMPK signaling in 3T3-L1 adipocytes. J. Food Biochem. 2022, 46, e14454. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kershaw, J.; Chen, C.-Y.; Komanetsky, S.M.; Zhu, Y.; Guo, X.; Myer, P.R.; Applegate, B.; Kim, K.-H. Piceatannol antagonizes lipolysis by promoting autophagy-lysosome-dependent degradation of lipolytic protein clusters in adipocytes. J. Nutr. Biochem. 2022, 105, 108998. [Google Scholar] [CrossRef]
- Lee, J.H.; Kong, J.; Jang, J.Y.; Han, J.S.; Ji, Y.; Lee, J.; Kim, J.B. Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans. Mol. Cell Biol. 2014, 34, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G.; Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Randriamampionona, D.; Beccaro, G.L. Phytochemical characterization and bioactivity evaluation of autumn olive (Elaeagnus umbellata Thunb.) pseudodrupes as potential sources of health-promoting compounds. Appl. Sci. 2020, 10, 4354. [Google Scholar] [CrossRef]
- Cyboran, S.; Strugała, P.; Włoch, A.; Oszmiański, J.; Kleszczyńska, y. Concentrated green tea supplement: Biological activity and molecular mechanisms. Life Sci. 2015, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Isemura, M. Catechin in human health and disease. MDPI: 2019; Vol. 24, p 528.
- Jiang, Y.; Ding, S.; Li, F.; Zhang, C.; Sun-Waterhouse, D.; Chen, Y.; Li, D. Effects of (+)-catechin on the differentiation and lipid metabolism of 3T3-L1 adipocytes. J. Funct. Foods 2019, 62, 103558. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; da Silva Oliveira, E.M.; de Brito, E.S.; Rodrigues, S.; Narain, N. Effect of freeze-and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp. J. Food Sci. Technol. 2018, 55, 2095–2102. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).