Preprint
Article
Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanics Patients Treated with Clopidogrel: Abundance and Functionality
Altmetrics
Downloads
196
Views
90
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.docx (24.63KB )
This version is not peer-reviewed
Submitted:
28 August 2024
Posted:
30 August 2024
You are already at the latest version
Alerts
Abstract
Clopidogrel; a prescription drug to reduce ischemic events in cardiovascular patients; has been extensively studied in mostly European individuals but not among Caribbean Hispanics. This study evaluated the low abundance and reduced activity of paraoxonase-1 (PON1) in clopidogrel-resistant patients as a predictive risk biomarker of poor responders and disease severity in this population. Thirty-six patients on clopidogrel (cases; divided into poor and normal responders) were enrolled along with 11 cardiovascular patients with no clopidogrel indications (positive control) and 13 healthy volunteers (negative control). Residual on-treatment platelet function (PRU); PON1 abundance by western blotting and PON1 activity by enzymatic assays were measured. PON1 genotyping and computational haplotype phasing were performed on 512 DNA specimens for two genetic loci (rs662 and rs854560). No statistical differences in mean relative PON1 abundance were found among the groups (p> 0.05). However; a significantly lower enzymatic activity was found in poor responders (10.57 ± 6.79 µU/mL) when compared to controls (22.66 ± 8.30 µU/mL and 22.21 ± 9.66 µU/mL; p= 0.004). PON1 activity among carriers of the most prevalent PON1 haplotype (AA|AA) was significantly lower than in wild-types (7.90 µU/mL vs 22.03 µU/mL; p= 0.005). Our findings suggested that PON1 is a potential biomarker of cardiovascular disease severity in Caribbean Hispanics.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
Caribbean Hispanics are underrepresented in pharmacogenomics studies, exacerbating health disparities in this admixed minority population. Clopidogrel is a commonly prescribed antiplatelet drug to prevent and reduce the incidence rate of acute ST-segment elevation myocardial infarction (STEMI) and non-ST segment elevation myocardial infarction (NSTEMI), unstable angina, stroke, and established peripheral artery disease (PAD), alone or in combination with aspirin as a daily dual antiplatelet therapy (DAPT) [1]. Although clinical studies have shown that clopidogrel can effectively reduce the risk of an ischemic event, significant inter-individual variability in response to treatment has been reported [2,3]. This variability results from multiple factors such as demographics, genetic variations, drug-drug interaction, patient’s compliance to therapy, and/or comorbidities [4]. Clopidogrel response variability has become increasingly important as it can be partially associated with poor clinical outcomes [5,6]. Patients who are resistant to clopidogrel (~33%) are labeled as subjects with a high on-treatment platelet reactivity (HTPR) [7]. If the measured platelet reactivity unit (PRU) is above a threshold (e.g., 230), then the patient is at increased risk of major adverse cardiac and cerebrovascular events (MACCEs) occurrence, including an ischemic event.
PON1 is a calcium-dependent enzyme that is associated with high-density lipoprotein (HDL) in circulation to protect against oxidized lipids accumulation and prevents cardiovascular diseases (CVD). Besides its capability of detoxifying organophosphate compounds, it can also have an antioxidant effect as it can prevent low-density lipoprotein (LDL) oxidation that contributes to the initiation and progression of atherosclerosis [8,9]. Consequently, PON1 has been described as a risk factor for developing coronary artery disease (CAD) [10]. Previous studies have shown that PON1 p.Q192R polymorphism (rs662) is associated with enhanced enzymatic function while L55M (rs854560) polymorphism is associated with a lower serum concentration affecting its activity [9,11]. However, literature findings are conflicting as p.Q192R has also been implicated in decreased enzymatic activity [12]. In addition, some studies favor the idea that PON1 activity is associated with individual’s genotypes, but others reject this argument and postulate that PON1 activity is independent of genotypes [12,13]. The disagreement among findings can be explained by the heterogeneity of the population. Allele frequencies and their effect sizes, as well as haplotype blocks and linkage disequilibrium (LD) patterns, vary across different ancestries and, therefore, such allelic heterogeneity can affect the enzyme catalytic capability among different populations worldwide [14,15]. Furthermore, PON1 has previously been postulated as a key factor for the bioactivation and clinical activity of clopidogrel [16].
In this study we aimed to validate the low abundance and ascertain enzymatic activity of PON1 protein in poor responders to clopidogrel, using western blot analysis and enzyme activity assays in Caribbean Hispanic patients. Also, we tested the hypothesis that participants having the PON1 p.Q192R but not the p.L55M polymorphisms will have a higher enzymatic capability and a higher concentration of PON1 in plasma, leading to a better response. Our experimental design sought to determine the PON1 activity and genotypes of clopidogrel-treated patients and compare them with positive and negative controls.
2. Materials and Methods
2.1. Study Population
Plasma specimens from thirty-six cardiovascular patients on clopidogrel (75 mg/day for at least 6 months), alone or as part of DAPT for acute coronary syndrome (ACS), stable coronary artery disease (CAD) or peripheral artery disease (PAD), were used for the experimental procedures described in the study. These patients were randomly selected from a larger study cohort of 512 participants in a clinical protocol (Clinical Trial Registration Unique Identifier: NCT03419325). Furthermore, additional plasma samples from thirteen healthy volunteers and eleven cardiovascular patients without an indication for clopidogrel were also used as negative and positive controls, respectively. All participants were originally recruited between January 2018 and July 2020 at two medical facilities in the Commonwealth of Puerto Rico (i.e., University hospital at Carolina, Cardiovascular Center of Puerto Rico and the Caribbean). Patients who met the inclusion/exclusion criteria (Table 1) and signed an IRB-approved written broad informed consent for future studies (protocol number A4070417) were enrolled. Demographical and clinical data were collected from medical records and/or during interviews, along with blood samples in two 3.0 mL 3.2% sodium citrate tubes.
2.2. Sample Processing
The collected blood samples from participants were used to measure PRU in plasma using the VerifyNow®® ex-vivo P2Y12 platelet function assay. The individual results of this test are used to identify poor (PRU ≥230) or normal (PRU< 230) responders to clopidogrel. For DNA isolation, 200 µL of blood were placed in a 2 mL Eppendorf tube, and samples were processed in the QIAcube following the QIAamp DNA Blood Mini Kit Protocol from QIAGEN. DNA quantification was performed using the NanoDrop 2000 Spectrophotometer and samples were stored at -20°C. To obtain plasma samples, blood was centrifuged at 3,000 rpm for 10 minutes and stored at -80°C.
2.3. PON1 Function Assay
Paraoxonase 1 Activity Assay Kit protocol (ab241044) was used to determine the enzymatic activity of PON1 (µU/mL) on each sample. Briefly, standards were prepared by diluting the Fluorescence Standard with the Paraoxonase Assay Buffer to obtain concentrations within a range from 0 to 1,000 pmol/well. The plasma samples were diluted (1:10) in Paraoxonase Assay Buffer in each technical (n = 3) and biological replicates (i.e., negative controls, n= 13; positive controls, n= 11; normal responders, n= 20; and poor responders, n= 16). Additionally, each sample was combined with an Inhibitor Mix for each technical replicate (n = 3) with the purpose of specific activity validation. The Background Mix was added as a negative control. A Positive Control Mix and a Positive Control Mix with the Inhibitor Mix were also present. All reactions were done in 96 well plates and controls had technical replicates (n = 3).
The 96 well plate was incubated for 10 minutes to allow the interaction between PON1 protein and its inhibitor. For all samples and controls 20 µL of 5x PON1 Substrate solution were added. SpectraMax M3 instrument (Ex/Em = 368/460) was used to read fluorescence on the microplate for 1 hour after adding the fluorogenic substrate. Data were obtained using SoftMax Pro 6.2.1 Software. PON1 enzymatic activity was calculated according to protocol [17].
2.4. Western Blot Analysis
For Western blot analysis, the existing plasma samples from normal (n= 20), poor responders (n= 16), healthy volunteers (negative control, n = 13) and cardiovascular patients without an indication for clopidogrel (positive control, n = 11) were used. To determine plasma protein concentration, we used the DC protein assay kit (Bio-Rad) and bovine serum albumin (BSA) as standard protein. A total of 25 µg of protein were loaded into a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel for electrophoretic separation, and the proteins were transferred into a 0.45 µm polyvinylidene fluoride (PVDF) membrane. The PVDF membranes were blocked with Intercept ®® blocking buffer for an hour followed by incubation with PON1 (4G8D3) mouse monoclonal antibody (dilution 1:1,000; Santa Cruz Biotechnology) overnight at 4°C in a shaker. The membranes were incubated with the secondary antibody, anti-mouse IRDye 680RD antibody (1;7,500; LI-COR), in the shaker at room temperature. For loading control, transferrin (TF) (101) rabbit monoclonal antibody (dilution 1: 1,000; Thermo Fischer) and goat anti-rabbit IRDye 680RD (1: 15,000; LI-COR) were used. Due to possible inter-assay variability, we used a pool control to normalize the samples. Briefly, 25 µg of protein from the 36 samples were mixed as a pool and quantified for plasma protein concentration as described above. From the pool, 25 µg were loaded into each of the 10% SDS polyacrylamide gels and Western blot protocol was performed as mentioned above. The membranes were scanned in the Odyssey®® CLx Imager in AUTO (680 nm and 800 nm channels), and the immune-fluorescent bands were quantified with the Image StudioTM Software. Data were expressed relative to the cardiovascular group.
2.5. Genotyping Microarray
Genotyping microarrays were run on 512 DNA specimens from our biorepository using the Infinium®® Multi-Ethnic AMR/AFR BeadChip (illumina®®) following the manufactures protocol. Briefly, 4 µL of genomic DNA were used for amplification during an incubation period of 20 to 24 hours. DNA was fragmented, precipitated, and re-suspended for hybridization of the DNA to the BeadChip overnight. The DNA BeadChip was washed with PB1 and DNA was extended and stained for imaging through the iScan instrument. GenomeStudio Software v2.0 was used to analyze the results from the iScan instrument using GRCh37 as the reference genome build.
2.6. Haplotype Phasing for PON1 Gene
Individual PON1 haplotypes of variants rs662 and rs854560 were determined using available data from genotyping microarray and the SHAPEIT software. The results from the Multi-Ethnic BeadChip were obtained as pedigree information (.ped) and variant information (.map) files by using the PLINK Report Plug-in v2.1.4 in Genome Studio Software. The files were converted to VCF files through the PLINK v1.07 software [18]. Appropriate data wrangling was applied by using manipulating and managing tools such as bcftools v1.14, VCFftools v0.1.13, and command-line shell. SHAPEIT v2 software [19] was executed according to the protocol for estimation of computational haplotype phasing for chromosome 7. The phased dataset was filtered for PON1 variants rs662 (position- 94937446) and rs854560 (position-94946084). The phased and filtered datasets were compressed, indexed, and normalized due to inverted reference and alternate alleles. Further details can be found in Appendix A (Supplementary Materials).
2.7. Statistical Analysis
Sample size was calculated for western blot analysis by using one-way balanced ANOVA test form the R package pwr. Due to multiple comparisons, a Bonferroni correction was made to the alpha value of 0.05 to reduce the false positive rate (type I error). With an effect size of 1.1 and a significance level of 0.0125, a minimum of five (n= 5) plasma samples for each of the four groups were required to achieve 80% of statistical power. Sample size was calculated for enzymatic activity assays in the same manner. For this purpose, with an effect size of 1.4 and a significance level of 0.0125, a minimum of four plasma samples (n= 4) were required to achieve 80% of statistical power.
Statistical analyses were carried out by GraphPad Prism 9.4.0. After testing the normality of data by Shapiro Wilkins Test, a one-way ANOVA was run as a variance test for group comparisons in the Western blot and PON1 activity results. Tukey’s post hoc test was applied if there was a significant difference between the means among groups to determine which group pair was statistically significant. Continuous variables were presented as mean ±SD. A chi-square test was performed to assess the genotype frequency. The 95% confidence interval (95% CI) was calculated using the Wilson-Brown method.
3. Results
3.1. PON1 Enzymatic Activity
Table 2 summarizes demographics, genetic and clinical data of participants in this study, including CYP2C19*2 minor allele frequency (MAF), comorbidities, clopidogrel indications (i.e., ACS, CAD, PAD), rates of MI (STEMI/ NSTEMIs) occurrence, coronary artery stents, and co-medications from individuals on clopidogrel whose plasma samples were used to perform the PON1 enzyme activity assays (i.e., functionality). The groups were balanced with data showing no significant difference between normal and poor responders, including their CYP2C19*2 MAFs, a well-known genetic marker of poor clopidogrel response (p= 0.35). Notably, the PON1 enzymatic activity among poor responders was significantly lower (10.57 µu/mL) than that in the negative control group (22.21 µU/mL, *p= 0.0134; Figure 1). Also, the poor responders group had a significantly lower enzymatic PON1 activity) than that among the participants in the positive control group (22.66 µU/mL, *p= 0.0101; Figure 1). Despite being younger than cases, other baseline characteristics of controls were comparable to those of cases (i.e., 53.8% females with BMI of 28.9 ± 6.2 among negative controls without CVD; 54.5% females with BMI of 30.1 ± 5.5 among positive CVD controls).
Plasma samples were diluted (1:10) with paraoxonase assay buffer. Standard curves were used to determine the amount of metabolites in each sample. PON1 enzymatic activity was calculated with the amount of metabolite, the time change for a linear phase, the dilution factor, and the volume of each sample on the wells according to protocol (ab241044) [17]. Shapiro-Wilk test was run to determine normality of data distribution. One-way ANOVA was used to test significant differences among groups means (p= 0.0044). Tukey’s post-hoc test was performed as multiple comparison (i.e., negative control versus poor responders, *p= 0.0101; positive control versus poor responders, *p= 0.0134). Dots represent enzyme activity per sample and bars are the means of enzyme activity per group, with vertical bars representing ±SD. The group of healthy volunteers (negative controls) is also known as “without cardiovascular disease (w/o CVD)”, while the positive control group is also identified as cardiovascular [CVD].
3.2. PON1 Relative Protein Abundance
PON1 protein was identified with a band detected at 43 kDa (Figure 2A). The band of the loading control TF was detected at 75 kDa (Figure 2A). Our findings indicate no statistical differences between the groups means (i.e., negative control= 1.11; positive control= 1.00; normal responders= 1.01; poor responders= 0.89) when performing validation of PON1 protein abundance for a combination of the initial sample set in the TMT1 and an independent sample cohort (Figure 2). However, when using the initial tandem mass tag-mass spectrometry (TMT-MS) sample set (n = 9), there was a significant decrease (*p= 0.0180) in the relative abundance of PON1 protein among poor responders compared to the CVD control group (Figure 3, CVD = 1.00 and poor responders= 0.71; *p= 0.0256 post-hoc test).
A total of 25 µg of protein were quantified from plasma samples and electrophoresed in a 10% polyacrylamide gel. The proteins were transferred from the gel into a PVDF membrane. A. Representative membranes from a technical replicate of Western Blot analysis for PON1 protein in plasma samples from normal (n = 20) and poor responders (n = 16) to clopidogrel and the controls with (n = 11) and without cardiovascular diseases (n = 13). PON1 protein has a molecular weight of 43 kDa while TF has 75 kDa. A pool control of all samples was included in each membrane to normalize the signal. B. Densitometry analysis of PON1 protein relative abundance normalized to pool from three independent Western Blots. The comparison was made between the following groups: negative “w/o CVD” control (n= 13); positive “CVD” control (n= 11); normal (n= 20) and poor responders (n= 16). Shapiro-Wilk test was performed to assess data distribution normality. One-way ANOVA was performed to test significant difference among the means of the groups (p= 0.6926). Values are the means with vertical bars representing ± SD.
A total of 25 µg of protein were quantified from plasma samples and electrophoresed in a 10% polyacrylamide gel. The proteins were transferred from the gel into a PVDF membrane. Each sample with PON1 signal was normalized with the pool control signal from three independent Western blots. The samples set used for this analysis (n = 9; representing the CVD control group, normal and poor responders, three samples each) were the same from the previous proteomic studies where the PON1 protein was found to be downregulated among poor responders. Shapiro-Wilk test was performed to assess data distribution normality. One-way ANOVA was performed to test significant differences among the means of the groups (p= 0.0180). Tukey’s post-hoc test was performed as a multiple comparison test (CVD versus normal responders, *p= 0.03; CVD versus poor responders; *p= 0.0256). Values are the means with vertical bars representing ± SD.
3.3. Genotypes and Haplotype Phasing
Table 3 summarizes the PON1 genotypes and MAFs of rs662 and rs854560 polymorphisms by groups (n = 60). For the purpose of this study, we extracted the PON1 genotypes specifically for the randomly selected 36 cases in our analysis, excluding the remaining reports in our database of 512 individuals (dbGaP Study Accession: phs003236.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gapprev/gap/cgi-bin/study.cgi?study_id=phs003236.v1.p1). The PON1 rs662 MAFs in poor and normal responders, as well as in the negative (without CVD) and positive (CVD) control groups were 53%, 57%, 42%, and 36%, respectively. On the other hand, PON1 rs854560 MAFs for these groups were 32%, 19%, 15%, and 36%, respectively. No significant differences were found between groups for both variants (p= 0.4866 for the rs662 single nucleotide polymorphism (SNP), and p= 0.4998 for the rs854560 SNP).
Control groups are defined as healthy volunteers (negative control, w/o CVD) and cardiovascular patients without an indication for clopidogrel (positive control). PON1 wild-types are denoted by the amino acid glutamine (Q) in position 192 and leucine (L) in position 55. The rs662 and rs854560 polymorphisms are defined as a change of Q in position 192 to arginine (R) and L in position 55 to methionine (M), respectively. Genotype data are presented as counts and proportions (n, %). Chi-square test was performed and no significant difference were found after comparing the PON1 rss662 (p= 0.4866) and PON1 rs854560 (p= 0.4866) MAFs between the groups. 95% confidence intervals (95% CI) of the corresponding MAFs for these two SNPs in each group were calculated using the Wilson-Brown method.
Genotype data is limited to only inform whether a given patient is homozygous for the variant of interest (i.e., carrier status), or heterozygous without discriminating from which chromosome the variant came from. Determining haplotypes has a beneficial clinical implication as it can predict the severity of a disease. Therefore, applying haplotype phasing based on computational algorithms allows us to estimate which haplotype a patient will have by assigning alleles to chromosomes. We observed a higher frequency of the TA|TA haplotype (no variations) in the control groups, while the experimental groups had a higher frequency of the *AA|*AA haplotype (*- asterisk represents the base allelic variant) that carried the variant allele on rs662 locus. Comparison of enzymatic activity by haplotypes showed a significant difference among the groups (*p = 0.0372). A comparison was made between controls and cases for TA|TA vs *AA|*AA haplotype. We found an association between haplotypes and the severity of cardiovascular disease (Figure 4A; ***p= 0.0001). In Figure 4B, comparison of PON1 enzymatic activity between the two haplotypes showed a significant (**p= 0.0050) lower enzymatic activity for those individuals harboring the haplotype with the rs662 variant in both chromosomes (*AA|*AA). Therefore, *AA|*AA haplotype was found more frequently among patients with more severe cardiovascular diseases (i.e., ACS, stable CAD or PAD patients).
Haplotype proportions in controls (n = 24) and cases (n = 36). Haplotypes are presented as a proportion of the total number of participants, with vertical bars representing 95%CI. B. PON1 enzymatic activity (µU/mL) of participants harboring the two most frequent haplotypes. DNA base changes are represented with an asterisk (*). Dots represent enzyme activity per sample and bars are the means of enzyme activity per group, with vertical bars representing ±SD. For statistical analysis to test association between control and cases, Fischer’s test was performed (p= 0.0001). Shapiro-Wilk test determined the normality of PON1 activity data. Mann Whitney test was used to compare differences between groups (p= 0.0050). Note: Individuals harboring the *AA/*AA haplotype are more likely to be poor responders (PRU > 230) as demonstrated in a previous work [20].
4. Discussion
A TMT-MS proteomic analysis was performed in these Caribbean Hispanic patients on clopidogrel to preliminarily identify circulating risk biomarkers that predict resistance to clopidogrel (response variability) and severity of CVD [21]. This study revealed that PON1 is downregulated in plasma of patients who are resistant to clopidogrel (i.e., low abundance among poor responders: -49.50) when compared to other cardiovascular controls without an indication for clopidogrel [21].
This is relevant because PON1 has previously been related to atherosclerosis and clopidogrel bioactivation pathways [8,16]. PON1 cysteine-284 interacts with oxidized LDL to reduce the accumulation of oxidized LDL in the sub-endothelial layer and prevent atherosclerosis progression. Therefore, PON1 has been described as a risk factor for developing cardiovascular diseases [9,22,23,24,25,26]. In this study, we validated PON1 protein in the initial sample set from the TMT-MS, where PON1 had a lower abundance in patients who were resistant to treatment with clopidogrel. These results suggest that PON1 could be a possible predictive biomarker of clopidogrel resistance in this population. A potential mechanism for this observation is that PON1 is participating in the biotransformation step of clopidogrel into the active metabolite. Therefore, due to a lower abundance of PON1 in patients from the poor responders group, they show a higher platelet reactivity, meaning that clopidogrel active metabolite is not adequately inhibiting platelet aggregation. This is supported by a study where PON1 was identified as a rate limiting step for the conversion of clopidogrel into its active metabolite, with PON1 p.Q192R polymorphism as a higher metabolite yielder [16]. Hence, this could suggest that the lower abundance of the PON1 protein in this group is causing the resistance to clopidogrel. However, multiple studies since then have failed to replicate these findings by Bouman et al. Therefore, this recent data outweighs the hypothesis that PON1 could be a rate limiting step for clopidogrel bioactivation and that it could serve as a biomarker of resistance to treatment [27,28,29,30,31].
We also found that genotypes concerning the PON1 p.L55M variant were not affecting the corresponding protein abundance in plasma. This finding differs from previous studies where the PON1 p.L55M polymorphism was found to be associated with reduced levels of gene expression, and hence, lower protein abundance in serum [32]. These results also suggest that the concentration of PON1 is independent from genotypes of the L55M variant in this locus. A possible explanation may be linkage disequilibrium (LD). LD is the non-random association of alleles at different loci, therefore, two SNPs in LD have a high probability of being inherited together in a population. It has been shown in the literature that a SNP associated with a particular feature is not necessarily the causative variant, [33] but rather, it is a close SNP that is in linkage disequilibrium with the associated SNP. In addition, the allelic frequency can change in admixed populations, generating different genetic variants with LD. For example, the tag-SNP CYP2C9 rs202201137 was found to be in LD with SNPs associated with low doses of warfarin [34]. This tag-SNP has only been found in the Puerto Rican population and the haplotype was named CYP2C9*61. In our study, a potential explanation is that PON1 p.L55M is not the tag-SNP or the genetic variant that is in LD with the causal variant of decreasing concentration of PON1. Genetic polymorphisms within the regulatory region of this gene have been early identified to alter the expression levels of PON1 protein [22,35,36]. The promoter region -108C/T polymorphism has previously been associated with a reduced PON1 concentration in plasma [35]. Therefore, further analysis of variants in the regulatory regions of the PON1 gene is needed to elucidate whether protein abundance is affected.
Published methods recommend validating quantitative proteomic results (TMT-MS) by using samples from an independent cohort that is different from the one initially used to conduct the proteomic studies [37]. Western blotting is a standard procedure to confirm our previous TMT-MS findings [38,39,40]. In our PON1 validation study, we added a negative control group of subjects without any diagnosed CVD and new samples across all the groups under study. Our results showed no statistical differences among groups under our experimental conditions, and thus, the previously observed low abundance of the PON1 protein in patients who are resistant to clopidogrel was not validated by using additional samples from an independent cohort. However, a nominal but non-significant trend towards lower PON1abundance was observed in this group (Figure 2). The low abundance of PON1 in the poor responders could also be a direct consequence of the severity of cardiovascular diseases. Most (80%) of the patients in the poor responders group reported having dyslipidemia, however, only 3% were in therapy with statins. This could promote atherosclerosis in this group of patients and have a negative impact on their clinical outcomes. Another factor that is affecting the poor responders is the BMI. The BMI for the normal responders falls under the category of overweight, while the BMI from the poor responders group falls under obesity. It has been demonstrated that obesity enhances atherosclerotic disease and stimulate inflammation [41]. Taken together, this evidence suggests that the risk factors for CVD create an imbalance of oxidants and antioxidants molecules leading to a possible state of systemic oxidative stress in the poor responders [42,43,44]. In addition, it is known that the HDL-associated PON1 protein can lower its protective capacity against atherosclerosis when exposed to inflammation [45]. These findings are consistent with previous studies showing that PON1 protein concentration is reduced in CVD [46,47,48,49]. Therefore, PON1 could serve as a possible predictive biomarker of the severity of the CVD.
Since PON1 polymorphisms could affect the proteolytic capability of the enzyme, we investigated if PON1 activity was also decreased in the poor responders group. We have previously found a significant association between the PON1 genotype status and PRU values in this study cohort [20]. Therefore, we already know that those showing a poor response to clopidogrel (PRU > 230) are more likely to harbor the *AA/*AA haplotype. However, no distinction was made between poor and normal responders for the association analysis because no difference was found between these two subgroups with respect to the PON1 activity (Figure 1). Our data showed an enzymatic activity in patients who are resistant to clopidogrel (i.e., poor responders) that is significantly lower than that in control groups (Figure 1). This may suggest that PON1 in poor responders has a reduced ability to act as an antioxidant enzyme, further increasing the severity of cardiovascular disease. In addition, poor responders had a higher incidence of NSTEMI than normal responders. Moreover, patients in the CVD positive control group had less severe conditions such as controlled hypertension or valve prolapse. Taken together, our results provide further evidence in favor of the role of PON1 as a potential predictive biomarker of cardiovascular disease severity in Caribbean Hispanics.
Genotyping data showed an overall MAF of ~50% for the rs662 variant (p.Q192R), while rs854560 (p.L55M) had a MAF of 25.8%. Allele frequencies vary substantially from one ancestral group to another, causing a wide variance in their frequency distribution across populations. It has been reported that p.Q192R is 60% for South African ancestry, 65% for East Asians, 43% for South Asians, and 31% from European ancestry; and that p.L55M is 4% for East Asians, 14% for Africans, 19% for South Asians, and 38% for Europeans [50,51]. The rs662 and rs854560 polymorphism of PON1 have been described in the literature as to changing the enzyme catalytic capability and modulating the enzyme expression, respectively [11,52]. To see how the combination of both major PON1 polymorphisms could affect enzymatic activity, we performed haplotype phasing. We obtained 11 estimated haplotypes among the without CVD, with CVD, normal responders, and poor responders. This finding is consistent with another study in a cohort of Latino mothers and their newborns, where authors found 32 different haplotypes comprising multiple combinations of PON1 polymorphisms in both regulatory (-909C>G, -162A>G, and -108C>T) and coding regions (p.Q192R and p.L55M) [53].
The most frequent haplotype was TA|TA (without SNPs) among the control groups, while *AA|*AA (*rs662 SNP) was the most frequent among patients in treatment with clopidogrel. As mentioned earlier, patients in the CVD control group mostly have less severe cardiovascular conditions, while the groups of patients in treatment with clopidogrel display more serious conditions including ACS. Therefore, our results show an association between the haplotypes and the severity of the disease. Contrary to our hypothesis, we found that patients with severe CVD and with PON1 p.Q192R polymorphism had a lower enzymatic activity than those patients with less severe CVD and without the SNP. Results on earlier studies have indicated that having PON1 p.Q192R caused a higher enzymatic activity and as a result, a better clinical outcome [54]. However, results are inconsistent as opposing evidence has been reported where PON1 p.Q192R has been associated with a lower enzymatic activity and linked as a CVD risk factor [10,12]. The latter result reiterates what we have found in this study. Reported literature differences in PON1 activity can be a result of a causal variant in LD with PON1-rs662. In addition to supporting our results, Mackness et al. showed that a combination of PON1 protein concentration and activity is reduced in patients with CHD [13]. However, they did not find an association of the genotype with PON1 concentration and activity [13]. Our results differ, since we explored beyond the PON1 p.Q192R genotype to determine the haplotype among groups and observed an association of the haplotype PON1 p.Q192R and the enzymatic activity. This is supported by studies that found an association of PON1 p.Q192R polymorphism with CHD [12].
Estrogens play a relevant role in PON1 abundance and activity. Postmenopausal women have reduced PON1 activity, and estrogen replacement therapy reverses these effects [55]. However, we do not anticipate a significant role of varying estrogens levels as a confounder in our study because sex as a variable was not found to be significantly different between groups (p-value: 0.5152). Instead, women were fairly distributed in similar proportions between poor and normal responders. Furthermore, age did not vary significantly between groups (67 ±10; p-value: 0.9817); therefore, no differences in the relative proportion of pre- and postmenopausal women between groups are expected. The study has some limitations, including the lack of a large external validation cohort, control by multiple covariates and potential confounders
Studies have demonstrated a relationship between carrying the PON1 p.L55M variant and having more susceptibility to CAD [56,57]. On the contrary, our results showed that PON1 p.L55M was neither associated with the disease nor the activity. This could be explained by the argument that different populations have different expression of genes and epigenetic changes influenced by environmental risk factors such as diet and exercise [58,59,60,61]. This idea is supported by other studies where populations yield inter-variability with respect to PON1 polymorphism distribution and the alteration of its phenotype by consequence [51,62,63].
5. Conclusions
In conclusion, inclusion of minority populations in pharmacogenomics and pharmacoproteomic studies is limited. The necessity for identifying valid biomarkers in an admixed population is increasingly important, especially because of the high prevalence of cardiovascular disease. We have demonstrated that there is a trend towards lower PON1 abundance in plasma, along with its diminished enzymatic activity, of patients who are carriers of the rs662 polymorphism and resistant to clopidogrel. The results presented herein indicate that PON1 could potentially serve as a useful predictive biomarker of the cardiovascular diseases severity in Caribbean Hispanic patients suffering from ACS or stable CAD. Our findings also provide the foundations for future studies on valid clinical “omic” biomarkers for CVD and help reduce the knowledge gap in cardiovascular pharmacogenomics/proteomics of the Caribbean Hispanic population. A larger study is warranted to further support our major findings and concluding remarks.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Appendix A: explanations of experimental details.
Author Contributions
Writing – original draft preparation, data curation, methodology, formal analysis, M.M.-P.; data curation, methodology, formal analysis, E.S., K.C.-C., and J.Y.R.; conceptualization, resources, supervision, validation, I.B.R.; conceptualization, resources, funding acquisition, supervision, project administration, writing – review and editing, A.R.-L. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by grant 2 U54 MD007600-31 from the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health and by the National Institute of General Medical Sciences (NIGMS)-Research Training Initiative for Student Enhancement (RISE) Program grant R25 GM061838.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB protocol number A4070417) from the University of Puerto Rico, Medical Sciences Campus. All subjects participated voluntarily and signed an informed consent. Written informed consent has been obtained from the patient(s) to publish this paper.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
For imputations and computational haplotype phasing, the data used as the reference populations are Surui and Karitiana in Brazil, Piapoco and Colombian in Colombia, and Maya and Pima in Mexico, found at https://www.internationalgenome.org/data-portal/data-collection/hgdp (accessed on 7 October 2021). IBS and YRI populations are from the NHGRI Sample Repository for Human Genetic Research of the Coriell Institute found at https://www.internationalgenome.org/data-portal/population (accessed on 7 October 2021). The datasets from the Caribbean Hispanics analyzed during the current study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank all the study participants for their contribution. We also want to thank Dr. Emilee Colón. Dr. Sylvette Ayala and Dr. Susan Corey for their advice.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- FDA. Highlights of Prescribing Information. www.fda.gov/medwatch. (2016).
- Gent, M. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348, 1329–1339 (1996).
- Quinn, M. J. & Fitzgerald, D. J. Ticlopidine and clopidogrel. Circulation 100, 1667–1672 (1999). [CrossRef]
- Jiang, X.-L., Samant, S., Lesko, L. J. & Schmidt, S. Clinical Pharmacokinetics and Pharmacodynamics of Clopidogrel. Physiol Behav 176, 139–148 (2017). [CrossRef]
- Lee, C. R. et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin Pharmacol Ther (2022). [CrossRef]
- Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, Kimmel SE, McDonough CW, Gong Y, Dave CV, Pratt VM, Alestock TD, Anderson RD, Alsip J, Ardati AK, Brott BC, Brown L, Chumnumwat S, Clare-Salzler MJ, Coons JC, Denny JC, Dillon C, Elsey AR, Hamadeh IS, Harada S, Hillegass WB, Hines L, Horenstein RB, Howell LA, Jeng LJB, Kelemen MD, Lee YM, Magvanjav O, Montasser M, Nelson DR, Nutescu EA, Nwaba DC, Pakyz RE, Palmer K, Peterson JF, Pollin TI, Quinn AH, Robinson SW, Schub J, Skaar TC, Smith DM, Sriramoju VB, Starostik P, Stys TP, Stevenson JM, Varunok N, Vesely MR, Wake DT, Weck KE, Weitzel KW, Wilke RA, Willig J, Zhao RY, Kreutz RP, Stouffer GA, Empey PE, Limdi NA, Shuldiner AR, Winterstein AG, Johnson JA; IGNITE Network. Multisite Investigation of Outcomes with Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2018;11(2):181-191. https://doi.org/10.1016/j.jcin.2017.07.022. PMCID: PMC5775044.
- Garabedian, T. & Alam, S. High residual platelet reactivity on clopidogrel: Its significance and therapeutic challenges overcoming clopidogrel resistance. Cardiovasc Diagn Ther 3, 23–37 (2013). [CrossRef]
- Mackness, M. I., Arrol, S. & Durringtorm, P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Journal 286, 152–154 (1991). [CrossRef]
- Mackness, M. & Mackness, B. Human Paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 567, 12–21 (2016). [CrossRef]
- Serrato, M. & Marian, A. J. A Variant of Human Paraoxonase/Arylesterase (HUMPONA) Gene Is a Risk Factor for Coronary Artery Disease. Journal of Clinical Investigation 96, 3005–3008 (1995). [CrossRef]
- Mackness, B., Mackness, M. I., Arrol, S., Turkie, W. & Durrington, P. N. Effect of the molecular polymorphisms of human paraoxonase (PON1) on the rate of hydrolysis of paraoxon. Br J Pharmacol 122, 265–268 (1997). [CrossRef]
- Ruiz, J. et al. Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. The Lancet 346, 869–872 (1995). [CrossRef]
- Mackness, B. et al. Paraoxonase Status in Coronary Heart Disease Are Activity and Concentration More Important Than Genotype? Arterioscler Thromb Vasc Biol 21, 1451–1457 (2001).
- Norris, E. T. et al. Genetic ancestry, admixture and health determinants in Latin America. BMC Genomics 19, 861 (2018). [CrossRef]
- Sirugo G, Williams SM, Tishkoff SA. The Missing Diversity in Human Genetic Studies. Cell. 2019;177(1):26-31. [CrossRef]
- Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhäuser C, Waldmann C, Schmalz HG, ten Berg JM, Taubert D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17(1), 110-116 (2011). Erratum in: Nat Med 17(9), 1153 (2011). [CrossRef]
- Abcam. ab241044 Paraoxonase 1 Activity Assay Kit. 1–20 Preprint at (2018).
- Purcell, S. et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet 81, (2007).
- Delaneau, O., Zagury, J. F. & Marchini, J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 10, 5–6 (2013). [CrossRef]
- Hernandez-Suarez DF, Botton MR, Scott SA, Tomey MI, Garcia MJ, Wiley J, Villablanca PA, Melin K, Lopez-Candales A, Renta JY, Duconge J. Pharmacogenetic association study on clopidogrel response in Puerto Rican Hispanics with cardiovascular disease: A novel characterization of a Caribbean population. Pharmgenomics Pers Med 11, 95-106 (2018). [CrossRef]
- Santiago-Cartagena, E. et al. Quantitative Proteomic Profile of Caribbean Hispanics with Resistance to Clopidogrel. The FASEB Journal 34, 1 (2020). [CrossRef]
- Mackness, B., Turkie, W. & Mackness, M. Paraoxonase-1 (PON1) promoter region polymorphisms, serum PON1 status and coronary heart disease. Archives of Medical Science 9, 8–13 (2013). [CrossRef]
- Shunmoogam, N., Naidoo, P. & Chilton, R. Paraoxonase (PON)-1: A brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc Health Risk Manag 14, 137–143 (2018). [CrossRef]
- Aviram, M. et al. Human serum paraoxonase (PON1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med 26, 892–904 (1999). [CrossRef]
- Shih, D. M. et al. Mice lacking serum paraoxonase are susceptible to organophosphatetoxicity and atherosclerosis. Nature 394, 284–287 (1998).
- Mackness, B., Hunt, R., Durrington, P. N. & Mackness, M. I. Increased Immunolocalization of Paraoxonase, Clusterin, and Apolipoprotein A-I in the Human Artery Wall with the Progression of Atherosclerosis. Arterioscler Thromb Vasc Biol 17, (1997). [CrossRef]
- Gong, I. Y. et al. Clarifying the importance of CYP2C19 and PON1 in the mechanism of clopidogrel bioactivation and in vivo antiplatelet response. Eur Heart J 33, 2856–2864 (2012). [CrossRef]
- Hulot, J. S. et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv 4, 422–428 (2011). [CrossRef]
- Lewis, J. P. et al. Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin Pharmacol Ther 90, 568–574 (2011). [CrossRef]
- Sibbing, D. et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J 32, 1605–1613 (2011). [CrossRef]
- Tresukosol, D. et al. Effects of cytochrome P450 2C19 and paraoxonase 1 polymorphisms on antiplatelet response to clopidogrel therapy in patients with coronary artery disease. PLoS ONE 9, (2014). [CrossRef]
- Leviev, I., Negro, F. & James, R. W. Two Alleles of the Human Paraoxonase Gene Produce Different Amounts of mRNA: An Explanation for Differences in Serum Concentrations of Paraoxonase Associated with the (Leu-Met54) Polymorphism. Arterioscler Thromb Vasc Biol 17, 2935–2939 (1997).
- Altman, N. & Krzywinski, M. Association, correlation and causation. Nat Methods 12, 899–900 (2015).
- Claudio-Campos, K. I. et al. CYP2C9∗61, a rare missense variant identified in a Puerto Rican patient with low warfarin dose requirements. Pharmacogenomics 20, 3–8 (2019). [CrossRef]
- Brophy, V. H. et al. Effects of 5′ regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. Am J Hum Genet 68, 1428–1436 (2001). [CrossRef]
- Leviev, I. & James, R. W. Promoter Polymorphisms of Human Paraoxonase PON1 Gene and Serum Paraoxonase Activities and Concentrations. Arterioscler Thromb Vasc Biol 20, 516–521 (2000). [CrossRef]
- Zhou, C. et al. Statistical considerations of optimal study design for human plasma proteomics and biomarker discovery. J Proteome Res 11, 2103–2113 (2012). [CrossRef]
- Handler DC, Pascovici D, Mirzaei M, Gupta V, Salekdeh GH, Haynes PA. The Art of Validating Quantitative Proteomics Data. Proteomics. 2018; 18(23): e1800222. [CrossRef]
- Geyer PE, Holdt LM, Teupser D, Mann M. Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol. 2017;13(9):942. [CrossRef]
- Mölleken C, Sitek B, Henkel C, Poschmann G, Sipos B, Wiese S, Warscheid B, Broelsch C, Reiser M, Friedman SL, Tornøe I, Schlosser A, Klöppel G, Schmiegel W, Meyer HE, Holmskov U, Stühler K. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology. 2009; 49(4):1257-66. [CrossRef]
- Powell-Wiley, T. M. et al. Obesity and Cardiovascular Disease A Scientific Statement From the American Heart Association. Circulation 143, E984–E1010 (2021). [CrossRef]
- Abu-Saleh, N., Aviram, M. & Hayek, T. Aqueous or lipid components of atherosclerotic lesion increase macrophage oxidation and lipid accumulation. Life Sci 154, 1–14 (2016). [CrossRef]
- Keaney, J. F. et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham study. Arterioscler Thromb Vasc Biol 23, 434–439 (2003).
- Münzel, T. et al. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. Journal of the American College of Cardiology vol. 70 212–229 Preprint at https://doi.org/10.1016/j.jacc.2017.05.035 (2017).
- Meisinger, C., Freuer, D., Bub, A. & Linseisen, J. Association between inflammatory markers and serum paraoxonase and arylesterase activities in the general population: A cross-sectional study. Lipids Health Dis 20, (2021). [CrossRef]
- Ikeda, Y. et al. Low human paraoxonase predicts cardiovascular events in Japanese patients with type 2 diabetes. Acta Diabetol 46, 239–242 (2009). [CrossRef]
- Murillo González, F. E. et al. PON1 concentration and high-density lipoprotein characteristics as cardiovascular biomarkers. Archives of Medical Science – Atherosclerotic Diseases 4, 47–54 (2019). [CrossRef]
- Zhao, Y. et al. Association between PON1 activity and coronary heart disease risk: A meta-analysis based on 43 studies. Mol Genet Metab 105, 141–148 (2012). [CrossRef]
- Chen, H. et al. PON1 L55M and Q192R gene polymorphisms and CAD risks in patients with hyperlipidemia: Clinical study of possible associations. Herz 43, 642–648 (2018).
- Luo, J. Q., Ren, H., Liu, M. Z., Fang, P. F. & Xiang, D. X. European versus Asian differences for the associations between paraoxonase-1 genetic polymorphisms and susceptibility to type 2 diabetes mellitus. J Cell Mol Med 22, 1720–1732 (2018). [CrossRef]
- Macharia, M., Kengne, A. P., Blackhurst, D. M., Erasmus, R. T. & Matsha, T. E. Paraoxonase1 genetic polymorphisms in a mixed ancestry African population. Mediators Inflamm 2014, (2014). [CrossRef]
- Garin, M.-C. B. et al. Paraoxonase Polymorphism Met-Leu54 Is Associated with Modified Serum Concentrations of the Enzyme A Possible Link between the Paraoxonase Gene and Increased Risk of Cardiovascular Disease in Diabetes. Journal of Clinical Investigation 99, 62–66 (1997). [CrossRef]
- Holland, N. et al. Paraoxonase polymorphisms, haplotypes, and enyzme activity in Latino mothers and newborns. Environ Health Perspect 114, 985–991 (2006).
- Bhattacharyya, T. et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA - Journal of the American Medical Association 299, 1265–1276 (2008). [CrossRef]
- Kumru S, Aydin S, Aras A, Gursu MF, Gulcu F. Effects of surgical menopause and estrogen replacement therapy on serum paraoxonase activity and plasma malondialdehyde concentration. Gynecol Obstet Invest. 2005;59(2):108-12. [CrossRef]
- Taşkiran, P. et al. The relationship between paraoxanase gene Leu-Met (55) and Gln-Arg (192) polymorphisms and coronary artery disease. Turk Kardiyol Dern Ars 37, 473—478 (2009).
- Watzinger, N. et al. Human Paraoxonase1 Gene Polymorphisms and the Risk of Coronary Heart Disease: A Community-Based Study. Cardiology 98, 116–122 (2002). [CrossRef]
- Barrès, R. et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15, 405–411 (2012). [CrossRef]
- Bonder, M. J. et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat Genet 49, 131–138 (2017). [CrossRef]
- Breton, C. v. et al. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med 180, 462–467 (2009). [CrossRef]
- Waterland, R. A. & Jirtle, R. L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol Cell Biol 23, 5293–5300 (2003). [CrossRef]
- Gamboa, R. et al. Distribution of paraoxonase PON1 gene polymorphisms in Mexican populations. Its role in the lipid profile. Exp Mol Pathol 80, 85–90 (2006). [CrossRef]
- Ginsberg, G. et al. Genetic polymorphism in paraoxonase 1 (PON1): Population distribution of PON1 activity. J Toxicol Environ Health B Crit Rev 12, 473–507 (2009). [CrossRef]
Figure 1.
Enzymatic activity of PON1 (µU/mL) in plasma from participants in the control groups with and without cardiovascular diseases (i.e., positive and negative controls, respectively), normal and poor responders to clopidogrel.
Figure 1.
Enzymatic activity of PON1 (µU/mL) in plasma from participants in the control groups with and without cardiovascular diseases (i.e., positive and negative controls, respectively), normal and poor responders to clopidogrel.
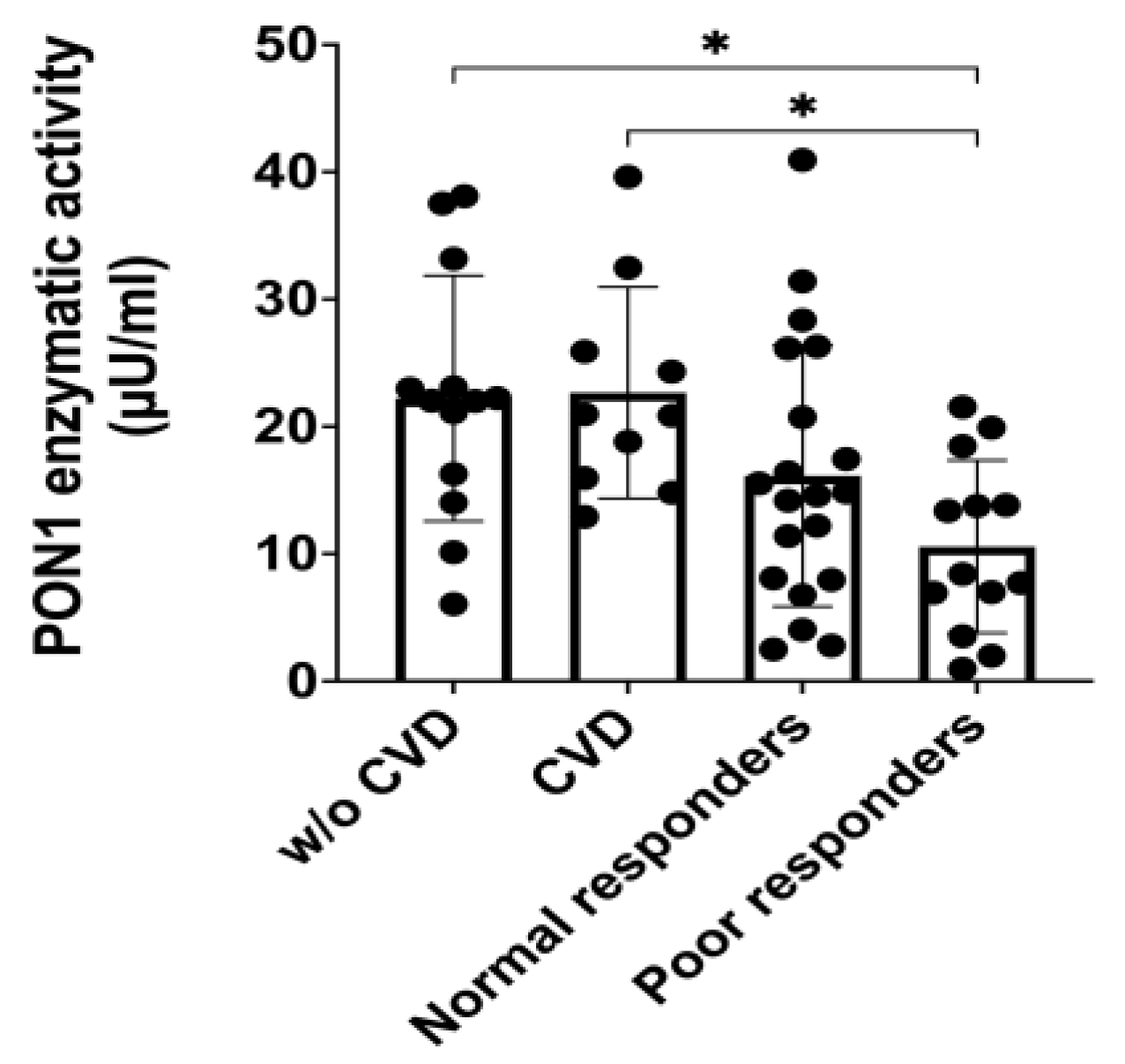
Figure 2.
Western blot analysis for the validation of low abundance PON1 protein in patients’ resistant to clopidogrel.
Figure 2.
Western blot analysis for the validation of low abundance PON1 protein in patients’ resistant to clopidogrel.
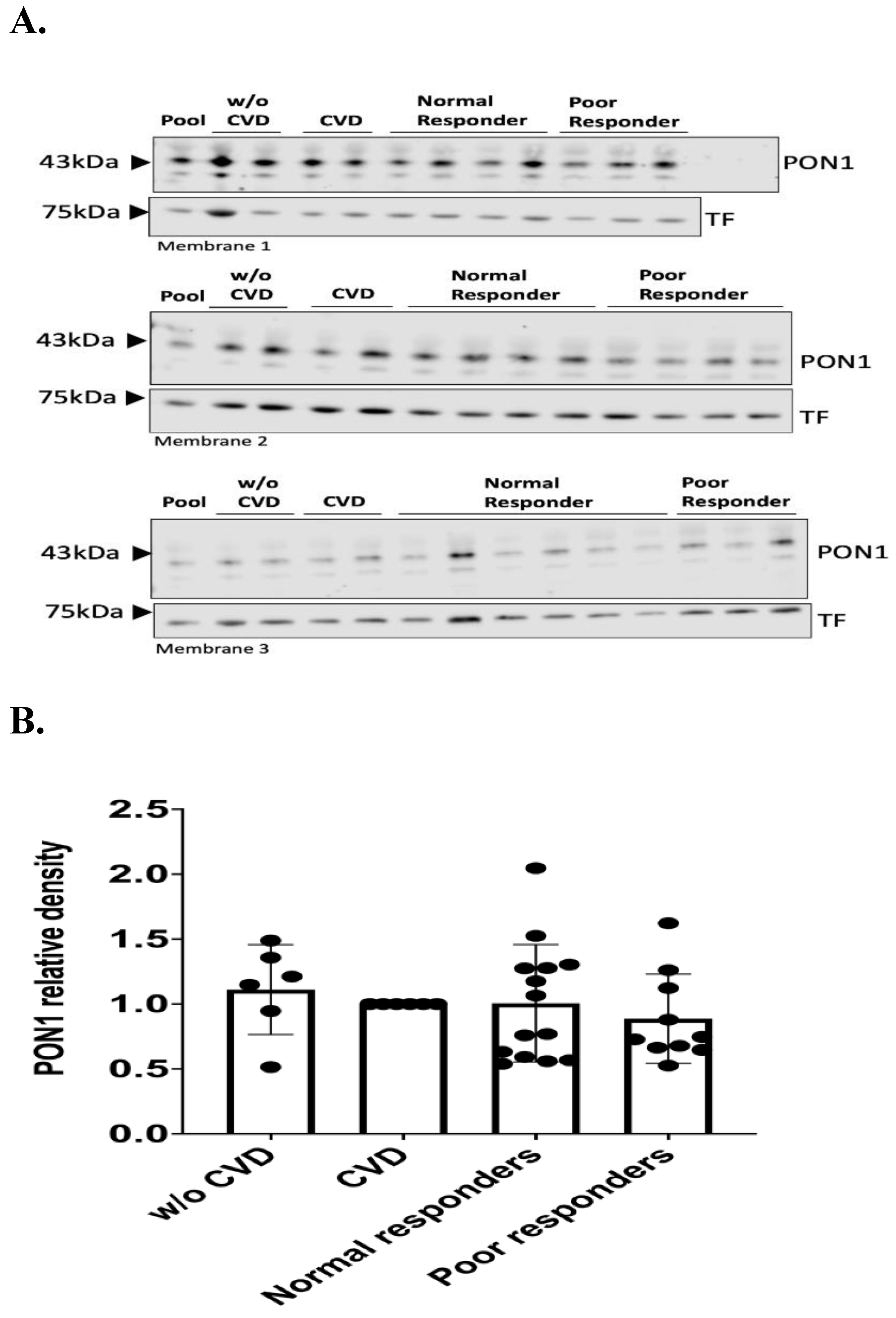
Figure 3.
Densitometry analysis of PON1 protein relative abundance with the samples previously used in the proteomic study.
Figure 3.
Densitometry analysis of PON1 protein relative abundance with the samples previously used in the proteomic study.
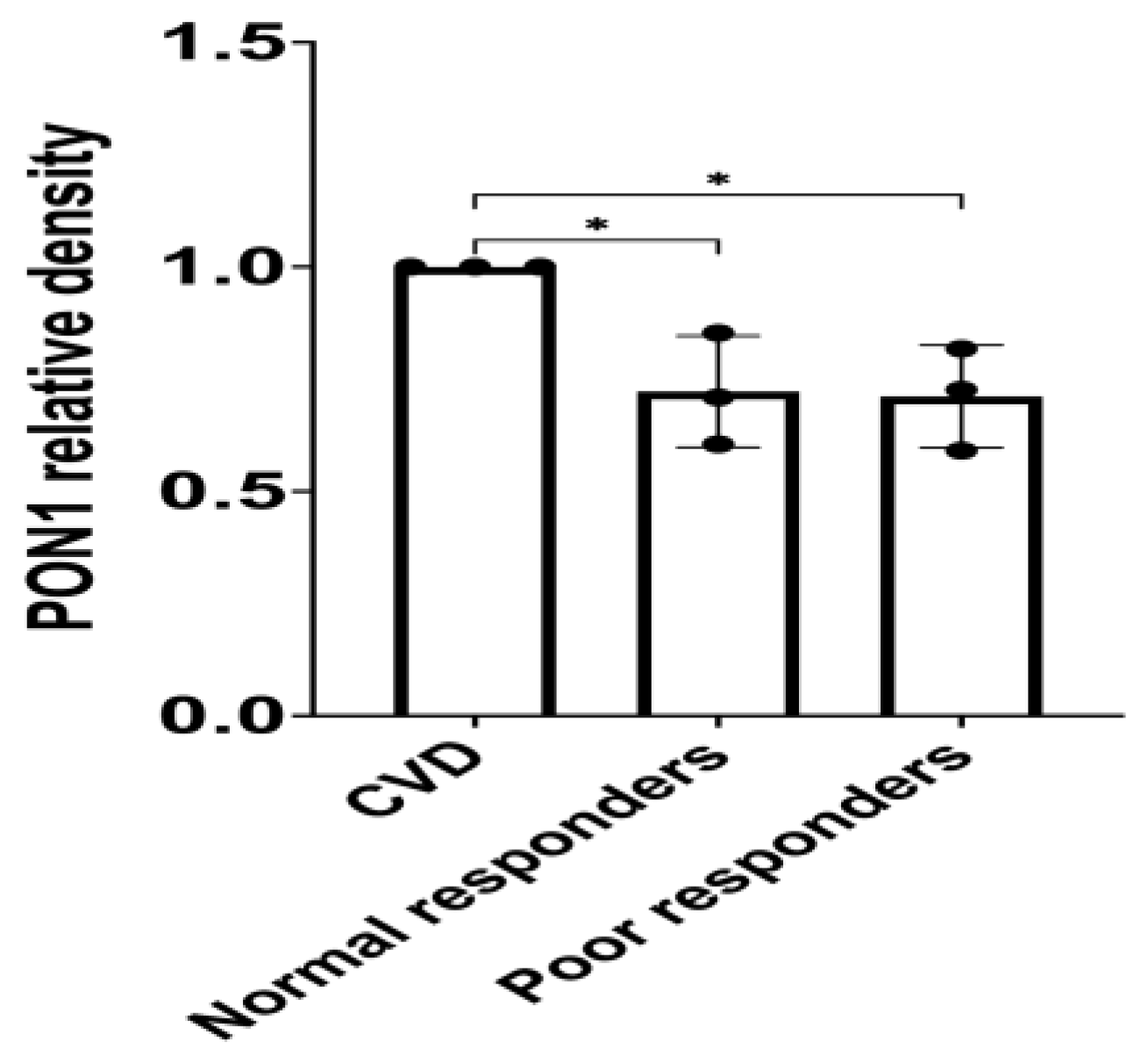
Figure 4.
Association between TA|TA and *AA|*AA haplotypes and the severity of cardiovascular disease. A.
Figure 4.
Association between TA|TA and *AA|*AA haplotypes and the severity of cardiovascular disease. A.
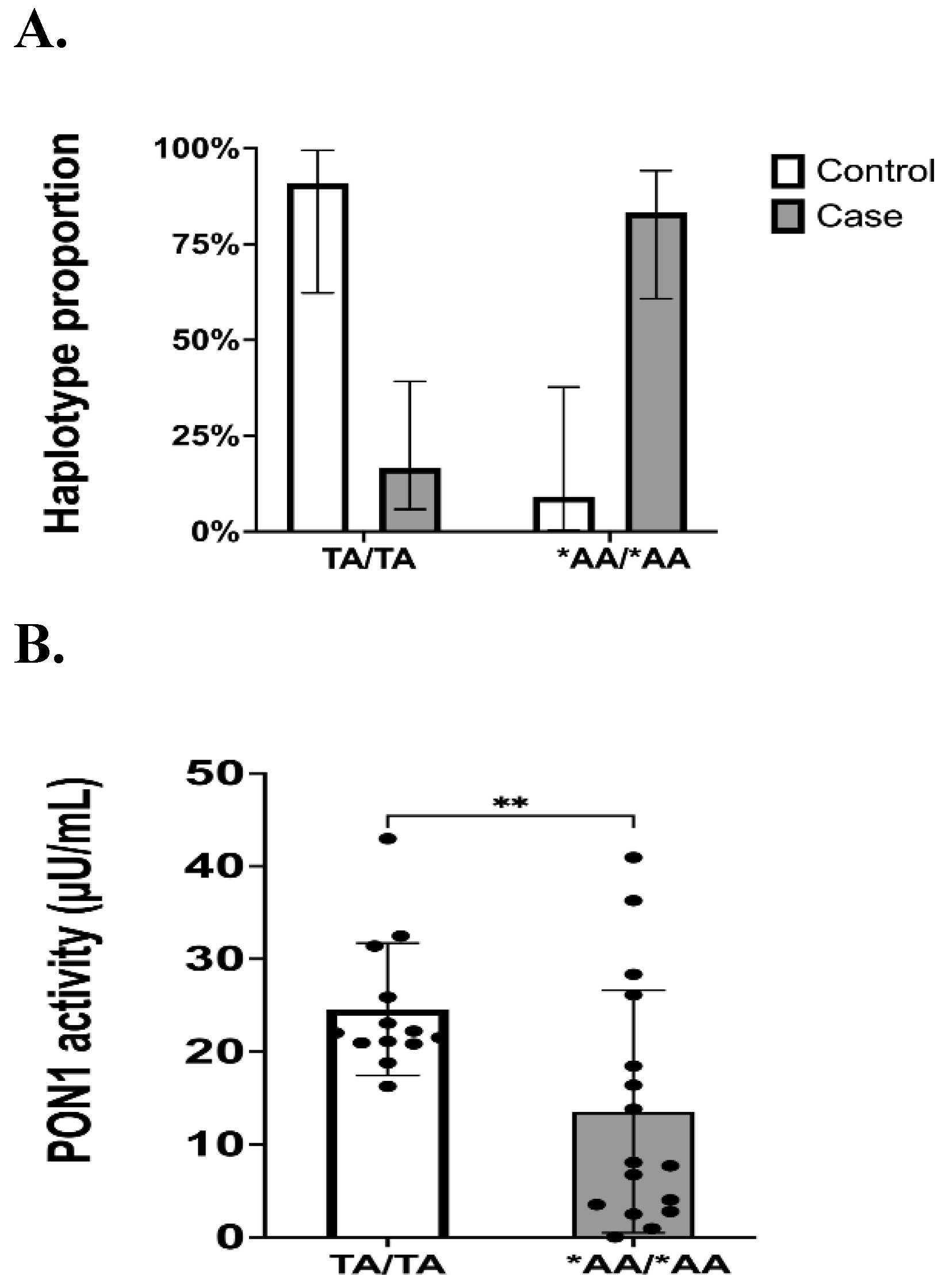
Table 1.
Inclusion and Exclusion Criteria for Patients.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Caribbean Hispanics residing in Puerto Rico Both sexes (i.e., males/females) Age ≥ 21 years old Receiving clopidogrel (75 mg/day) for therapeutic indications (ACS, stable CAD, PAD) over at least 6 months. No clinically active hepatic abnormality. The ability to understand the requirements of the study. The ability to comply with the study procedures and protocols. A female patient is eligible to enter the study if she is of child-bearing potential and not pregnant or nursing, or not of child-bearing potential. |
Non-Hispanic patients Currently enrolled in another active research protocols BUN > 30 and creatinine > 2.0 mg/dL Hematocrit (Hct) ≤ 25% Nasogastric or enteral feedings Acute illness (e.g., sepsis, infection, anemia) HIV/AIDS, Hepatitis B patients Alcoholism and drug abuse Patients with any cognitive and mental health impairment Sickle cell patients Active malignancy Patients taking another antiplatelet |
Table 2.
Demographics, genetics and clinical data of Caribbean Hispanic controls (i.e., negative and positive) and cardiovascular patients on treatment with clopidogrel (75 mg/daily) who donor plasma specimens for PON1 enzyme activity assays and Western blot analyses.
Table 2.
Demographics, genetics and clinical data of Caribbean Hispanic controls (i.e., negative and positive) and cardiovascular patients on treatment with clopidogrel (75 mg/daily) who donor plasma specimens for PON1 enzyme activity assays and Western blot analyses.
| Characteristics | All Patients (n = 36) | Normal Responders (n= 20) | Poor Responders (n= 16) | p value | Negative Controls (n = 13) | Positive Controls (n = 11) |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 67 ± 10 | 67 ± 9 | 68 ± 10 | 0.9817 | 48 ± 11 | 53 ± 18 |
| Sex (%, n) | 0.5152 | |||||
| Female | 56% (20) | 50% (10) | 63% (10) | 53.8% (7) | 54.5% (6) | |
| Male | 44% (16) | 50% (10) | 38% (6) | 46.2% (6) | 45.5% (5) | |
| BMI | 30.9 ± 5.9 | 29.6 ± 5.2 | 32.6 ± 6.5 | 0.1291 | 28.9 ± 6.2 | 30.1 ± 5.5 |
| Current smoker (%, n) | 19% (7) | 20% (4) | 19% (3) | > 0.9999 | 15.4% (2) | 18.2% (2) |
| Diabetes Mellitus (%, n) | 92% (33) | 90% (18) | 94% (15) | > 0.9999 | 91% (10) | |
| Dyslipidemia (%, n) | 92% (33) | 95% (19) | 88% (14) | 0.5742 | 91% (10) | |
| Hypertension (%, n) | 97% (35) | 95% (19) | 100% (16) | > 0.9999 | 100% (11) | |
| Clopidogrel indication (%, n) | ||||||
| Stable CAD or ACS | 72.2% (26) | 75% (15) | 68.75% (11) | |||
| PAD | 27.78% (10) | 25% (5) | 31.25% (5) | 0.7225 | ||
| MI, STEMI & NSTEMI (%, n) | 2.77% (1) | 0% (0) | 6.25% (1) | 0.4444 | ||
| Coronary artery stents (%, n)# | 36.1% (13) | 40% (8) | 31.3% (5) | 0.7314 | ||
| Aspirin Users (%, n) | 64% (23) | 60% (12) | 69% (11) | 0.7314 | 0% (0) | |
| CCB Users (%, n) | 31% (11) | 35% (7) | 25% (4) | 0.7182 | 27.3% (3) | |
| Cilostazol Users (%, n) | 14% (5) | 15% (3) | 13% (2) | > 0.999 | ||
| PPI Users (%, n) | 28% (10) | 20% (4) | 38% (6) | 0.2853 | 18.2% (2) | |
| Statins Users (%, n) | 81% (29) | 90% (18) | 69% (11) | 0.2036 | 82% (9) | |
| CYP2C19*2 status (MAF, %, n)* | 13.9% (10) | 12.5% (5) | 15.6% (5) | 0.7426 | 15.4% (4) | 13.6% (3) |
Body mass index (BMI), myocardial infarction (MI), calcium channel blockers (CCB), and proton pump inhibitors (PPI). Age in years old. BMI data is shown as kg/m2. Statistical analysis was performed to compare normal versus poor responders using Fisher’s exact test, t-Student’s test and z-test for proportions. #All patients with ACS and some with stable CAD underwent PCI (i.e., percutaneous coronary interventions with stent deployment). * n refers to the total # of minor alleles detected in the study groups.
Table 3.
PON1 genotypes and MAFs at the two polymorphic sites of interest (rs662 and rs854560) in samples used to run the Western blot analysis.
Table 3.
PON1 genotypes and MAFs at the two polymorphic sites of interest (rs662 and rs854560) in samples used to run the Western blot analysis.
| Variable | Negative Controls (n = 13) | Positive Controls (n = 11) | Normal Responders (n = 20) | Poor Responders (n = 16) |
|---|---|---|---|---|
| rs662 (p.Q192R) | ||||
| 4 (31%) | 5 (45.5%) | 3 (15%) | 2 (12.5%) | |
| QR | 7 (54%) | 4 (36.4%) | 11 (55%) | 11 (68.75%) |
| RR | 2 (15%) | 2 (18.1%) | 6 (30%) | 3 (18.75%) |
| MAF | 42.31% | 36.4% | 57.5% | 53.12% |
| 95%CI | 19.33 - 68.05 | 13.81 - 60.94 | 39.07 - 73.50 | 34.21 - 74.18 |
| rs854560 (p.L55M) | ||||
| LL | 9 (69.2%) | 6 (54.5%) | 10 (50%) | 10 (62.5%) |
| LM | 4 (30.8%) | 2 (18.2%) | 7 (35%) | 6 (37.5%) |
| MM | 0 (0%) | 3 (27.3%) | 3 (15%) | 0 (0%) |
| MAF | 15.38% | 36.4% | 32.5% | 18.75% |
| 95%CI | 2.96 - 44.80 | 19.33 - 68.05 | 17.93 - 50.66 | 8.07 - 41.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








