1. Introduction
Glycogen is a primary storage form of glucose. It is an efficient source of energy that is readily available for decomposition to glucose when needed by cells. Glycogen is associated with various enzymes and regulatory proteins involved in its metabolism resulting in the glycogen-protein complex called glycogen granule (GG) or glycosome [
1]. The initiation of glycogen synthesis occurs through the action of glycogenin, the central priming protein, which catalyzes auto-glucosylation of its conserved tyrosine residue. This covalent binding process results in the production of a short chain oligomer of approximately 12 glucose residues linked by α-1,4-glycosidic bonds. Subsequently, these short chain oligosaccharides covalently bound to glycogenin molecules are further extended via α-1,4-glycosidic linkages and spread out via α-1,6-glycosidic bonds at branch points by additional enzymes of glycogen biosynthesis pathway [
2,
3].
In
Saccharomyces cerevisiae, there are two isoforms of glycogenin, Glg1 and Glg2, which emerged after the whole genome duplication [
4,
5]. In contrast, another popular yeast model,
Komagataella phaffii (formerly
Pichia pastoris), has only one glycogenin (called Glg1 here), which exhibits a high sequence similarity with
S.
cerevisiae proteins. Notably, both
S.
cerevisiae glycogenins contain a conserved tyrosine residue, Tyr
232, which forms the glucose-1-
O-tyrosyl linkage when subjected to incubation with UDP-glucose as glucose donor [
5,
6]. Once
S.
cerevisiae glycogenins initiated glycogen synthesis and formed short chain glucose oligomers on their Tyr
232, glycogen synthases along with the branching enzyme produce a large, branched polysaccharide known as glycogen [
1].
Glycogen is present in many organisms from bacteria and archaea to eukaryotes, including humans [
3]. Since glycogen is a primary intracellular reserve polymer, glycogen biosynthesis represents a major strategy to cope with starvation conditions [
6]. Eventually, starvation leads to the autophagic degradation of GGs inside lysosomes and a non-autophagic glycogen breakdown in the cytosol [
6,
7]. In mammals, the autophagic accumulation of GGs inside lysosomes lacking acid α-glucosidase, the sole lysosomal glycogen hydrolase, contributes to the lysosomal storage disorder known as Pompe disease [
8]. Recently, there was some progress in understanding the trafficking of GGs to lysosomes: the autophagic delivery of GGs to lysosomes was suggested to be selective and called “glycophagy” to distinguish it from non-selective autophagy. However, glycophagy selectivity factors that package specifically GGs into autophagic vesicles, autophagosomes, remain largely unclear, except for the starch binding domain-containing protein 1 (Stbd1) [
9,
10]. Nevertheless, what is clear is that autophagosomes play an important role in the transport of GGs from the cytosol to lysosomes in mammalian cells [
8].
As yeast in general (and K. phaffii in particular) is such a good autophagic model, we used K. phaffii cells to clarify the type of autophagy responsible for the catabolism of GGs. First, we developed K. phaffii as a model to study autophagic delivery and degradation of GGs under nitrogen starvation conditions. For this, we turned Glg1, the protein marker of GGs, into the Glg1-GFP autophagic reporter and measured the cytoplasm-to-vacuole delivery and vacuolar degradation of GGs in autophagic, vacuolar, and glycogenin mutants. All our findings here unequivocally suggest that GGs are delivered to the vacuole for degradation by a non-selective autophagy.
2. Materials and Methods
2.1. Strains and Plasmids
K.
phaffii strains and plasmids used in this study are described in
Table 1. All the plasmids, which were cloned using polymerase chain reaction (PCR) fragments, were verified by sequencing. Recipient strains were transformed with all the plasmids by electroporation [
11]. All the plasmids were linearized with endonucleases of restriction (see below for details) before transformation for directed integration into the yeast genome.
The pRK22 plasmid with the Glg1-GFP expression cassette has 500 bp promoter and open reading frame (ORF) without STOP codon of
GLG1 gene. They were amplified by PCR using the genomic DNA of wild-type (WT) strain and cloned as XmaI-PstI fragment into the pRK1 vector [
16]. The pNW11 plasmid is the site-directed mutagenesis product of pRK22 and encodes the Glg1-GFP (Y212F) variant of Glg1-GFP. The pNW10 plasmid was created using pRK22 and pRK35 plasmids. First, we built pRK35 containing the Pgk1-GFP expression cassette with 500 bp promoter and ORF without STOP codon of
PGK1 gene. They were PCR amplified using the genomic DNA of WT strain and cloned as SpeI-PstI fragment into pRK1. Then, the promoter of
PGK1 on pRK35 was replaced with the
GLG1 promoter to make pNW10. For this, we substituted the pRK35 XmaI-PstI fragment carrying the entire Pgk1-GFP expression cassette with two fragments: (1) XmaI-SpeI fragment with the
GLG1 promoter amplified by PCR from pRK22, and (2) SpeI-PstI fragment with the
PGK1 ORF without STOP codon PCR amplified from pRK35.
All HIS4 plasmids, including pRK22, pNW11, and pNW10, were linearized in the HIS4 selection marker with EcoNI before transformation for their integration into his4 locus of recipient strains. His+-transformants of strains with these plasmids were selected on SD+DOM-His plates (1.7 g/L yeast nitrogen base [YNB] without amino acids and ammonium sulfate, 5 g/L ammonium sulfate, 1.92 g/L drop-out mix synthetic minus histidine, 20 g/L dextrose, and 20 g/L agar) and screened for the expression of GFP-fusion proteins by western blotting with mouse GFP antibodies (see below).
The pNW9 plasmid with the K. phaffii GLG1 (PAS_chr4_0847) deletion cassette was built by inserting the 1,000 bp 3′-untranslated region (3’-UTR) of GLG1 as SpeI-NotI fragment into the ZeocinR vector, pAP1, to create an intermediate plasmid, pNW8, and then, by inserting the 1,000 bp 5′-UTR of GLG1 as KpnI-SalI fragment into pNW8 to create pNW9. The GLG1 deletion cassette containing the 5’-UTR of GLG1, ZeocinR gene and 3’-UTR of GLG1 was released from pNW9 by double digestion with KpnI and NotI before transformation. The glg1 deletion mutant was selected as a ZeocinR-transformant of WT strain on YPD+Zeocin plates (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose, 20 g/L agar and 100 mg/L Zeocin) and verified by PCR.
2.2. Iodine Staining for Glycogen
The glycogen content of yeast biomass was assessed by the iodine staining method [
17] with modifications described in [
18]. Briefly, strains were grown as patches on YPD plates (in duplicate) for 2 d at 30°C. Then, plates were inverted over iodine crystals under the fume hood for 2 min. Patches synthesizing glycogen were stained brown.
2.3. Biochemical Analysis
For biochemical analysis, cells were grown in 1 ml of YPD medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose) for 1 d at 30°C. Then, 1.5 ODs of cells were taken for analysis. Alternatively, 3 ODs of cells were washed twice with 1 ml of sterile 1x YNB solution (1.7 g/L YNB without amino acids and ammonium sulfate) and shifted to 3 ml of SD-N medium (1.7 g/L YNB without amino acids and ammonium sulfate, and 20 g/L dextrose) with the starting OD
600 = 1. Then, 1 ml of cell culture was taken at 0 and 24 h time-points from SD-N medium. Protein lysates were prepared by trichloroacetic acid (TCA) precipitation [
19] and assayed by western blotting. Samples were subjected to 10% SDS-PAGE after heating in a heat block for 10 min at 95°C. The 15 µL of each sample was loaded into the gel and run at 115 V. Proteins were transferred from gels to nitrocellulose membranes for 1 h at 90 V. The membranes were stained with the Revert 700 total protein stain (926-11021, LI-COR Biosciences) for 5 min, de-stained for 1 min and imaged in the Odyssey CLx imager (LI-COR Biosciences) in 700 nm channel. Then, membranes were blocked in blocking solution (Tris-buffered saline with Tween-20 [TBS-T] containing 5% nonfat dry milk) for 1 h. After blocking, they were incubated with mouse GFP antibodies (11814460001, Roche Diagnostics; 1:2,000 dilution in blocking solution) overnight at 4°C. Next day, membranes were washed three times for 5 min each with 1x TBS-T. Then, they were incubated with IRDye 800CW goat anti-mouse antibodies (926-32210, LI-COR Biosciences; 1:15,000 dilution in TBS-T) for 1 h at room temperature and washed twice (5 min each) with 1x TBS-T and once (5 min) with 1x TBS. Finally, membranes were imaged in 700 and 800 nm channels, and images were quantified using the LI-COR Image Studio Lite v5.2 software. All experiments were performed at least twice in duplicate.
2.4. Fluorescence Microscopy
For fluorescence microscopy, cells were either grown only in YPD medium or grown in YPD medium, washed, and transferred to SD-N medium, as described in Biochemical Analysis (see above) with the following changes. After a fraction of cells was washed and transferred to SD-N medium (if necessary), the rest of YPD cultures was stained for vacuolar lumen with 10 mM stock solution of the CellTracker blue CMAC dye (C2110, Invitrogen) in DMSO (the final concentration of the dye in culture tube was 10 µM) for 30 min at 30°C and imaged as “0 min” or “0 h” time-point. The last 30 min of SD-N cultures was the incubation of cells with CMAC dye before imaging them as “30 min”, “9 h” or “24 h” time-point. For imaging, cells at all time-points were immobilized in 1% low-melt agarose. For this, 2 µl of cell culture was placed on a slide and merged with 5 µl of 1% low-melt agarose at 37°C placed on a coverslip. Cells in 5 non-overlapping fields of view were imaged on the Eclipse Ti2-E inverted microscope equipped with the CFI Plan Apochromat Lambda 100x oil objective and operated by the NIS Elements AR v5.20 software (Nikon Instruments Inc.). All experiments were performed at least twice in duplicate.
2.5. Statistical Analysis
Statistical analysis was performed in the Microsoft Excel 2016 software on data obtained from at least three independent experiments in duplicate (N = 6). Statistical results are presented as average ± standard deviation. The Student’s t-test (two-tailed distribution, two-sample unequal variance) was used to probe for statistical significance. Differences between two groups of samples were considered statistically significant if p < 0.05.
3. Results
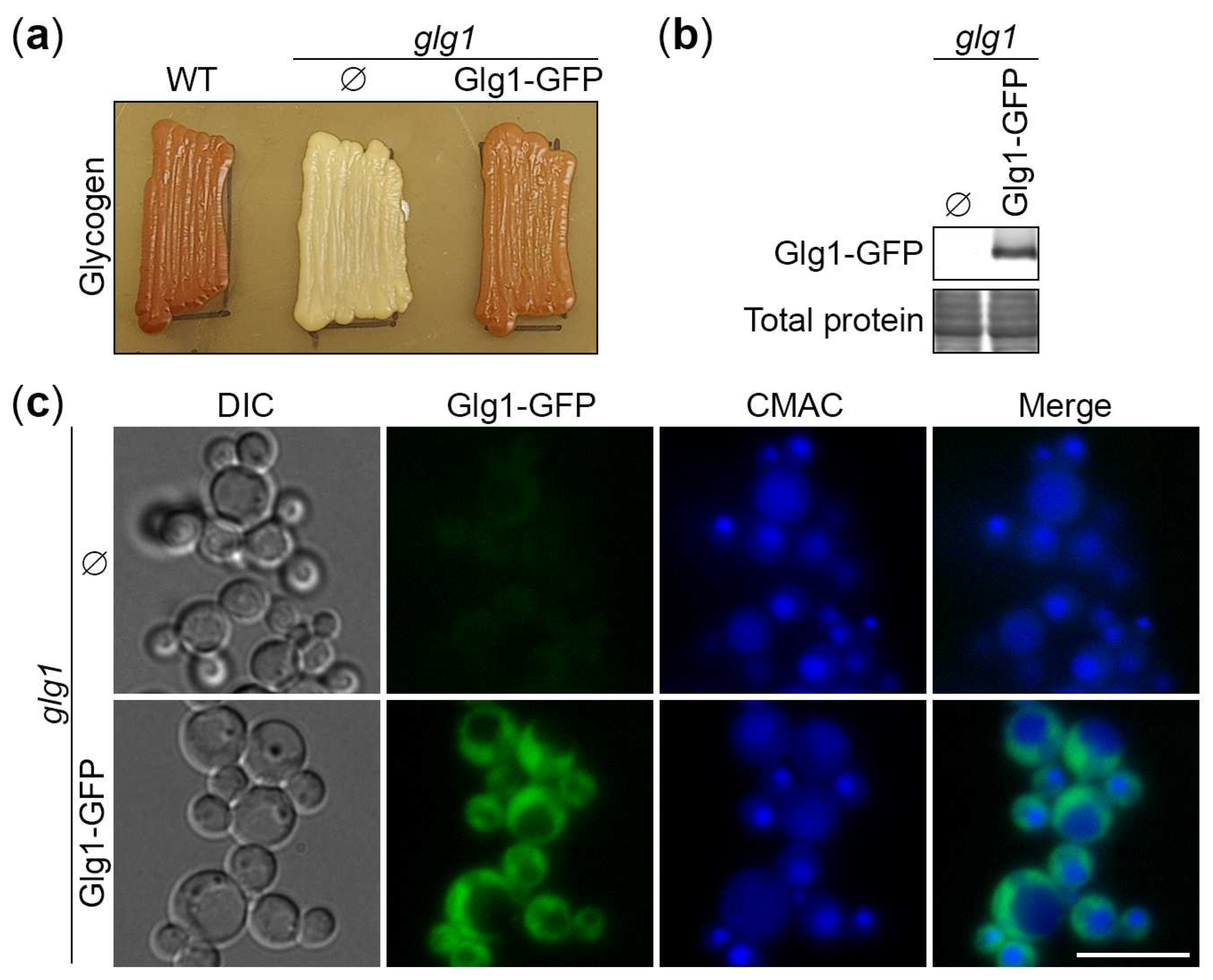
3.1. Glg1-GFP Fusion Protein is Functional in Glycogen Synthesis
To develop
K.
phaffii as a model for autophagy of GGs, we first tested if Glg1 is indeed a glycogenin responsible for the initiation of glycogen synthesis in this yeast species. For this, we constructed the
glg1 deletion mutant by gene replacement method (see Strains and Plasmids for details). Then, we studied glycogen synthesis in this strain on YPD plates using the iodine staining for glycogen and found that
glg1 cells are indeed fully deficient in the production of glycogen relative to WT strain (
Figure 1a), as expected.
Next, we used Glg1, which is a
bona fide marker of GGs because of its covalent linkage with glycogen, to create the Glg1-GFP fusion protein. For this, we generated the integrative plasmid, pRK22, with the P
GLG1-
GLG1-
GFP expression cassette (see Strains and Plasmids for details). We transformed this plasmid into
glg1 mutant and confirmed the expression of Glg1-GFP in YPD medium by western blotting using GFP antibodies (
Figure 1b). Also, we studied the localization of Glg1-GFP relative to the vacuole stained with CMAC dye [
20] by fluorescence microscopy (
Figure 1c). This experiment in YPD medium showed a diffuse cytosolic localization of Glg1-GFP, as expected. Finally, we tested if Glg1-GFP could rescue glycogen synthesis in
glg1 mutant and found that it was able to restore the production of glycogen in this glycogen-deficient strain (
Figure 1a). This suggests that Glg1-GFP is functional (i.e., capable of self-glucosylation for initiation of glycogen synthesis). As such, it can be used as a fluorescent marker of GGs.
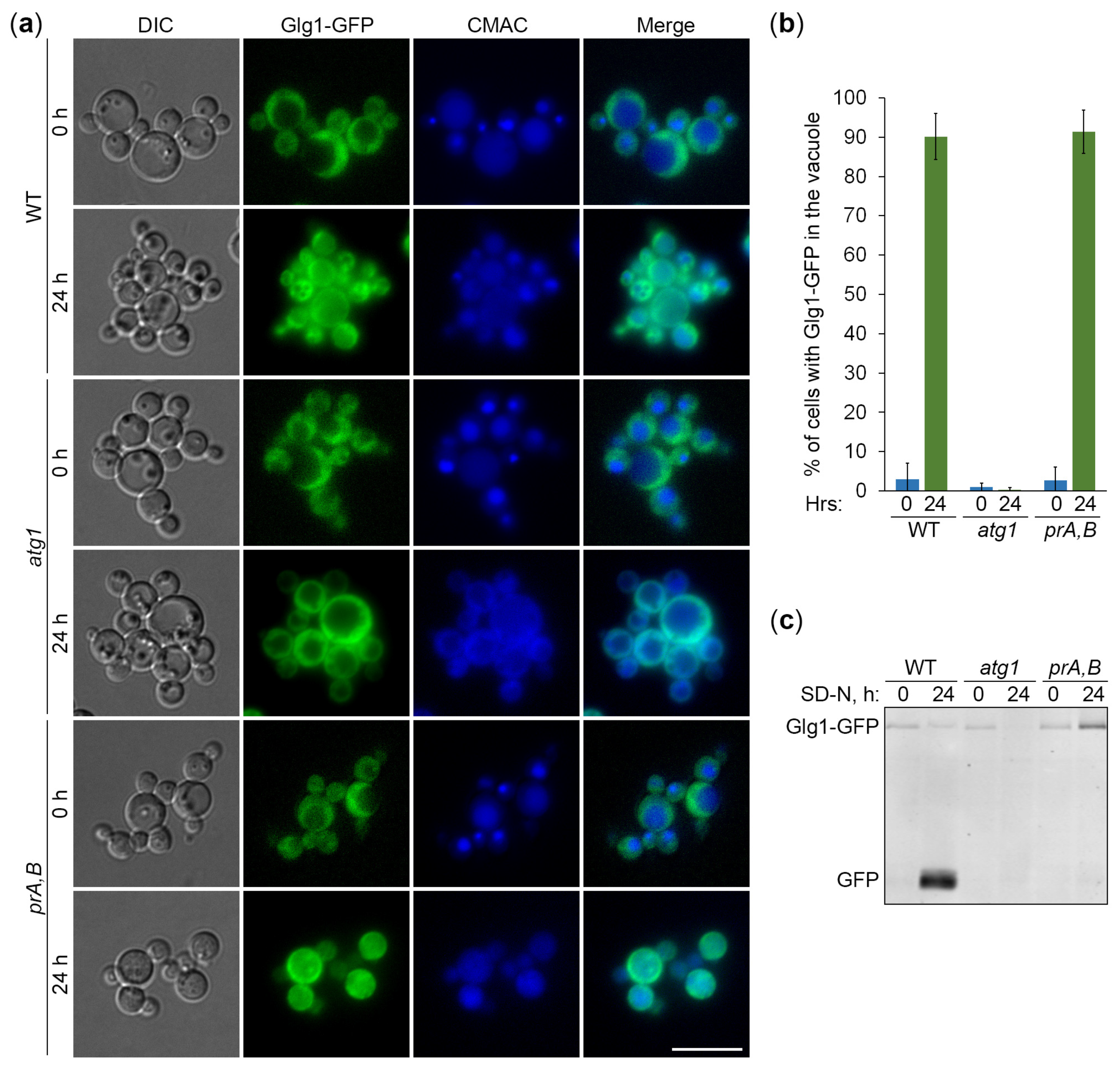
3.2. Degradation of Glycogen Granules Depends on Autophagy and Vacuole
Nitrogen starvation in SD-N medium is a strong inducer of both selective and non-selective autophagic pathways in yeast. Previously, we developed
K.
phaffii as a model for the nitrogen starvation-induced lipophagy, the selective autophagy of lipid droplets [
21]. To study the mechanism of GG degradation in the nitrogen starved
K.
phaffii, we employed Glg1-GFP and mutants deficient in autophagy (
atg1) and vacuolar proteolysis (
pep4 prb1, also known as
prA,B). In the first experiment, we monitored the delivery of Glg1-GFP tagged GGs to the vacuole by fluorescence microscopy (
Figure 2a). The cells of WT,
atg1 and
prA,B strains that express Glg1-GFP were grown for 1 d in YPD medium. First, a fraction of cells was transferred to SD-N medium at OD
600 = 1 for 24 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. At this time-point, all the strains had Glg1-GFP localized in the cytosol, as expected (
Figure 2a). The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “24 h”. At that time-point, we observed a remarkable redistribution of Glg1-GFP from the cytosol to the vacuole in WT and
prA,B strains, but not in
atg1 mutant (
Figure 2a). These results were confirmed by the quantification of images (
Figure 2b). The percentage of cells with Glg1-GFP inside the vacuole increased sharply after 24 h of nitrogen starvation for WT and
prA,B strains, but not for
atg1 mutant. Therefore, we concluded that GGs are delivered to the vacuole by autophagy.
In the second experiment, we examined the degradation of Glg1-GFP tagged GGs in the vacuole. The same strains were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD
600 = 1. At 0 and 24 h, equal volumes of cultures were taken from SD-N for immunoblotting with GFP antibodies (
Figure 2c). The immunoblotting showed that Glg1-GFP is efficiently processed to free GFP in nitrogen starved WT, but not
atg1 and
prA,B cells. The GFP processing assay is widely used in yeast autophagy studies because accumulation of proteolytically stable free GFP indicates delivery of the GFP-fusion protein to the vacuole and its partial degradation therein. The lack of free GFP in
atg1 cells (
Figure 2c) is consistent with the delivery block of GGs to the vacuole in
atg1 cells (
Figure 2a), while the lack of free GFP in
prA,B cells (
Figure 2c) is consistent with the vacuolar degradation of Glg1 by proteinases A and B under nitrogen starvation conditions. Collectively, in these experiments, we established that degradation of GGs in nitrogen starved
K.
phaffii cells depends on both autophagy and the vacuole.
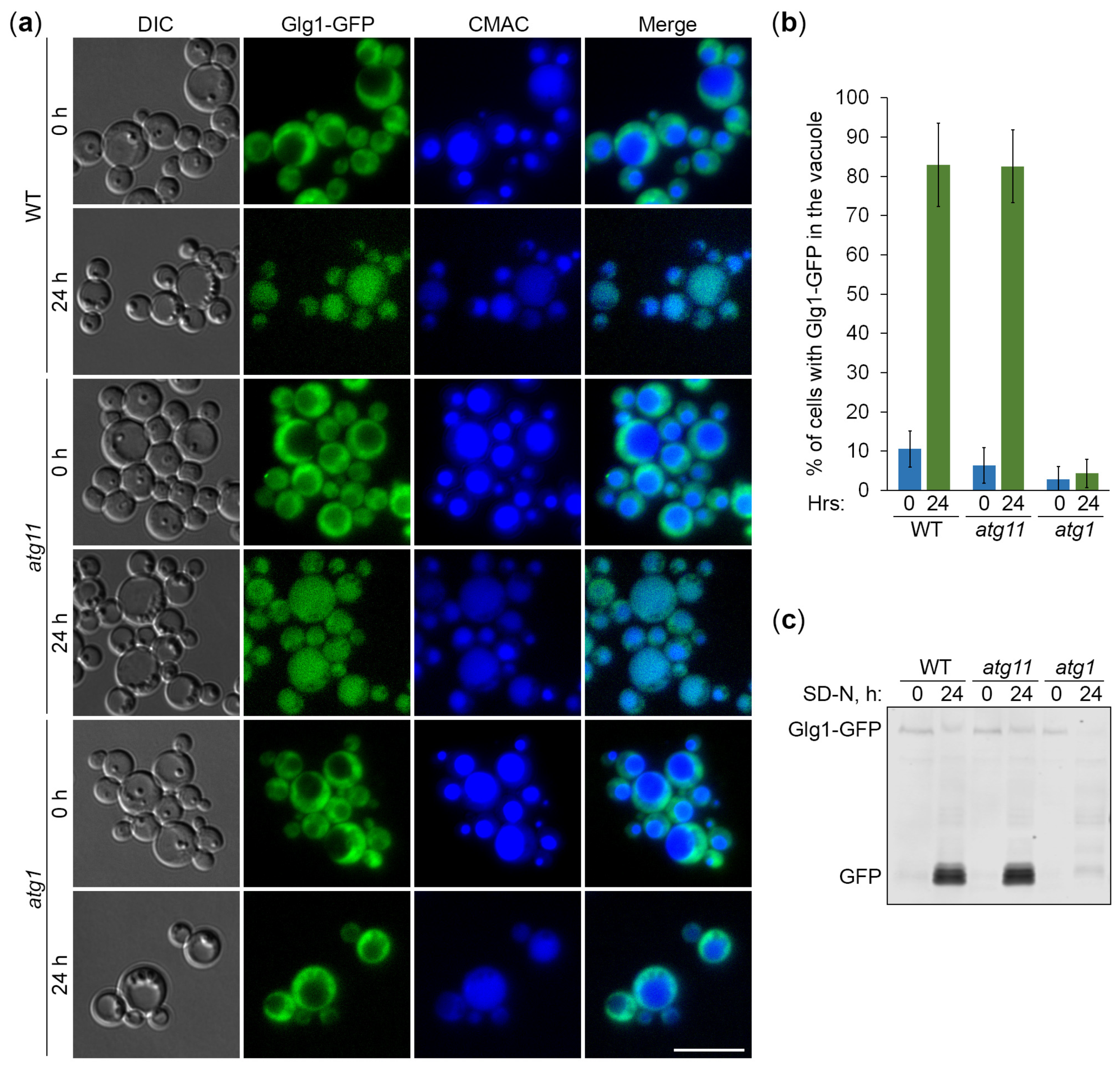
3.3. Autophagy of Glycogen Granules is Independent of Atg11
There are two major types of autophagy based on the specificity of cargo sequestration by autophagosomes, selective and non-selective autophagy. Most selective autophagy pathways utilize the autophagic scaffold protein, Atg11, which is dispensable for a non-selective autophagy [
22]. To gain insight into the specificity of GG autophagy in nitrogen starved
K.
phaffii, we used the Glgl-GFP autophagic reporter and
atg11 mutant. First, we monitored the delivery of GGs to the vacuole by microscopy (
Figure 3a). The cells of WT,
atg11 and
atg1 strains that express Glg1-GFP were grown in YPD, transferred to SD-N, and stained with CMAC, as above. At 0 h time-point, Glg1-GFP was diffused in the cytosol in all the strains, as expected, but after 24 h in SD-N, Glg1-GFP moved from the cytosol to the vacuole in WT and
atg11, but not in
atg1, cells (
Figure 3a). Quantification of images confirmed these observations (
Figure 3b). The percentage of cells with Glg1-GFP inside the vacuole increased dramatically after 24 h of nitrogen starvation for both WT and
atg11, but not
atg1. These results suggest that the autophagic delivery of GGs to the vacuole is an Atg11-independent process.
Next, we examined the degradation of GGs in the vacuole (
Figure 3c). The same strains with Glg1-GFP were grown in YPD, transferred to SD-N, and sampled for immunoblotting, as above. The immunoblotting showed that Glg1-GFP is efficiently processed to free GFP in nitrogen starved WT and
atg11, but not
atg1, cells (
Figure 3c). The presence of free GFP in
atg11 cells (
Figure 3c) is consistent with the vacuolar delivery of GGs in
atg11 cells (
Figure 3a) under nitrogen starvation conditions. Altogether, these results suggest that autophagy of GGs in nitrogen starved
K.
phaffii cells is independent of Atg11 raising a possibility that it might belong to a non-selective type of autophagy.
3.4. Autophagy of Glycogen Granules is a Non-Selective Process
To gain further insight into the specificity of Atg11-independent autophagy of GGs, we created two additional expression cassettes: (1) P
GLG1-
GLG1Y212F-
GFP on pNW11 plasmid, and (2) P
GLG1-
PGK1-
GFP on pNW10 plasmid (see Strains and Plasmids for details). The GFP-fusion proteins are expressed in all cassettes under the same (
GLG1) promoter (
Figure 4a). While the Y212F mutation in Glg1-GFP fusion creates glycogenin that lacks a conserved tyrosine residue essential for self-glucosylation [
5] what makes it effectively a cytosolic (not GG-bound) protein, the Pgk1-GFP fusion is a
bona fide cytosolic marker and an established reporter for a non-selective autophagy [
23]. We transformed pNW11 and pNW10 plasmids into
glg1 cells and confirmed that the mutated Glg1-GFP (Y212F) variant is indeed unable to rescue glycogen synthesis in these glycogen-deficient cells (similar to Pgk1-GFP, but in contrast to the non-mutated Glg1-GFP variant) (
Figure 4b). As such, these new cytosolic GFP-fusions can help clarify the type of autophagy of GGs.
To compare the delivery of GGs and cytosol to the vacuole,
glg1 strains that express GG-bound (Glg1-GFP) and cytosolic (Glg1-GFP [Y212F] and Pgk1-GFP) GFP-fusions were grown in YPD, transferred to SD-N, and stained with CMAC. At 0 h of nitrogen starvation, Glg1-GFP and Pgk1-GFP were diffused in the cytosol, as expected, but Glg1-GFP (Y212F) accumulated on dot-like structures (
Figure 4c). However, these Glg1-GFP (Y212F) dots that were present in 81% of cells at 0 min of nitrogen starvation quickly dissolved making cytosolic localization of this GFP-fusion evident just 30 min into nitrogen starvation before any bulk delivery of the protein to the vacuole (
Figure 4e,f). After 9 h in SD-N, all three GFP-fusions moved from the cytosol to the vacuole (
Figure 4c). Quantification of images showed that vacuolar deliveries of GG-bound Glg1-GFP and cytosolic Glg1-GFP (Y212F) were no more efficient than a non-selective vacuolar delivery of Pgk1-GFP (
Figure 4d). Therefore, GGs are delivered to the vacuole by a non-selective autophagy.
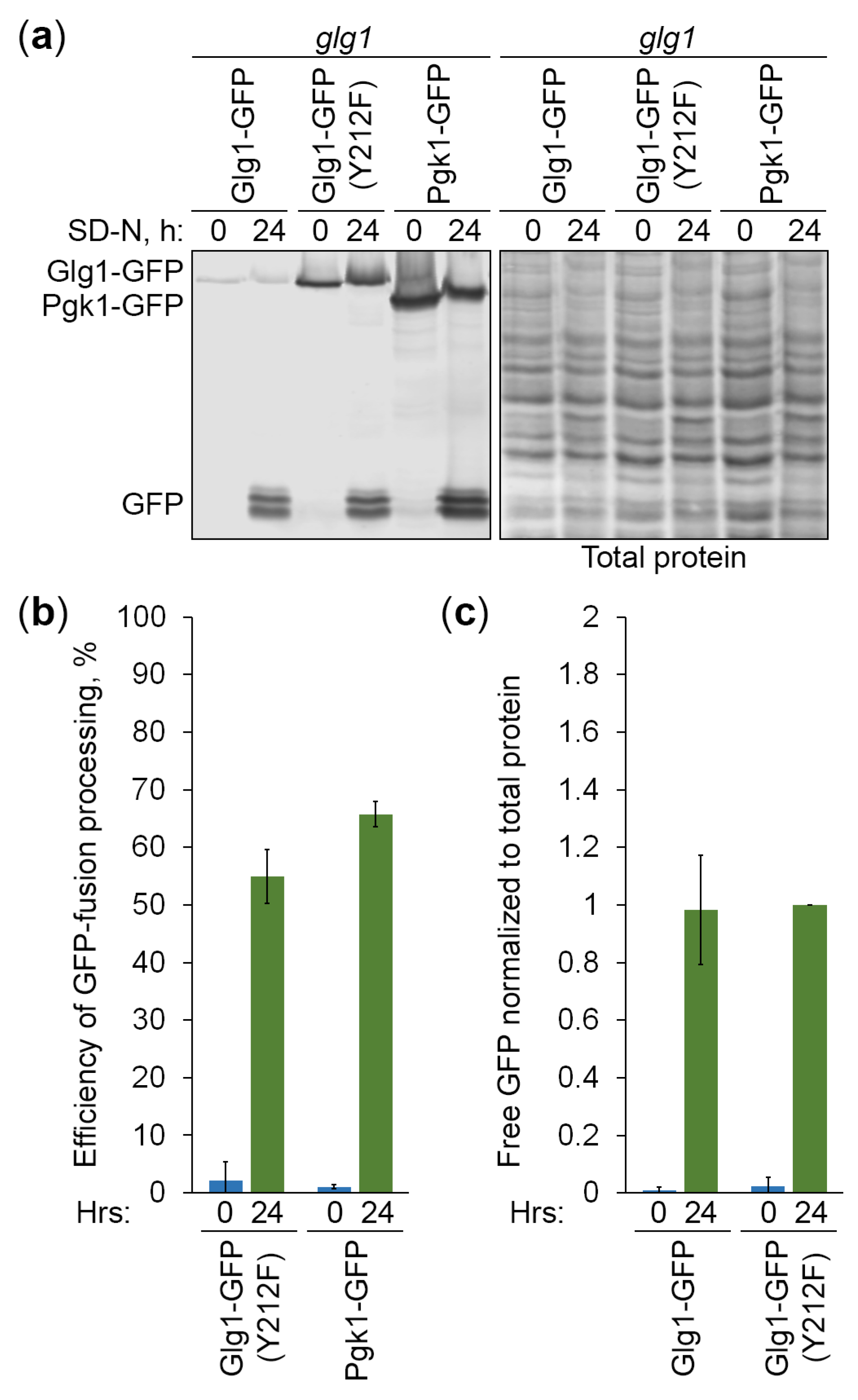
To compare the vacuolar degradation of GGs and cytosol, the above strains that express GG-bound and cytosolic GFP-fusions were grown in YPD, transferred to SD-N, and sampled for immunoblotting. Despite the same promoter was used to express GFP-fusions, the immunoblotting with GFP antibodies showed that protein levels were quite different (
Figure 5a). They increased in the order from Glg1-GFP to Glg1-GFP (Y212F) to Pgk1-GFP. Higher levels of Glg1-GFP (Y212F) than Glg1-GFP is most probably due to a better TCA precipitation of the non-glucosylated (mutated) protein than protein that is covalently bound to GGs. Indeed, a sizable portion of GGs is acid-soluble [
1] leading to the loss of GG-bound Glg1-GFP during protein lysate preparation by TCA precipitation. As such, the efficiency of GFP-fusion processing (GFP to [GFP + GFP-fusion] ratio) cannot be accurately assessed for Glg1-GFP, because levels of Glg1-GFP are underestimated. Despite the levels of Pgk1-GFP were slightly higher than those of Glg1-GFP (Y212F), the efficiencies of GFP-fusion processing were comparable for two proteins (
Figure 5b). This suggests that cytosolic Glg1-GFP (Y212F) is degraded by a non-selective autophagy, like Pgk1-GFP, in agreement with comparable vacuolar delivery of two proteins (
Figure 4d).
Even though we could precipitate only minute amounts of GG-bound Glg1-GFP, its delivery to the vacuole and vacuolar processing released free GFP that is precipitated by TCA same as GFP released from other GFP-fusions. Therefore, free GFP levels liberated from different GFP-fusions could be compared after their normalization to total protein levels. We excluded the free GFP from Pgk1-GFP fusion from this analysis, because Pgk1-GFP had higher expression levels, which caused higher free GFP levels. The free GFP levels from other two GFP-fusions normalized to corresponding total protein levels were nearly identical (
Figure 5c). Therefore, we concluded that GG-bound Glg1-GFP is degraded by a non-selective autophagy, like cytosolic Glg1-GFP (Y212F), in agreement with comparable efficiencies of their delivery to the vacuole (
Figure 4d).
4. Discussion
In this study, we developed
K. phaffii yeast as the first simple model to study autophagy of GGs and addressed the type of autophagy responsible for their elimination under nitrogen starvation conditions. To monitor the delivery of GGs to the vacuole and their degradation therein, we took advantage of the covalent linkage between GG and glycogenin (Glg1) protein and created the Glg1-GFP fusion protein. First, we proved that Glg1-GFP fusion is functional (i.e., able to synthesize glycogen in
glg1 mutant, which is fully devoid of this storage polysaccharide) (
Figure 1). Then, we showed that the degradation of GGs in the nitrogen starved
K. phaffii cells depends on autophagy and the vacuole (
Figure 2). However, it does not depend on the most common autophagic selectivity factor, Atg11 (
Figure 3) questioning the selectivity of GG autophagy. To clarify the type of GG autophagy, we compared efficiencies of vacuolar delivery and degradation for GGs and cytosol, which is known to be degraded by a non-selective autophagy. For this, we used the established autophagic reporter for cytosol, Pgk1-GFP. Also, we disrupted the ability of Glg1-GFP to generate GGs by introducing Y212F mutation making this GFP-fusion a cytosolic protein. The experiments with these additional autophagic reporters and non-mutated Glg1-GFP showed that GGs, similar to the cytosol, are delivered to the vacuole (
Figure 4) and degraded there (
Figure 5) by a non-selective autophagy. Therefore, autophagy of GGs is a non-selective process in the nitrogen starved
K. phaffii cells.
It will be interesting to see if same is true in
K. phaffii cells under other experimental conditions, e.g. during gradual carbon starvation in the stationary phase of growth. Previously, we showed that the nitrogen starvation lipophagy and stationary phase lipophagy have distinct molecular mechanisms [
21]. Therefore, we cannot exclude the possibility that autophagy of GGs is a selective process in
K. phaffii cells under other circumstances, like carbon starvation. Moreover, studies in nitrogen starved cells of other yeast species, e.g.
S. cerevisiae, must be conducted to clarify if a non-selective autophagy of GGs is a generic response of yeasts to nitrogen starvation. It would be even more important to re-evaluate the autophagy of GGs in mammalian cells because it contributes to Pompe disease, which is a lysosomal storage disorder with overaccumulation of GGs inside lysosomes [
8]. There is evidence of selectivity for the delivery of GGs to lysosomes in mouse liver where it is mediated by Stbd1 protein, but Stbd1 does not contribute to this process in cardiac and skeletal muscles [
24,
25]. Hence, it is possible that in non-hepatic tissues, autophagy of GGs is a non-selective process, like in
K. phaffii cells.
Author Contributions
Conceptualization, R.K. and T.Y.N.; methodology, N.V.W., R.K. and T.Y.N.; validation, N.V.W. and T.Y.N.; formal analysis, N.V.W. and T.Y.N; investigation, N.V.W.; resources, T.Y.N.; data curation, N.V.W.; writing—original draft preparation, N.V.W. and T.Y.N.; writing—review and editing, R.K.; visualization, N.V.W. and T.Y.N.; supervision, T.Y.N.; project administration, T.Y.N.; funding acquisition, T.Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH, grant number GM119571. It was also funded by GSU Research Initiation Grant. N.V.W. was also supported by GSU Molecular Basis of Disease fellowship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Prats, C.; Graham, T.E.; Shearer, J. The dynamic life of the glycogen granule. J Biol Chem 2018, 293, 7089-7098. [CrossRef]
- Li, C.; Hu, Z. Is liver glycogen fragility a possible drug target for diabetes? FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2020, 34, 3-15. [CrossRef]
- Liu, Q.H.; Tang, J.W.; Wen, P.B.; Wang, M.M.; Zhang, X.; Wang, L. From Prokaryotes to Eukaryotes: Insights Into the Molecular Structure of Glycogen Particles. Frontiers in molecular biosciences 2021, 8, 673315. [CrossRef]
- Cheng, C.; Mu, J.; Farkas, I.; Huang, D.; Goebl, M.G.; Roach, P.J. Requirement of the self-glucosylating initiator proteins Glg1p and Glg2p for glycogen accumulation in Saccharomyces cerevisiae. Molecular and cellular biology 1995, 15, 6632-6640. [CrossRef]
- Mu, J.; Cheng, C.; Roach, P.J. Initiation of glycogen synthesis in yeast. Requirement of multiple tyrosine residues for function of the self-glucosylating Glg proteins in vivo. J Biol Chem 1996, 271, 26554-26560. [CrossRef]
- Wilson, W.A.; Roach, P.J.; Montero, M.; Baroja-Fernández, E.; Muñoz, F.J.; Eydallin, G.; Viale, A.M.; Pozueta-Romero, J. Regulation of glycogen metabolism in yeast and bacteria. FEMS microbiology reviews 2010, 34, 952-985. [CrossRef]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA clinical 2016, 5, 85-100. [CrossRef]
- Raben, N.; Schreiner, C.; Baum, R.; Takikita, S.; Xu, S.; Xie, T.; Myerowitz, R.; Komatsu, M.; Van der Meulen, J.H.; Nagaraju, K.; et al. Suppression of autophagy permits successful enzyme replacement therapy in a lysosomal storage disorder--murine Pompe disease. Autophagy 2010, 6, 1078-1089. [CrossRef]
- Jiang, S.; Heller, B.; Tagliabracci, V.S.; Zhai, L.; Irimia, J.M.; DePaoli-Roach, A.A.; Wells, C.D.; Skurat, A.V.; Roach, P.J. Starch binding domain-containing protein 1/genethonin 1 is a novel participant in glycogen metabolism. J Biol Chem 2010, 285, 34960-34971. [CrossRef]
- Jiang, S.; Wells, C.D.; Roach, P.J. Starch-binding domain-containing protein 1 (Stbd1) and glycogen metabolism: Identification of the Atg8 family interacting motif (AIM) in Stbd1 required for interaction with GABARAPL1. Biochemical and biophysical research communications 2011, 413, 420-425. [CrossRef]
- Cregg, J.M.; Russell, K.A. Transformation. Methods Mol Biol 1998, 103, 27-39. [CrossRef]
- Gould, S.J.; McCollum, D.; Spong, A.P.; Heyman, J.A.; Subramani, S. Development of the yeast Pichia pastoris as a model organism for a genetic and molecular analysis of peroxisome assembly. Yeast 1992, 8, 613-628. [CrossRef]
- Stromhaug, P.E.; Bevan, A.; Dunn, W.A., Jr. GSA11 encodes a unique 208-kDa protein required for pexophagy and autophagy in Pichia pastoris. J Biol Chem 2001, 276, 42422-42435. [CrossRef]
- Kim, J.; Kamada, Y.; Stromhaug, P.E.; Guan, J.; Hefner-Gravink, A.; Baba, M.; Scott, S.V.; Ohsumi, Y.; Dunn, W.A., Jr.; Klionsky, D.J. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J Cell Biol 2001, 153, 381-396. [CrossRef]
- Tuttle, D.L.; Dunn, W.A., Jr. Divergent modes of autophagy in the methylotrophic yeast Pichia pastoris. J Cell Sci 1995, 108 ( Pt 1), 25-35. [CrossRef]
- Kumar, R.; Shroff, A.; Nazarko, T.Y. Komagataella phaffii Cue5 Piggybacks on Lipid Droplets for Its Vacuolar Degradation during Stationary Phase Lipophagy. Cells 2022, 11. [CrossRef]
- Enjalbert, B.; Parrou, J.L.; Vincent, O.; François, J. Mitochondrial respiratory mutants of Saccharomyces cerevisiae accumulate glycogen and readily mobilize it in a glucose-depleted medium. Microbiology (Reading, England) 2000, 146 ( Pt 10), 2685-2694. [CrossRef]
- Torija, M.J.; Novo, M.; Lemassu, A.; Wilson, W.; Roach, P.J.; François, J.; Parrou, J.L. Glycogen synthesis in the absence of glycogenin in the yeast Saccharomyces cerevisiae. FEBS letters 2005, 579, 3999-4004. [CrossRef]
- Baerends, R.J.; Faber, K.N.; Kram, A.M.; Kiel, J.A.; van der Klei, I.J.; Veenhuis, M. A stretch of positively charged amino acids at the N terminus of Hansenula polymorpha Pex3p is involved in incorporation of the protein into the peroxisomal membrane. J Biol Chem 2000, 275, 9986-9995. [CrossRef]
- Stefan, C.J.; Blumer, K.J. A syntaxin homolog encoded by VAM3 mediates down-regulation of a yeast G protein-coupled receptor. J Biol Chem 1999, 274, 1835-1841. [CrossRef]
- Kumar, R.; Rahman, M.A.; Nazarko, T.Y. Nitrogen Starvation and Stationary Phase Lipophagy Have Distinct Molecular Mechanisms. Int J Mol Sci 2020, 21. [CrossRef]
- Zientara-Rytter, K.; Subramani, S. Mechanistic Insights into the Role of Atg11 in Selective Autophagy. J Mol Biol 2020, 432, 104-122. [CrossRef]
- Welter, E.; Thumm, M.; Krick, R. Quantification of nonselective bulk autophagy in S. cerevisiae using Pgk1-GFP. Autophagy 2010, 6, 794-797. [CrossRef]
- Yi, H.; Fredrickson, K.B.; Das, S.; Kishnani, P.S.; Sun, B. Stbd1 is highly elevated in skeletal muscle of Pompe disease mice but suppression of its expression does not affect lysosomal glycogen accumulation. Mol Genet Metab 2013, 109, 312-314. [CrossRef]
- Sun, T.; Yi, H.; Yang, C.; Kishnani, P.S.; Sun, B. Starch Binding Domain-containing Protein 1 Plays a Dominant Role in Glycogen Transport to Lysosomes in Liver. J Biol Chem 2016, 291, 16479-16484. [CrossRef]
Figure 1.
Glg1-GFP fusion protein is functional in glycogen synthesis. (a) Glycogen synthesis in glg1 cells with the Glg1-GFP fusion protein. Patches of WT, empty glg1 and glg1 rescued with Glg1-GFP plasmid were grown for 2 d on YPD plate and exposed to the vapor of iodine crystals for glycogen staining. Patches synthesizing glycogen were stained brown. (b) Expression of the Glg1-GFP fusion protein. The cells of glg1 mutant without and with Glg1-GFP plasmid were grown for 1 d in YPD medium and studied for Glg1-GFP expression by immunoblotting with GFP antibodies. The total protein staining was used as a loading control. (c) Localization of the Glg1-GFP fusion protein. The same strains were grown for 1 d in YPD medium and studied for Glg1-GFP localization relative to the vacuole stained with CMAC dye. Scale bar, 10 µm.
Figure 1.
Glg1-GFP fusion protein is functional in glycogen synthesis. (a) Glycogen synthesis in glg1 cells with the Glg1-GFP fusion protein. Patches of WT, empty glg1 and glg1 rescued with Glg1-GFP plasmid were grown for 2 d on YPD plate and exposed to the vapor of iodine crystals for glycogen staining. Patches synthesizing glycogen were stained brown. (b) Expression of the Glg1-GFP fusion protein. The cells of glg1 mutant without and with Glg1-GFP plasmid were grown for 1 d in YPD medium and studied for Glg1-GFP expression by immunoblotting with GFP antibodies. The total protein staining was used as a loading control. (c) Localization of the Glg1-GFP fusion protein. The same strains were grown for 1 d in YPD medium and studied for Glg1-GFP localization relative to the vacuole stained with CMAC dye. Scale bar, 10 µm.
Figure 2.
Degradation of glycogen granules depends on autophagy and vacuole. (a) Delivery of glycogen granules to the vacuole. The cells of WT, atg1 and prA,B (pep4 prb1) strains that express Glg1-GFP were grown for 1 d in YPD. First, a fraction of cells was transferred to SD-N at OD600 = 1 for 24 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “24 h”. Scale bar, 10 µm. (b) Quantification of images in (a). Displayed are averages and standard deviations. (c) Degradation of glycogen granules in the vacuole. The cells of WT, atg1 and prA,B strains that express Glg1-GFP were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD600 = 1. At 0 and 24 h, equal volumes of cultures (not equal numbers of cells; therefore, loading control is not applicable) were taken from SD-N for immunoblotting with GFP antibodies.
Figure 2.
Degradation of glycogen granules depends on autophagy and vacuole. (a) Delivery of glycogen granules to the vacuole. The cells of WT, atg1 and prA,B (pep4 prb1) strains that express Glg1-GFP were grown for 1 d in YPD. First, a fraction of cells was transferred to SD-N at OD600 = 1 for 24 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “24 h”. Scale bar, 10 µm. (b) Quantification of images in (a). Displayed are averages and standard deviations. (c) Degradation of glycogen granules in the vacuole. The cells of WT, atg1 and prA,B strains that express Glg1-GFP were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD600 = 1. At 0 and 24 h, equal volumes of cultures (not equal numbers of cells; therefore, loading control is not applicable) were taken from SD-N for immunoblotting with GFP antibodies.
Figure 3.
Autophagy of glycogen granules is independent of Atg11. (a) Delivery of glycogen granules to the vacuole. The cells of WT, atg11 and atg1 strains that express Glg1-GFP were grown for 1 d in YPD. First, a fraction of cells was transferred to SD-N at OD600 = 1 for 24 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “24 h”. Scale bar, 10 µm. (b) Quantification of images in (a). Displayed are averages and standard deviations. (c) Degradation of glycogen granules in the vacuole. The cells of WT, atg11 and atg1 strains that express Glg1-GFP were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD600 = 1. At 0 and 24 h, equal volumes of cultures (not equal numbers of cells; therefore, loading control is not applicable) were taken from SD-N for immunoblotting with GFP antibodies.
Figure 3.
Autophagy of glycogen granules is independent of Atg11. (a) Delivery of glycogen granules to the vacuole. The cells of WT, atg11 and atg1 strains that express Glg1-GFP were grown for 1 d in YPD. First, a fraction of cells was transferred to SD-N at OD600 = 1 for 24 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “24 h”. Scale bar, 10 µm. (b) Quantification of images in (a). Displayed are averages and standard deviations. (c) Degradation of glycogen granules in the vacuole. The cells of WT, atg11 and atg1 strains that express Glg1-GFP were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD600 = 1. At 0 and 24 h, equal volumes of cultures (not equal numbers of cells; therefore, loading control is not applicable) were taken from SD-N for immunoblotting with GFP antibodies.
Figure 4.
Glycogen granules are delivered to the vacuole by a non-selective autophagy. (a) Cassettes for the expression of glycogen granule-bound (Glg1-GFP) and cytosolic (Glg1-GFP [Y212F] and Pgk1-GFP) GFP-fusion proteins under the same (GLG1) promoter. (b) Glycogen synthesis in glg1 cells with GFP-fusion proteins. Patches of indicated strains were grown for 2 d on YPD plate and exposed to the vapor of iodine crystals for glycogen staining (stained brown). (c) Delivery of GFP-fusion proteins to the vacuole. The glg1 cells that express indicated GFP-fusion proteins were grown for 1 d in YPD. First, a fraction of cells was transferred to SD-N at OD600 = 1 for 9 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “9 h”. Scale bar, 10 µm. (d) Quantification of images in (c). Displayed are averages and standard deviations. (e) Dissipation of Glg1-GFP (Y212F) dots before its bulk delivery to the vacuole. The glg1 cells that express Glg1-GFP (Y212F) were grown for 1 d in YPD. A fraction of cells was transferred to SD-N with CMAC for 30 min and imaged as “30 min” of nitrogen starvation. The rest of YPD culture was stained for 30 min with CMAC and imaged as “0 min” of nitrogen starvation. Scale bar, 10 µm. (f) Quantification of images in (e). Displayed are averages and standard deviations.
Figure 4.
Glycogen granules are delivered to the vacuole by a non-selective autophagy. (a) Cassettes for the expression of glycogen granule-bound (Glg1-GFP) and cytosolic (Glg1-GFP [Y212F] and Pgk1-GFP) GFP-fusion proteins under the same (GLG1) promoter. (b) Glycogen synthesis in glg1 cells with GFP-fusion proteins. Patches of indicated strains were grown for 2 d on YPD plate and exposed to the vapor of iodine crystals for glycogen staining (stained brown). (c) Delivery of GFP-fusion proteins to the vacuole. The glg1 cells that express indicated GFP-fusion proteins were grown for 1 d in YPD. First, a fraction of cells was transferred to SD-N at OD600 = 1 for 9 h. Then, the rest of YPD cultures was stained for 30 min with CMAC and imaged as “0 h”. The last 30 min of SD-N cultures was incubation with CMAC before imaging them as “9 h”. Scale bar, 10 µm. (d) Quantification of images in (c). Displayed are averages and standard deviations. (e) Dissipation of Glg1-GFP (Y212F) dots before its bulk delivery to the vacuole. The glg1 cells that express Glg1-GFP (Y212F) were grown for 1 d in YPD. A fraction of cells was transferred to SD-N with CMAC for 30 min and imaged as “30 min” of nitrogen starvation. The rest of YPD culture was stained for 30 min with CMAC and imaged as “0 min” of nitrogen starvation. Scale bar, 10 µm. (f) Quantification of images in (e). Displayed are averages and standard deviations.
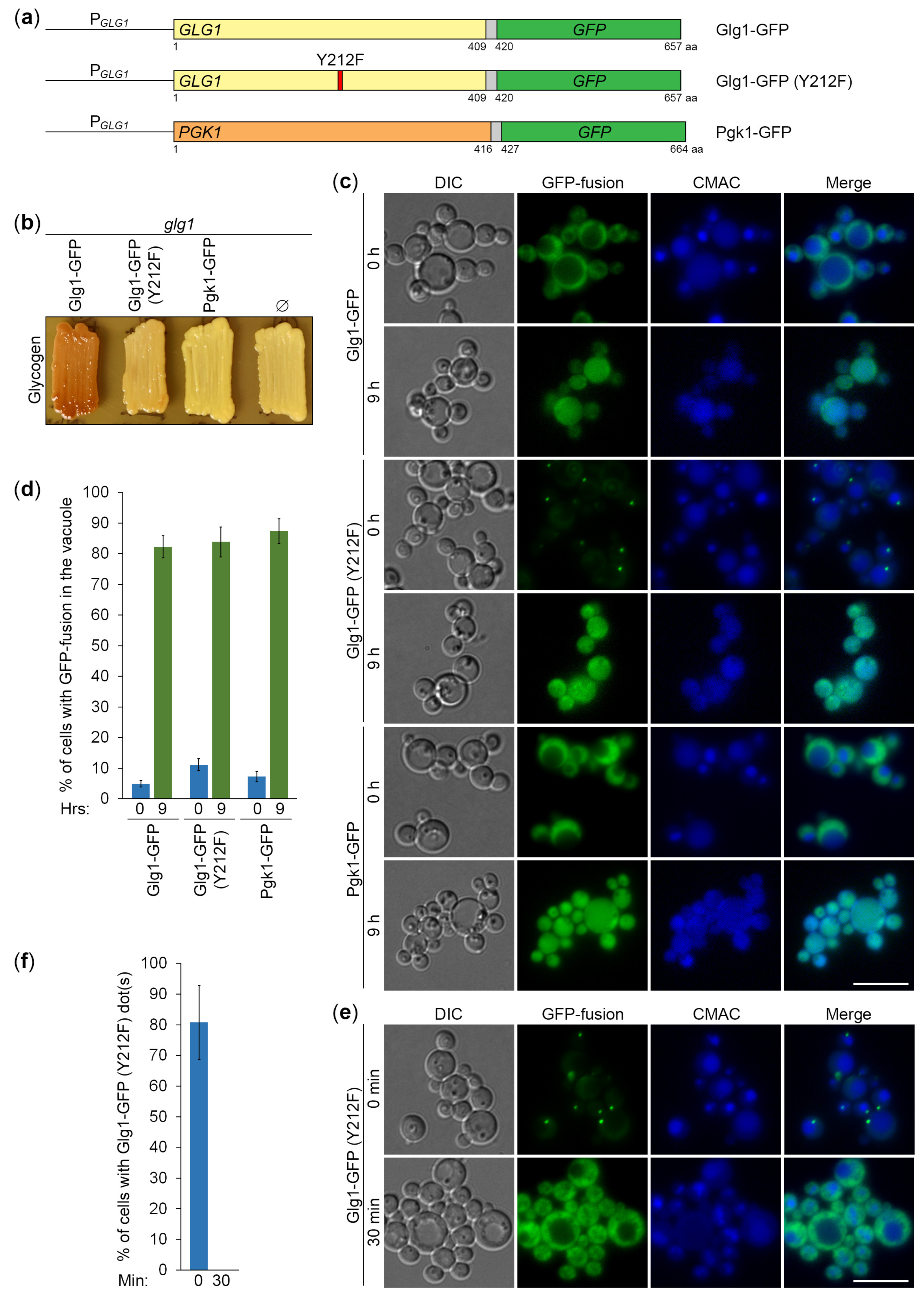
Figure 5.
Glycogen granules are degraded in the vacuole by a non-selective autophagy. (a) Processing of GFP-fusion proteins in the vacuole. The glg1 cells that express indicated GFP-fusion proteins were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD600 = 1. At 0 and 24 h, equal volumes of cultures (not equal numbers of cells; therefore, loading control is not applicable) were taken from SD-N for immunoblotting with GFP antibodies. The same membrane was also stained for total protein, which was used in the quantification below. (b, c) Quantifications of immunoblotting in (a). Displayed are averages and standard deviations.
Figure 5.
Glycogen granules are degraded in the vacuole by a non-selective autophagy. (a) Processing of GFP-fusion proteins in the vacuole. The glg1 cells that express indicated GFP-fusion proteins were grown for 1 d in YPD. Then, a fraction of cells was transferred to SD-N at OD600 = 1. At 0 and 24 h, equal volumes of cultures (not equal numbers of cells; therefore, loading control is not applicable) were taken from SD-N for immunoblotting with GFP antibodies. The same membrane was also stained for total protein, which was used in the quantification below. (b, c) Quantifications of immunoblotting in (a). Displayed are averages and standard deviations.
Table 1.
K. phaffii strains and plasmids used in this study.
Table 1.
K. phaffii strains and plasmids used in this study.
| Mutant |
Strain |
Background |
Genotype and Plasmid |
Source |
| WT |
PPY12h |
PPY12h |
arg4 his4 |
[12] |
| WT |
SRK147 |
PPY12h |
his4::pRK22 (PGLG1-GLG1-GFP, HIS4)
|
This study |
| atg1 |
R12 |
GS115 |
atg1-1::ZeocinR his4
|
[13] |
| atg1 |
SRK149 |
R12 |
his4::pRK22 (PGLG1-GLG1-GFP, HIS4)
|
This study |
| atg11 |
R8 |
GS115 |
atg11-2::ZeocinR his4
|
[14] |
| atg11 |
SNW7 |
R8 |
his4::pRK22 (PGLG1-GLG1-GFP, HIS4)
|
This study |
| glg1 |
SNW49 |
PPY12h |
Δglg1::ZeocinR (pNW9) |
This study |
| glg1 |
SNW65 |
SNW49 |
his4::pRK22 (PGLG1-GLG1-GFP, HIS4)
|
This study |
| glg1 |
SNW80 |
SNW49 |
his4::pNW11 (PGLG1-GLG1Y212F-GFP, HIS4)
|
This study |
| glg1 |
SNW82 |
SNW49 |
his4::pNW10 (PGLG1-PGK1-GFP, HIS4)
|
This study |
| pep4 prb1 |
SMD1163 |
GS115 |
pep4 prb1 his4 |
[15] |
| pep4 prb1 |
SRK151 |
SMD1163 |
his4::pRK22 (PGLG1-GLG1-GFP, HIS4)
|
This study |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).