1. Introduction
Anisakiosis is a parasitic infection caused by nematode parasites belonging to the family Anisakidae, genera
Anisakis,
Pseudoterranova and
Contracaecum. Human anisakiosis is most frequently caused by species of the genus
Anisakis, i.e.,
Anisakis simplex sensu stricto (s.s.),
Anisakis pegreffii,
Anisakis physeteris, and
Anisakis berlandi [
1].
Anisakis spp. has an indirect life cycle with marine mammals, i.e., porpoises, dolphins and whales as the definitive hosts. Adult
Anisakis spp. parasitise the gastrointestinal tract of these animals and produce eggs that are shed via the faeces into the water, where the developed larvae hatch and infect the intermediate hosts, i.e., various marine crustaceans. When consumed, the third-stage larvae (L3) in marine crustaceans are infective for the final hosts. Sea fish and cephalopods, that consume infected crustaceans, can harbour the infective L3 in their tissues, serving as paratenic hosts of the parasite. In fact, the infective larvae may have several passages from smaller to bigger fish, following the trophic chain in the sea until they reach their definitive host, where they eventually develop into adult nematodes.
Humans and fish-eating terrestrial animals are accidental and usually dead-end paratenic hosts of these parasites. Thus, humans acquire the infection by ingesting raw or undercooked seafood containing the infective L3 [
2], which invades the gastrointestinal mucosa causing direct gastric and intestinal damage or, rarely, extra-gastrointestinal inflammatory changes that can evolve either to acute abdomen necessitating surgical intervention or phlegmonous lesions and granulomatous changes in the peritoneum that can be misdiagnosed as tumours [
3,
4]. The clinical presentation of patients with anisakiosis includes abdominal pain, nausea, vomiting and allergic reactions ranging from urticaria to anaphylaxis [
3,
5,
6]. Allergic reactions can also occur in the presence of the parasite’s antigens, even if the parasites are consumed deactivated or dead [
1,
2].
The majority of human cases (over 90%) originate from countries of the Far East, particularly Japan, due to the food preparation habits in this area of the world. However, an increasing number of cases diagnosed in Europe and the American continent in the last decades can be attributed to the altered eating habits in Western countries [
3,
5,
7,
8]. In the present article, a case of invasive intestinal and ectopic anisakiosis is reported, in a young patient, who has been repeatedly exposed to the parasite by consuming homemade raw fish. The infection caused symptoms of subacute abdomen and mimicked intraperitoneal malignancy. Definitive diagnosis and management were accomplished by laparotomy. To the best of the authors' knowledge, this is the first report of clinical human anisakiosis in Greece.
2. Case description
A 22-year-old male patient, with unremarkable past medical history, presented to the emergency department of Papageorgiou General Hospital, Thessaloniki, Greece, complaining of abdominal pain located in the left lower quadrant that started about 12 hours before. The pain was associated with nausea and shivering, without vomiting or fever. The patient’s vital signs were normal and physical examination of the abdomen revealed tenderness in the left iliac region. An elevated white blood cell count (13.1 x 109/L) and slightly elevated CRP (1.2 mg/dl, with normal value <0.5 mg/dl) were found in the laboratory test and the patient was admitted to the surgical department for further evaluation.
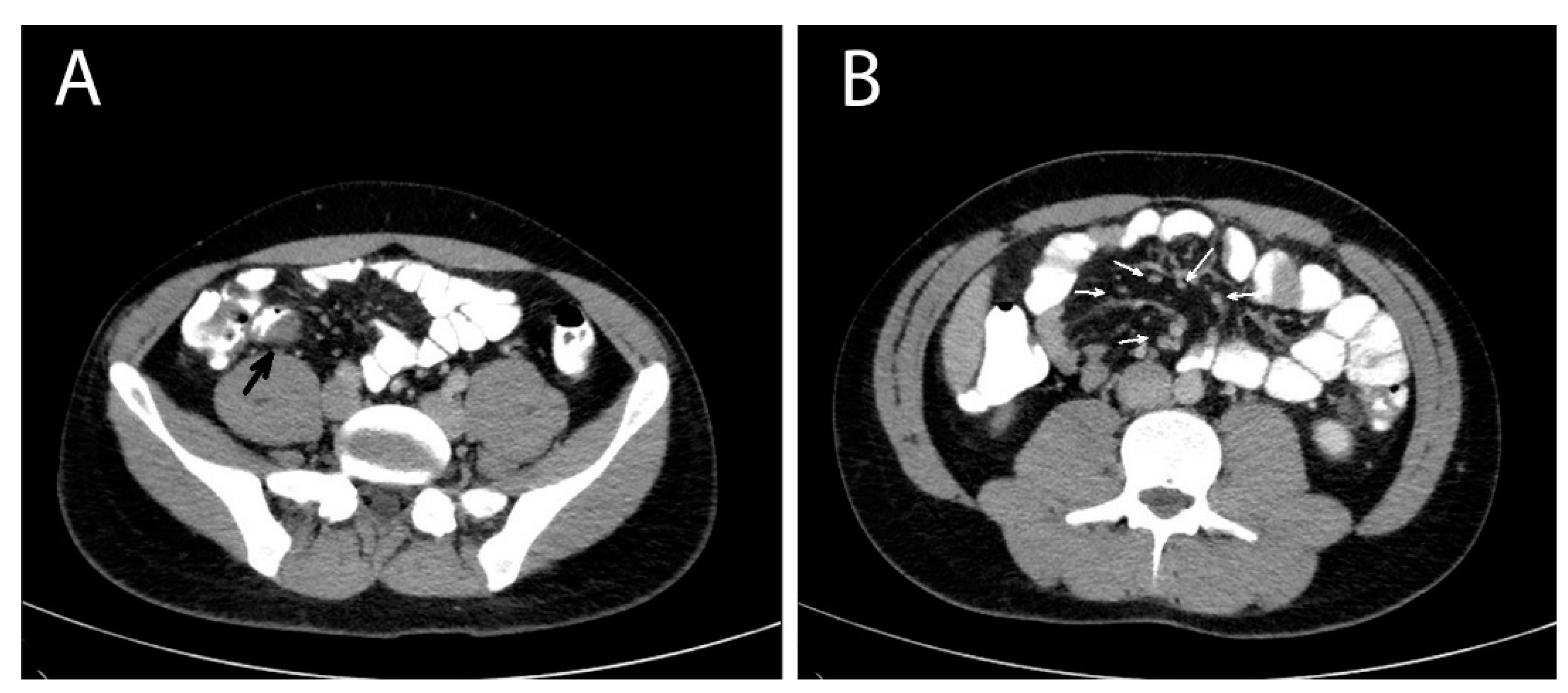
A contrast-enhanced computed tomography (CT) of the abdomen was performed the next day that showed multiple masses with soft tissue densities at the gastrocolic ligament (max ~1.1 cm), the left part of greater omentum (max ~1.5 cm), right paracolic gutter (max ~0.7 cm) and the left pararenal space (max ~1.7 cm), with multiple enlarged mesenteric lymph nodes along the ileocolic vessels (max ~1 cm), and at the aortic hiatus of the diaphragm (max ~1.2 cm). A thickening at the wall of the distal ileum right before the ileocolic junction was also depicted (
Figure 1). The CT findings suggested a neoplasmatic disease and endoscopy of the upper and lower gastrointestinal tract was decided. Oesophagogastroscopy was normal and colonoscopy revealed mild oedema of the mucosa of the distal ileum and a sessile polyp of the distal sigmoid. The investigation was completed with a computed tomography (CT) scan of the thorax, which was normal, and a contrast-enhanced MRI of the abdomen that depicted the same nodules and enlarged lymph nodes showed in the CT scan, in addition to a small quantity of pelvic fluid (ascites). During the investigation, the patient remained afebrile and hemodynamically stable, with however persistent abdominal symptoms and signs.
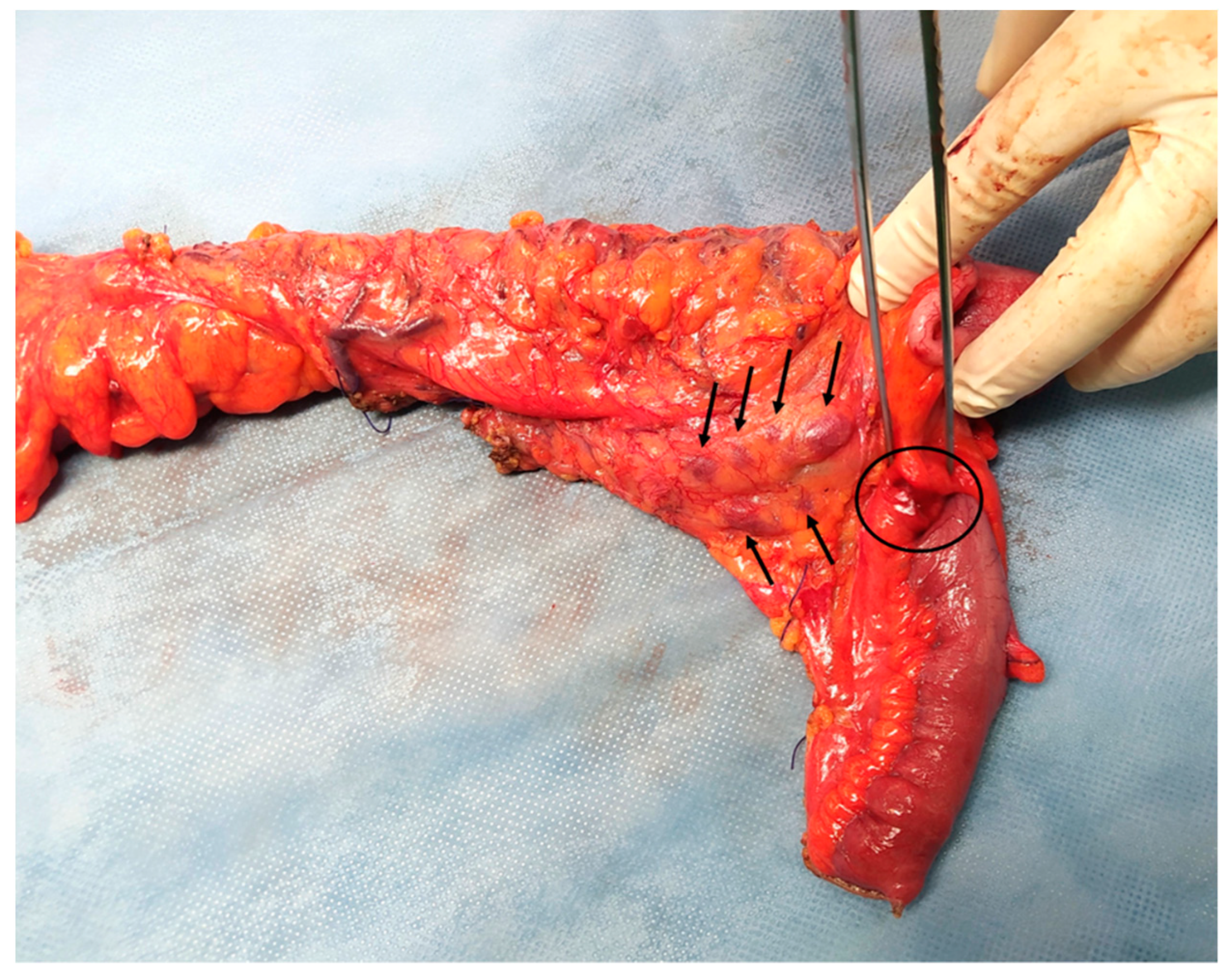
Considering the abovementioned findings, the young age of the patient and the absence of a definitive diagnosis, a diagnostic laparoscopy was performed, revealing dilated terminal ileum with localized oedema and a firm round nodule of ~1 cm in diameter in the mesenteric border of the distal ileum, about 2 cm from the ileocolic junction. Many enlarged lymph nodes were found along the ileocolic and middle colic vessels and the greater omentum. Another firm nodule was found in the mesocolic border of the descending colon approximately 2 cm in diameter (
Figure 2). There were no signs of diffuse peritoneal disease. Neoplasia was suspected and the operation converted to an open laparotomy through which an extended right colectomy and omentectomy were performed. A side-to-side ileotransverse anastomosis re-established the continuity of the gastrointestinal tract. The nodule of the descending colon was also excised. The patient had an uneventful course and was discharged on postoperative day 13.
Pathology of the surgical specimens revealed granulomatous tissue with eosinophilic infiltration at the masses at the terminal ileum, descending colon and omentum and granulomatous lymphadenitis with no sign of malignancy. Parasitosis was regarded as the most possible cause of the lesions. Accordingly, a faecal sample, blood serum and histology preparation were sent to the Laboratory of Parasitology and Parasitic Diseases, in the School of Veterinary Medicine, of the Aristotle University of Thessaloniki for further examinations. Treatment with albendazole (400 mg/kg of body weight, BID) for 28 days was initiated. Two weeks after the completion of antiparasitic therapy a CT scan of the abdomen confirmed no residual disease.
3. Parasitological, molecular, and serological examinations and results
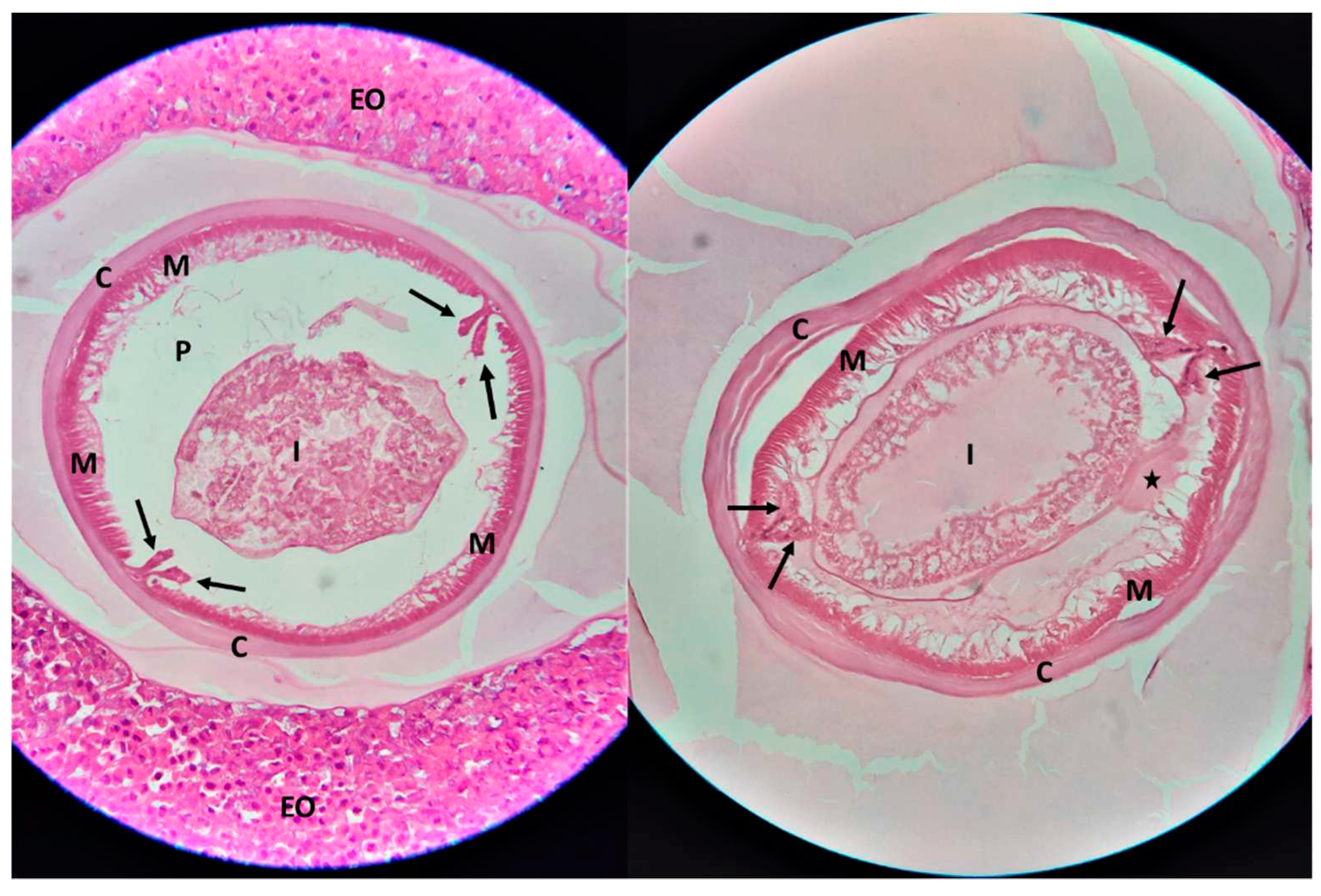
At the microscopical examination of the histological samples, cross sections of a nematode parasite were observed, in the middle of granulomatous lesions. The morphological characteristics of the nematode included a smooth cuticle, polymyarian/coelomyarian type muscle layer, prominent Y-shaped (clover-shaped) lateral chords, and a structure resembling the eosinophilic excretory organ (Renett cells) (
Figure 3). According to the morphological characteristics, the parasite was identified as
Anisakis spp. [
9,
10].
Sections from paraffin-embedded tissue biopsy underwent DNA extraction. Paraffin was first removed via xylene deparaffinization as follows: sections were placed in a microcentrifuge tube and were mixed with 1 ml of xylene, followed by incubation at room temperature for 30 min. After centrifugation (11,000 x g, 3 min) and discarding the supernatant, sections were subjected to a series of wash steps, using 100% and 75% ethanol, and PBS (1 ml each), respectively. All subsequent steps of the DNA extraction process were performed utilizing a commercially available DNA extraction kit (NucleoSpin Tissue; Macherey-Nagel, Düren, Germany).
Detection of
Anisakis spp. genome was performed via a previously published [
11] real-time PCR protocol targeting the internal transcriber spacer 1 (ITS1) region. The PCR reaction (25 μl) was comprised by QuantiFast SYBR Green PCR Master Mix (Qiagen, Hilden, Germany), 1 μM of each of the primers (Anir18F and Anir18R), 1 μl of Template DNA and nuclease-free water. A CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) was used. The following cycling conditions were applied: 95 °C for 5 min (initial denaturation and Taq polymerase activation), and 40 cycles in 2 steps: i) 95 °C for 10 sec (denaturation), and ii) 56 °C for 30 sec (annealing/extension), followed by fluorescence measurement. Subsequently, a melting curve was generated by heating the tubes from 65°C to 90°C in 0.2°C increments. Fluorescence data were analysed using the CFX Maestro Software (v4.1; Bio-Rad Laboratories, Hercules, CA, USA). Real-time PCR yielded a positive result with a melting curve analysis revealing a temperature peak of approx. 80°C, specific for the ITS1 of
Anisakis spp.
A serum sample collected on the day of operation from the patient was examined by an in-house ELISA, using crude antigen of
Anisakis spp., prepared as described before [
12] from parasites isolated from fish in a previous study [
13]. Specific anti-
Anisakis IgG [0.750 optical density units (OD), with a negative control at 0.120 OD] were detected. The serological examination was repeated 2 and 5 months after the completion of treatment with albendazole, scoring negative for specific anti-
Anisakis-antibodies.
The parasitological examination of faeces (ZnSO4 flotation and formalin-ether sedimentation methods) scored negative.
4. Discussion
The case described herein is the first clinical human anisakiosis reported in Greece. Following the confirmation of the nematode parasite as the cause of the excised nodular lesions and targeted questions directed at the patient, it was disclosed that he regularly consumed raw fish (sushi), nearly on a daily basis. A noteworthy detail of the anamnesis was that a considerable portion of the sushi he consumed was self-prepared, with fresh fish procured from the local fish market. Indeed, in the Greek market, the prevalence of
Anisakis infection in fish from the Aegean Sea has been documented to be between 18.8% and 98.8% [
13,
14].
Anisakiosis is a parasitic disease with a worldwide occurrence. The majority of cases originate from Japan (>90%), where eating raw fish, in the form of sushi and sashimi is very common [
8,
15]. It is estimated that approximately 20.000 new diagnoses of anisakiosis are made every year in Japanese hospitals [
16] and there is a rising number of reports from all over the world. [
3,
17,
18]. Among all countries reporting the disease, other than Japan, Spain, South Korea, and Italy have the highest number of published cases [
19]. The first case of anisakiosis in Europe was reported in the Netherlands in 1960 [
20], but currently, Spain has the highest load of anisakiosis incidents reported every year [
21].
Cases have been described in patients from 7 months to 85 years old, with the onset of symptoms ranging from direct (gastric anisakiosis) up to 2 months (intestinal and extraintestinal anisakiosis) after the consumption of raw or undercooked seafood. In some cases, the symptoms lasted up to 10 years [
5,
19].
Although anisakiosis is usually self-limiting, as the parasite survives in humans only for a few days, it may occasionally present as a severe parasitosis, with implications caused by the migratory, invasive behaviour of
Anisakis spp.-infective larvae [
8,
15,
22]. Accordingly, anisakiosis can present as gastric, intestinal, ectopic/extraintestinal or allergic reaction which can range from mild cutaneous manifestations to severe anaphylaxis [
3,
5,
8]. Symptoms include abdominal pain, nausea, vomiting and low-grade fever [
3]. When the stomach is affected, upper gastrointestinal endoscopy can offer both diagnosis and treatment, with the removal of the parasites [
3,
15,
23]. Intestinal and extraintestinal disease is challenging to diagnose because there are neither specific symptoms nor pathognomonic findings from common laboratory and imaging exams [
3,
5,
24]. The parasite invades the gastrointestinal wall, causing both direct damage to the tissue and allergic reactions, with an accumulation of neutrophils and eosinophils [
5]. Sometimes it can be misdiagnosed as acute intraabdominal inflammation (e.g., appendicitis, peritonitis), bowel obstruction or neoplasia. [
5,
23]. Indeed, the chronic form of the disease can result in granulomatous tissue formation, presenting as firm masses difficult to differentiate from neoplasm (mesenchymal tumours), radiologically and macroscopically [
3,
4,
15,
25,
26]. This form of the disease carries unfamiliar risks emerging serious diagnostic and therapeutic dilemmas. In such clinical case scenario, surgical exploration can decisively contribute to a definitive diagnosis and early identification of intraabdominal complications necessitating surgical intervention [
19], as in the present case.
Biochemical profile and imaging features of patients with anisakiosis are nonspecific, including leucocytosis, with or without eosinophilia, CRP elevation, gastric or bowel wall thickening, lymphadenopathy and ascites [
3,
5,
6,
27]. Imaging examinations can be more helpful but still not conclusive. Bowel wall thickening, lymphadenopathy and ascites are usually found in CT scan [
3,
5,
6,
27,
28]. Ultrasound examination can be also used for the evaluation of patients, but it requires a high rate of suspicion and is inferior to CT [
29,
30]. Serological confirmation of the diagnosis, with the detection of antibodies against
Anisakis spp. with ELISA is useful, especially for intestinal and ectopic disease, but is not widely available [
3,
15].
In the present case, neither biochemical nor radiological examinations led to a definitive diagnosis. Furthermore, the radiological report suggested a possible neoplasmatic cause of the disease while an endoscopic biopsy on the distal ileum lesions was not possible. Similarly, frozen section biopsies during the operation could not exclude neoplasia. Taking into account the extent of the lesions, the age of the patient and the possibility of neoplasia, in the absence of another possible diagnosis at that time, a right colectomy with a D2 lymphadenectomy and excision of the omentum and the descending colon nodule was decided. Pathology report of the specimen suggested granulomatous tissue and because of the eosinophilic infiltration of the lesions, parasitosis was regarded as the most probable cause. Microscopic examination of the nodules of the greater omentum, revealed nematode parasites in the centre of the lesions. The morphological identification of the parasite as Anisakis spp., the detection of specific antibodies in the patient’s blood serum, and ultimately, the molecular confirmation of the causative agent by real-time PCR, led to the unequivocal diagnosis of intestinal and ectopic anisakiosis in the present case.
Patients usually require only conservative treatment with fluid replantation and analgesics, as the disease has a self-limiting course and parasites commonly survive in the human body only for a few days [
8,
15,
22]. Rarely, when complications occur due to gastrointestinal wall penetration and perforation, bowel wall obstruction not amenable to conservative treatment, or neoplasia cannot be excluded, surgery is needed [
22,
24,
31]. Ectopic and chronic forms of the disease are rare and can pose serious diagnostic and therapeutic challenges. Albendazole (400-800 mg daily for 6-21 days) has been successfully used for the treatment of intestinal anisakiosis [
32,
33]. In the present case, administration of albendazole 400 mg twice daily for 28 days proved effective in clearing any remaining parasites, as after one circle of treatment the patient had negative serological testing and no radiological signs of residual disease.
Appropriate processing of seafood is paramount to prevent human infection by parasites of the family Anisakidae. European Food Safety Authority suggests freezing fishery products at –20 °C for at least 24h if they are to be consumed raw or almost raw. Heating seafood above 60 ᵒC and salting at high concentrations and for prolonged time can also kill the larvae [
18]. Nevertheless, freezing and heating do not eliminate
Anisakis spp. antigens, therefore, may not prevent allergic reactions or sensitization [
34].
5. Conclusion
Anisakiosis is a zoonotic disease with a self-limiting course. Thus, therapeutic operation is generally unnecessary. When certain implications occur, surgery is needed. Apart from being underappreciated, it should also be considered that in extremely rare cases, extra-gastrointestinal Anisakis larva localisation may cause serious intra-abdominal complications with practically unknown risks, posing a diagnostic and therapeutic dilemma. Surgical exploration can be helpful in aetiological diagnosis and early detection of intra-abdominal complications. Herein, it is described the first case of anisakiosis in Greece in a 22-year-old patient, subjected to right hemicolectomy for suspected mesenchymal neoplasia with peritoneal dissemination and diffuse lymphadenopathy. Due to the nonspecific symptoms, laboratory, and radiologic findings, and the high prevalence of Anisakis spp. infection of fish from the Aegean Sea, it is possible that the disease is underdiagnosed.
Author Contributions
SD, AD and KV designed the study; SD, KV, AT, AX and CP performed the surgery; AD performed the parasitological and serological examinations; SC performed the molecular examinations; GK contributed to the serological examination and immunological evaluation of the patient; EM performed the histopathological examination; SD and AD wrote the manuscript. SD, AD, KV, SC, EM, GK, AT, AX and CP reviewed the manuscript. SD, AD and VK revised the manuscript. All authors have read and agreed with the final version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morozińska-Gogol, J. Anisakis spp. as etiological agent of zoonotic disease and allergy in European region – an overview. Ann Parasitol. 2019, 65, 303–314. [Google Scholar] [CrossRef]
- Audicana, M.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: dangerous--dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Hamer, D.H. Anisakidosis: Perils of the deep. Clin Infect Dis. 2010, 51, 806–812. [Google Scholar] [CrossRef]
- Céspedes, M.; Saez, A.; Rodríguez, I.; Pinto, J.M.; Rodríguez, R. Chronic anisakiasis presenting as a mesenteric mass. Abdom Imaging. 2000, 25, 548–550. [Google Scholar] [CrossRef]
- Takabayashi, T.; Mochizuki, T.; Otani, N.; Nishiyama, K.; Ishimatsu, S. Anisakiasis presenting to the ED: clinical manifestations, time course, hematologic tests, computed tomographic findings, and treatment. Am J Emerg Med. 2014, 32, 1485–1489. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, B.S.; Kim, S.H.; Park, J.K.; Choi, G.; Hwang, I.K.; Jeong, S.Y.; Hyun, C.L.; Song, H.J.; Chung, Y.B. Acute invasive small-bowel Anisakiasis: clinical and CT findings in 19 patients. Abdom Imaging. 2014, 39, 452–458. [Google Scholar] [CrossRef]
- Audicana, M.T. Anisakis, Something Is Moving inside the Fish. Pathogens. 2022, 11, 326. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef]
- Pampiglione, S.; Rivasi, F.; Criscuolo, M.; De Benedittis, A.; Gentile, A.; Russo, S.; Testini, M.; Villan, M. Human anisakiasis in Italy: a report of eleven new cases. Pathol Res Pract. 2002, 198, 429–434. [Google Scholar] [CrossRef]
- Baron, L.; Branca, G.; Trombetta, C.; Punzo, E.; Quarto, F.; Speciale, G.; Barresi, V. Intestinal anisakidosis: histopathological findings and differential diagnosis. Pathol Res Pract. 2014, 210, 746–750. [Google Scholar] [CrossRef]
- Najjari, M.; Sadjjadi, S.M.; Khodadadi, H.; Farzaneh, M.R.; Mattiucci, S. Anisakis spp, DNA detection in paraffin-embedded tissue biopsies recovered from patients with gastritis using real-time PCR in Bushehr, Persian Gulf, Iran. Mol Biochem Parasitol. 2022, 251, 111494. [Google Scholar] [CrossRef]
- Kouam, M.K.; Diakou, A.; Kantzoura, V.; Feidas, H.; Theodoropoulou, H.; Theodoropoulos, G. An analysis of seroprevalence and risk factors for parasitic infections of economic importance in small ruminants in Greece. Vet J. 2014, 202, 146–152. [Google Scholar] [CrossRef]
- Tantanasi, J.; Diakou, A.; Tamvakis, A.; Batjakas, I.E. Anisakis spp. burden in Trachurus trachurus. Helminthologia. 2012, 49, 16–20. [Google Scholar] [CrossRef]
- Chaligiannis, I.; Lalle, M.; Pozio, E.; Sotiraki, S. Anisakidae infection in fish of the Aegean Sea. Vet Parasitol. 2012, 184, 362–366. [Google Scholar] [CrossRef]
- Nawa, Y.; Hatz, C.; Blum, J. Sushi delights and parasites: the risk of fishborne and foodborne parasitic zoonoses in Asia. Clin Infect Dis. 2005, 41, 1297–1303. [Google Scholar] [CrossRef]
- Sugiyama, H.; Shiroyama, M.; Yamamoto, I.; Ishikawa, T.; Morishima, Y. Anisakiasis Annual Incidence and Causative Species, Japan, 2018-2019. Emerg Infect Dis. 2022, 28, 2105–2108. [Google Scholar] [CrossRef]
- Mladineo, I. Anisakiasis in Europe: emerging, neglected, misdiagnosed, or all of the above? VETERINARSKA STANICA. 2019, 50, 397–405. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on risk assessment of parasites in fishery products. EFSA Journal. 2010, 8, 1543.
- Shamsi, S.; Barton, D.P. A critical review of anisakidosis cases occurring globally. Parasitol Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- van Thiel, P.; Kuipers, F.C.; Roskam, R.T. A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med. 1960, 12, 97–113. [Google Scholar] [PubMed]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci Rep. 2017, 7, 43699. [Google Scholar] [CrossRef]
- Shibata, K.; Yoshida, Y.; Miyaoka, Y.; Emoto, S.; Kawai, T.; Kobayashi, S.; Ogasawara, K.; Taketomi, A. Intestinal anisakiasis with severe intestinal ischemia caused by extraluminal live larvae: a case report. Surg Case Rep. 2020, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, H.; Horiguchi, H.; Kuwabara, K.; Hashimoto, H.; Matsuda, S. Clinical features of bowel anisakiasis in Japan. Am J Trop Med Hyg. 2010, 83, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Usuki, S.; Mizokami, K.; Tanabe, M.; Machi, J. Intestinal anisakiasis as a rare cause of small bowel obstruction. Am J Emerg Med. 2013, 31, 1422–e1. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, T.; Marui, A.; Yokota, K.; Yamaguchi, S.; Fukudome, I.; Uemura, S.; Munekage, M.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; Hanazaki, K. Gastric Eosinophilic Granuloma Related to Anisakiasis Resected by Laparoscopic and Endoscopic Cooperative Surgery. Cancer Diagn Progn. 2021, 1, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Baek, D.H.; Kim, G.H.; Lee, B.E.; Lee, S.J.; Park, D.Y. Endosonographic Findings and the Natural Course of Chronic Gastric Anisakiasis: A Single-Center Experience. Gastroenterol Res Pract. 2018, 2018, 8562792. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Iida, M.; Murakami, M.; Kimura, Y.; Fujishima, M.; Yao, Y.; Tsuji, M. Intestinal anisakiasis: clinical and radiologic features. Radiology. 1985, 157, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Ueda, T.; Akaike, G.; Saida, Y. CT findings of gastric and intestinal anisakiasis. Abdom Imaging. 2014, 39, 257–261. [Google Scholar] [CrossRef]
- Ripollés, T.; López-Calderón, L.E.; Martínez-Pérez, M.J.; Salvador, J.; Vizuete, J.; Vila, R. Usefulness of Ultrasound in the Diagnosis of Intestinal Anisakiasis. J Ultrasound Med. 2020, 39, 1703–1708. [Google Scholar] [CrossRef]
- Ogata, M.; Tamura, S.; Matsunoya, M. Sonographic Diagnosis of Intestinal Anisakiasis Presenting as Small Bowel Obstruction. J Clin Ultrasound. 2015, 43, 283–287. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujiwara, T.; Katakura, K.; Gunji, N.; Yokokawa, A.; Sakamoto, A.; Hikichi, T.; Kono, K.; Ohira, H. Anisakiasis in the Small Intestine with Excessive Bleeding That Was Difficult to Diagnose Endoscopically. Intern Med. 2019, 58, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Pacios, E.; Arias-Diaz, J.; Zuloaga, J.; Gonzalez-Armengol, J.; Villarroel, P.; Balibrea, J.L. Albendazole for the treatment of anisakiasis ileus. Clin Infect Dis. 2005, 41, 1825–1826. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.A.; Girdwood, R.W.; Chiodini, P.L. Treatment of anisakiasis with albendazole. Lancet. 2002, 360, 54. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.T.; Tummolo, R.A.; Di Leo, E.; D'Ersasmo, M.; Arsieni, A. Immediate and cell-mediated reactions in parasitic infections by Anisakis simplex. J Investig Allergol Clin Immunol. 2008, 18, 253–259. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).