1. Introduction
Within the last decades there has been an increasing number of cancer patients who develop spinal metastases. Most likely, this development is caused by improvements in cancer treatment resulting in an increased life expectancy[
1]. Metastatic spinal cord compression (MSCC) is defined as a compression of the spinal cord or cauda equina due to metastatic tissue. This may result in pain, instability, and neurological disability, such as paresthesia, paraplegia, and loss of sphincter function [
2].
It has been widely accepted that in some patients the optimal treatment of MSCC is a combination of surgical stabilization with decompression and subsequent radiotherapy[
3,
4]. Titanium implants, which have been widely used due to their reliability and corrosion resistance, have a density significantly different from the density of the human body because of the larger atomic amount of titanium. These titanium-based implants are, consequently, known for creating a scattering effect on e.g., MRI resulting in artifacts that interfere with post-surgery imagining, and potentially also the radiation therapy. The presence of these artifacts can result in imprecise dose calculation and insufficient quality of follow-up imagining. This may result in increased risk of progression or a postponed discovery of tumor regrowth[
5].
Recently, spinal implants consisting of carbon material, have been introduced as an alternative to titanium implants. The CarboClear system consists of pedicle screws, rods and locking elements made entirely out of carbon-fiber reinforced polyetheretherketone (CFR-PEEK). One of the qualities of the CFR-PEEK system is its radiographic translucency caused by the lower atomic number of the carbon implants resulting in radiation properties more like those of human tissue. This is expected to decrease the deflection of radiation to the affected area of the spine and, consequently, improve not just the diagnostic imaging but also post-surgery radiotherapy potentially leading to a superior cancer treatment[
6,
7].
It has been demonstrated in several studies, that the mechanical properties of the CFR-PEEK system are comparable to those of titanium-based systems. This was determined via an in vitro mechanical evaluation examining fatigue resistance, torsional stiffness, axial pull strength and bending load of the CFR-PEEK system[
8]. Moreover, this study showed superior fatigue qualities in the CFR-PEEK system in comparison to those of the titanium systems. Additionally, a biomechanical revision of screw loosing comparing CFR-PEEK and titanium showed, that the carbon screws resisted an equivalent amount of load cycles compared to those of titanium[
6]. In patients with MSCC only few studies have studied the safety and effectiveness of using carbon instrumentation compared to titanium instrumentation[
5,
9,
10,
11,
12]. Previous studies are limited by lack of control group and small sample size.
Here, we designed a consecutive, one center, cohort study to examine the safety and effectiveness of spinal carbon instrumentation compared to titanium instrumentation in patients with MSCC.
2. Materials and Methods
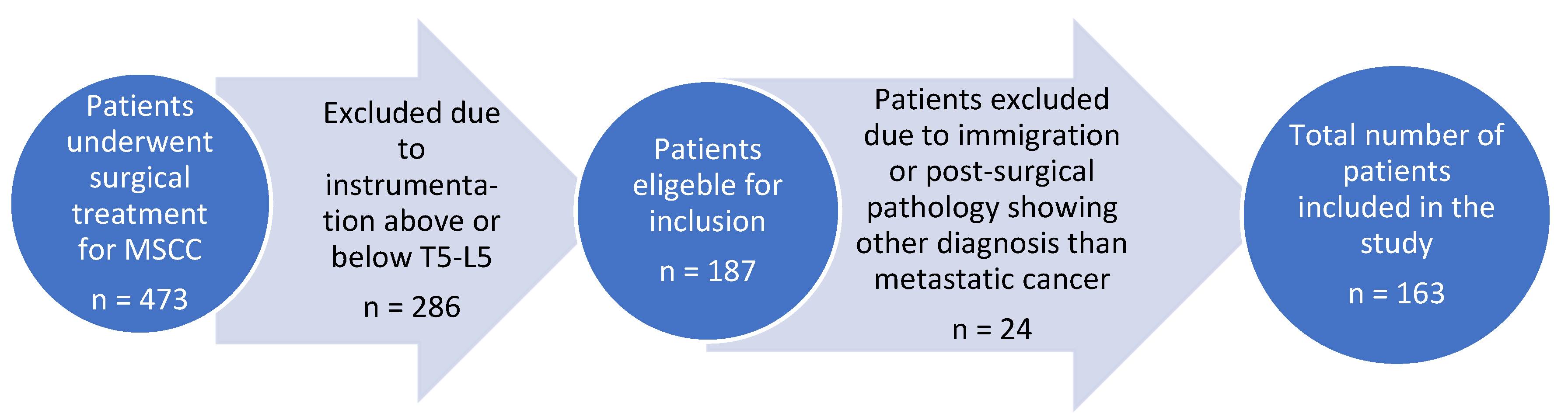
All patients included in this cohort study were surgically treated at the Spine Unit, Department of Orthopedic Surgery, Rigshospitalet, Denmark. From the 1st of January 2017 to the 31st of December 2021, a total of 473 patients underwent surgical treatment for MSCC and 163 were included in the study.
The inclusion criteria were: patient age >18 years and presence of metastatic spine tumors resulting in MSCC. Additionally, only patients where survival status could be obtained at the two-year follow up period and who had received surgical treatment with instrumentation between T5-L5 were included. Out of the original 473 MSCC patients 310 were excluded, and the final number of patients included in this cohort study was 163. A flowchart illustration the exclusion process is illustrated in
Figure 1.
80 of the elected patients were stabilized using CFR-PEEK implants (CI-group) while 83 patients were stabilized with titanium implants (TI-group). All patients were treated with posterior pedicle screw instrumentation and decompression at the metastatic level. The standard treatment was instrumentation 2 levels above and 2 levels below the metastatic level. The spinal cord was decompressed at the metastatic level with a wide laminectomy. By default, tissue samples were sent for analysis. The choice of the surgical treatment was at the discretion of the surgeon.
Patient information regarding age, gender, BMI, ASA-score, and primary oncologic diagnosis was collected at baseline. The outcome measures were surgical revision, postsurgical survival (days), peri-operative bleeding (ml) and surgery time (min).
Mann-Whitney and log rank tests were used to compare the groups and a p-value < 0.05 was considered statistically significant.
3. Results
Of the 163 patients included in this cohort study, 46,5% (n=76) were women and 53,4% (n=87) were men (p = 0,317). In the patient group who had been stabilized using CFR-PEEK implants (CI-group) 50% were women (n=40) and 50% were men (n=40), while the group that had been stabilized using titanium implants (TI-group) consisted of 43% women (n=36) and 57% (n=47) men. There were no significant differences between the groups with regards to average age, mean BMI, mean ASA-score or gender as illustrated in
Table 1.
Metastatic pulmonary cancer was the dominating primary cancer, followed by metastatic breast cancer, and metastatic renal cancer. The distribution of primary oncologic diagnosis in the two patient groups is illustrated in
Table 2.
The peri-operative blood loss in the CI-group was significantly lower than in the TI-group; the mean blood loss was 450 ml for the CI-group (range 100 ml-1800 ml) vs. 630 ml (range 150 ml-4100 ml) for the TI-group, (p = 0,024). The overall median duration of surgery was 121 min. The surgery time ranged from 73-202 min. for the CI-group, whereas the range in the TI-group was 67-329 min, (p = 0,990).
There were six surgical revisions in the CI-group and 10 in the TI-group, (p=0,386). An overview of the causes of surgical revision and the distribution between the two groups is illustrated in
Table 3.
In the CI-group the mean two-year survival post-surgery was 9.9 months with the survival time ranging from 0.4 to 40.1 months. Out of the original 80 patients in the CI-group 24 were still alive at the two-year follow-up mark. The mean two-year survival in the TI-group was 12.9 months, range 0.4- 38.7 months and 24 patients out the total 83 patients in the group were still alive at the two-year follow-up mark. The difference in survival time seems clinically relevant, but did not reach statistical significance, (p = 0.388).
4. Discussion
The results of the present study indicate that the use of CFR-PEEK spinal implants in patients with MSCC is equally safe and effective compared to titanium-based implants with regards to complications and survival. We found that the peri-operative blood loss in the CI-group was significantly lower than in the TI-group. It could be speculated that this was caused by the fact that the use of CI vs. TI was at the surgeon’s discretion, and that the more experienced surgeons tended to choose the CI implant. There were no significant differences between the groups in mean survival time or number of patients needing surgical revision speaking against surgical technique as the explanation for the reduced blood loss in the CI-group.
The aim of the present study was to examine the safety and effectiveness of carbon-based instrumentation for MSCC patients in a larger sample size than previously been published in the literature.
In a new systematic review by Khan et al. data were collected from all articles describing the treatment outcome on MSCC patients who underwent surgical stabilization with CFR-PEEK implants. In this study, they identified a total of 206 patients treated with CFR-PEEK implants and those were compared with 47 patients treated with titanium. Khan et al. concluded that there is need for direct comparable studies with larger sample sizes of patients which was the aim of our study[
11].
The largest comparable clinical study has been made by Cofano et al. The study is a retrospective, one center study of 78 patients who underwent surgery for cervical, thoracic, or lumbar metastatic lesions. Three patients were treated with cervical anterior instrumentation which is a different instrumentation than posterior instrumentation. Hence, the actual sample size was 75. The patients were divided into two groups with 35 patients treated with carbon instrumentation and 40 with titanium instrumentation[
16]. The study is well conducted but limited by the relatively small sample size. As in our study, the study showed no significant differences in terms of complications or survival between the two groups[
16].
A retrospective cohort study published in 2017 by Boriani
et al. included 34 patients with either thoracic or lumbar metastases or primary cancer in the spine requiring treatment with a combination of surgery and radiotherapy. Here it was also found that thoracic/lumbar spinal fixation using CFR-PEEK implants in MSCC patients is comparable to titanium regarding stability, functionality, and number of complications [
17].
One of the major differences between the previous studies and the current cohort study is the size of the patient population (163 vs. 75 and 34 respectively). Moreover, the difference in post-surgery mean survival in the two patient groups, which was found to be non-significant in this study, is not included in the above-mentioned studies. In the study by Cofano et al. , there was a more detailed data collection for each patient including grade of instability (SINS), grade of epidural compression (ESCC score) as well as pre-and postsurgical level of axial pain and neurological status, which should result in a more thorough patient evaluation. The results of this study were a longer mean surgical duration and a higher mean blood loss in the CI-group compared to the TI-group.. The reason for this was suggested to be a more complex closing system in the carbon-based implants and because more of the surgeries included circumferential decompression and debulking into the corpus vertebra. We found that the peri-operative blood loss in the CI-group was significantly lower than in the TI-group. The difference in the two groups amounted to a mean value of approximately 200 ml, and it could be speculated that this reduction in blood loss is beneficial in a fragile patient population. However, it is not known if the reduction in blood loss affected long term survival.
The mechanical properties of the CFR-PEEK system have been shown to be comparable to those of titanium-based systems and the overall benefit of CFR-PEEK in orthopedic implants in terms of durability has also been strongly supported in a systematic review from 2014 by Li
et al[
18].
The results from the current study underlines the safety of CFR-PEEK implants and their comparability to titanium in terms of perioperative bleeding, duration of surgery, post-surgical complications, and average survival time.
Strengths and weaknesses of the study
The primary strength of this cohort study is the relatively large patient population. Furthermore, there were no statistically significant difference between the two groups regarding gender, age, BMI, and ASA- score. This should minimize confounding and thereby make groups comparable regarding mortality, mean blood loss and duration of surgery, and complications. Another strength of the study is that all the patients were treated by a small and consistent group of surgeons at the same center. We did not specify the amount of experience of the surgeons who performed the procedures but all surgeons at the Spine Unit, Rigshospitalet have many years of experience in complex spine surgery. The surgeons’ high level of experience is supported by the fact that the surgery time did not change during the study period, indicating that there was no learning curve associated with the introduction of carbon-based implant.
A limitation of the study is that it is not randomized, and that surgeons’ preference decided if patients were treated with carbon-based or titanium-based implants.
Another limitation is that only the ASA-score was used for the preoperatively evaluation of the patients’ physiological status. The ASA score is a classification system created by The American Society of Anesthesiologists. It is used to categorize the physiological status of a patient pre-surgery based on the patient’s comorbidities. Although widely used, the score has certain weaknesses such as a possible significant variation in the classification assigned to patients as well as the assumption that psychical fitness and age is unrelated[
19]. A more detailed evaluation of the physiological status of the patients taking factors like comorbidities, grade of instability and epidural compression, pain level and neurological status of the patients into account, could result in a more thorough patient evaluation and hence more specific results. Another possible weakness could be that the Tokuhashi score used for estimating a patient’s survival time was not applied. Finally, the fact that the two patient groups were not matched can also be considered a limitation. Matched patient groups in terms of e.g., age, sex, primary oncological diagnosis and tumor level could result in a more exact comparison of the two groups and hence more representative results.
In future studies more focus should be on the clinical impact of using CFR-PEEK implants. With regards to benefits of better radiolucency and the radiotherapeutic effects of using CFR-PEEK implants as also stated by Khan and Takayanagi et al.[
11,
12].
5. Conclusions
Based on a large cohort we found that surgical treatment with CFR-PEEK for MSCC is safe and an equally sufficient treatment when compared to the more traditional titanium implants. This is in line with what previous studies has indicated. The use of CFR-PEEK could lead to improvements in the oncological treatment of patients surgically treated for MSCC.
Author Contributions
Conceptualization, Martin Gehrchen, Benny Dahl, and Søren Schmidt Morgen.; methodology, Martin Gehrchen and Søren Schmidt Morgen.; formal analysis, Søren Schmidt Morgen.; data curation, Anders Skive Veiland and Emma Benedikte Alfthan Madsen.; writing—original draft preparation, Emma Benedikte Alfthan Madsen and Søren Schmidt Morgen.; writing—review and editing, Martin Gehrchen, Benny Dahl, Emma Benedikte Alfthan Madsen and Søren Schmidt Morgen. All authors have read and agreed to the latest version of the manuscript.
Funding
This research received no direct external funding. Benny Dahl is Benny Dahl is supported by the Alfred Benzon foundation and is a consultant for Stryker. Martin Gehrchen received institutional grants from Cerapedics and Nuvasive
Institutional Review Board Statement
The study was approved by the Danish National Center for Ethics Case nr. 2301180, Document nr. 2570303.
Informed Consent Statement
Patient consent was waived due to the design of the study. Data Availability Statement: The data of this study are available upon reasonable request from the corresponding author. Conflicts of Interest: The authors declare no conflict of interest.
References
- Morgen, S. S. et al. A revision of the Tokuhashi revised score improves the prognostic ability in patients with metastatic spinal cord compression. J Cancer Res Clin Oncol 144, 33–38 (2018). [CrossRef]
- Boussios, S. et al. Metastatic spinal cord compression: Unraveling the diagnostic and therapeutic challenges. Anticancer Research vol. 38 4987–4997, (2018). [CrossRef]
- Sharan, A. D. et al. The integration of radiosurgery for the treatment of patients with metastatic spine diseases. J Am Acad Orthop Surg 22, 447–454 (2014). [CrossRef]
- Patchell, R. a et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 366, 643–8 (2005). [CrossRef]
- Tedesco, G., Gasbarrini, A., Bandiera, S., Ghermandi, R. & Boriani, S. Composite PEEK/Carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. Journal of Spine Surgery 3, 323–329 (2017). [CrossRef]
- Lindtner, R. A., Schmid, R., Nydegger, T., Konschake, M. & Schmoelz, W. Pedicle screw anchorage of carbon fiber-reinforced PEEK screws under cyclic loading. Eur Spine J 27, 1775–1784 (2018). [CrossRef]
- Krätzig, T. et al. Carbon fiber-reinforced PEEK versus titanium implants: an in vitro comparison of susceptibility artifacts in CT and MR imaging. [CrossRef]
- Uri, O., Folman, Y., Laufer, G. & Behrbalk, E. A Novel Spine Fixation System Made Entirely of Carbon-Fiber-Reinforced PEEK Composite: An In Vitro Mechanical Evaluation. Adv Orthop 2020, (2020). [CrossRef]
- Cofano, F. et al. Carbon fiber reinforced vs titanium implants for fixation in spinal metastases: A comparative clinical study about safety and effectiveness of the new ‘carbon-strategy’. J Clin Neurosci 75, 106–111 (2020). [CrossRef]
- Ringel, F. et al. Radiolucent Carbon Fiber–Reinforced Pedicle Screws for Treatment of Spinal Tumors: Advantages for Radiation Planning and Follow-Up Imaging. World Neurosurg 105, 294–301 (2017). [CrossRef]
- Khan, H. A. et al. Carbon fiber–reinforced PEEK spinal implants for primary and metastatic spine tumors: a systematic review on implant complications and radiotherapy benefits. J Neurosurg Spine 1–14 (2023). [CrossRef]
- Takayanagi, A. et al. Radiolucent Carbon Fiber–Reinforced Implants for Treatment of Spinal Tumors–Clinical, Radiographic, and Dosimetric Considerations. World Neurosurgery vol. 152 61–70, (2021). [CrossRef]
- Momin, A. A. et al. Epidemiology of primary malignant non-osseous spinal tumors in the United States. Spine Journal 22, 1325–1333 (2022). [CrossRef]
- Furlan, J. C., Wilson, J. R., Massicotte, E. M., Sahgal, A. & Fehlings, M. G. Recent advances and new discoveries in the pipeline of the treatment of primary spinal tumors and spinal metastases: a scoping review of registered clinical studies from 2000 to 2020. Neuro Oncol 24, 1–13 (2022). [CrossRef]
- Laufer, I. & Bilsky, M. H. Advances in the treatment of metastatic spine tumors: The future is not what it used to be. J Neurosurg Spine 30, 299–307 (2019). [CrossRef]
- Cofano, F. et al. Carbon fiber reinforced vs titanium implants for fixation in spinal metastases: A comparative clinical study about safety and effectiveness of the new “carbon-strategy”. Journal of Clinical Neuroscience 75, 106–111 (2020). [CrossRef]
- Boriani, S. et al. Carbon-fiber-reinforced PEEK fixation system in the treatment of spine tumors: a preliminary report. Eur Spine J 27, 874–881 (2018). [CrossRef]
- Li, C. S., Vannabouathong, C., Sprague, S. & Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin Med Insights Arthritis Musculoskelet Disord 8, 33–45 (2015). [CrossRef]
- DJ, D., JM, H. & EH, G. American Society of Anesthesiologists Classification. StatPearls (2022).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).