Submitted:
07 January 2024
Posted:
08 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Metformin
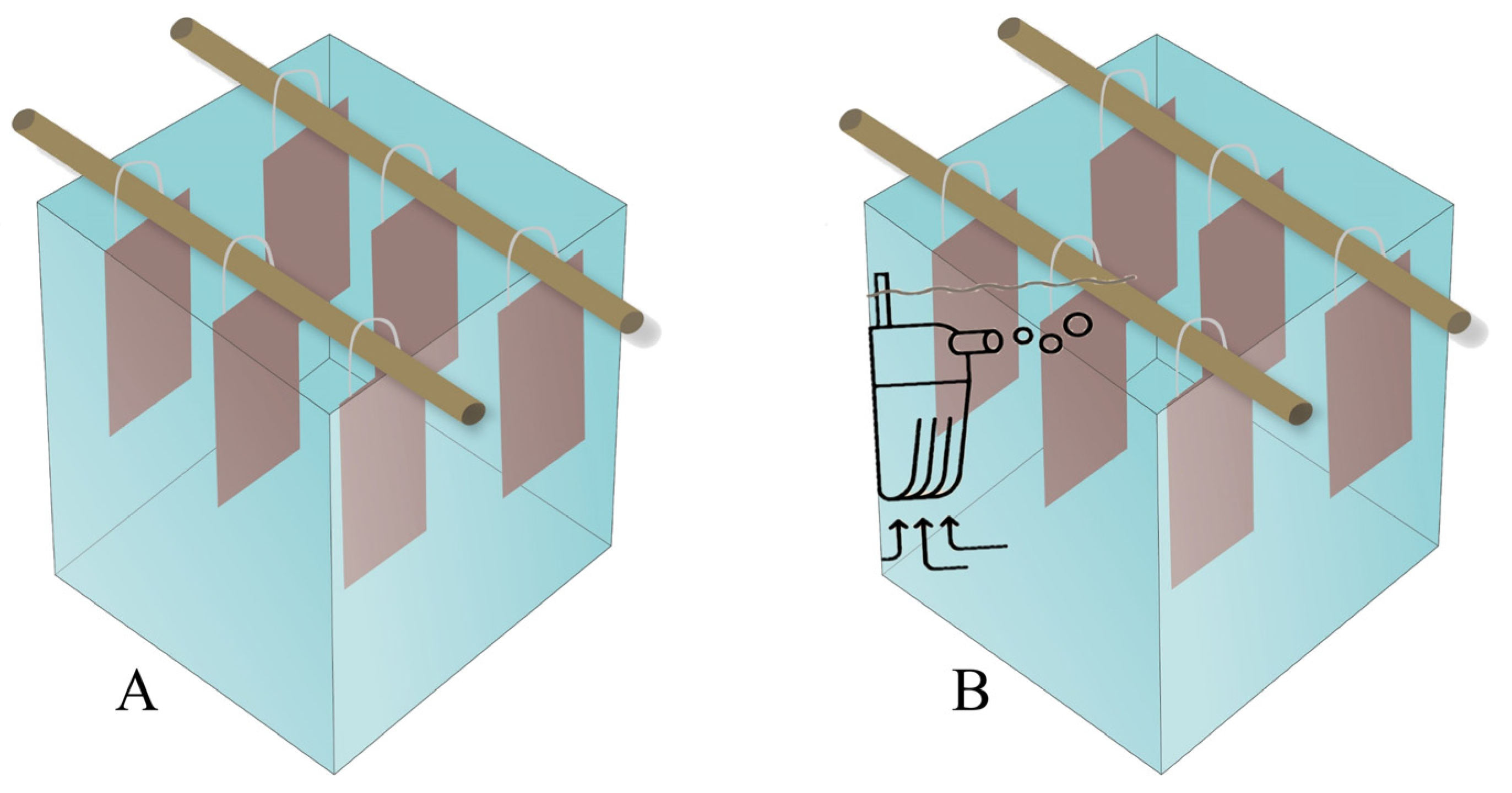
2.3. In vitro planktonic cell perturbation assay
2.4. In vitro biofilm formation assay
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Morais, V.C.; Soeder, J.; Özcan, E.; Gomes, V.T.; Lima, K.Y.G.; Vasconcelos, U. 2022. Different solutions prepared with over-the-counter and under prescription medicines alters germination and seedling growth of linseed (Linaceae). World J Pharm Pharm Sci. 2022, 11, 1-11.
- Valdéz-Carrillo, M.; Abrell, L.; Ramírez-Hernández, J.; Reyes-López, J.; Corréon-Diazconti, C. Pharmaceuticals as emerging contaminants in the aquatic environment of Latin America: a review. Environ Sci Pollut Res. 2020, 27, 44683–44891. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J Environ Manage. 2011, 277, 111485. [Google Scholar] [CrossRef] [PubMed]
- Kock, N.; Islam, N.F.; Sonowal, S.; Prasad, R.; Sarma, H. Environmental antibiotics and resistance genes as emerging contaminants: Methods of detection and bioremediation. Curr Res Microb Sci. 2020, 2: 100027. [CrossRef]
- United Nations Environment Programme. Environmental dimensions of antimicrobial resistance: Summary for policymakers. United Nations Environment Programme: Nairobi, Kenya, 2022.
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia. 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Drzewoski, J.; Hanefeld, M. The current and potential therapeutic use of metformin-The good old drug. Pharmaceuticals, 2021, 14, 122. [CrossRef]
- Executive Secretariat of the Drugs Market Regulation Chamber. Statistical yearbook of the pharmaceutical market. CMED: Brasília, Brazil, 2022.
- Dantas, P.; Azevedo, T.R.; Costa, H.P.; Rios, E.R.V.; Cavalcanti, M.G.; Oliveira, E.G. Off-Label use of metformin for the treatment of obesity: a risk or success in clinical practice? Biomed J Sci Tech Res. 2023, 48, 39158–39161. [Google Scholar]
- Cummings, B.M.; Needoba, J.A.; Peterson, T.D. Effect of metformin exposure on growth and photosynthetic performance in the unicellular freshwater chlorophyte, Chlorella vulgaris. PlosOne. 2018, 13, e0207041. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Berset, D.; Wolschke, H. Occurrence of the antidiabetic drug Metformin and its ultimate transformation product guanylurea in several compartments of the aquatic cycle. Environ Int. 2014, 70, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.O.; Caldwell, D.J.; Davidson, T.; D'Aco, V.; Kappler, K.; Robinson, P.F.; Simon-Hettich, B.; Tell, J. Environmental risk assessment of metformin and its transformation product guanylurea. I. Environmental fate. Chemosphere. 2019, 216, 844–854. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Ussery, E.; Nielsen, K.; Pandelides, Z.; Kirkwood, A.; Bonetta, D.; Venables, B.; Guchardi, J.; Holdway, D. Effects of environmentally relevant metformin exposure on Japanese medaka (Oryzias latipes). Aquat Toxicol. 2018, 205, 58–65. [Google Scholar] [CrossRef]
- Meherunisa; Saptna, J.; Vikas, S. Study of metformin effect on antimicrobial property. Int Arch Biomed Clin Res. 2018, 4, 85–87. [Google Scholar]
- Masadeh, M.M.; Alzoubi, K.H.; Masadeh, M.M.; Aburashed, Z.O. Metformin as a potential adjuvant antimicrobial agent against multidrug resistant bacteria. Clin Pharmacol. 2021, 13: 83-90.
- Wei, Z.; Wei, Y.; Li, H.; Shi, D.; Yang, D.; Yin, J.; Zhou, S.; Chen, T.; Li, J.; Jin, M. Emerging pollutant metformin in water promotes the development of multiple-antibiotic resistance in Escherichia coli via chromosome mutagenesis. J Hazard Mat. 2022, 430, 128474. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem. 2015, 80, 7–12. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O; Afolayan, A.J. In vitro antibacterial and time-kill assessment of crude methanolic stem bark extract of Acacia mearnsii De Wild against bacteria in shigellosis. Molecules. 2012, 17, 2103–2118. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Reyes-Lara, S.; Reyes-Mazzoco, R. Effect of hydraulic and organic loads on the mass removal of a structured packing in a trickling filter. Rev Mex Ing Quim. 2009, 8, 101–109. [Google Scholar]
- Ommen, P.; Zobek, N.; Meyer, R. Quantification of biofilm biomass by staining: Nontoxic safranin can replace the popular crystal violet. J Microbiol Methods. 2017, 141, 87–89. [Google Scholar] [CrossRef]
- Rossi, E.; Paroni, M.; Landini, P. Biofilm and motility in response to environmental and host-related signals in Gram negative opportunistic pathogens. Appl Microbiol. 2018, 125, 1587–1602. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Ma, J.; Chen, J.; Xie, B.; Yin, W.; Yang, Y.; Jia, W.; Xie, D.; Huang, F. Discrimination of Chemical Oxygen Demand pollution in surface water based on visible near-infrared spectroscopy. Water. 2022, 14, 3003. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Li, X.; Bian, W. Impact of organic matter on biofilm growth and microbial community diversity. Desalination Water Treat. 2017, 66, 10–16. [Google Scholar] [CrossRef]
- Ahmerkamp, S.; Jajaluddin, F.M.; Cui, Y.; Brumley, D.R.; Pacherres, C.O.; Berg, J.S.; Stocker, R.; Kuypers, M.M.M.; Koren, K.; Behrendt, L. Simultaneous visualization off low field sand oxygen concentrations to unravel transport and metabolic processes in biological systems. Cell Rep Method. 2022, 2, 100216. [Google Scholar] [CrossRef] [PubMed]
- Tsagkari, E.; Connelly, S.; Liu, Z.; McBride, A.; Sloan, W.T. . The role of shear dynamics in biofilm formation. NPJ Biofilms Microbiomes. 2022, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Manoel, C.M.; Nunes, O.C.; Melo, L.F. Dynamics of drinking water biofilm in flow/non-flow conditions. Water Res. 2007, 41: 551-562.
- Laspidou, C.S.; Rittmann, B.E.; Karamanos, S.A. Finite element modeling to expand the UMCCA model to describe biofilm mechanical behavior. Water Sci Technol. 2005, 52, 161e166. [Google Scholar] [CrossRef]
- Simunič, U.; Pipp, P.; Dular, M.; Stopar, D. The limitations of hydrodynamic removal of biofilms from the dead-ends in a model drinking water distribution system. Water Res. 2020, 178, 115838. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.L. Biofilm formation and its role in fixed film processes. In Handbook of Water and Wastewater Microbiology, 1st ed.; Mara, D., Horan, N., Eds.; Academic Press: London, United Kingdom, 2003; pp. 337–349. [Google Scholar]
- Liu, T.; Cheng, Y.F.; Sharma, M.; Voordouw, G. Effect of fluid flow on biofilm formation and microbiologically influenced corrosion of pipelines in oilfield produced water. J Petroleum Sci Eng. 2017, 156, 451–459. [Google Scholar] [CrossRef]
- Salgar-Chaparroa, S.J.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Nutrient level determines biofilm characteristics and subsequent impact on microbial corrosion and biocide effectiveness. Environ Microbiol. 2020, 86, e02885-19. [Google Scholar] [CrossRef] [PubMed]
- Legner, M.; McMillen, D.R.; Cvitkovitch, D.G. Role of dilution rate and nutrient availability in the formation of microbial biofilms. Front Microbiol. 2019, 10, 916. [Google Scholar] [CrossRef]
- Landini, P. Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res Microbiol. 2009, 160, 259–266. [Google Scholar] [CrossRef]
- Tsai, Y-P. Simulation of biofilm formation at different assimilable organic carbon concentrations under lower flow velocity condition. J Basic Microbiol. 2005, 45, 475–485. [Google Scholar] [CrossRef]
- Shaikh, S.; Rashid, N.; Onwusogh, U.; McKay, G.; Mackey, H.R. Effect of nutrients deficiency on biofilm formation and single cell protein production with a purple non-sulphur bacteria enriched culture. Biofilms. 2023, 5, 100098. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef] [PubMed]
- Elasri, M.O.; Miller, R.V. Study of the response of a biofilm bacterial community to UV radiation. Appl Environ Microbiol. 1999, 65, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Norwood, D.E.; Gilmour, A. The differential adherence capabilities of two Listeria monocytogenes strains in monoculture and multispecies biofilms as a function of temperature. Lett Appl Microbiol. 2001, 33, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Hostacká, A.; Ciznár, I.; Stefkovicová, M. Temperature and pH affect the production of bacterial biofilm. Folia Microbiol. 2010, 55, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival and persistence. Front Cell Infect Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Elabed, H.; Bakhrouf, A.; Hamza, R.; Gaddour, K. Evidence of the adaptive response in Pseudomonas aeruginosa to 14 years of incubation in seawater. Ann Microbiol. 2012, 62, 1385–1394. [Google Scholar] [CrossRef]
- O’Neal, L.; Baraquet, C.; Suo, Z.; Parsek, M.R. The Wsp system of Pseudomonas aeruginosa links surface sensing and cell envelope stress. PNAS. 2022, 119, e2117633119. [Google Scholar] [CrossRef]
- Melamed, J.; Kocev, A.; Torgov, V.; Veselovsky, V.; Brockhausen, I. Biosynthesis of the Pseudomonas aeruginosa common polysaccharide antigen by D-Rhamnosyltransferases WbpX and WbpY. Glycoconj J. 2022, 39, 393–411. [Google Scholar] [CrossRef]
- Azimi, S.; Thomas, J.; Cleland, S.E.; Curtis, J.E.; Goldberg, J.B.; Diggle, S.P. O-Specific Antigen-Dependent surface hydrophobicity mediates aggregate assembly type in Pseudomonas aeruginosa. mBio. 2021, 12, e0086021. [Google Scholar] [CrossRef]
- Silva, E.C.; Gomes, V.T.; Pragana, L.G.; Bandeira, J.A.C.; Santos, L.F.A.; Travassos, R.A.; Vasconcelos, U. Adherence reduction of Pseudomonas aeruginosa UFPEDA 416 under blue led light irradiation and curcumin exposure. Contemporary J. 2023, 3, 4437–4454. [Google Scholar] [CrossRef]
- Diaz, A.; Dixit, A.R.; Khodadad, CLM, Hummerick ME, Justiano-Velez Y-A, Li W, O’Rurke A. Biofilm formation is correlated with low nutrients and simulated microgravidity conditions in a Bulkholderia isolate from the ISS water processor assembly. Biofilm. 2023, 5, 100110. [Google Scholar] [CrossRef]
- Bernardi, S.; Anderson, A.; Macchiarelli, G.; Hellwig, E.; Cieplik, F.; Vach K, Al-Ahmad A. Subinhibitory antibiotic concentrations enhance biofilm formation of clinical Enterococcus faecalis isolates. Antibiotics. 2021, 10, 874. [Google Scholar] [CrossRef]
- Chada, J.; Khullar, L.; Gulati, P.; Chhibber, S.; Harjai, K. Anti-virulence prospects of Metformin against Pseudomonas aeruginosa: A new dimension to a multifaceted drug. Microb Pathog. 2013, 183, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.N.; Silveira, W.C.D.; Teixeira, L.E.M.; Araújo, I.D. Mechanisms of bacterial adhesion to biomaterials. Rev Med Minas Gerais. 2013, 23, 96–101. [Google Scholar] [CrossRef]
- Rosman, C.W.K.; van der Mei, H.C.; Sjollema, J. Influence of sub-inhibitory concentrations of antimicrobials on micrococcal nuclease and biofilm formation in Staphylococcus aureus. Sci Rep. 2021, 11, 13241. [Google Scholar] [CrossRef] [PubMed]
- Hathroubi, S.; Mekni, M.A.; Domenico, P.; Nguyen, D.; Jacques, M. Biofilms: Microbial shelters against antibiotics. Microb Drug Resist. 2017, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Tichaczek-Goska, D. Effect of sub-minimum inhibitory concentrations of ciprofloxacin, amikacin and colistin on biofilm formation and virulence factors of Escherichia coli planktonic and biofilm forms isolated from human urine. Braz J Microbiol. 2013, 44, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Elsherbini, A.M.; Shaldam, M.A. Repurposing metformin as a quorum sensing inhibitor in Pseudomonas aeruginosa. Afr Health Sci. 2017, 17, 808–819. [Google Scholar] [CrossRef]
- Zuo, J.; Shen, Y.; Wang, H.; Gao, S.; Yuan, S.; Song, D.; Wang, Y.; Wang, Y. Effects of metformin on Streptococcus suis LuxS/AI-2 quorum sensing system and biofilm formation. Microb Pathog. 2023, 181, 106183. [Google Scholar] [CrossRef]
| Test | Metformin (µg/mL) | COD (mg/L) | Flow rate (L/min) | Reduction (%) | Δ24-2h 1 | Type of adhesion (24h) | |
| 2h | 24h | ||||||
| 1 | 100 | 1000 | 0 | 60.00±0.02 | 72.64±0.02 | + 12.64 | Weakly |
| 2 | 100 | 1000 | 4 | 13.85±0.03 | 8.22±0.04 | – 5.63 | Highly |
| 3 | 100 | 10000 | 0 | 72.48±0.02 | 8.33±0.02 | – 64.15 | Highly |
| 4 | 100 | 10000 | 4 | 32.10±0.03 | 32.47±0.03 | + 0.37 | Moderately |
| 5 | 200 | 1000 | 0 | 65.88±0.02 | 67.92±0.02 | + 2.04 | Moderately |
| 6 | 200 | 1000 | 4 | 84.40±0.07* | 44.44±0.02 | – 39.96 | Moderately |
| 7 | 200 | 10000 | 0 | 75.38±0.01 | 49.32±0.03 | – 26.06 | Moderately |
| 8 | 200 | 10000 | 4 | 75.31±0.01 | 24.68±0.03 | – 50.63 | Weakly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





