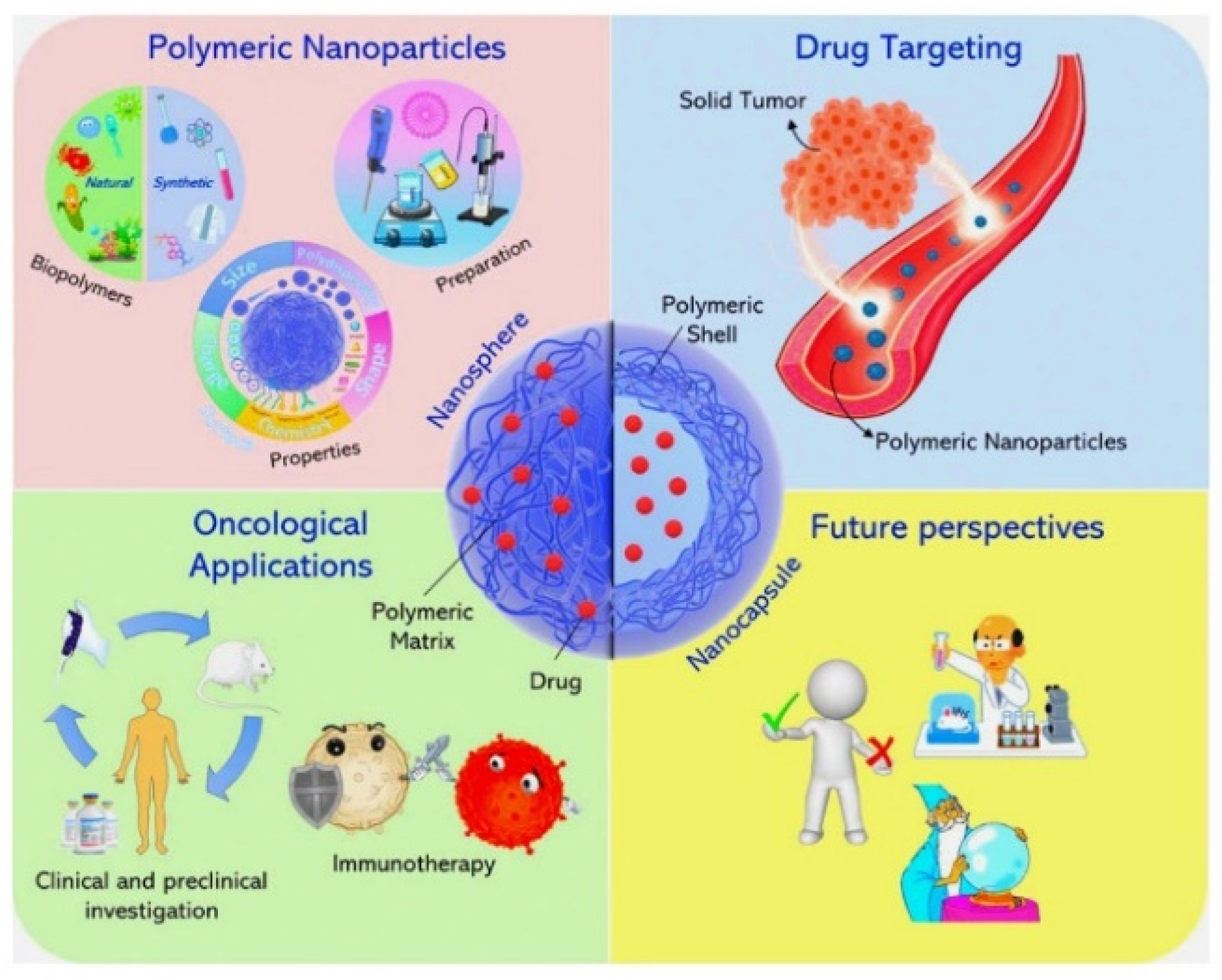
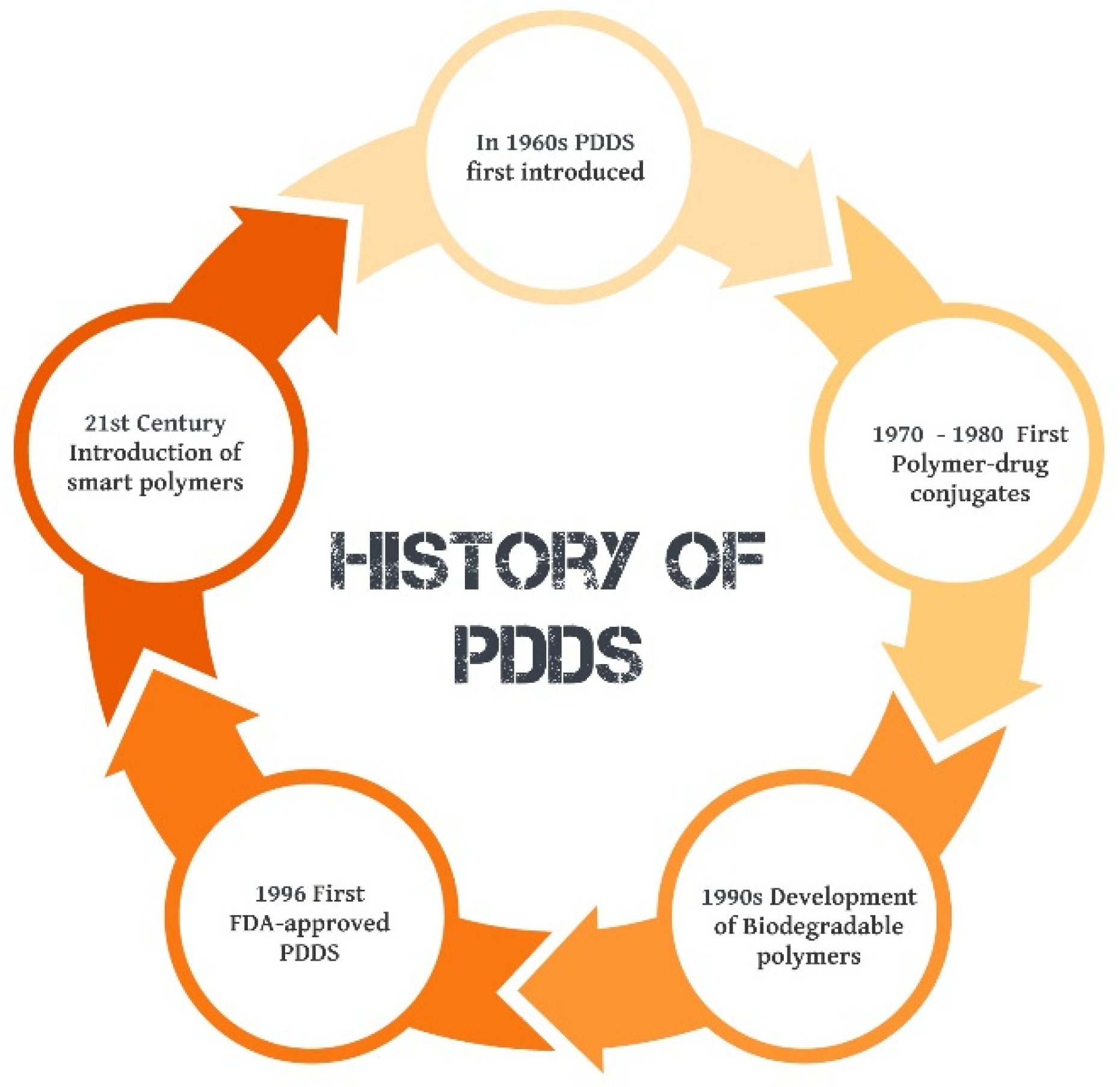
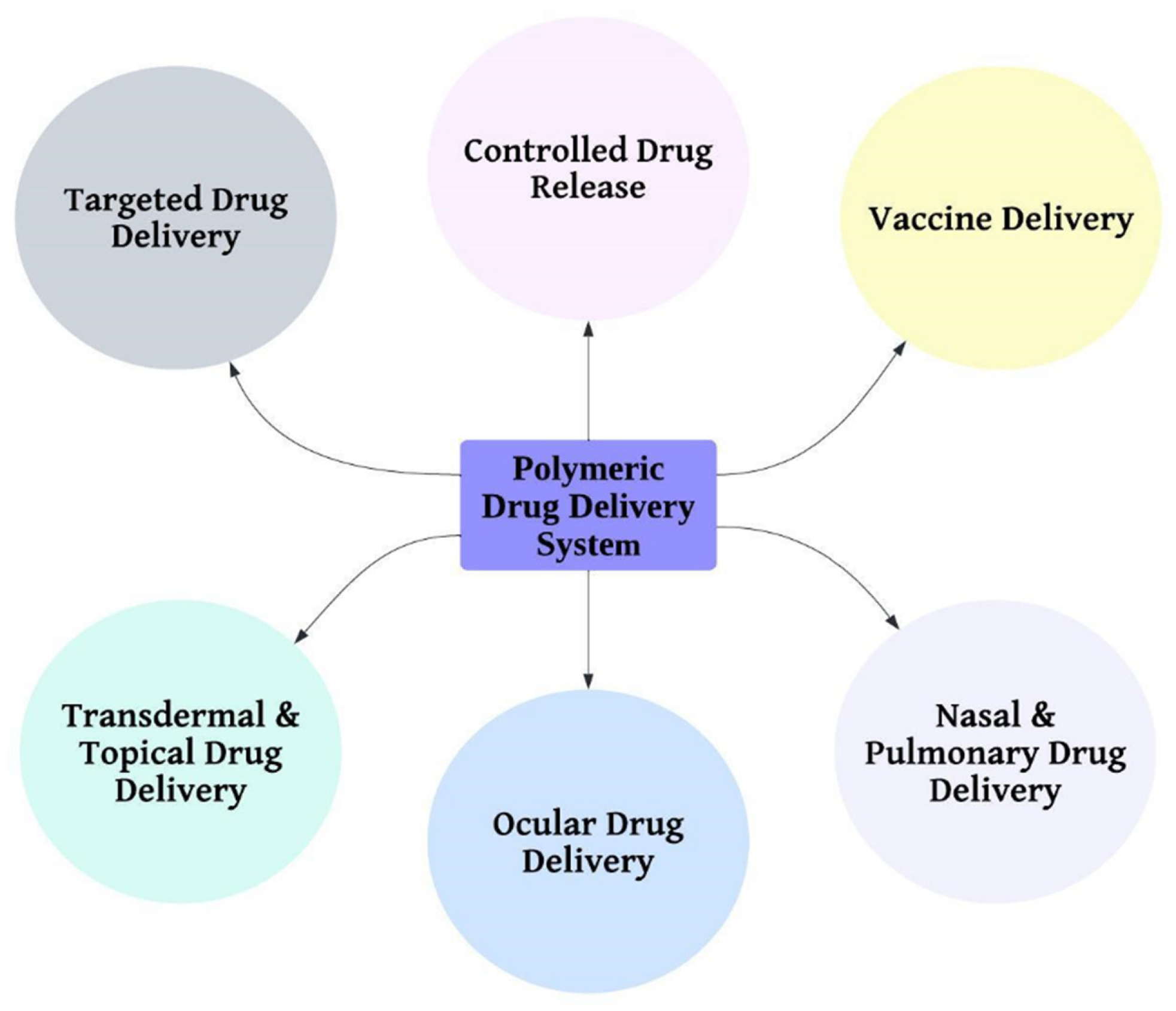
1. Introduction
Polymeric Drug Delivery Systems (PDDS) [
1] represent a significant advancement in the field of pharmaceutical sciences. They offer a novel approach to drug delivery by encapsulating the drug within a biodegradable polymer, allowing for controlled and sustained release of the drug over time as shown in
Figure 1. This not only enhances the therapeutic efficacy of the drug but also minimizes potential side effects and improves patient adherence to treatment regimens. The concept of Polymeric Drug Delivery Systems (PDDS) was first introduced in the 1960s, with the development of the first biodegradable polymer used for drug delivery. This was a breakthrough in the field of pharmaceutical sciences, paving the way for a new era of targeted and controlled drug delivery. Over the years, the technology has evolved and improved, with the introduction of various types of polymers, each with its unique properties and applications. In the 1970s and 1980s, researchers began to explore the use of PDDS for the delivery of anticancer drugs, leading to the development of the first polymer-drug conjugates. This was a significant milestone, as it allowed for the targeted delivery of drugs to cancer cells, minimizing damage to healthy tissues.
In the 1990s, the focus shifted towards the development of biodegradable polymers, which could be broken down and eliminated by the body after delivering the drug. This led to the creation of the first FDA-approved polymeric drug delivery system in 1996. The 21st century has seen further advancements in PDDS, with the introduction of smart polymers that can respond to specific stimuli such as pH, temperature [
5], and magnetic fields, allowing for even more precise control over drug release [
6], overview as shown in
Figure 3.
Today, PDDS are used in a wide range of applications, from the treatment of chronic diseases to the delivery of vaccines. The development and optimization of PDDS have been a focus of extensive research over the past few decades, leading to significant improvements in drug delivery technology and patient care. Looking forward, the field of PDDS is ripe with potential for further advancements. Researchers are currently exploring the use of nanotechnology to create even smaller and more efficient drug delivery systems [
7]. Additionally, the development of polymers that can respond to multiple stimuli simultaneously could allow for even more precise control over drug release. There is also great potential for the use of PDDS in personalized medicine, where the drug delivery system could be tailored to the individual patient's needs.
1.1. Biodegradable Polymers
When discussing Biodegradable Polymers in Polymeric Drug Delivery Systems, it's important to delve into the types of biodegradable polymers used, their properties, and how these properties influence drug delivery. A biodegradable polymer is a type of polymer material that can decompose and break down into simpler compounds under natural environmental conditions, such as exposure to sunlight, moisture, and microorganisms. This process is facilitated by the action of enzymes that can break the polymer chains into smaller fragments, which can then be further metabolized by microorganisms. The degradation of biodegradable polymers results in the breakdown of the polymer chains into smaller molecules, such as monomers or oligomers, through chemical or biological processes. This degradation process can be influenced by various factors, including temperature, pH, moisture, and the presence of enzymes or microorganisms.
Biodegradable polymers [
8], such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers (PLGA), are commonly used in PDDS due to their biocompatibility and tunable degradation rates. These polymers degrade into non-toxic monomers that can be metabolically eliminated from the body, reducing the risk of long-term accumulation and adverse reactions. The degradation rate of these polymers can be controlled by modifying their molecular weight and composition, allowing for the fine-tuning of drug release kinetics. For instance, a higher ratio of PGA to PLA in PLGA results in a faster degradation rate and hence a quicker drug release. Furthermore, the surface properties of these polymers can be modified to enhance drug absorption and distribution. For example, the addition of hydrophilic or hydrophobic groups can improve the polymer's interaction with the biological environment, affecting the drug's release rate and targeting ability. Biodegradable polymers [
9] can also be formulated into various drug delivery systems such as nanoparticles [
4], microparticles, hydrogels, and implants, each with their unique advantages and applications. For instance, biodegradable polymer nanoparticles
4 can enhance drug stability, improve bioavailability, and allow for targeted drug delivery, while biodegradable polymer implants can provide long-term, localized drug release. Moreover, the use of biodegradable polymers in PDDS has also opened up new possibilities for the delivery of sensitive drugs like proteins and genes. These drugs can be encapsulated within the polymer matrix, protecting them from degradation and allowing for their controlled release [
10]. In conclusion, biodegradable polymers play a crucial role in polymeric drug delivery systems. Their biocompatibility, degradability, and versatility make them an ideal material for the design and development of effective drug delivery systems. However, there are still challenges to overcome, such as the control of polymer degradation and drug release kinetics, the improvement of drug loading efficiency, and the enhancement of drug stability. Therefore, continued research and development in this field are essential to fully exploit the potential of biodegradable polymers in polymeric drug delivery systems.
2. Role of BCS Classification in PDDS
The Biopharmaceutics Classification System (BCS) is a crucial tool in pharmaceutical sciences, introduced by the U.S. Food and Drug Administration (FDA). It classifies drugs based on their solubility and intestinal permeability, which are key factors affecting the rate and extent of drug absorption. This system is particularly important in the development of immediate-release oral dosage forms, as it provides a scientific framework for drug dissolution and absorption studies.
BCS Classification: Explain the four classes of drugs according to the BCS.
The BCS divides drugs into four classes.
Class I drugs are high in both solubility and permeability, leading to high absorption rates.
Class II drugs have high permeability but low solubility, meaning their absorption can be limited by their solubility.
Class III drugs have high solubility but low permeability, so their absorption can be limited by their permeability. Lastly,
Class IV drugs have low solubility and low permeability, making them the most challenging to formulate into effective drug delivery systems.
The BCS plays a crucial role in the design of polymeric drug delivery systems. Based on the BCS classification of a drug, scientists can choose the most suitable type of polymer and design the most effective drug delivery system. For instance, for Class II drugs with low solubility [
11], polymers that can enhance drug solubility or create a sustained release profile may be used to improve drug absorption. On the other hand, for Class III drugs with low permeability, polymers that can enhance drug permeability or target specific absorption sites in the intestine may be used. Moreover, the BCS can also guide the choice of in vitro dissolution testing conditions for polymeric drug delivery systems. For example, for BCS Class II drugs, dissolution testing in various pH conditions may be necessary to fully understand the drug release behavior from the polymer.
While the BCS provides a valuable framework for the design and development of polymeric drug delivery systems, there are challenges in its application. One of the main challenges is that the BCS classification is based on the properties of the drug substance alone, not considering the influence of the drug delivery system. Therefore, the BCS may not accurately predict the in vivo performance of drugs formulated in polymeric delivery systems. Moreover, the BCS is primarily designed for immediate-release oral dosage forms, and its application to other types of dosage forms, such as controlled-release or targeted delivery systems, may not be straightforward. Therefore, modifications or extensions of the BCS may be needed to better guide the design and development of these advanced drug delivery systems. Looking forward, the integration of the BCS with other tools and concepts in pharmaceutical sciences, such as physiologically based pharmacokinetic (PBPK) modeling and the Quality by Design (QbD) approach, could provide a more comprehensive and effective framework for the development of polymeric drug delivery systems. This could lead to more efficient and patient-friendly drug delivery solutions, ultimately improving patient outcomes.
3. Applications of Polymeric Drug Delivery System
1. Targeted Drug Delivery: Polymeric drug delivery systems can be designed to deliver drugs directly to the site of action [
12], reducing systemic side effects. This is particularly useful for drugs that are toxic or have severe side effects. For example, polymeric nanoparticles can be used to deliver anticancer drugs directly to tumour cells [
13].
2. Controlled Drug Release: Polymers can be engineered to release drugs at a controlled rate over a prolonged period. This can improve the therapeutic efficacy of the drug, reduce dosing frequency, and improve patient adherence to treatment. For instance, biodegradable polymer implants can be used for the sustained release of contraceptives or antipsychotic drugs.
3. Delivery of Sensitive Drugs: Some drugs, such as proteins and genes, are sensitive to the harsh conditions in the gastrointestinal tract. Polymeric drug delivery systems can protect these drugs from degradation and allow for their controlled release. For example, polymeric nanoparticles can be used for the delivery of insulin or gene therapy.
4. Vaccine Delivery: Polymeric drug delivery systems can enhance the immune response to vaccines by providing sustained antigen release and/or adjuvant effect. For example, polymeric microspheres can be used for the delivery of subunit vaccines or DNA vaccines [
14].
5. Overcoming Drug Resistance: In some cases, polymeric drug delivery systems can help overcome drug resistance, a major issue in the treatment of diseases like cancer and infections. For example, polymeric nanoparticles can be used to co-deliver anticancer drugs and inhibitors of drug resistance pathways, enhancing the therapeutic efficacy of the treatment.
6. Transdermal and Topical Drug Delivery: Polymeric systems can also be used for the delivery of drugs through the skin or mucosal surfaces. For example, polymeric patches can be used for the sustained release of pain medication, while polymeric gels [
15] can be used for the localized delivery of anti-inflammatory or antimicrobial drugs.
7. Ocular Drug Delivery: Polymeric systems can enhance the delivery of drugs to the eye, a challenging task due to the eye's protective barriers. For example, polymeric nanoparticles or hydrogels can be used for the sustained release of drugs for the treatment of glaucoma or macular degeneration [
16].
8. Nasal and Pulmonary Drug Delivery: Polymeric systems can be used for the delivery of drugs to the respiratory tract, for the treatment of conditions like asthma or infections. For example, polymeric microspheres can be used for the sustained release of bronchodilators or antibiotics.
Polymeric drug delivery systems have a wide range of applications in the field of pharmaceutical sciences as shown in
Figure 3. They offer unique advantages in terms of drug targeting, controlled release, protection of sensitive drugs, vaccine delivery, overcoming drug resistance, and delivery through various routes. Their versatility and tunability make them an ideal platform for the design and development of effective drug delivery systems. However, there are still challenges to overcome, such as the control of polymer degradation and drug release kinetics, the improvement of drug loading efficiency, and the enhancement of drug stability and bioavailability. Therefore, continued research and development in this field are essential to fully exploit the potential of polymeric drug delivery systems. With ongoing advancements in polymer science and drug delivery technology, it is expected that polymeric drug delivery systems will continue to play a pivotal role in the treatment of a wide range of diseases and conditions, ultimately improving patient outcomes.
4. Methods of Preparation of Polymeric Drug Delivery System
There are several methods used to prepare PDDS, each with its unique advantages and applications.
1. Solvent Evaporation: This method is commonly used to prepare polymeric micro- and nanoparticles. The drug and polymer are dissolved in a volatile organic solvent, which is then evaporated to form a polymer-drug film. The film is then suspended in an aqueous solution to form particles. The size and morphology of the particles can be controlled by adjusting the polymer concentration, solvent type, and evaporation rate.
2. Emulsion Polymerization: This method is used to prepare polymeric nanoparticles. The drug and monomer are emulsified in an aqueous solution, and then a polymerization initiator is added to start the polymerization reaction. The size and morphology of the nanoparticles can be controlled by adjusting the emulsion conditions and polymerization parameters.
3. Coacervation: This method is used to prepare microcapsules. The drug is dispersed in a solution of the polymer, and then a coacervation agent is added to induce phase separation. The drug particles are encapsulated by the coacervate phase, forming microcapsules. The size and morphology of the microcapsules can be controlled by adjusting the coacervation conditions.
4. Solvent Casting: This method is used to prepare polymeric films and membranes. The drug and polymer are dissolved in a suitable solvent, and then the solvent is evaporated to form a thin film. The thickness and properties of the film can be controlled by adjusting the polymer concentration and evaporation conditions.
5. Melt Extrusion This method is used to prepare polymeric implants and tablets. The drug and polymer are mixed and heated until the polymer melts, and then the mixture is extruded through a die to form a solid dosage form. The size and properties of the dosage form can be controlled by adjusting the extrusion conditions.
6. Electrospinning: This method is used to prepare polymeric nanofibers. The drug and polymer are dissolved in a suitable solvent, and then the solution is subjected to a high voltage to form a jet. The solvent evaporates, leaving behind nanofibers. The size and morphology of the nanofibers can be controlled by adjusting the solution properties and electrospinning conditions.
7. Layer-by-Layer Assembly: This method is used to prepare multilayer polymeric systems. The drug and polymer are alternately deposited onto a substrate, forming a multilayer structure. The thickness and properties of the layers can be controlled by adjusting the deposition conditions.
8. Microfluidics: This method is used to prepare polymeric particles with precise control over size, shape, and composition. The drug and polymer are mixed in a microfluidic device, and the flow conditions are controlled to form particles.
The choice of preparation method for polymeric drug delivery systems depends on the properties of the drug and polymer, as well as the desired characteristics of the final product. Each method has its unique advantages and limitations, and the choice of method can significantly impact the performance of the drug delivery system. Therefore, a thorough understanding of these methods and their impact on the drug delivery system is crucial for the design and development of effective polymeric drug delivery systems. Moreover, continued research and development are needed to improve these methods and develop new ones, to meet the evolving needs of drug delivery. With ongoing advancements in polymer science and drug delivery technology, the methods of preparation for polymeric drug delivery systems will continue to evolve and improve, ultimately leading to more effective, safer, and more patient-friendly drug delivery solutions.
5. Current Status of Polymeric Drug Delivery System
The current status of polymeric drug delivery systems is that they are a widely used and highly researched method for drug delivery. These systems offer many advantages over others, such as controlled and sustained release of drugs, targeted delivery [
12,
17,
18], and the ability to deliver sensitive drugs like proteins and genes. They are used in a variety of applications, from the treatment of chronic diseases to the delivery of vaccines. There are several methods as discussed above which can be used to prepare polymeric drug delivery systems, including solvent evaporation, emulsion polymerization, coacervation, solvent casting, melt extrusion, electrospinning, layer-by-layer assembly, and microfluidics. Each method has its unique advantages and limitations, and the choice of method can significantly impact the performance of the drug delivery system as well. Despite the many advancements in polymeric drug delivery systems, there are still challenges to overcome. One of the main challenges is controlling the degradation and drug release kinetics of the polymer. Other challenges include improving drug loading efficiency and enhancing drug stability and bioavailability. Moreover, the application of the Biopharmaceutics Classification System (BCS) to polymeric drug delivery systems is another area of ongoing research. The BCS classifies drugs based on their solubility and intestinal permeability, which are key factors affecting the rate and extent of drug absorption. However, the BCS is based on the properties of the drug substance alone and does not consider the influence of the drug delivery system. Therefore, modifications or extensions of the BCS may be needed to better guide the design and development of polymeric drug delivery systems. In terms of regulatory approval, several polymeric drug delivery systems have been approved by the U.S. Food and Drug Administration (FDA) for use in humans. These include biodegradable polymer implants for the sustained release of contraceptives and antipsychotic drugs, polymeric nanoparticles for the delivery of anticancer drugs, and polymeric patches for the sustained release of pain medication. However, the regulatory pathway for polymeric drug delivery systems is complex and challenging, requiring extensive preclinical and clinical testing to demonstrate their safety and efficacy. Despite these challenges, the field of polymeric drug delivery systems is rapidly advancing, with numerous research studies and clinical trials underway. Areas of focus include the development of smart polymers that can respond to specific stimuli for controlled drug release, the use of nanotechnology to create smaller and more efficient drug delivery systems, and the application of polymeric drug delivery systems in personalized medicine where the drug delivery system could be tailored to the individual patient's needs. Furthermore, there is a growing interest in the use of natural polymers, such as proteins and polysaccharides, in drug delivery systems due to their biocompatibility and biodegradability. In conclusion, the current status of polymeric drug delivery systems is that they are at the forefront of drug delivery technology, offering promising solutions for the delivery of a wide range of drugs. However, there are still challenges to overcome, and continued research and development are needed to fully exploit their potential. With ongoing advancements in polymer science and drug delivery technology, it is expected that polymeric drug delivery systems will continue to evolve and improve, ultimately leading to more effective, safer, and more patient-friendly drug delivery solutions.
6. Drugs Encapsulated in Polymeric Drug Delivery System
Various drugs have been successfully encapsulated in polymeric drug delivery systems, including:
1. Anticancer Drugs [
19]: Polymeric drug delivery systems have been extensively used for the delivery of anticancer drugs. For example, Doxil® is a liposomal nanoparticle formulation of the anticancer drug doxorubicin [
19], which is encapsulated within a polymer coating. This allows for targeted delivery of the drug to the tumor, minimizing damage to healthy tissues.
2. Proteins and Peptides: Proteins and peptides are often sensitive to harsh conditions in the gastrointestinal tract and can be degraded before they reach their target site. Encapsulating these drugs in polymeric systems can protect them from degradation and allow for their controlled release. For instance, insulin, a peptide hormone used for the treatment of diabetes, has been encapsulated in polymeric nanoparticles for oral delivery.
3. Genes: Polymeric systems have been used for gene delivery in gene therapy. The gene is encapsulated within a polymeric vector, which can protect the gene from degradation and deliver it to the target cells. For example, polyethyleneimine (PEI) is a cationic polymer that has been widely used for gene delivery due to its ability to condense DNA into nanoparticles and facilitate its entry into cells.
4. Vaccines: Polymeric systems can enhance the immune response to vaccines by providing sustained antigen release and/or adjuvant effect. For example, poly(lactic-co-glycolic acid) (PLGA) microspheres have been used for the delivery of subunit vaccines or DNA vaccines. The encapsulation of the antigen within the polymer can protect it from degradation and allow for its controlled release, enhancing the immune response.
5. Antipsychotic Drugs: Polymeric drug delivery systems have been used for the sustained release of antipsychotic drugs, improving patient adherence to treatment. For instance, R Risperdal Consta® is a long-acting injectable formulation of the antipsychotic drug risperidone, which is encapsulated within biodegradable polymer microspheres. This allows for the sustained release of the drug for two weeks, reducing the need for daily medication and improving patient compliance.
6. Contraceptives: Polymeric drug delivery systems have also been used for the sustained release of contraceptives. For example, Nexplanon® is a contraceptive implant that contains the contraceptive drug etonogestrel encapsulated within a biodegradable polymer. Once implanted under the skin, the device releases the drug at a controlled rate over three years, providing long-term contraception without the need for daily medication.
7. Pain Medication: Polymeric patches have been used for the sustained release of pain medication. For example, Lidoderm® is a patch that contains the local anesthetic lidocaine encapsulated within a polymeric matrix. When applied to the skin, the patch releases the drug at a controlled rate, providing localized pain relief for up to 12 hours.
A wide range of drugs have been successfully encapsulated in polymeric drug delivery systems, demonstrating the versatility and potential of these systems. However, the encapsulation of drugs in polymeric systems is not without challenges. Issues such as the control of drug release kinetics, the stability of the drug within the polymer, and the bioavailability of the drug, need to be carefully considered and optimized for each specific drug and application. Therefore, continued research and development in this field are essential to fully exploit the potential of polymeric drug delivery systems. With ongoing advancements in polymer science and drug delivery technology, it is expected that more and more drugs will be successfully encapsulated in polymeric systems, leading to more effective, safer, and more patient-friendly drug delivery solutions.
7. Characterization of Polymeric Drug Delivery System
The characterization parameters for polymeric drug delivery systems are essential to understand their behavior and performance. These parameters can be broadly categorized into physical, chemical, and biological parameters.
1. Physical Parameters: These include size, shape, surface morphology, and mechanical properties of the polymeric system. Techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) can be used to determine these parameters. The size and shape of the system can greatly influence its drug-release behavior, biodistribution, and cellular uptake. The surface morphology can affect the interaction of the system with biological entities, while the mechanical properties can influence its stability and integrity.
2. Chemical Parameters: These include the composition, molecular weight, and degree of crosslinking of the polymer, as well as the drug loading efficiency and drug release kinetics. Techniques such as nuclear magnetic resonance (NMR) spectroscopy, gel permeation chromatography (GPC), and High-performance liquid chromatography (HPLC) can be used to determine these parameters. The composition and molecular weight of the polymer can affect its degradation rate and drug release behavior, while the degree of crosslinking can influence its mechanical properties and swelling behavior. The drug loading efficiency is a measure of the amount of drug that can be encapsulated in the system, while the drug release kinetics can determine the duration and rate of drug release.
3. Biological Parameters: These include the biocompatibility, biodistribution, cellular uptake, and therapeutic efficacy of the polymeric system. Techniques such as in vitro cytotoxicity assays, in vivo biodistribution studies, cellular uptake studies, and in vivo therapeutic efficacy studies can be used to determine these parameters. Biocompatibility is a measure of the system's safety, while the biodistribution can reveal its fate in the body. The cellular uptake can indicate its ability to deliver the drug to the target cells, while the therapeutic efficacy can demonstrate its effectiveness in treating the intended disease or condition.
The characterization of polymeric drug delivery systems is a crucial step in their development and optimization. It provides valuable information about their physical, chemical, and biological properties, which can greatly influence their performance as drug delivery systems. Therefore, a thorough understanding of these characterization parameters and the techniques used to determine them is essential for the design and development of effective polymeric drug delivery systems. Moreover, as the field of polymeric drug delivery systems continues to evolve, new characterization parameters and techniques may emerge, further enhancing our ability to design and optimize these systems. Therefore, continued research and development in this field are essential to fully exploit the potential of polymeric drug delivery systems. With ongoing advancements in polymer science and drug delivery technology, it is expected that the characterization of polymeric drug delivery systems will become increasingly sophisticated and comprehensive, ultimately leading to more effective, safer, and more patient-friendly drug delivery solutions.
8. Conclusion & Summary
In conclusion, the use of biodegradable polymers in Polymeric Drug Delivery Systems (PDDS) has revolutionized the field of drug delivery [
20]. From the initial breakthrough in the 1960s, through the development of targeted cancer treatments and FDA-approved systems, to the current exploration of smart polymers and nanotechnology, PDDS has continuously evolved to improve patient care. The ability of these systems to provide controlled, sustained release of drugs has significantly enhanced therapeutic efficacy and minimized side effects. The use of biodegradable polymers, in particular, has added an extra layer of safety and convenience, as these materials can be broken down and eliminated by the body after drug delivery. As we look to the future, the potential for further advancements in this field is immense. With ongoing research and development, PDDS could play an even more pivotal role in the treatment of a wide range of diseases and conditions. Personalized medicine, where drug delivery systems are tailored to individual patient needs, may become a reality with the continued advancement of PDDS. As such, the importance of biodegradable polymers in polymeric drug delivery systems cannot be overstated. Their continued development and optimization will undoubtedly lead to more effective, safer, and more patient-friendly drug delivery solutions in the future.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smyth HDC. A Review of: “Polymeric Drug Delivery Systems.” Drug Dev Ind Pharm. 2006;32(9):1111-1111. [CrossRef]
- Hu L, Sun Y, Wu Y. Advances in chitosan -based drug delivery vehicles. Nanoscale. 2013;5(8):3103-3111. [CrossRef]
- Safari J, Zarnegar Z. Advanced drug delivery systems: Nanotechnology of health design A review. J Saudi Chem Soc. 2014;18(2):85-99. [CrossRef]
- Gagliardi A, Giuliano E, Venkateswararao E, et al. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front Pharmacol. 2021;12:601626. [CrossRef]
- Bolla PK, Rodriguez VA, Kalhapure RS, Kolli CS, Andrews S, Renukuntla J. A review on pH and temperature responsive gels and other less explored drug delivery systems. J Drug Deliv Sci Technol. 2018;46:416-435. [CrossRef]
- Kalhapure RS, Renukuntla J. Thermo- and pH dual responsive polymeric micelles and nanoparticles. Chem-Biol Interact. 2018;295:20-37. [CrossRef]
- Alotaibi BS, Buabeid M, Ibrahim NA, et al. Potential of Nanocarrier-Based Drug Delivery Systems for Brain Targeting: A Current Review of Literature. Int J Nanomed. 2021;16:7517-7533. [CrossRef]
- Gopi S, Amalraj A, Sukumaran NP, Haponiuk JT, Thomas S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol Symp. [CrossRef]
- Park J, Ye M, Park K. Biodegradable Polymers for Microencapsulation of Drugs. Molecules. 2005;10(1):146-161. [CrossRef]
- Roy S, Prabhakar B. Bioadhesive polymeric platforms for transmucosal drug delivery systems – a review. Trop J Pharm Res. 2010;9(1). [CrossRef]
- Kesharwani R, Jaiswal P, Patel DK, Yadav PK. Lipid-Based Drug Delivery System (LBDDS): An Emerging Paradigm to Enhance Oral Bioavailability of Poorly Soluble Drugs. Biomed Mater Devices. 2023;1(2):648-663. [CrossRef]
- Domb A, Amselem S, Shah J, Maniar M. Degradable polymers for site-specific drug delivery. Polym Adv Technol. 1992;3(6):279-292. [CrossRef]
- Domb AJ, Ringel I. 10 Polymeric Drug Carrier Systems in the Brain. Methods Neurosci. 1994;21(Science2591993):169-183. [CrossRef]
- Han J, Zhao D, Li D, Wang X, Jin Z, Zhao K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers. 2018;10(1):31. [CrossRef]
- Xinming L, Yingde C, Lloyd AW, et al. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye. 2008;31(2):57-64. [CrossRef]
- Shadab S, Alam MA, Sharma PK, Paliwal D. Overcoming the Therapeutic Limitation of Ocular Drug Delivery with The Help of Novel Drug Carriers. Curr Nanomater. 2023;08. [CrossRef]
- Abasian P, Ghanavati S, Rahebi S, Khorasani SN, Khalili S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym Adv Technol. 2020;31(12):2939-2954. [CrossRef]
- Semwal R. Targeted Drug Nanoparticles: An Emphasis on Self-assembled Polymeric System. J Méd Sci. 2010;10(5):130-137. [CrossRef]
- Salari N, Faraji F, Torghabeh FM, et al. Polymer-based drug delivery systems for anticancer drugs: A systematic review. Cancer Treat Res Commun. 2022;32:100605. [CrossRef]
- Kapse A, Anup N, Patel V, Saraogi GK, Mishra DK, Tekade RK. Drug Delivery Systems. 2020;(Macromolecules 26 1993):235-289. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).