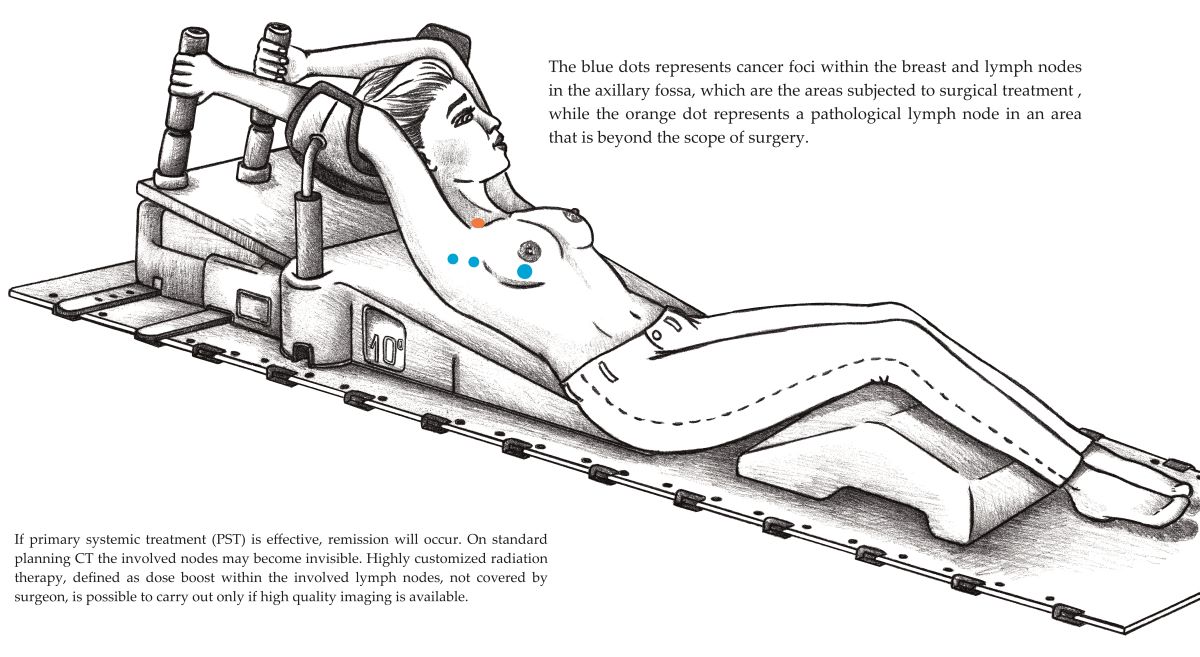
1. Introduction
Currently, systemic therapy is administered upfront in majority of triple-negative breast cancer (TNBC) and Her2-positive cases, to achieve pathological complete remission (pCR). Irradiation is carried out after surgical treatment to improve local control and overall survival in early and advanced breast cancer [
1]. When significant clinical response to systemic therapy is achieved, areas at highest risk of persistent disease will not be evident on the post-operative CT used for treatment planning. Standard imaging techniques for diagnosis and staging of breast cancer including mammography (MMG), ultrasound (US), and magnetic resonance imaging (MRI) have limited utility in personalizing radiation therapy (RT) because planning systems are mainly based on computed tomography (CT). MMG fails to show the lymph nodes clearly. It is more suitable for the evaluation of primary foci in the low mammographic density breasts of postmenopausal women than in the glandular breasts of younger patients [
2,
3], who are relatively frequently qualified for primary systemic therapy. Advanced RT techniques, such as intensity modulated radiation therapy (IMRT) require defining the desired dose to all target and non-target tissues on each slice of the planning CT. High-quality cross-sectional imaging that allows 3D visualisation, such as CT scan or positron emission tomography (PET-CT) are a necessary part of the procedure. Despite available data suggesting the utility of CT scan and PET-CT in breast cancer, they are not routinely performed [
4,
5,
6]. Medical imaging before systemic therapy and surgery, followed by planning CT, offers numerous possibilities for RT customization, such as increased dose in the non-operated anatomical area, where the pathological lymph nodes were observed (e.g. supraclavicular region or internal mammary lymph nodes). Additionally, it can aid in diagnosing of oligometastatic disease and facilitate the application of stereotactic body radiation therapy (SBRT). Scans performed before cancer treatment and planning CT done after surgery could be compared or superimposed on each other, providing a tool for RT individualisation (Supplmental Figure 7), this procedure is called image fusion. In our study, the radiation immobilization device was used to position patients during imaging (Supplmental Figure 3). The aim of this single-center study was comparison of the standard baseline imaging with extended radiological staging, in patients initially qualified for preoperative systemic treatment; investigation of whether CT scan and PET-CT reliably visualize the primary focus in the breast and pathological lymph nodes in the axilla, assessment if the nodes, recognized as involved actually contained metastases (correlation between the postoperative pathological report and the imaging). We also analyzed whether extended radiological staging had an additional diagnostic value. The secondary objective was to investigate how often, after receiving an additional examination result, the multidisciplinary team (MDT) has modified the originally planned treatment strategy.
2. Materials and Methods
The study design was prospective, cross-sectional, and observational (the assignment of medical interventions was not at the discretion of the investigator). Data were collected from patients' medical records. Eligible patients were, aged ≥18 years, with an established diagnosis of invasive breast adenocarcinoma defined by punch biopsy. Assessment of histological grade, Immunohistochemistry (IHC) evaluation of; Estrogen receptor (ER), Progesterone receptor (PgR), Ki67 and Human epidermal growth factor receptor 2 (HER2) status was required in the histopathology report. If the Her2 score was borderline, a FISH test was performed. Patients with the following; Eastern Co-operative Oncology Group Performance Status 0/2, no distant metastases and no clinically significant renal failure were recruited. All participants were selected for systemic preoperative therapy by a MDT at the Breast Unit of the Lower Silesian Oncology, Pulmonology, and Haematology Center. After carrying out preliminary radiological staging according to the recommendations of the Polish Society of Clinical Oncology (bilateral MMG, breast US, and chest X-ray [CXR] regardless of the stage, abdominal cavity imaging by US and/or CT scan, and bone scintigraphy in CS III) [
7], all eligible patients were offered an extended workup. Current Polish national recommendations suggest carrying out of additional imaging studies only as an option, routine inclusion of chest cross-sectional imaging (CT scan or PET-CT) prior to PST (primary systemic therapy) is not a common practice. Considering the estimated risk of spread of the neoplastic process, the decision to perform one of the imaging modalities was made by the MDT. High-risk patients underwent PET-CT; with no strict criteria for selecting additional imaging modality. Two cohorts of patients were analyzed separately: in the first, CT scan was performed, and in the second, PET-CT. All imaging and treatment results used in the study were considered a part of the patients’ diagnostic and therapeutic scheme.
In total, 132 participants were recruited between October 2015 and March 2020. After detailed verification, two were excluded. In one case, surgical biopsy of the breast tumour, turned out to be tumorectomy, and the breast lesion could not be measured. In the other case, the assumed period of 2 weeks from the beginning of systemic therapy to the CT scan was exceeded. Further analyses included 130 participants (128 woman and two men). All PET-CT examinations were performed before treatment and a CT scan was allowed up to 2 weeks after starting systemic therapy, assuming that during this time, there would be no significant tumour shrinkage in most patients. The COVID-19 pandemic interrupted the recruitment process; participation required an additional clinic visit, which could increase the risk of coronavirus transmission. The last CT scan was performed on 12.03.2020, and the collection of COVID-19 statistics in Poland began approximately on 14.03.2020 (according to the COVID-19 Data Repository of the Center for Systems Science and Engineering [CSSE] at Johns Hopkins University, there were 35 COVID-19 cases in Poland at that time). Thus, it may be concluded that CT scan and PET-CT results are free from COVID-19 bias, concerning the lung parenchyma, as well as possible vaccination-related lymphadenopathy [
8]. All scans were performed in the supine treatment position, were supervised by a radiation oncologist and experienced technicians, position was suited to the patient’s anatomical structure. The prone position is not optimal if lymph node radiation is planned, and the current guidelines and contouring atlases, which are helpful in daily practice, refer to the supine position [
9,
10,
11]. All CT scans were assessed by an experienced radiologist, and PET-CT by a nuclear medicine specialist. Tumour size measurements were also made by the above-mentioned professionals. CT was performed using an intravenous contrast agent and PET-CT using fluorodeoxyglucose (18F-FDG) as the radioactive tracer. The cross-sectional imaging result was attached to the patient's medical history and analyzed by MDT.
The age of the participants was similar in both cohorts, with a median of 51 and 50 years, respectively. A high-grade G3 histologic tumour was most common, and has been confirmed in 55.1% of cases in CT scan cohort and in 62.8% in PET-CT cohort, the G1 grade was observed in only one participant. High Ki67 expression prevailed and the median was 43.5 (7 ─ 90) in CT scan cohort and 52.5 (2 ─ 90) in PET-CT cohort (
Table 1). Participants who qualified for PET-CT according to the opinion of MDT had a higher risk of cancer spread, and were in many cases originally non-operable, cT4 clinical tumour stage has been established in 39.5% patients in this group. In the CT scan cohort, the majority, 58.6% presented cT2 features. The most common subtype, based on the receptor profile and Ki67 rate, according to the St. Gallen surrogate classification for breast cancer [
12], was the luminal-B-like, HER2 negative. Endocrine therapy as preoperative treatment was rare. Such management was applied in three patients in the PET-CT cohort, and in eight in the CT scan cohort. In 91.5 % of participants, PST was based on multi-drug chemotherapy. Anti-Her2 targeted therapy was administered when indicated. Optimal systemic therapy was selected by the MDT. The rate of pathologic complete response was rather low, slightly exceeding 22% in both the cohorts, due to a high representation of patients with T4 and N3 features and the dominant subtype being Luminal B (HER2-negative) [
13].
3. Results
3.1. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 25 software (IBM Corp., Armonk, N.Y., USA). Friedman test was used to evaluate the difference between largest dimension of the breast tumours in alternative medical imaging methods. Pairwise comparisons were performed using the post-hoc Dunn's test.
The following descriptive statistics were considered: mean, standard deviation, median, minimum, maximum, and the first and third quartiles. Hereby the distribution of the analyzed variables was presented in detail. Pair collation was made based on the median. We compared the largest dimension of the largest breast tumour focus (T/mm), between the standard MMG, US, and CT scans, as well as between MMG, US, and PET-CT. Chi-squared test was applied to estimate distribution of the variables pN+ (involved axillary nodes based on the histopathology report) and cN+ (involved axillary nodes based on imaging). Consistency between the clinical assessment by imaging and the pathology report was estimated separately in CT scan cohort and PET-CT cohort. The rate of patients was calculated in terms of an additional diagnostic value of extended radiological staging, which resulted in a modification of the clinical stage or change in the management strategy. The level of statistical significance was set at p<0.05.
3.2. Tumour Size Measurements
The largest dimension of the breast tumours was compared in millimetres (T/mm) using alternative medical imaging methods (
Table 2). In case of multifocal tumours, the largest lesion was taken into account. Friedman test was used to evaluate the difference. No statistically significant differences were observed between the median dimensions evaluated using CT scan and MMG. In PET-CT cohort, the measurement of the focus in the breast was made in low-dose CT (LDCT), which is a component of the PET-CT scan. When dimensions evaluated in PET-CT ware compared with US and MMG, there was no statistically significant difference in the size likewise. Only the dimensions evaluated in US turned out to be statistically significantly smaller (p = 0.02) than those evaluated in CT scan. It should be emphasised that US and MMG was performed before the diagnosis was confirmed by biopsy, whereas CT scan was performed after the initial workup and MDT meeting. Disease progression over a period of several weeks may be suspected in some patients.
3.3. Baseline Lymph Node Evaluation
Unambiguous results of lymph node assessment prevailed in all imaging techniques; these were further analysed (
Table 3).
3.4. Consistency between the Clinical Assessment (cN) with the Postoperative Pathology Report (pN)
Following the exclusion of unoperated patients and those reaching pCR, the consistency between the clinical assessment of axillary lymph nodes (cN) by CT scan and US with the postoperative pathology report (pN) was evaluated (
Table 4). This analysis is reliable because of the large cohort of participants with only a partial response, respectively 64 in CT scan cohort and 27 in PET-CT cohort. In 95.3 % of node positive cases, there was consistency between microscopic and CT scan evaluations. In only two cases (4.7 %), lymph nodes were assessed on CT scan as not involved, yet contained metastases. Similar results were obtained on comparing the clinical evaluation of lymph nodes by US and the pathology report. It may be assumed that the diagnostic values of CT scan and US are similar in terms of lymph node assessment. In 92.5 % of node positive cases, pathology reports and lymph node evaluations in US were consistent; however, in three cases (7.5%), lymph nodes were assessed as not involved, yet contained metastases (
Table 4). Chi-squared test confirmed consistency between the clinical assessment by imaging and the pathology report, for CT scan the result was χ2(1) = 18.98; p < 0.001.
Mirror analysis was performed in the PET-CT cohort (
Table 5). The clinical stage of the patients who qualified for PET-CT was more advanced. Noteworthily, there was no discordance between the nodes assessed, as not involved by PET-CT, and the postoperative pathology report. Although this cohort was smaller, the result of Chi-squared test confirmed consistency between the clinical assessment by imaging and the pathology report and for PET-CT was χ2(1) = 6.41; p = 0.03. All analyzed histopathological reports were postoperative and in patients after systemic treatment. It can be assumed that in some cases nodal pCR with simultaneous lack of pCR in the primary focus occurred. It is recognized that node-only pCR is present about twice as often as breast-only pCR [
14]. A small percentage of patients may also experience disease progression during systemic therapy. For this reason, the calculation of false negative and false positive rates of axillary status was abandoned.
3.5. Added Value of Extended Radiological Staging.
We also analysed whether CT scan or PET-CT have an additional diagnostic value, compared to that of the standard initial workup. The results confirmed that in 49.4 % of the participants, clinically significant information could be obtained from CT scan. Internal mammary lymph node involvement was detected in six patients. In two cases, the supraclavicular lymph nodes were found outside the anatomical boundaries suggested by the ESTRO guidelines for the delineation of lymph nodal areas. In one participant, the CT scan revealed a previously undiagnosed heart pathology (blood clot in the left atrium). Involved lymph nodes in the mediastinum were found in one participant (M1 feature). In ten cases, CT scan suggested satellite foci within the involved breast and in five pectoral muscle infiltration. In the PET-CT cohort, one-fourth of the cases ended with modification of the originally planned treatment strategy, and in 51.2 % of cases, the originally determined disease stage changed. PET-CT confirmed multifocal spread of the neoplastic process in seven patients and was helpful in diagnosing oligometastatic disease in two participants (
Table 6).
4. Discussion
In malignancies that are highly sensitive to systemic therapy, such as nasopharyngeal cancer, it is difficult to imagine modern RT planning without performing precise imaging before chemotherapy [
15,
16]. A thorough understanding of the original scope of the disease is required to determine the appropriate volume of irradiation. The effectiveness of systemic therapy is increasing in patients with breast cancer. Regarding HER2 positive and TNBC subtypes, pCR may be expected in nearly half of the treated population, even in patients with locally advanced disease [
17,
18]. The NCCN guidelines recommend an increased RT dose to the involved lymph nodes outside the surgeons’ reach [
19]. However, there are no suggestions on how to diagnose such clinical situations systematically. PET-CT is highly precise in evaluating breast cancer nodal involvement and was the basis of an anatomical atlas created in 2018 [
20]. A study published in 2012 demonstrated that a preoperative CT scan may facilitate and increase the precision of boost planning in the tumour bed [
21]. Recent experience in innovative imaging modalities, such as PET-prostate-specific membrane antigen (PSMA) in prostate cancer, has led to the assumption that in some cases, nodal metastases are outside the typical location covered in the current contouring guidelines [
22]. In terms of the radiation dose in breast cancer, hypofractionated schedules are considered state-of-the-art [
23]. However, significantly less attention has been paid to the complete radiation dose. Reportedly, an increased dose to the tumour bed doubles the local effectiveness of RT, yet the boost practice varies greatly in different cancer centers [
24,
25,
26]. For the majority of neoplasms, a hard-to-question paradigm in which local control depends on the total dose seems true. Modern irradiation techniques enable safe and precise application of high doses. It has been proven in a small group of breast cancer patients, that IMRT is effective enough to be considered as optional radical treatment, in those who do not want to undergo surgery [
27]. Data concerning increased doses in areas other than the tumour bed are scarce, but using a total dose higher than 60 Gy to involved lymph nodes improves local control [
28,
29,
30]. Particular attention should be paid to the internal mammary and supraclavicular nodes, because these are not routinely covered by surgical procedures [
31]. In the 130 patients in this study, a boost dose within the involved but unoperated lymph nodes was applied in nine patients, most often 63 Gy in 28 fractions. Boost to the internal mammary lymph nodes was applied in five patients, and in four patients, to the pathological lymph nodes within the part of the axilla not covered by the surgeon. Oligometastatic disease was diagnosed in two patients, and SBRT was carried out.
In the future, we may modify the dose, apart from the tumour bed boost, depending on the risk of anatomical area involvement. Such an approach is implemented in daily practice in RT for head and neck cancers, where one RT plan often involves three different doses to various areas: a gross disease dose to the volume of direct cancer infiltration, a high-risk subclinical disease dose to the areas that are the most common recurrence sites, and a low-risk dose to the elective lymph node areas [
32]. Similar methods may be used in the future for breast cancer in cases where high-quality imaging techniques are available.
This study has some limitations. The number of participants recruited was too small to analyze the impact of personalized RT on the progression-free survival (PFS) and overall survival (OS). This study was not a randomized but an observational trial; PET-CT was performed preferentially in the group of patients with an unfavorable prognosis. Therefore, it was not possible to compare the two additional imaging modalities. Despite these limitations, the results are encouraging.
5. Conclusions
The study results prove that both CT and PET-CT enable a detailed assessment of the location and size of the primary tumour in the breast and of the pathological lymph nodes. Contrary to MRI, which is most often performed in the prone position which significantly affects the anatomical conditions, both CT scan and PET-CT may be performed in the same position (therapeutic position) as that of the planning CT. The majority of recommendations suggest performing CT or PET-CT in cases of suspected spread of the disease [
33,
34]. However, considering the capabilities of modern RT, routine and accurate imaging should be performed in a systematic way. In our opinion, a patient’s selection by a MDT for systemic preoperative therapy, separates those who may benefit clinically from extended radiological staging.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization: Tomasz Borowiec and Rafał Matkowski, methodology: Tomasz Borowiec and Bożena Cybulska-Stopa, formal analysis: Tomasz Borowiec, investigation: Tomasz Borowiec, data curation: Tomasz Borowiec, writing—original draft preparation: Tomasz Borowiec, writing—review and editing, Tomasz Kuniej, Andrzej Kołodziejczyk and Dorota Dupla; supervision Adam Maciejczyk. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The trial was approved by the Ethics Committee of Wroclaw Medical University (No. KB – 571/2019). All procedures were performed in accordance with the principles for medical research of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent Statement
Written informed consent was obtained from all the participants.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions. Data were collected from patients' medical records.
Acknowledgments
With thanks to Michał Ordak for statistical elaboration and Izabela Pawińska for the drawings in the Supplementary Material. I would like to express my deepest gratitude to Orit Kaidar-Person and Wendy A. Woodward for brilliant suggestions and tips on academic writing.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CT scan (computed tomography scan, performed using intravenous contrast agent), CXR (chest X-ray), IMRT (intensity modulated radiation therapy), LDCT (low-dose computed tomography), MBC (metastatic breast cancer), MDT (multidisciplinary team), MMG (mammography), MRI (magnetic resonance imaging), NCCN (National Comprehensive Cancer Network), OS (overall survival), pCR (pathologic complete response), PET-CT (positron emission tomography), PFS (progression-free survival), planning CT (non-contrast-enhanced computer tomography, performed in supine treatment position), PST (primary systemic therapy), RT(radiation therapy), TNBC (triple-negative breast cancer), US (ultrasound), 18F-FDG (fluorodeoxyglucose).
References
- P.M. Poortmans, C. Weltens, C. Fortpied, C. Kirkove, K. Peignaux-Casasnovas, V. Budach, F. van der Leij, E. Vonk, N. Weidner, S. Rivera, G. van Tienhoven, A. Fourquet, G. Noel, M. Valli, M. Guckenberger, E. Koiter, S. Racadot, R. Abdah-Bortnyak, E.F. Van Limbergen, A. Engelen, P. De Brouwer, H. Struikmans, H. Bartelink, Internal mammary and medial supraclavicular lymph node chain irradiation in stage I–III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial, Lancet Oncol. 21 (2020) 1602–1610. [CrossRef]
- V. Dialani, D.F. James, P.J. Slanetz, A practical approach to imaging the axilla, Insights Imaging. 6 (2015) 217–229. [CrossRef]
- S.J. Vinnicombe, Breast density: why all the fuss?, Clin Radiol. 73 (2018) 334–357. [CrossRef]
- A. Sarno, G. Mettivier, P. Russo, Dedicated breast computed tomography: Basic aspects, Med Phys. 42 (2015) 2786–2804. [CrossRef]
- S.J. Ahn, Y.S. Kim, E.Y. Kim, H.K. Park, E.K. Cho, Y.K. Kim, Y.M. Sung, H.Y. Choi, The value of chest CT for prediction of breast tumor size: Comparison with pathology measurement, World J Surg Oncol. 11 (2013). [CrossRef]
- B.B. Koolen, F. Van Der Leij, W. V. Vogel, E.J.T. Rutgers, M.J.T.F.D. Vrancken Peeters, P.H.M. Elkhuizen, R.A. Valdés Olmos, Accuracy of 18F-FDG PET/CT for primary tumor visualization and staging in T1 breast cancer, Acta Oncol (Madr). 53 (2014) 50–57. [CrossRef]
- J. Jassem, M. Krzakowski Zespół autorski, M. Krzakowski, B. Bobek-Billewicz, R. Duchnowska, A. Jeziorski, W. Olszewski, E. Senkus-Konefka, H. Tchórzewska-Korba, P. Wysocki, WYTYCZNE POSTĘPOWANIA DIAGNOSTYCZNO-TERAPEUTYCZNEGO Rak piersi Breast cancer, (n.d.). [CrossRef]
- S. Wolfson, E. Kim, A. Plaunova, R. Bukhman, R.D. Sarmiento, N. Samreen, D. Awal, M.M. Sheth, H.B. Toth, L. Moy, B. Reig, Axillary Adenopathy after COVID-19 Vaccine: No Reason to Delay Screening Mammogram, Radiology. 303 (2022) 297–299. [CrossRef]
- O. Fargier-Bochaton, X. Wang, G. Dipasquale, M. Laouiti, M. Kountouri, O. Gorobets, N.P. Nguyen, R. Miralbell, V. Vinh-Hung, Prone versus supine free-breathing for right-sided whole breast radiotherapy, Sci Rep. 12 (2022). [CrossRef]
- N. Temme, R.M. Hermann, T. Hinsche, J.N. Becker, M. Sonnhoff, A. Kaltenborn, U.M. Carl, H. Christiansen, L. Geworski, M. Nitsche, Radiotherapy of Breast Cancer in Laterally Tilted Prone vs. Supine Position: What about the Internal Mammary Chain?, J Pers Med. 12 (2022). [CrossRef]
- B. V. Offersen, L.J. Boersma, C. Kirkove, S. Hol, M.C. Aznar, A. Biete Sola, Y.M. Kirova, J.P. Pignol, V. Remouchamps, K. Verhoeven, C. Weltens, M. Arenas, D. Gabrys, N. Kopek, M. Krause, D. Lundstedt, T. Marinko, A. Montero, J. Yarnold, P. Poortmans, ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, Radiotherapy and Oncology. 114 (2015) 3–10. [CrossRef]
- I. Vasconcelos, A. Hussainzada, S. Berger, E. Fietze, J. Linke, F. Siedentopf, W. Schoenegg, The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival, Breast. 29 (2016) 181–185. [CrossRef]
- C. Zarotti, B. Papassotiropoulos, C. Elfgen, K. Dedes, D. Vorburger, B. Pestalozzi, A. Trojan, Z. Varga, Biomarker dynamics and prognosis in breast cancer after neoadjuvant chemotherapy, Sci Rep. 12 (2022). [CrossRef]
- O.M. Fayanju, Y. Ren, S.M. Thomas, R.A. Greenup, J.K. Plichta, L.H. Rosenberger, N. Tamirisa, J. Force, J.C. Boughey, T. Hyslop, E.S. Hwang, The clinical significance of breast-only and node-only pathologic complete response (PCR) after neoadjuvant chemotherapy (NACT): A review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB), Ann Surg. 268 (2018) 591–601. [CrossRef]
- F. Xue, C. Hu, X. He, Induction chemotherapy followed by intensity-modulated radiotherapy with reduced gross tumor volume delineation for stage T3–4 nasopharyngeal carcinoma, Onco Targets Ther. 10 (2017) 3329–3336. [CrossRef]
- F. De Felice, D. Musio, V. Tombolini, Target volume delineation after induction chemotherapy in locally advanced head and neck cancer, Oral Oncol. 51 (2015) e81. [CrossRef]
- H. Reina Sofi, L. Gianni, T. Pienkowski, Y.-H. Im, L. Roman, L.-M. Tseng, M.-C. Liu, A. Lluch, E. Staroslawska, J. de la Haba-Rodriguez, S.-A. Im, J. Luiz Pedrini, B. Poirier, P. Morandi, V. Semiglazov, V. Srimuninnimit, G. Bianchi, T. Szado, J. Ratnayake, G. Ross, P. Valagussa, Effi cacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial, Articles Lancet Oncol. 13 (2012) 25–32. [CrossRef]
- T. Biswas, J.T. Efird, S. Prasad, C. Jindal, P.R. Walker, The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer, 2017. www.impactjournals.com/oncotarget. [CrossRef]
- Dwyer, Mary, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ® ) Breast Cancer NCCN.org NCCN Guidelines for Patients ® available at www.nccn.org/patients, 2023. https://www.nccn.
- K.J. Borm, J. Voppichler, M. Düsberg, M. Oechsner, T. Vag, W. Weber, S.E. Combs, M.N. Duma, FDG/PET-CT–Based Lymph Node Atlas in Breast Cancer Patients, Int J Radiat Oncol Biol Phys. 103 (2019) 574–582. [CrossRef]
- L.J. Boersma, T. Janssen, P.H.M. Elkhuizen, P. Poortmans, M. Van Der Sangen, A.N. Scholten, B. Hanbeukers, J.C. Duppen, C. Hurkmans, C. Van Vliet, Reducing interobserver variation of boost-CTV delineation in breast conserving radiation therapy using a pre-operative CT and delineation guidelines, in: Radiotherapy and Oncology, 2012: pp. 178–182. [CrossRef]
- K. Schiller, L. Stöhrer, M. Düsberg, K. Borm, M. Devecka, M.M.E. Vogel, R. Tauber, M.M. Heck, I. Rauscher, M. Eiber, J.E. Gschwend, M.N. Duma, S.E. Combs, PSMA-PET/CT-based Lymph Node Atlas for Prostate Cancer Patients Recurring After Primary Treatment: Clinical Implications for Salvage Radiation Therapy, Eur Urol Oncol. 4 (2021) 73–83. [CrossRef]
- F. De Rose, A. Fogliata, D. Franceschini, C. Iftode, P. Navarria, T. Comito, C. Franzese, B. Fernandes, G. Masci, R. Torrisi, C. Tinterri, A. Testori, A. Santoro, M. Scorsetti, Hypofractionation with simultaneous boost in breast cancer patients receiving adjuvant chemotherapy: A prospective evaluation of a case series and review of the literature, Breast. 42 (2018) 31–37. [CrossRef]
- C. Vrieling, E. Van Werkhoven, P. Maingon, P. Poortmans, C. Weltens, A. Fourquet, D. Schinagl, B. Oei, C.C. Rodenhuis, J.C. Horiot, H. Struikmans, E. Van Limbergen, Y. Kirova, P. Elkhuizen, R. Bongartz, R. Miralbell, D.A.L. Morgan, J.B. Dubois, V. Remouchamps, R.O. Mirimanoff, G. Hart, S. Collette, L. Collette, H. Bartelink, Prognostic factors for local control in breast cancer after long-term follow-up in the EORTC boost vs no boost trial: A randomized clinical trial, JAMA Oncol. 3 (2017) 42–48. [CrossRef]
- H.H. Lee, C.H. Chen, K.H. Luo, H.Y. Chuang, C.J. Huang, Y.K. Cheng, F. Chen, S.H. Kuo, M.Y. Huang, Five-year survival outcomes of intensity-modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) using forward IMRT or Tomotherapy for breast cancer, Sci Rep. 10 (2020). [CrossRef]
- D. Krug, R. Baumann, K. Krockenberger, R. Vonthein, A. Schreiber, A. Boicev, F. Würschmidt, E. Weinstrauch, K. Eilf, P. Andreas, U. Höller, S. Dinges, K. Piefel, J. Zimmer, K. Dellas, J. Dunst, Adjuvant hypofractionated radiotherapy with simultaneous integrated boost after breast-conserving surgery: results of a prospective trial, Strahlentherapie Und Onkologie. 197 (2021) 48–55. [CrossRef]
- Y. Shibamoto, T. Murai, K. Suzuki, C. Hashizume, K. Ohta, Y. Yamada, M. Niwa, A. Torii, M. Shimohira, Definitive radiotherapy with SBRT or IMRT boost for breast cancer: Excellent local control and cosmetic outcome, Technol Cancer Res Treat. 17 (2018) 1–7. [CrossRef]
- L.M. Andring, K. Diao, S. Sun, M. Patel, G.J. Whitman, P. Schlembach, I. Arzu, M.M. Joyner, S.F. Shaitelman, K. Hoffman, M.C. Stauder, B.D. Smith, W.A. Woodward, Locoregional Management and Prognostic Factors in Breast Cancer With Ipsilateral Internal Mammary and Axillary Lymph Node Involvement, Int J Radiat Oncol Biol Phys. 113 (2022) 552–560. [CrossRef]
- J.M. Purswani, C. Oh, J.R. Teruel, J. Xiao, D.L. Barbee, O.G. Maisonet, C.A. Perez, N.E. Huppert, N.K. Gerber, Definitive Radiation With Nodal Boost for Patients With Locally Advanced Breast Cancer, Pract Radiat Oncol. 13 (2023) e103–e114. [CrossRef]
- K. Diao, L.M. Andring, C.H. Barcenas, P. Singh, H. (Carisa) Le-Petross, V.K. Reed, J.P. Reddy, E.S. Bloom, N.R. Ahmad, L.L. Mayo, G.H. Perkins, M.P. Mitchell, K.T. Nead, W. Tereffe, B.D. Smith, W.A. Woodward, Contemporary Outcomes After Multimodality Therapy in Patients With Breast Cancer Presenting With Ipsilateral Supraclavicular Node Involvement, Int J Radiat Oncol Biol Phys. 112 (2022) 66–74. [CrossRef]
- K. Yang, H. Kim, D.H. Choi, W. Park, J.M. Noh, W.K. Cho, Optimal radiotherapy for patients with internal mammary lymph node metastasis from breast cancer, Radiation Oncology. 15 (2020). [CrossRef]
- J. WICHMANN, M. DURISIN, R.M. HERMANN, R. MERTEN, H. CHRISTIANSEN, Moderately hypofractionated intensity-modulated radiotherapy with a simultaneous integrated boost for locally advanced head and neck cancer - do modern techniques fulfil their promise?, In Vivo (Brooklyn). 35 (2021) 2801–2808. [CrossRef]
- T. Barrett, D.J. Bowden, D.C. Greenberg, C.H. Brown, G.C. Wishart, P.D. Britton, Radiological staging in breast cancer: Which asymptomatic patients to image and how, Br J Cancer. 101 (2009) 1522–1528. [CrossRef]
- F. Cardoso, S. Kyriakides, S. Ohno, F. Penault-Llorca, P. Poortmans, I.T. Rubio, S. Zackrisson, E. Senkus, Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology. 30 (2019) 1194–1220. [CrossRef]
Table 1.
Patients baseline characteristics.
Table 1.
Patients baseline characteristics.
| |
CT scan cohort (n=87) |
PET-CT cohort (n=43) |
| median age, years |
51 (25 ─ 80) |
50 (24 ─ 74) |
| sex |
Women |
85 (97.7%) |
43 (100%) |
| Men |
2 (2.3%) |
0 |
| clinical tumour stage (cT) |
cT1 |
3 (3.4%) |
0 |
| cT2 |
51 (58.6%) |
16 (37.2%) |
| cT3 |
25 (28.7%) |
10 (23.3%) |
| cT4 |
8 (9.2%) |
17 (39.5%) |
| clinical nodal stage (cN) |
cN0 |
24 (27.6%) |
7 (16.3%) |
| cN1 |
26 (29.9%) |
8 (18.6%) |
| cN2 |
26 (29.9%) |
14 (32.6%) |
| cN3 |
11 (12.6%) |
14 (32.6%) |
| histologic grade |
G1 |
0 |
1 (2.3 %) |
| G2 |
37 (42.5%) |
14 (32.6%) |
| G3 |
48 (55.1%) |
27 (62.8%) |
| not established |
2 (2.3%) |
1 (2.3%) |
| median Ki67 |
43,5 (7 ─ 90) |
52,5 (2 ─ 90) |
| St. Gallen surrogate classification for breast cancer |
Luminal A-like |
5 (5.7%) |
4 (9.3%) |
| Luminal B-like (HER2-negative) |
34 (39.1%) |
18 (41.9%) |
| Luminal B-like (HER2-positive) |
11 (12.6%) |
9 (20.9%) |
| HER2-positive (non-luminal) |
8 (9.2%) |
1 (2.3%) |
| Triple-negative |
29 (33.3%) |
11 (25.6%) |
| definitive surgery |
82 (94.3%) |
35 (81.4%) |
| no surgery |
5 (5.7%) |
8 (18.6%) |
| pathologic complete response (pCR) |
18 (22%) |
8 (22.9%) |
| no pathologic complete response |
64 (78%) |
27 (77.1%) |
Table 2.
Largest contiguous dimension of a tumour focus in alternative imaging methods.
Table 2.
Largest contiguous dimension of a tumour focus in alternative imaging methods.
| CT scan cohort |
M |
Me |
SD |
Min |
Max |
Q1 |
Q3 |
| T/mm MMG |
40.79 |
37.5 |
21.88 |
10 |
130 |
25 |
51.25 |
| T/mm US |
38.51 |
38 |
13.95 |
11 |
75 |
29 |
46 |
| T/mm CT scan |
43.48 |
40 |
19.2 |
13 |
115 |
30 |
54 |
| PET-CT cohort |
|
|
|
|
|
|
|
| T/mm MMG |
44.78 |
35 |
26.93 |
12 |
100 |
27 |
55 |
| T/mm US |
44.72 |
40 |
21.93 |
14 |
100 |
27 |
56 |
| T/mm PET-CT |
46.32 |
37.5 |
26.43 |
12 |
120 |
29.75 |
56 |
Table 3.
An unequivocal result was the one in which the nodes were recognized as pathological (cN+) or not involved (cN-). Results that reported suspicious or enlarged nodes were reckoned as dubious.
Table 3.
An unequivocal result was the one in which the nodes were recognized as pathological (cN+) or not involved (cN-). Results that reported suspicious or enlarged nodes were reckoned as dubious.
| no pathologic complete response |
CT scan cohort (n=64) |
| unequivocal lymph node evaluation by CT scan |
60 (94%) |
| dubious lymph node evaluation by CT scan |
4 (6%) |
| unequivocal lymph node evaluation by US |
59 (92%) |
| dubious lymph node evaluation by US |
5 (8%) |
| no pathologic complete response |
PET-CT cohort (n=27) |
| unequivocal lymph node evaluation by PET-CT |
26 (96 %) |
| dubious lymph node evaluation by PET-CT |
1 (4%) |
| unequivocal lymph node evaluation by US |
26 (96%) |
| dubious lymph node evaluation by US |
1 (4%) |
Table 4.
The consistency between clinical assessment of axillary lymph nodes (cN) by baseline CT scan, US, and the postoperative pathology report (pN). Only patients with no pathologic complete response and unequivocal lymph node evaluation by imaging were included in this analysis, refer to the
Table 3.
Table 4.
The consistency between clinical assessment of axillary lymph nodes (cN) by baseline CT scan, US, and the postoperative pathology report (pN). Only patients with no pathologic complete response and unequivocal lymph node evaluation by imaging were included in this analysis, refer to the
Table 3.
| Imaging vs pathology report |
cN evaluated by CT scan |
| cN-(n=11) |
cN+ (n=49) |
| n |
% |
n |
% |
| pN- (n=17) |
9 |
52.9 |
8 |
47.1 |
| pN+ (n=43) |
2 |
4.7 |
41 |
95.3 |
| cN evaluated by US |
|
cN- (n=16) cN+ (n=43)
|
| pN- (n=19) |
13 |
68.4 |
6 |
31.6 |
| pN+ (n=40) |
3 |
7.5 |
37 |
92.5 |
Table 5.
The consistency between clinical assessment of axillary lymph nodes (cN) by baseline PET-CT, US, and the postoperative pathology report (pN). Only patients with no pathologic complete response and unequivocal lymph node evaluation by imaging were included in this analysis, refer to the
Table 3.
Table 5.
The consistency between clinical assessment of axillary lymph nodes (cN) by baseline PET-CT, US, and the postoperative pathology report (pN). Only patients with no pathologic complete response and unequivocal lymph node evaluation by imaging were included in this analysis, refer to the
Table 3.
| Imaging vs pathology report |
cN evaluated by PET-CT |
| cN- (n=3) |
cN+ (n=23) |
| n |
% |
n |
% |
| pN- (n= 9) |
3 |
33.3 |
6 |
66.7 |
| pN+ (n=17) |
0 |
0 |
17 |
100 |
| cN evaluated by US |
|
cN- (n=5) cN+ (n=21)
|
| pN- (n=8) |
5 |
62.5 |
3 |
37.5 |
| pN+ (n=18) |
0 |
0 |
18 |
100 |
Table 6.
Impact of the CT scan and PET-CT on the multidisciplinary team’s decision. Modification of the management strategy was understood as withdrawal of surgery or personalized radiation therapy (e.g., boost within the internal mammary lymph nodes or stereotactic body radiation therapy [SBRT] in oligometastatic disease).
Table 6.
Impact of the CT scan and PET-CT on the multidisciplinary team’s decision. Modification of the management strategy was understood as withdrawal of surgery or personalized radiation therapy (e.g., boost within the internal mammary lymph nodes or stereotactic body radiation therapy [SBRT] in oligometastatic disease).
| CT scan cohort (n=87) |
n |
% |
| management strategy modification |
9 |
10.3 |
| clinical stage shift |
32 |
36.8 |
| other clinically significant findings |
2 |
2.3 |
| no added value of extended radiological staging |
44 |
50.6 |
| PET-CT cohort (n=43) |
n |
% |
| management strategy modification |
11 |
25.6 |
| clinical stage shift |
22 |
51.2 |
| no added value of extended radiological staging |
10 |
23.3 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).