1. Introduction
Endocrine-disrupting chemicals are becoming an increasing concern for human health due to their growing prevalence in the environment and their possible interactions with mammalian endocrine systems (Winz et al., 2023). They are toxic synthetic compounds that interfere with the body’s natural hormones, impacting critical human bodily functions. They can disrupt the normal functions of the endocrine system, like hormone production, release, and transportation, resulting in hormonal imbalances (Shanmugam et al., 2023; Barrios-Estrada et al., 2018). Their structural resemblance to hormones such as estrogen allows these chemicals to disrupt endocrine signalling, resulting in various harmful effects. EDCs, known for dysregulating various cell signalling pathways related to cellular metabolism, often mimic hormone structures and can erroneously trigger hormone signalling pathways by interacting with specific hormone receptors. Furthermore, they can alter enzymatic processes involved in hormone or receptor biosynthesis and metabolism (Winz et al., 2023; Turan, 2021; Rajesh et al., 2015).
Since the 1930s, human exposure to phthalates has been steadily growing, correlating with the rising prevalence of these substances in numerous products. These synthetic chemicals are recognized as key environmental contaminants and are a vital ingredient in the plastics industry, making them widespread in our surroundings and daily lives. Phthalates, used as plasticizers to enhance the flexibility of rigid polymers like polyvinyl chloride (PVC) and as solvents and additives in cosmetics and personal care items, are omnipresent in the environment. Their lack of covalent bonding to polymers allows them to seep into the environment and enter the human body through various exposure pathways. Once inside, phthalates act as endocrine disruptors, binding to molecular targets and disturbing hormonal balance. Their presence has been detected in food, water, air, consumer products, and biological samples such as urine, blood, faeces, meconium, breast milk, amniotic fluid, and saliva. Phthalates are linked to various health impairments in fetuses, infants, children, and adults, with adverse effects reported even at low exposure levels. This characteristic of endocrine-disrupting compounds (EDCs) is attributed to their non-monotonic response curve, where the effect does not linearly correlate with concentration increases (Mariana et al., 2023). In addition, the global production of plastics, essential in modern life, reached approximately 367 million metric tons in 2020, increasing annually. Phthalate production for industrial purposes is estimated at about 5.5 million metric tons each year (Tuan, 2022).
Di(2-ethylhexyl) phthalate (DEHP), a prevalent environmental endocrine disruptor, is extensively used in the plastics industry and widely found in various environments. It enters the human body via air, food, water, and skin contact. Once inside, DEHP quickly transforms into its more toxic metabolite, mono(2-ethylhexyl) phthalate (MEHP) (Zhang et al., 2022). DEHP can enter humans through multiple sources, including food, beverages, cosmetics, and medical devices. Its exposure has been linked to the development of various health issues. The widespread use of DEHP in plastics has sparked concerns about its continuous exposure and potential health risks for infants and adults. DEHP’s negative effects extend to metabolic and reproductive systems, embryonic development, gonadal formation, and sex differentiation (Sampson et al., 2011). Particularly vulnerable are females of all ages, at risk of reproductive health issues due to DEHP’s endocrine-disrupting activity. As a weak estrogenic compound, DEHP imitates estrogen, binds to estrogen receptors, and alters downstream signalling, disrupting estrogen homeostasis and causing lasting alterations in women’s reproductive systems (Pocar et al., 2017). DEHP is closely associated with ovarian toxicity and endometriosis (Béranger et al., 2012) and can cross the placental barrier and reach offspring through breast milk (Dostal et al., 1987). Further, recent research suggests that DEHP and MEHP exposure might impair placental development and function, adversely affecting fetal growth. Studies on various trophoblastic and placental models have demonstrated the detrimental effects of DEHP and MEHP on critical placental development processes such as implantation, differentiation, invasion, and angiogenesis. Furthermore, these substances have been found to alter placental functions, including nutrient transfer, immunomodulation, and oxidative stress response. Clinical-epidemiological evidence also indicates a correlation between DEHP exposure and negative pregnancy outcomes and related pathologies (Martínez-Razo et al., 2020). Additional adverse effects include liver, heart, lung damage, and renal dysfunction in both adults and offspring (Zarean et al., 2016).
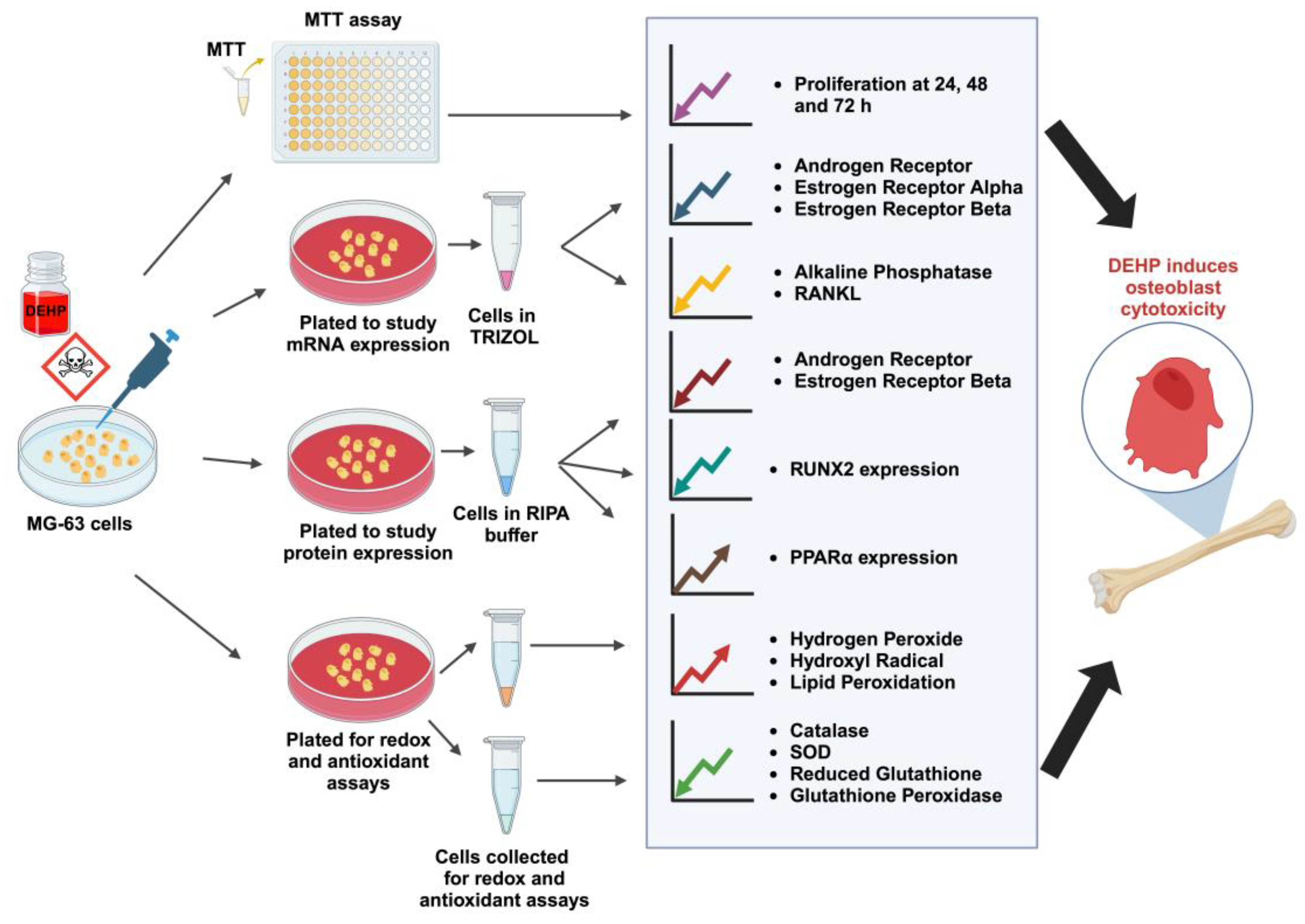
While extensive research has focused on DEHP’s harmful impact on various organs, limited literature specifically addresses its effects on bone health. As a dynamic organ, bone undergoes continuous remodelling, and disturbances in this process can compromise bone integrity. In the present study, we hypothesized that DEHP might alter bone remodelling and induce detrimental effects on the bone cells. To validate our hypothesis and decipher the effects of DEHP on bone cells and the possible molecular mechanisms, we designed the present study to ascertain and evaluate the impact of DEHP on human osteoblast-like cells (MG63). Our study clearly suggests that exposure to DEHP affects human osteoblast-like cells by altering the key genes and proteins involved in bone metabolism.
2. Materials and Methods
2.1. Chemicals, Antibodies, and Molecular Biology Reagents
Di(2-ethylhexyl) phthalate (DEHP), β-actin primary antibody and oligodeoxynucleotide primers for polymerase chain reaction (PCR) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Olive oil was used as a vehicle. Real-time PCR ready master mix was purchased from KAPA Biosystems, Wilmington, MA 0188, USA. Primary antibodies of androgen receptor (Ar), estrogen receptor α (Erα), estrogen receptor β (Erβ), Runt-related transcription factor 2 (Runx2) [also called as RUNX family transcription factor 2 (Runx2) or core-binding factor subunit alpha-1 (CBFA1)], and Peroxisome proliferator-activated receptor (PPAR) were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA) and Cell Signalling Technology (Danvers, Massachusetts, USA). The secondary antibodies, [horseradish peroxidase (HRP)-conjugated rabbit–anti-mouse immunoglobulin G (IgG) and HRP-conjugated goat–anti-rabbit IgG] were obtained from Genei (Karnataka, India). All the chemicals and reagents used in this study were of molecular biology and analytical grade, and they were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Sisco Research Laboratories (SRL) (Mumbai, Maharastra, India).
2.2. Cell Culture
MG-63 cell line was procured from the National Centre for Cell Science (NCCS), Pune. This cell line possesses different phenotypic and molecular characteristics, retaining characters specific to human osteoblastic-like cells. The MG-63 cells were cultured in a T25 flask using growth media (DMEM) supplemented with 10% FBS and antibiotic-antimycotic solution and maintained in an incubator at 37℃ supplemented with 5% CO2.
2.3. MTT Assay
MG-63 cells were treated with either vehicle or different concentrations of DEHP (1, 25, 50 and 100 µM) and assessed for cell viability by MTT assay at 24, 48 and 72 h.
2.4. Isolation of Total RNA
The total RNAs were isolated after exposing the MG-63 cells to DEHP for 48 h by using total RNA isolation reagent (TRIR) kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The osteoblasts were homogenized in 1 ml TRIR reagent in a mechanical homogenizer, and the samples were processed for total RNA isolation by following the method of Chomczynski and Sacchi (1987). The RNA pellets were then dissolved in 40 μl of 0.2% diethylpyrocarbonate (DEPC) treated water and stored at -80°C, until their subsequent use for reverse transcription (RT) and real-time polymerase chain reaction (PCR) experiments.
2.5. Estimation of Total RNA Levels
The total RNA concentrations were estimated by measuring their absorbance (A)/optical density (OD) at both 260 nm and 280 nm using the UV spectrophotometer. RNAs 260 nm absorbance (A)/optical density (OD) values were used to calculate concentrations of the total RNA samples, while the 260/280 nm ratio values of >1.8 showed that they were of good quality and free of protein contaminations (Fourney et al., 1988).
2.6. Reverse Transcription and Real-Time PCR Analysis
For reverse transcription reactions, 2 µg of total RNA from each sample was reverse transcribed using the commercial Superscript III first-strand complementary DNA (cDNA) synthesis kit (Invitrogen, Waltham, Massachusetts, USA) according to the manufacturer’s protocol. Then, the cDNAs generated from the total RNA isolated after exposing the MG-63 cells to DEHP for 48 h were analysed by real-time PCR using the (real-time PCR) machine CFX Manager Version 2.1 (Bio Rad, Hercules, California, USA). Reactions were performed using KAPA SYBR green fast PCR master mix PCR kit. It was comprised of all the PCR components along with SYBR green dye. The housekeeping Gapdh gene mRNA expression was used as the internal control to normalize the mRNA expressions of sex steroid receptors and bone markers. The primers used in the PCR reactions are given in
Table S1.
2.7. Total Protein Lysate Preparation
Total protein lysate from the MG-63 cells after exposing the DEHP for 48 h was prepared as described previously (Bennett and Tonks, 1997). The cells were collected in radioimmunoprecipitation assay (RIPA) buffer. The cells were then sonicated in RIPA buffer, using 1 ml of buffer (Proscientific, UK) at a precise low setting on ice. The homogenates were centrifuged at 1,300 X g for 10 min at 4ºC. Subsequently, the supernatant was centrifuged at 12,000 X g for 15 min at 4°C. The resultant supernatants were used as total protein samples for the western blot experiments.
2.8. Estimation of Total Protein
Protein concentrations in total protein lysates from the MG-63 cells after exposing them to DEHP for 48 h were estimated by the method of Lowry et al. (1951), using bovine serum albumin (BSA) as standard protein and measuring absorbance (A) of the standard BSA and sample proteins at 280 nm via the spectrophotometer (Pharmacia Biotech, Ontario, Canada).
2.9. Western Blot Analysis
The total protein lysates were used for the western blotting analysis. 50 g protein was loaded per well and subjected to 10% to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, the separated proteins on SDS-PAGE gels were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, Massachusetts, USA). The PVDF membranes were incubated with 5% skimmed milk for 2 h at room temperature to block the nonspecific binding. Then, membranes were incubated overnight at 40 C with primary antibodies at 1:500 to 1:1000 dilution in 5% skimmed milk against Ar, Erα, Erβ, Runx2, and PPAR proteins. Subsequently, the PVDF membranes were washed with Tris-buffered saline (TBS) and then incubated with the secondary (2⁰) antibody, [horseradish peroxidase (HRP)-conjugated rabbit–anti-mouse immunoglobulin G (IgG) or HRP-conjugated goat–anti-rabbit IgG] at a dilution of 1:10000 for 1 h at room temperature. Then, the protein bands were detected on the PVDF membranes using an enhanced chemiluminescence (ECL) system kit and quantified in Chemi Doc XRS Imaging System (Bio-Rad, Hercules, California). The western blot membranes were completely stripped and washed thoroughly off the earlier primary and secondary antibodies. Then, these membranes were re-probed as described above using the β-actin primary antibody at 1:1000 dilution. The β-actin protein bands are used as an internal control.
2.10. Estimation of Hydrogen Peroxide
The H2O2 levels of the MG-63 cells after exposing them to DEHP for 48 h were assayed using the spectrophotometer as described by Pick and Keisari (1981). The H2O2 concentration in the samples was measured at 610 nm and was expressed as µmol/min/mg protein.
2.11. Estimation of Hydroxyl Radical
The hydroxyl radical (HO•) levels of the MG-63 cells after exposing them to DEHP for 48 h were assessed using the spectrophotometer as described by Puntarulo and Cedarbaum (1988). The HO• in the sample was measured at 570 nm and expressed as µmol/min/mg protein.
2.12. Estimation of Lipid Peroxidation
The lipid peroxidation in the MG-63 cells after exposing them to DEHP for 48 h was assessed by the colorimetric measurement as described by Devasagayam and Tarachand (1987). The intensity of the colour developed in the tubes was measured at 532 nm, and the malonaldehyde content in the sample was expressed as moles of MDA formed/min/mg protein.
2.13. Estimation of Catalase Enzyme Activity
The catalase (CAT) activities in the MG-63 cells after exposure to DEHP for 48 h were measured by colorimetric measurement according to the manufacturer’s instruction (Cayman Chemical, Ann Arbor, MI, USA. The variations of the decomposition rate of H2O2 were determined by spectrophotometry at 540 nm. The activity of catalase was expressed as units/mg protein.
2.14. Estimation of Superoxide Dismutase Enzyme Activity
The superoxide dismutase (SOD) enzyme activities in the MG-63 cells after exposing them to DEHP for 48 h were performed as described by the manufacturer’s instruction (Cayman Chemical, Ann Arbor, MI, USA). The estimation was based on the generation of superoxide radicals measured at 550 nm by the degree of inhibition of this reaction. The activity of SOD was expressed as units/mg protein.
2.15. Quantification of Glutathione Peroxidase Enzyme Activity
The glutathione peroxidase (GPx) activities in the MG-63 cells after exposing them to DEHP for 48 h were measured using a spectrophotometer as described in the kit (Cayman Chemical, Ann Arbor, USA). The intensity was measured using a spectrophotometer at 340 nm. The glutathione peroxidase concentration in the sample was expressed as nmol/min/mg protein.
2.16. Statistical Analyses
The data obtained from the control and DEHP groups for the various parameters examined in our study were analyzed, compared and plotted as bar graphs using the computer-based GraphPad software (La Jolla, California, USA). Moreover, the data were subjected to statistical analysis using paired t-tests to assess the significance of individual variations between the control and DEHP-treated groups using the computer-based GraphPad software (La Jolla, California, USA). The data were represented as mean ± SD, and P < 0.05 was considered statistically significant.
3. Results
3.1. Impact of In Vitro DEHP Exposure on Osteoblast Viability
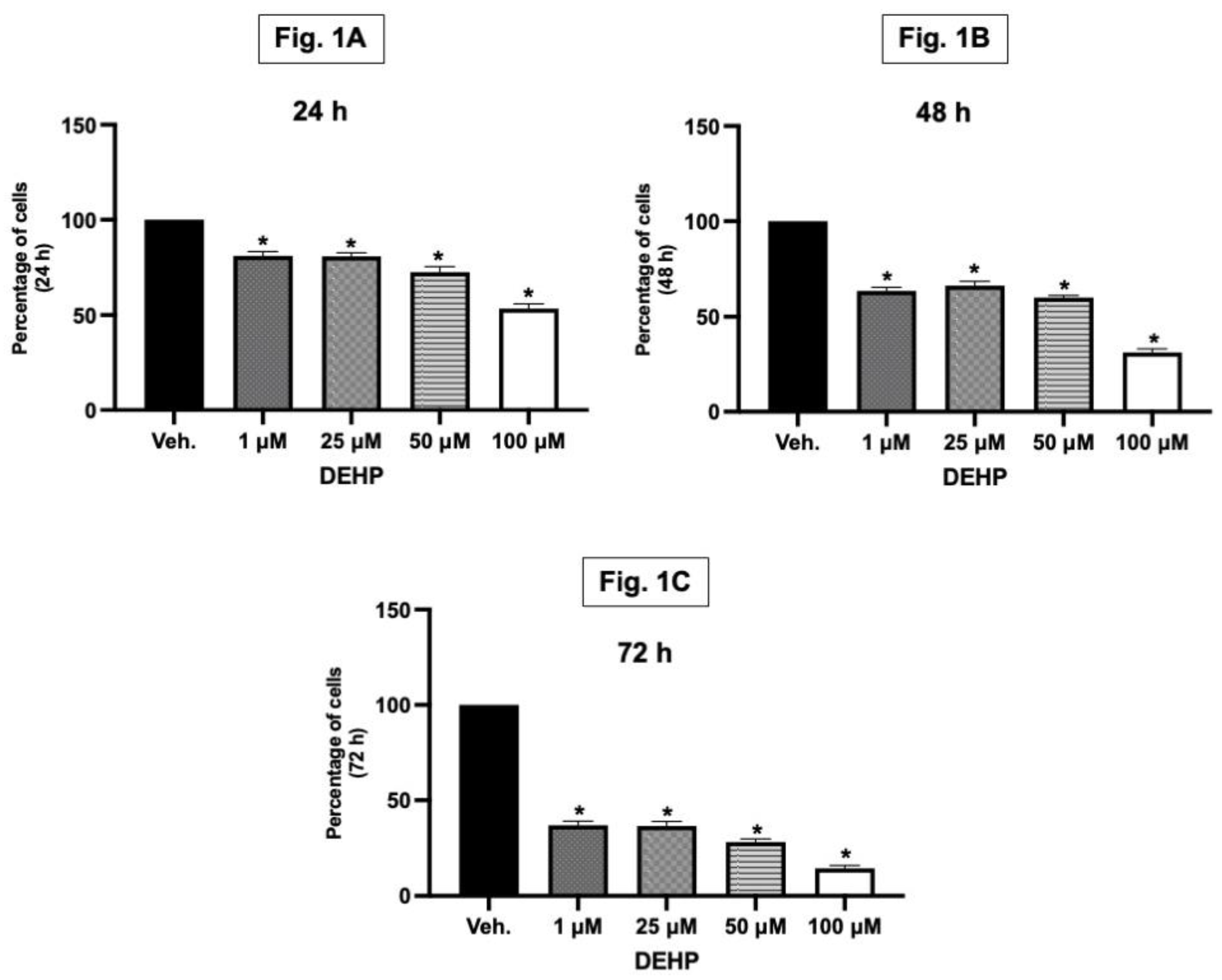
Bone formation is driven predominantly by the activity of osteoblasts, while the resorption of bone is largely reliant on osteoclast function. Thus, in the bone remodelling process, either stimulating the proliferation of osteoblasts or promoting their differentiation can effectively enhance bone formation (Liu et al., 2017). Our study demonstrated a significant reduction in the viability of MG-63 osteoblast cells when exposed to DEHP concentrations of 1, 25, 50, and 100 µM at different time points (24, 48, and 72 hours) as assessed by MTT assay (
Figure 1A–C). It is imporant to note that MTT assay can determine the viability as well as the proliferation of the cells. In this study, the lowest concentration (1 µM) as well as the highest concentration (100 µM) of DEHP used demonstrated reduction in the viability of the bone forming cells. This finding is particularly significant as the new bone formation is largely dependent on osteoblast survival and activity. Normally, enhancing osteoblast proliferation or differentiation is key to promote the bone formation process. Therefore, the inhibitory effect of DEHP on osteoblast viability/proliferation suggests a potential disruption in the bone formation process, which could have implications for overall bone health and remodelling. Based on the osteoblast viablity results, we used 1 and 100 µM for our further studies.
3.2. Effects of In Vitro DEHP Exposure on the mRNA and Protein Expression of Sex Steroid Receptors in Osteoblasts
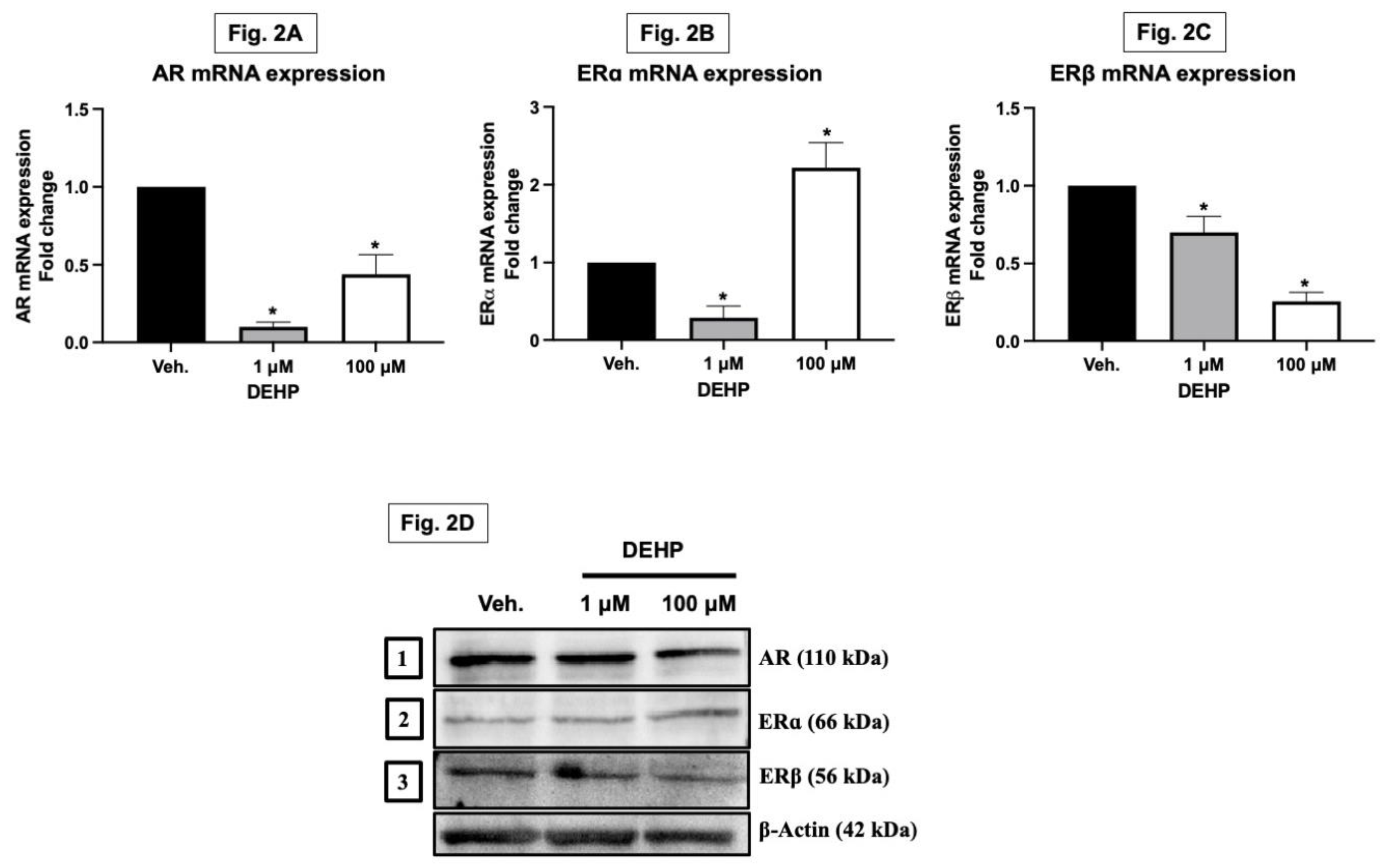
The adult skeleton undergoes periodic remodelling, a process significantly influenced by sex steroids like estrogens and androgens. These hormones play a crucial role in balancing bone formation and resorption. Estrogens and androgens slow down bone remodelling and are key in preventing bone loss. Their absence, particularly estrogen, leads to an increased remodelling rate, favouring bone resorption. Research has shown that estrogens and androgens reduce the frequency of remodelling cycles by diminishing the formation of osteoclasts and osteoblasts from progenitors. This effect is partly due to the transcriptional regulation of genes involved in osteoclastogenesis and mesenchymal cell differentiation. The sex steroid receptors interact with other transcription factors to exert these effects. Beyond influencing remodelling, sex steroids also affect the lifespan of mature bone cells. They have pro-apoptotic effects on osteoclasts but anti-apoptotic impacts on osteoblasts and osteocytes. This dual action involves the classical sex steroid receptors functioning both inside and outside the nucleus, activating specific signal transduction pathways. Interestingly, the estrogen and androgen receptors can transmit anti-apoptotic signals efficiently regardless of the ligand type. These non-genotropic, sex-nonspecific actions are mediated by the receptors’ ligand-binding domains and can be distinguished from transcriptional activity. With regard to sex steroid deficiency, the loss of transcriptional and non-genotropic anti-apoptotic effects leads to increased osteoclastogenesis and osteoblastogenesis, enhanced bone remodelling, and thus an imbalance between bone formation and resorption (Manolagas et al., 2002). In our study, DEHP exposure led to a significant downregulation in both mRNA and protein expression levels of androgen receptor and estrogen receptor beta in MG-63 cells. Interestingly, while estrogen receptor alpha mRNA expression was decreased, its protein expression was increased (
Figure 2A–D). This suggests that DEHP may disrupt the normal hormonal signalling pathways in osteoblasts through differential regulation of receptor expression.
3.3. Effects of In Vitro DEHP Exposure on the Expression of Key Bone Markers in Osteoblasts
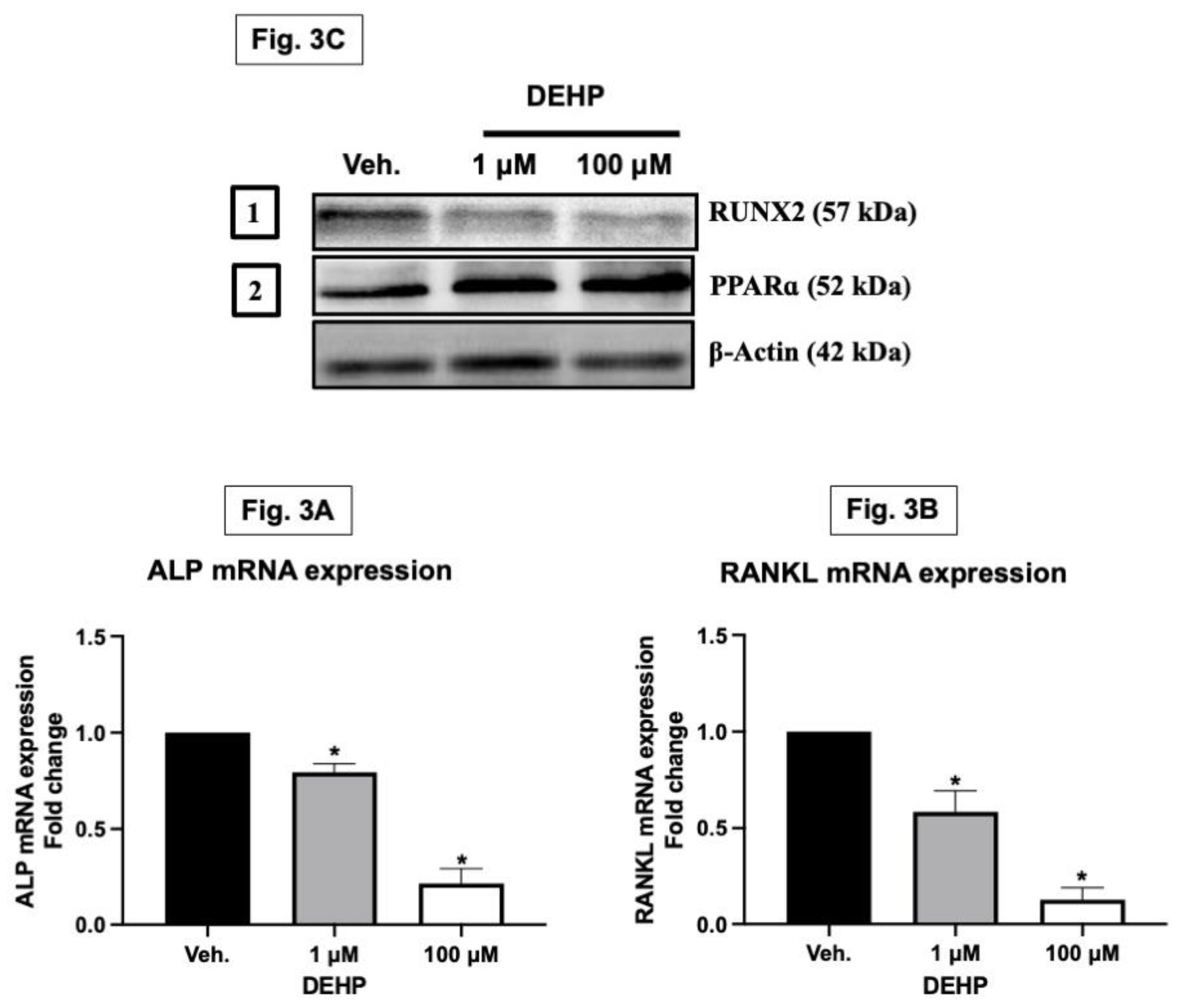
Alkaline phosphatase (ALP) is a membrane-bound glycoprotein recognized as an early marker of osteogenesis and bone calcification. Secreted by osteoblasts, ALP plays a key role in bone mineralization by increasing the phosphate concentration at the cell surface. Meanwhile, RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand), though present in low levels in bone marrow, is crucial for proper bone metabolism. This molecule, found on osteoblast surfaces, is vital for activating osteoclasts, which are integral to bone resorption. Furthermore, osteoblasts, not only synthesize the organic matrix of bones but also regulate their mineralization. A pivotal transcription factor in the development of osteoblasts is RUNX2. It controls the expression of various osteoblast-specific proteins, including osterix, osteopontin, bone sialoprotein, type I collagen, and osteocalcin, all of which are essential for bone formation and health (Yeniyol and Ricci, 2018; Cao, 2018; Komori, 2018). The exposure to DEHP significantly downregulated the mRNA expression of ALP and RANKL, important markers for bone formation and remodelling. Additionally, there was a decrease in the protein expression of RUNX2, a critical transcription factor for bone development, while PPAR alpha protein levels increased, indicating potential alterations in osteoblast differentiation and function (
Figure 3A–C).
3.4. In Vitro Exposure to DEHP Increases the ROS Levels in Osteoblasts
Reactive oxygen species (ROS) like superoxide anions, hydroxyl radicals, and hydrogen peroxide (H2O2) are capable of inflicting substantial damage to DNA, proteins, and lipids. The production of high levels of these oxidants, either through normal cellular activities such as mitochondrial electron transport or via external factors like cytokines and UV radiation, disrupts the cell’s normal redox state, leading to oxidative stress. This stress is a contributing factor to the development of various degenerative diseases, including diabetes, atherosclerosis, arthritis, cancer, and the aging process. On the cellular level, ROS function as secondary messengers in diverse signal transduction pathways, triggering a range of responses from cell proliferation and growth arrest to differentiation, senescence, and even cell death. This activation of key signalling pathways by ROS is critical in determining cell fate. Moreover, it’s established that ROS, including H2O2 and superoxide anion, play a role in diseases associated with bone loss by promoting osteoclast differentiation and enhancing bone resorption (Bai et al., 2005). Our findings showed increased levels of reactive oxygen species (ROS), such as hydrogen peroxide and hydroxyl radicals, in MG-63 cells exposed to 1 and 100 µM DEHP. This increase in ROS suggests that DEHP induces oxidative stress in osteoblasts, which can lead to cellular damage and impaired bone cell function (
Figure 4A–C).
3.5. In Vitro Exposure to DEHP Decreases the Enzymatic and Non-Enzymatic Antioxidant Levels in Osteoblasts
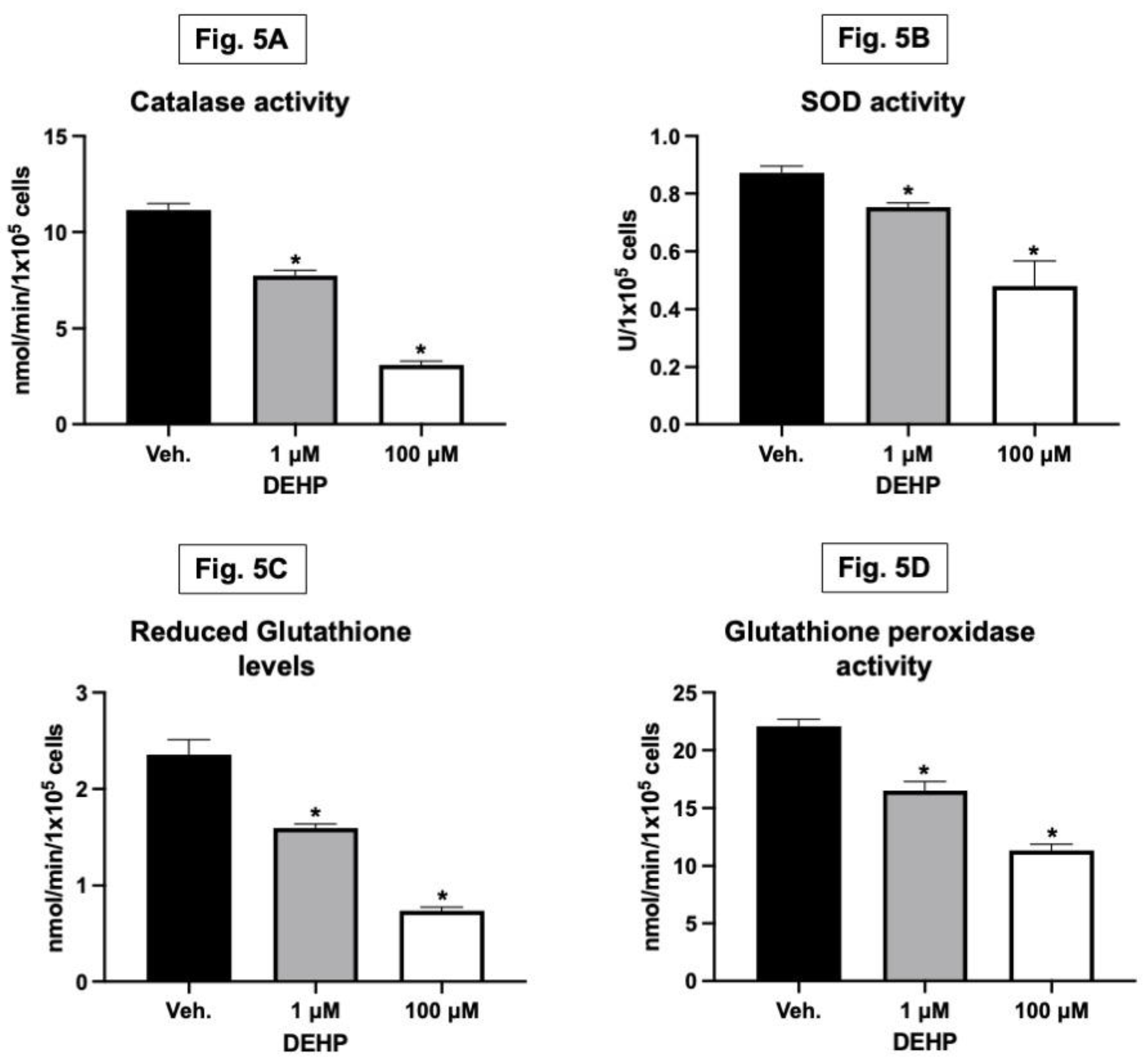
Antioxidants and redox enzymes play a crucial role in maintaining bone health. They act as protective agents against oxidative stress, adversely affecting bone cells (Kozakowska et al., 2015). These enzymes, including catalase, superoxide dismutase (SOD), glutathione peroxidase, and reduced glutathione, help neutralize reactive oxygen species (ROS) and prevent oxidative damage to cells. This protective mechanism is vital for preserving the function of osteoblasts, the cells responsible for bone formation. Alongside increasing ROS levels, DEHP exposure at 1 and 100 µM concentrations decreased antioxidant defences in MG-63 cells. This was evidenced by reduced levels of catalase, SOD (superoxide dismutase), glutathione, and glutathione peroxidase compared to the control group (
Figure 5A–D). The reduction in these critical antioxidant enzymes indicates a disruption in the cellular redox balance, further contributing to oxidative stress. When the activity of these antioxidant and redox enzymes is decreased, it can lead to an increase in oxidative stress within the bone. Elevated oxidative stress is known to impair the viability and function of osteoblasts, leading to reduced bone formation and potentially contributing to bone-related diseases. Therefore, the balance between ROS production and antioxidant defences is essential to ensure healthy bone remodelling and prevent bone loss or degenerative bone conditions.
4. Discussion
The health consequences caused by exposure to EDCs especially DEHP in humans have been extensively investigated in various studies, encompassing epidemiological, demographic, and metabolic research that covers nearly all organs (Praveena et al., 2018). However, the impact of DEHP on bone remains unclear and poorly understood. In the current study, in order to investigate the effects of DEHP on bone forming cells, we exposed the osteoblast cells to various concentrations of DEHP. Our in vitro study showed that exposure to various concentrations of DEHP decreased the viability of MG-63 cells at various time points. The decrease in viable cells can be attributed to two primary mechanisms: proliferation and/or suppression of cell metabolism, known as the cytostatic effect, or direct cell death., also referred to as the cytotoxic effect. It is necessary to differentiate between these two processes to understand the effect of any compounds or drugs. In our study, this distinction became clear as we observed a significant decrease in the viability of MG-63 cells in vitro, particularly at concentrations of 1 and 100 µM DEHP over 48 hours. This decline in cell viability at various concentrations and time intervals highlighted the cytotoxic effects of DEHP. In this context, Fang et al. (2019) showed that high-dose DEHP exposure (512 μM for 4 hours) to the differentiated human embryonic stem cells triggered the apoptosis by activating the PPARγ/PTEN/AKT pathway, which is critical for cell proliferation and survival. Complementing these findings, two other studies were conducted by Hannon et al. (2016) and Sun et al. (2018). They reported DEHP-induced cell death in various in vitro models, correlated with the increased expression of Bax and the activation of caspases 3 and 8. Additionally, Ha et al. (2016) documented increased apoptotic events in hepatocytes derived from Sprague-Dawley rats treated with DEHP at 250, 500, and 750 mg/kg for 30 days, attributing this to p53 overexpression induced by DEHP.
Studies conducted by Duarte et al. (2021) revealed that exposure to EDCs influenced the nuclear division index (NDI) of HepG2 cells. Consistent with prior research, oxidative DNA damage has been linked to cell cycle arrest. A lower NDI correlates with increased mononucleated cells as opposed to bi-, tri-, or polynucleated ones, indicating cell cycle arrest. The NDI serves as an indicator of the proliferative status of a viable cell population. A lower NDI than control suggests a higher count of cells with a single nucleus, indicative of cytostatic effects. Notably, in their study, the most significant changes in NDI occurred when cells were treated with combinations of Triclosan (TCS) and DEHP at all tested doses, highlighting DEHP’s cytostatic and toxic effects (Duarte et al., 2021). Consistent with these observations, our study demonstrates that DEHP reduce the osteoblast viability even at lower concentrations, as shown by increased cytotoxicity at 1, 25, 50 and 100 µM concentrations. This aligns with the broader understanding of DEHP as an EDC that can adversely affect cellular processes. Such an impact on osteoblasts is concerning, as these cells play a critical role in bone formation and maintenance.
Lipid peroxidation induced by reactive oxygen species (ROS) is a key factor in various forms of cell death, including apoptosis, autophagy, and ferroptosis. This process, deeply rooted in cellular biology, arises from an overabundance of ROS that targets biomembranes. This leads to a cascade of lipid peroxidation chain reactions, which then trigger different types of cell death. To counteract this oxidative damage, cells have developed an intricate antioxidant system. These mechanisms are essential in preventing oxidative stress, which, if uncontrolled, can significantly contribute to reduced cell proliferation (Su et al., 2019). In the present study, the increase ROS levels, such as hydrogen peroxide and hydroxyl radicals, in conjunction with the rise in TBARS levels upon DEHP exposure in both 1 and 100 µM concentrations, strongly indicates the oxidative stress in osteoblasts. This is further supported by the observed decrease in antioxidant enzymes like catalase, SOD, glutathione, and glutathione peroxidase in both 1 and 100 µM concentrations of DEHP exposure. In an in vivo study, exposure to DEHP has been shown to increase and accumulate intracellular lipid ROS and trigger ferroptosis in Sertoli cells. This process adversely affects the Blood-Testis Barrier (BTB) integrity and alters testosterone levels. The mechanism involves the activation of p38α through phosphorylation, which in turn phosphorylates p53. This phosphorylated p53 increases transcriptional activity, leading to the activation of the p53-SAT1-ALOX15 signalling pathway. As a result, the enhanced production of lipid ROS further stimulates the expression of p38α, creating a self-perpetuating cycle of cellular stress and damage (Yang et al., 2022). In another study involving five-week-old ICR male mice, the animals were administered either a vehicle, DEHP, or DINP (at doses of 0.05 and 4.8 mg/kg body weight) daily through gavage for a period of five weeks (Gu et al., 2021). The investigation revealed an increase in reactive oxygen species and malondialdehyde levels, coupled with a decrease in reduced glutathione in the kidneys at the higher doses of both chemicals, indicating oxidative damage. Additionally, there was an elevation in inflammatory cytokines, specifically tumour necrosis factor-α and interleukin-6 in the kidneys, pointing towards an inflammatory response resulting from exposure to high doses of both phthalates. Further, a targeted lipidomics analysis showed the most significant alterations in the kidney caused by the higher dose of DEHP. Nevertheless, DINP also brought about notable changes in phospholipids and diacylglycerides, which are linked to lipid accumulation in glomerular podocytes and inflammatory responses. The findings suggest that oxidative stress plays a role in the renal lipidomic disruption induced by DEHP and DINP (Gu et al., 2021).
In another study, 21-day-old ICR mice received DEHP at dosages of 0, 125, 250, and 375 mg/kg/day for 28 days through oral gavage. Subsequent examinations revealed mild liver tissue damage linked to DEHP exposure. There was a notable elevation in serum liver function indices, including aspartate transaminase (AST) and alanine transaminase (ALT). Furthermore, there was a significant increase in lipid peroxidation indicators, specifically reactive oxygen species (ROS) and malondialdehyde (MDA), while the activity of superoxide dismutase (SOD) in the liver of mice was markedly reduced. Additionally, DEHP exposure led to enhanced phosphorylation of JNK and p38MAPK proteins in the liver, increased p53 protein expression, and reduced DNA methylation levels in the p53 gene promoter region. These findings suggest that DEHP-induced hepatotoxicity in mice may be mediated by activating the JNK/p38MAPK/p53 signalling pathway, enhancing ROS generation, promoting lipid peroxidation, and subsequently decreasing the antioxidant levels in the liver (Huang et al., 2019).
Our findings align with previous research indicating that DEHP exposure leads to enhanced oxidative damage. This is evidenced by the increased levels of hydrogen peroxide, hydroxyl radicals, and TBARS (Thiobarbituric Acid Reactive Substances), coupled with a decrease in the activities of key antioxidant enzymes, including catalase, superoxide dismutase (SOD), glutathione, and glutathione peroxidase. Such alterations in oxidative status and antioxidant defences are known to contribute to cellular dysfunction and, potentially, cell death.
Sex steroids and their receptors are crucial in bone turnover regulation. Gonadectomy in both genders leads to increased bone remodelling, elevated bone resorption, and reduced bone formation, thereby speeding up bone loss. Recent studies have confirmed estrogen’s significant role in bone turnover regulation in both sexes. Research involving estrogen receptor, aromatase, or androgen receptor knockout mice has shed light on the in vivo effects of sex steroids on bone. The impact of sex steroids on bone-forming osteoblasts and bone-resorbing osteoclasts is increasingly well-understood at the cellular and molecular levels. Estrogen, for instance, reduces bone remodelling by inhibiting the formation of osteoblasts and osteoclasts from marrow precursors. Both estrogen and androgens limit bone resorption through their influence on the RANKL/RANK/osteoprotegerin system by decreasing the production of pro-resorptive cytokines and directly impacting osteoclast activity and lifespan. The influence of sex steroids on bone formation involves multiple mechanisms, including extending the lifespan of osteoblasts through non-genotropic means and affecting osteoblast differentiation and function. These diverse actions of sex steroids highlight the skeleton’s multifaceted role in providing structural support, enabling movement, and responding to the organism’s reproductive calcium requirements (Syed and Khosla, 2005). In this present study, we studied the mRNA and protein expression of AR, ERα and ERβ. We observed downregulation in the mRNA and protein expression of AR and ERα further corroborates the endocrine-disrupting potential of DEHP. The differential expression observed for estrogen receptor alpha, where mRNA expression decreased while protein expression increased, suggests a complex regulatory mechanism possibly triggered by DEHP exposure. In an experimental study, in order to understand the effect of di(2-ethylhexyl)phthalate (DEHP) on bone health, particularly in relation to bone mineral density (BMD) and bone remodelling, older female CD-1 mice were divided into four groups: a control group, a low-dose DEHP group, a high-dose DEHP group, and an estrogen group, with each group comprising five mice. All the mice underwent ovariectomy to simulate menopause and were then exposed to corn oil, DEHP, or estrogen for two months. They observed that the osteocalcin level, which is a marker for bone formation, decreased across all groups, with the high-dose DEHP group showing a significant reduction. Micro-CT results showed a significant drop in BMD in the high-dose DEHP group compared to the control group, suggesting more severe bone loss. The research findings indicated that high doses of DEHP have detrimental effects on bone health in mice subjected to ovariectomy. This underscores the harmful influence of DEHP and the critical role played by sex steroids and their receptors in preserving bone health.
We observed the reduced mRNA expression of ALP and RANKL, markers vital for bone health and remodelling. A study explored the use of bone marrow stromal cells (BMSCs) expanded ex vivo for in vivo bone tissue engineering. The cultured stromal cells, a mix of cells at various commitment and expansion stages, resulted in a heterogeneous population with varying potential for in vivo bone formation. The authors aimed to find parameters to predict this in vivo bone-forming capacity. To identify predictive parameters, the researchers established single colony-derived BMSC cultures using limiting dilution. Out of sixteen single colony-derived BMSC cultures produced from human bone marrow, only five successfully formed bone in vivo. These strains were evaluated for their proliferation and differentiation capacities in osteogenic, adipogenic, and chondrogenic pathways, including the expression of related markers. The study highlighted that the most reliable predictor of in vivo bone formation was the induction of alkaline phosphatase (ALP), specifically ALP mRNA levels and ALP activity, during osteogenic differentiation in vitro. The results suggest that an increase in ALP levels above baseline during in vitro osteogenic differentiation is a strong indicator of BMSCs’ performance in vivo (Prins et al., 2014). The downregulation of the ALP mRNA underscores the potential of DEHP to interfere with normal bone metabolism.
The physiological process of bone remodelling is regulated by a tri-molecular complex comprising Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL), osteoprotegerin (OPG), and Receptor Activator of Nuclear Factor Kappa-Β (RANK). This complex, which operates as receptors and ligands, is part of the tumour necrosis factor (TNF) superfamily. These molecules are vital for maintaining and renewing the bone matrix. In the process of bone remodelling, the synthesis of the bone matrix is carried out by osteoblasts, while the resorption of the extracellular bone matrix is executed by osteoclasts. These activities are intricately coordinated within the bone remodelling machinery. An imbalance between the functions of osteoblasts and osteoclasts can lead to immunopathological conditions characterized by either a decrease or an increase in bone mass mineral density. It is key to understand the fact that the RANKL is secreted by the osteoblasts (Kohli and Kohli, 2011). In this present study, the DEHP significantly decreased the mRNA expression of RANKL, suggesting a potential disruption in the critical balance of bone remodelling activities. In addition, the decrease in RUNX2 protein expression and the increase in PPAR alpha were observed following the exposure to DEHP. It suggests alterations in the regulatory pathways essential for bone development and lipid metabolism. The decreased expression of RUNX2 protein and increased expression of PPAR alpha observed in the study have significant implications for bone health. RUNX2 is a key factor in osteoblast differentiation and is essential for bone formation. A reduction in RUNX2 protein expression can impair osteoblast function, potentially resulting in weaker bone formation and compromised bone strength (Komori, 2017).
In another study, Bhat et al. (2013) demonstrated that DEHP can interfere with the differentiation and functioning of osteoblasts. This study showed that DEHP affects the expression of cell cycle proteins and differentiation markers, including Runx2 and its co-activator TAZ, in osteoblasts derived from neonatal rat calvaria. The study observed a notable reduction in the protein levels of cyclin D1 and CDK-2 following exposure to a high dose of DEHP (100 µM) for 24 hours. Additionally, DEHP treatment led to a significant decrease in ALP mRNA expression, TAZ mRNA and protein levels, and decreased protein expression of Runx2 in rat calvarial osteoblasts in vitro (Bhat et al., 2013). The downregulation of the ALP mRNA expression and decreased RUNX2 protein expression after DEHP exposure following 100 µM) corroborates with our study. On the other hand, the increased expression of Peroxisome Proliferator-Activated Receptor Alpha (PPAR alpha) in relation to bone health is a complex issue. PPAR alpha is primarily known for its role in lipid metabolism and energy homeostasis. Its overexpression could potentially shift the balance towards enhanced fat accumulation in the bone marrow, which may adversely affect bone quality and density (Varga et al., 2011). Therefore, the observed changes in RUNX2 and PPAR alpha expressions suggest a potential shift in the balance of bone remodelling towards reduced bone formation and possibly increased marrow adiposity, which could have detrimental effects on overall bone health.
In summary, our study highlights the deleterious effects of DEHP exposure on bone forming cells as observed by decreased osteoblast viability and alterations in key markers and proteins associated with bone metabolism in response to DEHP exposure. Notably, our study revealed a decrease in the expression of RANKL and RUNX2, essential factors in bone remodelling, and an increase in PPAR alpha, which may have implications on the bone quality. Our findings provide critical insights into the cytotoxic effects of DEHP, particularly in the context of osteoblast function and thus bone formation. The observed changes in sex steroid receptor expression, bone marker gene expression, and oxidative stress parameters suggest that DEHP exposure could pose significant risks as it induced cytotoxicity in osteoblasts (
Figure 6). This underscores the importance of further research into the mechanisms of DEHP toxicity and its broader health implications. Furthermore, our results contribute to the growing body of evidence on the harmful effects of EDCs namely DEHP on bone health, emphasizing the need for careful consideration of these substances in our environment and their potential impact on human health.
5. Conclusions
In conclusion, our study demonstrate for the first time that DEHP-induced cytotoxicity involves the reduction in osteoblast viability, alterations in sex steroid receptor expression and bone marker gene expression, and an imbalance in the redox-antioxidnat system, providing key insights into the deleterious effects of DEHP on bone forming cells. Our findings highlight the potential risks of DEHP exposure to bone health and thus underscore the need for further research to evaluate the osteotoxicity of DEHP.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1. Forward and Reverse primer sequences.
Author Contributions
Conceptualization, Gautham Chengizkhan and Ilangovan Ramachandran; Data curation, Gautham Chengizkhan, Manju Mohan and Shabana Thabassum Mohammed Rafi; Formal analysis, Gautham Chengizkhan, Manju Mohan and Shabana Thabassum Mohammed Rafi; Funding acquisition, Ilangovan Ramachandran; Investigation, Gautham Chengizkhan, Manju Mohan and Shabana Thabassum Mohammed Rafi; Methodology, Gautham Chengizkhan; Project administration, Ilangovan Ramachandran; Resources, Ilangovan Ramachandran; Supervision, Ilangovan Ramachandran; Validation, Gautham Chengizkhan, Manju Mohan and Shabana Thabassum Mohammed Rafi; Writing – original draft, Gautham Chengizkhan; Writing – review & editing, Sridhar Muthusami, Satish Ramalingam, Lurdes Queimado, R. Ileng Kumaran, Srinivasan Narasimhan and Ilangovan Ramachandran. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support provided by Science and Engineering Research Board (SERB) [ECR/2015/000277], Government of India, to Dr. Ilangovan Ramachandran is gratefully acknowledged. We also thank the funding support of UGC-SAP-DSA-1 (Sanction order no: F.5-4/2015/DSA-1 (SAP-II).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A (Abbreviations)
Actin beta (ACTB)/β-actin, Alkaline phosphatase (ALP), Androgen receptor (AR), Catalase (CAT), Di(2-ethylhexyl) phthalate (DEHP), Endocrine disrupting compound (EDC), Estrogen receptor α (ERα), Estrogen receptor β (ERβ), Glutathione peroxidase (GPx), Horse radish peroxidase (HRP), Hydrogen peroxide (H2O2), Hydroxyl radical (HO•), Mono(2-ethylhexyl) Phthalate (MEHP), Receptor activator of nuclear factor kappa B ligand (RANKL) [also named or called as TNF superfamily member 11 (TNFSF11)], Runt-related transcription factor 2 (RUNX2) [also named/called as RUNX family transcription factor 2 (RUNX2) or core-binding factor subunit alpha-1 (CBFA1)].
References
- Winz, C.; Zong, W.X.; Suh, N. Endocrine-disrupting compounds and metabolomic reprogramming in breast cancer. J. Biochem. Mol. Toxicol. 2023, 37, e23506. [Google Scholar] [CrossRef]
- Shanmugam, D.A.S.; Dhatchanamurthy, S.; Leela, K.A.; Bhaskaran, R.S. Maternal exposure to di(2-ethylhexyl) phthalate (DEHP) causes multigenerational adverse effects on the uterus of F1 and F2 offspring rats. Reprod. Toxicol. 2023, 115, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Estrada, C.; de Jesús Rostro-Alanis, M.; Muñoz-Gutiérrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldívar, R. Emergent contaminants: endocrine disruptors and their laccase-assisted degradation-a review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef]
- Turan, S. Endocrine disrupting chemicals and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Balasubramanian, K. Gestational exposure to di (2-ethylhexyl) phthalate (DEHP) impairs pancreatic Œ≤-cell function in F1 rat offspring. Toxicol. Lett. 2015, 232, 46–57. [Google Scholar] [CrossRef]
- Mariana, M.; Castelo-Branco, M.; Soares, A.M.; Cairrao, E. Phthalates’ exposure leads to an increasing concern on cardiovascular health. J. Hazard Mater. 2023, 457, 131680. [Google Scholar] [CrossRef]
- Tuan Tran, H.; Lin, C.; Bui, X.T.; Ky Nguyen, M.; Dan Thanh Cao, N.; Mukhtar, H.; Giang Hoang, H.; Varjani, S.; Hao Ngo, H.; Nghiem, L.D. Phthalates in the environment: characteristics, fate and transport, and advanced wastewater treatment technologies. Bioresour. Technol. 2022, 344, 126249. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, W.; Xu, Q.; Li, X.; Zhou, L.; Ye, L. Di(2-ethylhexyl) phthalate (DEHP) and thyroid: biological mechanisms of interference and possible clinical implications. Environ. Sci. Pollut. Res. Int. 2022, 29, 1634–1644. [Google Scholar] [CrossRef]
- Sampson, J.; De Korte, D. DEHP‚Äö√Ñ√™plasticised PVC: relevance to blood services. Transfus. Med. 2011, 21, 73–83. [Google Scholar] [CrossRef]
- Pocar, P.; Fiandanese, N.; Berrini, A.; Secchi, C.; Borromeo, V. Maternal exposure to di (2-ethylhexyl) phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol. Appl. Pharmacol. 2017, 322, 113–121. [Google Scholar] [CrossRef]
- Béranger, R.; Hoffmann, P.; Christin-Maitre, S.; Bonneterre, V. Occupational exposures to chemicals as a possible etiology in premature ovarian failure: a critical analysis of the literature. Reprod. Toxicol. 2012, 33, 269–279. [Google Scholar] [CrossRef]
- Dostal, L.A.; Weaver, R.P.; Schwetz, B.A. Transfer of di (2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxicol. Appl. Pharmacol. 1987, 91, 315–325. [Google Scholar] [CrossRef]
- Martínez-Razo, L.D.; Martínez-Ibarra, A.; Vázquez-Martínez, E.R.; Cerbón, M. The impact of Di-(2-ethylhexyl) Phthalate and Mono(2-ethylhexyl) Phthalate in placental development, function, and pathophysiology. Environ. Int. 2021, 146, 106228. [Google Scholar] [CrossRef]
- Zarean, M.; Keikha, M.; Poursafa, P.; Khalighinejad, P.; Amin, M.; Kelishadi, R. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ. Sci. Pollut. Res. 2016, 23, 24642–24693. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, H.; Ma, Y.; Cheng, J.; Hua, Z.; Huang, G. Osteoblasts Proliferation and Differentiation Stimulating Activities of the Main Components of Epimedii folium. Pharmacogn. Mag. 2017, 13, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Kousteni, S.; Jilka, R.L. Sex steroids and bone. Recent Prog. Horm. Res. 2002, 57, 385–409. [Google Scholar] [CrossRef]
- Yeniyol, S.; Ricci, J.L. Alkaline phosphatase levels of murine pre-osteoblastic cells on anodized and annealed titanium surfaces. Eur. Oral. Res. 2018, 52, 12–19. [Google Scholar] [CrossRef]
- Cao, X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res. 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell. Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Liu, A.L.; Zhang, Z.M.; Li, X.M.; Zou, Z.P.; Zeng, W.S.; Cheng, B.L.; Luo, S.Q. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J. Biol. Chem. 2005, 280, 17497–506. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–93. [Google Scholar] [CrossRef]
- Praveena, S.M.; Teh, S.W.; Rajendran, R.K.; Kannan, N.; Lin, C.C.; Abdullah, R.; Kumar, S. Recent updates on phthalate exposure and human health: a special focus on liver toxicity and stem cell regeneration. Environ. Sci. Pollut. Res. 2018, 25, 11333–11342. [Google Scholar] [CrossRef]
- Fang, H.; Fang, W.; Cao, H.; Luo, S.; Cong, J.; Liu, S.; Pan, F.; Jia, X. Di-(2-ethylhexyl)-phthalate induces apoptosis via the PPARγ/PTEN/AKT pathway in differentiated human embryonic stem cells. Food. Chem. Toxicol. 2019, 131, 110552. [Google Scholar] [CrossRef]
- Hannon, P.R.; Niermann, S.; Flaws, J.A. Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol. Sci. 2016, 150, 97–108. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, J.; Zeng, L.; Yang, D.; Shao, S.; Wang, J.; Wei, J.; Xiong, J.; Chen, J. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ. Pollut. 2018, 243, 563–572. [Google Scholar] [CrossRef]
- Ha, M.; Wei, L.; Guan, X.; Li, L.; Liu, C. p53-dependent apoptosis contributes to di-(2-ethylhexyl) phthalate-induced hepatotoxicity. Environ. Pollut. 2016, 208, 416–25. [Google Scholar] [CrossRef]
- Duarte, N.A.A.; de Lima, L.E.; Maraslis, F.T.; Kundi, M.; Nunes, E.A.; Barcelos, G.R.M. Acute Toxicity and DNA Instability Induced by Exposure to Low Doses of Triclosan and Phthalate DEHP, and Their Combinations, in vitro. Front. Genet. 2021, 12, 649845. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, L.; Sun, X.; Li, J.; Wang, N.; Liu, X.; Yao, X.; Zhang, C.; Deng, H.; Wang, S.; Yang, G. DEHP induces ferroptosis in testes via p38α-lipid ROS circulation and destroys the BTB integrity. Food Chem. Toxicol. 2022, 164, 113046. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Gao, M.; Zhang, W.; Yan, L.; Shao, F.; Zhou, J. Exposure to phthalates DEHP and DINP May lead to oxidative damage and lipidomic disruptions in mouse kidney. Chemosphere 2021, 271, 129740. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, C.; Ye, Y.; Zeng, J.; Zhu, J.; Li, Y.; Wang, W.; Zhang, W.; Chen, Y.; Xie, H.; Zhang, H.; Liu, J. The Increase of ROS Caused by the Interference of DEHP with JNK/p38/p53 Pathway as the Reason for Hepatotoxicity. Int. J. Environ. Res. Public Health 2019, 16, 356. [Google Scholar] [CrossRef]
- Syed, F.; Khosla, S. Mechanisms of sex steroid effects on bone. Biochem. Biophys Res. Commun. 2005, 328, 688–96. [Google Scholar] [CrossRef]
- Prins, H.J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem. Cell Res. 2014, 12, 428–40. [Google Scholar] [CrossRef]
- Kohli, S.S.; Kohli, V.S. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–81. [Google Scholar] [CrossRef]
- Komori, T. Roles of Runx2 in Skeletal Development. Adv. Exp. Med. Biol. 2017, 962, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bhat, F.A.; Ramajayam, G.; Parameswari, S.; Vignesh, R.C.; Karthikeyan, S.; Senthilkumar, K.; Karthikeyan, G.D.; Balasubramanian, K.; Arunakaran, J.; Srinivasan, N. Di 2-ethyl hexyl phthalate affects differentiation and matrix mineralization of rat calvarial osteoblasts--in vitro. Toxicol. Vitro 2013, 27, 250–6. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys Acta. 2011, 1812, 1007–22. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).