1. Introduction
Bacterial infections and sepsis represent a frequent complication in neonatal intensive care unit (NICU), due to the immune immaturity of preterm neonates and the frequent need for the use of invasive devices such as endotracheal tubes, drainages or central venous catheters [
1]. Signs and symptoms of sepsis that become evident during the first 72 hours of life indicate an early onset sepsis (EOS), typically caused by vertical transmission of bacteria colonizing the low genital tract of the mother, carried by contaminated amniotic fluid or infecting the baby during vaginal delivery [
2]. A sepsis that occur after 72 hours of life is called late onset sepsis (LOS) and is a common event especially in very low birth weight (VLBW) infants, affecting about 20 to 30% of them at least once during their hospitalization [
3] and being a significant cause of morbidity and mortality in this population [
4,
5]. Vertical transmission of mother’s pathogens (e.g. mother’s milk contaminated transmission) is a possible etiology even in this kind of sepsis, although rarely implied [
6]. LOS usually derives from a horizontal transmission of environmental pathogens, occurring despite strict hygiene measures and prevention strategies [
7]. Healthcare associated infections (HAI) represent the prevalent cause of LOS in NICU, and may be caused by the longer dwell time of invasive devices and the repeated need for invasive procedures in this vulnerable population [
3]. The temporal peak of LOS incidence occurs between the 10th and the 22nd day of life [
8]. LOS in NICU can be caused by several different pathogens, among which skin commensal
coagulase-negative staphylococci (CoNS) are the most commonly involved, accounting for at least half of the cases; other possible implicated pathogens are
Staphylococcus aureus, and gram-negative germs such as
Escherichia coli,
Klebsiella pneunoniae,
Acinetobacter baumanni [
5,
9,
10,
11,
12,
13,
14,
15].
If in the adult population the isolation of CoNS on blood culture is often considered a simple contamination, in the NICU setting the clinical significance of this finding has become increasingly evident over the years [
16,
17]. Over the last decades
Staphylococcus epidermidis has emerged as the prevalent CoNS causing LOS in preterm neonates [
9], but during the last few years several studies reported the isolation of different species of CoNS, including
Staphylococcus capitis becoming more and more common in preterm infants with a LOS [
18,
19].
In particular, a specific clone of
methicillin-resistant Staphylococcus capitis (pulsotype NRCS-A) has been isolated in hospitals from different countries worldwide (Australia, Belgium, France and the UK), showing a particular specificity for the NICU environment and a reduced susceptibility to vancomycin, one of the first line empiric antibiotics used in LOS in some Countries [
20,
21]. The reason for this widespread diffusion and persistence in such a specific setting has been investigated, and possible reservoirs have been identified in body care oil bottles [
22], stethoscopes and neonatal incubators, that were found to be colonized despite being repeatedly sanitized [
23]. Persistence on surfaces might seem to be linked with a decreased susceptibility towards some disinfectants [
23,
24]. Screening of caregivers colonization has also been performed, showing no chronic carriage of
S. capitis [
25] or isolation of a different clone than NRCS-A [
23].
All the
S. capitis isolated in NICUs usually present a multidrug resistant pattern, especially involving beta-lactams and aminoglycosides, molecules widely used in this setting [
20]. Resistance or heteroresistance to vancomycin has also been reported in several studies [
26,
27], even though this finding has not been encountered by all authors [
23,
28] and has in some cases been described as a specific characteristic only of the NRCS-A clone [
29].
As for all sepsis caused by CoNS, central venous catheters (CVCs) are often identified as the source of infection in
S. capitis LOS, supported by the ability of this pathogen to form biofilm [
30,
31,
32]. CoNS are the most common pathogens involved in central line-associated bloodstream infections (CLABSI) [
33], defined by the Center for Disease Control and Prevention (CDC) as a primary laboratory-confirmed BSI in a patient that had a central line within the 48-hour period before the development of the BSI and is not related to an infection at another site [
34]. This definition is mainly used for surveillance purposes, and differs from the more strict definition of CRBSI (catheter-related bloodstream infection), that requires the presence of clinical symptoms, along with a positive blood culture from a peripheral vein, and the same organism is detected from the catheter segment culture using any of the following methods: (i) semiquantitative or (ii) quantitative catheter culture with a positive result, or (iii) simultaneous quantitative cultures, with differential time to positivity of catheter vs peripheral blood [
35]. Central line removal in case of a CLABSI caused by a CoNS is controversial, and a conservative management is sometimes suggested, considering the relevance of having a secure vascular access for parenteral nutrition and therapy in preterm neonates [
36,
37].
The gastroenteric tract has also been hypothesized as source of infection, as mentioned in a case report of persistent LOS in a patient without a central line showing gut disorders [
38]. In order to investigate this hypothesis more thoroughly, the prevalence of
S. capitis gut colonization has been investigated; interestingly, presence of
S. capitis in the gut microbiota was not found to be a risk factor for LOS [
19].
The objective of our study is to describe and analyze S. capitis CLABSI that occurred in our NICU from the pathogen’s first isolation in 2019 until 2022, focusing on timing of central line removal and how it affected the outcome of the infection.
2. Materials and Methods
We revised the previously existent database of CLABSI of Buzzi Children’s Hospital NICU, which collected data from 2016, selecting patients who had a S. capitis infection. Demographic and clinical data were collected from clinical charts, including details on type of central line used, date of insertion and date of removal. Data on blood cultures (time to positivity, antibiogram, Minimum Inhibitory Concentration) were obtained from the electronic patient record system. Blood cultures were analyzed in Luigi Sacco Hospital microbiology laboratory as per regular practice, with antibiograms drafted according to the most recent EUCAST breakpoint tables.
Descriptive analyses were performed using Stata (version 17.0; StataCorp). All data are presented as medias, medians, and percentages.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the coordinating center in Milan (protocol number 30581/2023 of 03/07/2023)
3. Results
Based on the pre-existent CLABSI database, we found that the first catheter-related infection caused by
S. capitis in our NICU occurred in 2019. In that year, there were 5 cases of CLABSI caused by
S. capitis out of a total of 15 (33.3%). In 2020, patients with S. capitis related CLABSI were also 5, but out of a total of 24 (20.8%); in 2021 9 out of 25 (36%) and in 2022 6 out of 14 (42.9%) (
Table 1)
Within the total population of 25 newborns (
Table 2), 5 were late-preterm or term neonates needing a central catheter due to different clinical conditions requiring surgical intervention; the other 20 were all preterm babies necessitating prolonged insertion of a central vascular catheter for parenteral nutrition support or intravenous therapy.
The median gestational age of the infected neonates was 28 weeks (range 24+1-38+6 wks), with a median birthweight of 885 grams (range 491-2950 gr). The mean length of hospital stay was 88,3 days. One patient out of 25 had a subsequent fatal outcome, related to severe bronchopulmonary dysplasia complications and not directly to the event of S. capitis LOS.
Most of the central catheters in place during the onset of the infection were 1 French epicutaneo-caval polyurethane catheters (22 out of 25); 2 were centrally inserted central catheter (CICC), both in polyurethane, with a diameter of 3 and 4 French, inserted in the right internal jugular vein. Only in one patient the central vascular access involved was a 3.5 French umbilical venous catheter (UVC) in polyurethane.
The median dwell time of epicutaneo-caval catheters was 15 days (range 6–21 days) while of two CICCs was 11 and 14 days respectively and of the UVC of 6 days.
At the time the infection was suspected, and the blood culture performed, catheters were positioned by a mean of 8,84 day.
Bloodstream infections were identified performing blood cultures: they were drawn both from the catheter and from a peripheric vein only in 15 patients, due to the widespread use of epicutaneo-caval catheters of small diameter, not suitable for blood sampling. The other 10 patients had only peripheral blood culture available.
3.1. Catheter Removal
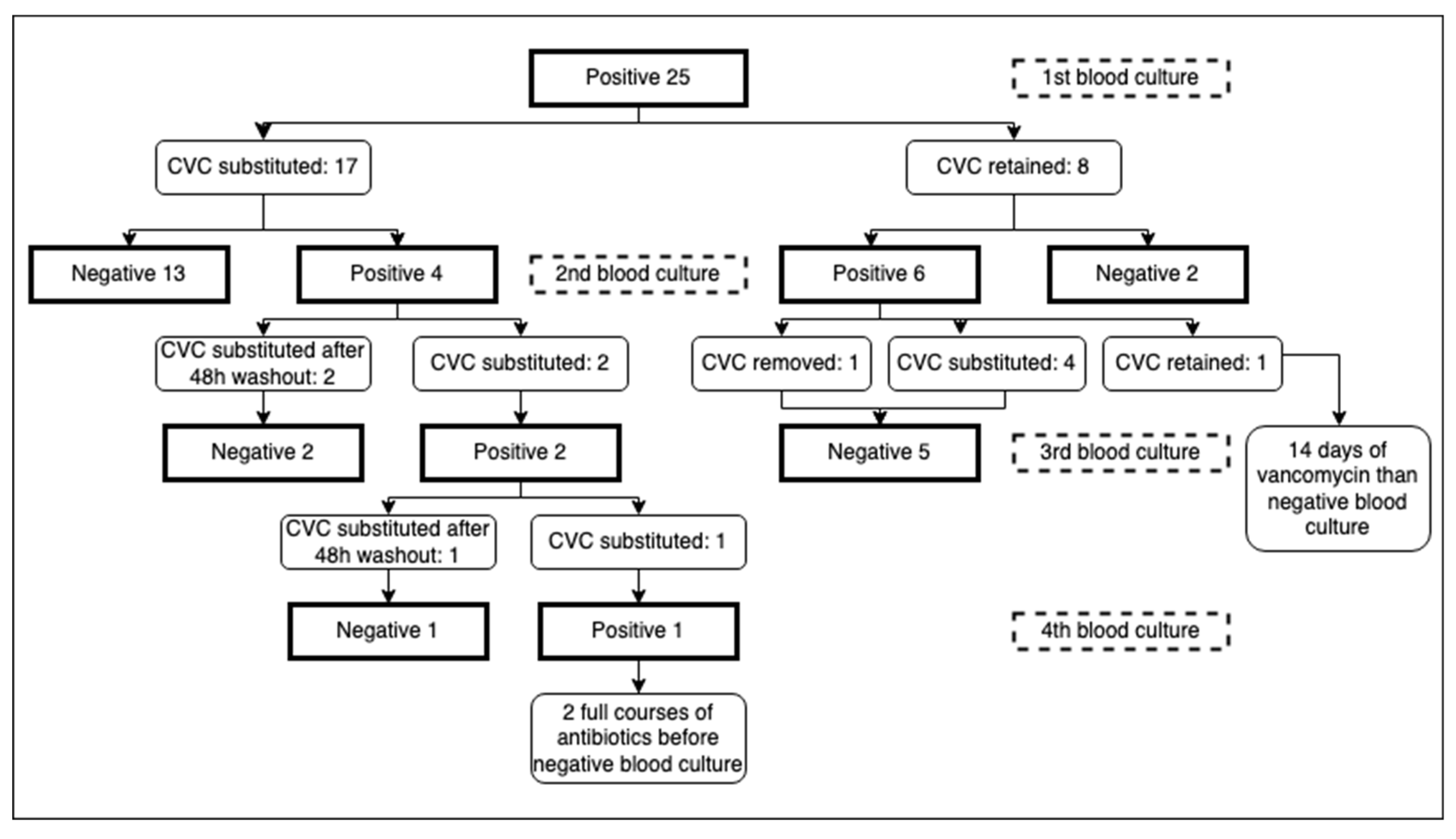
Concerning catheter removal, after the first positive blood culture for S. capitis, 17 out of 25 patients (68%) had their central catheter removed, with a mean time of 3,24 days from the day the blood culture was performed to the day of removal. Of these 17 patients, 13 (76,5%) had a subsequent negative blood culture, performed after a mean of 5,15 days from removal. 11 out of 13 had their catheter immediately substituted with a new one, while 2 patients remained without a central catheter as it was no longer necessary.
Two out of 17, conversely, had a positive blood culture after removal: they were left for 2 days without a central catheter, as a “washout” period. A new central catheter was then positioned and both blood cultures, performed respectively after 3 and 7 days from the previous one, resulted negative.
One of these 17 had his umbilical catheter substituted with an epicutaneo-caval catheter after the blood culture positivity (6 days after placement). Four days after, a second blood culture was performed, still positive: given the serious conditions of the patient requiring a stable central line, another central catheter was immediately placed. Blood cultures after 24 and 48 hours from positioning resulted positive. An attempt was then made to leave the patient for 2 days with just a peripheric catheter: blood culture drawn after 48 hours resulted negative, and a central catheter was then safely repositioned.
The last of the 17 patients who had the catheter replaced was a surgical patient who immediately positioned a new CICC and had his 14-days course of treatment with vancomycin, stabilizing his clinical condition without repeating a second blood culture. After 24 days, due to clinical instability, another blood culture was taken, finding a persistent positivity for S. capitis: a second course of vancomycin was performed, without removing the CICC, with a negative culture after 12 days of therapy.
On the other hand, of the 8 patients (32%) who did not remove the catheter, only 2 (25%) had a subsequent negative blood culture, respectively after 5 and 7 days of antimicrobial therapy. Five of them had their central catheter removed after the second positivity: one was no longer necessary while the other 4 were immediately replaced. All the five blood cultures taken after a mean of 4,8 days from removal were negative.
One patient kept the catheter in place even after the second positive culture, due to his very unstable conditions: after a full 14-days course of vancomycin the last blood culture taken was negative (
Figure 1).
3.2. Clinical Presentation
Clinical manifestation of LOS in our population was mainly constituted by respiratory instability (18 out of 25), represented by a sudden increase in desaturation episodes or frequent apneas, a few times requiring a higher level of respiratory assistance, not explained by a specific pulmonary acute condition. 10 patients presented with fever, 4 of them showing both respiratory instability and hyperpyrexia. Only 2 patients developed a hemodynamic instability with low blood pressure requiring crystalloid fluids in boluses to maintain an adequate perfusion; both of them also had fever and respiratory symptoms. For one patient clinical presentation details were not available.
3.3. Antimicrobial Therapy and Antibiogram
All patients started an empiric parenteral antibiotic therapy immediately after taking the blood culture, with oxacillin and amikacin, following our internal department protocol for the management of LOS. As soon as the antibiogram was available each patient switched to a treatment with vancomycin in continuous parenteral infusion for 10 to 14 days. All S. capitis isolated were, in fact, oxacillin and gentamicin resistant, but susceptible to vancomycin, with a stable MIC of 1 on every antibiogram throughout the years.
4. Discussion
Although CLABSI represents a common clinical situation encountered in our NICUs, there is a lack of available literature regarding the early removal or retention of catheters. BSI caused by
Staphylococcus aureus,
enterococci, Gram-negative bacilli or
Candida spp. have been reported in some observational studies to require prompt CVC removal in order to avoid complicated or persistent sepsis [
37,
39,
40,
41,
42]. Most recent Infectious Disease Society of America (IDSA) guidelines for management of catheter-related infections also identify BSI from
S. aureus, fungi and
P. aeruginosa as situations requiring removal of long-term catheter, while in case of isolation of coagulase-negative staphylococci immediate catheter removal is not suggested [
35].
A 2002 survey conducted across 34 neonatal intensive care units in the United States revealed that 61% of interviewed neonatologists would not routinely remove a peripherally inserted CVC upon detecting a positive blood culture for CoNS, eventually reevaluating their choice based on patient's clinical improvement or the persistence of positive cultures [
43]. This work underscores the consideration given to individual patient circumstances, with healthcare providers opting for catheter removal based on clinical indicators and microbial clearance rather than a standardized protocol.
In this retrospective observational study, we found that prompt catheter removal in
S. capitis CLABSI resulted in sepsis resolution in 76.5% of patients, whereas a first attempt to retain the catheter had success in 25% of patients. The 75% who retained the catheter and persisted positive eventually underwent catheter removal managing to resolve the infection: this is consistent with the IDSA guidelines, suggesting that in patients in whom treatment without catheter removal was attempted due to difficulty in finding an alternative access, a persistent or recurrent BSI should still lead to CVC removal [
35].
Few other similar retrospective studies are available: Karlowicz et al. [
44] reported a success rate with catheter retention during CoNS bacteremia of 46%, sensibly higher than our finding. This difference may be influenced by the lower retention rate found in our data: in their study CVCs were retained for more than 3 days in about half of patients (63 out of 119, 52%), while in our population only 8 out of 25 patients (32%) kept the central access in place. This tendency may be justified by the growing awareness in our department regarding
S. capitis widespread diffusion and persistence in the NICU setting, making it emerge among the other CoNS. The different success rate may as well be influenced by the pathogens involved: Karlowicz work considered all CoNS sepsis, while we only focused on
S. capitis, a pathogen possibly causing more protracted forms of CLABSI than other CoNS due to its described microbiological characteristics of colonization and persistence on surfaces and its heteroresistance to vancomycin [
23,
26].
Deshpande et al. [
45] found a catheter clearance rate for retained catheters during CLABSI caused by CoNS higher than the one described in our study (39% vs 25%): this work also considered all different CoNS together, therefore this difference might be explained by the same considerations exposed above.
Both studies did not report a difference in mortality or length of hospital stay between the groups that retained the CVC and those that had it removed during CoNS’s CLABSI [
44,
45]. Nevertheless, Deshpande et al. found that CVC retention increased bacteremia duration and use of systemic antibiotics [
45].
In our study, 3 cases with persistent positive blood cultures after CVC substitution (
Figure 1) were managed with a “washout” strategy, waiting for 48 hours between catheter removal and reinsertion of a new one. It is reported that immediate reinsertion of a CVC removed because of a CLABSI may cause persistent or recurrent infections [
46] or even a higher mortality [
47]; however, the washout strategy has been proven to be useful especially in fungal infection, while for other pathogens there is no strong evidence of efficacy [
48,
49]. Nevertheless, in our 3 patients, avoiding immediate reinsertion of CVC lead to negative blood cultures and eradication of the persistent blood infection, that in those cases couldn’t be obtained just by immediately changing the central line. Implementing this strategy in newborns is inherently challenging due to the unique characteristics of this patient population: neonates frequently rely on central access for vital medications, nutrition, and other life-sustaining therapies, making the temporary removal of a catheter a complex decision and sometimes an impossible option. The peculiarities of neonatal patients and their clinical needs can constitute an obstacle to the application of a 48-hour waiting period. In many cases, these infants may not tolerate prolonged periods without central access; consequently, the practicality of this strategy must be carefully weighed against the potential risks associated with interrupting essential medical support in this particular population or switching to a possibly less adequate support through a peripheric vein, when feasible. However, from our experience, we believe that in selected situation this management strategy needs to be taken in consideration in order to obtain the fundamental objective of sepsis eradication.
In our population, the clinical presentation was nonspecific and aligned with that commonly observed in cases of late-onset sepsis caused by coagulase-negative staphylococci; these infections are generally less severe and have a lower lethality compared to those induced by gram-negative pathogens [
50]. Our findings are consistent with existing literature, which suggests that sepsis attributed to S. capitis is typically associated with a milder clinical course [
18].
Regarding antimicrobial therapy and resistances, in our setting all
S. capitis isolated were found to be resistant to beta-lactams and aminoglycosides, while no resistance to vancomycin was detected. This is consistent to what is reported in literature: beta-lactams and aminoglycosides resistance is induced by the selective pressure of the NICU setting in which they are widely used [
51]. The absence of vancomycin resistance in the strains of Staphylococcus capitis identified in our department can be attributed to the fact that vancomycin is not routinely used as a first-line treatment, reducing the spread of
S. capitis clones with heteroresistance to vancomycin, similarly to what is reported in New Zealand [
23].
Several limitations warrant consideration when interpreting the findings of our study. Firstly, the retrospective observational design of our study introduces inherent biases and limits our ability to establish causal relationships. Moreover, our population size remains relatively little, potentially reducing the generalizability of our results. Additionally, due to limitations in laboratory methodologies, we were unable to ascertain the genotype of the S. capitis strains isolated, thus not knowing if we were dealing with NRCS-A clone.
5. Conclusions
S. capitis has emerged as an increasingly prevalent pathogen within our NICUs, often associated with infections related to CVCs. Its persistence in clinical settings poses challenges due to its recalcitrance to eradication. The removal of the central catheter in such cases seems to work as a strategy to facilitate pathogen elimination, potentially considering a 48-hour washout period if feasible. Presently, the decision to remove the CVC, after an established diagnosis of CLABSI, remains a subject of controversy and is evaluated on a case-by-case basis: further randomized controlled trials on larger populations could better substantiate the efficacy of a removal approach, possibly associated with a shorter antibiotic course, and try to establish standardized protocols for the management of these infections.
Author Contributions
Conceptualization, F.C., A.S. and V.P.; methodology, F.C..; formal analysis, A.S.; investigation, A.V. and C.V..; data curation, A.V., C.V. and A.S.; writing—original draft preparation, A.S.; writing—review and editing, F.C. and V.P.; supervision, G.L. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Milano Area 1 (protocol code 30581/2023 of 03/07/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bizzarro, M.J. Health Care–Associated Infections in the Neonatal Intensive Care Unit: Barriers to Continued Success. Seminars in Perinatology 2012, 36, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; COMMITTEE ON FETUS AND NEWBORN; COMMITTEE ON INFECTIOUS DISEASES; Cummings, J.; Juul, S.; Hand, I.; Eichenwald, E.; Poindexter, B.; et al. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182894. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, N.S.; Page, G.P.; Bell, E.F.; Stoll, B.J.; Murray, J.C.; Cotten, C.M.; Shankaran, S.; Walsh, M.C.; Laptook, A.R.; Newman, N.S.; et al. Late-Onset Sepsis in Very Low Birth Weight Infants from Singleton and Multiple-Gestation Births. The Journal of Pediatrics 2013, 162, 1120–1124.e1. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.A.; Korones, S.B.; Wright, L.L.; Verter, J.; Poland, R.L.; Bauer, C.R.; Tyson, J.E.; Philips, J.B.; Edwards, W.; Lucey, J.F.; et al. Incidence, Presenting Features, Risk Factors and Significance of Late Onset Septicemia in Very Low Birth Weight Infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 1998, 17, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Edwards, E.M.; Coggins, S.A.; Horbar, J.D.; Puopolo, K.M. Late-Onset Sepsis Among Very Preterm Infants. Pediatrics 2022, 150, e2022058813. [Google Scholar] [CrossRef] [PubMed]
- Glaser, M.A.; Hughes, L.M.; Jnah, A.; Newberry, D. Neonatal Sepsis: A Review of Pathophysiology and Current Management Strategies. Advances in Neonatal Care 2021, 21, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Luca, D.D.; Stronati, M.; Jacqz-Aigrain, E.; Ruffinazzi, G.; Luparia, M.; Tavella, E.; Boano, E.; Castagnola, E.; Mostert, M.; et al. Prevention of Nosocomial Infections in Neonatal Intensive Care Units. Am J Perinatol 2013, 30, 81–88. [Google Scholar] [CrossRef]
- Dong, Y.; Speer, C.P. Late-Onset Neonatal Sepsis: Recent Developments. Arch Dis Child Fetal Neonatal Ed 2015, 100, F257–263. [Google Scholar] [CrossRef]
- Dong, Y.; Speer, C.P. The Role of Staphylococcus Epidermidis in Neonatal Sepsis: Guarding Angel or Pathogenic Devil? International Journal of Medical Microbiology 2014, 304, 513–520. [Google Scholar] [CrossRef]
- Berardi, A.; Sforza, F.; Baroni, L.; Spada, C.; Ambretti, S.; Biasucci, G.; Bolognesi, S.; Capretti, M.; Carretto, E.; Ciccia, M.; et al. Epidemiology and Complications of Late-Onset Sepsis: An Italian Area-Based Study. PLoS One 2019, 14, e0225407. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Sousa, E.; Freitas, J.; Viana, M.; Miranda, F.; Silva, F.P. da Positive Blood Culture and Neonatal Sepsis – A Five-Year Study. NASCER E CRESCER - BIRTH AND GROWTH MEDICAL JOURNAL 2022, 31, 106–114. [Google Scholar] [CrossRef]
- Størdal, E.H.; Solevåg, A.L.; Bjørnholt, J.V.; Rønnestad, A.; Stensvold, H.J. Sepsis Treatment Options Identified by 10-Year Study of Microbial Isolates and Antibiotic Susceptibility in a Level-Four Neonatal Intensive Care Unit. Acta Paediatr 2022, 111, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Sands, K.; Carvalho, M.J.; Spiller, O.B.; Portal, E.A.R.; Thomson, K.; Watkins, W.J.; Mathias, J.; Dyer, C.; Akpulu, C.; Andrews, R.; et al. Characterisation of Staphylococci Species from Neonatal Blood Cultures in Low- and Middle-Income Countries. BMC Infect Dis 2022, 22, 593. [Google Scholar] [CrossRef]
- Okomo, U.; Akpalu, E.N.K.; Le Doare, K.; Roca, A.; Cousens, S.; Jarde, A.; Sharland, M.; Kampmann, B.; Lawn, J.E. Aetiology of Invasive Bacterial Infection and Antimicrobial Resistance in Neonates in Sub-Saharan Africa: A Systematic Review and Meta-Analysis in Line with the STROBE-NI Reporting Guidelines. Lancet Infect Dis 2019, 19, 1219–1234. [Google Scholar] [CrossRef]
- Medugu, N.; Iregbu, K.; Tam, P.-Y.I.; Obaro, S. Aetiology of Neonatal Sepsis in Nigeria, and Relevance of Group b Streptococcus: A Systematic Review. PLOS ONE 2018, 13, e0200350. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Platt, R.; Sidebottom, D.G.; Leclair, J.M.; Epstein, M.F.; Goldmann, D.A. Coagulase-Negative Staphylococcal Bacteremia in the Changing Neonatal Intensive Care Unit Population. Is There an Epidemic? JAMA 1987, 258, 2548–2552. [Google Scholar] [CrossRef]
- Healy, C.M.; Baker, C.J.; Palazzi, D.L.; Campbell, J.R.; Edwards, M.S. Distinguishing True Coagulase-Negative Staphylococcus Infections from Contaminants in the Neonatal Intensive Care Unit. J Perinatol 2013, 33, 52–58. [Google Scholar] [CrossRef]
- Ben Said, M.; Hays, S.; Bonfils, M.; Jourdes, E.; Rasigade, J.-P.; Laurent, F.; Picaud, J.-C. Late-Onset Sepsis Due to Staphylococcus Capitis ‘Neonatalis’ in Low-Birthweight Infants: A New Entity? Journal of Hospital Infection 2016, 94, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Butin, M.; Rasigade, J.-P.; Subtil, F.; Martins-Simões, P.; Pralong, C.; Freydière, A.-M.; Vandenesch, F.; Tigaud, S.; Picaud, J.-C.; Laurent, F. Vancomycin Treatment Is a Risk Factor for Vancomycin-Nonsusceptible Staphylococcus Capitis Sepsis in Preterm Neonates. Clinical Microbiology and Infection 2017, 23, 839–844. [Google Scholar] [CrossRef]
- Butin, M.; Rasigade, J.-P.; Martins-Simões, P.; Meugnier, H.; Lemriss, H.; Goering, R.V.; Kearns, A.; Deighton, M.A.; Denis, O.; Ibrahimi, A.; et al. Wide Geographical Dissemination of the Multiresistant Staphylococcus Capitis NRCS-A Clone in Neonatal Intensive-Care Units. Clinical Microbiology and Infection 2016, 22, 46–52. [Google Scholar] [CrossRef]
- Moore, G.; Barry, A.; Carter, J.; Ready, J.; Wan, Y.; Elsayed, M.; Haill, C.; Khashu, M.; Williams, O.M.; Brown, C.S.; et al. Detection, Survival, and Persistence of Staphylococcus Capitis NRCS-A in Neonatal Units in England. J Hosp Infect 2023, 140, 8–14. [Google Scholar] [CrossRef]
- Gras-Le Guen, C.; Fournier, S.; Andre-Richet, B.; Caillon, J.; Chamoux, C.; Espaze, E.; Richet, H.; Roze, J.C.; Lepelletier, D. Almond Oil Implicated in a Staphylococcus Capitis Outbreak in a Neonatal Intensive Care Unit. J Perinatol 2007, 27, 713–717. [Google Scholar] [CrossRef]
- Carter, G.P.; Ussher, J.E.; Da Silva, A.G.; Baines, S.L.; Heffernan, H.; Riley, T.V.; Broadbent, R.; van der Linden, A.; Lee, J.; Monk, I.R.; et al. Genomic Analysis of Multiresistant Staphylococcus Capitis Associated with Neonatal Sepsis. Antimicrob Agents Chemother 2018, 62, e00898-18. [Google Scholar] [CrossRef]
- Lepainteur, M.; Royer, G.; Bourrel, A.S.; Romain, O.; Duport, C.; Doucet-Populaire, F.; Decousser, J.-W. Prevalence of Resistance to Antiseptics and Mupirocin among Invasive Coagulase-Negative Staphylococci from Very Preterm Neonates in NICU: The Creeping Threat? J Hosp Infect 2013, 83, 333–336. [Google Scholar] [CrossRef]
- Butin, M.; Dumont, Y.; Monteix, A.; Raphard, A.; Roques, C.; Martins Simoes, P.; Picaud, J.-C.; Laurent, F. Sources and Reservoirs of Staphylococcus Capitis NRCS-A inside a NICU. Antimicrob Resist Infect Control 2019, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- D’mello, D.; Daley, A.J.; Rahman, M.S.; Qu, Y.; Garland, S.; Pearce, C.; Deighton, M.A. Vancomycin Heteroresistance in Bloodstream Isolates of Staphylococcus Capitis. Journal of Clinical Microbiology 2008, 46, 3124–3126. [Google Scholar] [CrossRef]
- Van Der Zwet, W.C.; Debets-Ossenkopp, Y.J.; Reinders, E.; Kapi, M.; Savelkoul, P.H.M.; Van Elburg, R.M.; Hiramatsu, K.; Vandenbroucke-Grauls, C.M.J.E. Nosocomial Spread of a Staphylococcus Capitis Strain with Heteroresistance to Vancomycin in a Neonatal Intensive Care Unit. Journal of Clinical Microbiology 2002, 40, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Decalonne, M.; Dos Santos, S.; Gimenes, R.; Goube, F.; Abadie, G.; Aberrane, S.; Ambrogi, V.; Baron, R.; Barthelemy, P.; Bauvin, I.; et al. Staphylococcus Capitis Isolated from Bloodstream Infections: A Nationwide 3-Month Survey in 38 Neonatal Intensive Care Units. Eur J Clin Microbiol Infect Dis 2020, 39, 2185–2194. [Google Scholar] [CrossRef]
- Rasigade, J.-P.; Raulin, O.; Picaud, J.-C.; Tellini, C.; Bes, M.; Grando, J.; Said, M.B.; Claris, O.; Etienne, J.; Tigaud, S.; et al. Methicillin-Resistant Staphylococcus Capitis with Reduced Vancomycin Susceptibility Causes Late-Onset Sepsis in Intensive Care Neonates. PLoS ONE 2012, 7, e31548–e31548. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Daley, A.J.; Istivan, T.S.; Garland, S.M.; Deighton, M.A. Antibiotic Susceptibility of Coagulase-Negative Staphylococci Isolated from Very Low Birth Weight Babies: Comprehensive Comparisons of Bacteria at Different Stages of Biofilm Formation. Ann Clin Microbiol Antimicrob 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Cui, B.; Smooker, P.M.; Rouch, D.A.; Daley, A.J.; Deighton, M.A. Differences between Two Clinical Staphylococcus Capitis Subspecies as Revealed by Biofilm, Antibiotic Resistance, and Pulsed-Field Gel Electrophoresis Profiling. J Clin Microbiol 2013, 51, 9–14. [Google Scholar] [CrossRef]
- França, A. The Role of Coagulase-Negative Staphylococci Biofilms on Late-Onset Sepsis: Current Challenges and Emerging Diagnostics and Therapies. Antibiotics (Basel) 2023, 12, 554. [Google Scholar] [CrossRef]
- Hocevar, S.N.; Edwards, J.R.; Horan, T.C.; Morrell, G.C.; Iwamoto, M.; Lessa, F.C. Device-Associated Infections among Neonatal Intensive Care Unit Patients: Incidence and Associated Pathogens Reported to the National Healthcare Safety Network, 2006-2008. Infect Control Hosp Epidemiol 2012, 33, 1200–1206. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am J Infect Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Vasudevan, C.; Oddie, S.J.; McGuire, W. Early Removal versus Expectant Management of Central Venous Catheters in Neonates with Bloodstream Infection. Cochrane Database of Systematic Reviews 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.K., Jr.; Miller, W.; Garges, H.; Benjamin, D.K.; McKinney, R.E., Jr.; Cotton, M.; Fisher, R.G.; Alexander, K.A. Bacteremia, Central Catheters, and Neonates: When to Pull the Line. Pediatrics 2001, 107, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Chow, V.C.Y.; Lee, C.H.; Ling, J.M.L.; Wong, H.L.; Chan, R.C.Y. PERSISTENT STAPHYLOCOCCUS CAPITIS SEPTICEMIA IN A PRETERM INFANT. Pediatric Infectious Disease Journal 2006, 25, 652–654. [Google Scholar] [CrossRef]
- Karlowicz, M.G.; Hashimoto, L.N.; Kelly, R.E.; Buescher, E.S. Should Central Venous Catheters Be Removed as Soon as Candidemia Is Detected in Neonates? Pediatrics 2000, 106, E63. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, K.J.; Buescher, E.S.; Kelly, R.E.; Karlowicz, M.G. Central Venous Catheter Removal versus in Situ Treatment in Neonates with Enterobacteriaceae Bacteremia. Pediatrics 2003, 111, e269–274. [Google Scholar] [CrossRef] [PubMed]
- Sc, E.; Jl, T.; Lt, G. Outcome of Treatment of Candidemia in Children Whose Central Catheters Were Removed or Retained. The Pediatric infectious disease journal 1989, 8. [Google Scholar]
- Boussamet, L.; Launay, E.; Thomas, E.; Leguen, C.G.; Lepelletier, D. Should Central Venous Catheters Be Rapidly Removed to Treat Staphylococcus Aureus Related-Catheter Bloodstream Infection (CR-BSI) in Neonates and Children? An 8-Year Period (2010–2017) Retrospective Analysis in a French University Hospital. Journal of Hospital Infection 2019, 103, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Sánchez, P.J.; Siegel, J.; Levine, G.; Saiman, L.; Jarvis, W.R.; the Pediatric Prevention Network. Evaluation and Treatment of Neonates With Suspected Late-Onset Sepsis: A Survey of Neonatologists’ Practices. Pediatrics 2002, 110, e42. [Google Scholar] [CrossRef]
- Karlowicz, M.G.; Furigay, P.J.; Croitoru, D.P.; Buescher, E.S. Central Venous Catheter Removal versus in Situ Treatment in Neonates with Coagulase-Negative Staphylococcal Bacteremia. Pediatr Infect Dis J 2002, 21, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Jain, A.; Shah, P.S. Outcomes Associated with Early Removal versus Retention of Peripherally Inserted Central Catheters after Diagnosis of Catheter-Associated Infections in Neonates. The Journal of Maternal-Fetal & Neonatal Medicine 2016, 29, 4082–4087. [Google Scholar] [CrossRef]
- İşgüder, R.; Devrim, İ.; Ceylan, G.; Kara, A.; Gülfidan, G.; Ağın, H. Risk Factors for Recurrent Central Line-Associated Bloodstream Infections in a Pediatric Intensive Care Unit. Turk J Med Sci 2017, 47, 1128–1136. [Google Scholar] [CrossRef]
- Zhong, Y.; Deng, L.; Zhou, L.; Liao, S.; Yue, L.; Wen, S.W.; Xie, R.; Lu, Y.; Zhang, L.; Tang, J.; et al. Association of Immediate Reinsertion of New Catheters with Subsequent Mortality among Patients with Suspected Catheter Infection: A Cohort Study. Annals of Intensive Care 2022, 12, 38. [Google Scholar] [CrossRef]
- Chin, B.S.; Han, S.H.; Lee, H.S.; Jeong, S.J.; Choi, H.; Kim, C.O.; Choi, J.Y.; Song, Y.G.; Kim, J.M. Risk Factors for Recurrent Catheter-Related Infections after Catheter-Related Bloodstream Infections. Int J Infect Dis 2010, 14, e16–21. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Ryu, B.-H.; Hong, S.I.; Cho, O.-H.; Hong, K.-W.; Bae, I.-G.; Kwack, W.G.; Kim, Y.J.; Chung, E.K.; Kim, D.Y.; et al. Clinical Impact of Early Reinsertion of a Central Venous Catheter after Catheter Removal in Patients with Catheter-Related Bloodstream Infections. Infection Control & Hospital Epidemiology 2021, 42, 162–168. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-Onset Sepsis in Very Low Birth Weight Neonates: The Experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef]
- Laurent, F.; Butin, M. Staphylococcus Capitis and NRCS-A Clone: The Story of an Unrecognized Pathogen in Neonatal Intensive Care Units. Clinical Microbiology and Infection 2019, 25, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).