1. Introduction
Restorative Reproductive Medicine (RRM) [
1] is a newly emerging “medical treatment process” for patients with infertility. Infertility can more accurately be described as a consequence of multiple chronic health conditions instead of an independent diagnosis. When these chronic issues are identified and treated, fertility can return without the need for more invasive assisted reproductive technology (ART). The process of RRM treats infertility as a chronic illness requiring multiple and sustained interventions over time [
2,
3]. Restorative treatments are guided with the help of “specialised cycle tracking of fertility biomarkers” such as bleeding, cervical mucus, and basal body temperature [
4]. Assisted reproductive technology on the other hand treats infertility as an acute condition, providing a procedure to bypass natural fertility with less attention to diagnosing and correcting the underlying causes of infertility. It is invasive, expensive and has more adverse obstetric and perinatal outcomes [
5]. Furthermore, at least 35% of couples undergoing IVF have unexplained infertility. IVF has not been proven to be better than expectant management (doing nothing) for this group of patients [
6]. Giving suitable couples the option of choosing RRM as an alternative or pre-requisite to IVF when further fertility treatment is advised would avoid more costly and invasive medical technology that can present challenges for some couples [
7].
Health coverage for infertility is a needed development given the prevalence of infertility, but any health policy program considering the provision of funding for infertility should not exclusively cover ART. As an example of how this type of health policy is being implemented, we cite a recent development in Ireland. In 2023, the HSE has introduced publicly funded fertility services for people who need fertility treatment, including IUI, IVF and ICSI. This is delivered by “approved private providers” on behalf of the HSE [
8]. To access state funded fertility treatment, patients must first attend their GP for referral to one of six regional fertility hubs, based in public maternity hospitals. The hub provides all necessary fertility investigations, including blood tests, semen analysis and fertility-related surgeries and medical management of fertility challenges including ovulation induction with follicle tracking. If further advanced treatment is recommended by a Reproductive Medical Consultant and the patients meet the access criteria, the hubs will refer patients to an HSE-approved private provider of their choosing. There is no cost to patients for the recommended treatment through private providers. Criteria to access one free cycle of IVF, ICSI or IUI treatment, include having no children, no more than one previous attempt with IVF, a BMI less than 30 and female age 40 or less.
Because the HSE has selectively funded IVF treatment for infertility patients, but not RRM, access to the less invasive option is reduced. We believe that if funding for RRM was included by the HSE, it would offer a less expensive and more effective treatment, with likely better birth outcomes compared to IVF for many patients. Therefore, to demonstrate the potential opportunity, we retrospectively compared success rates and costs of IVF in 2019 reported by the HFEA, to results obtained by an experienced RRM clinic, from 2019. This comparison provides preliminary evidence to support our claim. With this preliminary data we propose the urgent need for prospective comparisons between RRM and IVF for the treatment of infertility. When RRM and IVF are both funded it would be an ideal opportunity to prospectively collect data on all outcomes for fertility patients and babies resulting from these procedures.
2. Materials and Methods
The RRM approach used at the NeoFertility clinic has been developed over the past 25 years. It includes 3 phases. Phase one is finding the problem and it lasts about 2 months, phase 2 is fixing the problem and lasts about 2 months and phase 3 is attempting natural conception for 1-18 months.
The key components of phase one are collecting a good history and teaching the patients how to accurately chart their menstrual cycle using the ChartNeo App. Correct charting of the menstrual cycle is vital to determine cycle health and confirmation of the stage of the cycle which is subsequently used for proper blood sampling and medication timing. ChartNeo uses proven elements from established fertility awareness-based methods including the Billings Ovulation Method®, the Creighton Model Fertility System™, and the Sympto-thermal method [
4]. Using biomarkers of fertility such as cervical mucus and basal body temperature, the woman enters cycle observations into the app which can be used prospectively to determine the fertile window and the phases of the menstrual cycle. Precise timing of blood sampling can be conducted during the phases of the cycle for diagnostic evaluation and subsequent therapeutic intervention. By keeping a record of the observations along with the corresponding diagnostic results, a retrospective evaluation of women’s cycle health can be ascertained from the app. Key diagnostic evaluation of day 3, and post- peak day 7 levels of estradiol and progesterone along with ultrasound assessment of follicle development and rupture determines the quality of ovulation. Additional evaluation of underlying health issues is also performed including hypothalamic, pituitary, adrenal, and thyroid function. Metabolic assessment is performed when indicated. If symptoms of endometriosis, poly-cystic ovaries, sleep apnea, chronic fatigue or stress, low mood, premenstrual syndrome, insulin resistance, or auto-immune disease are present, these are treated with a combination of conventional and holistic approaches during phase 2. Interventions during phase 2 may include diet, supplements and symptom determined medications. Medications, include DHEA, progesterone, levothyroxine, low-dose naltrexone, metformin, prednisolone, and cycle stimulation drugs such as letrozole, clomiphene, FSH and HCG and more details can be found in
Table 1. These medications are monitored and managed until reproductive hormones are balanced, follicle rupture is confirmed by ultrasound, and adequate luteal support is available. Male fertility is also assessed by semen analysis using current WHO criteria, and DNA fragmentation testing. Underlying male health issues are investigated and treated in the same way as his female partner. If varicocele or other anatomic abnormality is found, they are referred to a competent urologist for surgery. This is similar to the way endometriosis or tubal blockage is referred for gynecological surgery. Only the most severe and persistent male factor or uterine/tubal abnormalities are not suitable for RRM. Phase 3 then begins up to 12 cycles of attempted natural conception during the fertile period informed by using the ChartNeo app. If ovarian stimulation is necessary to achieve ovulation and adequate hormone levels it is monitored closely using ultrasound and the stimulation drugs are titrated to achieve follicular recruitment of one mature follicle, which is the usual outcome. Additional details on the application of RRM using the NeoFertility treatment plan have been previously published [
9,
10].
To compare our results to IVF success rates we retrospectively collected the live birth rate data from our patient records in 2019 and then compared our results to one treatment cycle of IVF (using patient’s own eggs and their partners sperm) reported by the HFEA, for 2019. The HFEA data were extracted from their website [
11].
The NeoFertility clinic, located in Dublin, Ireland had two physicians providing services in 2019. All data used for this evaluation were routinely collected from patients during their visits or from phone, mail, email, or survey and placed in the chart. Data was then abstracted, anonymized, and entered into a database using a standardized system called STORRM (
Surveillance of
Treatment
Outcomes from
RRM) [
12], an international registry template developed by the International Institute for Restorative Reproductive Medicine to be like the IVF data registry. A total of 193 new patient couples started the treatment plan for infertility in 2019 at the NeoFertility clinic and were included in our analysis.
We then statistically compared the live birth rates between the two data sets using a two-sample test of proportions on STATA. Multiple pregnancy rates, weeks’ gestation, and weight at birth were reported for RRM data but were not available from HFEA data. A cost analysis was performed using prices listed on the website of an Irish IVF clinic [
13] and the rates listed on the NeoFertility RRM clinic’s website [
14].
3. Results
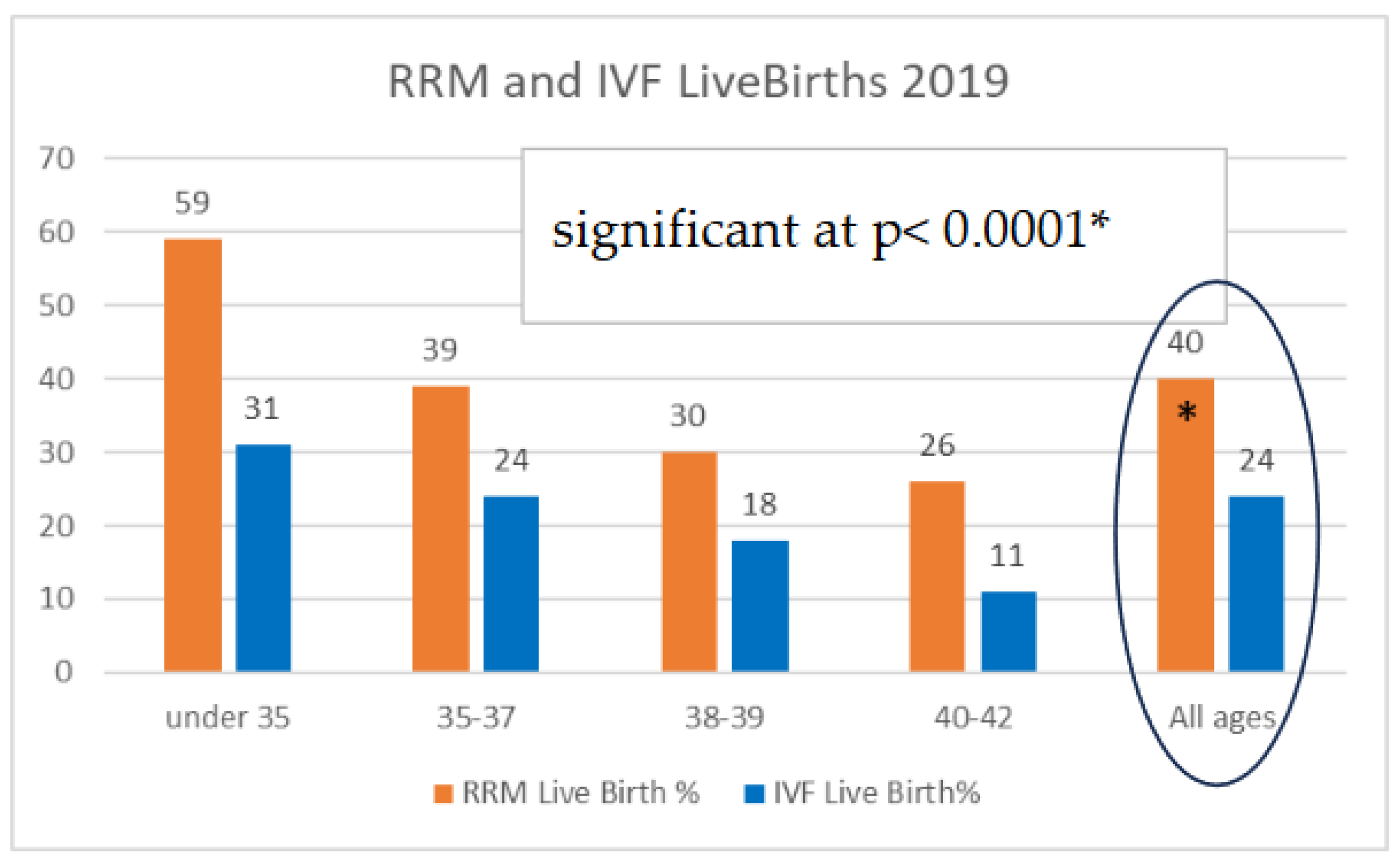
Official HFEA data from 2019, published in 2021, reported the live birth rate “per treatment cycle” for couples using their own eggs and partner sperm on a fresh cycle. A total of 32,502 couples were treated, resulting in 7920 live births for women aged 42 years and younger. Overall, this was a (7,920/32,502) 24.4% live birth rate. The twin live birth rate was 7%. The live birth rate was highest for females under 35 years at 31% and lowest for females age 43+. The average female age was 34.9 years. Data on weeks’ gestation or infant weight for IVF patients was not available.
STORRM data extracted for the RRM practice cohort in 2019 revealed that 193 couples were treated, resulting in 78 live births for all age groups. There were 20 couples who conceived but subsequently experienced miscarriage and there were 95 couples who never conceived. Overall, this showed a (78/193) 40.4% live birth rate which was significantly higher than IVF at p < 0.0001. The twin birth rate was (2/78) 2.5%. Consistent with the IVF data, the live birth rate was highest for females under 35 years (59%) and lower for older females (
Figure 1).
The average female age for RRM patients was 36.4 years which was 1.5 years older than the average IVF patient in this comparison. Couples were trying to conceive for an average of 2.5 years. Remarkably, 35 couples in the RRM cohort (18%) previously had IVF totaling 80 combined cycles of previous unsuccessful IVF treatment. The average weeks’ gestation at birth was 39 weeks with a birth weight of 3466g (7lb 10oz) for singleton pregnancies. The 2 sets of twins were delivered at 35 and 36 weeks with an average birth weight 2,343g (5lb 2oz). There were no deliveries before 35 weeks and no babies had a birth weight below 2,000g.
Currently, the cost for a single IVF cycle without additional laboratory procedures such as sperm injection, embryo biopsy or freezing is listed at €5150. It includes all the cycle monitoring tests but not any additional diagnostic testing, surgery, or medication costs. The RRM medical management plan is offered for €2000 and similarly does not include diagnostic testing, surgery, or medications.
4. Discussion
This is a retrospective cohort study comparing outcome data between STORRM (RRM) and HFEA (IVF) data registries. Although the 2019 STORRM data set is small compared to HFEA, it shows a statistically significant result in favour of RRM treatment for couples with infertility, (P<0.0001). The average age of IVF patients was 1.5 years younger than the RRM group. Despite this advantage, IVF had a lower live birth rate and a higher incidence of twins. In addition, IVF pregnancies generally have an increased incidence of gestational complications such as diabetes, hypertension, pre-eclampsia, abnormal birth weight, and premature delivery [
5]. Children born from IVF have a higher rate of neo-natal complications and birth defects [
15].
Cochrane data [
6] indicates there is no good evidence to show IVF is any better than expectant management for couples with unexplained infertility, which accounts for over 35% of all couples who try IVF. Most infertile couples are suitable for RRM treatment apart from females with untreatable bilateral blocked fallopian tubes and men with severe male factor infertility, (total motile sperm count, TMSC < 5 x10
6).
An argument can be made that comparing 1 treatment cycle of IVF to 12 months of attempted natural conception is not equivalent. We used that comparison simply because that was what was being funded in our Irish example. Additional treatment cycles of IVF along with frozen embryo transfer attempts can increase success rates but this also comes with additional costs. Additional costs include those of the treatment cycles but also should include the obstetrical and peri-natal care of the women and their offspring. More difficult to capture is the cost of not identifying and correcting underlying health issues in the parents in a timely manner when conception using IVF is the primary goal.
The RRM treatment plan can run for up to 12 balanced cycles, at a cost of €2000 which is less than half the cost of a single IVF treatment cycle. Using this information, if we compare outcomes for a budget of 1 million Euro spent on IVF or RRM we have a striking contrast. For IVF, this provides funding for 194 couples, resulting in 47 live births at a cost of €21,277 per baby. 1 million euro spent on RRM provides funding for 500 couples, resulting in 202 live births at a cost of €4,950 per baby. There are further savings due to a lower incidence of low birth weight and premature delivery with RRM, because fewer babies require neonatal care unit services. A direct comparison between IVF and RRM for all peri-natal outcomes, birth defects and health of the offspring is needed.
5. Conclusions
These data show that IVF babies are over 4 times more expensive than RRM babies, and IVF success rates are lower. RRM has a higher live birth rate (40.4% vs 24.4%), and due to the low incidence of adverse outcomes, RRM likely has better peri-natal outcomes compared to IVF. Any government health policy that provides exclusive funding for IVF threatens access to RRM treatment despite evidence that RRM may be a better treatment for many patients. Using RRM as a prerequisite option in suitable patients would save thousands and avoid the use of invasive procedures that often are not necessary. We provide a comparison of costs and effectiveness from a clinic in Ireland for illustrative purposes, and we propose a change in the Irish HSE funding policy to include the option of RRM treatment for infertile couples. We recommend urgent prospective data collection and analysis to compare outcomes on multiple levels between IVF and RRM. This will likely make fertility treatment available to more couples at a lower cost, with less intervention, consistent with HSE objectives [
8].
6. Patents
Dr Phil Boyle is co-developer of the ChartNeo fertility tracking app which is available in the app store. Copyright © 2023 Neo Fertility App LTD.
Author Contributions
Conceptualization, Boyle, and Turczynski; methodology, Boyle; formal analysis, Boyle, and Turczynski; investigation, Boyle, Toth, ONeill; resources, Boyle.; data curation, Boyle and Turczynski.; writing—original draft preparation, Boyle and Turczynski.; writing—Boyle, Turczynski, and Toth. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Beacon Hospital.
Informed Consent Statement
Patient consent was waived due to the data source including de-identified patient records retrospectively collected.
Conflicts of Interest
Phil C. Boyle has declared that he is co-developer of the ChartNeo fertility tracking app available for sale on the App store. The other authors declare no conflict of interest.
References
- Boyle, P.C.; de Groot, T.; Andralojc, K.M.; Parnell, T.A. Healthy Singleton Pregnancies from Restorative Reproductive Medicine (RRM) After Failed IVF. Front. Med. 2018, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.B.; Carpentier, P.A.; Meier, B.L.; Rollo, M.; Tingey, B. Restorative reproductive medicine for infertility in two family medicine clinics in New England, an observational study. BMC Pregnancy Childbirth. 2021, 21, 495. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.B.; Parnell, T.; Kantor, K.; Reeder, M.R.; Najmabadi, S.; Johnson, K.; Musso, I.; Hartman, H.; Tham, E.; Winter, I.; Galczynski, K.; Carus, A.; Sherlock, A.; Golden Tevald, J.; Barczentewicz, M.; Meier, B.; Carpentier, P.; Poehailos, K.; Chasuk, R.; Danis, P.; Lipscomb, L. International Natural Procreative Technology Evaluation and Surveillance of Treatment for Subfertility (iNEST): enrollment and methods. Hum Reprod Open 2022, 2022, hoac033. [Google Scholar] [CrossRef] [PubMed]
- Duane, M.; Stanford, J.B.; Porucznik, C.A.; Vigil, P. Fertility Awareness-Based Methods for Women's Health and Family Planning. Front. Med. 2022, 9, 858977. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Pyke, C.; Senapati, S.; Mainigi, M.; Barnhart, K. IVF and adverse Obstetric and Perinatal Outcomes. Semin Perinatol. 2017, 345–353. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Kamath, M.S.; Pandian, Z.; Gibreel, A.; Bhattacharya, S. In vitro fertilisation for unexplained subfertility. Cochrane Database of Systematic Reviews TBD, Issue TBD. Art. No.: CD003357. [CrossRef]
- Brezina, P.R.; Zhao, Y. The ethical, legal, and social issues impacted by modern assisted reproductive technologies. Obstet. Gynecol. Int. 2012, 2012, 686253. [Google Scholar] [CrossRef] [PubMed]
- “HSE-funded Fertility Treatment Services to start in September” (no date) Our Health Service. Available online: https://www.hse.ie/eng/services/news/media/pressrel/hse-funded-fertility-treatment-services-to-start-in-september.html (accessed on 25 October 2023).
- Boyle, P.; Androlojc, K.; van der Velden, S.; Najmabadi, S.; de Groot, T.; Turczynski, C.; Stanford, J. Restoration of Serum Estradiol and Reduced Incidence of Miscarriage in Patients with Low Serum Estradiol During Pregnancy: A Retrospective Cohort Study Using a Multifactorial Protocol Including DHEA. Front. Reprod. Health 2023, 5. In-Press. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.C.; Stanford, J.B.; Zecevic, I. Successful pregnancy with restorative reproductive medicine after 16 years of infertility, three recurrent miscarriages, and eight unsuccessful embryo transfers with in vitro fertilization/intracytoplasmic sperm injection: a case report. J. Med. Case Rep. 2022, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- “Fertility Treatment 2019: Trends and Figures.” (no date). Human Fertilisation & Embryology Authority- HFEA, Table 14. Available online: https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2019-trends-and-figures/ (accessed on 25 October 2023).
- “The Surveillance of Treatment Outcomes in Restorative Reproductive Medicine” (no date) International Institute for Restorative Reproductive Medicine. Available online: https://iirrm.org/surveillance-of-treatment-and-outcomes-in-restorative-reproductive-medicine-storrm/ (accessed on 25 October 2023).
- Available online: https://www.sims.ie/about-sims-ivf/prices/how-much-does-ivf-cost-main-treatment-pricing (accessed on 20 December 2023).
- Available online: https://neofertility.ie/clinic-pricing/ (accessed on 20 December 2023).
- Luke, B.; Brown, M.; Wantman, E.; Forestieri, N.; Browne, M.; Fisher, S.; et al. The Risk of Birth Defects with Conception by, A.R.T. Hum. Reprod. 2021, 36, 116–129. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).