Submitted:
08 January 2024
Posted:
09 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Chemical and microbial composition of fresh alfalfa samples
2.2. Chemical composition and fermentation quality analysis of alfalfa silage
2.3. Analysis of microbial community diversity and population change in alfalfa silage
2.4. Relationship between chemical composition, fermentation quality, and bacterial community
3. Materials and Methods
3.1. Materials and chemicals
3.2. Sample collection and measurements
3.3. Sequencing and analysis of microbial population
3.4. Statistical analysis
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of Metabolome and Bacterial Community in Whole Crop Corn Silage by Inoculating Homofermentative Lactobacillus plantarum and Heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Wang, Z.; Bao, J.; Zhao, M.; Ge, G.; Jia, Y.; Du, S. Effects of Isolated LAB on Chemical Composition, Fermentation Quality and Bacterial Community of Stipa grandis Silage. Microorganisms 2022, 10, 2463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Z.; Li, J.; Chen, L.; Shao, T. Effects of storage temperature and combined microbial inoculants on fermentation end products and microbial populations of Italian ryegrass (Lolium multiflorumLam.) silage. J. Appl. Microbiol. 2018, 125, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Adesogan, A. T. (2006). Factors affecting corn silage quality in hot and humid climates. Florida Ruminant Nutrition Symposium.
- Zhou, Y.; Drouin, P.; Lafrenière, C. Effect of temperature (5-25°C) on epiphytic lactic acid bacteria populations and fermentation of whole-plant corn silage. J. Appl. Microbiol. 2016, 121, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ding, Z.; Ke, W.; Xu, D.; Zhang, P.; Bai, J.; Mudassar, S.; Muhammad, I.; Guo, X. Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 2019, 282, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Adesogan, A. Influence of Ensiling Temperature, Simulated Rainfall, and Delayed Sealing on Fermentation Characteristics and Aerobic Stability of Corn Silage. J. Dairy Sci. 2006, 89, 3122–3132. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A. (1989). Role of Yeasts and Clostridia in Silage Deterioration: Identification and Ecology.
- Driehuis, F.; Oude Elferink, S.J.W.H. The impact of the quality of silage on animal health and food safety: A review. Vet. Q. 2000, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Anésio, A.; Santos, M.; da Silva, L.; Silveira, R.; Braz, T.; Pereira, R. Effects of ensiling density on chemical and microbiological characteristics of sorghum silage. J. Anim. Feed. Sci. 2017, 26, 65–69. [Google Scholar] [CrossRef]
- Cai,Y.M.,Benno,Y.,Ogawa,M.,Ohmomo,S.,Kumal,S.,Nakase,T.(1998).Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from Forage Crops on Silage Fermentation.Applied and Environmental Microbiology.Aug. 1998, p. 2982–2987.
- Wang, S.; Dong, Z.; Li, J.; Chen, L.; Shao, T. Effects of storage temperature and combined microbial inoculants on fermentation end products and microbial populations of Italian ryegrass (Lolium multiflorumLam.) silage. J. Appl. Microbiol. 2018, 125, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Du, S.; Ge, G.; Wan, T.; Jia, Y. Selection of lactic acid bacteria from native grass silage and its effects as inoculant on silage fermentation. Agron. J. 2021, 113, 3169–3177. [Google Scholar] [CrossRef]
- Ruppel,R. A.,pitt,R.E.,Chase,L.E.,Galton,D.M. Bunker Silo Management and Its Relationship to Forage preservation on Dairy Farms. J Dairy Sci 1995, 78, 141–153. [Google Scholar] [CrossRef]
- Sucu, E.; Kalkan, H.; Canbolat, O.; Filya, I. Effects of ensiling density on nutritive value of maize and sorghum silages. Rev. Bras. de Zootec. 2016, 45, 596–603. [Google Scholar] [CrossRef]
- Han,K. J.,Collins,M.,Vanzant,E.S.,Dougherty,C.T. Bale Density and Moisture Effects on Alfalfa Round Bale Silage. CROp SCIENCE 2004, 44, 914–919.
- Li, P.; Gou, W.; Zhang, Y.; Yang, F.; You, M.; Bai, S.; Shen, Y. Fluctuant storage temperature increased the heterogeneous distributions of pH and fermentation products in large round bale silage. Grassl. Sci. 2019, 65, 155–161. [Google Scholar] [CrossRef]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z.; et al. Impact of Packing Density on the Bacterial Community, Fermentation, and In Vitro Digestibility of Whole-Crop Barley Silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- Green, O.; Bartzanas, T.; Løkke, M.M.; Bochtis, D.D.; Sørensen, C.G.; Jørgensen, O.J.; Tortajada, V.G. Spatial and temporal variation of temperature and oxygen concentration inside silage stacks. Biosyst. Eng. 2012, 111, 155–165. [Google Scholar] [CrossRef]
- Ke-Li, L. , Ju-Lin, G., & Shu-Guo, L. U. (2004). Studies on Characteristics of Silage production of Different Types Maize. Journal of Maize Sciences.
- Williams,A. G.,Lowe,J.F.,Rees,D.V. The effect of oxygen concentration on changes in the microhial population, temperature and dry-matter content in grass silage. Grass Forage Sci. 1994, 49, 183–191. [Google Scholar] [CrossRef]
- Muller,C. E. Fermentation patterns of small-bale silage and haylage produced as a feed for horses. Grass Forage Sci. 2005, 60, 109–118. [Google Scholar] [CrossRef]
- Yuan, X.; Dong, Z.; Li, J.; Shao, T. Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 2019, 75, 37–44. [Google Scholar] [CrossRef]
- McGarvey, J.; Franco, R.; Palumbo, J.; Hnasko, R.; Stanker, L.; Mitloehner, F. Bacterial population dynamics during the ensiling of Medicago sativa (alfalfa) and subsequent exposure to air. J. Appl. Microbiol. 2013, 114, 1661–1670. [Google Scholar] [CrossRef]
- Muck,R.E.(2010).Silage microbiology and its control through additives.Revista Brasileira de Zootecnia.p.183-191, 2010 (supl. especial).
- Elferink, S.J.W.H.O.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed. Sci. Technol. 2021, 275, 114766. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.; Li, X.; Li, X.; Shuai, Y.; Feng, G.; Ran, Q.; Cai, Y.; Li, Y.; Zhang, X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, W.; Chen, X.; Zhang, Q. Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 2021, 329, 129792. [Google Scholar] [CrossRef]
- Ávila, C.; Carvalho, B. Silage fermentation—updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2019, 128, 966–984. [Google Scholar] [CrossRef]
- Kampfer,K. ,Rainey,F.A.,Andersson,M.A.,Lassila,E.L.N.,Ulrych,U.,Busse,H.J.,Weiss,N.,Mikkola,R. Frigoribacterium faeni gen. nov., sp. nov., a novel psychrophilic genus of the family Microbacteriaceae. Int. J. Syst. Evol. Microbiol. 2000, 50, 355–363. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.A.P.; Kim, D.H.; de Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial Diversity and Composition of Alfalfa Silage as Analyzed by Illumina MiSeq Sequencing: Effects of Escherichia Coli O157:H7 and Silage Additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, B.; Nishino, N.; Wang, X.; Yu, Z. Fermentation and microbial population dynamics during the ensiling of native grass and subsequent exposure to air. Anim. Sci. J. 2016, 87, 389–397. [Google Scholar] [CrossRef]
- Thomas, T.A. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- You, S.; Du, S.; Ge, G.; Wan, T.; Jia, Y. Selection of lactic acid bacteria from native grass silage and its effects as inoculant on silage fermentation. Agron. J. 2021, 113, 3169–3177. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Sun, L.; Hou, M.; Hao, J.; Lu, Q.; Liu, T.; Ren, X.; Jia, Y.; Wang, Z.; Ge, G. Effects of different harvest frequencies on microbial community and metabolomic properties of annual ryegrass silage. Front. Microbiol. 2022, 13, 971449. [Google Scholar] [CrossRef] [PubMed]

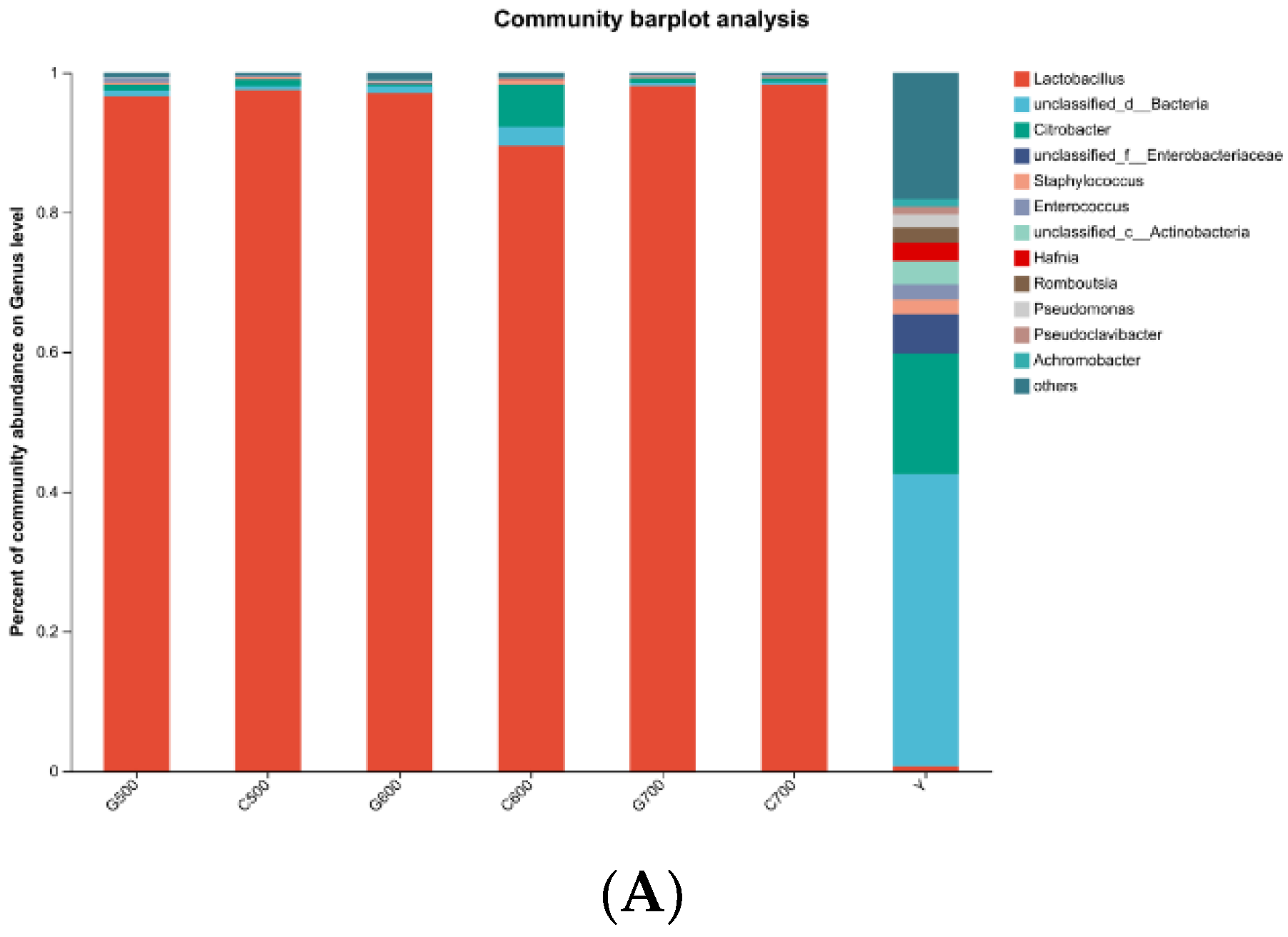
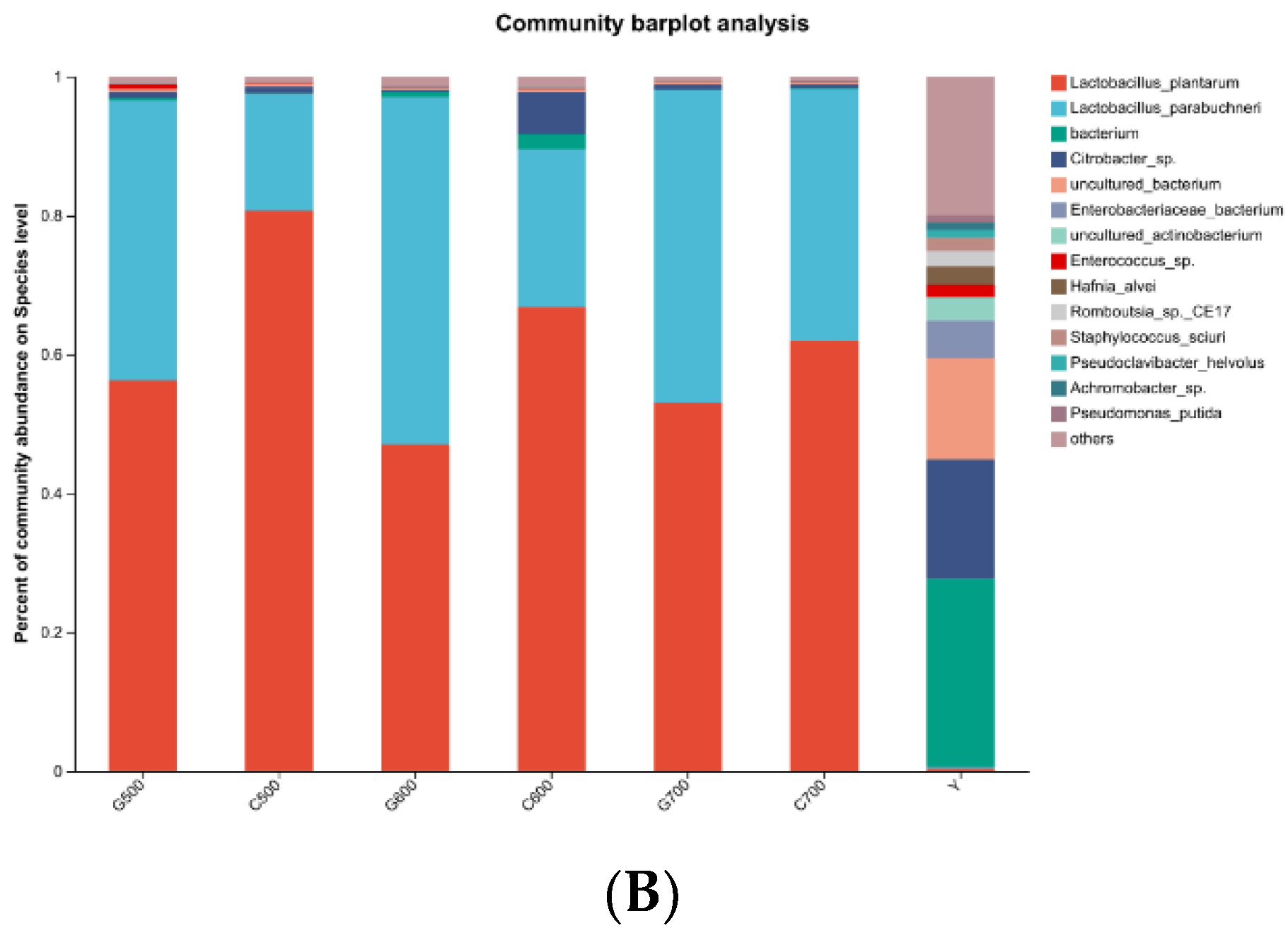
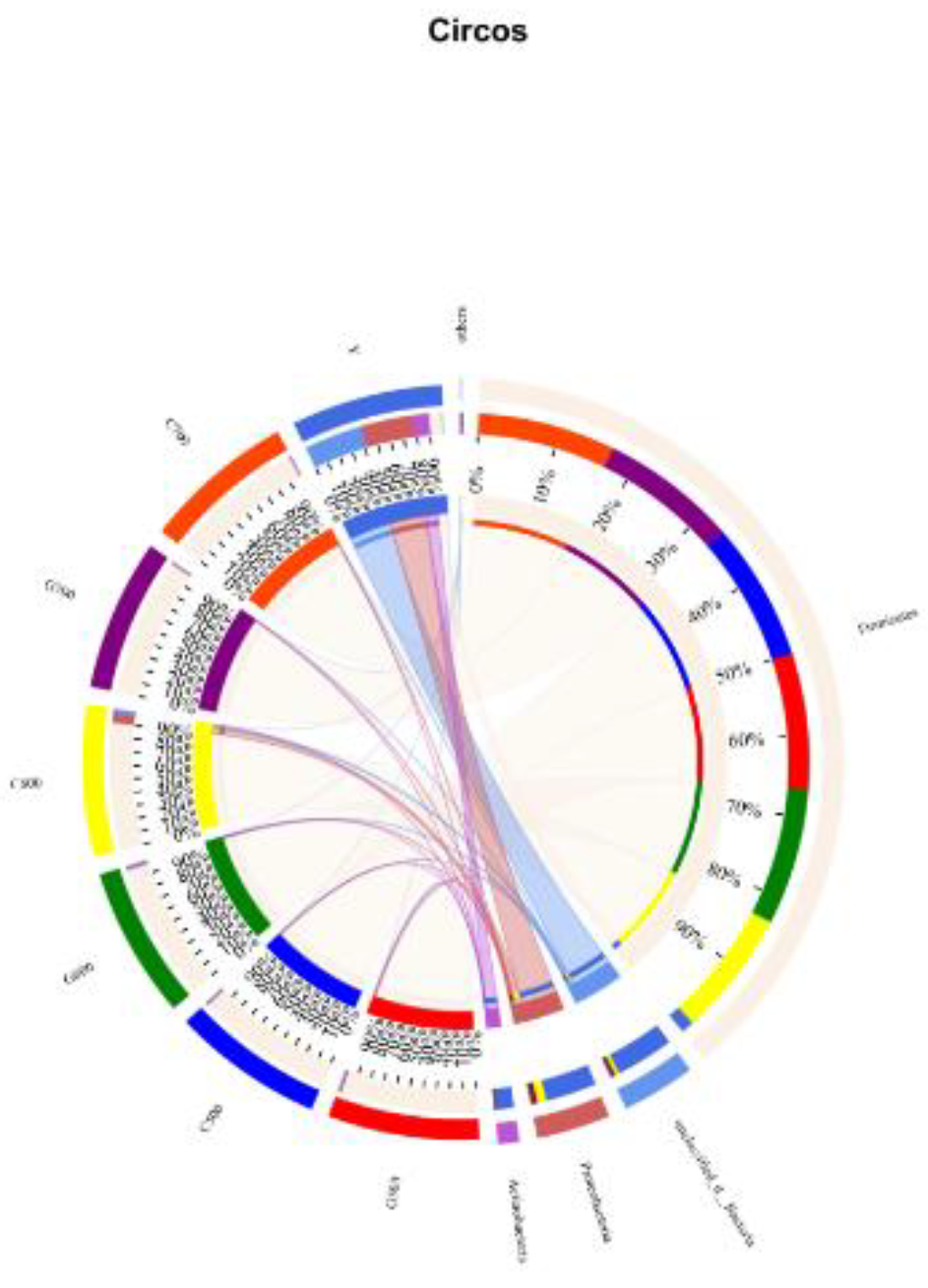
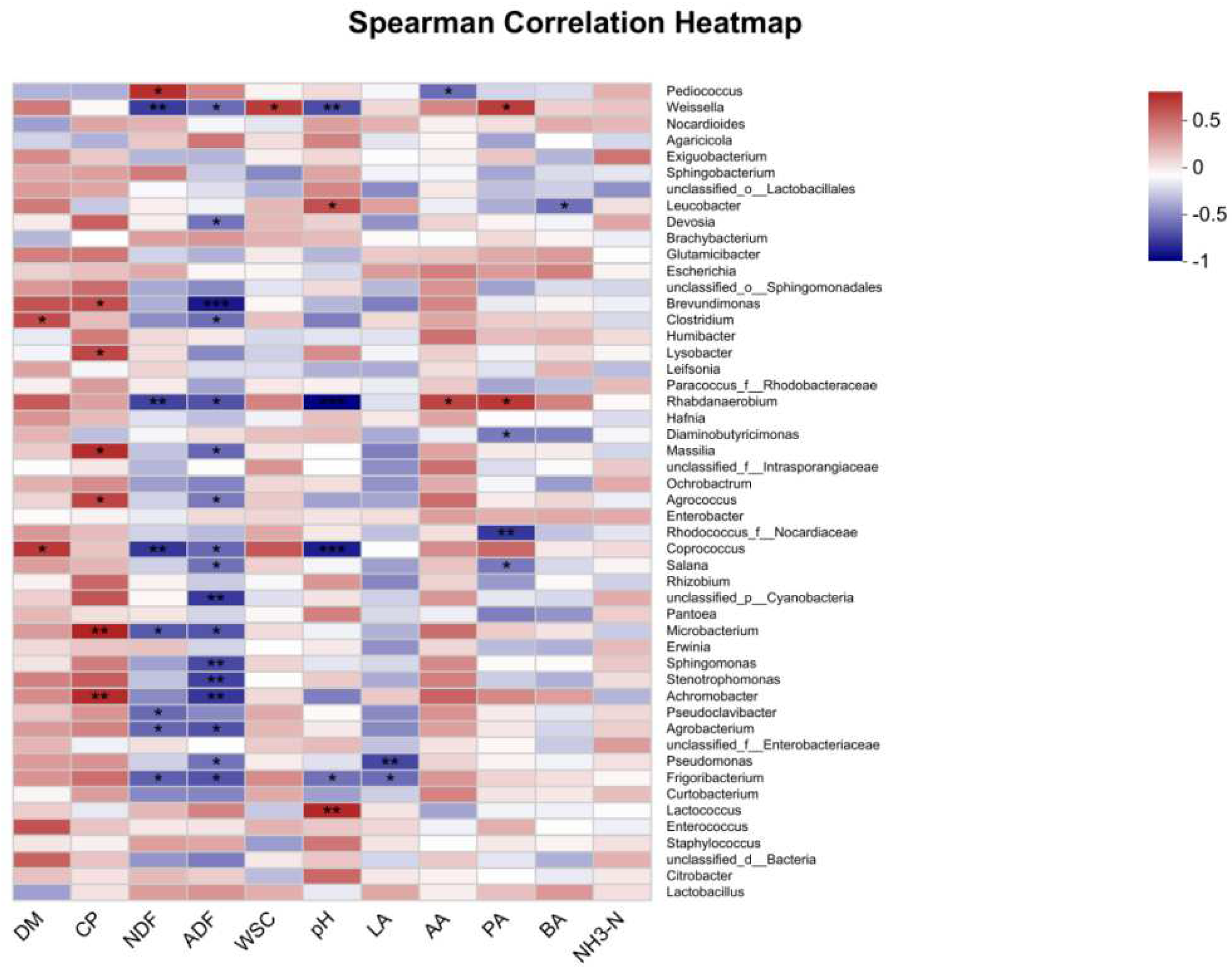
| Items | Stipa grandis |
| Dry matter (g/kg FW) | 42.57±0.98 |
| Crude protein (g/kg DM) | 20.52±1.23 |
| Neutral detergent fiber (g/kg DM) | 38.29±1.54 |
| Acid detergent fiber (g/kg DM) | 31.30±0.71 |
| Water-soluble carbohydrates (g/kg DM) | 1.59±0.05 |
| LAB (log10 cfu/g FM) | 5.41±1.87 |
| Aerobic bacteria (log10 cfu/g FM) | 6.33±0.43 |
| Coliform bacteria (log10 cfu/g FM) | 2.77±2.66 |
| Yeasts (log10 cfu/g FM) | 5.96±0.19 |
| Mold (log10 cfu/g FM) | 3.16±0.28 |
| Temperature | 500 | 600 | 700 | SEM | Temperature | Density | The interaction effect of temperature and density | |
| DM (%) | G | 39.33±0.73Aa | 40.00±1.2Aa | 35.09±1.46Ba | 0.500 | p=0.9083 | s<0.0001 | p*S=0.4116 |
| C | 39.28±0.40Aa | 39.16±0.93Aa | 35.82±0.48Ba | p<0.0001 | ||||
| Cp (% DM) | G | 19.4±4.68Aa | 21.75±1.42Aa | 20.56±1.65Aa | 0.473 | p=0.6597 | s=0.4891 | p*S=0.8245 |
| C | 20.86±0.69Aa | 21.73±0.22Aa | 20.6±1.13Aa | p=0.4891 | ||||
| ADF (% DM) | G | 32.87±2.68Ba | 31.82±0.86Bb | 38.39±0.91Aa | 0.611 | p=0.0217 | s=0.0217 | p*s=0.0073 |
| C | 36.88±0.80Aa | 35.26±0.83Ba | 36.36±0.56ABb | p=0.0027 | ||||
| NDF (% DM) | G | 37.29±1.79Ba | 36.75±0.61Ba | 40.04±0.20Ab | 0.45 | p=0.0211 | s=0.0484 | p*s=0.4067 |
| C | 39.15±0.41Aa | 39.87±2.79Aa | 40.76±0.29Aa | p=0.0484 | ||||
| EE(% DM) | G | 1.27±0.27Ab | 1.53±0.29Aa | 1.72±0.04Ab | 0.079 | p=0.0183 | s=0.2025 | p*s=0.2331 |
| C | 1.92±0.25Aa | 1.67±0.48Aa | 1.97±0.02Aa | p=0.2025 | ||||
| WSC (% DM) | G | 1.37±0.03Aa | 1.21±0.20Aa | 1.14±0.22Aa | 0.035 | p=0.1825 | S=0.0065 | p*s=0.8064 |
| C | 1.29±0.07Aa | 1.19±0.09Aa | 1.05±0.04Ba | p=0.0065 | ||||
| pH | G | 4.59±0.02Ab | 4.56±0.04Ab | 4.69±0.07Aa | 0.036 | p<0.0001 | s=0.1924 | p*s=0.4116 |
| C | 4.87±0.01Aa | 4.84±0.06Aa | 4.89±0.05Aa | |||||
| Lactic acid (g/kg) | G | 0.27±0.07Aa | 0.00±0.00Ba | 0.07±0.07Ba | 0.032 | p=0.2413 | s=0.1569 | p*s=0.0656 |
| C | 0.13±0.03Aa | 0.13±0.09Aa | 0.27±0.07Aa | |||||
| Acetic acid (g/kg) | G | 0.60±0.31Aa | 0.33±0.03Aa | 0.37±0.13Aa | 0.061 | p=0.0758 | s=0.7140 | p*s=0.5474 |
| C | 0.17±0.09Aa | 0.23±0.03Aa | 0.17±0.03Aa | |||||
| propionic acid (g/kg) | G | 0.40±0.06Aa | 0.40±0.23Aa | 0.43±0.15Aa | 0.05 | p=0.0837 | S=0.9857 | p*S=0.9050 |
| C | 0.27±0.12Aa | 0.23±0.03Aa | 0.20±0.10Aa | |||||
| Butyric acid (g/kg) | G | 0.00±0.00Aa | 0.10±0.10Aa | 0.20±0.12Aa | 0.028 | p=0.0563 | s=0.2589 | p*s=0.2589 |
| C | 0.00±0.00Aa | 0.00±0.00Aa | 0.00±0.00Aa | |||||
| NH3-N | G | 4.40±0.15Aa | 4.73±0.94Aa | 4.07±0.18Aa | 0.148 | p=0.7395 | s=0.9713 | p*s=0.3757 |
| C | 4.23±0.07Ba | 4.07±0.03Ca | 4.57±0.03Aa | |||||
| LAB (log10 cfu/g FM) | G | 4.37±2.19Aa | 4.89±0.04Ab | 4.68±1.03Aa | 0.422 | p=0.0938 | s=0.8155 | p*s=0.9201 |
| C | 6.22±0.61Aa | 6.92±0.37Aa | 5.85±0.46Aa | |||||
| Aerobic bacteria (log10 cfu/g FM) | G | 5.28±1.21Aa | 3.80±0.63Aa | 4.35±0.73Aa | 0.309 | p=0.0527 | s=0.3687 | p*s=0.4100 |
| C | 5.46±0.31Aa | 5.21±0.55Aa | 6.37±0.23Aa | |||||
| Yeasts (log10 cfu/g FM) | G | 4.34±0.03Ab | 4.81±0.15Ab | 5.20±0.67Aa | 0.238 | p<0.0001 | s=0.3266 | p*s=0.5158 |
| C | 6.38±0.08Aa | 6.55±0.25Aa | 6.49±0.08Aa | |||||
| Coliform bacteria (log10 cfu/g FM) | G | ND | ND | ND | ||||
| C | ND | ND | ND | |||||
| Mold (log10 cfu/g FM) | G | ND | ND | ND | ||||
| C | ND | ND | ND |
| Sample\Estimators | ace | chao1 | shannon | sobs | coverage |
| Y | 800.55 | 764.76 | 3.39 | 728.67 | 0.998 |
| C500 | 125.13 | 103.65 | 0.63 | 64.00 | 0.999 |
| C600 | 108.39 | 106.94 | 0.89 | 85.67 | 0.999 |
| C700 | 102.24 | 94.36 | 0.76 | 69.00 | 0.999 |
| G500 | 184.05 | 147.27 | 0.88 | 99.33 | 0.999 |
| G600 | 148.02 | 136.74 | 0.82 | 103.00 | 0.999 |
| G700 | 110.37 | 102.20 | 0.81 | 74.00 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





