Submitted:
08 January 2024
Posted:
09 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Parasites and culture
2.2. Mammalian cell lines’ culture
2.3. Antiparasitic assays
2.3.1. Antikinetoplastid activity
Assay of Trypanosoma brucei bloodstream and Leishmania donovani promastigote inhibition
Antiamastigote assay
2.3.2. Antiplasmodial assay
2.4. Cytotoxicity assay
2.5. In silico prediction of physicochemical and pharmacokinetic properties
2.6. Data analysis
3. Results and discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; Abraham, J.; Ackerman, I.; Aggarwal, R.; Ahn, S.Y.; Ali, M.K.; AlMazroa, M.A.; Alvarado, M.; Anderson, H.R.; Lopez, A.D. and Collaborators. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 2012, 380 (9859), 2197-2223. [CrossRef]
- The World Health Organization (WHO). (2023a). Malaria. https://www.who.int/news-room/fact-sheets/detail/malaria.
- The World Health Organization (WHO) (2022). World Malaria Report 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022.
- The World Health Organization (WHO) (2021). WHO recommends groundbreaking malaria vaccine for children at risk. https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk.
- The World Health Organization (WHO) (2010). First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases. https://www.who.int/publications/i/item/9789241564090.
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. (2017). Leishmaniasis: A review. F1000 Research, 6 (May), 1-15.
- The World Health Organization (WHO) (2023b). Leishmaniasis. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- Silva-Jardim, I.; Thiemann, O.H.; Anibal, F.F. (2014). Leishmaniasis and Chagas disease chemotherapy: A critical review. J. Braz. Chem. Soc. 25 (10), 1810-1823. [CrossRef]
- The World Health Organization (WHO) (2023c). Trypanosomiasis (Sleeping sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness).
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology, 2016, 143 (14), 1862-1889. [CrossRef]
- Food and Agriculture Organization (FAO) (2023). Programme Against Africa, Trypanosomiasis. https://www.fao.org/paat/the-programme/the-disease/en/.
- Fairlamb, A.H. Chemotherapy of human African trypanosomiasis: Current and future prospects. Trends Parasitol. 2003, 19 (11), 488-494. [CrossRef]
- Betu Kumeso, V.K.; Kalonji, W.M.; Rembry, S.; Mordt, O.V.; Tete, D.N.; Prêtre, A.; Delhomme, S.; Kyhi, M.I.W.; Camara, M.; Catusse, J.; Schneitter, S.; Nusbaumer, M.; Miaka, E.M.; Mbembo, H.M.; Mayawula, J.M.; Camara, M..; Massa, F.A.; Badibabi, L.K.; Bonama, A.K.; Lukula, P.K.; Kalonji, S.M.; Philemon, P.M.; Nganyonyi, R.M.; Mankiara, H.E.; Nguba, A.A.A.; Muanza, V.K.; Nasandhel, E.M.; Bambuwu, A.F.N.; Scherrer, B.; Strub-Wourgaft, N.; Tarral, A. Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: a multicenter, open-label, single-arm, phase 2/3 trial. Lancet Infect. Dis. 2023, 23 (4), 463-470.
- Christensen, S.B. Natural products that changed society. Biomedicines 2021, 9(5), 472. [CrossRef]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The birth of artemisinin. Pharmacol. Ther. 2020, 216:107658. [CrossRef]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67 (11), 2576-2597. [CrossRef]
- Pinto-Martinez, A.K.; Rodriguez-Durán, J.; Serrano-Martin, X.; Hernandez-Rodriguez, V.; Benaim, G. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob. Agents Chemother. 2017, 62(1), e01614-17. [CrossRef]
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazué, G.; Bray, M.A.; Pécoul, B. Fexinidazole--a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4(12):e923, 1-15. [CrossRef]
- Bilsland, E.; van Vliet, L.; Williams, K.; Feltham, J.; Carrasco, M.P.; Fotoran, W.L.; Cubillos, E.F.G.; Wunderlich, G.; Grøtli, M.; Hollfelder, F.; Jackson, V.; King, R.D.; Oliver, S.G. 2018. Plasmodium dihydrofolate reductase is a second enzyme target for the antimalarial action of triclosan. Sci. Rep. 2018, 8:1038, 1-8. [CrossRef]
- Shamshad, H.; Bakri, R.; Mirza, A.Z. Dihydrofolate reductase, thymidylate synthase, and serine hydroxy methyltransferase: successful targets against some infectious diseases. Mol. Biol. Rep. 2022, 49(7), 6659-6691. [CrossRef]
- Ellekvist, P.; Mlambo, G.; Kumar, N.; Klaerke, D.A. Functional characterization of malaria parasites deficient in the K+ channel Kch2. Biochem. Biophys. Res. Commun. 2017, 493 (1) 690-696. [CrossRef]
- Serrano-Martín, X.; Payares, G.; Mendoza-León, A. Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2006, 50 (12) 4214-4216. [CrossRef]
- Schmidt, R.S.; Macedo, J.P.; Steinmann, M.E.; Salgado, A.G.; Butikofer, P.; Sigel, E.; Rentsch, D.; Maser, P. Transporters of Trypanosoma brucei-phylogeny, physiology, pharmacology. The FEBS Journal. 2018, 285, 1012-1023.
- Müller, J.; Hemphill, A. Drug target identification in protozoan parasites Drug target identification in protozoan parasites. Expert Opin. Drug Discov. 2016, 11(8), 815-824.
- Kotake, Y.; Iijima, A.; Yoshimatau, K.; Tamai, N.; Ozawa, Y.; Koyanagi, N.; Kitoh, K.; Nomura, H. Synthesis and antitumor activities of novel 6-5 fused ring heterocycle antifolates: N-[4-[o-(2-amino-4-substituted-6,7-dihydrocyclopenta[~pyrimidin-5-yl)alkyl]benzoyl]-L-glutamic Acids. J. Med. Chem., 1994, 37(11), 1616-1624.
- Kotake, Y.; Okauchi, T.; Iuima, A.; Yoshimatau, K.; Nomura, H. Novel 6-5 fused ring heterocycle antifolates with potent antitumor activity: bridge modifications and heterocyclic Benzoyl isosters of 2,4-diamino-6.7-difydro-5H-cyclopenta[d]pyrimidine antifolate. Chem. Pharm. Bull. 1995, 43 (5), 829-841. [CrossRef]
- Mcguire, J.J.; Bergoltz, V.V.; Heitzman, K.J.; Haile, W.H.; Russell, C.A., Bolanowska, E. Novel 6,5-fused ring heterocyclic antifolates : Biochemical and biological characterization. Cancer Res. 1994, 54, 2673-2680.
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science, 1976, 193 (4254), 673-675. [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75 (6), 985-989. [CrossRef]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human african trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262-270. [CrossRef]
- Siqueira-Neto, J.L.; Song, O.R.; Oh, H.; Sohn, J.H.; Yang, G.; Nam, J.; Jang, J.; Cechetto, J.; Lee, C. B.; Moon, S.; Genovesio, A.; Chatelain, E.; Christophe, T.; Freitas-Junior, L.H. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl. Trop. Dis. 2010, 4 (5), 1-9. [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. Journal of Visualized Experiments (JoVE) 2012, 70, 1-14.
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48 (5), 1803-1806. [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58 (9), 4066-4072. [CrossRef]
- Andrade, E.L.; Bento, A.F.; Cavalli, J.; Oliveira, S.K.; Freitas, C.S.; Marcon, R.; Schwanke, R.C.; Siqueira, J.M., Calixto, J.B. Non-clinical studies required for new drug development – Part I : early in silico and in vitro studies , new target discovery and validation , proof of principles and robustness of animal studies. Braz. J. Med. Biol. Res. 2016, 49, 1-9.
- Gangjee, A.; Kurup, S.; Namjoshi, O. Dihydrofolate reductase as a target for chemotherapy in parasites. Curr. Pharm. Des. 2007, 13(6), 609-639. [CrossRef]
- Sharma, M.; Chauhan, M. Dihydrofolate reductase as a therapeutic target for infectious diseases : opportunities and challenges. Future Med. Chem. 2012, 4 (10), 1335-1365.
- Choudhury, A.A.K.; Vinayagam, S.; Adhikari, N.; Ghosh, S.K.; Sattu, K. Microwave synthesis and antimalarial screening of novel 4-amino benzoic acid (PABA)-substituted pyrimidine derivatives as Plasmodium falciparum dihydrofolate reductase inhibitors. 3 Biotech. 2022, 12(8), 170. [CrossRef]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR inhibitors: Reading the past for discovering novel anticancer agents. Molecules. 2019, 24(6) 1140. [CrossRef]
- Wróbel, A.; Drozdowska, D. Recent design and structure-activity relationship studies on the modifications of DHFR inhibitors as anticancer agents. Curr. Med. Chem. 2021, 28(5), 910-939. [CrossRef]
- Yuthavong, Y.; Tarnchompoo, B.; Vilaivan, T.; Chitnumsub, P.; Kamchonwongpaisan, S.; Charman, S.A.; McLennan, D.N.; White, K.L.; Vivas, L.; Bongard, E.; Thongphanchang, C.; Taweechai, S.; Vanichtanankul, J.; Rattanajak, R.; Arwon, U.; Fantauzzi, P.; Yuvaniyama, J.; Charman, W.N.; Matthews, D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc. Natl. Acad. Sci. U S A. 2012, 109 (42), 16823-16828. [CrossRef]
- Gilbert, I.H. Inhibitors of dihydrofolate reductase in leishmania and trypanosomes. Biochim. Biophys Acta Mol. Basis Dis. 2002, 1587 (2-3), 249-257. [CrossRef]
- Teixeira, B.V.F.; Teles, A.L.B.; Silva, S.G.D.; Brito, C.C.B.; Freitas, H.F.; Pires, A.B.L.; Froes, T.Q.; Castilho, M.S. Dual and selective inhibitors of pteridine reductase 1 (PTR1) and dihydrofolate reductase-thymidylate synthase (DHFR-TS) from Leishmania chagasi. J. Enzyme Inhib. Med. Chem. 2019, 34(1), 1439-1450. [CrossRef]
- Dize, D.; Tata, R.B.; Keumoe, R.; Kouipou Toghueo, R.M.; Tchatat, M.B.; Njanpa, C.N.; Tchuenguia, V.C.; Yamthe, L.T.; Fokou, P.V.T.; Laleu, B.; Duffy, J.; Bishop, O.T.; Boyom, F.F. Preliminary structure–activity relationship study of the MMV pathogen box compound MMV675968 (2,4-diaminoquinazoline) unveils novel inhibitors of Trypanosoma brucei brucei. Molecules, 2022, 27 (19), 1-38.
- Waller, K.L.; Kim, K.; McDonald, T.V. Plasmodium falciparum: Growth response to potassium channel blocking compounds. Exp. Parasitol. 2008, 120 (3), 280-285. [CrossRef]
- Mosimann, M.; Goshima, S.; Wenzler, T.; Lu, A.; Uozumi, N.; Ma, P. (2010). A Trk/HKT-Type K+ transporter from Trypanosoma brucei. Eukaryot. Cell. 2010, 9 (4), 539-546.
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. 2015, 72, 3677-3693. [CrossRef]
- Paul, A.; Mubashra; Singh, S. Identification of a novel calcium activated potassium channel from Leishmania donovani and in silico predictions of its antigenic features. Acta Trop. 2021, 220, 105922. [CrossRef]
- Barteselli, A.; Casagrande, M.; Basilico, N.; Parapini, S.; Rusconi, C.M.; Tonelli, M.; Boido, V.; Taramelli, D.; Sparatore, F.; Sparatore, A. Clofazimine analogs with antileishmanial and antiplasmodial activity. Bioorg. Med. Chem. 2015, 23 (1), 55-65. [CrossRef]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and dengue. Phytother. Res. 2019, 33 (10), 2473-2517. [CrossRef]
- Tchatat Tali, M.B.; Boniface, P.K.; Jean Claude, T.; Fabrice, F.F. 2023. Current developments on the antimalarial, antileishmanial, and antitrypanosomal potential and mechanisms of action of Terminalia spp. S. Afr. J. Bot. 2023, 156, 309-333.
- Pérez-Silanes, S.; Berrade, L.; García-Sánchez, R.N.; Mendoza, A.; Galiano, S.; Pérez-Solórzano, B.M.; Nogal-Ruiz, J.J.; Martínez-Fernández, A.R.; Aldana, I.; Monge, A. New 1-aryl-3-substituted propanol derivatives as antimalarial agents. Molecules. 2009, 14(10), 4120-4135. [CrossRef]
- Ravindar, L.; Hasbullah, S.A. ; Rakesh, K.P. ; Hassan, N.I. Recent developments in antimalarial activities of 4-aminoquinoline derivatives. Eur. J. Med. Chem. 2023, 256, 115458. [CrossRef]
- Chen, X.; Li. H.; Tian. L.; Li, Q.; Luo, J.; Zhang, Y. Analysis of the physicochemical properties of Acaricides based on Lipinski's Rule of five. J. Comput. Biol. 2020, 27(9), 1397-1406.
- Rai, M.; Singh, A.V.; Paudel, N.; Kanase, A.; Falletta, E.; Kerkar, P.; Heyda, J.; Barghash, R.F.; Pratap Singh, S.; Soos, M. Herbal concoction unveiled: A computational analysis of phytochemicals' pharmacokinetic and toxicological profiles using novel approach methodologies (NAMs). Curr. Res. Toxicol. 2023, 5:100118. [CrossRef]
- Guenfoud, F.; Khaoua, O.; Cherak, Z.; Loucif, L.; Boussebaa, W.; Benbellat, N.; Laabassi, M.; Mosset, P. 2024. Synthesis, antimicrobial, DFT, and in silico pharmacokinetic profiling of nitroaldol quinoline derivatives: A comprehensive exploration for designing potential oral antibacterial agents targeting DNA-gyrase. J. Mol. Struct. 1300, 137293. [CrossRef]
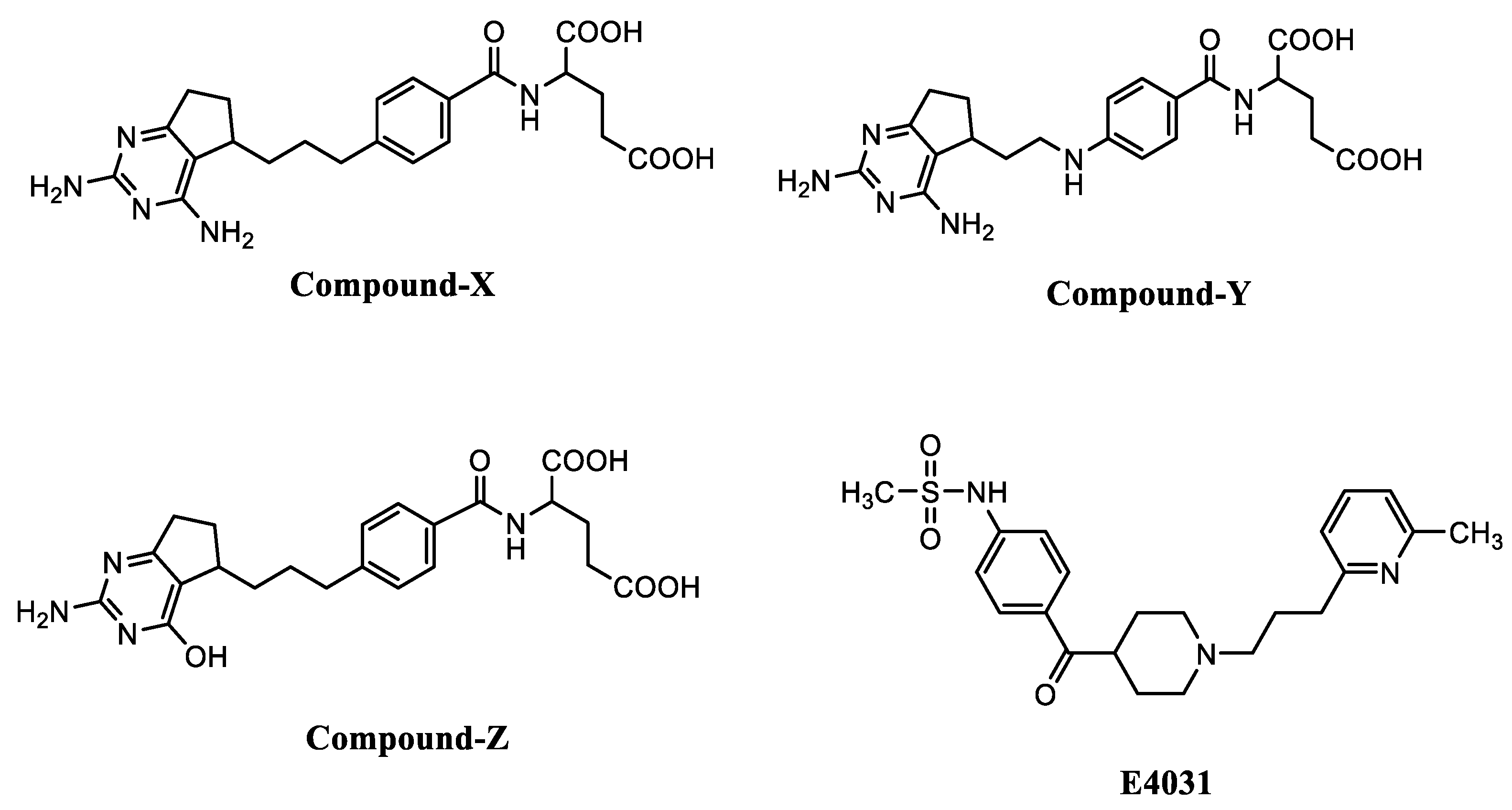
| Compounds | *IC50 (nM)_DHFR | References | ||
|---|---|---|---|---|
| Bovine liver | P388 | CCRF-CEM | ||
| X | 2.5 | 7.1 | 0.6 | [25, 26, 27] |
| Y | 5.9 | - | 0.8 | [26,27] |
| Z | 60 000 | - | - | [25] |
| Compounds ID | IC50±SD (µM) | CC 50± SD (µM) | SI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. b. brucei |
L. donovani prom |
L. donovani ama |
Pf_3D7 | ||||||||||||
| T. b. brucei |
L. donovani Prom |
L. donovani ama |
Pf_3D7 | Raw264.7 | Vero | HepG-2 | Raw264.7 | Vero HepG-2 |
Raw264.7 | Vero/ HepG-2 |
Raw264.7 | Vero/ HepG-2 |
Raw264.7 | Vero/ HepG-2 |
|
| Compound-X | 6.49±0.4 | ˃10 | NT | 0.0052 | 1.91±0.09 | ˃50 | ˃50 | 0.29 | ˃7.7 | 366.6 | ˃9596 | ||||
| Compound-Y | 0.81±0.00 | 12.47±3.04 | 4.28±0.12 | 0.028 | 33.58±5.5 | ˃50 | ˃50 | 41 | ˃61.4 | 2.69 | ˃4 | 7.85 | ˃11.7 | 1179 | ˃1756 |
| Compound-Z | ˃10 | ˃10 | NT | ˃10 | ˃50 | ˃50 | ˃50 | - | - | - | - | - | - | - | - |
| E4031 | ˃10 | ˃10 | NT | ˃10 | ˃50 | ˃50 | ˃50 | - | - | - | - | - | - | - | - |
| Pentamidine | 0.006±0.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Artemisinin | - | - | - | 0.03±0.004 | - | - | - | - | - | - | - | - | - | - | - |
| Amphotericin B | - | 20±1.63 | 247.812±24 | ND | - | - | - | - | - | - | - | - | - | - | - |
| Compounds | Compound-X | Compound-Y | Compound-Z | E4031 | |
|---|---|---|---|---|---|
| Physicochemical properties | MW | 441.488 | 442.472 | 442.476 | 415.559 |
| ClogP | 1.7415 | 1.8649 | 1.2208 | 3.28902 | |
| Rotatable Bonds | 10 | 10 | 10 | 8 | |
| Acceptors | 7 | 7 | 8 | 5 | |
| Donors | 5 | 5 | 6 | 1 | |
| TPSA | 184.604 | 184.058 | 183.789 | 172.949 | |
| Absorption | Water solubility (log mol/L) | -2.907 | -2.911 | -2.903 | -4.414 |
| Caco2 permeability (log Papp) | -0.822 | -0.758 | -0.797 | 1.093 | |
| Intestinal absorption (%) | 28.627 | 25.758 | 22.436 | 92.885 | |
| Skin Permeability (log Kp) | -2.735 | -2.735 | -2.735 | -3.35 | |
| P-gp substrate | Yes | Yes | Yes | Yes | |
| P-gp I inhibitor | No | No | No | No | |
| P-gp II inhibitor | No | No | No | Yes | |
| Distribution | VDss (human) (log L/kg) | -0.62 | -0.25 | -0.224 | 0.677 |
| Fraction unbound | 0.282 | 0.204 | 0.369 | 0.286 | |
| BBB permeability (log BB) | -1.544 | -1.67 | -1.649 | -0.448 | |
| CNS permeability (log PS) | -3.528 | -3.545 | -3.654 | -2.916 | |
| Metabolism | CYP2D6 substrate | No | No | Yes | No |
| CYP3A4 substrate | No | No | No | Yes | |
| CYP1A2 inhibitior | No | No | No | No | |
| CYP2C19 inhibitior | No | No | No | No | |
| CYP2C9 inhibitior | No | No | No | No | |
| CYP2D6 inhibitior | No | No | No | No | |
| CYP3A4 inhibitior | No | No | No | Yes | |
| Excretion | Total Clearance (log ml/min/kg) | 0.689 | 0.722 | 0.481 | 0.744 |
| Renal OCT2 substrate | No | No | No | No | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





