1. Introduction
Since late 2021, the SARS-CoV-2 epidemiologic landscape has been dominated by successive cycles of emergent Omicron sublineages [
1], some with the ability to evade infection or vaccine-acquired host immunity [
2]. In August 2022, Omicron sublineages XBB and XBB.1 were identified [
3], and by early 2023, Omicron XBB.1.5 was predominant globally [
4]. Omicron sublineages continue to emerge: in October 2023, EG.5.1 was predominant globally and as of the end of November 2023, BA.2.86 and JN.1 are expanding rapidly [
5,
6,
7]. Because vaccination remains critical to protect against the serious consequences of COVID-19 [
8], vaccines will likely need to continue to adapt to this ongoing antigenic drift [
2,
9].
BNT162b2 is an mRNA-based COVID-19 vaccine that, as of December 2022, had been approved or authorized in more than 149 countries for individuals from 6 months of age [
10,
11,
12]. The original version of BNT162b2 encodes the ancestral Wuhan-Hu-1 strain spike glycoprotein [
13], and provided protection against COVID-19 caused by the SARS-CoV-2 ancestral strain and early variants, especially against severe disease [
14,
15,
16]. The original vaccine was less protective against the Omicron variant [
17,
18], and Omicron-adapted vaccines, which contained mRNA components for the ancestral strain of the virus in combination with those encoding the Omicron BA.4/BA.5 spike protein were subsequently widely introduced for the 2022 season [
19]. Omicron BA.4/BA.5-adapted mRNA vaccines provide early effective protection against Omicron-related COVID-19, including against the XBB/XBB.1.5 sublineage [
20,
21]. Moreover, an additional BA.4/BA.5-adapted mRNA vaccine dose has been reported to have higher efficacy against severe Omicron-related illness than the original vaccine [
22], indicating that better strain-matched vaccines improve protection against COVID-19. However, waning effectiveness of COVID-19 vaccines has been observed 2 to 6 months after vaccination, including against severe disease [
22,
23,
24].
In response to the continued evolution of SARS-CoV-2 Omicron sublineages, in May and June 2023, the World Health Organization and the US Food and Drug Administration (FDA), respectively, recommended that an XBB.1 monovalent variant vaccine be developed for the 2023/2024 season [
25,
26]. One approach suggested by the FDA was to use an XBB.1 descendent virus, such as XBB.1.5 [
25]. Subsequently, in September 2023, the FDA authorized an updated monovalent BNT162b2 vaccine encoding the viral spike protein of SARS-CoV-2 Omicron XBB.1.5 (XBB.1.5-adapted BNT162b2) for use in individuals ≥6 months of age [
11,
27].
The aim of this analysis is to provide 1-month safety and preliminary immunogenicity data from a clinical trial investigating the safety and immunogenicity of the XBB.1.5-adapted BNT162b2. The trial consists of 2 substudies; 1 in vaccine-experienced individuals and the other in previously unvaccinated individuals. Here we present results in a population of vaccine-experienced individuals.
2. Materials and Methods
2.1. Study Design and Participants
In this ongoing, open-label, phase 2/3 study, participants received a single, 30-μg dose of XBB.1.5-adapted BNT162b2 (ClinicalTrials.gov NCT05997290). It was planned that approximately 400 participants would be enrolled (200 participants 12 to 55 years of age [including ≤50 participants 12- to 17-years] and 200 participants >55 years of age). This report provides 1 month safety data and preliminary immunogenicity data from a substudy that included only vaccine-experienced individuals. The study enrolled healthy individuals who were at least 12 years old and who had previously received at least 3 doses of an mRNA COVID-19 vaccine that was authorized by the US Food and Drug Administration. The most recent dose must have been the bivalent Omicron BA.4/BA.5-adapted vaccine, given at least 150 days before study vaccination. Immunocompromised individuals, those with a history of severe reaction associated with vaccination, and pregnant and breastfeeding individuals were excluded.
An institutional review board or independent ethics committee reviewed and approved relevant study documents including the protocol. Study conduct adhered to the Declaration of Helsinki principles, the Council for International Organizations of Medical Sciences international ethical guidelines, and all applicable laws and regulations. Written informed consent was obtained from all participants; if a child or adolescent participant’s parent(s) or legal guardian(s) provided consent, the participant’s assent was also obtained if the participant was capable of providing assent. If study participants reached adulthood or the age of assent (per local institutional review board or ethics committee requirements) during the study, the child or adolescent then provided the appropriate consent or assent to document their willingness to continue in the study.
2.2. Objectives, Endpoints, and Assessments
Describing the tolerability and safety profile of XBB.1.5-adapted BNT162b2 at the 30-μg dose level in vaccine-experienced ≥12-year-old participants was the primary safety objective. The associated tolerability and safety endpoints included the proportions of participants reporting local reactions and systemic events up to 7 days following vaccination, adverse events through 1 month following vaccination, and serious adverse events through 6 months following vaccination. Described here are serious adverse events reported through 1 month following vaccination. Participants recorded local reaction and systemic event data using an electronic diary. If these local reactions or systemic events were not recorded in the electronic diary, they were reported as adverse events. Table S1 summarizes the grading scales used to describe the severity of local reactions and systemic events.
Protocol-specified adverse events of special interest included confirmed diagnoses of either myocarditis or pericarditis that occurred within 6 weeks after vaccination and potential menstrual cycle disturbances. Additional adverse events were reported as being of special interest as specified in a targeted medical event list that considered identified pharmacology and toxicology findings and possible class effects, from sources such as the published literature and safety data assessment signals. A participant reporting any symptom(s) possibly indicative of myocarditis or pericarditis, such as acute chest pain, shortness of breath, or palpitations, within 6 weeks after receiving the study vaccination was to undergo cardiologist evaluation for diagnosis of potential myocarditis or pericarditis. Any diagnosis of myocarditis or pericarditis would be considered an important medical event and reported as a serious adverse event. Any study participant who reported any symptoms that may indicate a disturbance of their normal menstrual cycle (including, but not exclusively, heavy menstrual bleeding, amenorrhea, irregular periods) following receipt of study intervention until 6 months after vaccination would be specifically evaluated by the investigator. The symptoms, menstrual history, and any investigative results were recorded. Surveillance occurred throughout the study for any potential COVID-19 cases in all participants and multisystem inflammatory syndrome in children (MIS-C) in participants younger than 21 years.
In this analysis, immunogenicity was evaluated in a subset (the variant neutralization subset) of 40 baseline SARS-CoV-2–positive participants (20 participants 18–55 years old and 20 participants >55 years old) who were selected at random. A qualified fluorescent focus reduction neutralization test (FFRNT) evaluated SARS-CoV-2 serum neutralization titers against Omicron XBB.1.5, EG.5.1, and BA.2.86 viruses. Immunogenicity endpoints included geometric mean titers (GMTs) 7 days and 1 month after vaccination, geometric mean fold rises (GMFRs) from before to 7 days and from before to 1 month after vaccination, and percentages of participants with seroresponses (defined as ≥4-fold rise from baseline, or ≥4 × lower limit of quantitation [LLOQ] for baseline measurements <LLOQ) 7 days and 1 month after vaccination. Seven-day immunogenicity was also evaluated in a group of 40 baseline SARS-CoV-2–positive participants who received bivalent BA.4/BA.5-adapted BNT162b2 in a previous study (NCT05472038). The 7-day and 1-month samples were tested at different times; baseline samples were retested with the 1-month samples. Participants were matched by age and sex to those in the current analysis. The inclusion criteria for the BA.4/BA.5-adapted cohort required the participant to have received 3 or 4 previous doses of original BNT162b2 30 μg with the last dose being 150 to 365 days before study vaccination.
2.3. Statistical Analysis
Descriptive statistics, which includes the counts and percentages of participants as well as the associated 95% CIs determined by the Clopper-Pearson method, are provided for each reactogenicity endpoint by age subgroup (12−17 years, 18−55 years, >55 years) and overall. Adverse events and serious adverse events were categorized using terminology of the Medical Dictionary for Regulatory Activities v26.0 and presented descriptively by age subgroup and overall. All participants receiving the study vaccine are included in the safety population.
The GMTs were calculated by exponentiating the mean and the GMFRs by exponentiating the mean of the difference of the logarithmically transformed assay results and corresponding Student t distribution-based 2-sided 95% CIs. The percentages of participants with a seroresponse are presented descriptively with associated Clopper-Pearson 95% CIs. The evaluable immunogenicity population was used for the 1-month analysis and comprised individuals from the variant neutralization subset who received the study vaccine, had at least 1 valid and determinate immunogenicity result using the blood sample which was collected within 28 to 42 days after vaccination, and who had no other clinician-determined important protocol deviations. The all-available immunogenicity population was used for the 7-day analysis and comprised participants who received the study vaccine and who had a valid and determinate post-vaccination immunogenicity result. An evaluable immunogenicity population was not defined for the 7-day analysis.
3. Results
3.1. Participants
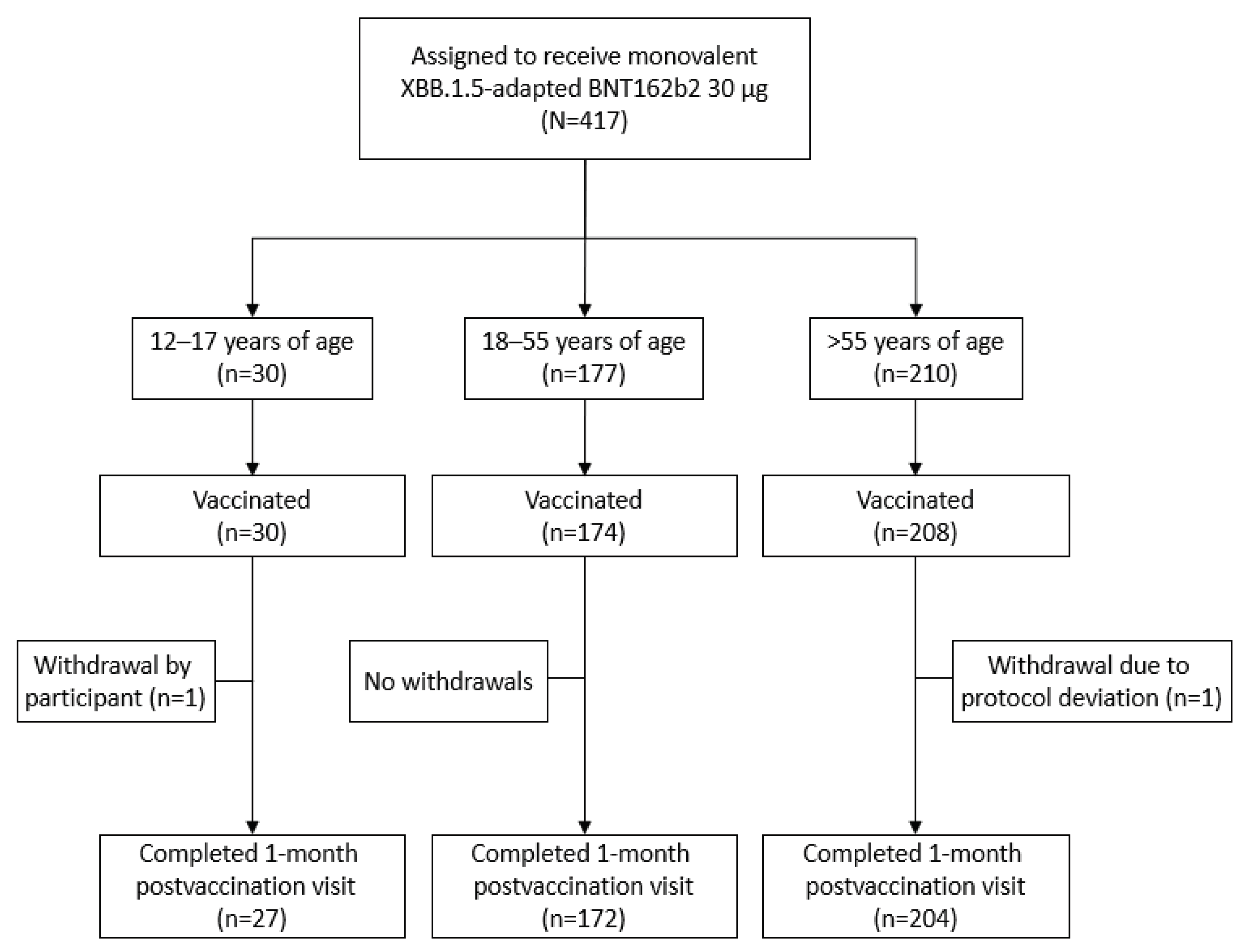
This substudy was conducted at 20 sites in the United States. From August 10, 2023, to data cutoff (September 27, 2023), 412 participants received the XBB.1.5-adapted BNT162b2 30 μg (
Figure 1; 12‒17 years of age, N=30; 18‒55 years of age, N=174; >55 years of age, N=208).
Overall, 58.7% of participants were female, 79.1% were White, 12.6% were Black, and 18.2% were Hispanic or Latino (
Table 1). Median age at vaccination was 14.0 years in the 12- to 17-year-old group, 42.0 years in the 18- to 55-year-old group, and 68.5 years in the >55-year-old group. Overall, 78.2% of participants were baseline SARS-CoV-2-positive, and the median time from the last mRNA COVID-19 vaccination to study vaccination was 303 days (range, 154‒675 days). All participants had received at least 3 previous mRNA COVID-19 vaccine doses; 92.0%, 30.1%, and 1.2% of participants had received 4, 5, or 6 previous doses, respectively. Demographic results for the variant neutralization subset are provided in Table S2.
3.2. Safety
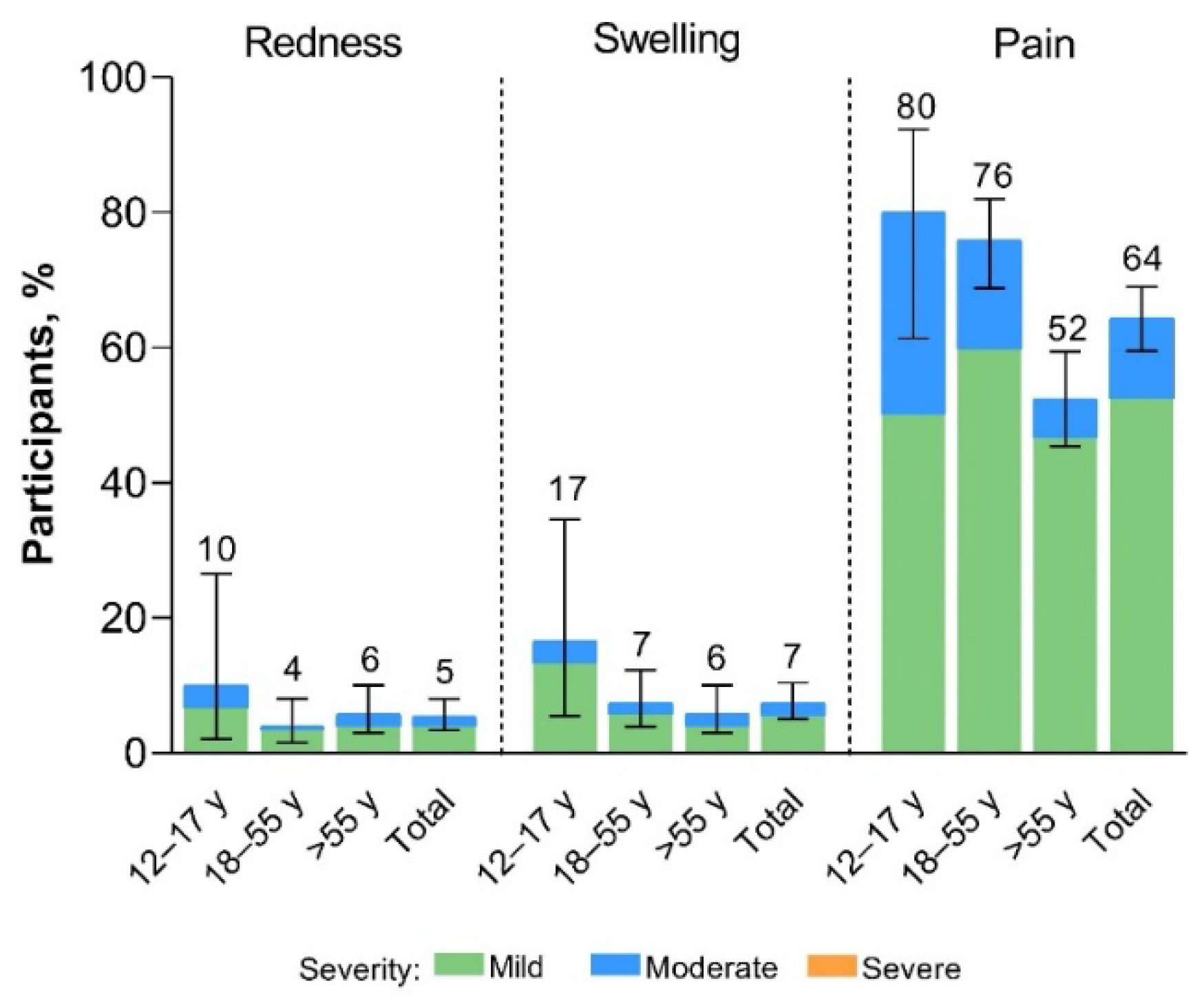
Local reactions reported within 7 days of the XBB.1.5-adapted BNT162b2 vaccination were mild to moderate in severity (
Figure 2). The most common local reaction was injection site pain, which was less frequently reported among >55-year-old participants (52%) than in 12- to 17-year-old (80%) and 18- to 55-year-old (76%) participants. Median onset and duration of local reactions was 1 to 2 days and 1 to 3 days, respectively.
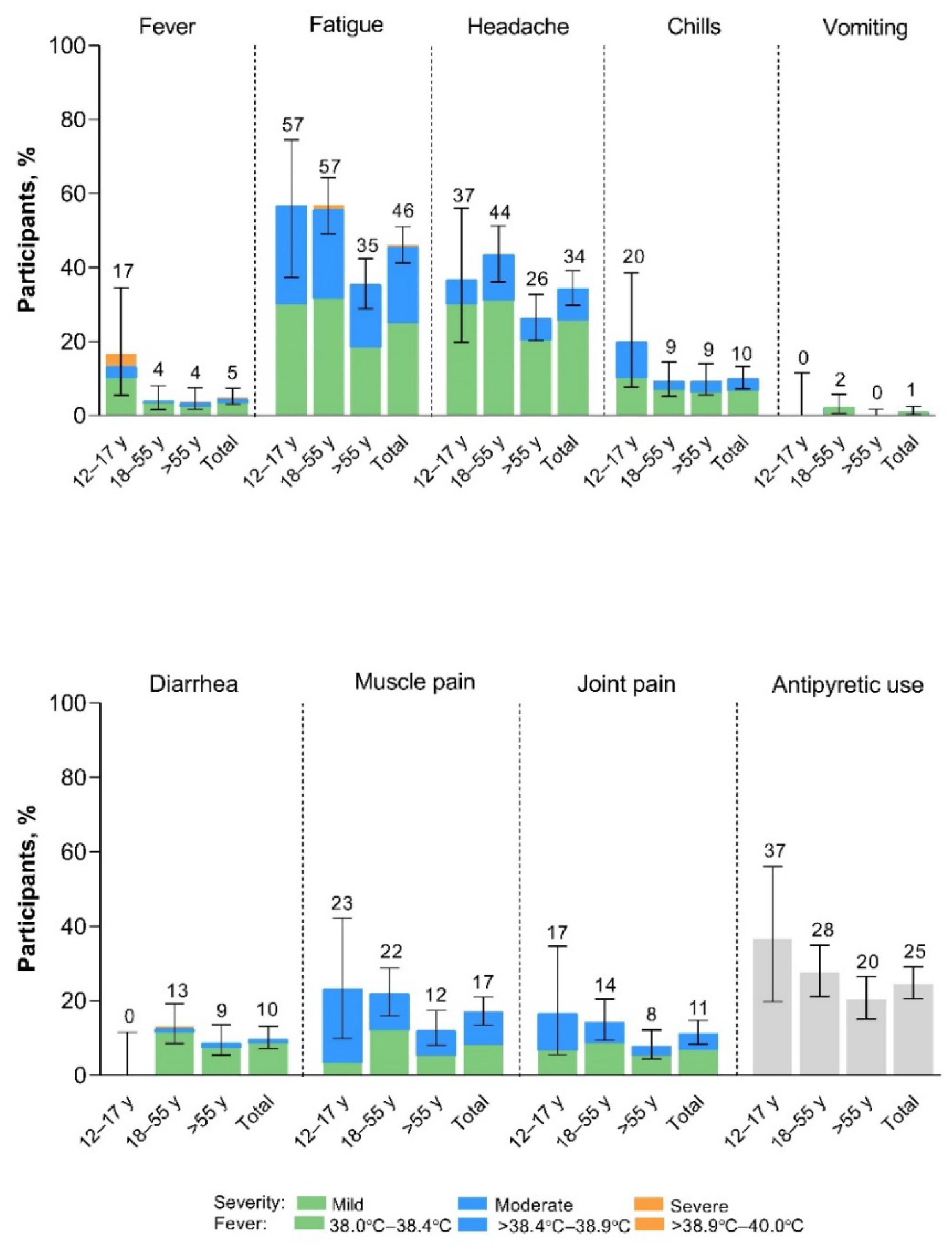
Within 7 days of vaccination with XBB.1.5-adapted BNT162b2, systemic events were predominantly mild to moderate in severity (
Figure 3). Fatigue and headache were the most common systemic events, and both were reported less frequently among >55-year-old participants (35% and 26%, respectively) than among 12- to 17-year-old (57% and 37%) and 18- to 55-year-old (57% and 44%) participants. Fever was more frequently reported in 12- to 17-year-old participants (5/30 participants [17%]) than in >18-year-old participants (15/380 [4%]); however due to the small number of 12- to 17-year-old participants included in the study, the point estimate lacks precision. Overall, 2 participants (0.5%) experienced fever >38.9°C to 40.0°C (39.1°C fever reported in the 12- to 17-year-old group and 39.0°C fever in the >55-year-old group); the fevers occurred on Day 2 after vaccination and resolved after 1 and 2 days, respectively. No fevers >40.0°C were reported. A further 3 participants reported severe systemic events, all in the 18- to 55-year-old group: 2 participants reported severe fatigue starting on Day 2 which resolved after 1 and 3 days, respectively, and 1 participant reported severe diarrhea starting Day 5 after vaccination. This event had been moderate from Day 2 after vaccination and returned to moderate severity on Day 6 but did not resolve until 20 days after onset. Median onset and duration of systemic events was 2 days and 1 to 2 days, respectively.
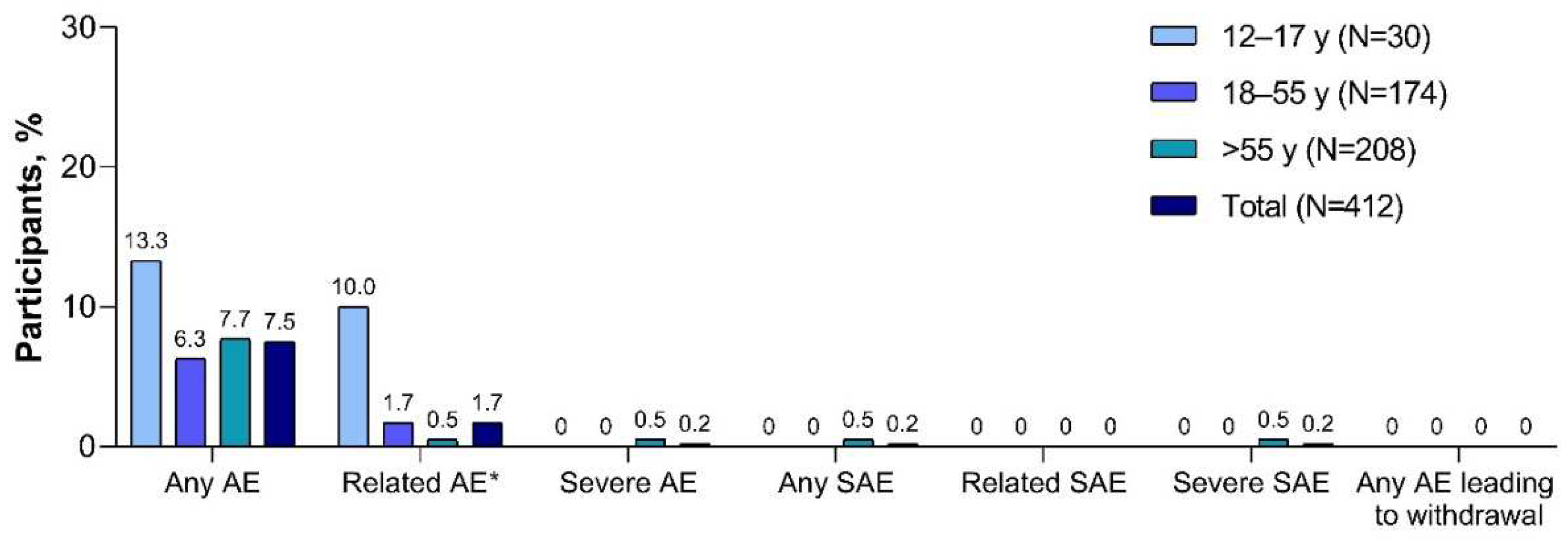
Adverse events reported within 1 month of the XBB.1.5-adapted BNT162b2 vaccination are summarized in
Figure 4. Adverse events were infrequent (7.5% in the total population) and none led to study withdrawal. One participant >55 years of age with a history of paroxysmal nocturnal hemoglobinuria (PNH) who received 5 previous doses of mRNA COVID-19 vaccine reported serious adverse events of hyponatremia, acute kidney injury, and PNH after the XBB.1.5-adapted BNT162b2 vaccination; none of the events was considered related to study vaccination as determined by the investigator. At least 1 adverse event of special interest was reported by 5 participants (1.2%) through 1 month after receipt of the XBB.1.5-adapted BNT162b2; these included single reports of arthralgia, chest discomfort, chest pain (considered possibly vaccine related by the investigator), dyspnea, heavy menstrual bleeding (considered possibly vaccine related by the investigator), and PNH (as described above). No confirmed myocarditis or pericarditis reports and no cases of severe COVID-19 or MIS-C were reported at the time of this analysis.
3.3. Immunogenicity
The evaluable immunogenicity population comprised 37 participants; 3 participants from the variant neutralization subset were excluded from this population because of protocol deviations on or before the 1 month post-vaccination visit (18- to 55-year-old group, n=1; >55-year-old group, n=2).
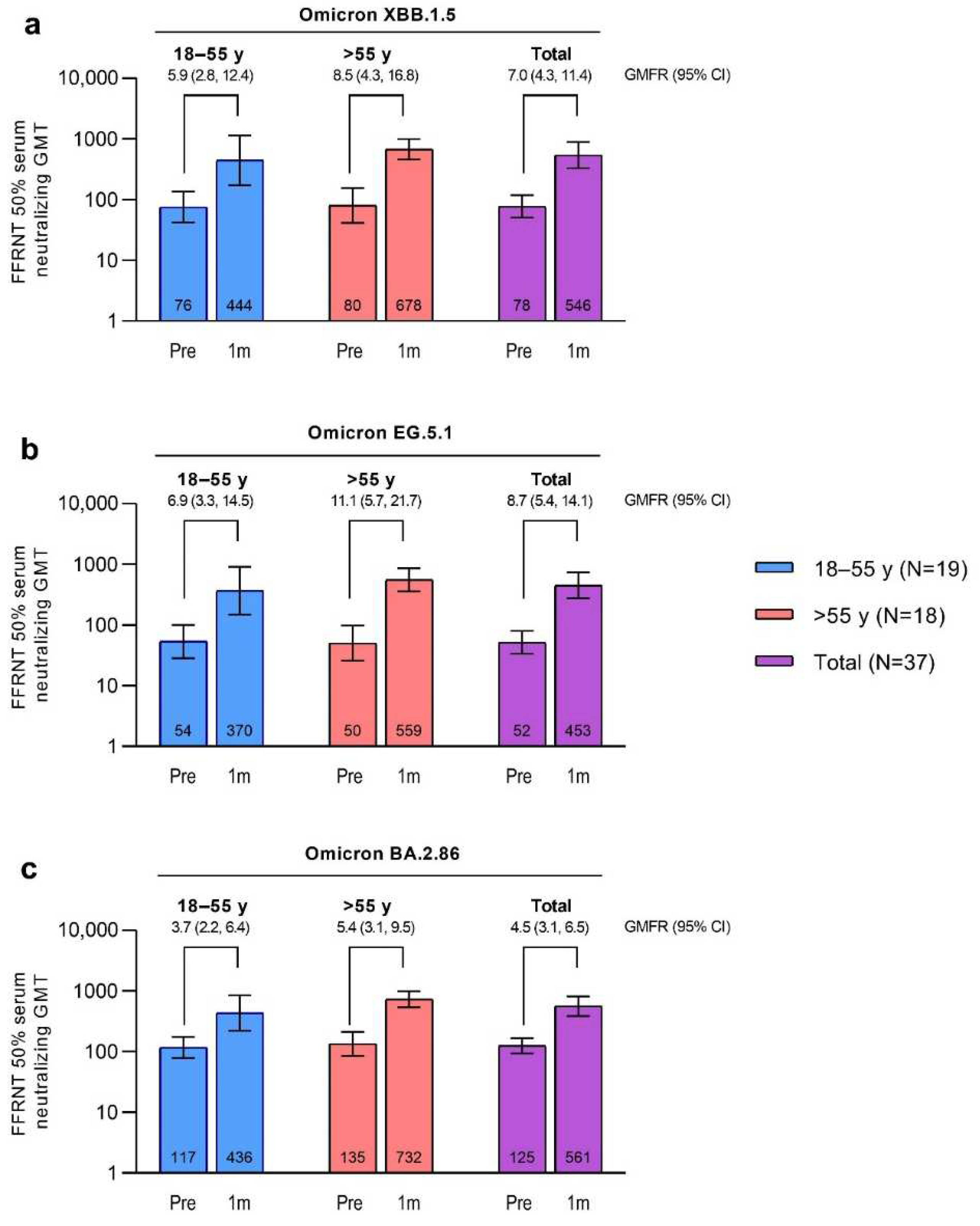
The SARS-CoV-2 FFRNT 50% neutralizing titers against Omicron XBB.1.5, EG.5.1, and BA.2.86 were increased 7 days after vaccination with the XBB.1.5-adapted BNT162b2 compared with baseline levels and numerically higher than those in the matched comparator group of participants who had received the BA.4/BA.5-adapted BNT162b2 (Figure S1). The SARS-CoV-2 FFRNT 50% neutralizing titers against Omicron XBB.1.5, EG.5.1, and BA.2.86 were further increased 1 month after vaccination with the XBB.1.5-adapted BNT162b2 compared with baseline levels (GMTs and GMFRs are shown in
Figure 5). GMTs at 1 month were similar for all 3 sublineages (GMT range overall, 452.5‒561.3). Baseline GMTs were higher for Omicron BA.2.86 compared to XBB.1.5 and EG.5.1; GMFRs from baseline to 1 month after vaccination were similar for XBB.1.5 (overall GMFR, 7.0 [95% CI, 4.3, 11.4]) and EG.5.1 (8.7 [5.4, 14.1]) and slightly lower for BA.2.86 (4.5 [3.1, 6.5]). Postvaccination titers were generally higher in participants >55 years of age (GMT range, 559.3‒732.3) compared with those 18 to 55 years of age (370.3‒444.4), while the baseline titers were generally similar in the 2 age groups.
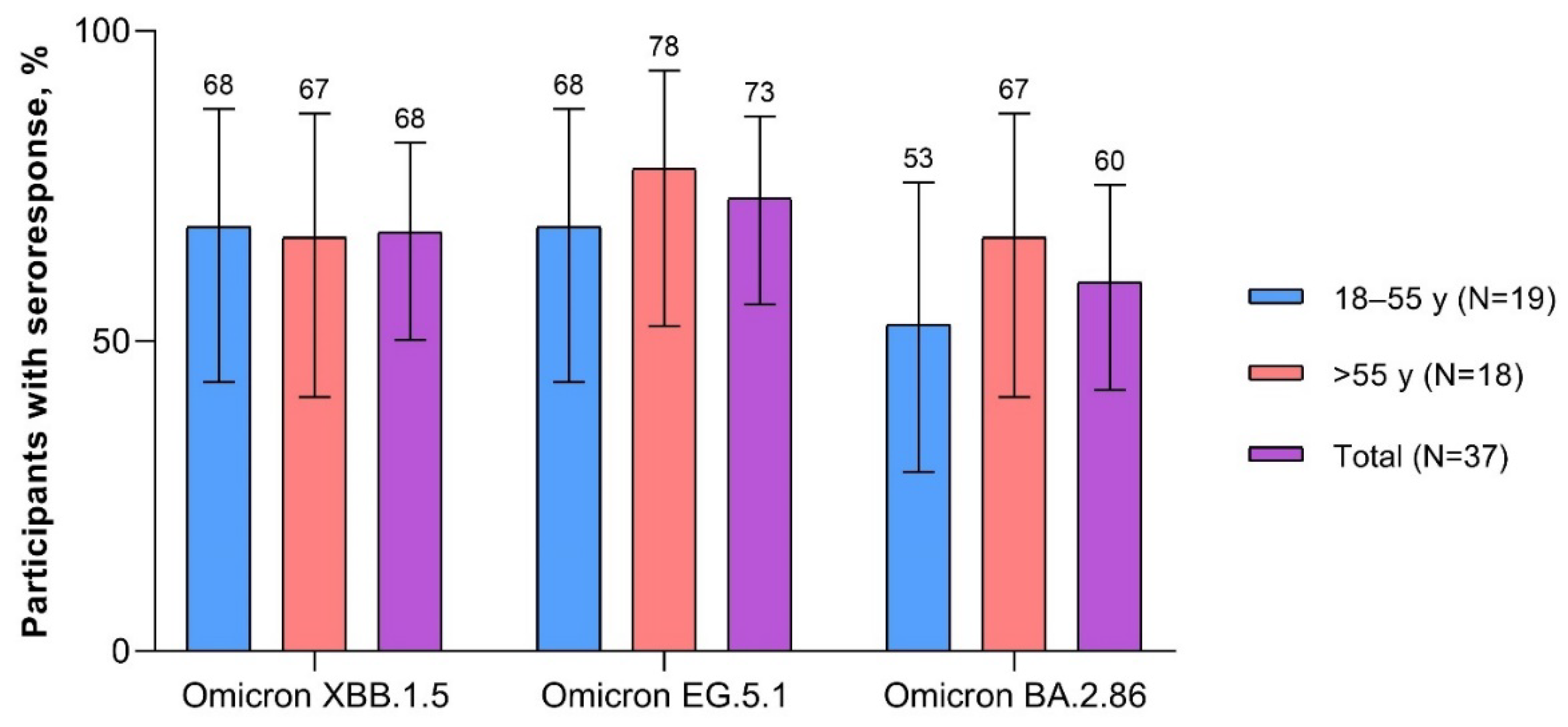
Seven days after vaccination, the overall percentage of participants with seroresponse was numerically higher after the XBB.1.5-adapted BNT162b2 (overall range 45.0%‒62.5%) than after the BA.4/BA.5-adapted BNT162b2 (17.5%‒25.0%; Figure S2). One month after vaccination with the XBB.1.5-adapted BNT162b2, the percentage of participants overall with seroresponse to Omicron XBB.1.5 and EG.5.1 were similar (67.6% and 73.0%, respectively) and slightly higher than to BA.2.86 (59.5%;
Figure 6). The percentage of participants with seroresponse to Omicron XBB.1.5 was similar in 18- to 55-year-old and >55-year-old participants (68.4% and 66.7%, respectively). The percentage of participants with seroresponse to Omicron EG.5.1 and BA.2.86 was slightly lower for 18- to 55-year-olds (68.4% and 52.6%, respectively) than for >55-year-olds (77.8% and 66.7%).
4. Discussion
In this analysis, the XBB.1.5-adapted BNT162b2 in COVID-19 vaccine-experienced individuals ≥12 years of age had a safety and tolerability profile similar to that seen with original and the BA.5/BA.5-adapted and BA.1-adapted BNT162b2 vaccines [
28,
29,
30,
31] and induced substantial increases in neutralizing responses against Omicron XBB.1.5, EG.5.1, and BA.2.86.
The Omicron sublineages have predominated the SARS-CoV-2 epidemiologic landscape since November 2021, although variability in geographic distribution has been observed and attributed to social networking patterns as well as the biological properties of the specific sublineage [
1]. The SARS-CoV-2 Omicron XBB.1.5 sublineage has increased transmissibility compared with previous circulating sublineages, most likely because of immune escape and enhanced infectivity compared with previously circulating strains [
1,
32]. Other Omicron sublineages have more recently emerged, including EG.5 and its sublineages EG.5.1, EG.5.1.1, and EG.5.2 in February 2023, BA.2.86 in July 2023, and JN.1 in August 2023 [
6,
7,
33,
34,
35]. Characterization of the biological properties of these more recent sublineages are forthcoming, including clarification on their transmissibility and virulence. Together, these observations emphasize the continued and rapid evolution of SARS-CoV-2 [
36] and underscore the importance of continued surveillance and viral characterization as well as the availability of safe and effective vaccines to protect against the serious consequences of COVID-19.
The XBB.1.5-adapted BNT162b2 vaccine was safe and tolerable, with local reactions and systemic events within 7 days of vaccination of mild to moderate severity, adverse events that were infrequent and none of which led to study withdrawal, and no confirmed reports of myocarditis or pericarditis. The safety and tolerability profile of the XBB.1.5-adapted BNT162b2 is consistent with the profiles of original BNT162b2 and the BA.4/BA.5-adapted BNT162b2 [
30,
31]. In safety surveillance from the US Centers for Disease Control (CDC) using V-safe and the CDC and FDA Vaccine Adverse Event Reporting System (VAERS) from September 22, 2021, to February 6, 2022, in individuals 18 years and older who received more than 330,000 booster doses of original BNT162b2, the rates of local reactions and systemic events were consistent with those reported in our analysis of this current substudy of a clinical trial of the XBB.1.5-adapted BNT162b2 [
30]. Additionally, in safety surveillance using the same databases in the first 7 weeks of the BA.4/BA.5-adapted BNT162b2 vaccine availability (ie, August 31 to October 23, 2022), at which time approximately 14.4 million individuals 12 years and older had received a BA.4/BA.5-adapted BNT162b2 booster dose, the frequency of local reactions and systemic events decreased with increasing age [
31]; this is consistent with our analysis of the XBB.1.5-adapted BNT162b2. Longer-term follow-up in our trial and further uptake of the XBB.1.5-adapted BNT162b2 within global vaccination programs will provide further characterization of the safety and tolerability profile of the vaccine.
In September 2022, it was estimated that more than 96% of individuals ≥16 years of age in the United States had SARS-CoV-2 antibodies from previous infection or vaccination, including 23% from infection alone, 26% from vaccination alone, and 48% from hybrid immunity [
37]. In spite of the epidemiologic setting of high population seropositivity, it is likely that vaccine-induced immunity will be reduced whenever antigenic drift causes a mismatch between the composition of COVID-19 vaccines and circulating SARS-CoV-2 strains [
9]. Encouraging data in a subset of participants in this study indicate that the XBB.1.5-adapted BNT162b2 vaccine induces robust neutralizing responses to Omicron XBB.1.5 and EG.5.1 and slightly lower but still robust responses to BA.2.86. Seven days after vaccination, GMTs, GMFRs and seroresponses were numerically higher for all 3 sublineages following the XBB.1.5-adapted BNT162b2 than for the matched participants who had received BA.4/BA.5-adapted BNT162b2. Although the analysis was not powered to detect a difference between groups, the results are encouraging and in line with a preclinical study reporting higher neutralizing titers with the XBB.1.5-adapted BNT162b2 compared with the BA.4/BA.5-adapted BNT162b2 for XBB.1.5, EG.5.1, BA.2.8 6 and other sublineages [
38]. One-month postvaccination titers against all 3 strains tended to be higher in participants >55 years of age compared with those 18 to 55 years of age; the reasons for this have not been elucidated, and the sample size is relatively small. Immunogenicity differences between age groups will be further explored when data are available for all participants.
Limitations of this study include the short follow-up for both safety and immunogenicity. However, it is important to disseminate early and timely results to understand the potential benefit of the XBB.1.5-adapted BNT162b2, especially given that vaccination with the XBB.1.5-adapted vaccine is underway. Longer-term follow-up from this and other studies is ongoing and will be supplemented with real-world effectiveness investigations. The immunogenicity analyses were conducted in a small subset of participants using an exploratory SARS-CoV-2 neutralization assay. Comprehensive immunogenicity results with a larger number of participants will be published later. Additionally, the study population was predominantly White, exclusively from the United States, and 12 years and older. Phase 3 trials of the XBB.1.5-adapted BNT162b2 are underway in children 5 to <12 years old and planned in infants and children 6 months to <5 years of age (NCT05543616). Previous trials in individuals from 6 months of age evaluating BNT162b2 variant-adapted vaccines have shown a similar safety and tolerability profile to the initial primary series with original BNT162b2 [
39,
40,
41].
In conclusion, these safety and immunogenicity data support administration of the XBB.1.5-adapted BNT162b2 in vaccine-experienced individuals ≥12 years of age. Safe and effective variant-adapted COVID-19 vaccines closely matched to circulating strains will likely remain critical to protect the vulnerable against serious COVID-19 outcomes and to reduce the burden on healthcare systems.
Author Contributions
Conceptualization, J.G, F.S.L, X.Xu, V.B, F.M, D.C, K.K, A.S.A, K.A.S, K.M, A.G, N.K; Methodology, J.G, F.S.L, X.Xu, V.B, J.Z, X.Xie, Y.H, C.L, M.C., T.B, K.M, N.K; Data Acquisition, O.D, F.S.L, J.Z, X.Xie, Y.H; Data Interpretation, O.D, F.S.L, X.Xu, V.B, F.M, J.Z, X.Xie, Y.H, K.K, A.S.A, Ö.T, U.Ş, K.A.S, K.M, A.G, N.K; Formal Analysis, X.Xu, V.B, C.L, M.C., T.B; Writing – Original Draft Preparation, J.G; Writing – Review and Editing, all authors; Project Administration, J.G. .
Funding
This study was sponsored by BioNTech and funded by Pfizer and BioNTech.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the WCG IRB (Puyallup, WA 98374, USA) on July 28, 2023 (Protocol C4591054). ClinicalTrials.gov ID: NCT05997290.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See
https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Acknowledgments
Editorial/medical writing support was provided by Sheena Hunt, PhD, and Tricia Newell, PhD, of ICON (Blue Bell, PA, USA) and was funded by Pfizer Inc. We thank the participants who volunteered for this study and the study site personnel for their contributions to this study; Cynthia Ingerick-Holt, Helen Smith, Rucha Dadhe, Pascale Nantermet, Georgina Keep, Ninika Kamra, Prajakta Chivate, Marie Hodrinsky, Pankaj Bhoir, Prathamesh Rode, Kat Vollmer, Julia Usenko, Karen Rosenzweig, Kim Cotton-West, Caroline Westgarth, Izabela Bialach-Lastowiecka, Sara Wren, Richa Kala, Naren Surampali, Jeff Lu, Carolyn Ryan from Pfizer; Shon Remich, Nadine Salisch, Nicola Charpentier, and Ruben Rizzi from BioNTech; and the Pfizer and BioNTech colleagues not named here who contributed to the success of this trial.
Conflicts of Interest
Özlem Türeci, Uǧur Şahin, and Federico Mensa are BioNTech employes and may hold stock or stock options. Özlem Türeci, Uǧur Şahin, Kena Swanson and Kayvon Modjarrad report holding an interest in a patent relevant to this manuscript. Jing Zou, Xuping Xie, and Yanping Hu have received funding from Pfizer. All other authors are Pfizer employees and may hold stock or stock options.
References
- Velavan, T.P.; Ntoumi, F.; Kremsner, P.G.; Lee, S.S.; Meyer, C.G. Emergence and geographic dominance of Omicron subvariants XBB/XBB.1.5 and BF.7 - the public health challenges. Int. J. Infect. Dis. 2023, 128, 307-309. [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162-177. [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Chopra, H.; Islam, M.A.; Saikumar, G.; Dhama, K. The SARS-CoV-2 Omicron recombinant subvariants XBB, XBB.1, and XBB.1.5 are expanding rapidly with unique mutations, antibody evasion, and immune escape properties - an alarming global threat of a surge in COVID-19 cases again? Int. J. Surg. 2023, 109, 1041-1043. [CrossRef]
- World Health Organization. XBB.1.5 Updated Risk Assessment, 20 June 2023. Available online: https://www.who.int/docs/default-source/coronaviruse/20230620xbb.1.5.pdf?sfvrsn=fff6f686_3 (accessed November 19, 2023).
- Lassaunière, R.; Polacek, C.; Utko, M.; Sørensen, K.M.; Baig, S.; Ellegaard, K.; Escobar-Herrera, L.A.; Fomsgaard, A.; Spiess, K.; Gunalan, V., et al. Virus isolation and neutralisation of SARS-CoV-2 variants BA.2.86 and EG.5.1. Lancet Infect. Dis. 2023, [Epub ahead of print; doi:10.1016/s1473-3099(23)00682-5].
- GISAID. Tracking of hCoV-19 Variants. Available online: https://gisaid.org/hcov19-variants/ (accessed November 24, 2023).
- Yang, S.; Yu, Y.; Xu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Wang, P.; Wang, J.; Liu, J.; Yu, L., et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. bioRxiv 2023, 2023.11.13.566860.
- World Health Organization. Vulnerable? Vaccinate. Protecting the unprotected from COVID-19 and influenza. Available online: https://www.who.int/europe/news/item/09-10-2023-vulnerable--vaccinate.-protecting-the-unprotected-from-covid-19-and-influenza (accessed November 10, 2023).
- Pather, S.; Muik, A.; Rizzi, R.; Mensa, F. Clinical development of variant-adapted BNT162b2 COVID-19 vaccines: the early Omicron era. Expert Rev Vaccines 2023, 22, 650-661. [CrossRef]
- COVID-19 Vaccine Tracker. Pfizer/BioNTech: Comirnaty. Available online: https://covid19.trackvaccines.org/vaccines/6/ (accessed November 10, 2023).
- US Food and Drug Administration. Fact sheet for healthcare providers administering vaccine: emergency use authorization of Pfizer-BioNTech COVID-19 vaccine (2023-2024 formula), for 6 months through 11 years of age. Available online: https://www.fda.gov/media/167211/download (accessed November 14, 2023).
- COMIRNATY®(COVID-19 vaccine mRNA) Highlights of Prescribing Information. Pfizer Inc, New York, NY; 2023.
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Sarkar, R., et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N. Engl. J. Med. 2021.
- Singer, S.R.; Angulo, F.J.; Swerdlow, D.L.; McLaughlin, J.M.; Hazan, I.; Ginish, N.; Anis, E.; Mendelson, E.; Mor, O.; Zuckerman, N.S., et al. Effectiveness of BNT162b2 mRNA COVID-19 vaccine against SARS-CoV-2 variant Beta (B.1.351) among persons identified through contact tracing in Israel: A prospective cohort study. EClinicalMedicine 2021, 42, 101190. [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N. Engl. J. Med. 2021, 385, 585-594. https://doi:.org/10.1056/nejmoa2108891.
- Barda, N.; Dagan, N.; Cohen, C.; Hernan, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021, 398, 2093-2100. [CrossRef]
- Collie, S.; Nayager, J.; Bamford, L.; Bekker, L.G.; Zylstra, M.; Gray, G. Effectiveness and durability of the BNT162b2 vaccine against Omicron sublineages in South Africa. N. Engl. J. Med. 2022, 387, 1332-1333. [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712-1720. [CrossRef]
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose in Younger Age Groups. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-bivalent-covid-19-vaccines (accessed December 5, 2022 ).
- Link-Gelles, R.; Ciesla, A.A.; Roper, L.E.; Scobie, H.M.; Ali, A.R.; Miller, J.D.; Wiegand, R.E.; Accorsi, E.K.; Verani, J.R.; Shang, N., et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults: increasing community access to testing program, United States, December 2022-January 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 119-124.
- Tenforde, M.W.; Weber, Z.A.; Natarajan, K.; Klein, N.P.; Kharbanda, A.B.; Stenehjem, E.; Embi, P.J.; Reese, S.E.; Naleway, A.L.; Grannis, S.J., et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults - VISION Network, Nine States, September-November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 71, 1637-1646.
- Lin, D.Y.; Xu, Y.; Gu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med. 2023, 388, 764-766. [CrossRef]
- Poukka, E.; Nohynek, H.; Goebeler, S.; Leino, T.; Baum, U. Bivalent booster effectiveness against severe COVID-19 outcomes in Finland, September 2022 – March 2023. medRxiv. Preprint posted online May 8, 2023. [CrossRef]
- Link-Gelles, R. COVID-19 vaccine effectiveness updates, 19 April 2023. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-04-19/05-COVID-Link-Gelles-508.pdf (accessed June 19, 2023).
- US Food and Drug Administration. FDA Briefing Document: Vaccines and Related Biological Products Advisory Committee Meeting, June 15, 2023. Available online: https://www.fda.gov/media/169378/download (accessed July 19, 2023).
- World Health Organization. Statement on the antigen composition of COVID-19 vaccines. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed June 19, 2023).
- US Food and Drug Administration. FDA Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating (accessed November 9, 2023).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603-2615. [CrossRef]
- Winokur, P.; Gayed, J.; Fitz-Patrick, D.; Thomas, S.J.; Diya, O.; Lockhart, S.; Xu, X.; Zhang, Y.; Bangad, V.; Schwartz, H.I., et al. Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years. N. Engl. J. Med. 2023, 388, 214-227. [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Su, J.R.; Blanc, P.G.; Gwira Baumblatt, J.A.; Woo, E.J.; Gee, J.; Shimabukuro, T.T., et al. Safety monitoring of COVID-19 vaccine booster doses among adults - United States, September 22, 2021-February 6, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 249-254.
- Hause, A.M.; Baggs, J.; Marquez, P.; Abara, W.E.; Baumblatt, J.; Blanc, P.G.; Su, J.R.; Hugueley, B.; Parker, C.; Myers, T.R., et al. Safety monitoring of COVID-19 mRNA vaccine second booster doses among adults aged ≥50 years - United States, March 29, 2022-July 10, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 971-976.
- Uriu, K.; Ito, J.; Zahradnik, J.; Fujita, S.; Kosugi, Y.; Schreiber, G.; Sato, K. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 2023, 23, 280-281.
- Qu, P.; Xu, K.; Faraone, J.N.; Goodarzi, N.; Zheng, Y.-M.; Carlin, C.; Bednash, J.S.; Horowitz, J.C.; Mallampalli, R.K.; Saif, L.J., et al. Immune evasion, infectivity, and fusogenicity of SARS-CoV-2 Omicron BA.2.86 and FLip variants. bioRxiv. Preprint posted online September 12 2023. [CrossRef]
- Parums, D.V. Editorial: A rapid global increase in COVID-19 is due to the emergence of the EG.5 (Eris) subvariant of Omicron SARS-CoV-2. Med. Sci. Monit. 2023, 29, e942244. [CrossRef]
- Wang, Q.; Guo, Y.; Liu, L.; Schwanz, L.T.; Li, Z.; Nair, M.S.; Ho, J.; Zhang, R.M.; Iketani, S.; Yu, J., et al. Antigenicity and receptor affinity of SARS-CoV-2 BA.2.86 spike. Nature 2023. [CrossRef]
- Attar Cohen, H.; Mesfin, S.; Ikejezie, J.; Kassamali, Z.; Campbell, F.; Adele, S.; Guinko, N.; Idoko, F.; Mirembe, B.B.; Mitri, M.E., et al. Surveillance for variants of SARS-CoV-2 to inform risk assessments. Bull. World Health Organ. 2023, 101, 707-716. [CrossRef]
- Jones, J.M.; Manrique, I.M.; Stone, M.S.; Grebe, E.; Saa, P.; Germanio, C.D.; Spencer, B.R.; Notari, E.; Bravo, M.; Lanteri, M.C., et al. Estimates of SARS-CoV-2 Seroprevalence and Incidence of Primary SARS-CoV-2 Infections Among Blood Donors, by COVID-19 Vaccination Status - United States, April 2021-September 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 601-605.
- Modjarrad, K.; Che, Y.; Chen, W.; Wu, H.; Cadima, C.I.; Muik, A.; Maddur, M.S.; Tompkins, K.R.; Martinez, L.T.; Cai, H., et al. Preclinical Characterization of the Omicron XBB.1.5-Adapted BNT162b2 COVID-19 Vaccine. bioRxiv 2023, 2023.11.17.567633. [CrossRef]
- Muñoz, F.M.; Sher, L.D.; Sabharwal, C.; Gurtman, A.; Xu, X.; Kitchin, N.; Lockhart, S.; Riesenberg, R.; Sexter, J.M.; Czajka, H., et al. Evaluation of BNT162b2 Covid-19 Vaccine in Children Younger than 5 Years of Age. N. Engl. J. Med. 2023, 388, 621-634. [CrossRef]
- Sher, L.; Sabharwal, C.; Kitchin, N.; Kofi Boakya-Appiah, J.; Xu, X.; Walter, E.; Maldonado, Y.A.; Munoz, F.M.; Englund, J.A.; Talaat, K.R., et al. 362. Safety and Immunogenicity of a Variant-adapted Bivalent (Original/Omicron BA.4/BA.5) BNT162b2 COVID-19 Vaccine Given as a Booster (Dose 4) to Toddlers and Children 6 Months to < 5 Years of Age Who Previously Received Original BNT162b2 as a 3-Dose Primary Series. Open Forum Infectious Diseases 2023, 10. [CrossRef]
- Paulsen, G.C.; Sher, L.; Sabharwal, C.; Kitchin, N.; Hill, S.; Wasserman, E.; Xu, X.; Maldonado, Y.A.; Barnett, E.; Englund, J.A., et al. 385. Safety and Immunogenicity of a Variant-adapted Bivalent (Original/Omicron BA.4/BA.5) BNT162b2 COVID-19 Vaccine Given as a Booster (Dose 4) to 5- to 11-Year-Old Children Who Previously Received 3 Doses of Original BNT162b2. Open Forum Infectious Diseases 2023, 10. [CrossRef]
Figure 1.
Participant disposition. At data cutoff (September 27, 2023), some participants had not reached the 1-month postvaccination visit.
Figure 1.
Participant disposition. At data cutoff (September 27, 2023), some participants had not reached the 1-month postvaccination visit.
Figure 2.
Local reactions occurring within 7 days after receipt of XBB.1.5-adapted BNT162b2 30 μg (safety population). Error bars and numbers above the error bars denote the 95% CIs and the percentage of participants in each group reporting the specified local reaction, respectively. The N values are 30, 174, 206, and 410 for 12–17-year-olds, 18–55-year-olds, >55-year-olds, and the total population, respectively.
Figure 2.
Local reactions occurring within 7 days after receipt of XBB.1.5-adapted BNT162b2 30 μg (safety population). Error bars and numbers above the error bars denote the 95% CIs and the percentage of participants in each group reporting the specified local reaction, respectively. The N values are 30, 174, 206, and 410 for 12–17-year-olds, 18–55-year-olds, >55-year-olds, and the total population, respectively.
Figure 3.
Systemic events occurring within 7 days after receipt of XBB.1.5-adapted BNT162b2 30 μg (safety population). Error bars and numbers above the error bars denote the 95% CIs and the percentage of participants in each group reporting the specified systemic event, respectively. The N values are 30, 174, 206, and 410 in 12–17-year-olds, 18–55-year-olds, >55-year-olds, and the total population, respectively.
Figure 3.
Systemic events occurring within 7 days after receipt of XBB.1.5-adapted BNT162b2 30 μg (safety population). Error bars and numbers above the error bars denote the 95% CIs and the percentage of participants in each group reporting the specified systemic event, respectively. The N values are 30, 174, 206, and 410 in 12–17-year-olds, 18–55-year-olds, >55-year-olds, and the total population, respectively.
Figure 4.
Adverse events reported within 1 month of XBB.1.5-adapted BNT162b2 30 μg (safety population). AE=adverse event; SAE=serious adverse event. *Related AEs were as determined by the investigator.
Figure 4.
Adverse events reported within 1 month of XBB.1.5-adapted BNT162b2 30 μg (safety population). AE=adverse event; SAE=serious adverse event. *Related AEs were as determined by the investigator.
Figure 5.
Serum neutralizing GMTs (95% CIs) before and 1 month after vaccination with XBB.1.5-adapted BNT162b2 30 μg and GMFRs (95% CIs) from before to 1 month after vaccination to Omicron XBB.1.5 (a), EG.5.1 (b), and BA.2.86 (c). Data are for the evaluable immunogenicity population. Assay results <LLOQ were set to 0.5 × LLOQ. Numbers within the bars are the GMTs. 1m=1 month after vaccination; FFRNT=fluorescent focus reduction neutralization test; GMFR=geometric mean fold rise; GMT=geometric mean titer; LLOQ=lower limit of quantitation; Pre=before vaccination.
Figure 5.
Serum neutralizing GMTs (95% CIs) before and 1 month after vaccination with XBB.1.5-adapted BNT162b2 30 μg and GMFRs (95% CIs) from before to 1 month after vaccination to Omicron XBB.1.5 (a), EG.5.1 (b), and BA.2.86 (c). Data are for the evaluable immunogenicity population. Assay results <LLOQ were set to 0.5 × LLOQ. Numbers within the bars are the GMTs. 1m=1 month after vaccination; FFRNT=fluorescent focus reduction neutralization test; GMFR=geometric mean fold rise; GMT=geometric mean titer; LLOQ=lower limit of quantitation; Pre=before vaccination.
Figure 6.
Participants achieving seroresponse (95% CIs) 1 month after vaccination with XBB.1.5-adapted BNT162b2 30 μg (evaluable immunogenicity population). A ≥4-fold rise from before study vaccination in FFRNT 50% serum neutralizing titers was considered a seroresponse. For participants with a baseline measurement <LLOQ, a postvaccination assay result of ≥4 × LLOQ was considered a seroresponse. FFRNT=fluorescent focus reduction neutralization test; LLOQ=lower limit of quantitation.
Figure 6.
Participants achieving seroresponse (95% CIs) 1 month after vaccination with XBB.1.5-adapted BNT162b2 30 μg (evaluable immunogenicity population). A ≥4-fold rise from before study vaccination in FFRNT 50% serum neutralizing titers was considered a seroresponse. For participants with a baseline measurement <LLOQ, a postvaccination assay result of ≥4 × LLOQ was considered a seroresponse. FFRNT=fluorescent focus reduction neutralization test; LLOQ=lower limit of quantitation.
Table 1.
Participant demographic and baseline clinical characteristics.
Table 1.
Participant demographic and baseline clinical characteristics.
| Characteristic |
12–17 Years Old
(Na=30) |
18–55 Years Old
(Na=174) |
>55 Years Old
(Na=208) |
Total
(Na=412) |
| Sex, nb (%) |
|
|
|
|
| Male |
11 (36.7) |
74 (42.5) |
85 (40.9) |
170 (41.3) |
| Female |
19 (63.3) |
100 (57.5) |
123 (59.1) |
242 (58.7) |
| Race, nb (%) |
|
|
|
|
| White |
26 (86.7) |
135 (77.6) |
165 (79.3) |
326 (79.1) |
| Black |
3 (10.0) |
23 (13.2) |
26 (12.5) |
52 (12.6) |
| American Indian or Alaska Native |
0 |
0 |
1 (0.5) |
1 (0.2) |
| Asian |
1 (3.3) |
11 (6.3) |
10 (4.8) |
22 (5.3) |
| Native Hawaiian or other Pacific Islander |
0 |
0 |
2 (1.0) |
2 (0.5) |
| Multiracial or unknown |
0 |
5 (2.9) |
4 (1.9) |
9 (2.1) |
| Ethnicity, nb (%) |
|
|
|
|
| Hispanic/Latino |
6 (20.0) |
35 (20.1) |
34 (16.3) |
75 (18.2) |
| Age at vaccination, years |
|
|
|
|
| Mean (SD) |
14.0 (1.74) |
40.4 (9.82) |
68.6 (6.74) |
52.7 (19.12) |
| Median (range) |
14.0 (12–17) |
42.0 (18–55) |
68.5 (56–88) |
56.0 (12–88) |
| Baseline SARS-CoV-2 status, nb (%) |
|
|
|
|
| Positivec
|
23 (76.7) |
144 (82.8) |
155 (74.5) |
322 (78.2) |
| Negatived
|
7 (23.3) |
30 (17.2) |
53 (25.5) |
90 (21.8) |
| Prior COVID-19 vaccine doses,e nb (%) |
|
|
|
|
| 3 doses |
30 (100.0) |
174 (100.0) |
208 (100.0) |
412 (100.0) |
| 4 doses |
26 (86.7) |
154 (88.5) |
199 (95.7) |
379 (92.0) |
| 5 doses |
1 (3.3) |
19 (10.9) |
104 (50.0) |
124 (30.1) |
| 6 doses |
0 |
1 (0.6) |
4 (1.9) |
5 (1.2) |
| 7 doses |
0 |
0 |
1 (0.5) |
1 (0.2) |
| Time from last dose of mRNA COVID-19 vaccinee to the study vaccination, monthsf
|
|
|
|
|
| Mean (SD) |
10.2 (1.95) |
10.4 (2.33) |
10.4 (1.82) |
10.4 (2.05) |
| Median (range) |
10.3 (6.7–12.6) |
10.9 (5.5–24.1) |
10.8 (5.8–22.6) |
10.8 (5.5–24.1) |
| 5 to <7, nb (%) |
1 (3.3) |
12 (6.9) |
11 (5.3) |
24 (5.8) |
| 7 to <9, nb (%) |
8 (26.7) |
31 (17.8) |
31 (14.9) |
70 (17.0) |
| 9 to 12, nb (%) |
10 (33.3) |
108 (62.1) |
155 (74.5) |
273 (66.3) |
| >12, nb (%) |
11 (36.7) |
23 (13.2) |
11 (5.3) |
45 (10.9) |
| Time from last dose of mRNA COVID-19 vaccinee to the study vaccination, days |
|
|
|
|
| Mean (SD) |
286.4 (54.50) |
292.1 (65.24) |
290.2 (50.95) |
290.8 (57.54) |
| Median (range) |
289.0 (188–352) |
305.0 (154–675) |
303.0 (162–634) |
303.0 (154–675) |
| BMI, ≥16 y of age, nb (%) |
|
|
|
|
| Number of participantsg
|
5 |
174 |
208 |
387 |
| Underweight (<18.5 kg/m2) |
0 |
4 (2.3) |
2 (1.0) |
6 (1.6) |
| Normal weight (≥18.5–24.9 kg/m2) |
4 (80.0) |
52 (29.9) |
53 (25.5) |
109 (28.2) |
| Overweight (≥25.0–29.9 kg/m2) |
0 |
56 (32.2) |
78 (37.5) |
134 (34.6) |
| Obese (≥30.0 kg/m2) |
1 (20.0) |
62 (35.6) |
75 (36.1) |
138 (35.7) |
| BMI, 12–15 y of age/obeseh, nb (%) |
|
|
|
|
| Number of participantsg |
25 |
- |
- |
25 |
| Not obese |
22 (88.0) |
- |
- |
22 (88.0) |
| Obese |
3 (12.0) |
- |
- |
3 (12.0) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).