Submitted:
06 January 2024
Posted:
09 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
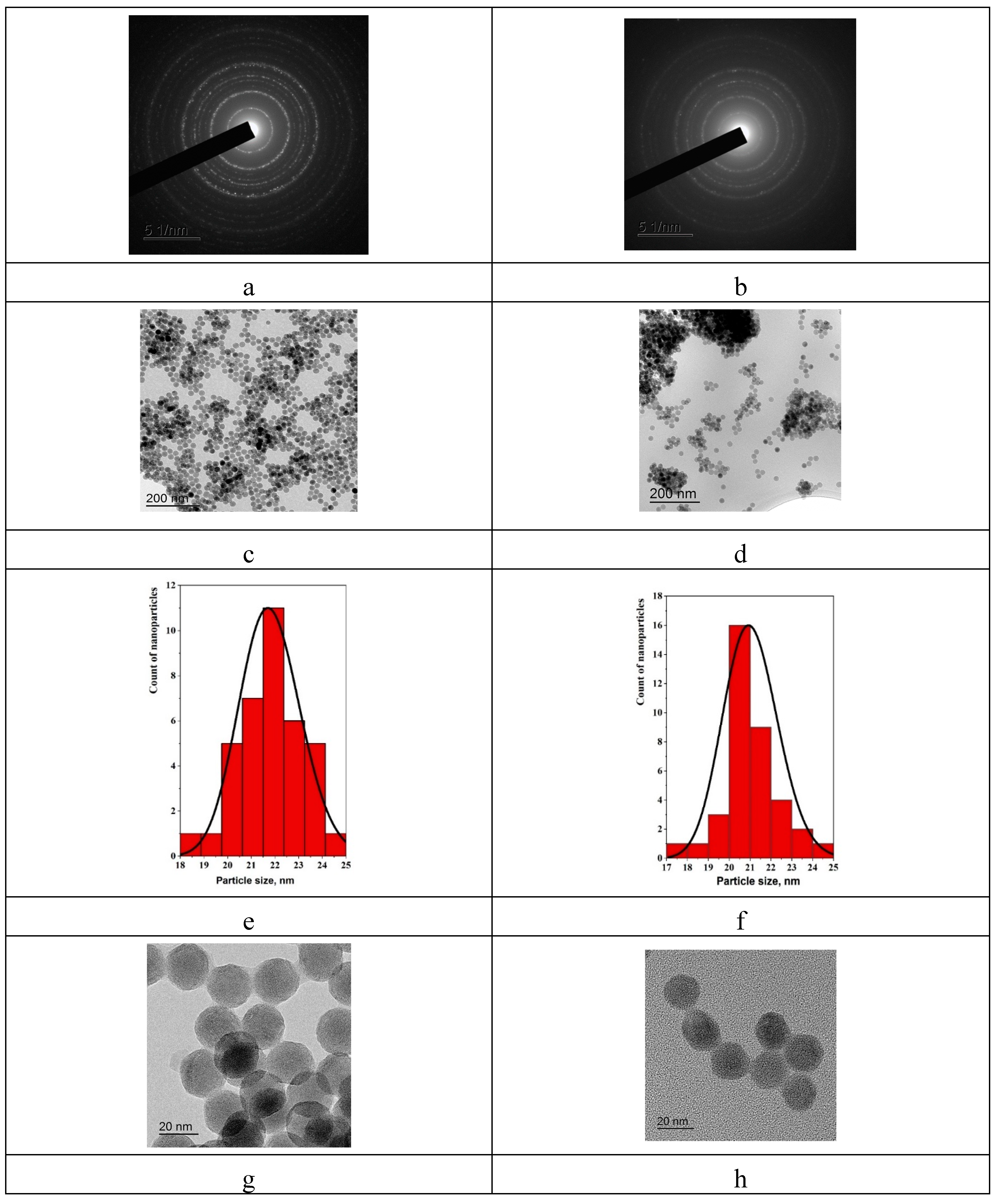
2.1. Synthesis and characterization of the samples
2.2. Luminescent spectroscopy of colloids β-NaYF4:Yb3+/Tm3+ in DMSO
3. Results and discussion
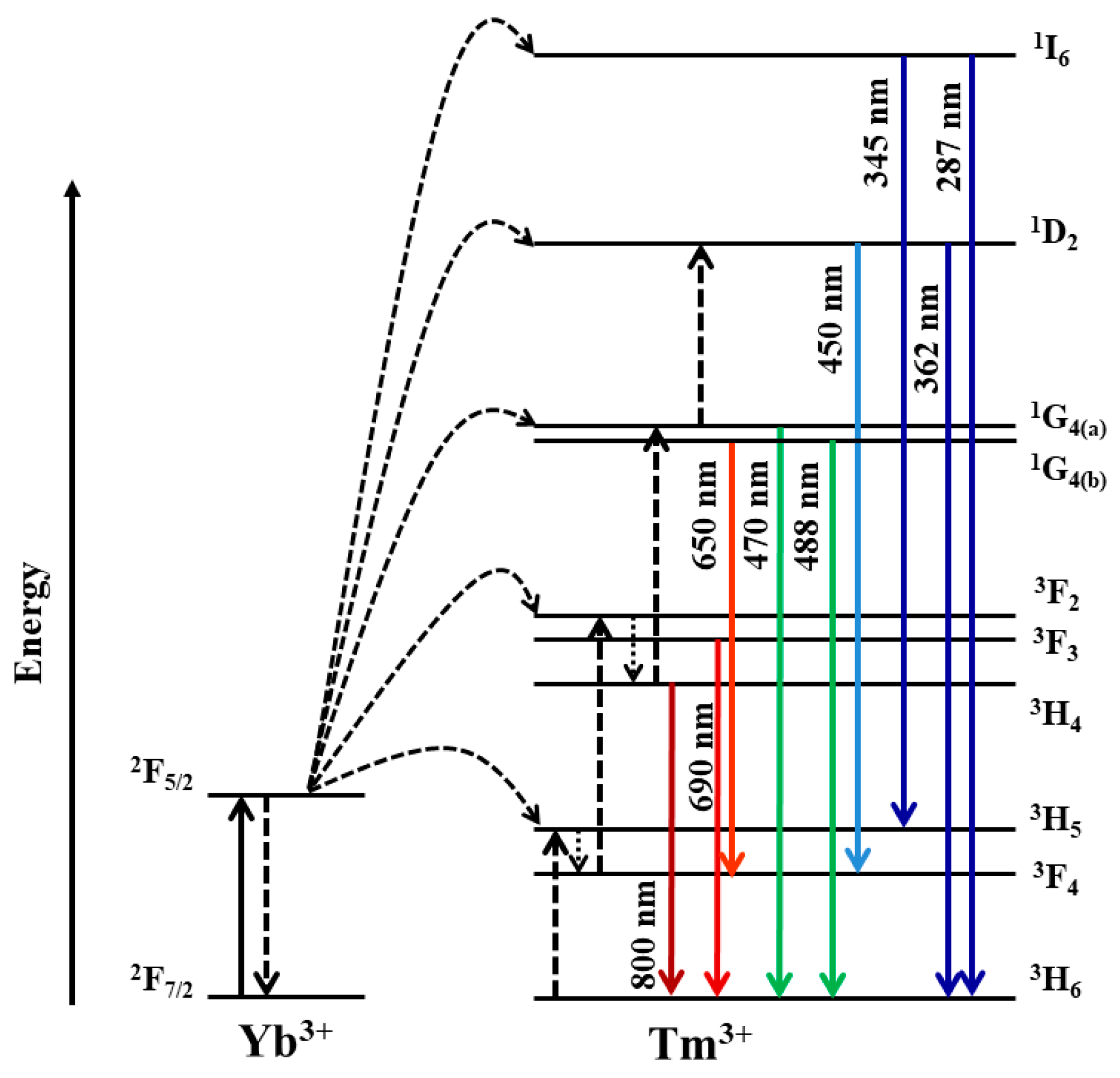
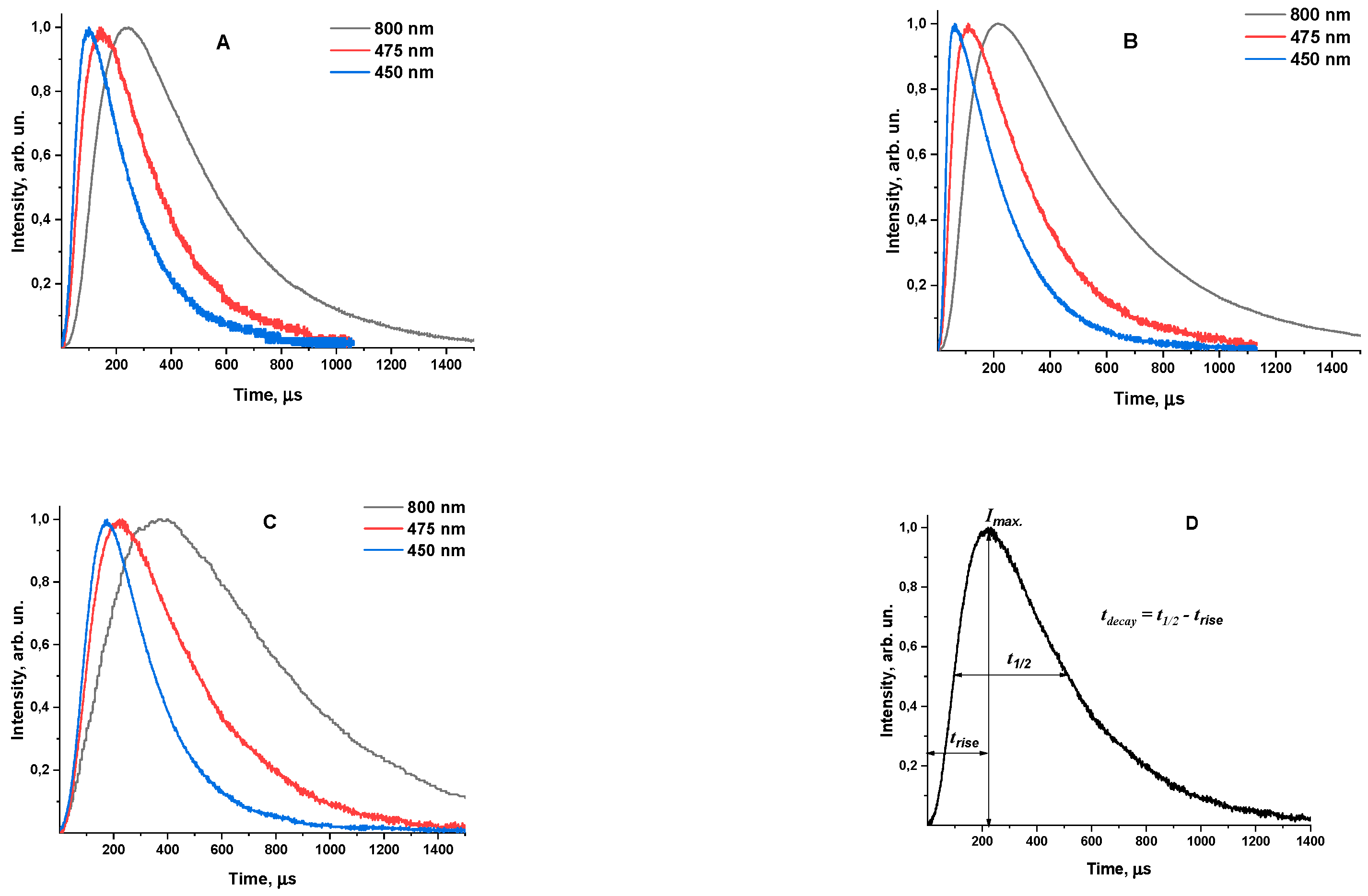
3.1. Investigation of the kinetics of β-NaYF4:Yb3+/Tm3+ luminescence
3.2. Rise time of β-NaYF4:Yb3+/Tm3+ luminescence
3.3. Decay time of β-NaYF4:Yb3+/Tm3+ luminescence
4. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2003, 104, 139–174. [Google Scholar] [CrossRef]
- Bloembergen, N. Solid State Infrared Quantum Counters. Phys. Rev. Lett. 1959, 2, 84–85. [Google Scholar] [CrossRef]
- Ovsyankin, V.V.; Feofilov, P.P. Mechanism of Summation of Electronic Excitations in Activated Crystals. JETP Lett. 1966, 3, 322. [Google Scholar]
- Salley, G. M.; Valiente, R.; Guedel, H. U. Luminescence Upconversion Mechanisms in Yb3+–Tb3+ Systems. J. Lumin. 2001, 94–95, 305–309. [Google Scholar] [CrossRef]
- You, M.; Zhong, J.; Hong, Y.; Duan, Z.; Lin, M.; Xu, F. Inkjet Printing of Upconversion Nanoparticles for Anti-Counterfeit Applications. Nanoscale 2015, 7, 4423–4431. [Google Scholar] [CrossRef]
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing Luminescence in Lanthanide-Doped Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 11702–11715. [Google Scholar] [CrossRef]
- Escudero, A.; Becerro, A. I.; Carrillo-Carrión, C.; Núñez, N. O.; Zyuzin, M. V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W. J. Rare Earth Based Nanostructured Materials: Synthesis, Functionalization, Properties and Bioimaging and Biosensing Applications. Nanophotonics 2017, 6, 881–921. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent Progress in the Development of Upconversion Nanomaterials in Bioimaging and Disease Treatment. Journal of Nanobiotechnology 2020, 18, 154. [Google Scholar] [CrossRef]
- Reddy, K. L.; Rai, M.; Prabhakar, N.; Arppe, R.; Rai, S. B.; Singh, S. K.; Rosenholm, J. M.; Krishnan, V. Controlled Synthesis, Bioimaging and Toxicity Assessments in Strong Red Emitting Mn2+ Doped NaYF4:Yb3+/Ho3+ Nanophosphors. RSC Adv. 2016, 6, 53698–53704. [Google Scholar] [CrossRef]
- Jaque, D.; Vetrone, F. Luminescence Nanothermometry. Nanoscale 2012, 4, 4301. [Google Scholar] [CrossRef]
- Sarmanova, O. E.; Burikov, S. A.; Laptinskiy, K. A.; Kotova, O. D.; Filippova, E. A.; Dolenko, T. A. In Vitro Temperature Sensing with Up-Conversion NaYF4:Yb3+/Tm3+-Based Nanocomposites: Peculiarities and Pitfalls. Spectrochim. Acta A 2020, 241, 118627. [Google Scholar] [CrossRef]
- Runowski, M.; Woźny, P.; Lis, S.; Lavín, V.; Martín, I. R. Optical Vacuum Sensor Based on Lanthanide Upconversion—Luminescence Thermometry as a Tool for Ultralow Pressure Sensing. Adv. Mater. Technol 2020, 5, 1901091. [Google Scholar] [CrossRef]
- Singh, R.; Madirov, E.; Busko, D.; Hossain, I. M.; Konyushkin, V. A.; Nakladov, A. N.; Kuznetsov, S. V.; Farooq, A.; Gharibzadeh, S.; Paetzold, U. W.; et al. Harvesting Sub-Bandgap Photons via Upconversion for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 54874–54883. [Google Scholar] [CrossRef]
- Karimov, D. N.; Demina, P. A.; Koshelev, A. V.; Rocheva, V. V.; Sokovikov, A. V.; Generalova, A. N.; Zubov, V. P.; Khaydukov, E. V.; Koval’chuk, M. V.; Panchenko, V. Ya. Upconversion Nanoparticles: Synthesis, Photoluminescence Properties, and Applications. Nanotechnol Russ. 2020, 15, 655–678. [Google Scholar] [CrossRef]
- Dobretsova, E. A.; Xia, X.; Pant, A.; Lim, M. B.; De Siena, M. C.; Boldyrev, K. N.; Molchanova, A. D.; Novikova, N. N.; Klimin, S. A.; Popova, M. N.; et al. Hydrothermal Synthesis of Yb3+: LuLiF4 Microcrystals and Laser Refrigeration of Yb3+: LuLiF4/Silicon-Nitride Composite Nanostructures. Laser Photonics Rev. 2021, 15, 2100019. [Google Scholar] [CrossRef]
- Scheps, R. Upconversion Laser Processes. Prog. Quantum. Electron. 1996, 20, 271–358. [Google Scholar] [CrossRef]
- Chen, G.; Ohulchanskyy, T. Y.; Kumar, R.; Ågren, H.; Prasad, P. N. Ultrasmall Monodisperse NaYF4:Yb3+/Tm3+ Nanocrystals with Enhanced Near-Infrared to Near-Infrared Upconversion Photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Tian, L.; Yu, Y.; Kong, X.; Zhao, J.; Zhang, H. Controlled Synthesis and Morphology Dependent Upconversion Luminescence of NaYF4:Yb, Er Nanocrystals. Nanotechnology 2007, 18, 275609. [Google Scholar] [CrossRef]
- Krämer, K. W.; Biner, D.; Frei, G.; Güdel, H. U.; Hehlen, M. P.; Lüthi, S. R. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Upconversion Phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2014, 115, 395–465. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Y.; Li, Y.; Sun, S.; Huo, J.; Zhao, X. A Facile Synthesis of NaYF4:Yb3+/Er3+Nanoparticles with Tunable Multicolor Upconversion Luminescence Properties for Cell Imaging. RSC Adv. 2014, 4, 43653–43660. [Google Scholar] [CrossRef]
- Kormshchikov, I. D.; Voronov, V. V.; Burikov, S. A.; Dolenko, T. A.; Kuznetsov, S. V. Study of Stability of Luminescence Intensity of B-NaGdF4:Yb:Er Nanoparticle Colloids in Aqueous Solution. Nanosyst.: Phys. Chem. Math. 2021, 12, 218–223. [Google Scholar] [CrossRef]
- Bazhukova, I. N.; Pustovarov, V. A.; Myshkina, A. V.; Ulitko, M. V. Luminescent Nanomaterials Doped with Rare Earth Ions and Prospects for Their Biomedical Applications (A Review). Opt Spectrosc 2020, 128, 2050–2068. [Google Scholar] [CrossRef]
- Kuznetsov, S.; Ermakova, Yu.; Voronov, V.; Fedorov, P.; Busko, D.; Howard, I. A.; Richards, B. S.; Turshatov, A. Up-Conversion Quantum Yields of SrF2:Yb3+,Er3+ Sub-Micron Particles Prepared by Precipitation from Aqueous Solution. J. Mater. Chem. C 2018, 6, 598–604. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D. R.; Lüthi, S. R.; Güdel, H. U.; Hehlen, M. P. Power Dependence of Upconversion Luminescence in Lanthanide and Transition-Metal-Ion Systems. Phys. Rev. B 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Burikov, S.A.; Filippova, E.A.; Fedyanina, A.A.; Kuznetsov, S.V.; Proydakova V., Yu.; Voronov, V.V.; Dolenko, T.A. Influence of the Intensity of Exciting Radiation on the Luminescent Properties of Nanopowders NaYF-=SUB=-4-=/SUB=- : Yb/Tm. Opt Spectrosc 2022, 130, 655. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Zhang, Z.; Fu, Z.; Zheng, H. Luminescence Thermometry with Rare Earth Doped Nanoparticles: Status and Challenges. J. Lumin. 2022, 250, 119110. [Google Scholar] [CrossRef]
- Cong, T.; Ding, Y.; Xin, S.; Hong, X.; Zhang, H.; Liu, Y. Solvent-Induced Luminescence Variation of Upconversion Nanoparticles. Langmuir 2016, 32, 13200–13206. [Google Scholar] [CrossRef] [PubMed]
- Rozhnova, Yu. A.; Kuznetsov, S. V.; Luginina, A. A.; Voronov, V. V.; Ryabova, A. V.; Pominova, D. V.; Ermakov, R. P.; Usachev, V. A.; Kononenko, N. E.; Baranchikov, A. E.; et al. New Sr1−x−zRx(NH4)zF2+x−z (R = Yb, Er) Solid Solution as Precursor for High Efficiency up-Conversion Luminophor and Optical Ceramics on the Base of Strontium Fluoride. Mater. Chem. Phys. 2016, 172, 150–157. [Google Scholar] [CrossRef]
- Kuznetsov, S. V.; Burikov, S. A.; Fedyanina, A. A.; Filippova, E. A.; Proydakova, V. Yu.; Voronov, V. V.; Tabachkova, N. Yu.; Fedorov, P. P.; Dolenko, T. A. Impact of Sensitizer Yb and Activator Tm on Luminescence Intensity of Beta-NaYF4:Yb/Tm Nanoluminophores. Nanosyst.: Phys. Chem. Math. 2022, 13, 331–341. [Google Scholar] [CrossRef]
- Pisarenko, V.F. Rare-earth scandoborates as new laser materials. Soros. Obrazovat. Zh. (in Rus.) 1996, 11, 111–116. [Google Scholar]
- Pilch, A.; Wawrzyńczyk, D.; Kurnatowska, M.; Czaban, B.; Samoć, M.; Strek, W.; Bednarkiewicz, A. The Concentration Dependent Up-Conversion Luminescence of Ho3+ and Yb3+ Co-Doped β-NaYF4. J. Lumin. 2017, 182, 114–122. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Chen, R.; Goggi, J.; Ren, N.; Huang, L.; Bhakoo, K. K.; Sun, H.; Tan, T. T. Y. Cross Relaxation Induced Pure Red Upconversion in Activator- and Sensitizer-Rich Lanthanide Nanoparticles. Chem. Mater. 2014, 26, 5183–5186. [Google Scholar] [CrossRef]
- Kong, J.; Shang, X.; Zheng, W.; Chen, X.; Tu, D.; Wang, M.; Song, J.; Qu, J. Revisiting the Luminescence Decay Kinetics of Energy Transfer Upconversion. J. Phys. Chem. Lett. 2020, 11, 3672–3680. [Google Scholar] [CrossRef]
- Gamelin, D. R.; Gudel, H. U. Upconversion Processes in Transition Metal and Rare Earth Metal Systems. In Transition Metal and Rare Earth Compounds; Yersin, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–56. [Google Scholar] [CrossRef]
- Liu, H.; Jayakumar, M. K. G.; Huang, K.; Wang, Z.; Zheng, X.; Ågren, H.; Zhang, Y. Phase Angle Encoded Upconversion Luminescent Nanocrystals for Multiplexing Applications. Nanoscale 2017, 9, 1676–1686. [Google Scholar] [CrossRef]
- Naccache, R.; Vetrone, F.; Speghini, A.; Bettinelli, M.; Capobianco, J. A. Cross-Relaxation and Upconversion Processes in Pr3+ Singly Doped and Pr3+/Yb3+ Codoped Nanocrystalline Gd3Ga5O12: The Sensitizer/Activator Relationship. J. Phys. Chem. C 2008, 112, 7750–7756. [Google Scholar] [CrossRef]
- Dexter, D. L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Nadort, A.; Zhao, J.; Goldys, E. M. Lanthanide Upconversion Luminescence at the Nanoscale: Fundamentals and Optical Properties. Nanoscale 2016, 8, 13099–13130. [Google Scholar] [CrossRef]
- Villanueva-Delgado, P.; Krämer, K. W.; Valiente, R. Simulating Energy Transfer and Upconversion in β-NaYF4: Yb3+, Tm3+. J. Phys. Chem. C 2015, 119, 23648–23657. [Google Scholar] [CrossRef]
- Hong-Wei, S.; Hai-Ping, X.; Bao-Juan, S.; Shao-Zhe, L.; Zhong-Xin, L.; Li-Xin, Y. Upconversion Luminescence Dynamics in Er 3+ /Yb 3+ Codoped Nanocrystalline Yttria. Chin. Phys. Lett. 2006, 23, 474–477. [Google Scholar] [CrossRef]
- Chen, X. Y.; Zhuang, H. Z.; Liu, G. K.; Li, S.; Niedbala, R. S. Confinement on Energy Transfer between Luminescent Centers in Nanocrystals. J. Appl. Phys. 2003, 94, 5559–5565. [Google Scholar] [CrossRef]
- Martín-Rodríguez, R.; Rabouw, F. T.; Trevisani, M.; Bettinelli, M.; Meijerink, A. Upconversion Dynamics in Er3+-Doped Gd2O2S: Influence of Excitation Power, Er3+ Concentration, and Defects. Adv. Opt. Mater. 2015, 3, 558–567. [Google Scholar] [CrossRef]
- Misiak, M.; Prorok, K.; Cichy, B.; Bednarkiewicz, A.; Stręk, W. Thulium Concentration Quenching in the Up-Converting α-Tm3+/Yb3+ NaYF4 Colloidal Nanocrystals. Opt. Mater. 2013, 35, 1124–1128. [Google Scholar] [CrossRef]
- Pominova, D.; Proydakova, V.; Romanishkin, I.; Ryabova, A.; Kuznetsov, S.; Uvarov, O.; Fedorov, P.; Loschenov, V. Temperature Sensing in the Short-Wave Infrared Spectral Region Using Core-Shell NaGdF4:Yb3+, Ho3+, Er3+@NaYF4 Nanothermometers. Nanomaterials 2020, 10, 1992. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, G.; Hao, S.; Yang, C. Sub-6 Nm Monodisperse Hexagonal Core/Shell NaGdF4Nanocrystals with Enhanced Upconversion Photoluminescence. Nanoscale 2017, 9, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wade, S. A.; Collins, S. F.; Baxter, G. W. Fluorescence Intensity Ratio Technique for Optical Fiber Point Temperature Sensing. J. Appl. Phys. 2003, 94, 4743–4756. [Google Scholar] [CrossRef]
- Alyatkin, S.; Asharchuk, I.; Khaydukov, K.; Nechaev, A.; Lebedev, O.; Vainer, Y.; Semchishen, V.; Khaydukov, E. The Influence of Energy Migration on Luminescence Kinetics Parameters in Upconversion Nanoparticles. Nanotechnology 2016, 28, 035401. [Google Scholar] [CrossRef]
- Buisson, R.; Vial, J. C. Transfer inside Pairs of Pr3+ in LaF3 Studied by Up-Conversion Fluorescence. Journal de Physique Lettres 1981, 42, 115–118. [Google Scholar] [CrossRef]
- Mikheev, A. V.; Kazakov, B. N. Rise Kinetics of Up-Conversion Luminescence under Pulsed Excitation. Probabilistic Model and Experiment. J. Lumin. 2019, 205, 167–178. [Google Scholar] [CrossRef]
- Mikheev, A. V.; Kazakov, B. N. Rise Kinetics of Up-Conversion Luminescence of the LiY0.8Yb0.2F4:Tm3+ (0.2 at %) Crystal with Pulsed Excitation. Phys. Solid State 2019, 61, 860–866. [Google Scholar] [CrossRef]
- Clegg, R. M. Chapter 1 Förster Resonance Energy Transfer—FRET What Is It, Why Do It, and How It’s Done. Fret and Flim Techniques 2009, 1–57. [Google Scholar] [CrossRef]
- Lemmetyinen, H.; Tkachenko, N. V.; Valeur, B.; Hotta, J.; Ameloot, M.; Ernsting, N. P.; Gustavsson, T.; Boens, N. Time-Resolved Fluorescence Methods (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 1969–1998. [Google Scholar] [CrossRef]

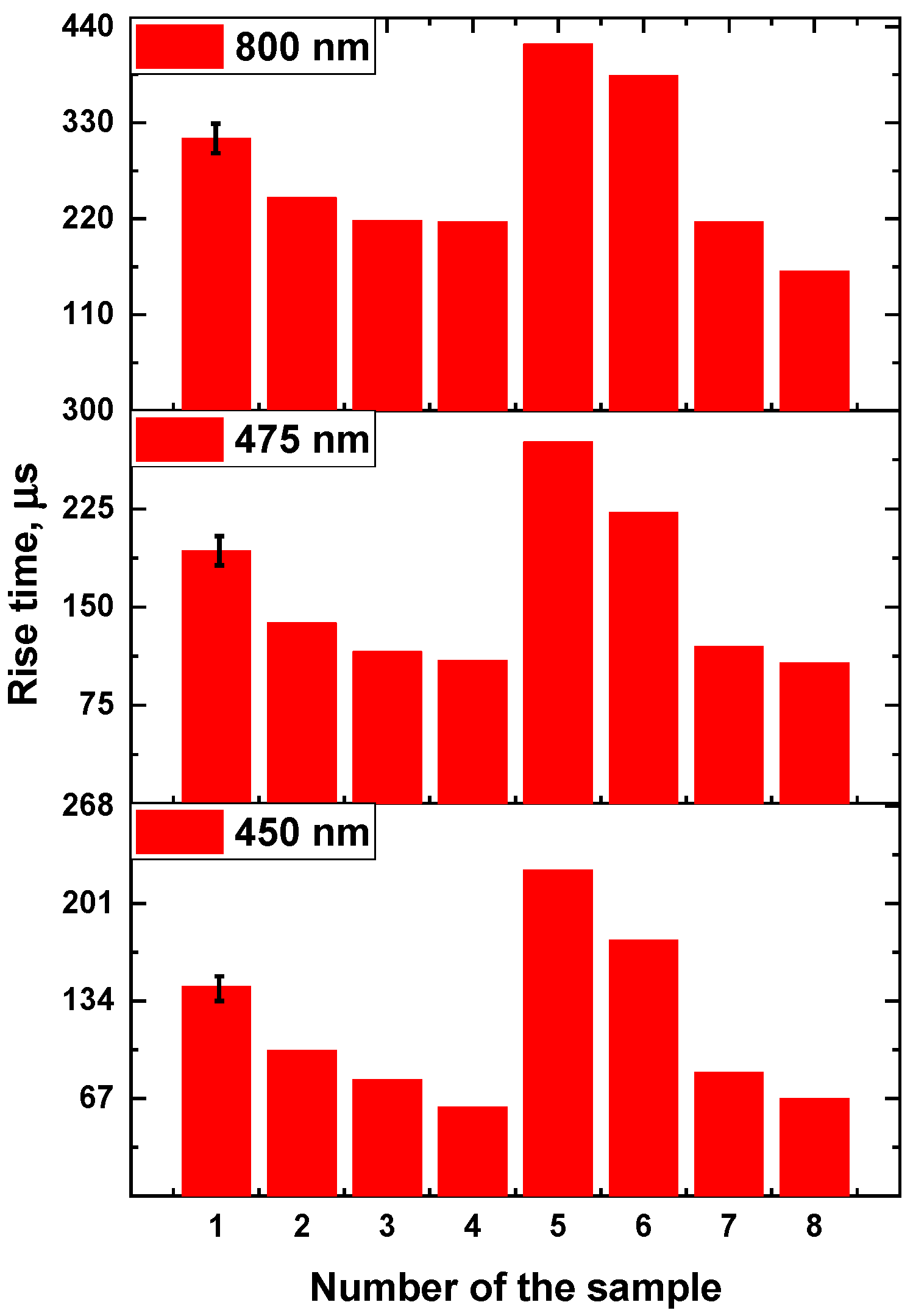
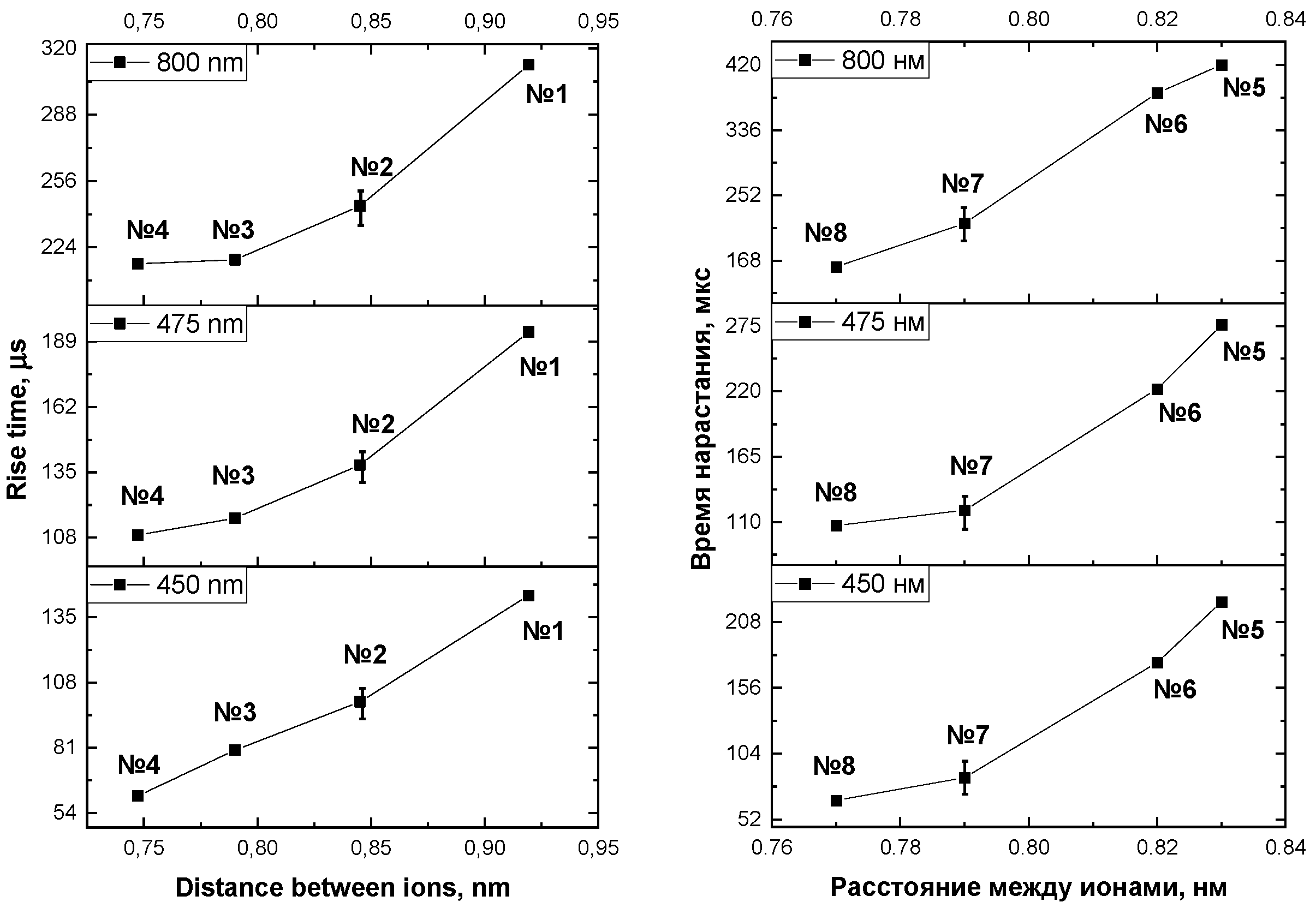
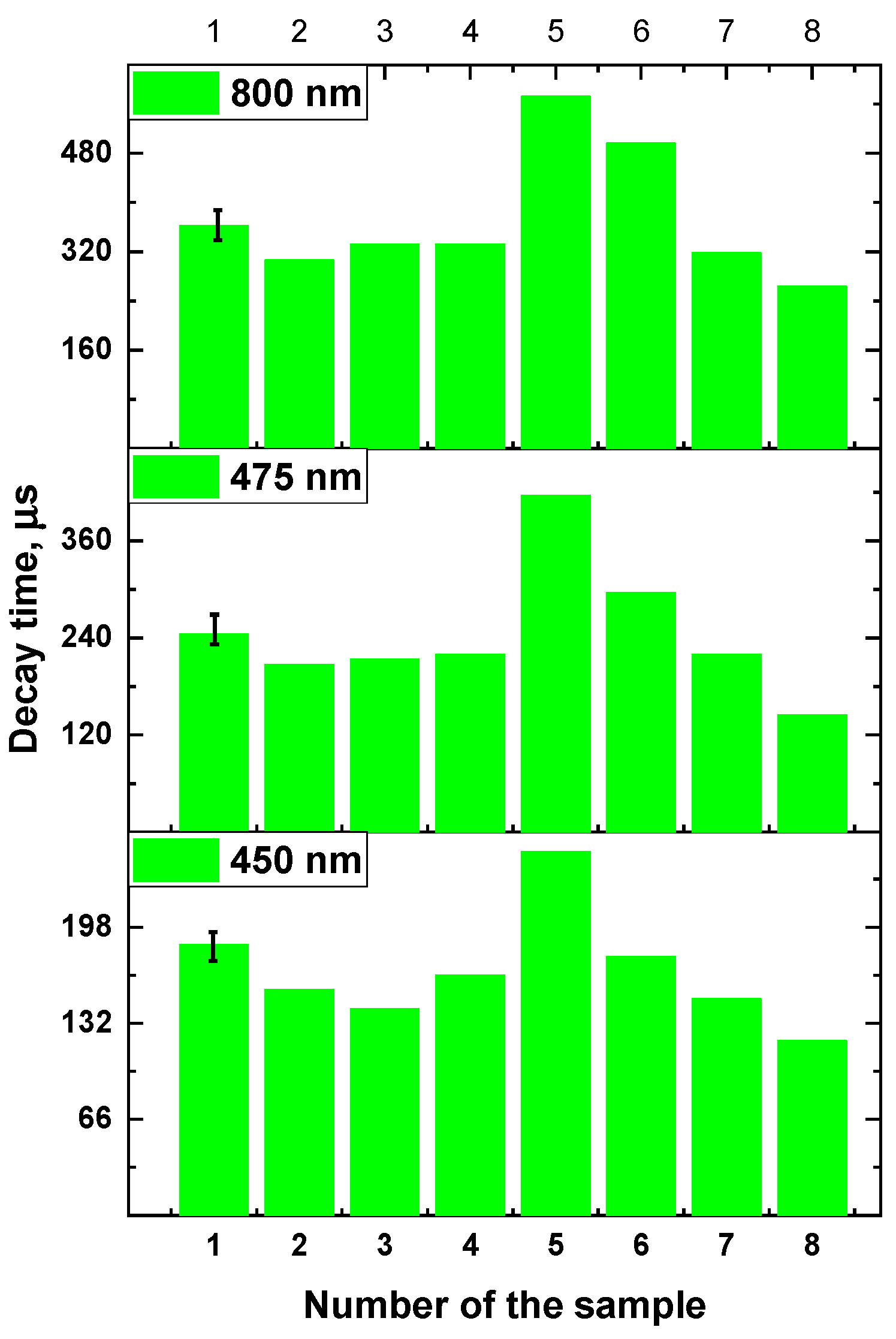
| Number of the sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Concentration Yb, mol.% | 10 | 14 | 18 | 22 | 18 | 18 | 18 | 18 |
| Concentration Tm, mol. % | 4 | 4 | 4 | 4 | 1 | 2 | 4 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





