Submitted:
08 January 2024
Posted:
09 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1 Sources of the Materials
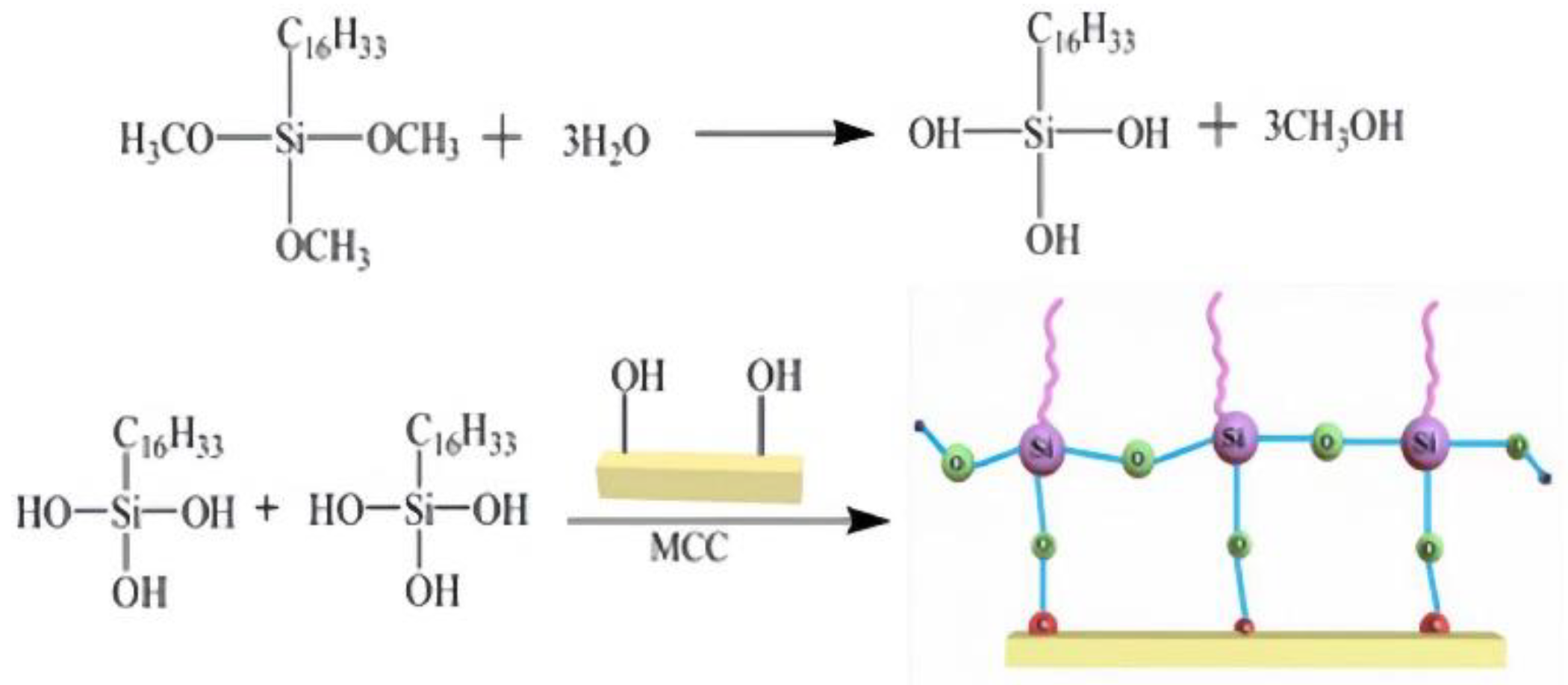
2.2 Synthesis of SG
2.3 Preparation of PBAT/SG composites
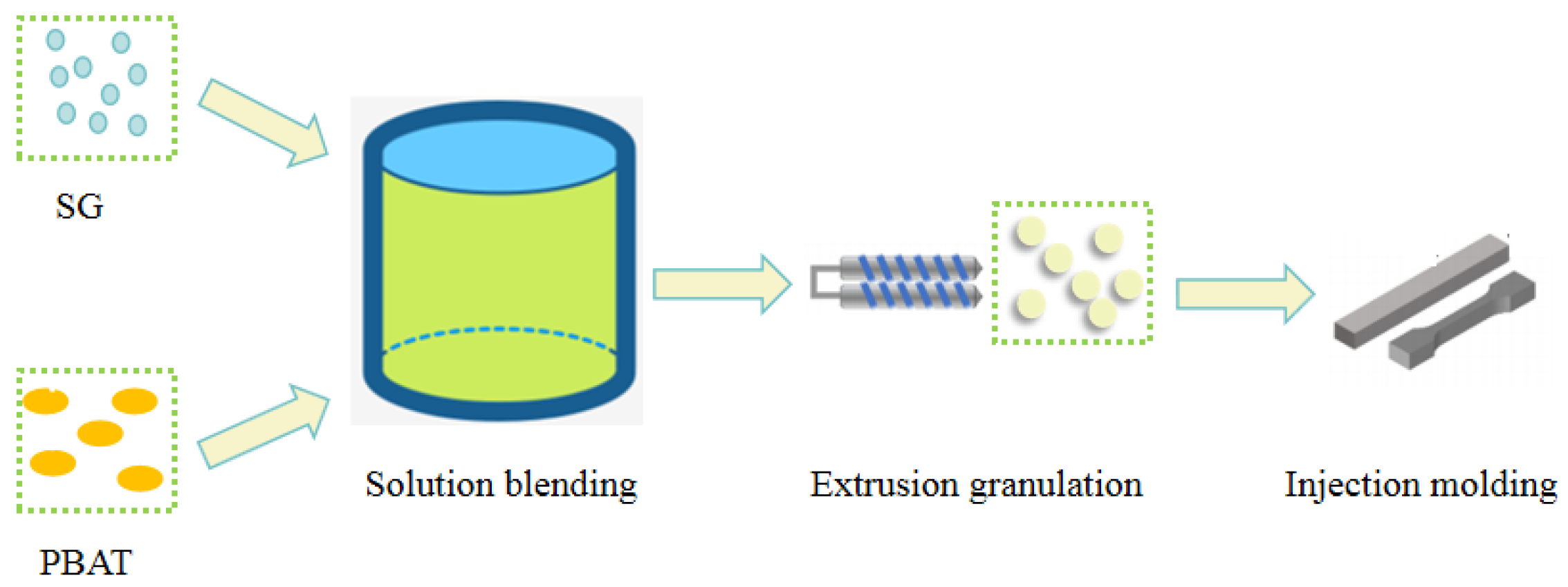
2.4 Measurements and characterizations
3. Results and Discussions
3.1. Synthesis and characterization of SG
3.2. Performance analysis of composite materials
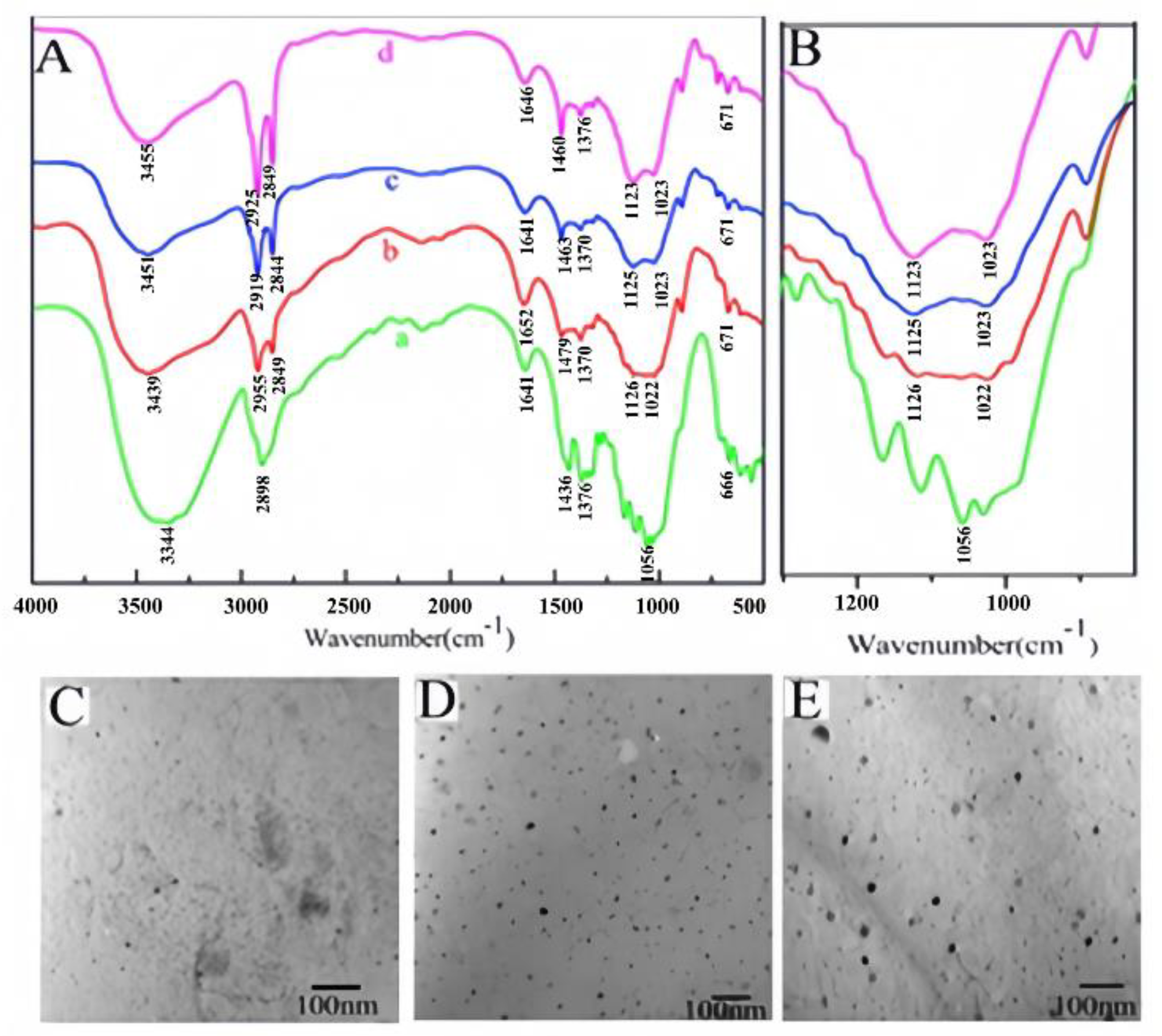
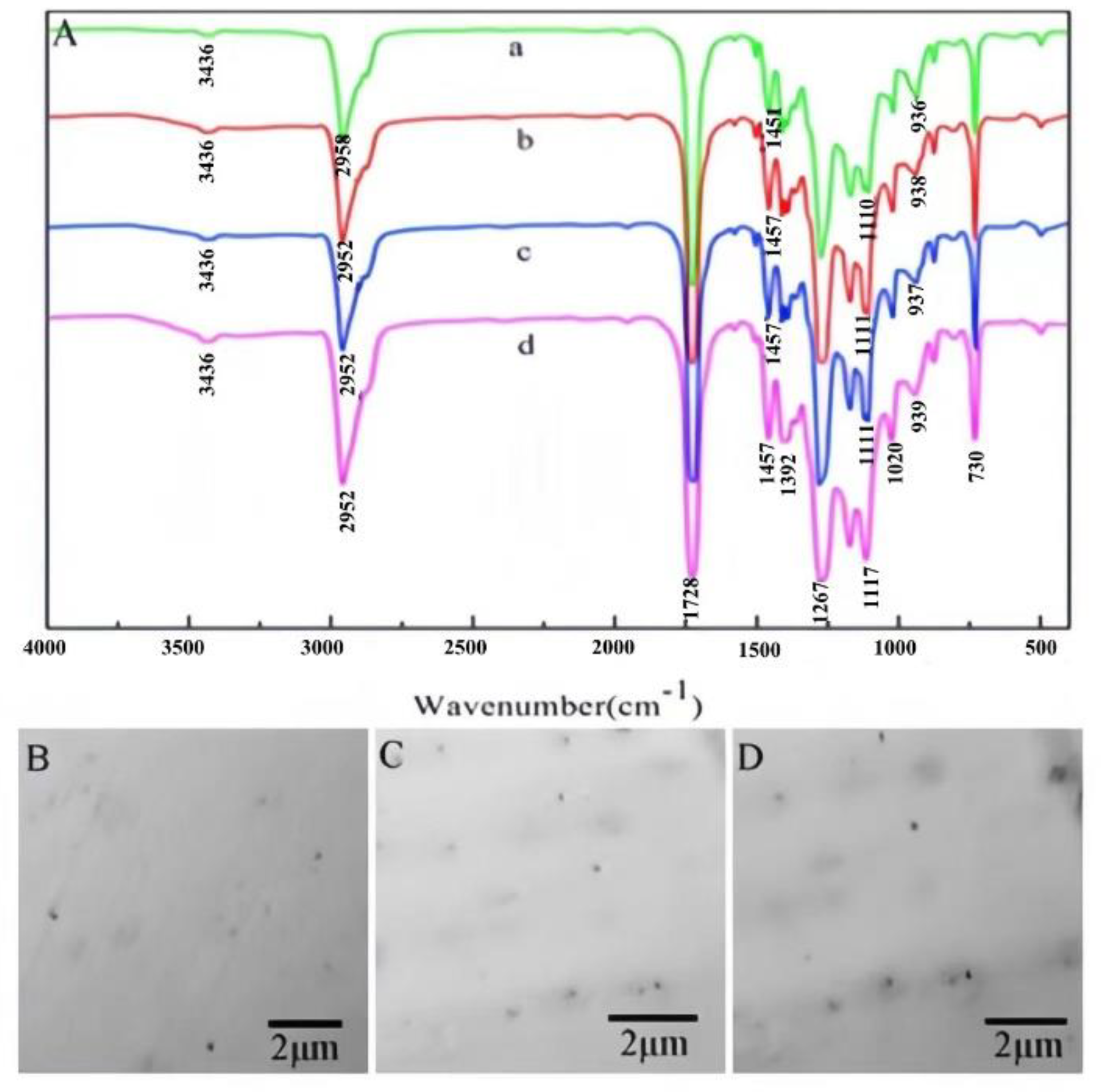
3.2.1. IR and TEM analysis of composite materials
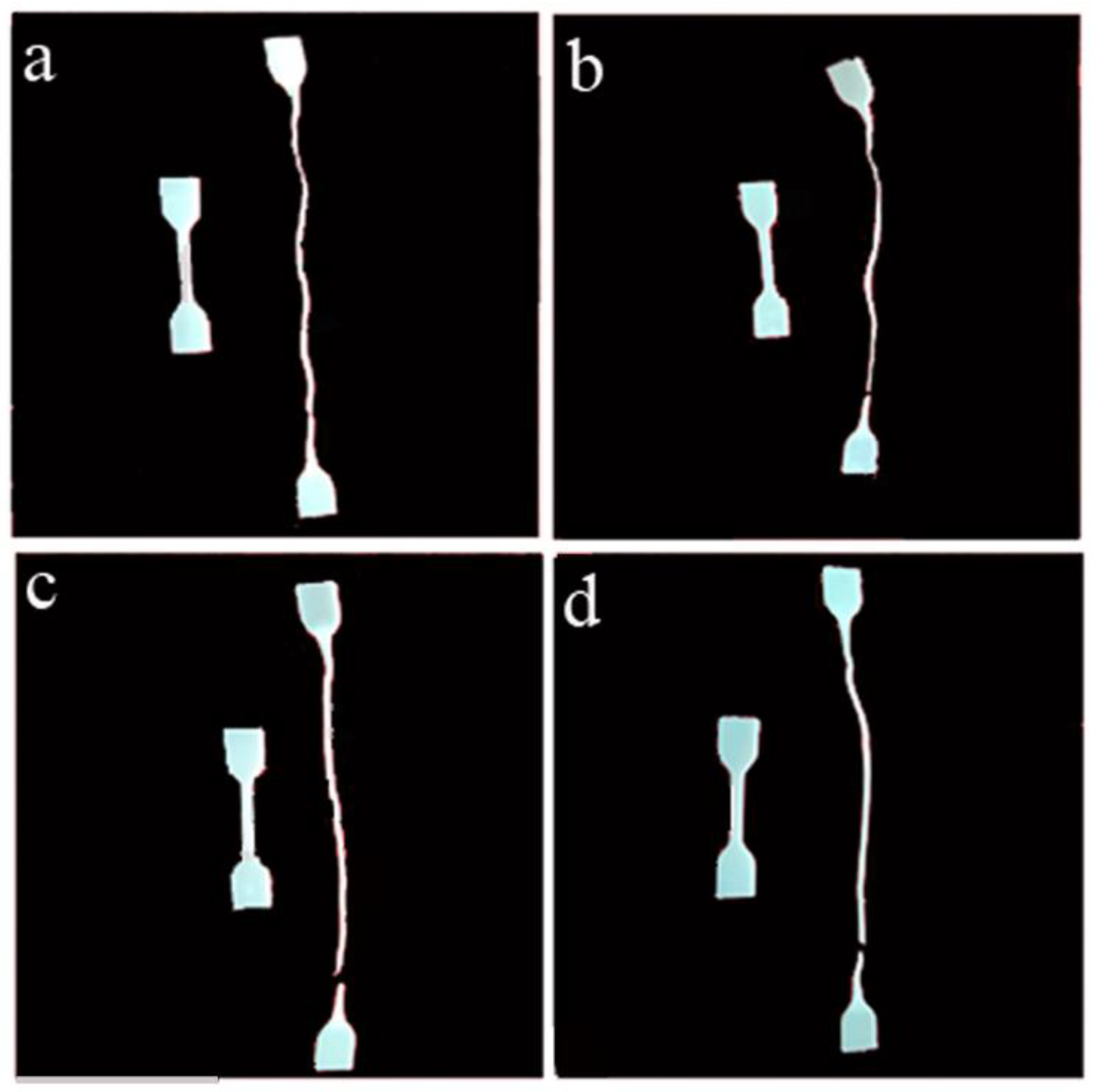
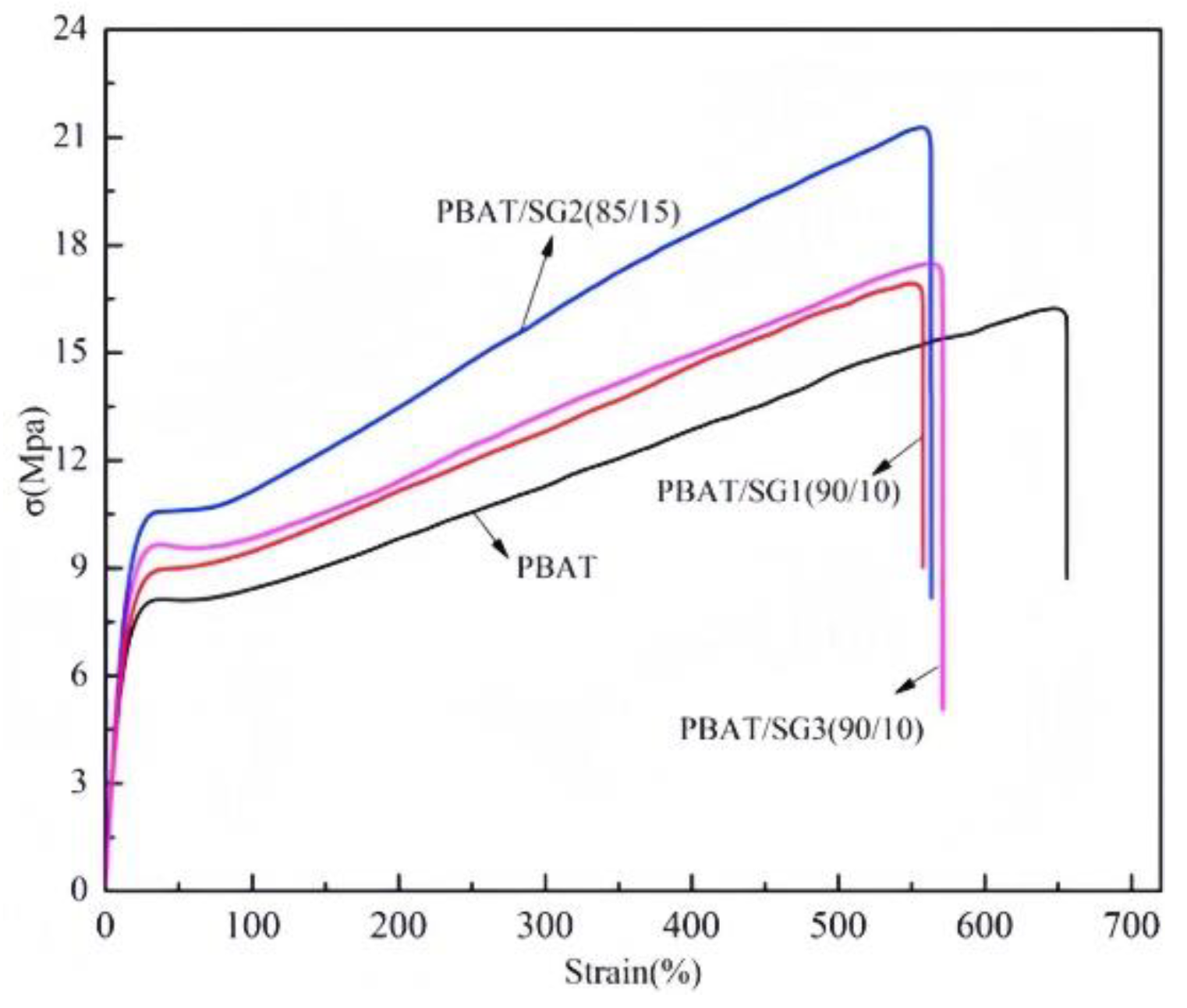
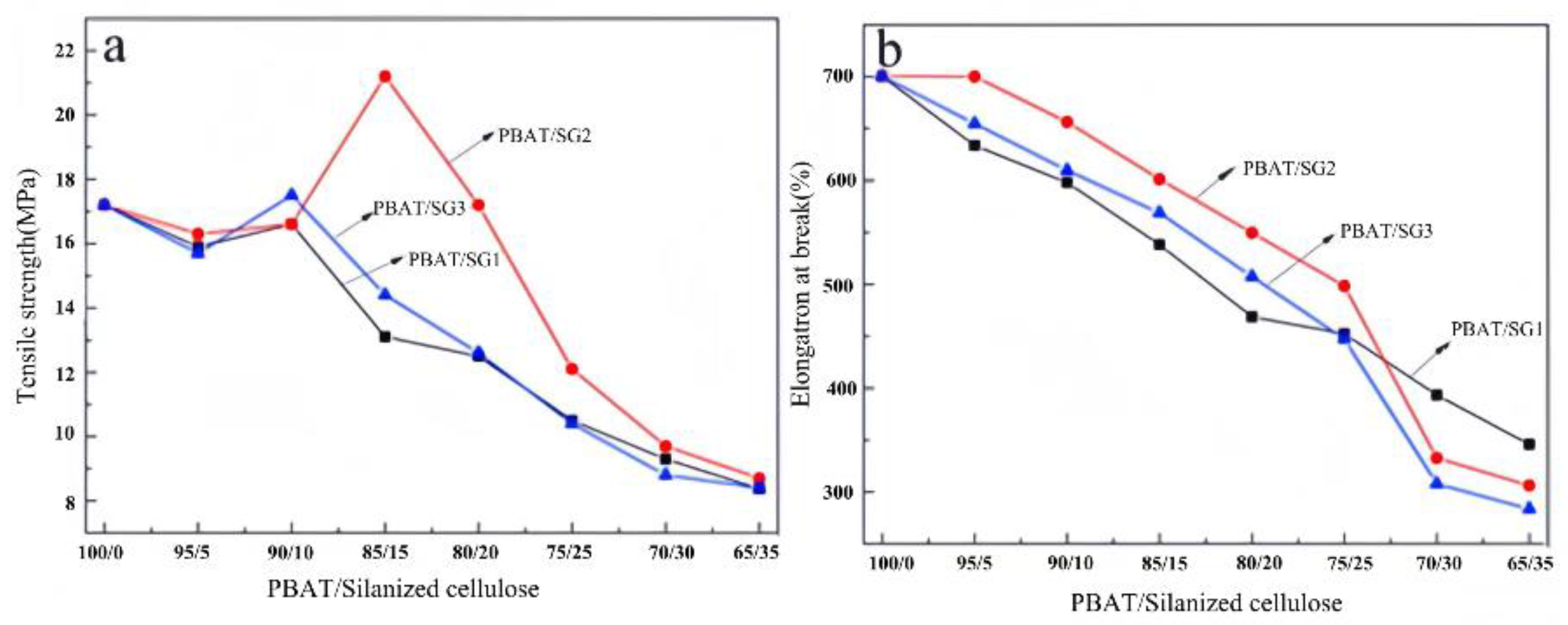
3.2.2. Influence of the SG dosage on the mechanical properties of the composite materials
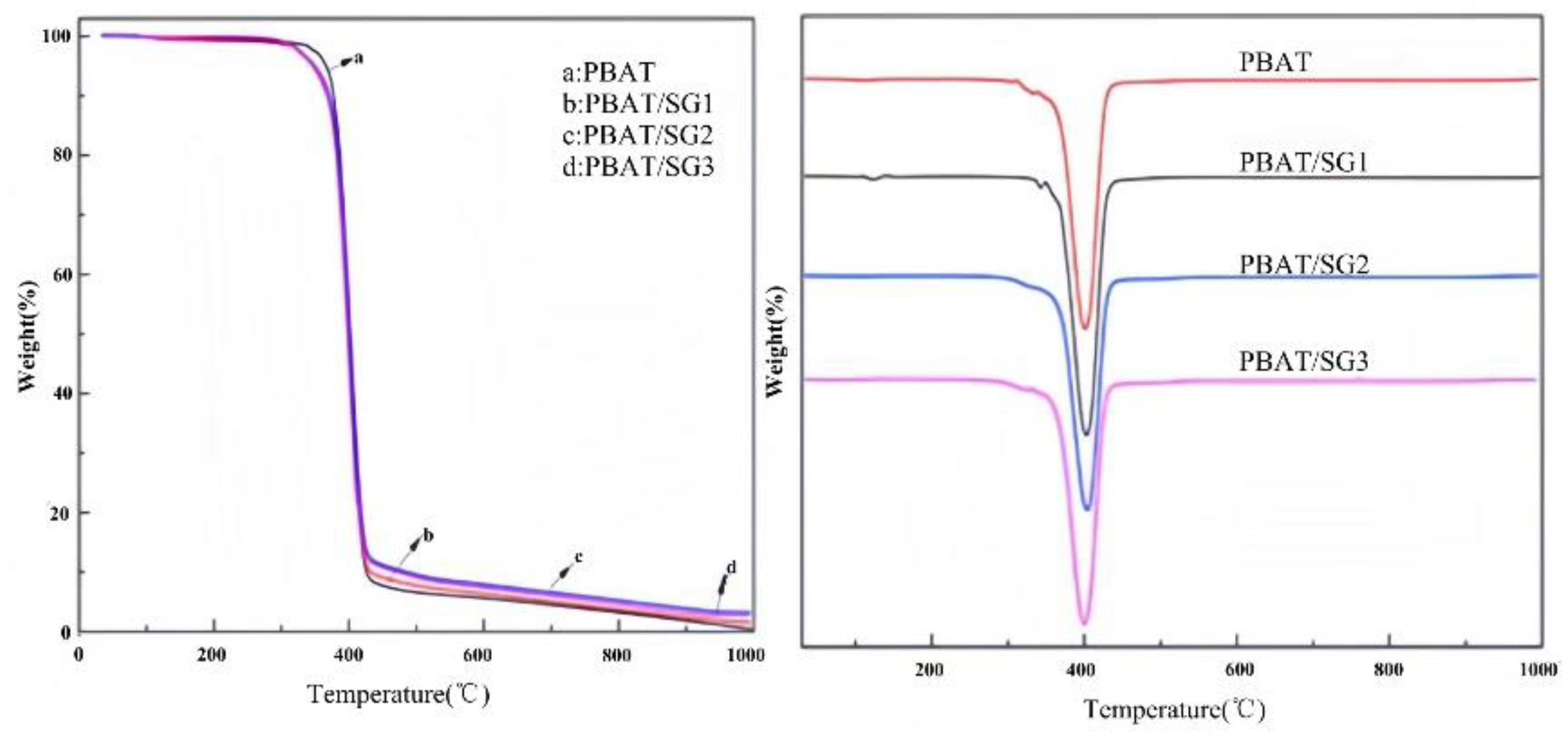
3.2.3. Thermal behavior analysis of different kinds of composite materials
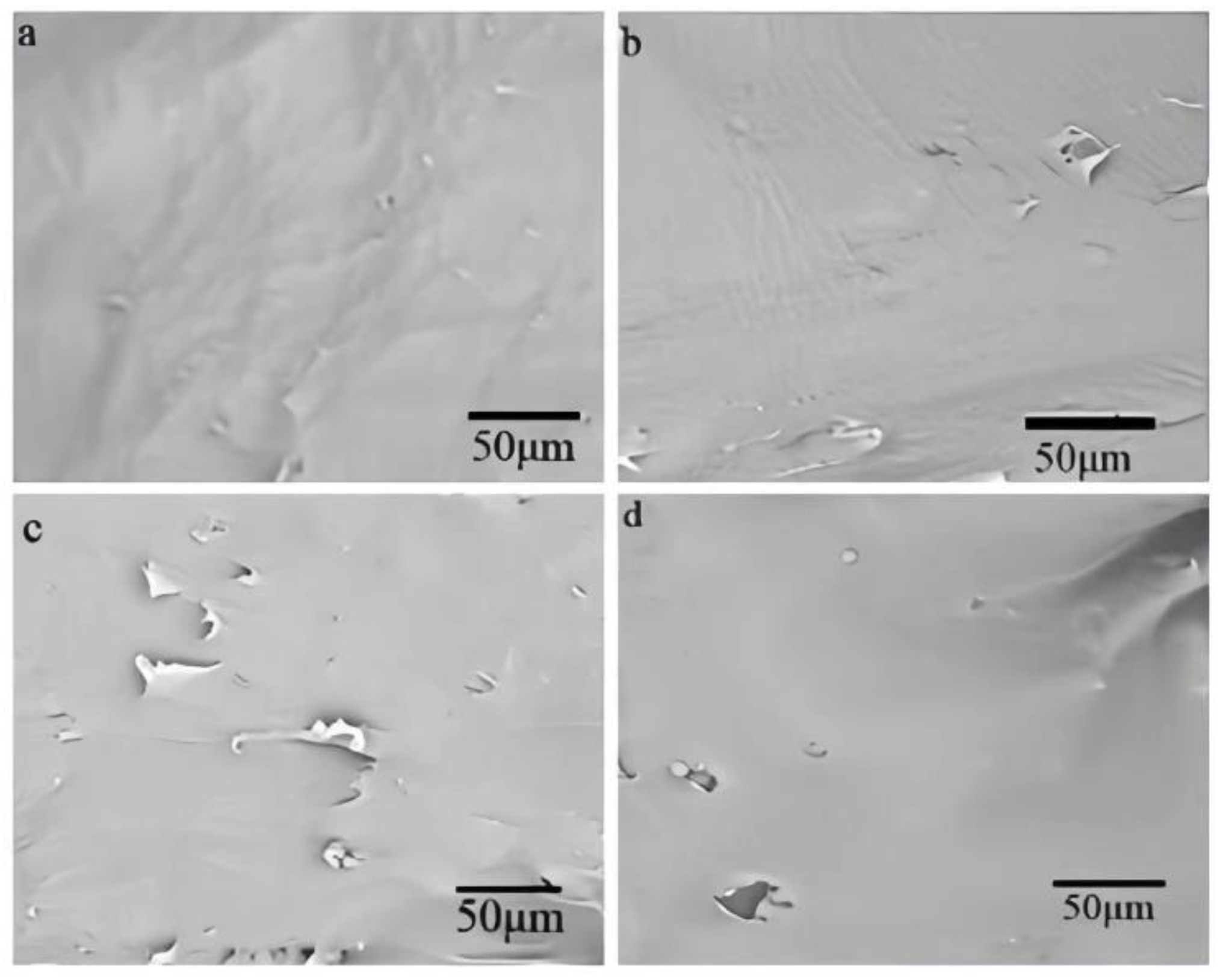
3.2.4. SEM analysis of different kinds of composite materials
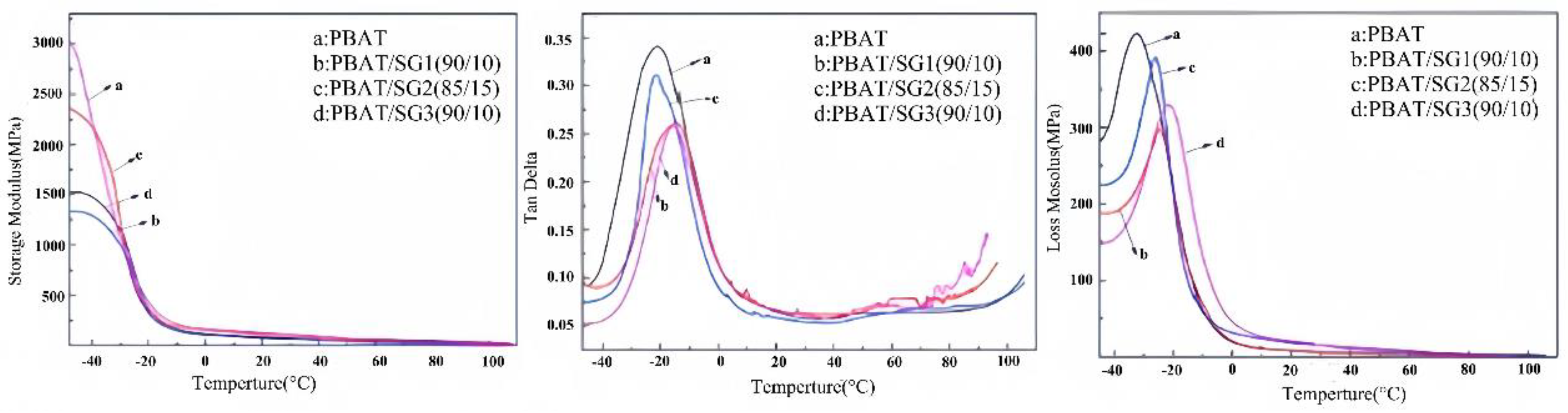
3.2.5. DMA analysis of different kinds of composite materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dufresne, A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr Opin Colloin In. 2017, 29, 1-8. [CrossRef]
- Chaves, R.P.; Fechine, G.J.M. Thermo stabilisation of poly(butylene adipate-co- terephthalate). Polimeros. 2016, 26, 102-105. [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F. R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Inter. Ed. 2019, 58, 50-62. [CrossRef]
- Bruno, V.M.; Aline, S.; Gabriela, F.S.; Luana, M. R.; Fernanda, R.; Anderson, O. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly(butylene adipate-co-terephthalate) fibers. Mat Sci Eng C-Mater. 2016, 59, 782-791.
- Roldán-San Antonio, J.E.; Martín, M. Optimal Integrated Plant for Biodegradable Polymer Production. ACS Sustain Chem Eng. 2023, 11, 2172-2185. [CrossRef]
- Kargarzadeh, H.; Galeski, A.; Pawlak, A. PBAT green composites: Effects of kraft lignin particles on the morphological, thermal, crystalline, macro and micromechanical properties. Polymer. 2020, 203, 122748. [CrossRef]
- Liu, T.; Lian, X.; Li, L.; Peng, X.; Kuang, T. Facile fabrication of fully biodegradable and biorenewable poly (lactic acid)/poly (butylene adipate-co-terephthalate) in-situ nanofbrillar composites with high strength, good toughness and excellent heat resistance-ScienceDirect. Polym Degrad Stab. 2020, 171, 109044.
- Yang, K.; Liu, W.; Zhang, S.; Yu, W.; Shi, J.; Lin, Z.; Zheng, Q. Influence of the aggregated structures of layered double hydroxide nanoparticles on the degradation behavior of poly(butyleneadipate-co-terephthalate) composites. Appl Clay Sci. 2022, 230, 106713. [CrossRef]
- Caligiuri, V.; Tedeschi, G.; Palei, M.; Miscuglio, M.; MartinGarcia, B.; Guzman-Puyol, S.; Hedayati, M.K.; Kristensen, A.; Athanassiou, A.; Cingolani,R.; Sorger,V. J.; Salerno, M.; Bonaccorso, F.; Krahne, R.; Heredia-Guerrero, J.A. Biodegradable and Insoluble Cellulose Photonic Crystals and Metasurfaces. ACS Nano. 2020, 14, 9502-9511. [CrossRef]
- Shanker, R.; Ravi Anusuyadevi, P.; Gamage, S.; Hallberg, T.; Kariis, H.; Banerjee, D.; Svagan, A.J.; Jonsson, M.P. Structurally Colored Cellulose Nanocrystal Films as Transreflective Radiative Coolers. ACS Nano. 2022, 16, 10156-10162. [CrossRef]
- Han, D., Wang, H., Lu, T., Cao, L., Dai, Y., Cao, H., Yu, X. Scalable manufacturing green core-shell structure flame retardant,with enhanced mechanical and flame-retardant performances of polylactic acid. J Polym Environ. 2022, 30, 2516-2533. [CrossRef]
- Yeh, J.; Tsou, C.; Huang, C.; Chen. K.; Wu, C.; Chai, W. Compatible and crystallization properties of poly(Iactic acid)/poly(butylene adipate-co-terephthalate) blends. J Appl Polym Sci. 2010, 116, 680-687.
- Chen, X.; Zeng, Z., Ju, Y., Zhou, M., Bai, H., Fu, Q. Design of biodegradable PLA/PBAT blends with balanced toughness and strength via interfacial compatibilization and dynamic vulcanization. Polymer, 2023, 266 . [CrossRef]
- Wu, C. S. Antibacterial and static dissipating composites of poly(butyleneadipate-co-terephthalate) and multi-walled carbon nanotubes. Carbon. 2009, 47, 3091-3098.
- Zhao, X.; Yu, J.; Wang, X.; Huang, Z.; Zhou, W.; Peng, S. Strong synergistic toughenin and compatibilization enhancement of carbon nanotubes and multi-functional epoxy compatibilizer in high toughened polylactic acid (PLA)/poly (butylene adipate-co- terephthalate) (PBAT) blends. Int J Biol Macromol. 2023, 250, 126204.
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Thermal properties and cold crystallization kinetics of surface-treated banana fiber (BF)-reinforced poly(lactic acid) (PLA) nanocomposites. J Therm Anal Calorim. 2013, 114, 1265–1278. [CrossRef]
- Liu, Z,; Meng, F.; Tang, X.; Su, C.; Mu,Q.; Ju,G. Research on Properties of PBAT/CaCO3 Composite Films Modified with Titanate Coupling Agent. Polymers. 2023, 15, 2379. [CrossRef]
- Lo Re,G.; Engel, E.R.; Björn, L.; Sicairos, M.G.; Liebi, M.; Wahlberg, J.; Jonasson, K.; Larsson, P.A. Melt processable cellulose fibres engineered for replacing oil-based thermoplastics. Chem. Eng. J. 2023, 458, 141372. [CrossRef]
- Zhou, K.; Zhang, M.; Zhang, X.; Wang, T.; Wang, H.; Wang, Z.; Tang, X.; Bai, M.; Li, S.; Wang, Z.; Yue, M. cellulose reinforced polymer composite electrolyte for the wide-temperature-range solid lithium batteries. Chem. Eng. J. 2023, 464, 142537. [CrossRef]
- Malkapuram, R.; Kumar, V.; Negi, Y.S. Recent development in natural fiber reinforced polypropylene composites. J Reinf Plast Comp. 2009, 28, 1169-1189. [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibers, biodegradable polymers and biocomposites: An overview. Macromol Mater Eng. 2000, 276, 1-24.
- Arif, M.F.; Yusoff, P.S.; Ahmad, M.F. Effects of chemical treatment on oil palmempty fruit bunch reinforced high density polyethylene composites. J Reinf Plast Comp. 2010, 29, 2105–2118.
- Araujo, J.R.; Waldman, W.R.; De, M.A. Thermal properties of high densitypolyethylene composites with natural fibers: Coupling agent effect. Polym Degrad Stabil. 2008, 93, 1770-1775.
- Giri, J.; Lach, R.; Le, H.; Grellmann, W.; Saiter, J.; Henning, S.; Radusch H.; Adhikariet, R. Structural, thermal and mechanical properties of composites of poly (butylene adipate-co-terephthalate) with wheat straw microcrystalline cellulose. Polym Bull. 2020, 78, 4779-4795. [CrossRef]
- Hou, L.; Chen, J; Liu, J. Octadecylamine graft-modified cellulose nanofiber and its reinforcement to poly(butylene adipate-co-terephthalate) composites. Paper & Biomaterials. 2023, 7, 42-50.
- Sara, F.; Elena, M.; Cristina, M.; Pierluigi, M. Comparison of solution-blending and melt- intercalation for the preparation of poly(ethylene-co-acrylic acid)/organoclay nano- composites. Eur Polym J. 2008, 43, 1645-1659.
- Das, C.K.; Reddy, C.S. HLDPE/Organic Functionalized SiO2 Nanocomposites with Improved Thermal Stability and Mechanical Properties. Compos Interface. 2005, 11, 687-699. [CrossRef]
- Sankaraiah, S.; Lee, J.M.; Kim, J.H.; Choi, S.W. Preparation and characterization of surface-functionalized polysilsesquioxane hard spheres in aqueous medium. Macromolecules. 2008, 41, 6195-6204. [CrossRef]
- Liu J.; Huang W.; Xing Y.; Li, R.; Dai, J. Preparation of durable superhydrophobic surface by sol–gel method with water glass and citric acid. J Sol-Gel Sci Techn. 2011, 58, 18-23. [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A.; Gandini, A. Modificationof cellulose fibers with functionalized silanes: Effect of the fiber treatment on the mechanicalperformances of cellulose-thermoset composites. J Appl Polym Sci. 2005, 98, 974-984.
- Qu, P.; Zhou, Y.; Zhang, X.; Yao, S.; Zhang, L. Surface modification of cellulose nanofibrils for poly(lactic acid) composite application. J Appl Polym Sci. 2011, 125, 3084-3091. [CrossRef]
- Xu, L.; Zhang, W.; Xu, B.; Cai, Z. Fabrication of superhydrophobic cotton fabricsby silica hydrosol and hydrophobization. Appl Surf Sci. 2011, 257, 5491-5498.
- Banerjee, M.; Saraswatula, S.; Willows, L.G.; Woods, H.; Brettmann, B.Pharmaceutical crystallization in surface-modified nanocellulose organogels. J Matere Chem B. 2018, 6, 7317-7328. [CrossRef]
- Phosee, J.; Wittayakun, J.; Suppakarn, N. Mechanical properties and morphologies of rice husk silica (RHS)/poly(butylene adipate-co-terephthalate) (PBAT) composites: Effect of silane coupling agent. Advanced Materials Research. 2010, 978, 141-144.
| Sample | Mass ratio of HDTMS to MCC | Water absorption (%) |
|---|---|---|
| MCC | - | 82.80 |
| SG1 | 1:10 | 65.59 |
| SG2 | 3:10 | 30.11 |
| SG3 | 5:10 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




