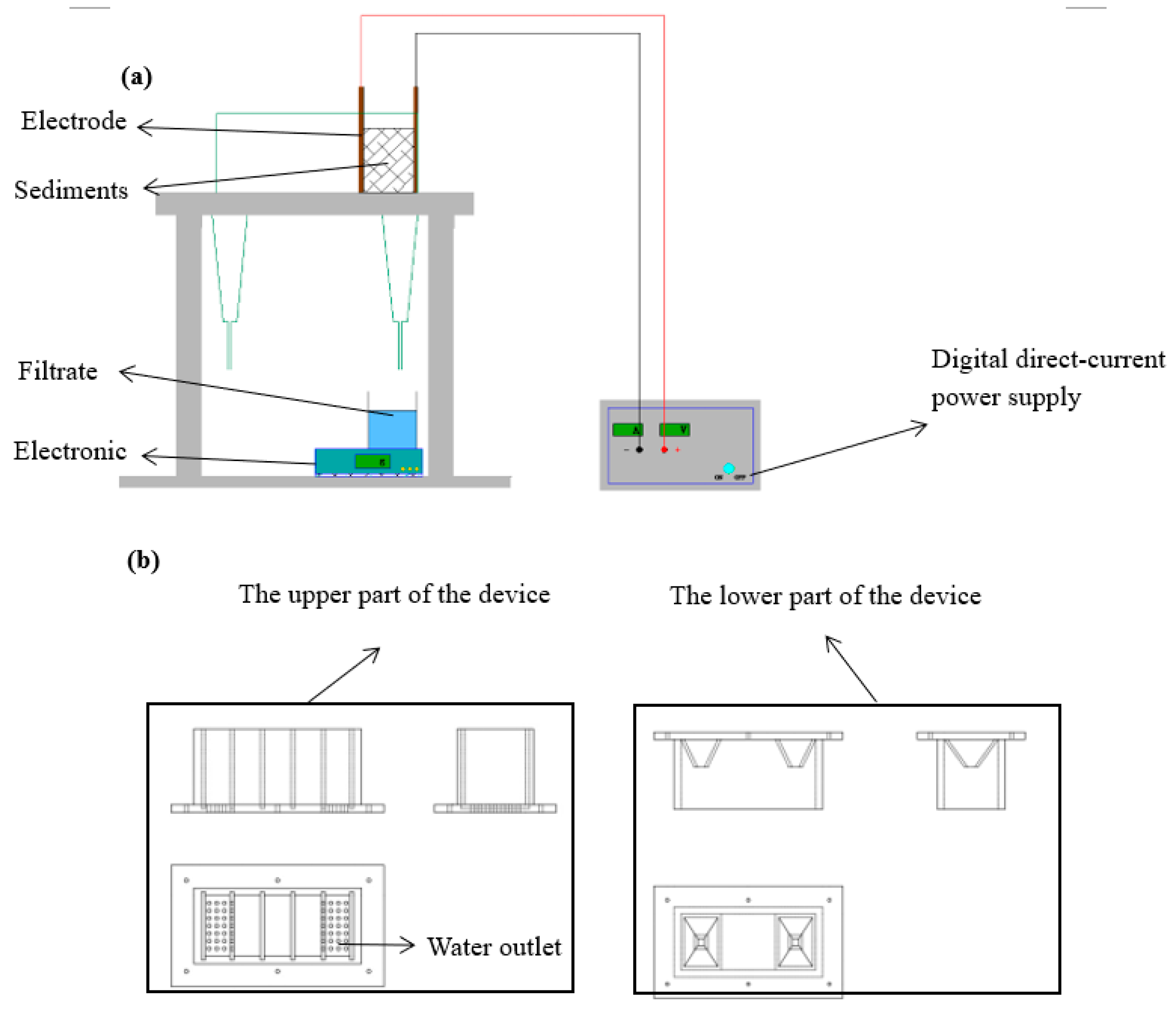
3.1. Effect of different electrode materials on dewatering efficiency
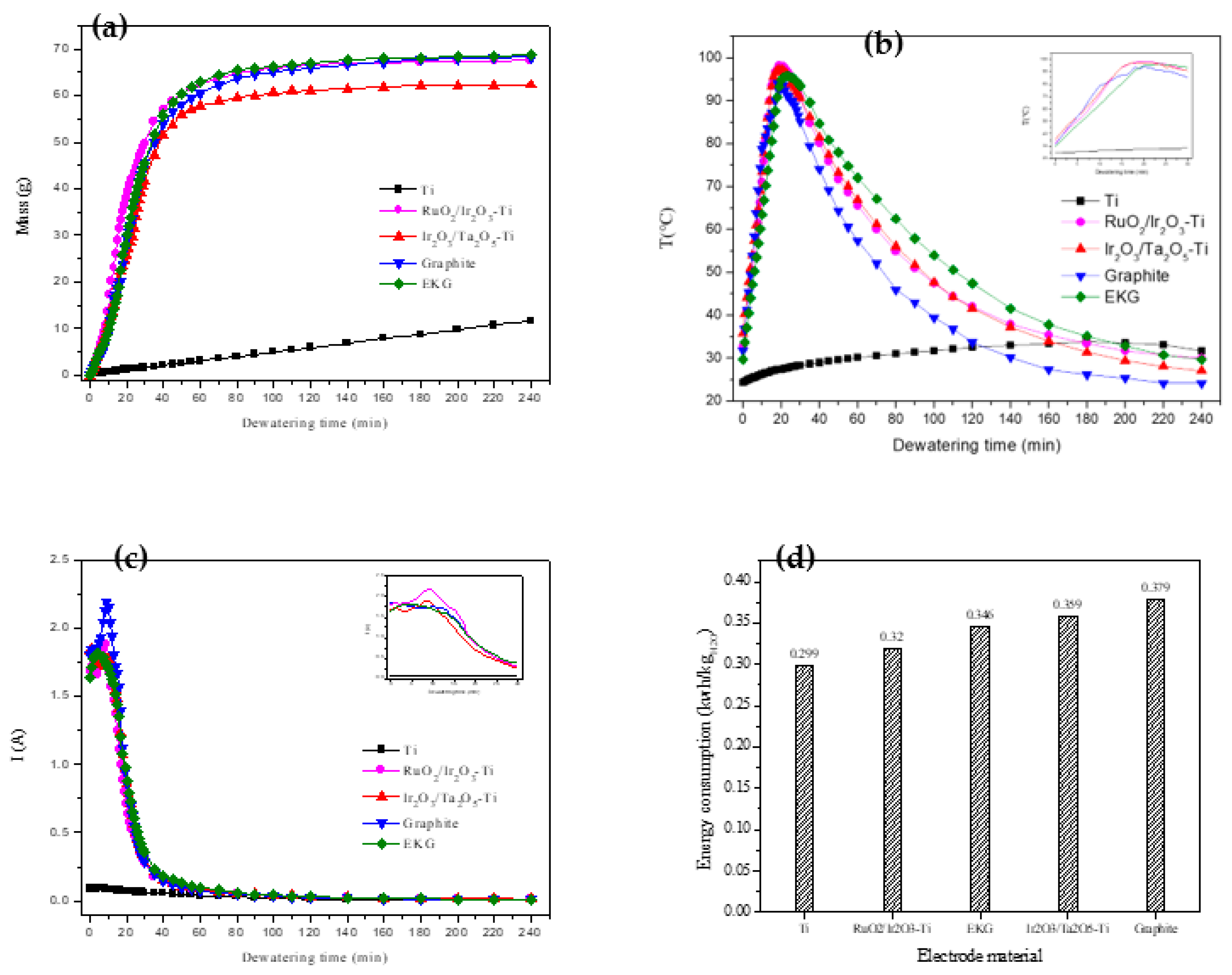
The changes in dehydration mass over time during the electroosmotic dewatering process with different electrode materials are shown in
Figure 2(a). Based on previous research [
30], the electroosmotic dewatering process can be divided into three stages: rapid dehydration, slow dehydration, and minimal dehydration. As can be seen from Fig. (a), in this experiment, Stage I (0-30 min) corresponds to the rapid dehydration phase, Stage II (30-140min) represents the slow dehydration phase, and Stage III (140-240 min) indicates the minimal dehydration phase, except for the Ti electrode.
This anomaly with the Ti electrode can be attributed to the characteristic nature of Ti as a threshold metal. When an electrical field is present, a dense metal oxide film rapidly forms on its anode, hindering further corrosion of the inner metal layer. Simultaneously, its resistance quickly increases, resulting in a rapid rise in the anode voltage drop. Consequently, the voltage drop used for the sludge cake decreases rapidly—an occurrence not conducive to dehydration.
After a comprehensive comparison of the dehydration effects of the five electrode materials, the final water removal quality was ranked as follows: EKG > Ir2O3/Ta2O5-Ti > RuO2/Ir2O3-Ti > Graphite > Ti. Compared to metal oxide-coated electrodes, the EKG electrode demonstrates a comparable, and slightly superior, dehydration performance. The Graphite electrode ranks next in efficiency, while the Ti electrode exhibits the least effective dehydration results.
Figure 2(b) illustrates the time-dependent changes in sludge temperature during the dehydration process with different anode materials. Irrespective of the material type, the sludge temperature saw a rapid rise within the initial 30 minutes after power activation, peaking between 15 and 25 minutes—a phase that aligns with earlier studies [
30].
Following this initial phase, a consistent decrease in temperature was observed. This can be attributed to the low resistance of the sediment at the onset, leading to high heat production rates and a rapid temperature rise. As dehydration proceeded, the sediment's moisture content dropped, resistance increased, and the rate of heat production slowed, causing a consequent decrease in sludge temperature.
Interestingly, the peak temperature for the electrode with the Ti-based coating occurred earlier and was higher than for the other materials. Conversely, the graphite electrode displayed the lowest peak temperature, accompanied by significant fluctuations around the peak. The EKG electrode's peak temperature was slightly delayed compared to those observed for the other electrodes, with the highest temperature lower than that of the Ti-coated electrode.
Prior research suggests that even though a temperature rise in the sediment can lead to greater energy consumption, it facilitates water removal [
31]. The increased temperature reduces soil viscosity to a certain extent, accelerating water molecule movement.
During the slow dehydration phase following the initial 30 minutes, the sludge temperature under the EKG electrode was consistently higher than that under the other electrodes. Upon examining
Figure 2(a), it can be seen that the EKG electrode also maintained a higher dehydration rate than the other electrodes during this stage, and it achieved the greatest volume of water removal by the end of the slow dehydration stage. These findings not only reaffirm that an increase in temperature facilitates water removal; they also demonstrate the superior effectiveness of the EKG electrode in electroosmotic dewatering.
Figure 2(c) presents the variation in current during the dewatering process for the four types of electrodes. Notably, barring the Ti anode, an initial brief increase in current was observed in the process, followed by a swift decline after peaking.
This initial surge in current can be primarily attributed to the fact that the voltage was predominantly applied to the sludge cake at the process's inception. At this stage, polar water molecules and electrolyte ions were moving rapidly. Concurrently, moisture was accumulating on the cathode, while it had not yet dissipated at the anode.
Such conditions led to a decrease in the resistance between the electrode plate and the soil. Consequently, the sludge cake's temperature skyrocketed, and the thermal motion of water molecules and other ions accelerated. This, in turn, reduced the sludge cake's resistance, causing a short-term increase in the current [
32,
33].
As the process continued, the moisture content of the mud cake decreased. This led to a drastic increase in resistance near the anode within the sludge, and the current consequently dropped significantly.
Figure 2(d) illustrates the energy consumption per unit mass of water removed after electroosmotic dewatering using the different electrode materials. The energy consumption for Ti, RuO
2/Ir
2O
3-Ti, EKG, Ir
2O
3/Ta
2O
5-Ti, and Graphite electrodes per unit mass of water removed were 0.299 kWh/kgH
2O, 0.359 kWh/kgH
2O, 0.346 kWh/kgH
2O, 0.359kWh/kgH
2O, and 0.379 kWh/kgH
2O, respectively. Lower energy consumption indicates higher energy utilization efficiency.
Therefore, the relative energy utilization efficiencies of the five types of electrodes during the electroosmotic dewatering process is as follows: Ti > RuO2/Ir2O3-Ti > EKG > Ta2O5-Ti > Graphite. Excluding the Ti electrode, the RuO2/Ir2O3-Ti and EKG electrodes demonstrated superior performance, followed by the Ir2O3/Ta2O5-Ti electrode, with the Graphite electrode performing the worst.
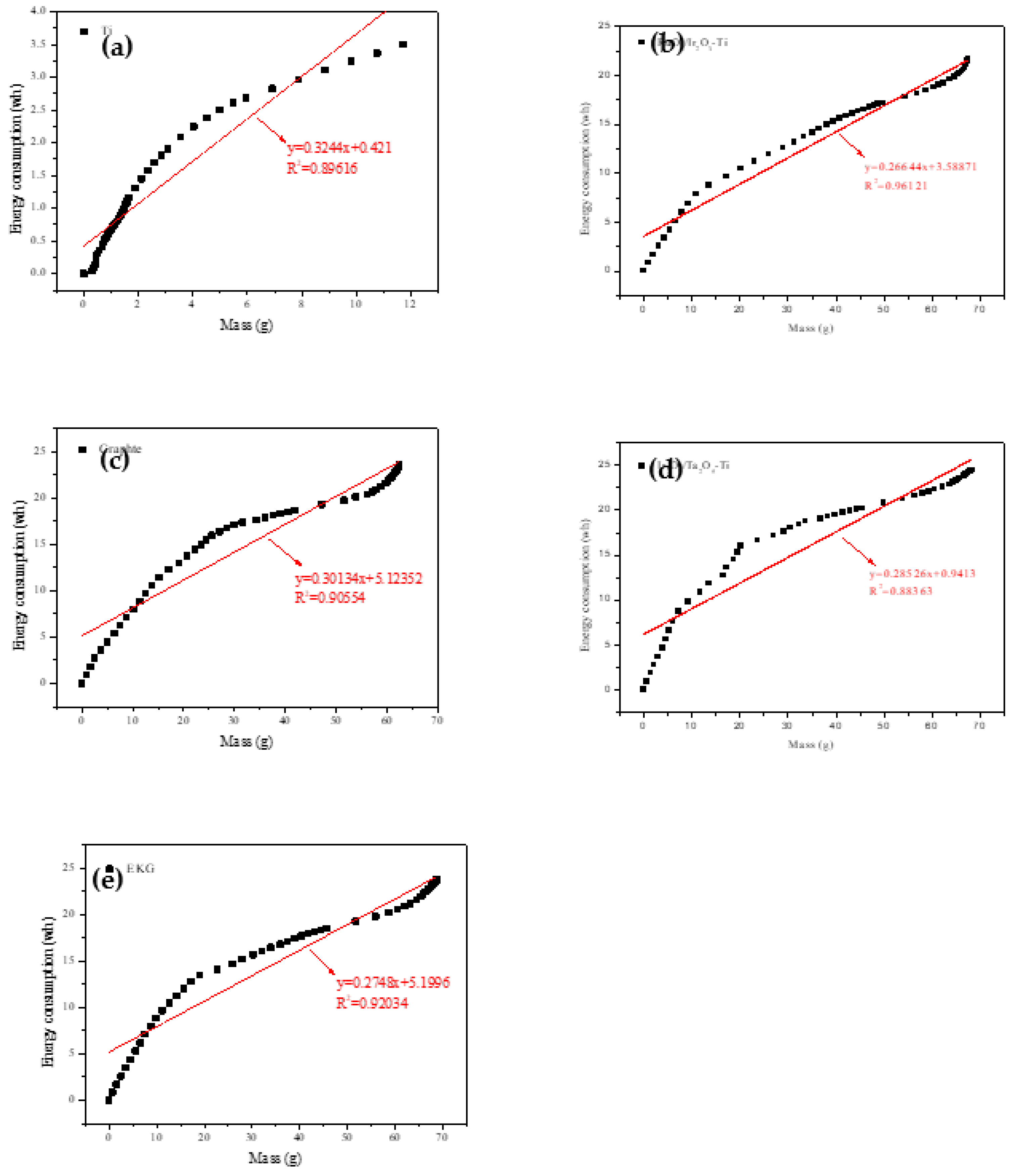
In the present study, we conducted a fitting of the energy consumption and water removal quantity during the dehydration process for the different electrode materials. This resulted in obtaining both actual change curves and fitting curves for dehydration energy consumption and dehydration quality for each electrode material.
Figure 3 presents these findings. Impressively, the curves for all five test groups demonstrated linear relationships, which can be expressed by (Eq. 3):
where ‘a’ and ‘b’ are fitting coefficients. These coefficients are influenced by the electrode material and the physicochemical properties of the underlying mud.
In the context of this study, the voltage applied directly to the two sides of the soil for electrical dehydration is defined as the effective voltage (Ve). Given the existence of an interface resistance between the polar plate and the soil, the expression for the effective voltage is given by (Eq. 4):
Here, V0, Vc, and Vd represent the power supply output voltage, the anode potential drop, and the cathode potential drop, respectively.
During the electrical dehydration process, the slope of the fitted curve is inversely proportional to the energy consumption utilization rate [
33]. According to Eq. (2), if the effective voltage (V
e) increases while the power supply output voltage (V
0) remains unchanged, a smaller anode potential drop (V
c) would indicate a more stable electrode.
Interestingly, the fitting results shown in
Figure 3 reveal that the order of the slopes of the curve is RuO
2/Ir
2O
3-Ti < EKG < Ir
2O
3/Ta
2O
5-Ti < Graphite < Ti. Therefore, the RuO
2 / Ir
2O
3-Ti and EKG electrodes exhibited the greatest stability.
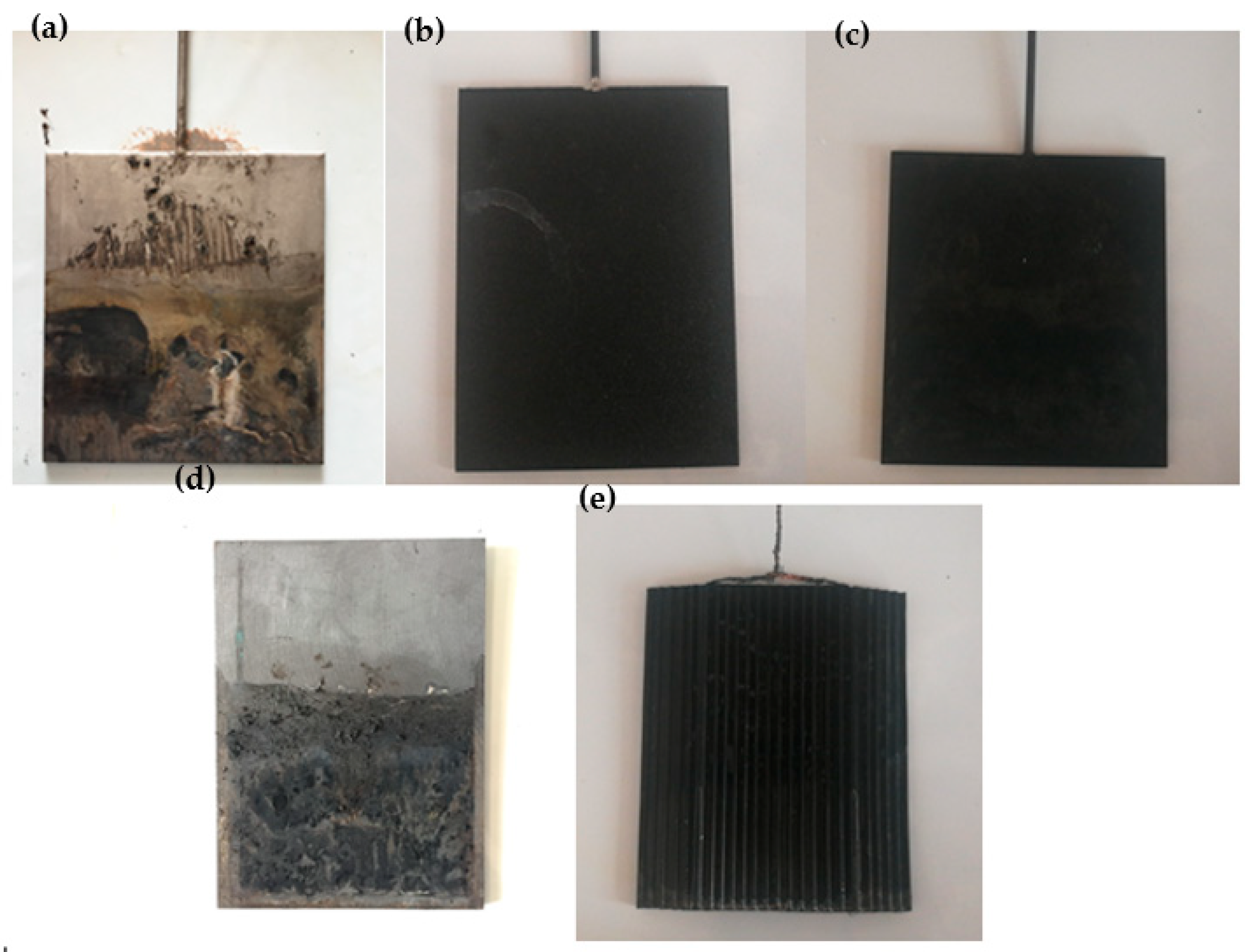
Figure 4 provides a visual representation of the corrosion observed in several anode anti-corrosion electrodes after being energized for 240 minutes. Notably, the Ti electrode underwent a considerable color change on its surface. This alteration was attributed to the formation of an oxide film [
33], which effectively prevented internal corrosion. In contrast, the RuO
2 / Ir
2O
3-Ti– and Ir
2O
3 / Ta
2O
5-Ti–coated electrodes demonstrated negligible corrosion. This observation underscores the superior anti-corrosion properties of these electrodes, a characteristic that has earned them frequent use in the field of electrochemistry. However, the graphite electrodes displayed a significantly higher degree of corrosion. Evidence of extensive pitting was apparent, likely due to fluctuating current during the dehydration process. Last but not least, the EKG materials showed no discernible corrosion on the electrode. This result is comparable to that of the precious metal titanium-based coated electrode, further emphasizing the robustness of EKG materials in resisting corrosion.
3.2. Influence of electric-field strength on EDW
Analysis of
Figure 5 suggests a tripartite division of the dewatering process into distinct stages of rapid dehydration, slow dehydration, and minimal dehydration. The rate of dehydration, denoted by the curve's slope, progressively diminishes, culminating in a plateau of water removal and a stabilization of the final dehydration effect. Concurrently, the electric current exhibits a transient initial increase followed by a swift decrease, eventually tapering off to zero. The temperature profile is characterized by a rapid ascendancy, subsequent decline, and ultimate stabilization around ambient conditions.
A close examination of the trend across these three parameters reveals a noteworthy alignment between the rapid dehydration stage and the periods of swift temperature surge and current decline in the sludge. The transition to the slow dehydration phase coincides with the temperature peaking, mirrored by a slower decrement in the electric current. As dehydration enters the negligible stage, both the temperature and the current approach a state of stability. This is attributed to an escalated resistance in the sludge cake, consequent to water removal and sludge cracking, leading to a reduction in current and deceleration of heat generation.
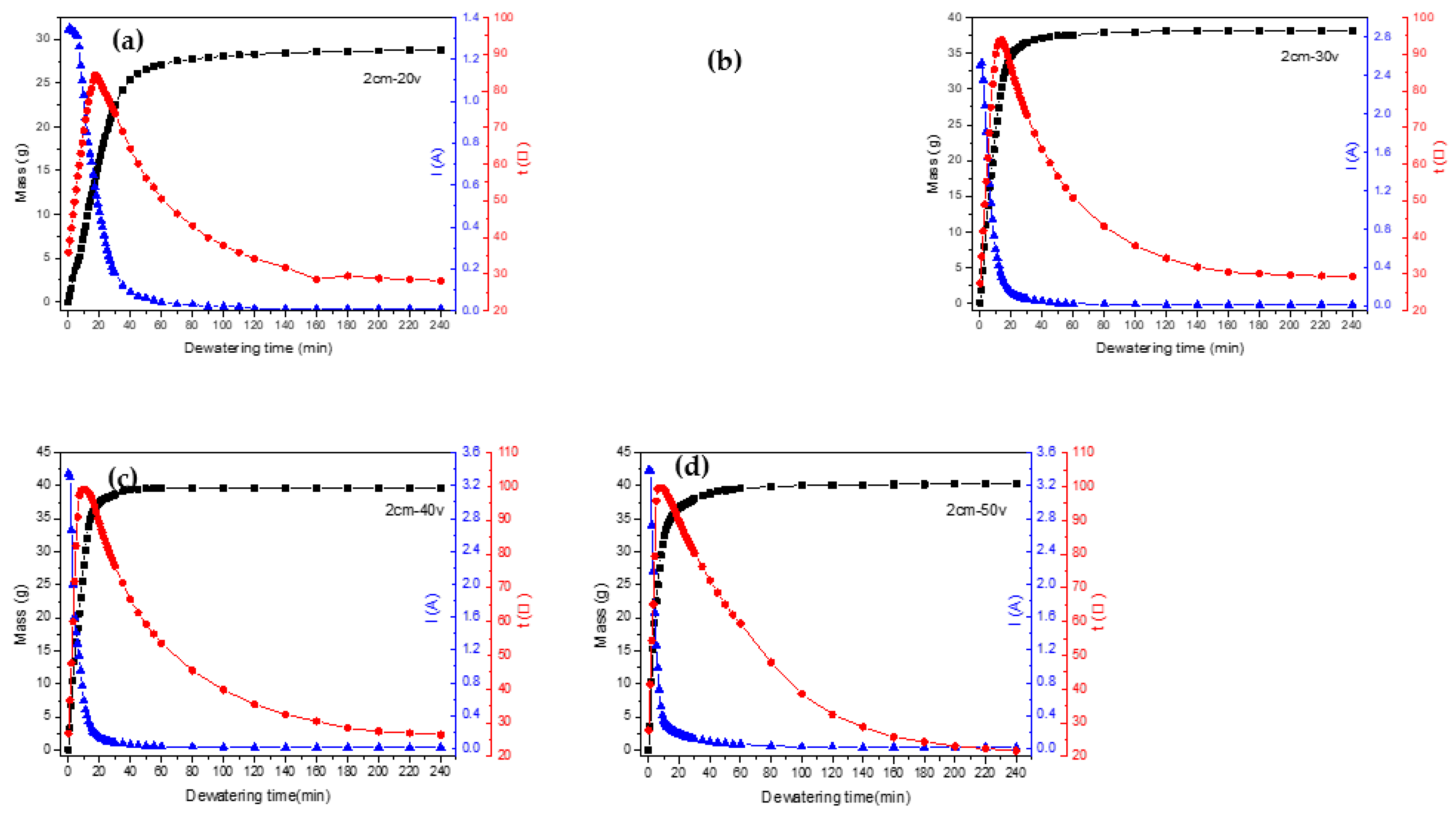
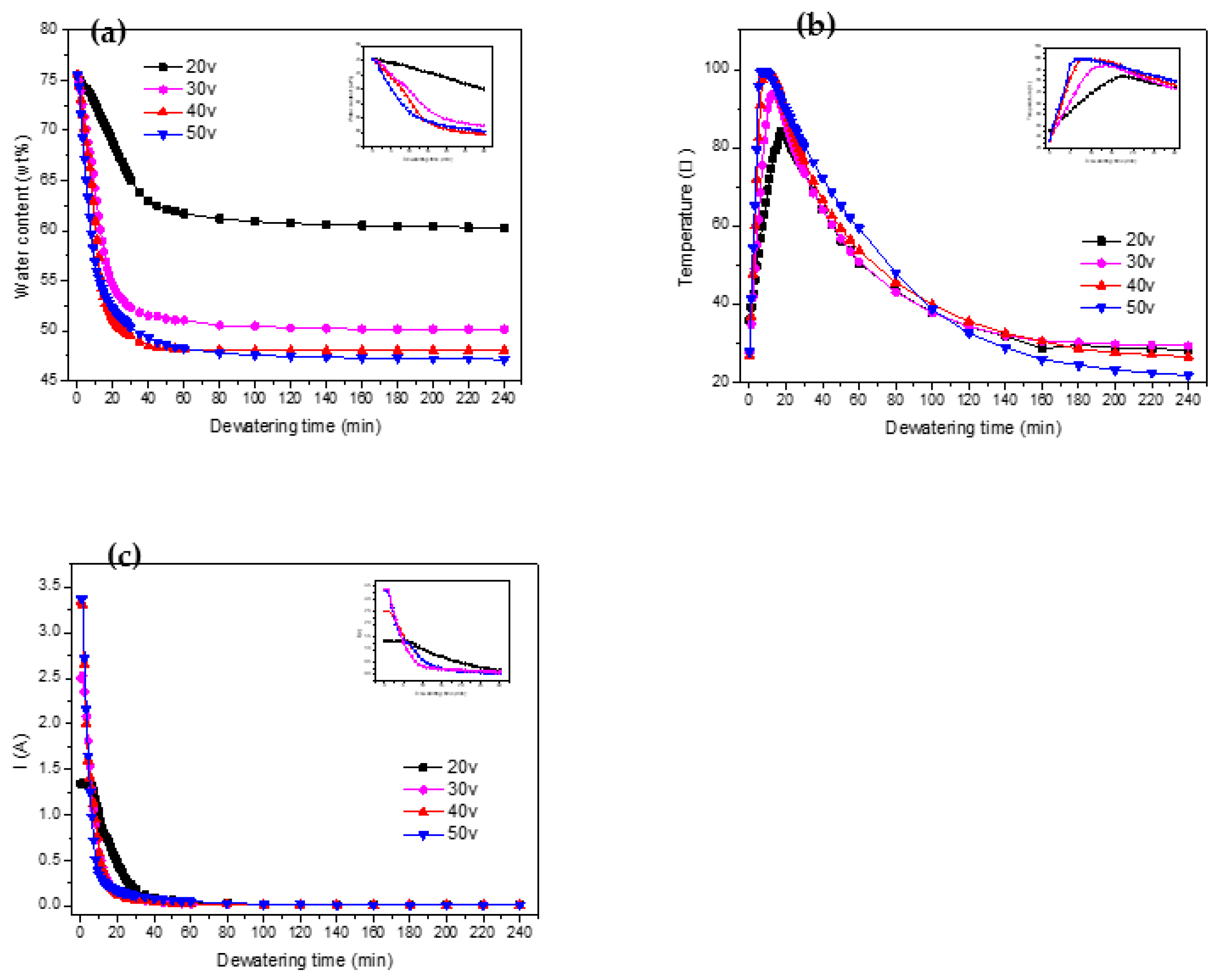
We can see from
Figure 6(a) that with an increase in voltage, the initial rate of the electrodynamic dehydration stage also rose. When the voltages were 20 V, 30 V, 40 V, and 50 V, the times required to reach the basic non-dehydration stage were 100 min, 80 min, 55 min, and 100 min, respectively. The final moisture contents of the four groups of experimental samples were 60.3%, 50.16%, 48.05%, and 47.12%. It can be seen that as the voltage increased, the final moisture content of the sediment decreased, indicating that larger electric-field strength contributed to the final electrodynamic dehydration and curing effect. As the voltage increased from 20 V to 30 V, the rate of dewatering increased by 10.14%. However, when the voltage increased from 30 V to 40 V and from 40 V to 50 V, the rate of dehydration increased by 2.11% and 0.93% respectively. According to the experimental results depicted in
Figure 6(a), during the dewatering process, if the voltage continued to increase at the higher voltages, the improvement in dehydration rate gradually decreased, perhaps because the sludge layer near the anode dried and cracked under the large electric-field strength. One possible explanation for this result is that high voltage could lead to excessive current, which might cause a large amount of gas near the anode drying electrode to increase the resistance. Another possibility is that the cracking of the sludge near the anode led to a reduction in the contact area between the sludge cake and the electrode plate [
23].
Figure 6(b) shows the temperature variation curve for the sediment in the electric dehydration process. The times required to reach the highest temperature, for the four groups of experimental samples, were 17 min, 14 min, 10 min, and 7 min. The highest temperatures reached were 84.3℃, 93.9℃, 99.1℃, and 99.5℃. It can be seen that it took more time to reach the peak temperature when the voltage was higher. The peak temperature itself also rose with the voltage. When the voltage increased from 20 V to 30 V, the peak temperature increased by 9.6℃, while when the voltage increased from 30 V to 40 V and from 40 V to 50 V, the peak temperatures increased by 5.2℃ and 0.4℃, respectively. It can be concluded, then, that "40 V" was the extreme point in this experiment. After this point, the temperature increased less as the voltage rose. As mentioned above, dewatering occurred mainly in the rapid dehydration stage, as expected for the rapid temperature rise. The increase in temperature was beneficial to the removal of water. The experiment also showed that as the voltage increases, the temperature drops more sharply from its peak value, and the improvement in the dehydration rate decreases. The higher voltage improves the dehydration effect as the water content decreases, but the drying out of the anode then causes the soil to crack, increasing the resistance (Ma et al., 2020; Jing et al., 2014; Glendinning et al., 2010).
Figure 6(c) outlines the variation in the loop current over time under different voltage settings. The initial currents for the voltages of 20 V, 30 V, 40 V, and 50 V were 1.34 A, 2.50 A, 3.35 A, and 3.38 A, respectively. This indicates a direct relationship between voltage and initial current, with the latter escalating as the voltage increases. In the first 60 minutes of the experiment, a rapid decrease in the current was observed across all groups, particularly in the first half hour. Beyond the 60-minute mark, the currents for all four groups stabilized, gravitating toward 0.01 A. However, a deviation was observed during the initial 60-minute period at different voltages. The initial current values were higher at 40 V and 50 V, but these also experienced a sharper decline. This rapid decrease was especially pronounced at 50 V, where the accelerated dewatering process caused the sediments around the anode to dry out quickly, leading to significant soil cracking. This soil cracking increased the sediment resistance, which subsequently resulted in a decrease in the loop current. Thus, it can be concluded that as the voltage increased, the current decayed more quickly.
We employed MATLAB software and incorporated the Wild Horse algorithm to fit the curve representing the change in sediment moisture content over time in the electrodynamic sediment dewatering test. After several iterations, an effective fit was achieved using both exponential and Gaussian functions. The results of this fitting process are shown in
Table 2.
Upon analysis, it was evident that the degree of fit (R²) for the 2-dimensional Gaussian function was higher than the exponential function across all groups in the dewatering experiment tests conducted at various voltages. This observation suggests that the relationship between water content and time during the process of electrodynamic dehydration of sediment better aligns with the 2-dimensional Gaussian function. The empirical model for this relationship is presented in Eq. (5), where a
1, b
1, a
2, and b
2 are empirical constants.
Leveraging this equation, we were able to obtain real-time predictions of the sediment's water content. These findings not only highlight the effectiveness of our approach but also provide useful insights for future investigations in this area.
Several previous studies have found a linear relationship between the dehydration rate and the current [
34,
35]. Consistent with this, [
36] discovered that the electrodynamic dehydration effect was more pronounced at higher applied current densities.
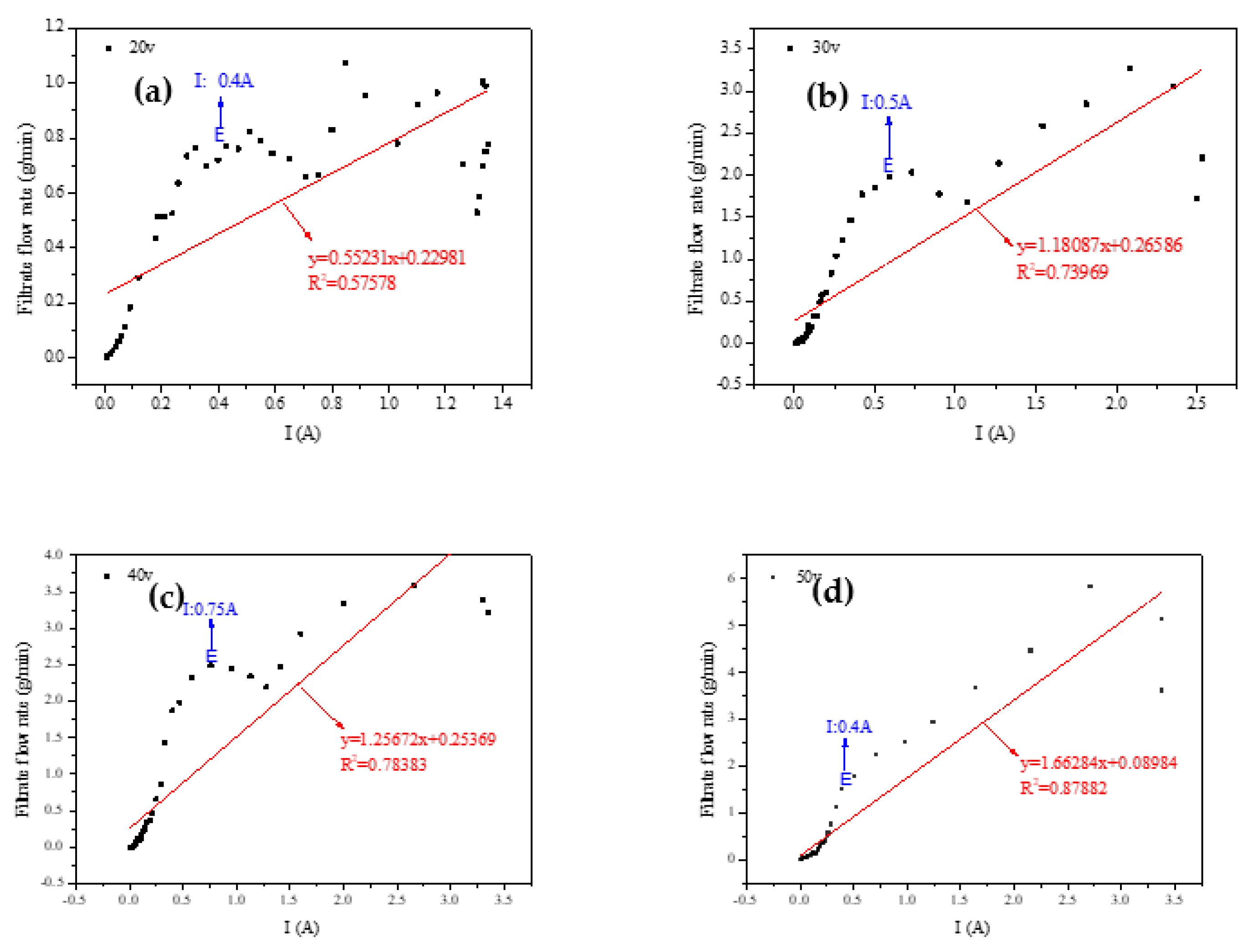
In our study, the observation of
Figure 7 reveals an obvious trend: the degree of curve fitting increases with the voltage. A closer look at the four fitting curves demonstrates that the actual flow rate near the current 0.5 A exceeds the midpoint of the fitting curve. When we juxtapose this with
Figure 5, it becomes apparent that a current of 0.5 A corresponds to the peak temperature of the sediment.
Based on these observations, we conjectured that the dehydration rate is influenced not just by the current density, but also by the temperature of the sediment [
34].
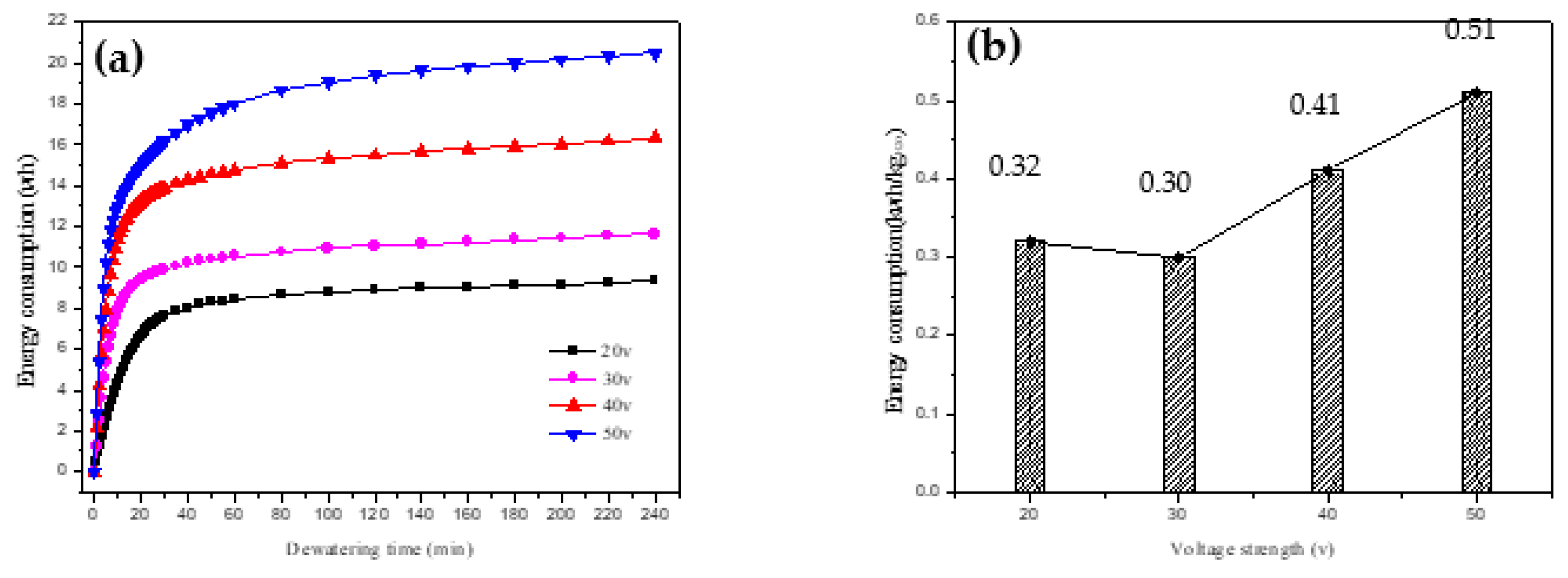
As depicted in
Figure 8(a), the total energy consumption and unit water energy consumption for 240 minutes of dehydration vary with different voltages. The overall energy consumption during dehydration is significantly influenced by the voltage, indicating that the electric-field strength is a critical factor in the energy consumption of electric-power dehydration.
Given a constant plate spacing, an increase in voltage benefits the dehydration process. However, it also results in elevated energy consumption. Thus, in line with the findings of [
35], the selection of an appropriate voltage requires a careful balance between energy consumption and the effectiveness of dehydration.
Further analysis of
Figure 8(b) reveals that the least energy per kilogram of water was consumed at a voltage of 30 V.
3.3. Construction of prediction model
In the field of sludge dewatering, controlling and optimizing operational conditions is essential, to enhance efficiency and achieve desirable results. In this context, we established a response model that accounts for two key variables: electric-field strength and plate spacing.
The electric-field strength, in our model, ranges from 0 to 25 V/cm, and the plate spacing varies from 2 to 8 cm. These ranges were determined based on our experimental setup and the constraints of our equipment.
We implemented the Wild Horse algorithm in our model to predict the response interval. This algorithm, known for its robustness and accuracy, helps us understand the impact of changing operational conditions on the final solid content capacity and dewatering yield.
To ensure the validity of our model, we conducted a model verification process. This involved comparing the model's predictions against actual experimental results, in order to ascertain the model's predictive capability.
We also carried out a significance analysis to ascertain the statistical significance of our findings, to help us ensure that the observed effects were not merely due to chance.
Drawing on insights from the response model, we can optimize the operational conditions for electrodynamic sediment dewatering. By adjusting the electric-field strength and plate spacing according to the model's predictions, we can maximize the final solid content capacity and dewatering yield.
This approach not only improves the efficiency of the dewatering process but also provides a methodological framework that other researchers can use to optimize similar systems. Beyond its immediate application, this model serves as a valuable contribution to the broader field of sediment management and environmental engineering.
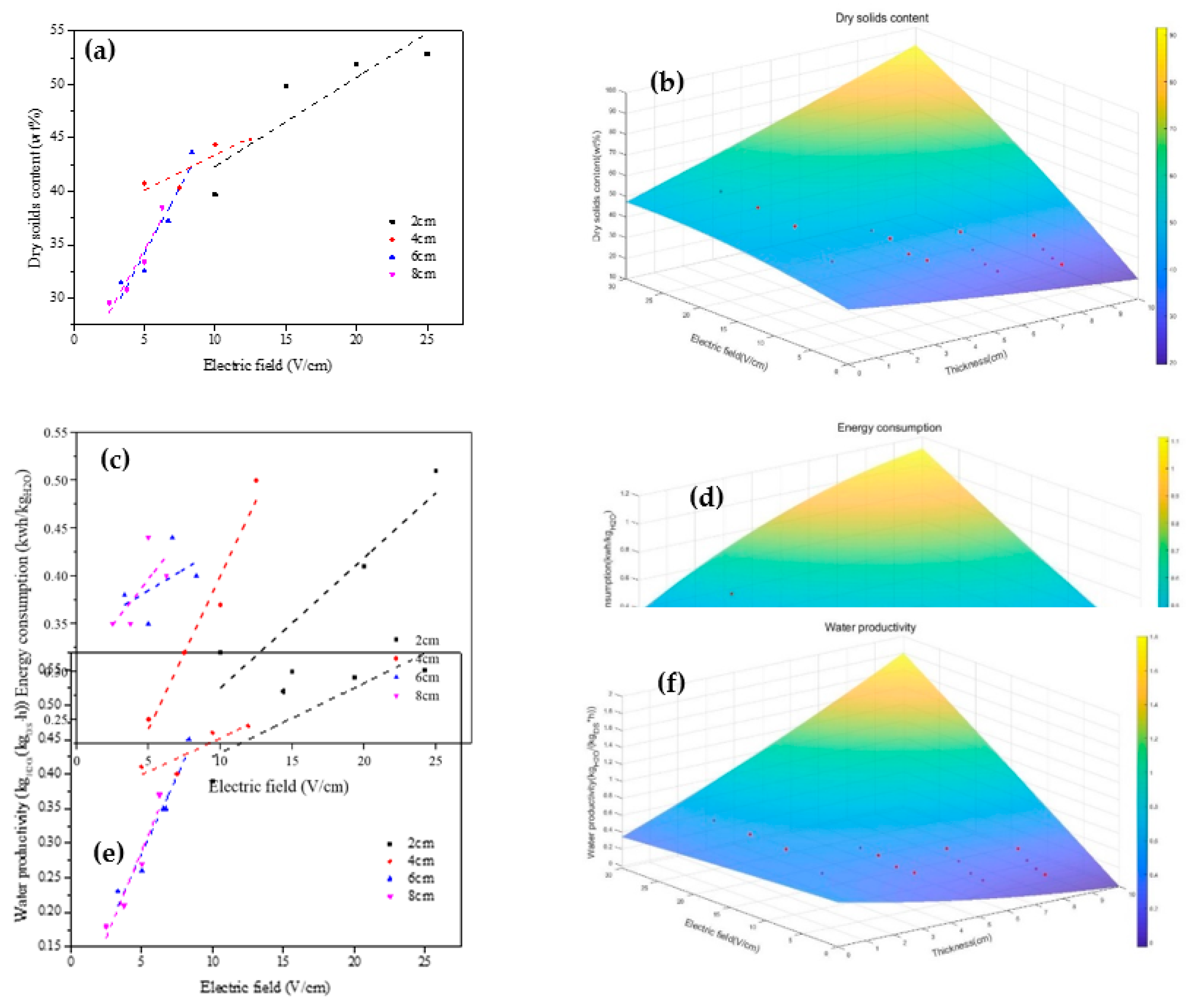
Figure 9(a) illustrates the variation curve of the final solid content and the electric-field intensity at different plate spacings. It becomes evident that with specific plate spacing, the final solid content of the sediments undergoing electrical dewatering increases with the electric-field intensity. This relationship, however, is not linear.
In the initial stages, substantial increases in final solid content are observed with increments in electric-field intensity. For instance, when the electric-field intensity is increased from 10 V/cm to 15 V/cm, the dry solid content is boosted from 39.7% to 49.84%, marking an increase of 25.54%.
However, beyond an electric-field intensity of 10 V/cm, further increases in field intensity result in relatively smaller increments in the final solid content. As an example, a rise in electric-field strength to 20V leads to a mere 4.23% increase in dry solid content (49.84% to 51.95%).
This indicates, as supported by [
15], that the impact of increasing electric-field strength on the final solid content is significant at low electric-field strength but diminishes as the field strength increases beyond a certain point. It reaffirms the necessity of optimizing electric-field intensity to achieve efficient sediment dewatering.
Figure 9(b) shows the response model of the final solid content affected by the electric-field intensity and plate spacing, and its functional relationship is shown in Eq. (6):
(R2=0.9286947, MSE=3.7321915)
where x and y represent plate spacing and electric-field strength, respectively, and Z represents the final solid content. From Eq. (6), it can be found that without considering the expression of quadratic and interaction terms, Z is negatively correlated with x and positively correlated with X, indicating that the final solid content is negatively correlated with plate spacing and positively correlated with electric-field intensity. Therefore, increasing plate spacing is not conducive to improving the final solid content, but increasing electric-field intensity is.
As depicted in
Figure 9(c), the relationship between energy consumption per unit mass of water and electric-field intensity is demonstrated at different plate spacings. For a given plate spacing, the energy consumption of electro-dynamic dehydration for sediment escalates with increasing electric-field intensity. Similarly, at a constant electric-field intensity, the energy consumption of electro-dynamic dehydration rises with an increase in plate spacing.
The practicality of electro-dynamic dehydration predominantly hinges on the cost of power. When energy consumption becomes too high, the technology loses its practicality, rendering it an inefficient solution. Consequently, the application of a low electric-field intensity holds practical significance for enhancing the cost efficiency of electrodynamic dehydration.
This observation aligns with the findings of [
37], who established that the appropriate application of electric fields can result in significant energy savings. Therefore, by optimizing the electric-field intensity and plate spacing, we can strike a balance between operational efficiency and energy consumption, thereby improving the overall cost efficiency of sediment dewatering processes.
Figure 9(d) shows the response model of dehydration energy consumption affected by electric-field intensity and plate spacing, and its functional relationship is shown in Eq. (7):
(R2=0.7174657, MSE=0.0012978)
where x and y represent plate spacing and electric-field intensity respectively, and Z represents the energy consumption per unit mass of water removed. From Eq. (7), it can be found that without considering the expression of quadratic and interaction terms, Z and x are positively correlated, indicating that the final solid content is positively correlated with plate spacing and electric-field intensity. Therefore, increasing plate spacing and electric-field intensity will increase energy consumption, which is not conducive to energy saving or consumption reduction.
The rate of water yield is defined as the mass unit of filtrate (kgH
2O/(kg·h) per kilogram of solid content per hour. The relationship between water yield and electric-field strength under different plate spacings is shown in
Figure 9(e), which is similar to
Figure 9(a). It can be seen that the higher the electric-field strength, the higher the rate of dehydration. After the electric-field strength rose above 10 V/cm, the dehydration yield grew more slowly with the increase of electric-field strength. Therefore, increasing the electric-field strength at low electric-field intensity (< 10 V/cm) was more effective for improving the dehydration yield.
Figure 9(f) shows the response model of water loss rate affected by electric-field intensity and plate spacing, and its function relationship is shown in Eq. (8):
(R2=0.9304939, MSE=0.0009287)
where x and y represent plate spacing and electric-field intensity respectively, and Z is the dehydration rate. It can be found from Eq. (8) that when quadratic and interactive expressions are not considered, Z is negatively correlated with x and positively correlated with y, which indicates that the water removal rate is negatively correlated with plate spacing and positively correlated with electric-field intensity. Therefore, neither increasing plate spacing nor reducing electric-field intensity is conducive to improving the water removal rate.