Submitted:
08 January 2024
Posted:
09 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Patients
PET/CT scans and image reconstruction
Imaging analysis
Statistical analysis
3. Results
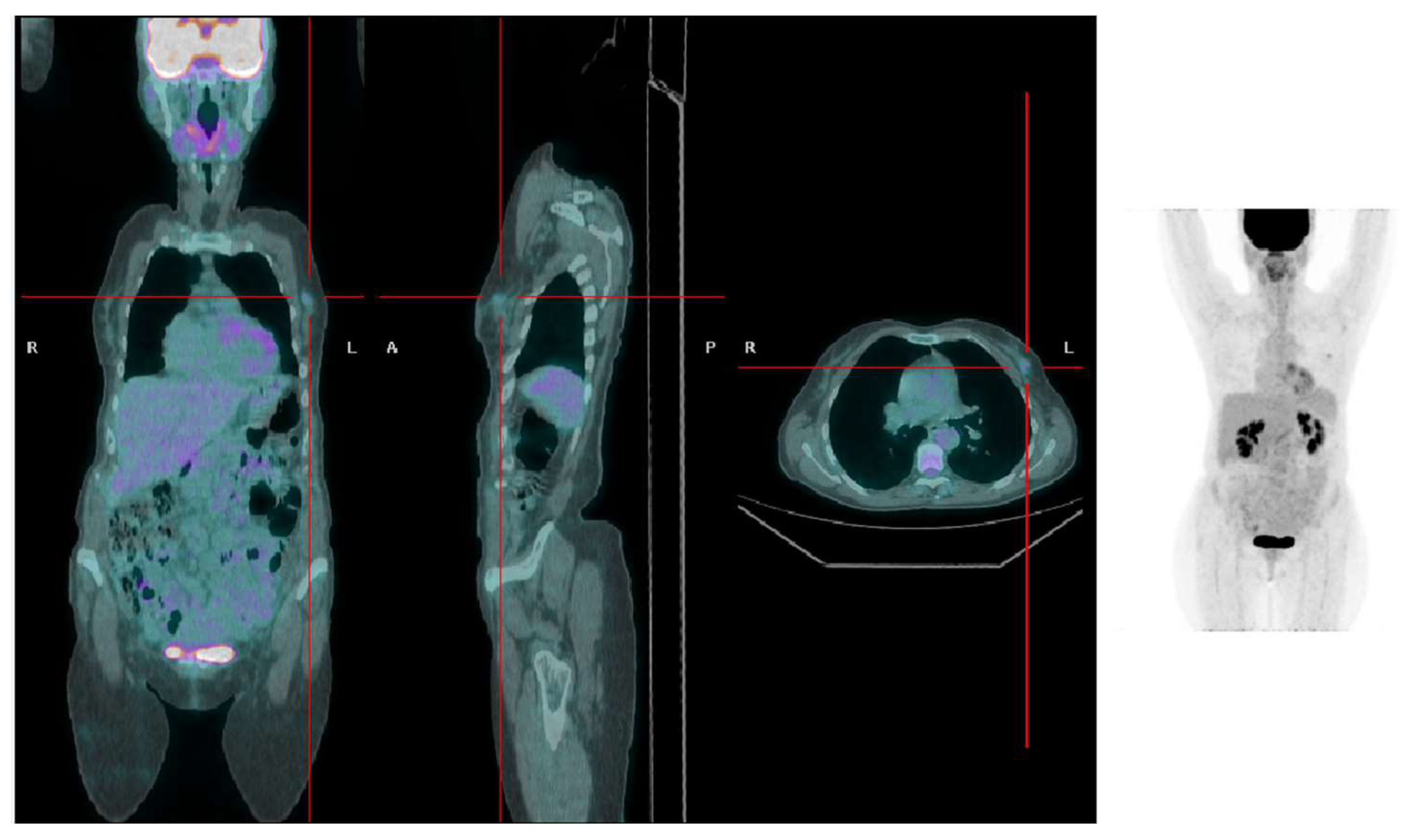
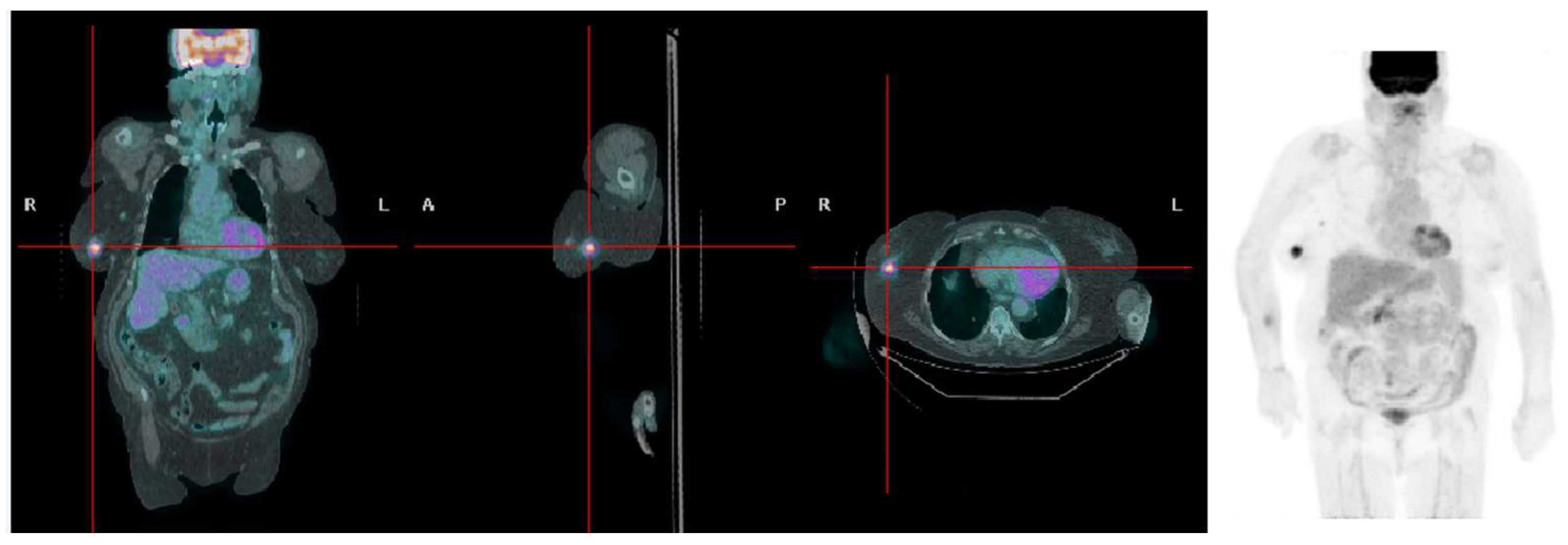
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global cancer observatory: Cancer today. International agency for research on cancer, lyon. 2020.
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the prevention and treatment of obesity-driven effects in breast cancers. Frontiers in oncology 2022, 12, 820968. [Google Scholar] [CrossRef]
- Fakhri, N.; Chad, M.A.; Lahkim, M.; Houari, A.; Dehbi, H.; Belmouden, A.; El Kadmiri, N. Risk factors for breast cancer in women: An update review. Medical oncology (Northwood, London, England) 2022, 39, 197. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Yugawa, K.; Itoh, S.; Iseda, N.; Kurihara, T.; Kitamura, Y.; Toshima, T.; Harada, N.; Kohashi, K.; Baba, S.; Ishigami, K.; et al. Obesity is a risk factor for intrahepatic cholangiocarcinoma progression associated with alterations of metabolic activity and immune status. Sci Rep 2021, 11, 5845. [Google Scholar] [CrossRef]
- Pahk, K.; Joung, C.; Kim, S. Visceral fat metabolic activity evaluated by preoperative (18)f-fdg pet/ct significantly affects axillary lymph node metastasis in postmenopausal luminal breast cancer. Sci Rep 2020, 10, 1348. [Google Scholar] [CrossRef]
- Prentice, A.M. Body mass index standards for children. Are useful for clinicians but not yet for epidemiologists. Bmj 1998, 317, 1401–1402. [Google Scholar] [CrossRef]
- Taieb, A.B.; Roberts, E.; Luckevich, M.; Larsen, S.; le Roux, C.W.; de Freitas, P.G.; Wolfert, D. Understanding the risk of developing weight-related complications associated with different body mass index categories: A systematic review. Diabetology & Metabolic Syndrome 2022, 14, 186. [Google Scholar] [CrossRef]
- Vitolins, M.Z.; Kimmick, G.G.; Case, L.D. Bmi influences prognosis following surgery and adjuvant chemotherapy for lymph node positive breast cancer. The breast journal 2008, 14, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Biglia, N.; Peano, E.; Sgandurra, P.; Moggio, G.; Pecchio, S.; Maggiorotto, F.; Sismondi, P. Body mass index (bmi) and breast cancer: Impact on tumor histopatologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecological Endocrinology 2013, 29, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose-response meta-analysis. Cancer management and research 2018, 10, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Balma, M.; Liberini, V.; Racca, M.; Laudicella, R.; Bauckneht, M.; Buschiazzo, A.; Nicolotti, D.G.; Peano, S.; Bianchi, A.; Albano, G.; et al. Non-conventional and investigational pet radiotracers for breast cancer: A systematic review. Front Med (Lausanne) 2022, 9, 881551. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N.; Alongi, P.; Urso, L.; Ortolan, N.; Borgia, F.; Bartolomei, M.; Arnone, G.; Evangelista, L. 18f-fdg pet-derived volume-based parameters to predict disease-free survival in patients with grade iii breast cancer of different molecular subtypes candidates to neoadjuvant chemotherapy. Cancers 2023, 15, 2715. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up†. Annals of oncology : official journal of the European Society for Medical Oncology 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Leitner, B.P.; Perry, R.J. The impact of obesity on tumor glucose uptake in breast and lung cancer. JNCI Cancer Spectr 2020, 4, pkaa007. [Google Scholar] [CrossRef] [PubMed]
- Balma, M.; Liberini, V.; Buschiazzo, A.; Racca, M.; Rizzo, A.; Nicolotti, D.G.; Laudicella, R.; Quartuccio, N.; Longo, M.; Perlo, G.; et al. The role of theragnostics in breast cancer: A systematic review of the last 12 years. Current medical imaging 2023, 19, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Obesity: Preventing and managing the global epidemic : Report of a who consultation. World Health Organization: 2000.
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. Fdg pet/ct: Eanm procedure guidelines for tumour imaging: Version 2.0. European journal of nuclear medicine and molecular imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Eheman, C.; Henley, S.J.; Ballard-Barbash, R.; Jacobs, E.J.; Schymura, M.J.; Noone, A.M.; Pan, L.; Anderson, R.N.; Fulton, J.E.; Kohler, B.A.; et al. Annual report to the nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012, 118, 2338–2366. [Google Scholar] [CrossRef] [PubMed]
- Saxe, G.A.; Rock, C.L.; Wicha, M.S.; Schottenfeld, D. Diet and risk for breast cancer recurrence and survival. Breast Cancer Research and Treatment 1999, 53, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Lee, J.Y.; Sterner, C.J.; Solomon, A.C.; Pant, D.K.; Shen, F.; Peraza, J.; Vaught, L.; Mahendra, S.; Belka, G.K.; et al. Impact of obesity on breast cancer recurrence and minimal residual disease. Breast Cancer Research 2019, 21, 41. [Google Scholar] [CrossRef]
- Yuan, Q.; Du, M.; Loehrer, E.; Johnson, B.E.; Gainor, J.F.; Lanuti, M.; Li, Y.; Christiani, D.C. Postdiagnosis bmi change is associated with non-small cell lung cancer survival. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2022, 31, 262–268. [Google Scholar] [CrossRef]
- Hatami, S.; Frye, S.; McMunn, A.; Botkin, C.; Muzaffar, R.; Christopher, K.; Osman, M. Added value of digital over analog pet/ct: More significant as image field of view and body mass index increase. J Nucl Med Technol 2020, 48, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Koopman, D.; van Dalen, J.A.; Stevens, H.; Slump, C.H.; Knollema, S.; Jager, P.L. Performance of digital pet compared with high-resolution conventional pet in patients with cancer. J Nucl Med 2020, 61, 1448–1454. [Google Scholar] [CrossRef]
- Merath, K.; Mehta, R.; Hyer, J.M.; Bagante, F.; Sahara, K.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; et al. Impact of body mass index on tumor recurrence among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma- a multi-institutional international analysis. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2019, 45, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Pahk, K.; Ryu, K.J.; Joung, C.; Kwon, H.W.; Lee, S.; Park, H.; Kim, T.; Song, J.Y.; Kim, S. Metabolic activity of visceral adipose tissue is associated with metastatic status of lymph nodes in endometrial cancer: A (18)f-fdg pet/ct study. International journal of environmental research and public health 2021, 19. [Google Scholar] [CrossRef]
- Shinkawa, H.; Tanaka, S.; Takemura, S.; Ito, T.; Aota, T.; Koda, M.; Miyazaki, T.; Yamamoto, T.; Kubo, S. Obesity and recurrence-free survival in patients with hepatocellular carcinoma after achieving sustained virological response to interferon therapy for chronic hepatitis c. Annals of gastroenterological surgery 2018, 2, 319–326. [Google Scholar] [CrossRef]
| Overweight | Normal weight | p. | |
| n. | 84 | 58 | |
| BMI | 31.43±5.59 | 22.91±2.55 | <0.001 |
| age | 66.01±11.11 | 60.5±11.45 | 0.005 |
| Any recurrence | 35 | 13 | 0,45 |
| T_rec | 15 | 2 | 0.018 |
| SUVmax T_rec | 4.74±2.9 | 1.85±0.63 | 0.09 |
| N_rec | 21 | 7 | 0.18 |
| SUVmax N_rec | 6.57±4.08 | 3.14±1.62 | 0.004 |
| M_rec | 26 | 10 | 0.27 |
| SUVmax M_rec | 5.94±4.45 | 5.71±4.58 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





