3.1. What is energy? And why is energy conserved?
The following derivation of momentum and energy conservation from Newton’s laws is for the conservation of momentum similar to what is found in literature, while for energy conservation it follows an approach different e.g. from Feynman’s approach. Further on the steps are rearranged, to build the basis for the following synthesis of conservation of momentum, energy, and forces. The synthesis then in turn leads to a consistent understanding of forces, momentum, and energy. These issues, treated here in sections 3.1.1 to 3.1.3, have already been treated in Mehling 2019. They are here refined as then required to answer the new questions stated in the goal of this work.
3.1.1. Momentum and energy conservation as consequences of Newton’s laws
As basis, Newton’s laws of motion are briefly repeated and discussed regarding their meaning. The formulas here correspond to the original statements which were in text form (Newton 1687), where “quantity of matter” corresponds to mass and “quantity of motion” is mass times velocity. For constant mass the “quantity of motion” corresponds to the more general term momentum p
Newton’s 1
st law, also called law of inertia, states that if the net force F acting on a body is zero then its product of mass m and velocity v (incl. direction) does not change with time t, and reverse, no change in motion means that no force is acting. It can then be written as
Newton’s 1
st law is a special case of his 2
nd law, which goes significantly further in its statement. Newton’s 2
nd law, also called principle of action, gives a quantitative correlation between the net force F acting on a body and the change of its motion
Newton’s 3
rd law, also called principle of reaction, then states that the force on body 1 by body 2, F
(2→)1, is equal in magnitude but opposite in direction to that on 2 by 1
Thus, in a mutual interaction the sum of the forces (called action and reaction forces) is zero.
Newton’s laws of motion describe nature by forces, in the framework called classical mechanics. But Newton’s laws cannot be used in all situations, so e.g. quantum mechanics was developed. Still, Newton’s laws have a meaning that holds beyond their applicability in classical mechanics. A basic model to describe nature is by particles that interact, and are affected by the interaction. It is crucial that forces and their laws are a concept developed for a mathematical description. Newton’s 1st law says that if the net force F acting on a body is zero, then its motion is unchanged, and reverse. A net zero force F means there is no interaction with another body. Therefore, Newton’s 1st law means without interaction motion does not change, and with interaction it does. Newton’s 2nd law means there is proportionality between the interaction on a body and the magnitude of the change of its motion. And Newton’s 3rd law means that in a mutual interaction between two bodies the effect on the bodies is always such that it affects them symmetrically.
Despite the drawback that forces and Newton’s laws cannot be used in all situations, they are still a good starting point as they are easy to comprehend, keeping in mind what they actually mean. Besides, none of the other concepts developed by physics is useful in all situations; all of them have their limits.
Forces and Newton’s laws can be used to derive conservation of linear momentum by looking at two objects that are interacting in a collision. This was as already done by Newton (Newton 1687), and in a similar way later repeated by Feynman (Feynman 1961 – 1963 b) as well as many others. Starting with Newton’s 3
rd law (Eq. 4), inserting Newton’s 2
nd law (Eq. 3) on both sides gives
Eliminating the forces (which describe the mutual interaction) from the equation gives
This is mathematically equivalent to saying that the individual changes of momentum cancel out
that the sum of the momentum does not change
or that the sum of the momentum is “conserved”
Feynman (Feynman 1961 – 1963 b) already stated that conservation of momentum is a consequence of Newton’s laws. Essentially it is conserved as a consequence of Newton’s 3rd law. Symmetry of space is not required, or more precise, symmetry of space is not obvious this way. The derivation holds for any pair of objects that are in mutual interaction, and consequently momentum is also conserved for any number of objects as long as the interactions are only mutual. It is noteworthy that conservation here does not imply that “momentum” is a real part of nature, in addition to the objects and their interactions.
That energy conservation is also a consequence of Newton’s laws was also stated by Feynman (Feynman 1961 – 1963 b). He showed that it can be traced back to Newton’s 3
rd and 2
nd law using the example of free fall, and suggested this as the most simple example (Feynman 1961 – 1963 c). However, free fall combines kinetic and gravitational energy, therefore conversion of energy, precisely conversion between motion and position, as observed by Galilei, Leibnitz, and others. But the collision of two objects, observed by Huygens, is much simpler as it is without conversion (for this it is assumed that the collision is elastic, an assumption investigated then in section 3.1.4.) The conservation of energy can then be derived by a slight modification of the derivation of the conservation of linear momentum (Mehling 2019). For this the terms in Eq. 5 are rearranged to
That changes of momentum cancel out is a consequence of Newton’s 3
rd law, now multiplied by the time dt the forces act, which is the same in a mutual interaction (the same dt on both sides). The change of linear momentum is due to what is called impulse (force times the time it acts), also called impulse - linear momentum theorem. The “transfer” or “exchange” of momentum between both objects in Eq. 10 is by impulse. Linear momentum is conserved because the impulse on both objects in a mutual interaction cancels out; the explanation for this is Newton’s 3
rd law. Newton’s 3
rd law holds however not just at any time t of a mutual interaction, as used in Eq. 10, but also anywhere on the path s of the interaction. Then, with the same ds on both sides, follows
This is the basis of energy conservation. Using Newton’s 2
nd law (Eq. 2) gives for constant mass
The change of the kinetic energy is by what is called work, the force times the distance it acts. Eq. 12 is also called the work - kinetic energy theorem. Using it on both sides of Eq. 11 gives
It can be understood as “transfer” or “exchange” of energy between both objects, here by work. Eq. 13 shows that kinetic energy is conserved because work in mutual interactions cancels out. Except for a factor ½ the result was already described by Huygens in his “laws of collision”. Another most simple example hides in Eq. 11. It is the “golden rule of mechanics”, stated by Galilei as "the driving force times the driving distance is equal to the load times the load distance". It was used in the Middle Ages to correlate forces and distances. If using a lever to lift a stone the force on one side of Eq. 11 is the force exerted by gravitation on the mass of the lifted stone
The corresponding term is called a potential energy, being energy related to a change of position. An easy way to get a more general description is now looking again at a collision of two objects, however now not horizontal but instead vertical, such that gravitational energy is included
Huygens laws of collision as well as the golden rule of mechanics are rarely used as examples of an energy balance as they just combine changes of motion respectively just changes of position. But it is exactly the focus on conservation, without conversion, which reveals the natural cause. At the same time, this focus is the reason for their use being limited to only few special situations. The range of situations increased dramatically with the later integration of conversion between motion and position, then allowing to describe e.g. the flow of fluids or the swing of a pendulum.
Despite that using Newton’s laws is widely accepted to explain the conservation of momentum, to do the same to explain the conservation of energy is surprisingly not commonly accepted at all. Of course, Newton’s laws are only applicable in classical mechanics, thus Newton’s 3rd law is not yet the natural cause of conservation of energy and momentum, and that holds equally for both.
3.1.2. Common basis for conservation of momentum and energy
The derivation using Newton’s laws, despite that it is only applicable in classical mechanics, can be the starting point for further conclusions. Thus, it is summarized in
Figure 1 in a suitable way.
Crucial is that the derivation from Newton’s laws simplifies many things, and is comprehensible. The previous discussion has shown that force, momentum, and energy, are related. Specifically, the conservation of linear momentum is a consequence of Newton’s 3rd law, resulting if it is multiplied by the time an interaction acts (Eq. 10), and conservation of energy too, resulting if it is multiplied by the distance an interaction acts (Eq. 11, and Eq. 13 for the case of kinetic energy). Newton’s 3rd law is therefore at the core of the conservation of both, momentum as well as energy. Moreover, forces, momentum, and energy, all are not independent but instead are tightly related.
3.1.3. Common nature of forces, momentum, and energy, and their conservation
Force, momentum, particularly energy, are often comprehended as independent physical realities. The previous discussion shows that, in the same way as a force is not an own physical reality, momentum and energy are also no own physical realities. Instead, all are just different concepts to describe the effect of interactions between objects resulting in changes of motion and position. And it has to be that way. If we assume that everything can be described by objects and the interactions between them, then force, momentum, and energy cannot be independent of them. What should be considered “real” is the minimum set of things that is needed to describe nature, which is objects and their interactions, while force, momentum, and energy, are mathematical concepts to describe what happens, used to “explain” why it happens. The most intuitive concept, closest to common experience (so well known to everybody) and as a result also investigated first, is that of forces. The “origin” of forces are interactions; forces describe the effect of interactions. In simple situations, e.g. two objects, forces describe the effect of interactions between objects on motion and position of the objects. Newton’s laws of motion describe mathematically the most basic features of interactions: if there is no interaction nothing changes (and the same reverse), the effect of an interaction, and that interactions always act such that effects overall cancel out. The latter can also be understood as law of conservation of forces for only mutual interactions. The effect of interactions between objects on objects can however also be expressed by the concept of momentum or energy, being developed later. Each has advantages, and limitations. Momentum and energy allow a description of interactions also when things change dynamically in space and time, also when objects do not have a precise location as in quantum mechanics, even in situations when objects have no mass like photons in electrodynamics, actually even in situations where objects after an interaction are completely different ones like in an electron-positron annihilation resulting in photon creation. This is a consequence of eliminating forces, distances and locations, and times, by multiplication of Newton’s 3rd law (Eq. 10, Eq. 11, Eq. 13) and eliminating the terms, with just the conservation laws remaining. Similarly, Newton’s 3rd law multiplied by specific ds enforced by certain constraints leads to the principle of d’Alembert, which is the basis for the Langrange function and formalism (see Goldstein 1987).
Based on the previous results and synthesis a consistent and clear definition of energy is possible. Energy is one of several concepts in physics to describe nature, specifically to mathematically describe the effect of interactions between objects on the objects. Energy can be converted while it is overall conserved, thereby correlating changes of a wide variety, e.g. change of motion and position of individual objects, for many objects bulk deformation, and lots more. That energy connects changes is widely accepted (Lehrman 1973, Feynman 1961 - 1963 a, Walker et al. 2014). The investigation here showed what energy is, a mathematical concept, and its root in interactions.
3.1.4. Natural cause of conservation of forces, momentum, and energy
The issues investigated and discussed in sections 3.1.1 to 3.1.3, that conservation of momentum and energy are consequences of Newton’s 3rd law, that they have thus a common basis, and that momentum and energy are even of the same nature as forces being mathematical concepts to describe how interactions between objects affect them, was already presented in Mehling 2019. The previous derivation of conservation of momentum and energy in mutual interactions holds where Newton's laws are applicable, and then conservation is a consequence of Newton's 3rd law. That Newton's 3rd law is the correct “origin” can be concluded as this way also results in a single, common explanation for conservation of momentum and energy as e.g. required for an ideal gas. But the limited validity of Newton’s laws also makes clear it is the origin only within some limits. Newton originally wrote “To every action there is always opposed an equal reaction: or the mutual interactions of two bodies upon each other are always equal, and directed to contrary parts.” Thus, Eq. 4 is a mathematical way to express a general property of mutual interactions regarding action and reaction using forces; that it expresses something that has general validity is widely accepted. The question that is still unanswered, what exactly that general property is, is now investigated.
The easiest application of Newton’s 3
rd law is in static situations. A simple example is a spring that pulls on the walls of a box (
Figure 2, right). The force by the spring on the right wall by its support on the left is equal in magnitude and opposite in direction to the force on the left wall by its support on the right. It must be that way, simply because the situation is mirror symmetric. Also, if gravitation acts between two equal masses (
Figure 2, centre) it acts mirror symmetric. Generally, however, to include things like the spin of a particle, forces must be of same magnitude and opposite direction but not necessarily on the same line (Goldstein 1987). This is compatible with Newton’s 3
rd law. Consequently, the general case that interactions act symmetric with the same magnitude and opposite direction describes a point symmetry to the centre of the interaction (
Figure 2, right).
That interactions act symmetric does however not cover all issues yet. In the previous derivation the requirement of having “elastic” collisions was used. What happens if collisions are not elastic? In a completely inelastic collision of two objects of the same mass and velocity their motion stops, consequently the kinetic energy as well as the momentum of each object after the collision is zero. The sum of the kinetic energy is zero too, however the total momentum as vector is still conserved. To analyse what happens it helps to imagine a direct collision, and interaction to be by a spring. In an elastic collision the objects approach each other, when the spring acts they slow down as energy of their motion is converted to energy in the interaction (deformation energy of the spring), and then converted back to energy of motion; no energy is lost by conversion to something else. If not elastic then during the interaction energy is at least partly converted, e.g. to thermal energy, and not back. If described by a force this means that the force on the back conversion way is less. That an interaction is elastic can be expressed saying all energy is converted in the desired way; the reason is the interaction is the same forward and backward, so it does not change with time. If two masses interact by gravitation, and only by it, then there is obviously no way for conversion.
Physics knows 4 fundamental interactions: besides gravitation the electromagnetic interaction, and the strong and the weak nuclear interaction. The electromagnetic interaction can be split up into electrostatic and magnetic interaction, where the latter is just a relativistic effect of the first. That the fundamental interactions fulfil the requirements, meaning acting symmetric in space and acting independent of time, is thus the natural cause for conservation of energy and momentum. Because everything can be described by fundamental particles and fundamental interactions, consequently follows that energy and momentum must be conserved in general.
For an arbitrary interaction the previous derivation of the conservation laws holds too if the interaction fulfils the same requirements of acting symmetric in space and independent of time. This was inherently included before: the symmetric effect is what is stated by Newton’s 3rd law, and that the interaction does not change with time was included by assuming an elastic collision. The natural cause for conservation of energy and momentum is thus that mutual interactions between objects act symmetric with respect to the effect, and that their effect is time independent. It is the natural cause, generally valid. It removes all inconsistencies identified in the introduction. The cause are the mutual interactions and their symmetries with respect to space and also to time. A general symmetry of space and of time is nowhere required. Before investigating what these symmetries “have to do” with the conservation laws it is helpful to investigate first another issue, which then explains what it means if a situation is not symmetric with respect to space or time.
3.1.5. Conditions for conservation of forces, momentum, and energy in systems
As shown, the cause of the conservation laws is that mutual interactions have a symmetry in space and are independent of time; both is the case for the fundamental interactions known in physics. This became clear looking at the simplest situation, two interacting objects in mutual interaction. Knowing that complex situations are then simply composed of many simple situations it is clear that the conservation laws hold in general, precisely if comprising complete mutual interactions.
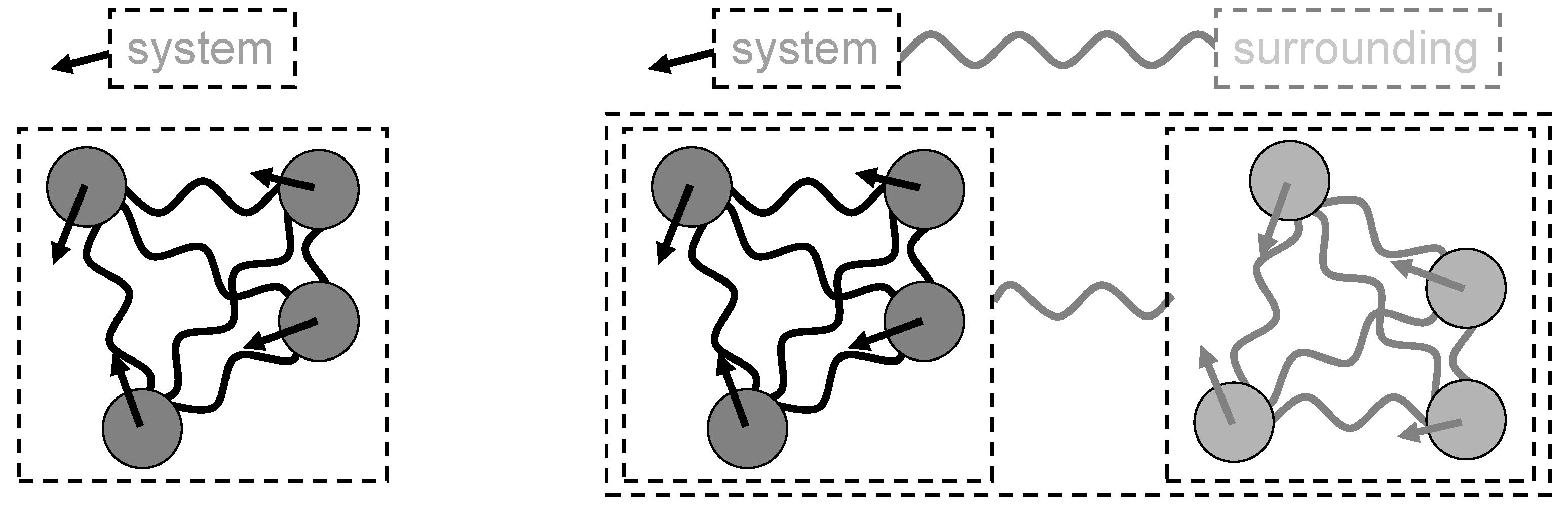
Looking at the interior of a system (
Figure 3, left), the energy associated with the motion of the objects and mutual interactions is called the internal energy. As there are only mutual interactions, the sum of all internal forces in the system cancels out; their sum is conserved (it is always zero), also momentum is conserved, as well as energy. They are always conserved together, never alone (energy conservation is only “violated” if conversion, e.g. by friction, is not correctly included). Only mutual interactions means no interaction with the surrounding.
If there are not only mutual interactions but also interactions with the surrounding of the system, so another system, the momentum as well as the energy of the system is not generally conserved. For linear momentum this case was discussed in detail by Feynman (Feynman 1961 – 1963 b). For energy this is discussed in detail in Mehling 2019, distinguishing two cases. The first case is an external interaction acts on the interior of the system, affects motion and position of its objects, therefore the energy related to the motion and position, thus changes the system’s internal energy. This case is reflected in the first law of thermodynamics, saying the change of the internal energy of a system is equal to the energy exchanged by work and heat. Work and heat denote energy exchanged between a system and its surrounding by interaction without respectively with entropy. The second case is the external interaction acts on the whole system, leading to external energy. For example, an electric field acting on a system of charges, e.g. a salt, changes the internal energy by dragging charges of opposite sign into different directions, and if the salt sample has some net charge the external field also changes its external energy by attracting or repelling the salt sample. Another example is the interaction between earth and moon: the gravitational field of the moon attracts the earth, but because of its inhomogeneity at the location of the earth it also pulls different parts of the earth differently and thereby causes a change of the internal energy, e.g. by the tides.
Strictly speaking there is a second condition for the conservation laws, besides that the system comprises all mutual interactions. The condition is that the system is in an inertial reference frame. An accelerated reference frame will cause motion in the system, thus change the momentum as well as the kinetic energy of objects in the system, even if they have only mutual interactions. The motion of the objects in the system is then not due to a real force, but by the reference frame. This connects to the principle of relativity, as stated first by Newton in Corollary V to the laws of motion (Newton 1687) as “The motions of bodies included in a given space are the same among themselves, whether that space is at rest or moves uniformly forward in a straight line.”
3.1.6. Connection between conservation laws and symmetry of space and time
The connection between conservation laws and symmetry of space and time is investigated next. It is crucial that in this context symmetry of space and time refers to a system, and that the previous discussion of systems without / with an interaction with their surrounding showed differences.
For systems which are not interacting with their surrounding all conservation laws hold, always. For systems that are interacting with their surrounding this is not the case. Thus, it is the interaction with the surrounding that seems to determine if a system has a symmetry or not. Fließbach 2020 states that external influences on a system lead in general to a system not being invariant under certain transformations; thus, the conclusion here is already accepted in literature. It is important to remember that the formulas used in physics to describe the behaviour of systems are merely a mathematical tool to describe the observations of the behaviour of systems in nature. If a system has a symmetry in space or time the symmetry is reflected in the system’s behaviour, and consequently it must be reflected then also in the formulas that are used to describe the system.
If a system behaves the same no matter where it is located then there is no effect of the location. This means that the formulas used to describe the behaviour of the system need to show the same independence of location, mathematically this requires a translation symmetry regarding space. Thus, no absolute space coordinates can be involved, just differentials or just relative coordinates. It is common in discussions of this topic to use T for kinetic energy, and V for potential energy. Looking at internal energy of a system comprising more than one objects in mutual interactions, it is possible to derive under which conditions energy and momentum conservation would hold. Goldstein 1987 looks at energy of a system of objects as the sum of kinetic and potential energy, the potential energy causing changes of the kinetic energy by work, via the forces that it causes. He derives that for energy to be conserved the forces of a mutual interaction must be caused by a potential energy that is a function of the relative distances only, so the resulting forces are then of the same size and have opposite direction; this is exactly the same as stated by Newton’s 3
rd law. The result derived by Goldstein is the same as derived here, just with a different starting point. Mutual interactions are symmetric with respect to translations of space or time, so in agreement. The reason to be not symmetric must be, as derived before, an interaction with the surrounding. For simplicity only one space dimension is used. The kinetic energy T of the system is then
and the potential energy V, derived from a force acting on it, e.g.
The kinetic energy T, being a function of dx/dt, is not changed by a translation of space or time. The potential energy V, being a function of x, is changed by translation of space unless V = const. Since F = dV/dx, follows that symmetry with respect to translation in x means that no force acts, and consequently the momentum of the system is conserved; it is not if there is an external force. The force represents an interaction between the system and its surrounding, as discussed before. Regarding energy conservation, as discussed before it must be the same way, an interaction with the surrounding, thus an external force, has to be the cause if energy conservation does not hold. A force acting on the system will change the system’s energy, which must show up in the potential energy being dependent on time thus that V = V(x, t) is then not symmetric with respect to time. Thus, for momentum as well as energy, if there is no interaction with the surrounding there is a conservation law and for each a corresponding symmetry exists. They are not independent causes.
The correct natural cause of the conservation laws, and what symmetry of space and time of a system have to do with the conservation laws is now clarified. Finally, left is to investigate why symmetry of space and time are commonly believed to be the real cause of the conservation laws. As the symmetries correspond to a conservation law with regard to all isolated systems, there seems to be the belief that therefore the symmetries must be a general property of space and time. However, as shown before, this neglects that the natural cause is not the symmetry of the system but what is behind it: the presence of only mutual interactions in a system and no interaction with the system’s surrounding; the latter is not mutual as the surrounding is not a part of the system. Since the universe has no surrounding, by its definition, all interactions in it are mutual; therefore, momentum and energy are conserved. This does not require a general symmetry of space or time.
3.1.7. Correlation between energy and momentum
The approach chosen here, to start looking at mutual interactions being the most simple situation, and using Newton’s laws as they are easy to comprehend, has already helped to answer several questions and remove initial inconsistencies. One issue left to investigate is that in an ideal gas there is a correlation between kinetic energy and momentum by Ekin = p2/(2∙m), thus that the conservation of momentum and of energy cannot be independent. The conditions for energy and momentum to be conserved together or not have been discussed before in sections 3.1.4 and 3.1.5. For mutual interactions the condition is that no conversion with another energy form takes place, which would result in energy gains or losses. Consequently, the work exchanged as well as the impulse exchanged in a mutual interaction cancel out, which then results in the conservation laws. This is fulfilled in an ideal gas, as example, because elastic collisions are one assumption for it. The specific case is now understood, but the question arises if there is a general correlation.
The summary in
Section 3.1.2, specifically in
Figure 1, allows to derive a more general correlation. For this, the focus is now only on one side of an interaction. If a force acts on an object, the work done is the product of the force times the distance it acts, and equal to the change of its energy
The impulse is the product of the force and the time it acts, and equal to the change of momentum
Division of both correlations, and eliminating the force that represents the interaction, results in
Thus, an object, by an interaction, changes its energy in a relation to the momentum equal to ds/dt.
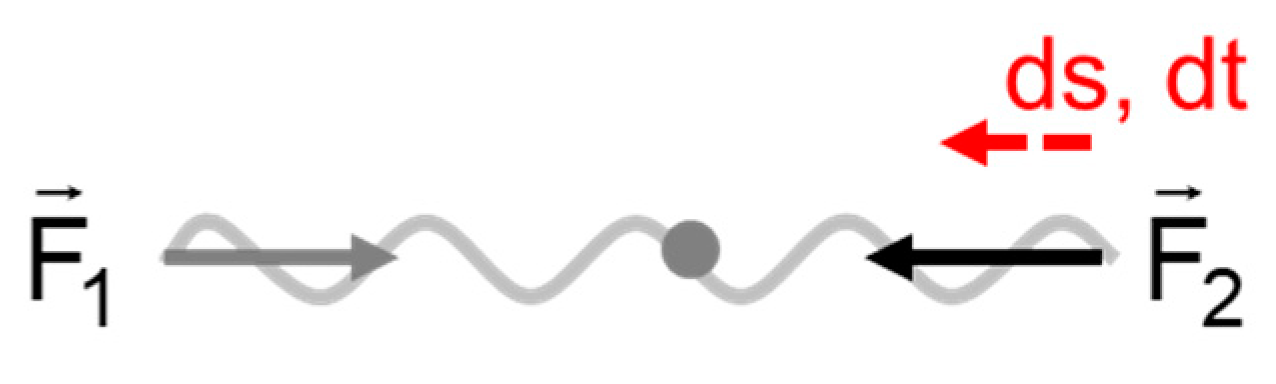
Interesting is the case where the origin of the interaction, in other words the centre of the mutual interaction, is at rest (
Figure 4). The ratio of the distance ds and time interval dt the interaction acts on the object is then equal to the velocity of the object. The meaning of Eq. 20 is then clear. The equation can easily be checked looking at objects in free motion, having only kinetic energy. In classical mechanics Eq. 20 is fulfilled; E
kin = ½∙m∙v
2 and p = m∙v, so E
kin = p
2/2∙m and the right side of Eq. 20 is then equal to the velocity v on the left. This is not a surprise as the derivation in section 3.1.1, summarized in section 3.1.2, was withing the framework of classical mechanics. The correlation in Eq. 20 is also fulfilled beyond classical mechanics however. For photons holds E = h∙c/λ and p = h/λ, thus that E = p∙c, so the right side is again equal to the velocity c on the left.
3.2. What is an energy form? Which energy forms exist?
3.2.1. External and internal energy, and fundamental interactions
The previous discussion now allows a bottom-up derivation of the energy of systems of objects. Starting from 2 objects which mutually interact, going to many objects is now straight forward.
It was already discussed in
Section 3.1.5, specifically
Figure 3, that a system of interacting objects has energy within, called internal energy, due to the motion and mutual interaction of the objects. Additionally, if the system interacts with its surrounding (actually another system), it has energy as a whole from its motion and interaction with respect to the surrounding, called external energy. The interaction with the surrounding acts on the individual objects within the system, thus affects motion and position of them, consequently an external interaction can change the internal energy.
The motion and position and related interaction of all objects completely describes a system state. Physics knows 4 fundamental interactions: gravitation, the electromagnetic interaction, and the strong and weak nuclear interaction. Taking kinetic energy associated with motion in addition, this altogether gives 5 “fundamental energy contributions” that cover all energy (Mehling 2019). If the interactions are not treated by the interacting particles and their positions, they can also be treated by the corresponding field energies, e.g. for wave propagation, or by exchange particles. No energy is counted twice, or is missed, unless a new fundamental interaction is discovered.
3.2.2. Systematic derivation of the energy forms
To use fundamental energy contributions to describe systems has however several weaknesses, specifically if looking at materials with many small objects, commonly called particles: treating many particles is difficult, often there is not even interest in the individual motion and position, and most important, in everyday life we do not see particles and thus not their motion and position. Historically energy as a concept was derived from visual observations, thus that a different way of description, not following fundamental energy contributions, was established: energy forms. Energy forms are energy contributions associated with changes that are macroscopic, in many cases directly visible, but with regard to the internal energy of materials have microscopic origin. The fundamental energy contributions now allow a systematic treatment of the energy forms too.
External energy forms are energy forms related to a macroscopic system, due to its macroscopic visible motion, or its position by a fundamental interaction with the surrounding. The energies can be given absolute values as we see the position and motion. The energy forms are
- Kinetic energy: energy associated with motion of a mass, e.g. of a pendulum, a car, etc.
- Gravitational energy: energy associated with gravitation and relative position, e.g. the mass of a pendulum or a stone by interaction with the earth. The latter is crucial but often forgotten: gravitational energy is not “due to” the height of an object or its position.
- Electric energy: energy associated with the electric interaction and relative position, e.g. fluff sticks to a charged plastic slab, or relative orientation, e.g. for electric dipoles from coils.
- Magnetic energy; energy associated with the magnetic interaction and relative orientation, e.g. a compass interacting with the magnetic field of the earth.
Kinetic energy and gravitational energy are well known, e.g. from the historic observations on the collisions of balls or the swing of a pendulum. Electric and magnetic energy are often missed, here uncovered by the systematic approach following fundamental interactions. Despite that both originate from a single fundamental interaction, it is practical for the description of observations to split both; this is also the reason why initially they were discovered separately. Regarding nuclear interactions there is no associated energy form; their range is too short, so they are not acting between macroscopic bodies, and the nuclear particles where they act are also not visible.
Internal energy forms are energy forms related to a macroscopic system of microscopic particles due to their motion or position by fundamental interactions, associated to macroscopic changes. As a start, the well-known function in thermodynamics for the change of the internal energy U
can be used. It leads to several energy forms, but is not complete as the systematic analysis shows.
- Thermal energy: energy change (T∙dS) associated with undirected, random changes of the motion and position of all particles, described by the temperature T and entropy change dS.
- Deformation energy: energy change (p∙dV) associated with a directed deformation, thus changing the motion (e.g. in an ideal gas) and position of all particles, causing a macroscopic volume change dV.
It is crucial to note that thermal and deformation energy refer to all particles in a macroscopic system, and split up energy to that associated with random and respectively directed change. Therefore, all options with regard to all particles and no specific type of interaction are covered. Next are energy forms which are due to a specific interaction, usually acting on specific particles.
- Electric energy: energy change (E∙dP) associated with a directed effect by an external electric field E acting on electric charges and dipoles and leading to a shift of their position or orientation such that an overall macroscopic polarization change dP results.
- Magnetic energy: energy change (B∙dM) associated with a directed effect by an external magnetic field B acting on magnetic dipoles and leading to a shift of their orientation such that an overall magnetization change dM results.
For gravitation there is no corresponding term as the effect on microscopic particles is negligible. And for nuclear interactions there is also no corresponding term as nuclear interactions are too short range to affect a macroscopic system from outside. Nuclear interactions only act in particles. The final group of energy forms refers to changes within particles, incl. leading to new particles.
- Chemically energy: energy change (μ∙dN) associated with a change of chemical composition, specifically expressed as being proportional to the change of amount of substance dN of the initial substance or the new one after a chemical process between atoms and molecules.
- Nuclear energy: energy change associated with a change of composition of nuclei. It can also be expressed as being proportional to the change of amount of substance dN of the initial substance or the new one, similar as chemical energy. It is crucial to note that nuclear energy as energy form is not the energy of nuclear interactions; in nuclei also the electromagnetic interaction is relevant, e.g. in nuclear decay.
- Ionization energy: energy change associated with the ionization of atoms or molecules.
- Excitation energy: energy change associated with the excitation of atoms, molecules, or ions.
Ionization and excitation energy need to be added to make the set of energy forms associated with changes within particles complete. They complete the systematic set of internal energy forms. Again, the complete, systematic set of energy forms assures that no energy contribution is counted twice, and none is missed. For more details and an in-depth discussion, see Mehling 2019.
Finally, it is necessary to discuss crucial differences between internal and external energy forms. The motion and position and related interaction of all objects completely describes a system state, therefore also its energy. This works if a system is described by fundamental energy contributions, and in the same way for the external energy forms, however not for all the internal energy forms. For example, the internal electric energy of a system of charges has an absolute value, calculated from the position of all charges and their electric interaction. Regarding the internal energy form, associated with the change of macroscopic polarization P due to an external electric field E, there is however only the change of electric energy observable. This holds for all internal energy forms. Internal energy forms refer to macroscopic changes, e.g. of entropy, volume, polarization etc., and thus are only associated with energy changes. But this is not all as thermodynamics shows. Deformation energy p∙dV has no fixed value because even for the same dV it depends also on p. The pressure p can be changed between compression and expansion by changing the temperature. Therefore, the energy change associated with a deformation is not a function of state of the system. The same applies to all internal energy forms. The difference to external energy forms is crucial. In engineering, in a closed thermodynamic cycle it allows conversion between heat exchange, changing the thermal energy of the system, and change of work, changing the deformation energy. The reason is that the integral over the closed cycle of the changes of thermal energy is not zero, and the same holds for deformation energy. The same is not possible with external energy; here, in a closed cycle, the change of the energy form is always zero. Currently definitions of energy forms in literature almost never take into account these differences. This is not really surprising; only the systematic overview on energy forms allows a comprehensive and detailed analysis.