1. Introduction
Africa and other low-resource countries numerous obstacles significantly impede the establishment or extension of sustainable capability in biosafety and biosecurity management [
1,
6]. Global mobility, urbanization, terrorist interest in weapons of mass destruction, and rapid technological breakthroughs, which may result in the creation and manipulation of diseases with pandemic potential, continue to amplify the risk of a catastrophic biological agent. However, many African governments lack the resources to deal with these risks. Africa CDC established an initiative in April 2019 to develop African Union Member States' biosecurity and biosafety systems in order to comply with the International Health Regulations (IHR) (2005), the Biological Weapons Convention (BWC), and UN Security Council Resolution (UNSCR) 1540 [
1].
Biosafety policies and practices are designed to prevent the unintentional or accidental release of specific biological agents and toxins, whereas
Biosecurity policies and practices are designed to prevent the intentional or negligent release of biological agents, as well as the acquisition of knowledge, tools, or techniques that could be used to cause harm [
1,
2].
African Nations and Governments must now be more conscious of the growing biological threat posed by bioterrorism and newly emerging infectious diseases, as well as by being ready to identify and contain those agents [
3,
4]. The goals of biosecurity and biosafety protocols are to reduce both intentional and unintentional biological risks that could have disastrous effects on a nation's health system, security, and political and economic stability. Regrettably, on a national, regional, and international level, biosecurity and biosafety are frequently given insufficient priority [
4].
Public health advancements rarely make headlines in Africa. Ebola, MERS-CoV, and other infectious disease outbreaks frequently make headlines around the world, feeding a myth that distorts the reality of the ongoing efforts to improve the health of people and animals in these areas. We can take into account the efforts made by biosafety organizations [
4,
5]
Bioterrorism and biological weapons were the original uses of the phrase "biosecurity." These days, it's applied to a wide range of fields, such as biological laboratory hazards and infectious disease control for both public and animal health [
4].
The capacity for diagnosis and research has expanded as a result of the growing need on a global scale for better disease identification and control. But especially in low-resource nations, the capacity to identify infectious diseases has not always increased in accordance with the capacity to ensure biosafety and biosecurity. In Africa in and other low-resource countries numerous obstacles significantly impede the establishment or extension of sustainable capability in biosafety and biosecurity management [
6].
In this mini review the current state, challenges, and future prospects of biosafety and biosecurity status of African nations discussed.
2. Biosafety and Biosecurity Challenges in Africa
Even though, Africa are home to a growing number of biosafety organisations that work to enhance public health in their nations by bringing attention to the significance of biosafety and biosecurity, Standard Biosafety and Biosecurity Measures to contain hazardous infections are missing across the African continent [
5].
The WHO JEE and GHS Index worldwide measurement matrices reveal that the biosafety and biosecurity capacities and capabilities of African Union member states have long been weak. Innovative strategies, such as adopting regional strategies that are readily transferable to national execution, are needed to overcome this. The Africa CDC Regional Biosafety and Biosecurity Initiative enables the development of standardised regional interventions that may be domesticated at the national level, as well as the identification of priorities through a consensus-building process [
4,
7]. These gaps clearly show that thorough and focused actions are required. Africa CDC held regional consultations between 2019 and 2021 to address these issues. A recurring shortcoming that emerged from these conversations was the scarcity of nationally accredited, standardised biosafety and biosecurity training courses. The lack of resources made it more difficult for the area to develop its potential, which highlights the urgency of taking quick action [
7].
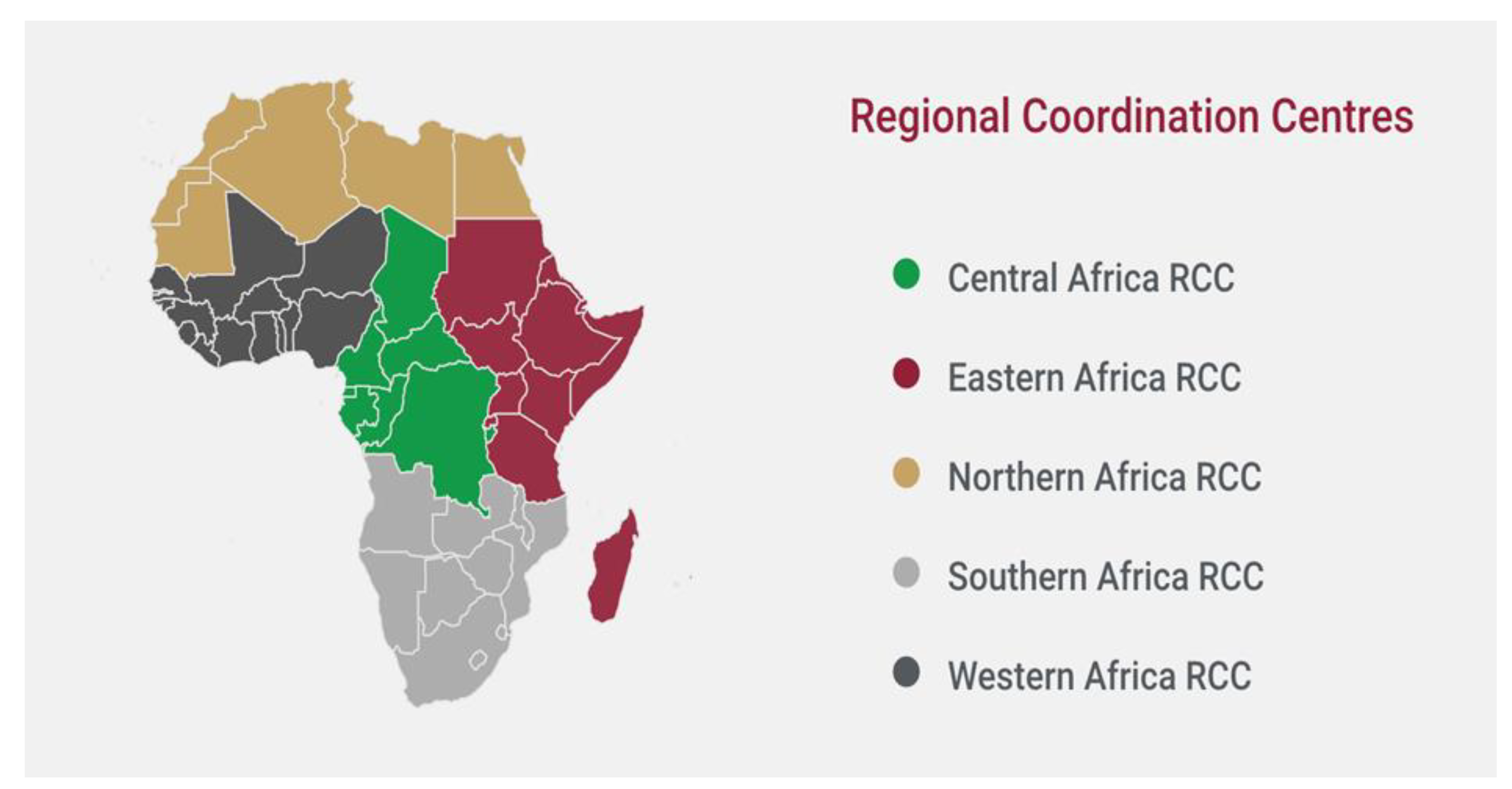
Figure 1.
African CDC Regional Collaborating Centres [
7].
Figure 1.
African CDC Regional Collaborating Centres [
7].
Between November 2020 and March 2021, five multisectoral Regional Biosafety and Biosecurity Technical Working Groups (RBB-TWGs) were created and put into operation for the regions of Central, East, North, Southern, and West Africa. To form the multi-sectoral and multi-expert RBB-TWGs, official nominations were invited from Member States. The groups included members of parliament, legal professionals, and institutions of higher learning, security, customs, human, animal, and environmental health. Every RBB-TWG has a chair who leads it. The chairs' tasks and responsibilities, membership, election and tenure, decision-making procedures, frequency, quorum, and meeting types are all outlined in their respective Terms of Reference [
6].
Over the past ten years, the North Africa Region has made a concentrated effort to raise broad knowledge of biosafety and biosecurity in the life sciences. The results of studies indicate that, up until now, most efforts have been directed on increasing public knowledge within the larger scientific community. For instance, in 2016 the study done by Khan
et al. indicate around 1832 biosafety/biosecurity activities in total were logged from 97 online links; of these, 70.68% (n = 1295) aimed to increase broad scientific community awareness of biosafety, biosecurity, and biocontainment. In biomedical and biotechnology laboratories, biorisk management was the most often mentioned topic of interest (13%; n = 239), followed by living modified organisms (LMOs) at 9.17% (n = 168). Of the efforts, 2.67% (n = 49) involved hands-on training. The results of the desktop review were supported by the online survey, albeit only 11% of respondents responded [
8].
Insufficient training for frontline workers in labs, farms, and other locations where potential global pandemics could originate is a result of the growing risk of zoonotic epidemics due to increased human-animal contact and travel. Developing nations such as Africa lack the resources, knowledge, and preparedness regarding biosafety and biosecurity among general health professionals and laboratory workers handling infectious disease agents [
9].
The majority of African governments have just lately begun to build national biosafety systems, and much like other safety-related fields, developing and enforcing science-based laws has proven to be a challenging undertaking. The Cartagena Protocol on Biosafety, which was ratified in 2003 as an addition to the Convention on Biological Diversity (CBD) and addresses the environmental effects of transboundary genetically modified organism (GMO) movement, management, and safe use, has established the foundation for the majority of them [
10].
Because of the endemic burden of disease and the numerous environmental and host factors that promote the establishment of biological threats, the African continent faces numerous health-related difficulties. The Global Emerging Pathogens Treatment Consortium (GET) played a key role in setting up the inaugural African Voices and Leadership conference on Ebola, which took place in Dakar in January 2015 in cooperation with regional partners in West Africa. Leading representatives from continental and regional health organisations (such as the World Food Programme, World Health Organisation, African Union, MSF, ECOWAS, WAHO, NEPAD, and MSF) as well as scientists, Ebola survivors, and medical professionals working in Ebola treatment centres attended the conference. During this meeting the plethora of socio-economic, infrastructural and capacity inadequacies that caused the Ebola spread to infect so many, were identified and crystalized into what became the Dakar Declaration [
11].
Concerns about the potential negative effects of genetically modified organisms (GMOs) on commerce and food security in the region were brought up at the 2001 Meeting of the COMESA Ministers of Agriculture. This led to research in Eastern and Southern Africa on a regional strategy for biotechnology and biosafety regulations. The COMESA Council of Ministers adopted a policy in 2014 recognizing the benefits and risks of Genetically Modified Organisms (GMOs), establishing a regional biosafety risk assessment system, and providing capacity building assistance to member states, marking the first regional effort in Africa [
12].
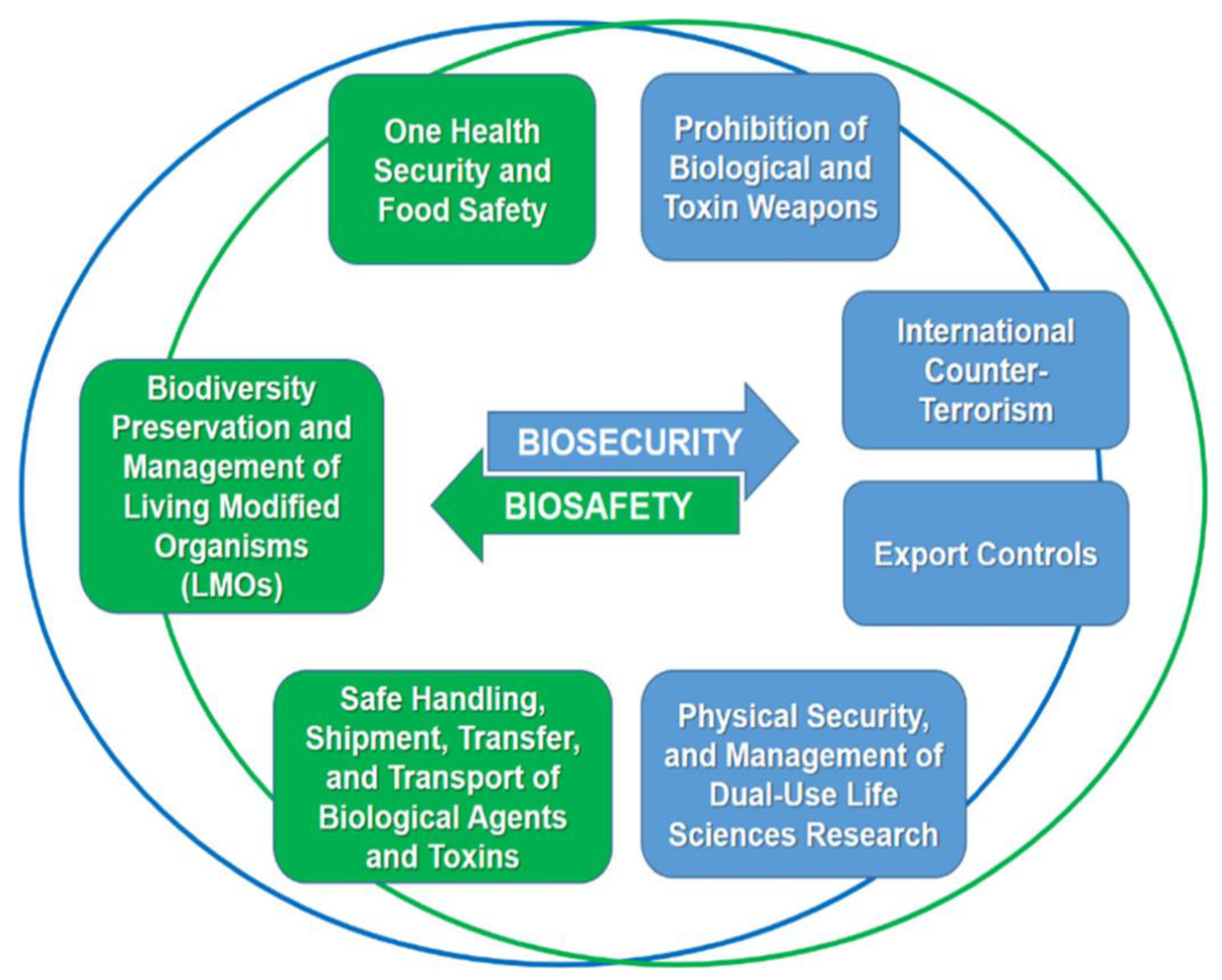
Figure 1.
The Web of Prevention for Biosafety and Biosecurity [
13].
Figure 1.
The Web of Prevention for Biosafety and Biosecurity [
13].
3. Conclusion and Future Perspectives
Africa are home to a growing number of biosafety organisations that work to enhance public health in their nations by bringing attention to the significance of biosafety and biosecurity. Since many African countries are low-resource countries, they frequently confront a number of obstacles that seriously limit their ability to create or grow a sustainable capacity for managing biosafety and biosecurity.
Inadequate training for frontline workers in laboratories, farms, and other locations where potential global pandemic could originate because of the growing potential for zoonotic epidemics as a result of increased human-animal contact and travel; Lack of preparedness, resources, and knowledge about biosafety and biosecurity among general health professionals and laboratory workers handling infectious disease agents in developing nations like Africa.
In order to overcome these obstacles African CDC, the World Organisation, international development partners, representatives from the highest ranks of local government, and the international biosafety community must work together to develop, finance, and implement sustainable capacity building strategies. Working together is necessary to create solutions that are suitable for the unique requirements and resources in each nation.
The current understanding of laboratory biosafety in Africa is restricted to a small group of researchers and need special focus to strengthen the status of Biosafety and biosecurity in the continent.
Funding
No funding was received for this work.
Conflicts of Interest
The authors declares that there is no conflict of interest.
References
- CDC, A. Biosafety and Biosecurity. 2019. [Google Scholar]
- Zhou, D.; et al. Biosafety and biosecurity. J Biosaf Biosecur 2019, 1(1), 15–18. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; et al. Biosafety and biosecurity challenges during the COVID-19 pandemic and beyond. Front Bioeng Biotechnol 2023, 11, 1117316. [Google Scholar] [CrossRef] [PubMed]
- Brizee, S.; et al. Accelerating Action in Global Health Security: Global Biosecurity Dialogue as a Model for Advancing the Global Health Security Agenda. Health Secur 2019, 17(6), 495–503. [Google Scholar] [CrossRef] [PubMed]
- GLOBAL, C. Biosecurity and Biosafety: Perspectives from the Middle East and Africa. 2023. Available online: https://www.crdfglobal.org/insights/biosecurity-and-biosafety-perspectives-middle-east-and-africa/.
- Heckert, R.A.; et al. International biosafety and biosecurity challenges: suggestions for developing sustainable capacity in low-resource countries. Applied Biosafety 2011, 16(4), 223–230. [Google Scholar] [CrossRef]
- CDC, A. Africa CDC Certification Program for Biosafety and Biosecurity Professionals. 2023. Available online: https://africacdc.org/news-item/africa-cdc-certification-program-for-biosafety-and-biosecurity-professionals/ (accessed on 1 August 2024).
- Khan, E.; et al. Biosafety initiatives in BMENA region: identification of gaps and advances. Frontiers in Public Health 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Rutebemberwa, E.; et al. Reasons for and barriers to biosafety and biosecurity training in health-related organizations in Africa, Middle East and Central Asia: findings from GIBACHT training needs assessments 2018-2019. Pan Afr Med J 2020, 37, 64. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.; et al. Biosafety regulatory reviews and leeway to operate: case studies from sub-Sahara Africa. Frontiers in Plant Science 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Abayomi, A.; et al. African civil society initiatives to drive a biobanking, biosecurity and infrastructure development agenda in the wake of the West African Ebola outbreak. Pan Afr Med J 2016, 24, 270. [Google Scholar] [CrossRef] [PubMed]
- Waithaka, M.; et al. Progress and challenges for implementation of the Common Market for Eastern and Southern Africa policy on biotechnology and biosafety. Frontiers in Bioengineering and Biotechnology 2015, 3, 109. [Google Scholar] [CrossRef] [PubMed]
- Novossiolova, T.; et al. Strengthening the biological and toxin weapons convention: the vital importance of a web of prevention for effective biosafety and biosecurity in the 21st century. 2019. Biological Weapons Convention.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).