Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy where the majority of patients diagnosed with PDAC will have unresectable, locally advanced or metastatic disease (collectively termed advanced PDAC) [
1]. The clinical course of PDAC is often characterized by cachexia, a multifactorial syndrome defined by weight loss, muscle wasting, and systemic inflammation. Nearly 80% of PDAC patients develop cachexia with enteral feeding having been used for nutritional support in select patient populations [
2]. Despite a multidisciplinary approach to treatment of PDAC cachexia including nutritional assessment, dietary counseling, nutritional supplementation, pancreatic enzyme replacement therapy, and pharmaceutical interventions, novel and effective anti-cachexia interventions are a high unmet need given the tendency of cachexia to contribute to increased morbidity and mortality and decreased quality-of-life in patients diagnosed with pancreatic cancer.
We previously conducted a single-institution, single-arm prospective clinical trial (PANCAX-1) enrolling subjects with advanced or locally advanced PDAC and cachexia, defined as greater than 5% unexplained weight loss within 6 months from screening, to receive three 28-day cycles of a semi-elemental peptide-based formula, administered through a jejunal or gastrojejunal feeding tube alongside standard-of-care chemotherapy [
3]. Out of 36 eligible subjects, 31 underwent jejunal tube placement and 16 subjects completed the full 12-week course of enteral feeding. The primary outcome of weight stability at 12 weeks was achieved in 10 patients (62.5%). Statistically significant improvements in lean body mass, appendicular lean mass, and multiple health-related quality-of-life metrics were observed in PDAC subjects with cachexia receiving enteral nutrition.
PANCAX-1 was uniquely designed to allow collections of stool and blood across multiple timepoints of enteral feeding for preplanned exploratory analyses in a cachectic population of advanced PDAC subjects all receiving enteral nutrition. The gut microbiome has been implicated in cancer cachexia through multiple mechanisms and manipulation of the microbiome to treat cancer cachexia is actively being explored [
4]. Given the possible links between cachexia, nutrition support, and the fecal microbiome, this study focused on microbiome changes in response to nutrition support as a cancer cachexia intervention. Specifically, we sought to characterize, for the first time, the stool microbiome composition in patients with advanced PDAC receiving enteral feeding for the treatment of cachexia.
Methods
The PANCAX-1 prospective trial (NCT02400398) enrolled 36 patients with advanced PDAC meeting consensus criteria for cachexia planned for standard-of-care chemotherapy to receive enteral feeding (Peptamen 1.5) over 12 weeks [
3]. As part of study correlatives, stool and blood samples were collected at baseline or time 0, 6 weeks, and 12 weeks of enteral feeding and chemotherapy. DNA extraction and sequencing of the 16S ribosomal RNA gene was performed for fecal samples as previously described [
5]. Metabolites were extracted from plasma and analyzed with mass spectrometry-liquid chromatography (Agilent Technologies, Santa Clara CA) where up to 219 polar metabolites within each sample were measured by relative area under the curve.
Patients (>18 years) diagnosed with locally advanced, unresectable or metastatic PDAC were enrolled into PANCAX-1 from outpatient gastrointestinal oncology clinic at Cedars-Sinai Medical Center after providing informed consent. Briefly, eligible patients included those with locally advanced, unresectable or metastatic PDAC referred for standard chemotherapy who fulfilled criteria for cachexia (defined as greater than 5% unexplained weight loss within 6 months prior to the screening visit). Enrolled patients received enteral feeding (Peptamen) through a jejunal or gastrojejunal tube placed prior to study initiation and were planned for standard-of-care chemotherapy. The design and primary results of PANCAX-1 have been described elsewhere [
3]. Out of 31 consenting patients from PANCAX-1, 16 subjects were evaluable for the primary outcome of weight stability. As part of study correlatives, 29 total stool samples were collected from the 16 evaluable patients enrolled in the study over 3 pre-defined time points over the 12 weeks of enteral feeding.
Association of microbial genera with clinical metadata were evaluated using DESeq2 in R and p-values for differential abundance were converted to q-values to correct for multiple hypothesis testing (<0.05 for significance). Differences in plasma metabolite levels were compared by fold change using two-sample t-tests (p<0.05 level of significance). Patients were stratified by weight stable (defined as weight change <0.1 kg/baseline BMI-unit over 12 weeks of enteral feeding) vs. weight unstable and high vs. low Veillonella abundance (defined by dichotomizing at the mean relative abundance in weight stable subjects).
Results
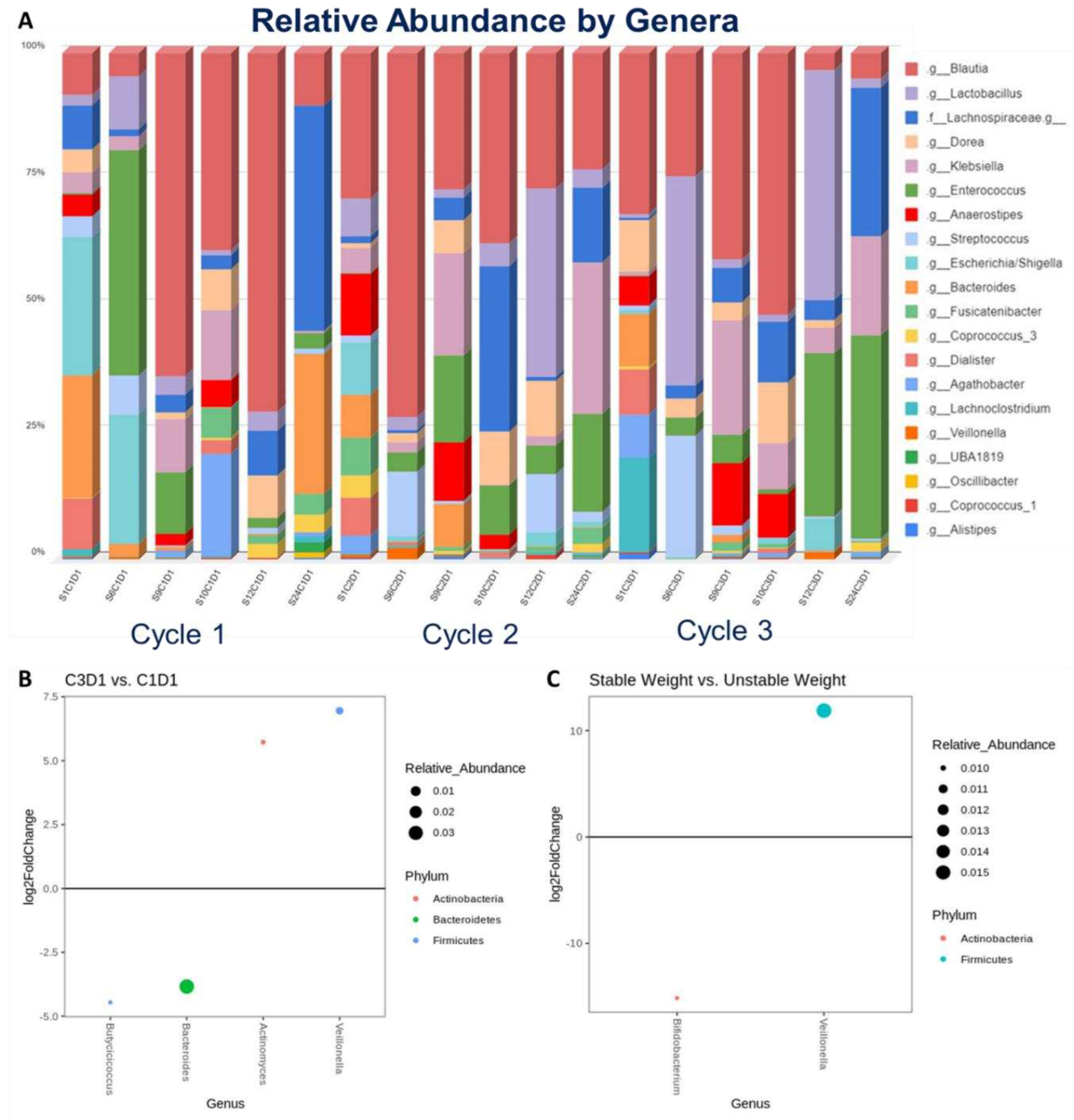
A total of 29 stool samples were prospectively collected from 16 subjects with advanced PDAC enrolled in PANCAX-1 who were evaluable for weight stability, defined as weight change <0.1 kg/baseline BMI-unit over 12 weeks of enteral feeding. Of these, 6 subjects had complete sets of stool samples collected at 0 (C1D1), 6 (C2D1), and 12 (C3D1) weeks of enteral feeding where changes in relative abundance by genera were observed over time (
Figure 1A). On differential abundance testing from C1D1 to C3D1, C3D1 samples were significantly associated with higher abundance of
Veillonella (p=0.0150) and A
ctinomyces (p=0.0390) and lower abundance of
Bacteroides (p=0.0150) and
Butyricicoccus (p=0.0390) (
Figure 1B). Stool samples collected at C1D1 (baseline) from 8 subjects were evaluated for microbiome associations to weight stability. Here, weight stability was significantly associated with greater abundance of
Veillonella (p=0.0006) (
Figure 1C).
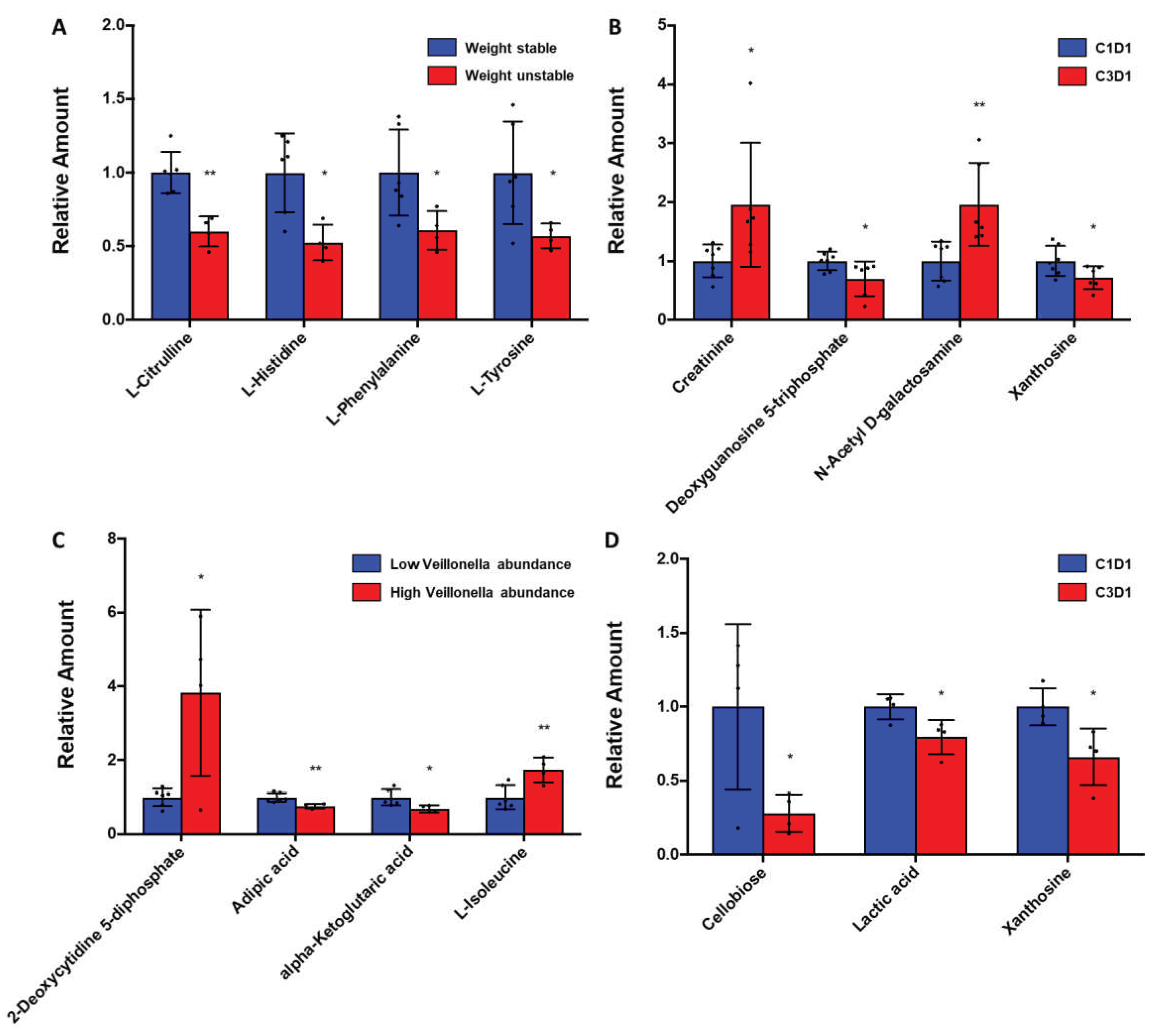
Analysis of plasma metabolites in subjects with advanced PDAC receiving enteral feeding and chemotherapy showed that weight stability was significantly associated with levels of specific essential/non-essential amino acid and nucleosides (
Figure 2A-B), while stool
Veillonella abundance was significantly associated with unique metabolic signatures as well (
Figure 2C-D). Specifically, plasma metabolomics in 10 patients showed that weight unstable subjects (n=4) had significantly decreased levels of essential amino acids (AAs, L-histidine, L-phenylalanine) and non-essential AAs (L-citrulline, L-tyrosine, all p<0.05) than weight stable subjects (n=6) at the end of 12 weeks of enteral feeding. In 7 weight stable subjects with complete serial sets of blood samples available, enteral feeding over 12 weeks was associated with increases in markers of muscle mass (creatinine) but decreases in nucleotide precursors (all p<0.05) compared to baseline. Comparison of baseline metabolites between 6 weight stable subjects with high stool
Veillonella abundance and 4 weight unstable subjects with low stool
Veillonella abundance showed that high stool
Veillonella abundance was associated with increases in the nucleotide 2-deoxycytidine 5-diphosphate and essential AA L-isoleucine but decreased TCA cycle metabolite alpha-ketoglutarate (all p<0.05). Decreases in lactic acid was observed at 12 weeks of enteral feeding in high stool
Veillonella abundance subjects when compared to baseline (p<0.05).
Discussion
In this retrospective analysis of prospectively collected stool samples from a cohort of advanced PDAC patients treated with enteral feeding and standard chemotherapy as part of the PANCAX-1 clinical trial, we sought to evaluate the changes in the stool microbiome and plasma metabolites throughout 12 weeks of nutrition intervention with enteral feeding in cachectic subjects with PDAC. Cancer cachexia remains highly prevalent among pancreatic cancer patients and is associated with poor survival outcomes [
2]. It is increasingly recognized that supportive care inclusive of nutritional assessment, counseling, pancreatic enzyme supplementation, and nutritional supplement replacement are evidence-based strategies that can stabilize or reverse weight loss in PDAC patients and improve outcomes [
2]. Thus, interventions to mitigate pancreatic cancer-related weight loss can be transformative, and we and others have shown that nutritional intervention through enteral feeding has shown potential to mitigate weight loss in specific PDAC patient subsets [
3]. However, enteral feeding is an invasive process and not all patients are likely to benefit from this intervention. Consequently, less invasive but innovative anti-cachexia strategies are welcomed and development of biomarkers to help identify those who could benefit from nutritional supplementation could prove indispensable in PDAC patients where cachexia is prevalent in early 85% of cases.
We are among the first to characterize the stool microbiome composition in advanced PDAC patients in a prospective clinical trial whereby all patients were enteral fed as their primary source of nutrition, thereby representing a homogenous (from a source of nutrition standpoint) and internally controlled population. Here, we identified a novel association between
Veillonella in cachectic patients whose abundance increased over time and was associated with greater weight stability. Furthermore, plasma metabolomics identified several metabolites of interest that were significantly associated with weight stability and high stool abundance of
Veillonella in this enteral-fed cohort.
Veillonella has been shown to be a protective microbe against PDAC in a separate prospective cohort [
6] and among the most abundant genera in cachectic PDAC patients where short-chain fatty acids may be a cachexia marker [
7]. Among the bacterial genera decreased in abundance over 12 weeks of enteral feeding was
Bacteroides. An abundance of
Bacteroides has been implicated in the pathogenesis of other gastrointestinal cancers, whereas in our enteral-fed PDAC cohort we observed a decrease abundance in this species over time [
8].
In analyses of plasma metabolites across subjects achieving weight stability vs. those who did not achieve weight stability, we observed significantly reduced levels of several essential and non-essential amino acids. This is not unsurprising as deficiencies in multiple intermediates of bioenergetic pathways have been characterized in states of cancer cachexia where there is a strong trend towards catabolism and negative protein-energy balance [
9,
10,
11,
12]. Multiple groups have recognized amino acid supplementation as a foundation of nutritional support approaches in patients with cancer cachexia [
9,
10,
11,
12]. Interestingly, we observed differences in several metabolites in subjects with high stool abundance of
Veillonella compared to low abundance of
Veillonella as well as over time with enteral feeding in those with high stool abundance of
Veillonella. A previous study identified a potential relationship between the genera
Veillonella and exercise performance, where inoculation of
Veillonella atypica isolated from stool samples of marathon runners was able to enhance exercise performance in mice [
13]. Furthermore, it was shown that the active metabolite, propionate, was sufficient to reproduce the enhanced exercise performance in these mouse models, suggesting that a breakdown product of lactate metabolism in
Veillonella could be contributing to the beneficial effects of this microbe towards exercise performance. Our plasma metabolomic findings are exploratory, but reinforce that further characterization of
Veillonella and its active metabolite(s) is warranted and could prove timely in ongoing efforts to modulate the gut microbiome to mitigate PDAC cachexia. Lastly, we have previously demonstrated feasibility in associating plasma metabolites to chemotherapy response in advanced PDAC subjects [
14]. Our findings are supportive of future efforts focused on identifying novel metabolic and microbial signatures that could serve as predictors of response to anti-cachexia interventions as well.
This study was limited by its small sample size. Although the uniqueness of our cohort lies in the fact that all PDAC patients were enteral fed with the same semi-elemental formula as the primary source of nutrition, heterogeneity exists in the chemotherapy regimen offered and disease severity. For example, some patients were treated in refractory settings (second-line treatment), while others received first-line standard chemotherapy. Future studies will thus be better served if the impact of the gut microbiome from chemotherapy could be characterized from those by enteral feeding in PDAC patients. Despite these limitations, our results provocatively suggest that certain gut microbiome and plasma metabolic signatures may be associated with improved weight stability in PDAC subjects treated with aggressive nutritional supplementation and standard chemotherapy. Further validation of our findings in larger, ideally prospective cohorts of PDAC patients is warranted particularly as data continues to build in therapeutic strategies to counteract PDAC cachexia. Furthermore, as clinical trials are now being conducted whereby gut microbiome modulation is induced by administering patients with specific bacterial species, our study is hypothesis-generating to support future research into the modulation of the gut microbiome to mitigate weight loss and overall improve outcomes in PDAC patients.
Conclusions
Our findings suggest that certain bacterial genera may be associated with weight stabilizing effects observed in cachectic patients with pancreatic cancer treated with 12 weeks of enteral feeding and standard-of-care chemotherapy. To our knowledge, this is the first study of its kind, evaluating changes in the stool microbiome and metabolomics in paired samples resultant from enteral feeding in PDAC patients with cachexia. Our findings support further investigation into the potential for future development of novel fecal and metabolic biomarkers of response to anti-cachexia therapies for cachectic patients with PDAC. Additionally, we have identified unique bacterial genera and related metabolites that warrant further study into anti-cachexia strategies focused on microbial modulation.
Author Contributions
A.H., J.G.: study conception and design; R.A., S.B., H.M., J.J., A.A., N.M., V.P.: data collection and analysis; All authors: manuscript preparation, editing, and approval of final manuscript.
Funding
This study was supported by funding from the UCLA Clinical and Translational Science Institute UL1TR001881 award.
Institutional Review Board Statement
All study activities were conducted with prior approval and oversight from the Cedars-Sinai Medical Center Institutional Research Ethics Board (IRB) under IRB protocol Pro00038239.
Informed Consent Statement
All subjects provided written informed consent and understanding of study activities prior to participation in this study.
Acknowledgements
We thank Dr. Venu Lagishetty from the UCLA Microbiome Center, Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine at UCLA for contributing to the stool microbiome analyses in this study.
Conflicts of Interest
A.H.: Consultant/advisory: Abbvie; Celgene; Ipsen; Novartis; Perthera; Research Funding: Ipsen. J.G.: Consultant/advisory: EMD Serono, Elsevier, Exelixis, QED Therapeutics, Natera, Basilea, HalioDx, Eisai, Janssen, Astellas, and Amgen.
References
- Gong, J.; Tuli, R.; Shinde, A.; Hendifar, A.E. Meta-analyses of treatment standards for pancreatic cancer. Mol Clin Oncol 2016, 4, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Petzel, M.Q.B.; Zimmers, T.A.; Denlinger, C.S.; Matrisian, L.M.; Picozzi, V.J.; Rahib, L. Pancreas Cancer-Associated Weight Loss. Oncologist 2019, 24, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Gresham, G.; Placencio-Hickok, V.R.; Lauzon, M.; Nguyen, T.; Kim, H.; Mehta, S.; Paski, S.; Pandol, S.J.; Osipov, A.; Gong, J.; et al. Feasibility and efficacy of enteral tube feeding on weight stability, lean body mass, and patient-reported outcomes in pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle, 2021. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The Microbiota and Cancer Cachexia. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lin, L.; Goudarzi, M.; Ruegger, P.; McGovern, D.P.; Fornace, A.J., Jr.; Borneman, J.; Xia, L.; Braun, J. Microbial, metabolomic, and immunologic dynamics in a relapsing genetic mouse model of colitis induced by T-synthase deficiency. Gut Microbes 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.L.; Li, M.; Li, G.Q.; Wang, X.; Hu, W.M.; Li, Z.L.; Yuan, J.; Liu, H.Y.; Zhou, L.L.; Li, K.; et al. Oral microbiome and pancreatic cancer. World J Gastroenterol 2020, 26, 7679–7692. [Google Scholar] [CrossRef] [PubMed]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; van Dijk, D.P.J.; Penders, J.; van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.; Baade-Corpelijn, L.; et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J Cachexia Sarcopenia Muscle, 2021. [Google Scholar] [CrossRef]
- Elsalem, L.; Jum'ah, A.A.; Alfaqih, M.A.; Aloudat, O. The Bacterial Microbiota of Gastrointestinal Cancers: Role in Cancer Pathogenesis and Therapeutic Perspectives. Clin Exp Gastroenterol 2020, 13, 151–185. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Braha, A.; Albai, A.; Timar, B.; Negru, Ș.; Sorin, S.; Roman, D.; Popovici, D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer-A Systematic Review. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, S.; Narimatsu, H. Nutritional Approach to Cancer Cachexia: A Proposal for Dietitians. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, H.; Hendifar, A.; Osipov, A.; Moshayedi, N.; Placencio-Hickok, V.; Tatonetti, N.; Stotland, A.; Parker, S.; Van Eyk, J.; Pandol, S.J.; et al. Plasma Metabolomics Predicts Chemotherapy Response in Advanced Pancreatic Cancer. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).