1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2, also known as the severe acute respiratory syndrome coronavirus 2 [
1]. When the new SARS-CoV-2 was first discovered in Chinese pregnant women, there was hope that, unlike other RNA respiratory viruses and previous coronavirus infections such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), vertical transmission would not happen [
2]. Recent research indicates that multiple case reports and series have provided compelling evidence of congenital transmission of SARS-CoV-2; as a result, placental pathology is of great interest [
3].
The maintenance and sustenance of fetal growth depend on the placenta, a special tissue that only forms during pregnancy. It acts as the primary barrier between the mother and her fetus during pregnancy and facilitates the exchange of gases, nutrients, waste products, and hormones [
4]. Compared to the cytotrophoblast and other cells within the chorionic villus core, which are extremely prone to infections, the syncytiotrophoblast layer, which comes into direct contact with maternal blood, is fairly resistant to most pathogens [
5]. The extravillous trophoblast cell layer of the membranous chorion comprises the outer wall which surrounds approximately 60–70% of the surface. Apart from the membranes' structural barrier, trophoblast uses the toll-like receptor TLR4 to identify bacterial products and signals nearby immune cells to preserve tolerance. Their role is to support innate immunity alongside the amnion by generating antimicrobial peptides to ward off infection [
6].
Cell entry and the spread of SARS-CoV-2 are widely thought to depend on the angiotensin-converting enzyme 2 (ACE2) receptor and the serine protease TMPRSS2 [
7]. In both the first and second trimesters of pregnancy, the placentas exhibit notable expression of each entry receptor. Lower levels of entry cofactor expression are seen in the placenta and chorioamniotic membranes at the maternal-fetal interface at term [
8].
Often referred to as "SARS-CoV-2 placentitis" the most common histopathological triad is represented by chronic histiocytic intervillositis (CHI), villous trophoblast necrosis, and intervillous fibrin deposition (IFD) [
9]. Other studies show that the more frequent features in the placentas of infected women are represented by maternal and deciduous thrombosis, increased intervillous fibrin and, in rare cases, fetal thrombosis [
10].
The aim of this study offers an approach on both histopathological and immunohistochemical observations of placentas from SARS-CoV-2 positive mothers compared to those who did not encounter the infection during pregnancy.
2. Results and discussion
The positive group consisted of 24 patients aged 21-38 years (mean 31.37 years) and the negative group consisted of 20 patients aged 20-40 years (mean 32.27). The SARS-CoV-2 patients gave birth between 37-41 weeks gestational age (mean 38.91 weeks). Their newborns had birth weights ranging from 2950 to 3800 grams (mean 3333.75 grams) and has no associated comorbidities. As for the control group, the mean gestational age was 39.2 weeks, mean birth weight 3250.56g with no associated comorbidities nor complications during pregnancy or birth.
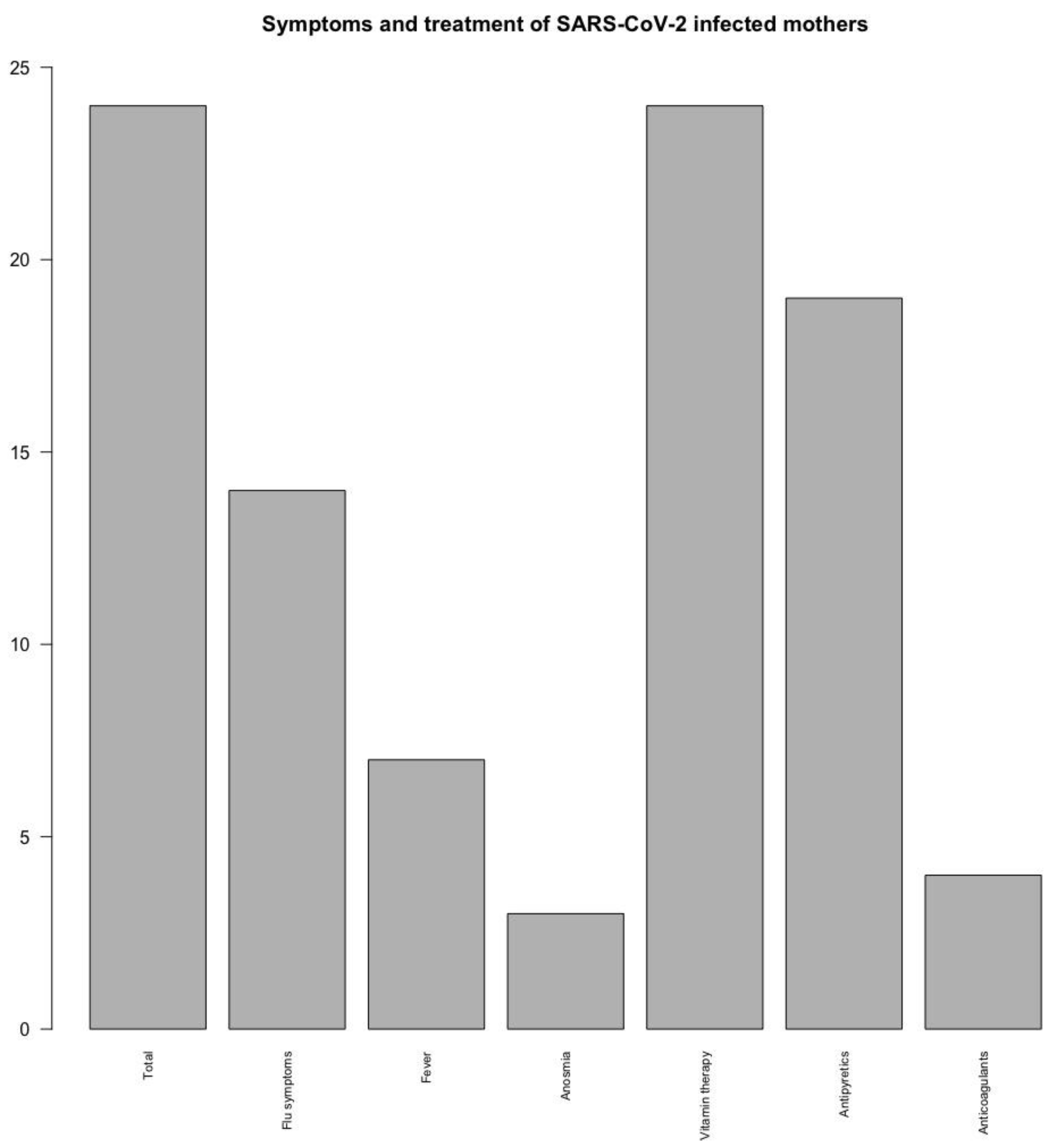
Maternal symptoms and treatment from the positive group were divided into several categories, as seen in
Figure 1. All 24 mothers received vitamin therapy during the infection period (100%), a large percent received antipyretics (19 out of 24, 79.1%) and only a few anticoagulant medication (4 patients, 16.6%). The most prevalent signs of disease were represented by flu-like symptoms (14 out of 24, 58.3%) such as fatigue, chills, pharyngitis, headaches, rhinorrhea, myalgia. Some patients developed fever (7, representing 29.1%) and only a few presented anosmia (3 patients, 12.5%).
Most SARS-CoV-2 maternal infections occurred during the third trimester of pregnancy (16 out of 24, 66.6%), 6 during the second trimester (25%) and 2 during the first trimester (8.3%).
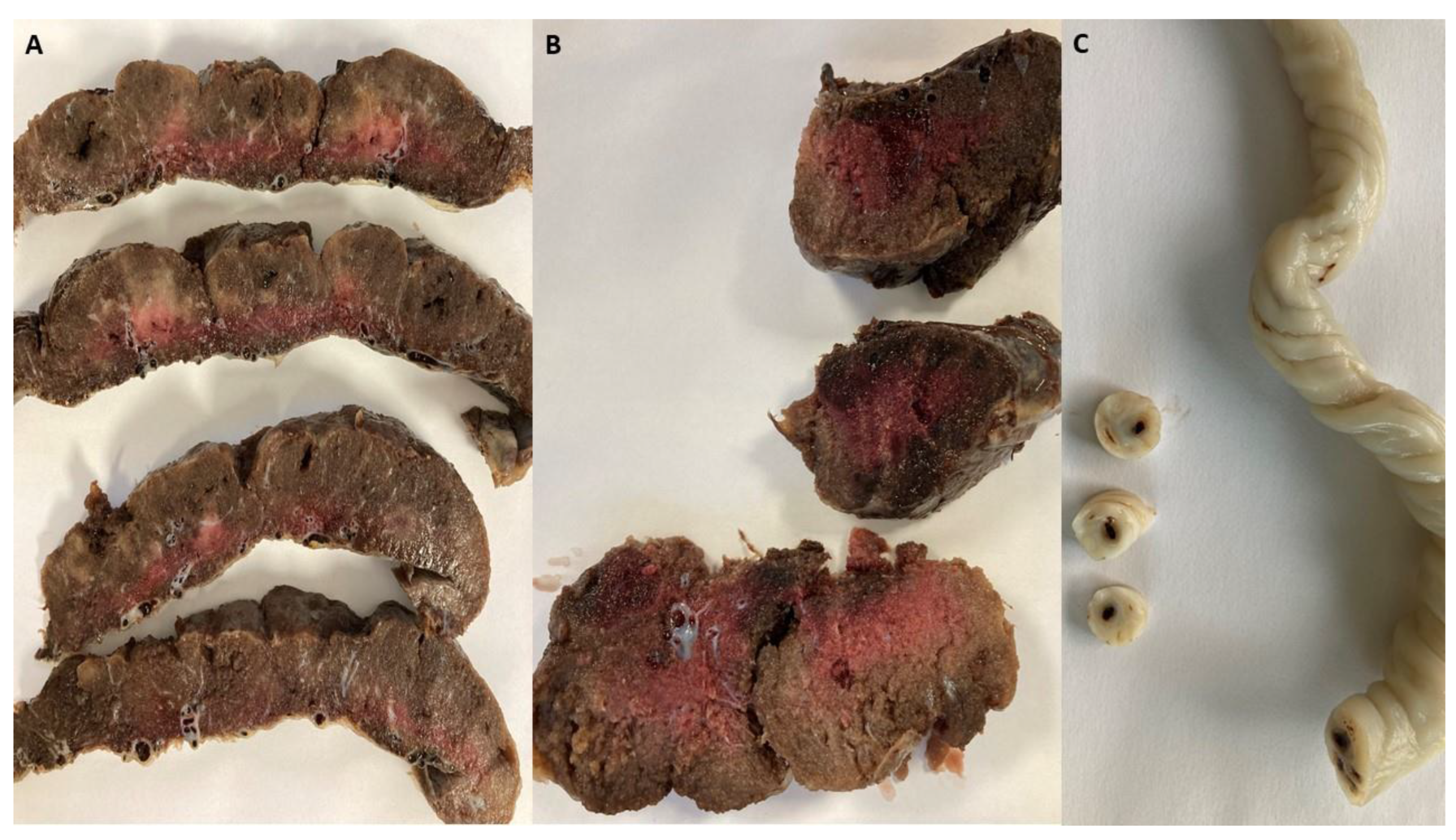
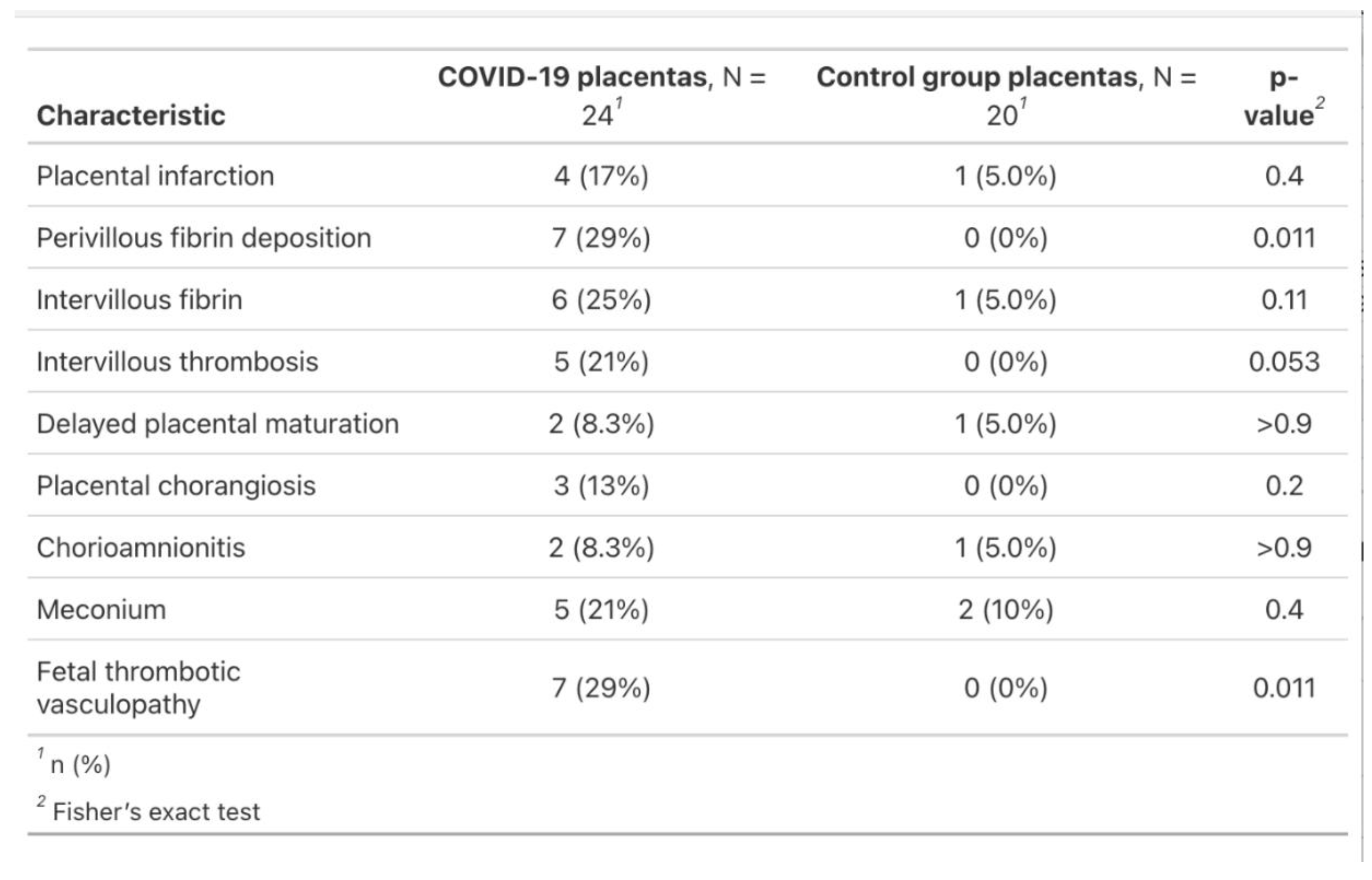
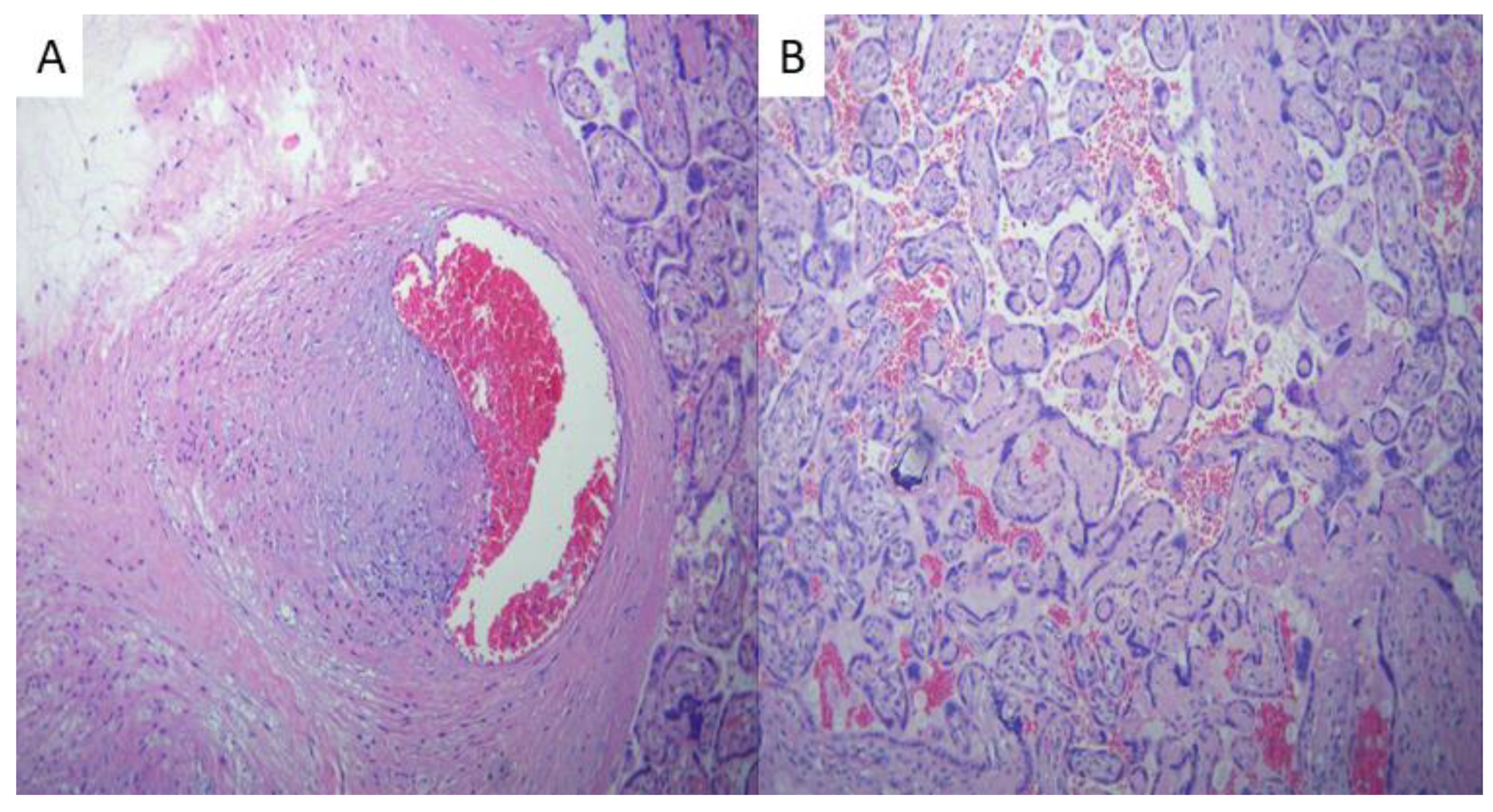
Histopathological examination of the placentas from the disease group revealed areas consistent with avascular villi (AV) and thrombi in the chorionic vessels and umbilical cord (
Figure 2), suggesting fetal vascular malperfusion (FVM). In the positive group, fetal thrombotic vasculopathy was seen in 29% of cases compared to the placentas from the control group (p=0.01), suggesting SARS-CoV-2 infection during pregnancy has a great impact on coagulation disorders, although coagulation can already be altered by pregnancy itself [
11]. One study found thrombo-hemorrhagic alterations with decidual microvascular thrombi being present in 3 cases, a significantly different frequency compared to the control cohort (
p = 0.003) [
12]. Glynn et al. describes that FVM lesions appeared more frequent among acute SARS-CoV-2 than either nonacute SARS-CoV-2 or control cases (53.8% vs. 18.8% vs. 13.2% for acute, nonacute and controls, respectively; P < 0.001) [
13]. What’s more, FVM was observed in 27.08% of cases (95% CI, 19.2−35.6) in another study conducted by Di Girolamo et al.
Intervillous thrombosis (
Figure 3) was also remarked during the evaluation of the placentas of SARS-CoV-2 infected patients, consisting of 21% of cases compared to the control group (p=0.053). Bouachba et al. Describes large intervillous thrombi that were disseminated throughout all the placental parenchyma and were not only confined to the basalplate [
15].
Our study revealed extensive microscopic involvement correlating with the gross placental appearances, similarly to the ones described by Fitzgerald et al. [
16].
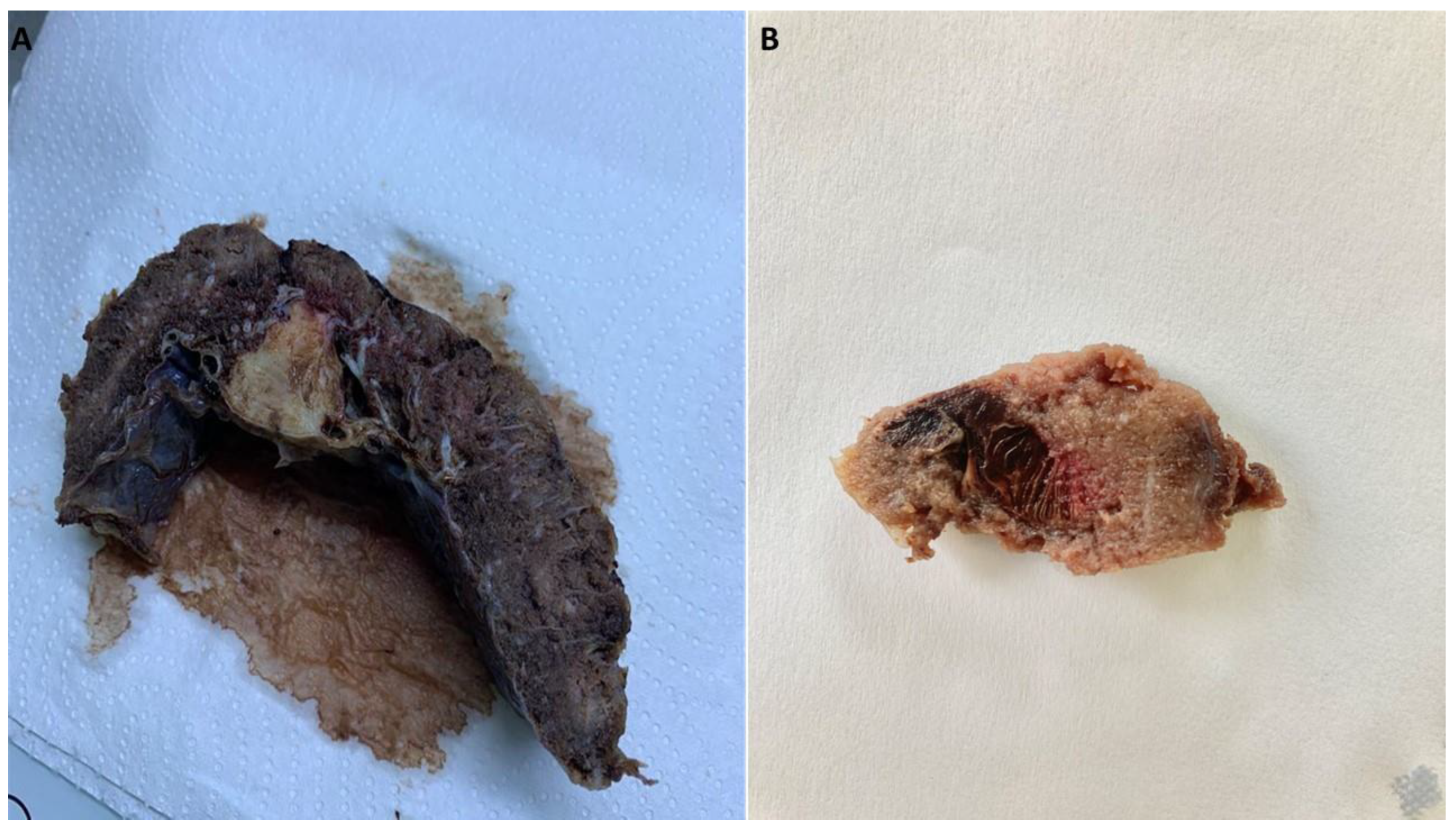
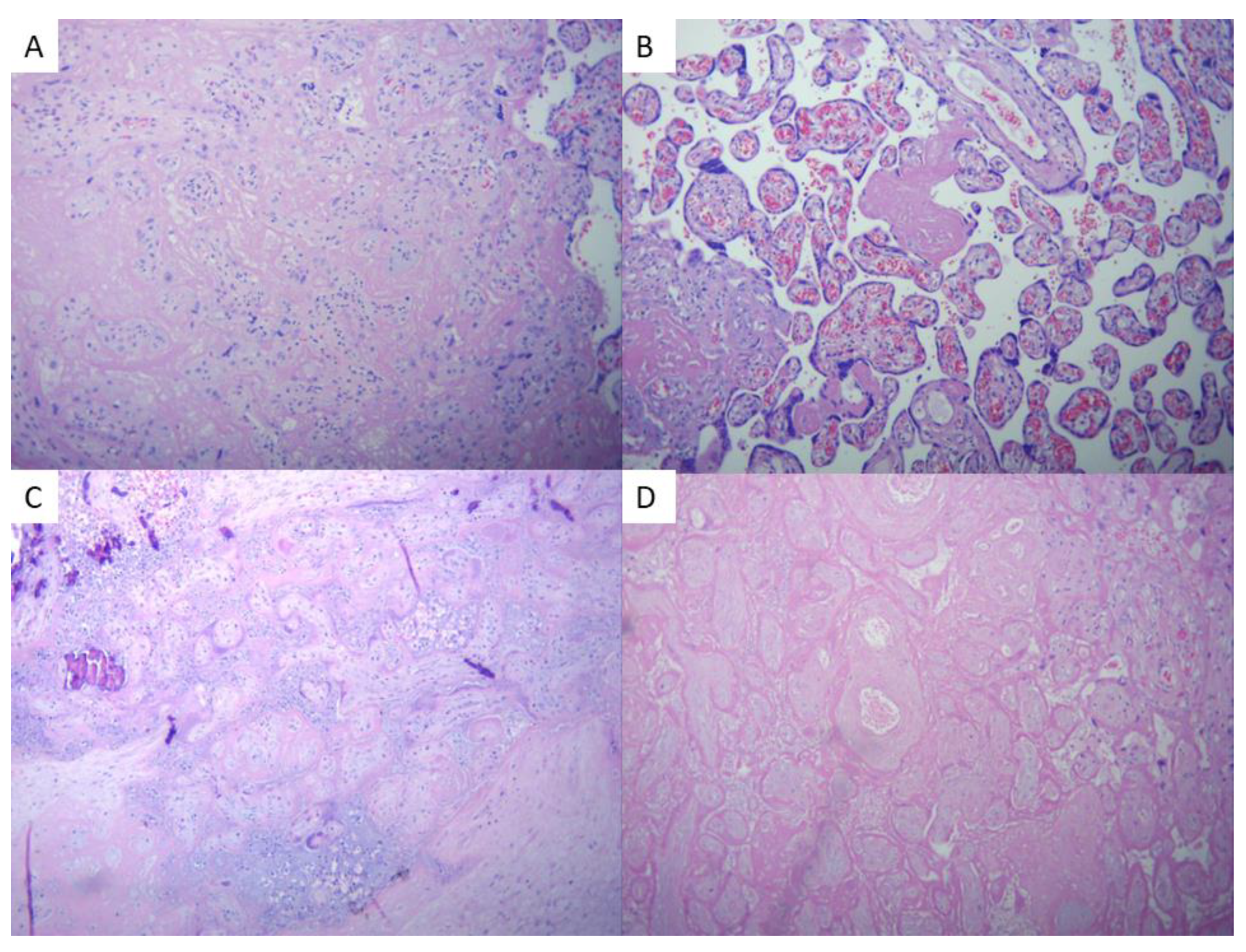
Other pathological features of the COVID-19 placentas were placental infarction (17%), perivillous fibrin deposition (29%), intervillous fibrin (25%), delayed placental maturation (8.3%), chorangiosis (13%), chorioamnionitis (8.3%) and meconium (21%). The negative control group revealed only one case of placental infarction (5%), intervillous fibrin (5%), delayed placental maturation (5%), chorioamnionitis (5%) and two cases of meconium (19%) (
Figure 4).
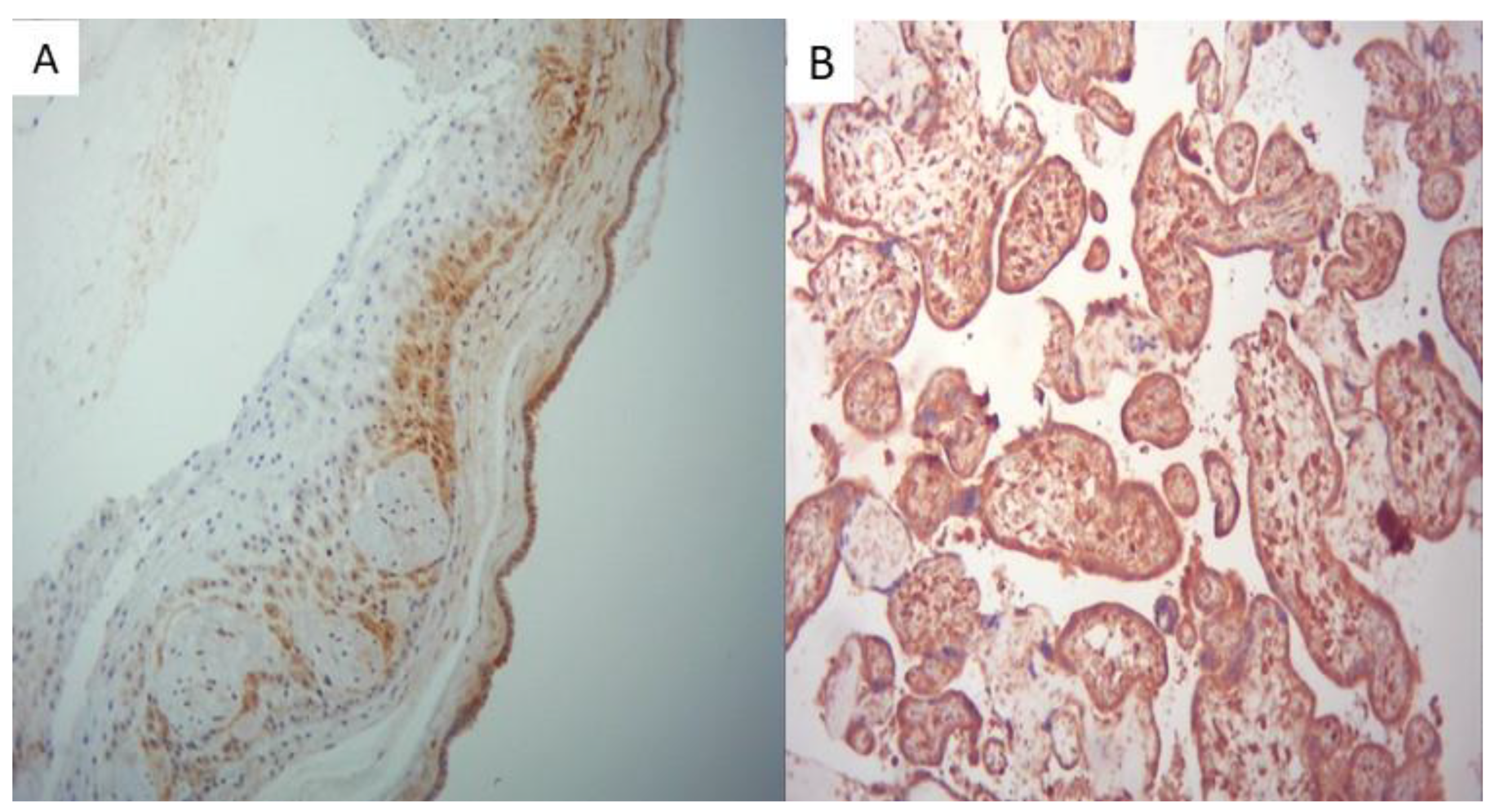
All placentas from the positive group showed strong SARS-CoV-2 positivity of the placental membranes, of the trophoblast and fetal villous macrophages, as seen in
Figure 5.
Microscopic findings of the COVID-19 group placentas were consistent with data described in literature, and have been briefly illustrated in
Figure 6 and
Figure 7.
Histopathological examination of the placentas revealed a spectrum of pathologically increased perivillous fibrinoid deposition counting for seven of the positive group (29%), versus none in the comparative group (p=0.01). Zaigham et al. found massive perivillous fibrinoid deposition (MPFD) in 13 of the 14 placentas (93%) [
3]. Argueta et al. observed that the intervillous spaces showed extensive infiltration of maternal immune cells, perivillous fibrin deposition, and clots with erythrocytes, mononuclear cells, and fibrin deposition, while none of the negative samples or negative controls displayed massive chronic intervillositis (MCI), chronic histiocytic intervillositis (CHI), or infiltration of maternal immune cells [
8].
Chronic placental hypoperfusion or low-grade tissue hypoxia was also a concern among pregnant patients with COVID-19 disease during pregnancy. Our study revealed 3 cases of chorangiosis (13%) in the positive group with none in the negative group, with similar data in literature. Ferraiolo et al. also observed that the terminal villi of the placentas collected from SARS-CoV-2 infected mothers were characterized by capillary congestion and focal microchorangiosis [
17]. Chorangiosis was also described by Levitan et al., but was not among the most prevalent characteristics for this group of patients [
1]. Hsu et al. describes an overall histology consistent with acute uterine hypoxia (subchorionic laminar necrosis) superimposed on chronic uterine hypoxia (extra-villous trophoblasts and focal chronic villitis), of which hypertrophic arteriolopathy may be partly responsible [
18].
Placental infarction, resulting from the interruption of blood supply, was seen in four of the positive group placentas (17%) and in only one case of the control group (5%). In an article written by Salvatore et al., placental infarcts in different stages of organization were observed in 192 samples (19.8%) [
9]. By contrast, Levitan et al. reported villous infarction predominated in the SARS-CoV-2–negative group (14 [16%]), suggesting this characteristic occurs sporadically in pregnancies with COVID-19 disease [
1].
Intervillous fibrin occurred in 6 of the 24 studies placentas of the positive group (25%), compared to one case from the negative control group (5%). Reagan-Steiner et al. noted increased villous fibrinoid necrosis, as a feature of villous trophoblastic injury in placentas of SARS-CoV-2 infected mothers [
19].
Meconium staining of the placenta was seen in 5 cases of the positive group (21%) and 2 of the negative group (10%) (p=0.4). Glynn et al. describe a distribution of the frequency of meconium staining of the placenta different across the 3 studied groups (65.4% vs. 40.6% vs. 36.2% for acute, nonacute and controls, respectively; P = 0.018) [
13].
Examination of placentas collected from SARS-CoV-2 positive mothers during pregnancy revealed that, while the features on the slides varied, they shared a common pattern, in accordance with findings described in literature, such as perivillous fibrin deposition, aggregation of villi with obliteration of the intervillous space and fetal thrombotic vasculopathy.
The main limitation encountered in our study was represented by a small sample size due to the short time interval for placental collection, preparation and data analysis.
3. Materials and Methods
We examined 44 placentas from patients who delivered between November 2021 and August 2022, collected from mothers who were not vaccinated against SARS-CoV-2 infection before/during pregnancy. We divided them into two groups, the positive one, consisting of 24 placentas which were collected and inspected from SARS-CoV-2 positive patients (confirmed using Polymerase chain reaction - PCR technique). In these cases, the infection was acquired during pregnancy or at birth. Inclusion criteria were represented by COVID-19 confirmation using the PCR technique. Exclusion criteria were based on vaccination during pregnancy or other pathologies that could impact placental blood circulation, such as pregnancy induced hypertension, diabetes mellitus, signs of impaired fetal growth. The negative lot consisted of 20 placentas gathered from SARS-CoV-2 negative patients throughout gestation. Inclusion criteria were defined based on negative SARS-CoV-2 symptoms and tests along pregnancy, while the same exclusion criteria applied for the first group were used, as a way to rule out bias.
Clinicopathological data and macroscopy pictures were obtained from the database of the Premiere Hospital of the Regina Maria Private Health Network, according to the protocols approved by the ethics committee (No. 330/18.11.2021), and the standards of the World Medical Association Declaration of Helsinki.
Tissue samples were fixed in 10% buffered formalin for a minimum of 48 hours and then embedded in paraffin. Placentas were weighed after formalin fixation and after removal of amniotic membranes and umbilical cord. The sections required for histological evaluation were obtained from the umbilical cord, amniotic membranes and some that included the entire thickness of the placental parenchyma. Subsequently, the segments were made from paraffin blocks, 5 micrometers thick and slides were stained using standard hematoxylin-eosin stain.
For performing the immunohistochemistry technique, representative components were taken from placentas, 3 micrometers thick. During the first phase, standard deparaffinization and dehydration of the sections was performed, followed by heat induced epitope retrieval for 20 minutes with citrate buffer at a pH of 6.0. Endogenous peroxidase blocking was performed with 3% hydrogen peroxide for 5 minutes. Incubation with primary monoclonal antibody specific for SARS-CoV-2 nucleocapsid, dilution 1:50 (model STJ11101210, St John's, UK) lasted 30 minutes. NovoLink Max Polymer Detection System was used for visualization. Counterstaining was performed using modified Lillie's Hematoxylin for 5 minutes. The entire immunohistochemical procedure was performed using the Leica Bond-Max Autostainer.
Placentas collected from patients confirmed with SARS-Cov-2 using PCR technique during pregnancy were considered positive controls and placentas collected from patients who did not gather the infection during pregnancy were considered negative controls.
Statistical tests
Statistical analysis was performed using Fisher’s exact test to determine the relationship between SARS-CoV-2 and clinical parameters and histological changes. Values less than 0.05 were considered statistically significant. Statistical tests were performed in RStudio for Mac, version 2023.09.1+494.
Microscopic evaluation was performed using the Nikon Eclipse E600 microscope and images were acquired and analyzed using Lucia G software.
4. Conclusions
This paper explains the differences found between placentas from COVID-19 disease during pregnancy and normal gestation. Infection throughout pregnancy occurred mostly during the third trimester of pregnancy (16 out of 24, 66.6%), 6 during the second trimester (25%) and 2 during the first trimester (8.3%). Overall, the most common lesions found in the SARS-CoV-2 positive group were consistent with literature findings, representing fetal thrombotic vasculopathy (29%, p=0.01), perivillous fibrin deposition (29%, p=0.01), followed by placental infarction (17%, p=0.4), intervillous fibrin (25%, p=0.11), delayed placental maturation (8.3%, p>0.9), chorangiosis (13%, p=0.2), chorioamnionitis (8.3%, p>0.9) and meconium stains (21%, p=0.4). Our study shows SARS-CoV-2 has an impact on coagulation, suggested by fetal thrombotic vasculopathy and fibrin deposition, although coagulation can already be altered by pregnancy itself. Further research is necessary to establish whether SARS-CoV-2 is the main cause of vascular and circulatory pathologies during pregnancy.
Author Contributions
Conceptualization, D.E.P. and I.R.; methodology, D.E.P.; software, A.M.C.J.; validation, D.E.P., I.R. and A.C.; formal analysis, O.P.; investigation, Z.L.P. and A.R.; resources, N.L.; data curation, D.E.P.; writing—original draft preparation, A.M.C.J.; writing—review and editing, D.E.P., I.R. and A.C.; visualization, N.L.; supervision, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee (No. 330/18.11.2021) of the Premiere Hospital of the Regina Maria Private Health Network.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Levitan D, London V, McLaren RA Jr, Mann JD, Cheng K, Silver M, et al. Histologic and Immunohistochemical Evaluation of 65 Placentas From Women With Polymerase Chain Reaction–Proven Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Arch Pathol Lab Med. 2021 Feb 17;145(6):648–56. [CrossRef]
- JDB | Free Full-Text | Molecular Pathology Demonstration of SARS-CoV-2 in Cytotrophoblast from Placental Tissue with Chronic Histiocytic Intervillositis, Trophoblast Necrosis and COVID-19 [Internet]. [cited 2023 Nov 6]. Available from: https://www.mdpi.com/2221-3759/9/3/33.
- Zaigham M, Gisselsson D, Sand A, Wikström AK, von Wowern E, Schwartz DA, et al. Clinical-pathological features in placentas of pregnancies with SARS-CoV-2 infection and adverse outcome: case series with and without congenital transmission. BJOG Int J Obstet Gynaecol. 2022;129(8):1361–74. [CrossRef]
- Delorme-Axford E, Sadovsky Y, Coyne CB. The Placenta as a Barrier to Viral Infections. Annu Rev Virol. 2014;1(1):133–46. [CrossRef]
- Yong HEJ, Chan SY, Chakraborty A, Rajaraman G, Ricardo S, Benharouga M, et al. Significance of the placental barrier in antenatal viral infections. Biochim Biophys Acta BBA - Mol Basis Dis. 2021 Dec 1;1867(12):166244. [CrossRef]
- Heerema-McKenney, A. Defense and infection of the human placenta. APMIS. 2018;126(7):570–88. [CrossRef]
- Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Parker SC, Bronner ME, editors. eLife. 2020 Jul 14;9:e58716. [CrossRef]
- Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Rendeiro AF, et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience. 2022 May 20;25(5):104223.
- Salvatore MA, Corsi Decenti E, Bonasoni MP, Botta G, Castiglione F, D’Armiento M, et al. Placental Characteristics of a Large Italian Cohort of SARS-CoV-2-Positive Pregnant Women. Microorganisms. 2022 Jul;10(7):1435. [CrossRef]
- Viruses | Free Full-Text | SARS-CoV-2, Placental Histopathology, Gravity of Infection and Immunopathology: Is There an Association? [Internet]. [cited 2023 Nov 6]. Available from: https://www.mdpi.com/1999-4915/14/6/1330.
- Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion | Pathobiology | Karger Publishers [Internet]. [cited 2023 Nov 6]. Available from: https://karger.com/pat/article/88/1/69/329210.
- Placenta histopathology in SARS-CoV-2 infection: analysis of a consecutive series and comparison with control cohorts | Virchows Archiv [Internet]. [cited 2023 Nov 6]. Available from: https://link.springer.com/article/10.1007/s00428-021-03097-3. [CrossRef]
- Glynn SM, Yang YJ, Thomas C, Friedlander RL, Cagino KA, Matthews KC, et al. SARS-CoV-2 and Placental Pathology. Am J Surg Pathol. 2022 Jan;46(1):51–7. [CrossRef]
- Di Girolamo R, Khalil A, Alameddine S, D’Angelo E, Galliani C, Matarrelli B, et al. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021 Nov 1;3(6):100468. [CrossRef]
- Bouachba A, Allias F, Nadaud B, Massardier J, Mekki Y, Bouscambert Duchamp M, et al. Placental lesions and SARS-Cov-2 infection: Diffuse placenta damage associated to poor fetal outcome. Placenta. 2021 Sep 1;112:97–104. [CrossRef]
- Fitzgerald B, O’Donoghue K, McEntagart N, Gillan JE, Kelehan P, O’Leary J, et al. Fetal Deaths in Ireland Due to SARS-CoV-2 Placentitis Caused by SARS-CoV-2 Alpha. Arch Pathol Lab Med. 2022 Jan 12;146(5):529–37. [CrossRef]
- Medicina | Free Full-Text | Report of Positive Placental Swabs for SARS-CoV-2 in an Asymptomatic Pregnant Woman with COVID-19 [Internet]. [cited 2023 Nov 6]. Available from: https://www.mdpi.com/1648-9144/56/6/306.
- Hsu AL, Guan M, Johannesen E, Stephens AJ, Khaleel N, Kagan N, et al. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J Med Virol. 2021;93(2):1038–44. [CrossRef]
- Reagan-Steiner S, Bhatnagar J, Martines RB, Milligan NS, Gisondo C, Williams FB, et al. Detection of SARS-CoV-2 in Neonatal Autopsy Tissues and Placenta. Emerg Infect Dis. 2022 Mar;28(3):510–7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).