1. Introduction
Idiopathic epiretinal membrane (iERM) is a frequently encountered vitreoretinal disorder affecting the macula, with an age-related increasing prevalence.[
1,
2] The advent of high-resolution imaging techniques, such as spectral domain- and swept source-optical coherence tomography (SD-OCT and SS-OCT), has significantly enhanced the understanding and evaluation of this condition.[
3,
4] Fundus oculi examination in patients with ERMs reveals evident vascular tortuosity and retinal contraction, although quantification of these changes poses challenges. The introduction of optical coherence tomography angiography (OCTA), which is able to evaluate the tangential traction while taking into account the depth of the retinal distortions caused by the epiretinal traction via the inner and outer retinal layers, allowed to measure various parameters, thanks to the en-face visualization of the retinal plexuses, assessing the extent of distortion and the impact of ERM contractions on the retinal microvasculature.[
5,
6]
The standard surgical approach for ERMs is vitrectomy combined with membrane peeling.[
7] Nevertheless, the development of postoperative cystoid macular edema (CME) is a well-known side effect, hindering the possibility to achieve optimal visual outcomes.[
8,
9] In order to prevent CME development, the use of intraoperative intravitreal dexamethasone implant (Ozurdex®, Allergan Inc., Irvine, CA, USA) has been proposed.[
10,
11] A recent meta-analysis compared ERM vitrectomy without and with intraoperative intravitreal Ozurdex, showing that the latter allowed for a better visual outcome at three months.[
12]
The evolution of vascular tortuosity after surgical ERM treatment has been studied with OCTA, showing that the reconstitution on vessel linearity around the macula was associated with better visual outcomes.[
13,
14] Similarly, Bacherini et al. showed significant changes in all retinal plexuses (superficial, deep and choriocapillaris), evolving in the six months after ERM surgery.[
15]
As far as we know, the application of OCTA in ERM vitrectomy combined with intraoperative dexamethasone implant, has not been explored yet. This paper aims to explore microvascular changes in patients undergoing Ozurdex-augmented ERM surgery, compared with standard surgical approach. Additionally, we investigated the anti-inflammatory and anti-angiogenic properties of dexamethasone to prevent postoperative CME.
2. Materials and Methods
In this prospective mono-centric interventional analysis, we enrolled 50 eyes of 50 patients affected by iERMs at Policlinico Universitario Agostino Gemelli between October 27, 2021, and June 20, 2023. The Policlinico Universitario Agostino Gemelli Institutional Ethics Committee approved the study, which followed the tenets of the Declaration of Helsinki. All patients who were recruited provided signed, fully informed consent.
The inclusion criteria for patient selection were as follows: age >18 years; clinical and instrumental diagnosis of primary epiretinal membrane (ERM) at stage 3-4 according to the OCT staging system proposed by Govetto et al.;[
16] preoperative pseudophakia.
Exclusion criteria included patients with any kind of secondary ERM, concomitant ocular or systemic diseases that could affect best-corrected visual acuity (BCVA) and/or central macular thickness (CMT), degenerative myopia, ambient opacities hindering imaging and history of ocular surgeries except for uncomplicated cataract surgery.
We divided our sample into two subgroups:
25 eyes underwent pars plana vitrectomy (PPV) combined intraoperative intravitreal administration of dexamethasone implant (Ozurdex) (Group A).
25 eyes underwent PPV and peeling without concomitant intravitreal corticosteroid administration (Group B).
Before the intervention, the participants' eyes underwent a complete ophtalmological examination, with BCVA measurement, tonometry, slit-lamp biomicroscopy and fundus examination. OCT and OCT-A scans were performed under pharmacologically induced mydriasis with 1% tropicamide solution. These procedures were repeated at the follow-up visit 3 months after surgery. This timing was chosen based on the duration of dexamethasone implant’s therapeutic effect, as reported in literature.[
17]
The main outcome of this research was to outline the changes in retinal plexuses after iERM surgery in the combined PPV-Ozurdex group compared to the PPV alone group. As secondary outcomes, we assessed changes in CMT, BCVA and the rate of postoperative CME between the two groups.
2.1. Scan protocol
The OCT/OCT-A scans were performed using the full-range OCT Solix system (Optovue Inc, Freemont, CA, USA). Scans with low-quality indices (<7/10) due to significant lens opacities, frequent eyelid blinking, excessive motion artifacts, and other factors were rejected. The OCTA images were acquired using the AngioVue Retina software, which automatically assessed the vascular density (VD) of the superficial and deep capillary plexuses (SCP, DCP) in the entire macular region corresponding to a 6.4 × 6.4 mm field centered on the fovea. Parameters such as Whole Image (the entire image) and Fovea (the area within the 1 mm central ring of the ETDRS grid) were evaluated. Additionally, the RetinaCube software evaluated the central macular thickness (CMT). For the SCP, en-face images were obtained using the automatic segmentation between the inner boundary (internal limiting membrane, ILM) and the outer boundary (inner plexiform layer). The DCP was identified between the outer plexiform layer and the inner plexiform layer. Each OCTA image underwent motion correction and 3D projection artifact removal technology to improve image quality.
2.2. Surgical procedure
Under peribulbar anesthesia, a standard 25-gauge 3-port pars plana vitrectomy was performed on each eye utilizing the Constellation Vision System (Alcon Laboratories Inc., Fort Worth, TX, USA) and a wide-angle noncontact viewing system (Resight®; Carl Zeiss Meditec AG, Jena, Germany). The ILM/ERM complex was dyed with MembraneBlue Dual, (TrypanBlue 0,15% + Brillian Blue G 0,025%, DORC, Zuidland, the Netherlands) and peeled using vitreoretinal forceps till reaching an area of two papillary diameters far from the fovea. For eyes with retinal tears or holes, peripheral retinal photocoagulation was done in addition to a full vitrectomy.
At the end of the surgery, in the first subgroup, following fluid-air exchange, the Ozurdex implant was injected through the inferotemporal sclerotomy after infusion cannula removal and repositioning in the superotemporal sclerotomy. The implant was always visualized in the vitreous cavity thanks to the wide angle viewing system.
In the non-Ozurdex subgroup, a fluid-air exchange was performed to terminate the surgery.
All surgeries were performed by two experienced surgeons (A.B. and S.R.).
2.3. Statistical analysis
Statistical analysis was conducted using GraphPad PRISM Software (Version 9.5; GraphPad, La Jolla, CA). The Shapiro-Wilk test was employed to assess the normality of the sample, with a p-value greater than 0.05 supporting the null hypothesis of normal distribution. For the comparison of continuous variables between baseline and postoperative data, Mann-Whitney U test was utilized with a 95% confidence interval (CI). Analysis of variance (ANOVA) was performed, and multiple comparison tests using matched pairs with Geisser-Greenhouse correction were conducted. To explore the relationship between quantitative OCTA values and clinical outcomes (BCVA and CMT) a Spearman correlation was performed. Mean standard deviation (SD) was used to present quantitative data, and a significance level of p<0.05 was employed to determine statistical significance.
3. Results
Demographic characteristics of the two subgroups are visible in
Table 1. No significant differences were reported in preoperative data.
Mean preoperative BCVA was 0.41±0.3 and 0.38±0.3 Snellen equivalent in Group A and group B, respectively, improving significantly to 0.79±0.2 and 0.71±0.3 Snellen equivalent at 3 months (p=0.008 and p=0.001 for Group A and B, respectively). The difference in terms of visual acuity was borderline significant between the two groups (p=0.054). Mean postoperative IOP showed mild but not significant elevation in Group A (from 14.1±2.9 to 17.6±3.1, range 13-22 mmHg, p=0.11), while remaining stable in Group B (from 15.0±4.3 to 15.8±5.1, p=0.88). One patient had mild ocular hypertension (IOP 22 mmHg) at 3 months but then fully resolved without medical treatment.
Mean CMT at baseline was 485±63 µm and 467±93 µm in Group A and B, respectively. At 3 months, a significative improvement was seen in either Group A (mean CMT 358±62 µm, p=0.001) and Group B (mean CMT 377±53 µm, p=0.009).
There were no complications related to Ozurdex implant injection procedure in an airfilled eye at the end of the surgery. After the injection, the implant was visible on the inferior retina with no apparent hemorrhage or trauma.
In Group A, we reported no cases of post-operative CME, while in Group B, 2 eyes had CME at the 3 months follow-up’s OCT scans.
3.1. OCTA findings
Concerning the SCP, in Group A we found a statistically significant increase of VD in either the whole image (42.1±4.1 vs. 45.6±4.3 % from baseline to the 3 months follow-up, p=0.01) and the fovea image (38.5±7.5 vs. 41.7±4.2% from baseline to the 3 months follow-up, p=0.03). In Group B, we reported no significant variations in the VD of the whole area (42.9±5.3% at baseline vs. 43.8±5.8% at the 3 months follow-up, p=0.69) and the fovea area (40.4±6.7 vs. 40.9±6.7% from baseline to the 3 months follow-up, p=0.25). The differences at three months between Group A and B were significant for the whole image’s VD (p=0.03), while borderline significant for the fovea image (p=0.06).
By measuring the VD of the DCP before and after surgery, in Group A we observed a significant improvement in the whole image (from 40.4±10.1% at baseline to 44.7±6.6% at the 3 months follow-up, p=0.005), but not in the fovea area (30.7±10.0 vs. 32.3±10.9% from baseline to the 3 months follow-up, p=0.14). In Group B, we reported no significant changes in both areas: 41.8±13.2 vs. 42.0±11.5% in the whole image from baseline to the 3 months follow-up, p=0.26; 30.0±7.7 vs. 31.0±8.9% in the fovea image from baseline to the 3 months follow-up, p=0.11. Overall, the differences between the final DCP parameters differed between the two groups in the whole area (p=0.02), but not in the fovea area (p=0.11).
Pre and post-operative OCTA of Group A and Group B patients are visible in
Figure 1 and
Figure 2.
Table 2. summarizes the differences between the two groups at 3 months.
3.2. Correlation analysis
Before surgery, negative correlations were found between the Govetto stages and VD of the SCP and the DCP whole area (r=−0.2108, p=0.04 for the SCP; r=−0.3591, p=0.02 for the DCP) of the entire cohort. Similar results were found for the fovea area (r=-0.3045 and r=-0.3869 for SCP and DCP, respectively) of the entire cohort.
Postoperative analysis in patients undergoing PPV+Ozurdex (Group A), highlighted no correlation between variations in SCP whole area’s VD and CMT (r: -0.1756, p, 0.29), neither when correlating variations in DCP whole area’s VD and CMT (r: 0.1137, p, 0.47). Conversely, SCP and DCP variations positively correlated with BCVA improvement, and this correlation was significant for the DCP (r: 0.3610, p, 0.08 for SCP vs. BCVA; r: 0.4520, p, 0.03 for DCP vs. BCVA).
3.3. Stage 3 vs. Stage 4
Successively, we performed a subgroup analysis based on the stage of the epiretinal membrane. The complete comparison is summarized in
Table 3.
In stage 4 ERMs, we reported significant changes in all parameters in Group A (all p<0.05). [
Figure 3]
Conversely, in Group B we found only significant changes in VD of the SCP in both the whole the fovea areas. [
Figure 4]
In stage 3 ERMs, Group B did not show significant changes in any of the studied parameters at 3 months (all p>0.05). Meantime, Group A showed a significant increase in whole images in both SCP and DCP’s VD.
4. Discussion
With the advent of OCTA, retinal microvasculature has been a subject of research in various retinal disorders, with several works analyzing microvascular changes caused by ERM and their evolution after surgical peeling.[
6,
15] In this study, we aimed to analyze the effects of intravitreal dexamethasone implant (Ozurdex), in the context of ERM surgery, on macular microvascular homeostasis. In the PPV+Ozurdex group, we highlighted significant increase in the vessel densities of either the SCP and DCP at the 3 months follow up, suggesting significant postoperative microvascular remodeling. Conversely, no significant changes in terms of vessel density were found in the PPV alone group.
In vitreoretinal interface disorders, such as ERMs, it is hypothesized that the macular distortion caused by the contractile membrane may initiate the inflammatory cascade.[
18] Within this context, the utilization of the intravitreal Ozurdex implant has been investigated, since dexamethasone (DEX) is recognized for its potent anti-inflammatory properties and favorable safety profile.[
19] The intravitreal dexamethasone implant is authorized for use in Europe for the management of posterior segment inflammation resulting from noninfectious uveitis, CME associated with retinal vein occlusion, and diabetic macular edema (DME).[
11] Furthermore, the use of dexamethasone has been documented in various other inflammatory conditions, including pseudophakic CME,[
10] inflammation following retinal detachment ab-externo repair surgery,[
20] and exacerbation of macular edema following cataract surgery.[
21]
One notable benefit of this sustained-release implant is its effectiveness in vitrectomized eyes, which may not be as responsive to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy due to a more rapid clearance or washout effect.[
22] Its use has been studied in the last few years in patients for ERM removal and showed promising results, both anatomically and functionally.[
12,
23,
24,
25,
26] In particular, Iovino et.al and Fallico et.al respectively demonstrated how eyes undergoing ERM vitrectomy combined with intraoperative dexamethasone implantation have better visual outcomes at 15-day follow-up and 3 months follow-up compared to those undergoing ERM vitrectomy without dexamethasone implantation, with a significant improvement in macular thickness in the immediate postoperative period correlating with better visual recovery.[
25,
27] Similarly, we reported better BCVA at three months in the PPV+Ozurdex group, even though the difference was only borderline significant.
Studies on OCTA showed that, in case of ERM development, SCP is significantly impaired, probably due to the ERM-associated direct mechanical stress affecting the inner retinal layer and causing progressive capillary subocclusion.[
28] Lin et al. demonstrated that the tractional force of ERM affected not only the tortuous SCP, but also the DCP, which presented focal areas of non-perfusion, and their results were confirmed by successive studies.[
29,
30] Mastropasqua et al. showed that the perfusion density (PD) and VD were statistically lower in ERM-affected eyes than that in the control group.[
31] Furthermore, according to Rommel et al., ERMs influence choroidal and choriocapillary networks, and damage in the DCP and SCP may therefore have an impact on the choriocapillaris' microvasculature.[
32]
In a 6 months study of ERMs undergoing PPV and peeling, Bacherini et al. observed a considerable rise in SCP’s VD, likely due to its increased sensitivity to the reopening of small vessels that were suboccluded during the preoperative period. Moreover, the DCP showed a marked increase in VD and PD during the follow-ups. The removal of the ERM seemed to induce a gradual and stable reopening of retinal vessels, which were emptied by tractional stretching forces, but vascular walls were conceptually unaltered. Therefore, the postoperative increase in blood flow may last for several months.[
15] Interestingly, in the first month after surgery, they reported a decrease in SCP and DCP’s VDs.[
15] Li et al. previously reported no changes in SCP and DCP 1 month after surgery, but reported an increased arterial oxygen saturation. They hypothesized that the removal of the vitreous body determines an improvement in retinal blood flow. As a consequence, there is a gradual increase in retinal arteriolar saturation, with better oxygen diffusion and transport from the anterior segment. Conversely, there was no corresponding change in venous saturation, suggesting an improved utilization of oxygen.[
33]
In our research, we reported an improvement in vascular parameters in the PPV+Ozurdex group, rather than the PPV alone group. Previous studies showed that Ozurdex determines a leukostasis inhibition, a VEGF reduction and a tightening of the vascular endothelial tight junctions with reduced capillary leakage, as well as a block of the inflammatory cascade.[
34,
35,
36] In a OCTA analysis, Minnella et al. highlighted significant microvascular remodeling after intravitreal dexamethasone implant in both DME and retinal vein occlusion (RVO). In particular, they reported vascular restoration mainly concerning the DCP in the DME group and both the SCP and DCP in eyes affected by RVO. (Minnella) Even though ERM-induced CME and microvascular changes does not rely on a capillary leakage mechanism, we hypothesize that dexamethasone implant, in addition to reducing the rate of post-vitrectomy CME (no cases in PPV+Ozurdex group vs. 2 cases in PPV alone group), may allow for faster microvascular restoration in either the SCP and DCP. We indeed reported higher SCP and DCP’s VD in the PPV+Ozurdex group, compared to the PPV alone group, and those values, specifically the DCP’s VD raise, positively correlated with postoperative BCVA improvement.
Consistently with the study of Bacherini et al., we found a negative correlation between SCP and DCP’s VD and the stage of the pathology, following Govetto’s classification.[
15,
16] Moreover, stage 4 ERMs, starting from lower VD values, showed a more significant microvascular improvement, suggesting an even more pronounced postoperative remodeling in these advanced conditions. Nevertheless, postoperative VD values remained in all instances lower than those of stage 3 ERMs.
Our study has several limitations. Firstly, the small sample size, which may have probably impacted statistically significance. However, this result represents an important starting point for expanding the study to a larger cohort of patients with the aim of using intravitreal Ozurdex implantation as a standard intraoperative protocol. Secondly, the short follow up period may have hampered the possibility of evaluating long term microvascular changes. Nevertheless, we consciously decided to evaluate three-months results to assess the effects of dexamethasone implant at the end of his period of action.
5. Conclusions
In conclusion, we can affirm that intraoperative intravitreal Ozurdex administration is associated with good functional and anatomical recovery after ERM surgery, limiting the occurrence of postoperative CME compared to the standard procedure. Furthermore, we hypothesize that the blockage of the cytokine cascade and the tight junction tightening are at the basis of the microvascular restoration we reported after Ozurdex implantation. Further studies with larger study population and longer follow-ups are needed to corroborate these findings.
Author Contributions
Conceptualization, A.B.; methodology, M.M.C. and L.V.; validation, G.G.. formal analysis, A.B. and M.M.C..; data acquisition, L.V. and C.M; writing—original draft preparation, M.M.C. and L.V.; writing—review and editing, G.G., F.M. and N.D.O..; supervision and project administration, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Fondazione Policlinico Universitario A. Gemelli-IRCCS Rome.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MMC, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meuer, S.M.; Myers, C.E.; Klein, B.E.; Swift, M.K.; Huang, Y.; Gangaputra, S.; Pak, J.W.; Danis, R.P.; Klein, R. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology 2015, 122, 787–795. [Google Scholar] [CrossRef]
- Zapata, M.A.; Figueroa, M.S.; Esteban Gonzalez, E.; Huguet, C.; Giralt, J.; Gallego Pinazo, R.; Abecia, E.; Group, S.-V.S. Prevalence of Vitreoretinal Interface Abnormalities on Spectral-Domain OCT in Healthy Participants over 45 Years of Age. Ophthalmol Retina 2017, 1, 249–254. [Google Scholar] [CrossRef]
- Do, D.V.; Cho, M.; Nguyen, Q.D.; Shah, S.M.; Handa, J.T.; Campochiaro, P.A.; Zimmer-Galler, I.; Sung, J.U.; Haller, J.A. The impact of optical coherence tomography on surgical decision making in epiretinal membrane and vitreomacular traction. Trans Am Ophthalmol Soc 2006, 104, 161–166. [Google Scholar] [CrossRef]
- Bae, K.; Choi, J.H.; Kim, K.T.; Kang, S.W. EN-FACE OPTICAL COHERENCE TOMOGRAPHY IN PATIENTS WITH EPIRETINAL MEMBRANE: Intuitive Method for Predicting Functional Outcomes. Retina 2020, 40, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, M.; Le Rouic, J.F.; Lesnoni, G.; Colecchio, L.; Catalano, S.; Lumbroso, B. Retinal surface en face optical coherence tomography: a new imaging approach in epiretinal membrane surgery. Retina 2012, 32, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, S.; Lee, J.Y.; Kim, J.G.; Yoon, Y.H. Macular capillary plexuses after epiretinal membrane surgery: an optical coherence tomography angiography study. Br J Ophthalmol 2018, 102, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, R.; Zhang, B. WITH OR WITHOUT INTERNAL LIMITING MEMBRANE PEELING FOR IDIOPATHIC EPIRETINAL MEMBRANE: A Meta-Analysis of Randomized Controlled Trials. Retina 2021, 41, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Martin, D.F.; Hubbard, G.B., 3rd; Srivastava, S.K.; Yan, J.; Bergstrom, C.S.; Aaberg, T.M., Sr. Incidence of postvitrectomy macular edema using optical coherence tomography. Ophthalmology 2009, 116, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.; Pinackatt, S.J.; Sartore, M.; Monfardini, A.; Baldi, A.; Cesana, B.M.; Semeraro, F.; Bratu, A.; Parolini, B. Cystoid macular edema after pars plana vitrectomy for idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol 2015, 253, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, D.; Pierre-Kahn, V.; Matonti, F.; Burillon, C.; Voirin, N.; Dot, C.; Akesbi, J.; Milazzo, S.; Baillif, S.; Soler, V.; et al. Effectiveness and safety of dexamethasone implants for postsurgical macular oedema including Irvine-Gass syndrome: the EPISODIC-2 study. Br J Ophthalmol 2017, 101, 333–341. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Reibaldi, M.; Fallico, M.; Russo, A.; Pizzo, A.; Fichera, S.; Rapisarda, C.; Macchi, I.; Avitabile, T.; Longo, A. Widening use of dexamethasone implant for the treatment of macular edema. Drug Des Devel Ther 2017, 11, 2359–2372. [Google Scholar] [CrossRef]
- Parisi, G.; Fallico, M.; Avitabile, T.; Longo, A.; Ortisi, E.; Russo, A.; Petrillo, F.; Maugeri, A.; Barchitta, M.; Bonfiglio, V.; et al. Intravitreal Dexamethasone Implant for Postoperative Macular Oedema Secondary to Vitrectomy for Epiretinal Membrane and Retinal Detachment: A Systematic Review and Meta-Analysis. J Ophthalmol 2021, 2021, 6627677. [Google Scholar] [CrossRef]
- Ulfik-Dembska, K.; Teper, S.; Dembski, M.; Nowinska, A.; Wylegala, E. Idiopathic Epiretinal Membrane: Microvasculature Analysis with Optical Coherence Tomography and Optical Coherence Tomography Angiography. Tomography 2022, 8, 189–199. [Google Scholar] [CrossRef]
- Miyazawa, K.; Sakimoto, S.; Kanai, M.; Shiraki, A.; Takahashi, S.; Shiraki, N.; Maruyama, K.; Sakaguchi, H.; Nishida, K. Vascular tortuosity analysis in eyes with epiretinal membrane imaged by optical coherence tomography angiography. BMC ophthalmology 2022, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Bacherini, D.; Dragotto, F.; Caporossi, T.; Lenzetti, C.; Finocchio, L.; Savastano, A.; Savastano, M.C.; Barca, F.; Dragotto, M.; Vannozzi, L.; et al. The Role of OCT Angiography in the Assessment of Epiretinal Macular Membrane. J Ophthalmol 2021, 2021, 8866407. [Google Scholar] [CrossRef]
- Govetto, A.; Lalane, R.A., 3rd; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. Am J Ophthalmol 2017, 175, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kishore, K.; Bhat, P.V.; Venkatesh, P.; Canizela, C.C. Dexamethasone Intravitreal Implant for the Treatment of Macular Edema and Uveitis: A Comprehensive Narrative Review. Clin Ophthalmol 2022, 16, 1019–1045. [Google Scholar] [CrossRef]
- Konstantinidis, L.; Berguiga, M.; Beknazar, E.; Wolfensberger, T.J. Anatomic and functional outcome after 23-gauge vitrectomy, peeling, and intravitreal triamcinolone for idiopathic macular epiretinal membrane. Retina 2009, 29, 1119–1127. [Google Scholar] [CrossRef]
- Bucolo, C.; Gozzo, L.; Longo, L.; Mansueto, S.; Vitale, D.C.; Drago, F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J Pharmacol Sci 2018, 138, 219–232. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Fallico, M.R.; Russo, A.; De Grande, V.; Longo, A.; Uva, M.G.; Reibaldi, M.; Avitabile, T. Intravitreal dexamethasone implant for cystoid macular edema and inflammation after scleral buckling. Eur J Ophthalmol 2015, 25, e98–e100. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Avitabile, T.; Castellino, N.; Longo, A.; Russo, A.; Bonfiglio, V.; Parisi, F.; Furino, C.; Panozzo, G.; Scorcia, V.; et al. Intravitreal dexamethasone implant one month before versus concomitant with cataract surgery in patients with diabetic macular oedema: the dexcat study. Acta Ophthalmol 2021, 99, e74–e80. [Google Scholar] [CrossRef]
- Boyer, D.S.; Faber, D.; Gupta, S.; Patel, S.S.; Tabandeh, H.; Li, X.Y.; Liu, C.C.; Lou, J.; Whitcup, S.M.; Ozurdex, C.S.G. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina 2011, 31, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.O.; Springer-Wanner, C.; Hoerauf, H.; Callizo, J.; Jungmann, S.; Brauns, T.; Fulle, G.; Eichel, S.; Koss, M.J.; Kuhli-Hattenbach, C. Intravitreal Sustained-Release Steroid Implants for the Treatment of Macular Edema following Surgical Removal of Epiretinal Membranes. Ophthalmologica 2017, 237, 232–237. [Google Scholar] [CrossRef]
- Furino, C.; Boscia, F.; Recchimurzo, N.; Sborgia, C.; Alessio, G. Intravitreal dexamethasone implant for refractory macular edema secondary to vitrectomy for macular pucker. Retina 2014, 34, 1612–1616. [Google Scholar] [CrossRef]
- Fallico, M.; Maugeri, A.; Romano, G.L.; Bucolo, C.; Longo, A.; Bonfiglio, V.; Russo, A.; Avitabile, T.; Barchitta, M.; Agodi, A. Epiretinal membrane vitrectomy with and without intraoperative intravitreal dexamethasone implant: A systematic review with meta-analysis. Frontiers in Pharmacology 2021, 12, 635101. [Google Scholar] [CrossRef]
- Hostovsky, A.; Muni, R.H.; Eng, K.T.; Mulhall, D.; Leung, C.; Kertes, P.J. Intraoperative Dexamethasone Intravitreal Implant (Ozurdex) in Vitrectomy Surgery for Epiretinal Membrane. Curr Eye Res 2020, 45, 737–741. [Google Scholar] [CrossRef]
- Iovino, C.; Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Braghiroli, M.; Caporossi, T.; Peiretti, E. Efficacy and Safety of Combined Vitrectomy with Intravitreal Dexamethasone Implant for Advanced Stage Epiretinal Membrane. Drug Des Devel Ther 2019, 13, 4107–4114. [Google Scholar] [CrossRef]
- Pierro, L.; Iuliano, L.; Marchese, A.; Arrigo, A.; Rabiolo, A.; Bandello, F. Reduced vascular perfusion density in idiopathic epiretinal membrane compared to macular pseudohole. Int Ophthalmol 2019, 39, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Chung, Y.C.; Lin, C.Y.; Lee, F.L.; Chen, S.J. Focal Nonperfusion of Deep Retinal Capillary Plexus in Eyes With Epiretinal Membranes Revealed by Optical Coherence Tomography Angiography. Ophthalmic Surg Lasers Imaging Retina 2016, 47, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Isik-Ericek, P.; Sizmaz, S.; Esen, E.; Demircan, N. The effect of epiretinal membrane surgery on macular microvasculature: an optical coherence tomography angiography study. Int Ophthalmol 2021, 41, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; D'Aloisio, R.; Viggiano, P.; Borrelli, E.; Iafigliola, C.; Di Nicola, M.; Aharrh-Gnama, A.; Di Marzio, G.; Toto, L.; Mariotti, C.; et al. Early retinal flow changes after vitreoretinal surgery in idiopathic epiretinal membrane using swept source optical coherence tomography angiography. J Clin Med 2019, 8. [Google Scholar] [CrossRef]
- Rommel, F.; Siegfried, F.; Sochurek, J.A.M.; Rothe, M.; Brinkmann, M.P.; Kurz, M.; Prasuhn, M.; Grisanti, S.; Ranjbar, M. Mapping diurnal variations in choroidal sublayer perfusion in patients with idiopathic epiretinal membrane: an optical coherence tomography angiography study. Int J Retina Vitreous 2019, 5, 12. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lin, T.; Peng, W.; Lu, L.; Hu, J. Macular vascular circulation and retinal oxygen saturation changes for idiopathic macular epiretinal membrane after vitrectomy. Acta Ophthalmol 2019, 97, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Hafiz, G.; Mir, T.A.; Scott, A.W.; Zimmer-Galler, I.; Shah, S.M.; Wenick, A.S.; Brady, C.J.; Han, I.; He, L.; et al. Pro-permeability Factors in Diabetic Macular Edema; the Diabetic Macular Edema Treated With Ozurdex Trial. Am J Ophthalmol 2016, 168, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Miyamoto, K.; Kiryu, J.; Miyahara, S.; Katsuta, H.; Hirose, F.; Musashi, K.; Yoshimura, N. Intravitreal injection of corticosteroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci 2005, 46, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Capelanes, N.C.; Malerbi, F.K.; Novais, E.A.; Regatieri, C.V.S. Optical Coherence Tomography Angiographic Evaluation of Macular Vessel Density in Diabetic Macular Edema After Intravitreal Dexamethasone Implants: A Prospective Interventional Trial. Ophthalmic Surg Lasers Imaging Retina 2023, 54, 174–182. [Google Scholar] [CrossRef]
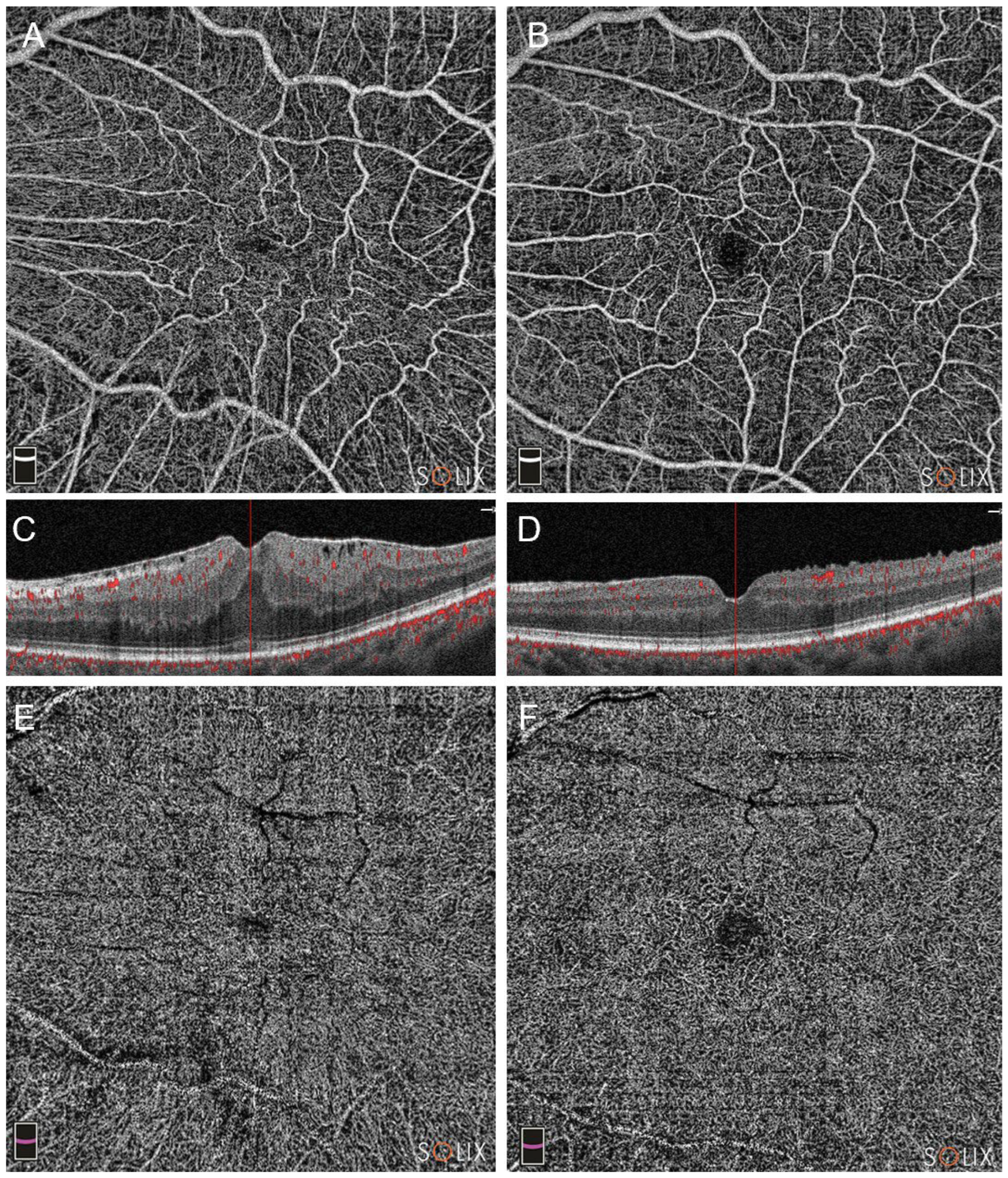
Figure 1.
Optical coherence tomography analysis (OCTA) of a patient in Group A (pars plana vitrectomy with intraoperative Ozurdex implantation), affected by a stage 3 epiretinal membrane (ERM). Preoperative (A, C and E) and postoperative scans at three months (B, D, F) are showed. Note the improvement in vascular network in either the superficial capillary plexus (SCP, images A and B) and the deep capillary plexus (DCP, E and F). The B-scans highlight a significant improvement, too (scans C and D).
Figure 1.
Optical coherence tomography analysis (OCTA) of a patient in Group A (pars plana vitrectomy with intraoperative Ozurdex implantation), affected by a stage 3 epiretinal membrane (ERM). Preoperative (A, C and E) and postoperative scans at three months (B, D, F) are showed. Note the improvement in vascular network in either the superficial capillary plexus (SCP, images A and B) and the deep capillary plexus (DCP, E and F). The B-scans highlight a significant improvement, too (scans C and D).
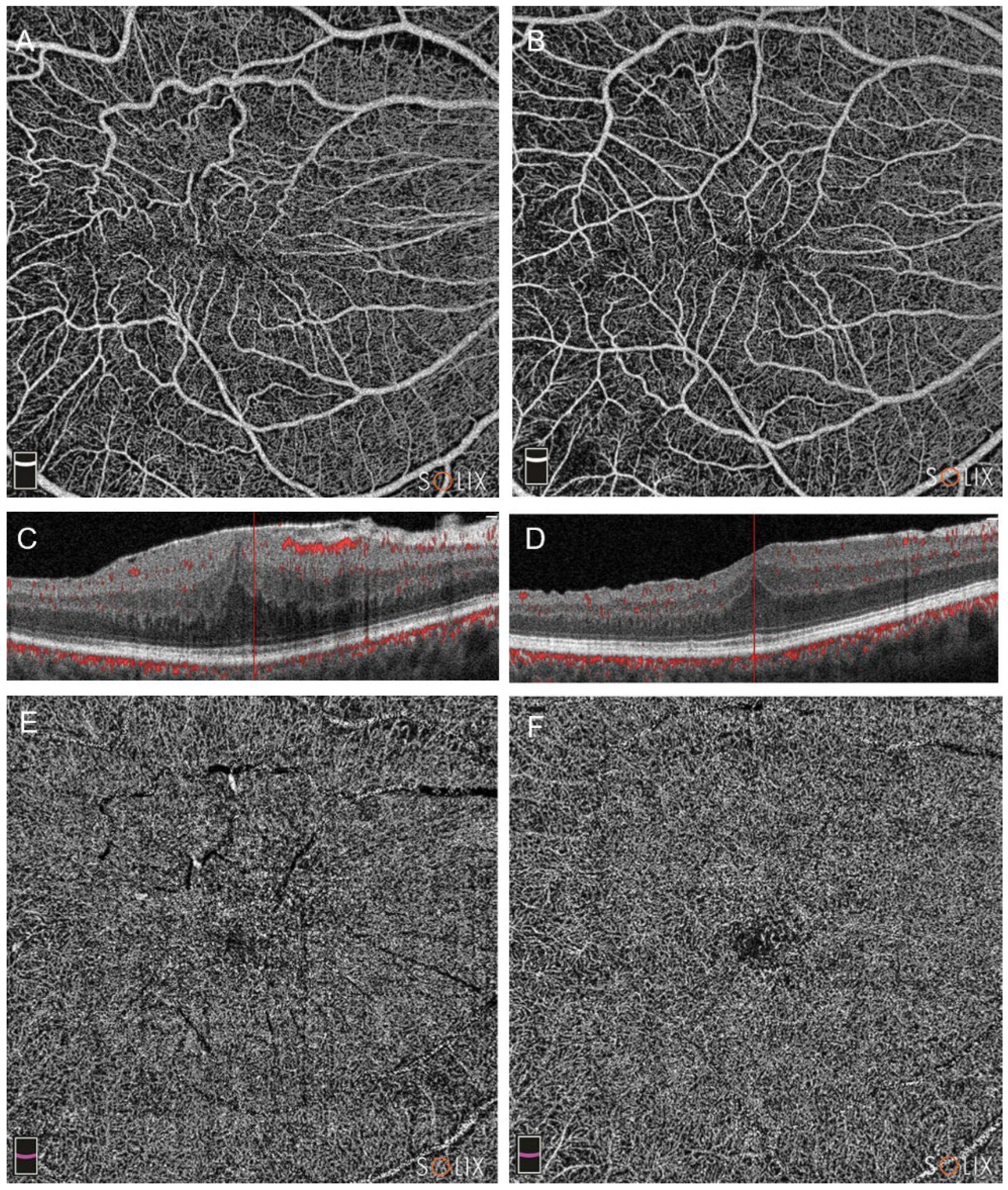
Figure 2.
Optical coherence tomography analysis (OCTA) of a patient in Group B (pars plana vitrectomy alone), affected by a stage 3 epiretinal membrane (ERM). Preoperative (A, C and E) and postoperative scans at three months (B, D, F) are showed, with the evolution of the superficial capillary plexus (SCP, images A and B), the deep capillary plexus (DCP, E and F) and the B-scans (C and D).
Figure 2.
Optical coherence tomography analysis (OCTA) of a patient in Group B (pars plana vitrectomy alone), affected by a stage 3 epiretinal membrane (ERM). Preoperative (A, C and E) and postoperative scans at three months (B, D, F) are showed, with the evolution of the superficial capillary plexus (SCP, images A and B), the deep capillary plexus (DCP, E and F) and the B-scans (C and D).
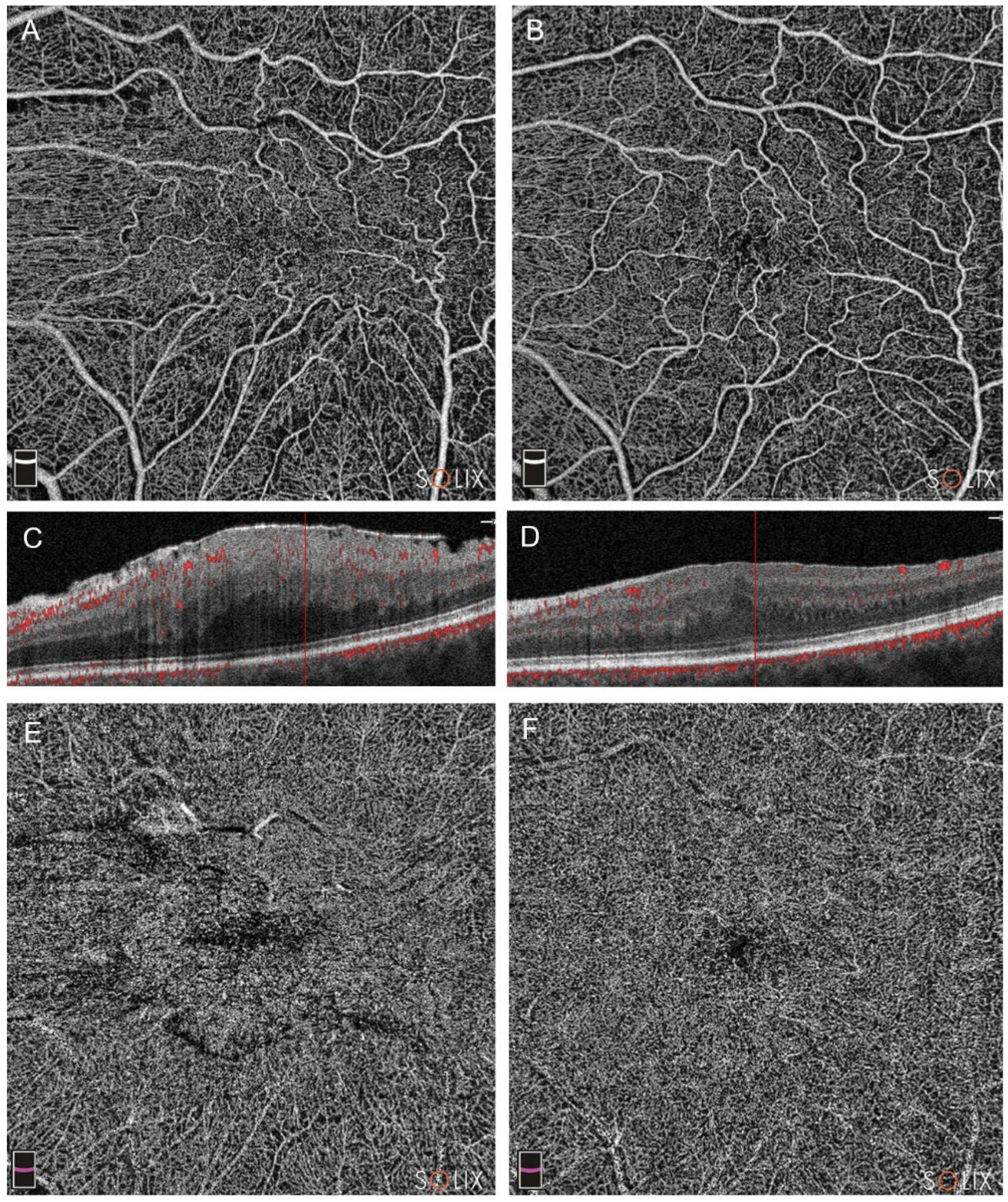
Figure 3.
Optical coherence tomography analysis (OCTA) of a patient in Group A (pars plana vitrectomy with intraoperative Ozurdex implantation), affected by a stage 4 epiretinal membrane (ERM), showing preoperative (A, C and E) and postoperative scans at three months (B, D, F). In patients with stage 4, a signficant microvascular restoration was reported, in either the superficial capillary plexus (SCP, images A and B) and the deep capillary plexus (DCP, E and F). Note the partial restoration of retinal layers in B-scans (scans C and D).
Figure 3.
Optical coherence tomography analysis (OCTA) of a patient in Group A (pars plana vitrectomy with intraoperative Ozurdex implantation), affected by a stage 4 epiretinal membrane (ERM), showing preoperative (A, C and E) and postoperative scans at three months (B, D, F). In patients with stage 4, a signficant microvascular restoration was reported, in either the superficial capillary plexus (SCP, images A and B) and the deep capillary plexus (DCP, E and F). Note the partial restoration of retinal layers in B-scans (scans C and D).
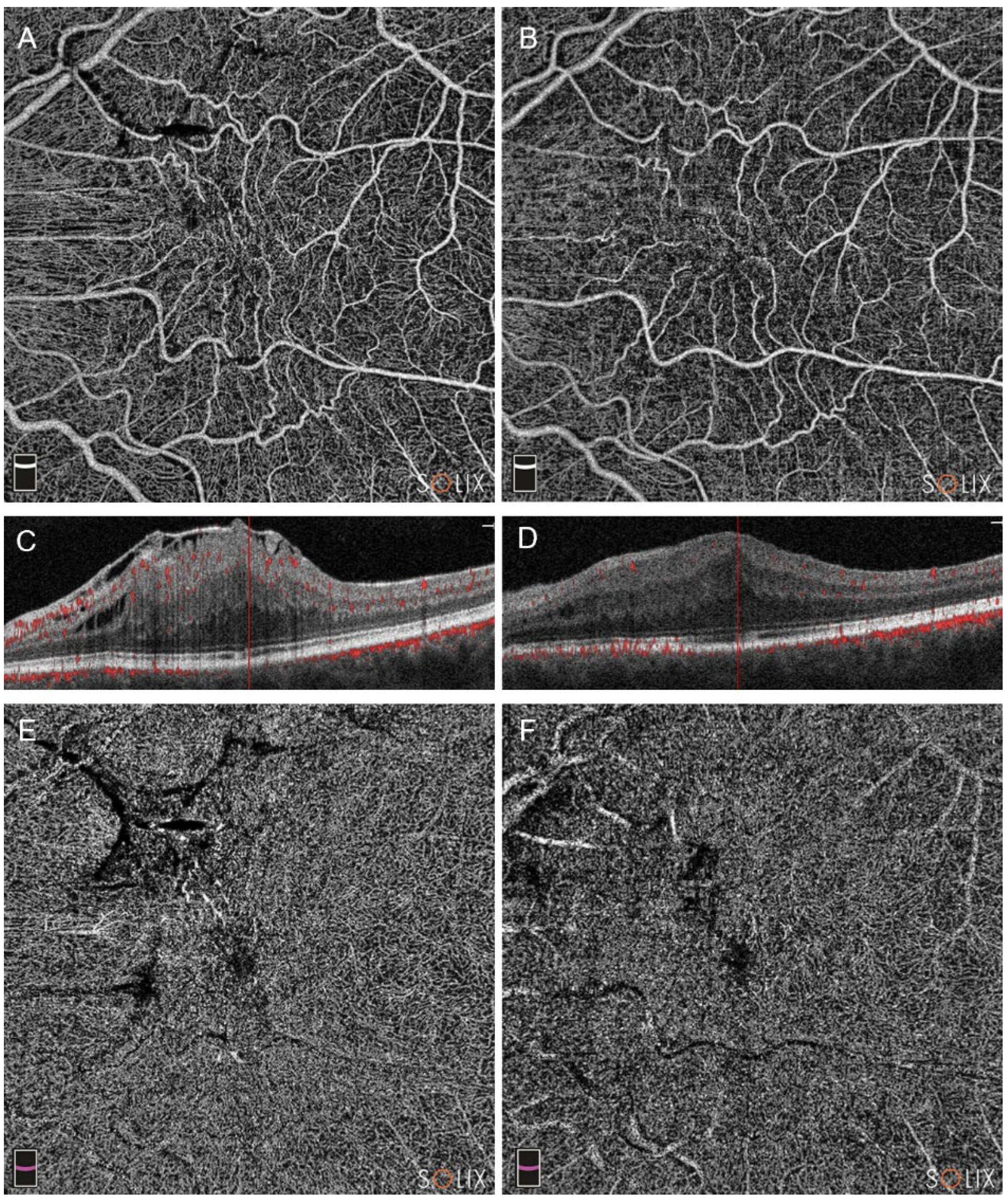
Figure 4.
Optical coherence tomography analysis (OCTA) of a patient in Group B (pars plana vitrectomy alone), affected by a stage 4 epiretinal membrane (ERM), showing preoperative (A, C and E) and postoperative scans at three months (B, D, F). Note the changes in superficial capillary plexus (SCP, images A and B), deep capillary plexus (DCP, E and F) and the partial restoration of retinal layers in B-scans (scans C and D).
Figure 4.
Optical coherence tomography analysis (OCTA) of a patient in Group B (pars plana vitrectomy alone), affected by a stage 4 epiretinal membrane (ERM), showing preoperative (A, C and E) and postoperative scans at three months (B, D, F). Note the changes in superficial capillary plexus (SCP, images A and B), deep capillary plexus (DCP, E and F) and the partial restoration of retinal layers in B-scans (scans C and D).
Table 1.
Baseline demographic and clinical characteristics of the study population.
Table 1.
Baseline demographic and clinical characteristics of the study population.
| Characteristics (mean±SD) |
PPV+Ozurdex (n=25) |
PPV alone (n=25) |
p |
| Study eye, OD, no (%) |
12 (48%) |
13 (52%) |
0.81 |
| Age, yrs |
68.2±4.8 |
69.7±5.3 |
0.57 |
| Gender, male, no (%) |
11 (44%) |
14 (56%) |
0.49 |
| Preoperative BCVA, decimals |
0.41±0.3 |
0.38±0.3 |
0.53 |
| Preoperative IOP, mmHg |
14.1±2.9 |
15.0±4.3 |
0.64 |
| Stage 3/4 (%/%) |
14/11 (56%/44%) |
12/13 (48%/52%) |
0.18 |
| OCT/OCTA features |
|
|
|
| CMT, µm |
485±63 |
467±93 |
0.21 |
| SCP whole VD, % |
42.1±4.1 |
42.9±5.3 |
0.57 |
| SCP fovea VD, % |
38.5±7.5 |
40.4±6.7 |
0.09 |
| DCP whole VD, % |
40.4±10.1 |
41.8±13.2 |
0.23 |
| DCP fovea VD, % |
30.7±10.0 |
30.0±7.7 |
0.14 |
Table 2.
OCTA parameters at the 3 months follow up, compared between Group A (PPV+Ozurdex) and Group B (PPV alone).
Table 2.
OCTA parameters at the 3 months follow up, compared between Group A (PPV+Ozurdex) and Group B (PPV alone).
| OCTA parameters at 3 months (mean±SD) |
PPV+Ozurdex (n=25) |
PPV alone (n=25) |
p |
| SCP whole VD, % |
45.6±4.3 |
43.8±5.8 |
0.03* |
| SCP fovea VD, % |
41.7±4.2 |
40.9±6.7 |
0.06 |
| DCP whole VD, % |
44.7±6.6 |
42.0±11.5 |
0.02* |
| DCP fovea VD, % |
32.3±10.9 |
31.0±8.9 |
0.11 |
Table 3.
Baseline demographic and clinical characteristics of the study population.
Table 3.
Baseline demographic and clinical characteristics of the study population.
| Characteristics (mean±SD) |
PPV+Ozurdex (n=25) |
p |
PPV alone (n=25) |
p |
| Stage 3 |
Baseline |
3 months |
|
Baseline |
3 months |
|
| SCP whole VD, % |
43.5±6.1 |
46.3±8.3 |
0.01* |
43.9±5.2 |
44.7±5.6 |
0.36 |
| SCP fovea VD, % |
40.5±9.5 |
42.1±8.4 |
0.10 |
41.3±6.4 |
41.0±6.2 |
0.64 |
| DCP whole VD, % |
41.3±11.3 |
45.9±7.8 |
0.04* |
42.4±12.8 |
42.1±11.5 |
0.44 |
| DCP fovea VD, % |
31.7±10.3 |
32.6±9.1 |
0.21 |
30.9±11.7 |
31.5±10.2 |
0.22 |
| Stage 4 |
|
|
|
|
|
|
| SCP whole VD, % |
40.8±4.3 |
44.7±5.3 |
0.003* |
41.3±4.3 |
42.9±4.8 |
0.03* |
| SCP fovea VD, % |
36.9±6.1 |
40.4±8.2 |
0.02* |
39.9±7.0 |
40.8±5.5 |
0.04* |
| DCP whole VD, % |
39.0±8.9 |
43.7±5.4 |
0.01* |
41.2±11.2 |
41.9±9.5 |
0.12 |
| DCP fovea VD, % |
29.8±9.6 |
31.6±6.5 |
0.03* |
29.3±9.0 |
30.6±7.2 |
0.08 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).