1. Introduction
In many disciplines of life science, bioimpedance is known as a non-invasive and easy-to-use method for the assessment of body composition and fluid status of the human body [
1,
2]. Recent innovations in the field of micro-electronics have led to the miniaturization of bioimpedance devices accompanied by a longer lifespan of batteries [
3]. This evolution enabled the development of wearable devices that can record bioimpedance signals semi-continuously. The thoracic region is a body-segment of specific interest to measure bioimpedance because of the presence of heart, lungs and large blood vessels which determine central hemodynamic changes (i.e. blood pressure and fluid changes) [
4,
5,
6,
7,
8].
Patients treated with hemodialysis are exposed to frequent fluid changes which contribute to their high cardiovascular morbidity and mortality [
9]. To avoid low blood pressure during hemodialysis and fluid overload during the interdialytic interval, monitoring fluid dynamics such as changes in blood pressure and fluid volume, is highly needed. Nowadays, clinicians rely on estimations of fluid status obtained by interdialytic weight gain or by a single-point (predialytic), whole-body bioimpedance measurement. In addition, fluid dynamics during hemodialysis can be monitored by estimating changes in intravascular volume. However, these methods' feasibility and accuracy remain subject to debate [
10,
11,
12,
13]. During the interdialytic interval, the use of hemodynamic monitoring is very limited [
14]. Given that wearable devices have the theoretical ability to measure bioimpedance semi-continuously, the thoracic bioimpedance technique emerges as an alternative to longitudinally monitor fluid dynamics in hemodialysis patients [
3,
15].
The use of a wearable device measuring thoracic bioimpedance in relation to hemodynamic parameters of dialysis patients has been investigated before [
5,
16,
17]. In these studies, repeated single-point thoracic bioimpedance measurements (i.e. pre-dialysis, every half hour or every hour during dialysis, and post-dialysis) at single-frequency (8 kHz) already showed their potential to accurately track fluid changes during hemodialysis [
5,
16]. Moreover, changes in thoracic bioimpedance were moderately related to changes in systolic blood pressure [
18]. However, an extension to semi-continuous thoracic recordings (i.e. every ten minutes), both during dialysis and during the interdialytic interval, may offer a more detailed look into the longitudinal time pattern of the bioimpedance signal. Hence, semi-continuous recordings could predict clinical endpoints, such as dialysis-related hypotension or acute pulmonary oedema during the interdialytic interval, through longitudinal and remote hemodynamic monitoring during fluid shifts. Moreover, it is known that multifrequency bioimpedance signals are more accurate in determining fluid volumes compared to single-frequency signals [
19,
20]. As such, introducing a multifrequency electrical current to the wearable device may provide in-depth physiological knowledge on fluid changes within and between compartments. To date, semi-continuous measurements of multifrequency bioimpedance by a wearable device are lacking. Therefore, this study aims to investigate the feasibility of a wearable device measuring semi-continuous multifrequency thoracic bioimpedance in hemodialysis patients. We hypothesize to observe a different (non-linear) time pattern in bioimpedance signals obtained by a few single-point versus semi-continuous measurements; and to observe a different time pattern in low versus high frequency thoracic bioimpedance signals obtained by semi-continuous recordings.
2. Methods
2.1. Study Design
This study was designed as a multicenter prospective cohort and was conducted in the dialysis units of the tertiary care centers Ziekenhuis Oost-Limburg (Genk, Belgium) and Jessa Ziekenhuis (Hasselt, Belgium). Hemodialysis patients who were over 18 years old and able to provide informed consent were eligible to participate. Limb amputation, the need for acute hemodialysis, or the long-interval dialysis session were the exclusion criteria. Each patient underwent thoracic bioimpedance measurements during three consecutive days. On the first day, thoracic bioimpedance was recorded semi-continuously (i.e. every 10 minutes) during a 4-hour hemodialysis session. The second day was the interdialytic day. Patients were visited at home and one single-point measurement was obtained. On the third day, which was the following hemodialysis day, the exactly same measurement protocol was applied as during the first hemodialysis.
Prior to study enrollment, written informed consent was obtained from each patient. The study complies with the Declaration of Helsinki and the study protocol was approved by the local committee on human research (eudract/B-number B371201628917) of Ziekenhuis Oost-Limburg (Genk, Belgium), Jessa Ziekenhuis (Hasselt, Belgium) and Hasselt University (Hasselt, Belgium).
2.2. Data Collection
Patients’ medical history and dialysis data were collected from the electronic medical records. Hemodialysis prescriptions were checked to determine the number of sessions per week, the duration of the treatment, patients’ target weight, and the dialysis efficacy, expressed as standard Kt/V. Patients were weighed before and after each dialysis session, and during the home-visit. As in standard clinical practice, net ultrafiltration volume was derived from the interdialytic weight gain and adjusted by the treating nephrologist based on clinical examination.
Thoracic bioimpedance was performed by a wearable device developed by imec the Netherlands (Eindhoven, the Netherlands) [
21]. The wearable device can emit an alternating current at nine frequencies (i.e. 8, 10, 13, 16, 20, 26, 40, 80, 160 kHz). Technical information has been published previously [
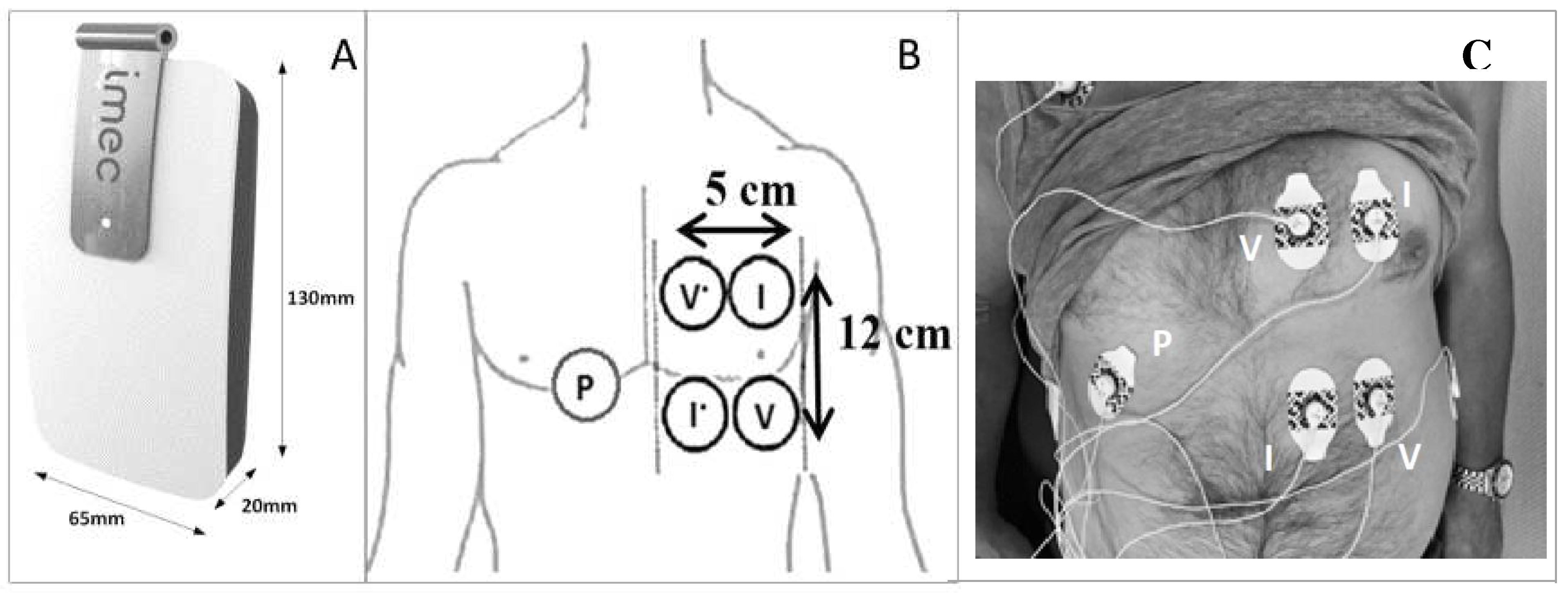
5]. The electrodes used to perform the thoracic bioimpedance were attached on the patient’s left chest (or right when the vascular access catheter was located on the left side) before the first dialysis session and removed after the second session to eliminate changes in electrode position (
Figure 1). The device itself was attached by cables to the electrodes before the start of dialysis and removed at the end of each session. Measurements during hemodialysis were taken in the supine position. During the home-measurements, patients were asked to lay in the supine position for 20 minutes, mimicking their position during hemodialysis. The software in the wearable device was programmed to render bioimpedance data at nine different frequencies every ten minutes.
2.3. Bioimpedance Signal Processing
Bioimpedance measurements are dependent on the posture and the level of movement of the patient [
22]. Therefore, only bioimpedance data recorded under the same posture and during periods of low movement intensity were selected for analysis. To do so, the accelerometer data of the thoracic device were used to derive the static posture and the dynamic movement of the subject at the time of measurement. Secondly, as the resistance component of bioimpedance represents the total body water volume, and as the volume changes in dialysis patients are of main interest in our research, most changes are expected in the resistance component. Therefore, solely the resistance data were selected for further analysis. Thirdly, outliers of the bioimpedance data were detected and removed. Herefore, data were normalized to the median of each session to eliminate inter-subject variability. The normalized values were computed by expressing each measurement as percentage of the median of that session. Outliers in the normalized data were defined as data points that were outside the normal range of the measurements (mean of all data normalized to the median ± 3 * standard deviation).
2.4. Statistical Analysis
Baseline characteristics of the population are expressed as mean ± standard deviation if normally distributed and median [25th – 75th percentile] for skewed data. Dichotomous data are expressed as the absolute number and frequency (%).
The data were approached in three methods. First, a global description of the evolution of the resistance over time, at all frequencies, was performed. Second, to investigate the time pattern of semi-continuous and multifrequency measurements, linear slopes were fitted to a selection of measurements during the first 180 minutes of the dialysis sessions, specifically at the lowest and the highest frequency of the resistance data. Third, to integrate all semi-continuous measurements and the home-measurement, a statistical model was built, taking missing values and the hierarchical structure of the data into account, with a focus on 8 and 160 kHz.
2.4.1. Descriptive Approach: The Global Evolution during and In-Between The Hemodialysis Sessions
The evolution of the resistance over time, at all frequencies, was described as mean ± standard deviation. Hereto, four intervals were considered: from pre- to end-dialysis session 1 (expressed in hours as ∆T0 – T4), from end-dialysis session 1 to the home-measurement (expressed in hours as ∆T4 – T24), from the home-measurement to predialysis session 2 (expressed in hours as ∆T24 – T48), and from pre- to end-dialysis session 2 (expressed in hours as ∆T48 – T52). The evolution of resistance was visually compared to the evolution of weight. Furthermore, to zoom in on the differences within each interval, the number of increasing or decreasing signals per interval was described.
2.4.2. Selective Approach: Comparing the Slopes of 8 and 160 kHz during the First 180 Minutes of Hemodialysis Based on Single-Point Measurements
During hemodialysis, visual inspection of the average trend of the semi-continuous resistance data suggested a different time pattern between the frequencies, which was most clear between 8 and 160 kHz (
Supplementary Figure S2). To further explore these time patterns, the resistance data were selectively approached as single-point measurements. To fit a slope and explore the time patterns, the most valuable single-point measurements during hemodialysis were determined. For this, each dialysis session was divided into two parts, based on four different cut-off points according to the visual inspection: 30, 50, 90 and 100 minutes after the start of dialysis. A selection of measurements up to 180 minutes after the start of dialysis was considered to avoid extrapolation since only three patients completed all 24 measurements during the 240 minutes of session 1, and five patients during session 2. For each part (before and after the cut-off point) and each frequency (focus on 8 and 160 kHz), the mean slope of the resistance of all sessions was calculated, ignoring the correlation between two sessions of a single patient and not considering the home measurement in the analysis. For example, the slope
before 30 minutes is based on the single-point measurements at 0 and 30 minutes after the start of dialysis, and the slope
after 30 minutes is based on the single-point measurements at 30 and 180 minutes after the start of dialysis. Thereafter, the slope of the part
before the cut-off point was compared to the slope of the part
after the cut-off point by a paired t-test. The assumptions (normality and equal variance) underlying the paired t-test were checked. The latter was verified by the Brown-Forsyth test. A significant difference between the average slope
before and
after the cut-off point could indicate a non-linear time pattern of the resistance signal at a specific frequency during dialysis. Similarly, to investigate the added value of multifrequency against single-frequency measurements, the slope of the part before the cut-off point at 8 kHz was compared to that at 160 kHz, and likewise for the slope of the part after the cut-off point. A significant difference between the slope of 8 and 160 kHz could advocate multifrequency measurements instead of single-frequency.
2.4.3. Integrated Approach: Analyzing Semi-Continuous Measurements during Hemodialysis and the Interdialytic Measurement
To integrate all measurements at frequencies 8 and 160 kHz, a statistical model was built incorporating the different dialysis sessions up until 240 min after the start of dialysis as well as the home measurement that was performed. The linear mixed model takes the following form:
Were is a dummy variable for the first dialysis session, is a dummy variable for the second dialysis session. Consequently, the home measurement is comprised in the intercept. is 1 if the bioimpedance measurement is taken at a frequency of 8 kHz, if the frequency is 160 kHz then takes the value 0. represents the time that ranges from 0 to 24, i.e. the dialysis time in minutes divided by 10. Two subject-specific intercepts (, ) and slopes () were added to the model, one for each dialysis session. represents the bioimpedance signal for patient i at time point j. The error term is i.i.d. and the random effects , , follow a , with being an unstructured variance-covariance matrix. Comparing evolutions between session or frequencies, as well as comparing the starting point and endpoint of sessions, was done through the construction of linear combinations of the parameters in the linear mixed model, also referred to as contrasts.
A significance level of 0.05 was used for all tests. Statistical software R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 (Cary, NC, USA) were used to analyze the data.
3. Results
3.1. Baseline Characteristics
The initial cohort consisted of 68 subjects who were measured during three consecutive days, including two dialysis sessions and an interdialytic day. The clinical characteristics of the study participants are represented in
Table 1.
3.2. Bioimpedance Data Quality
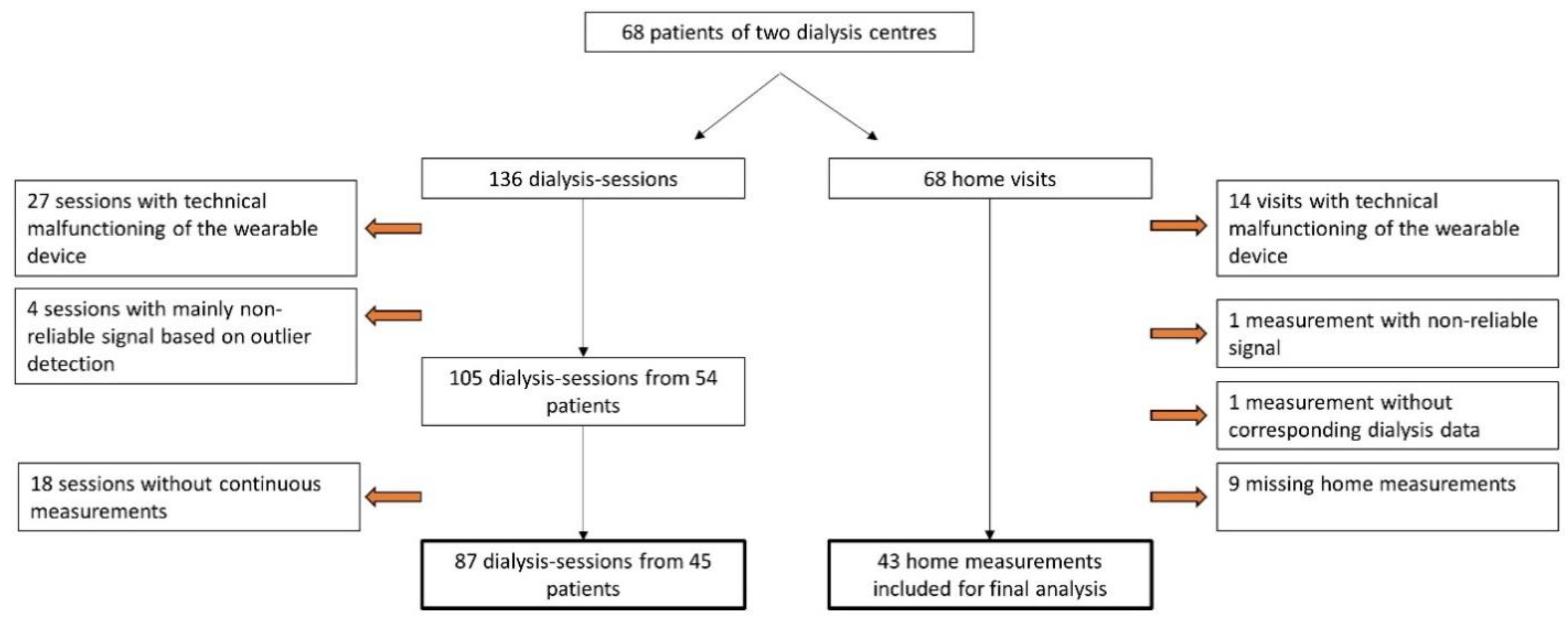
The flow chart in
Figure 2 describes the in- and exclusion procedure of the data. Due to technical impediments, 19.9% (27/136) of the dialysis sessions and 20.6% (14/68) of the home measurements could not be executed. Semi-continuous measurements during two dialysis sessions at nine frequencies resulted in a total of 17.452 bioimpedance signals in the study population. Outlier detection marked 1.7% (289/17.452) of the measurements during dialysis as non-reliable. An example of the outlier detection for the measurements at 160 kHz during the first dialysis session is represented in
Supplementary Figure S1. In-between the two dialysis sessions, patients were visited at home and similar measurements were performed. In 2.3% (9/369) of the home measurements, the device gave a non-reliable signal based on the outlier detection.
3.3. Descriptive Characteristics of the Bioimpedance Signal over Time
Semi-continuous measurements visualised the immediate changes in resistance occurring from the start of dialysis, and this in all frequencies (
Supplementary Figure S2).
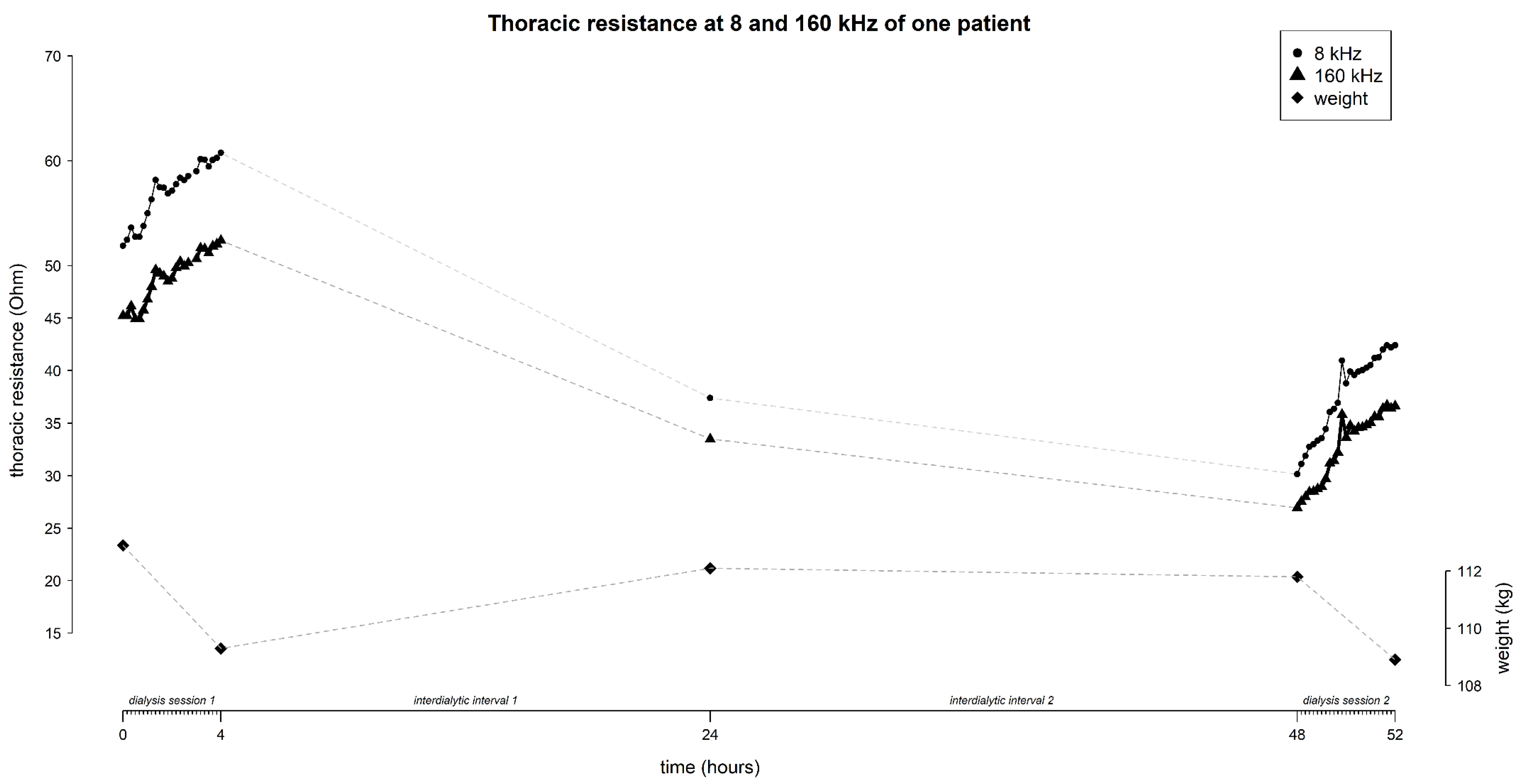
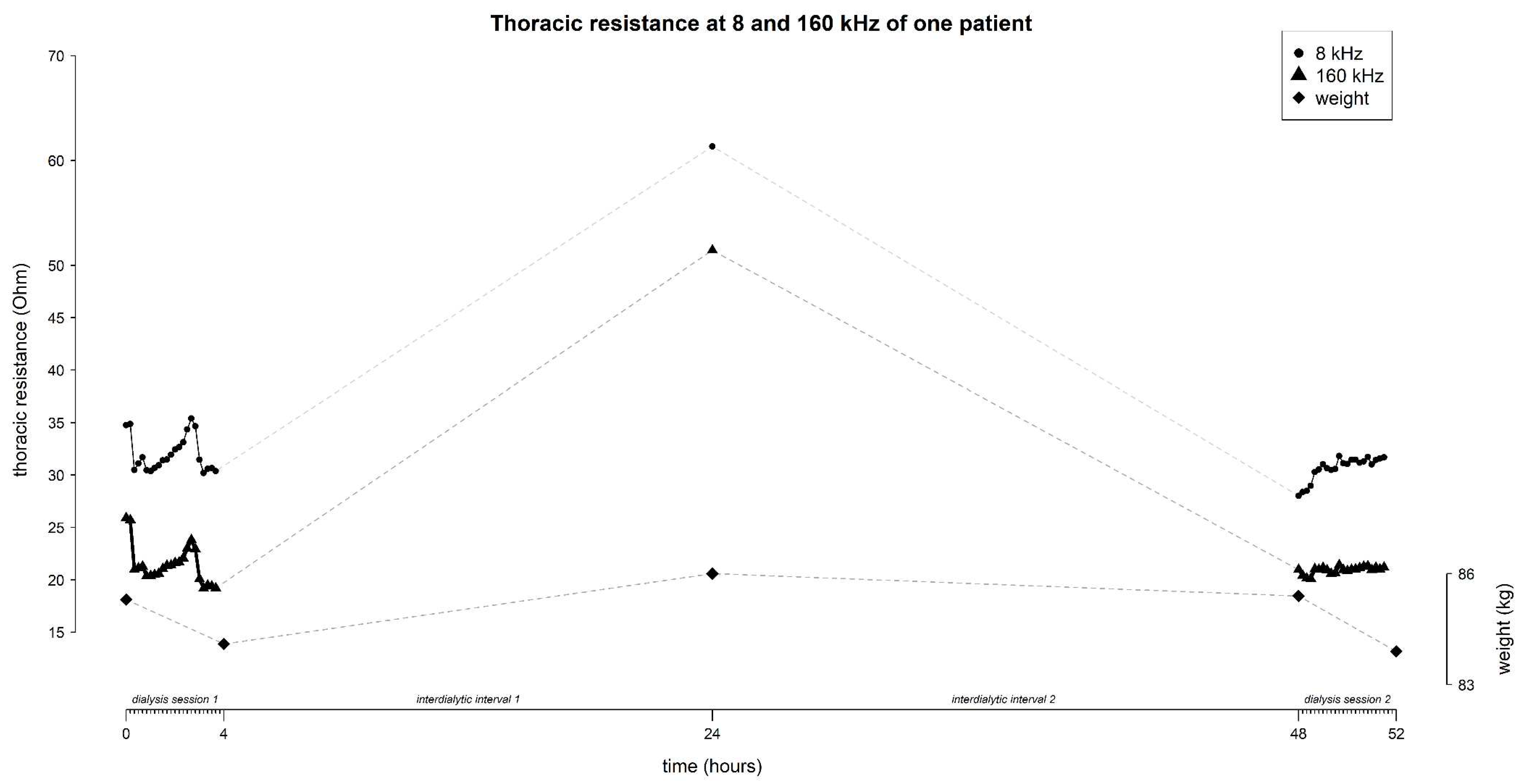
The mean resistance at 8 and 160 kHz in function of time is represented in
Figure 3 (for all frequencies see
Supplementary Figure S2). On average, the thoracic resistance increased over time during fluid removal by hemodialysis and decreased during the interdialytic interval which is characterized by fluid gain due to food- and beverage intake.
At 8 kHz, mean thoracic resistance increased from 36.9 ± 16.1 Ω to 69.5 ± 10.4 Ω (∆ 32.6 Ω) during session 1, decreased to 41.9 ± 16.8 Ω (∆ -27.6 Ω) at the home-visit, and decreased further towards the start of session 2 to 37.3 ± 14.9 Ω (∆ -4.6 Ω), to increase again to 47.3 ± 18.7 Ω (∆ 10 Ω) during session 2 (
Figure 3).
Likewise, at 160 kHz, mean thoracic resistance increased from 31.1 ± 15.7 Ω to 60.6 ± 9.9 Ω (∆ 29.5 Ω) during session 1, decreased to 36.6 ± 16.9 Ω (∆ -24 Ω) at the home-visit, and decreased further towards the start of session 2 to 32.2 ± 14.9 Ω (∆ -4.4 Ω), to increase again during session 2 to 37.3 ± 19.2 Ω (∆ 5.1 Ω) (
Figure 3).
The mean weight of the study-population evolved from 75.4 ± 15.3 kg to 73.9 ± 15.2 kg (∆ -1.5 kg) during session 1, increased to 74.5 ± 15.5 kg (∆ 0.6 kg) during the home-visit, and increased further towards the start of session 2 to 75.1 ± 15.4 kg (∆ 0.6 kg), to decrease again during session 2 to 73.8 ± 15.2 kg (∆ -1.3 kg) (
Figure 3).
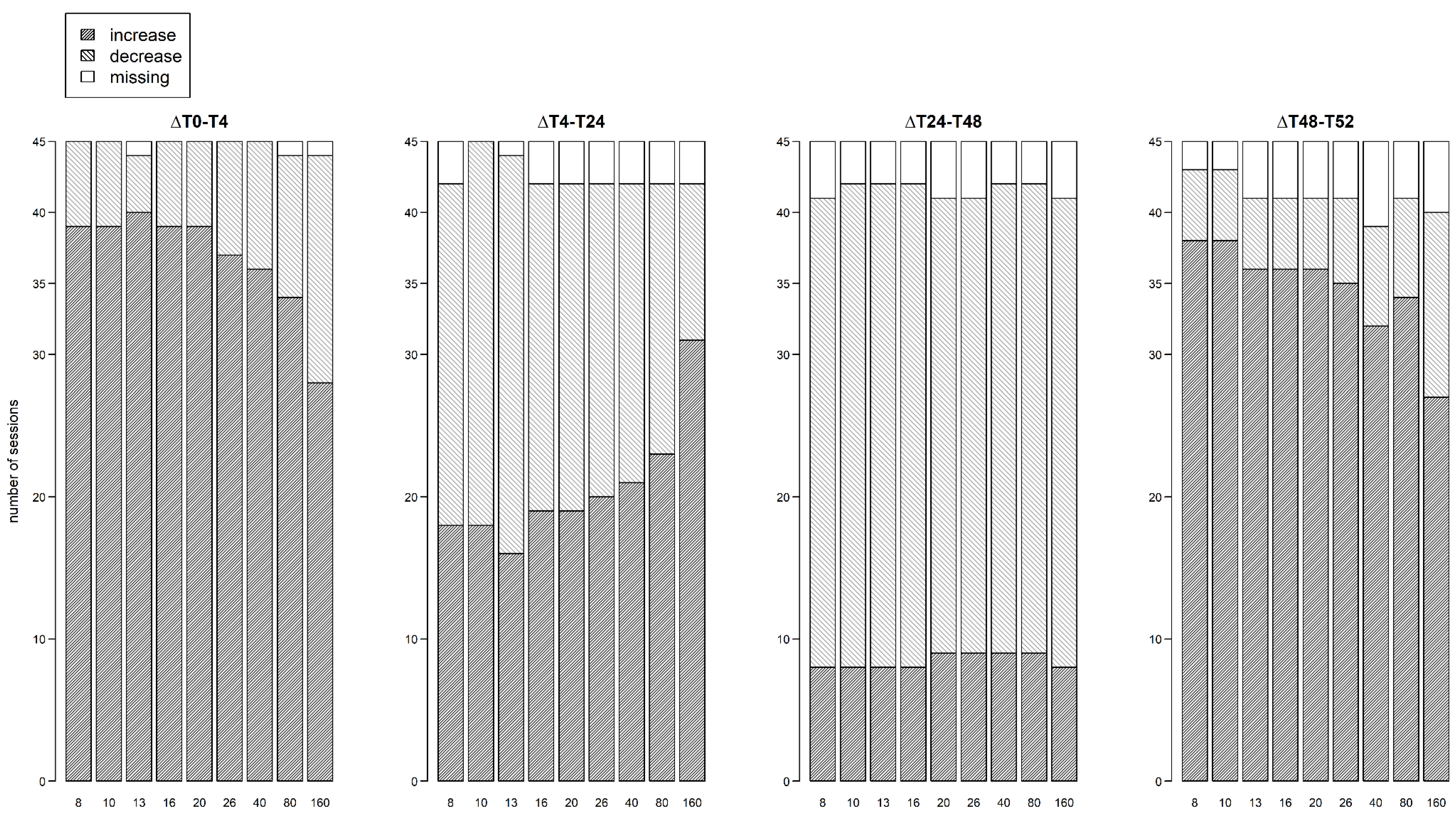
During hemodialysis (∆T0 – T4 and ∆T48 – T52), the percentage of subjects with an increasing resistance signal was higher at 8 kHz compared to 160 kHz (session 1: 86.7% vs 64.4%; session 2: 84.4% vs 62.2% respectively,
Figure 4). Visa versa, the percentage of subjects with a decreasing resistance signal during dialysis was lower at 8 kHz compared to 160 kHz (session 1: 13.3% vs 35.6%; session 2: 11.1% vs 28.9% respectively,
Figure 4). To study the subject-specific behaviour of the increasing and decreasing resistance signals at 8 kHz and 160 kHz, individual profiles were visualised as seen in
Figure 5 and
Figure 6.
During the first interdialytic interval (∆T4 – T24), half of the subjects showed a decreasing signal at 8 kHz (53.3%), while at 160 kHz the majority of subjects still showed an increasing signal (68.9%) (
Figure 4).
During the second interdialytic interval (∆T24 – T48), the decrease in resistance was present in most of the subjects both at 8 kHz (73.3%), and 160 kHz (73.3%) (
Figure 4).
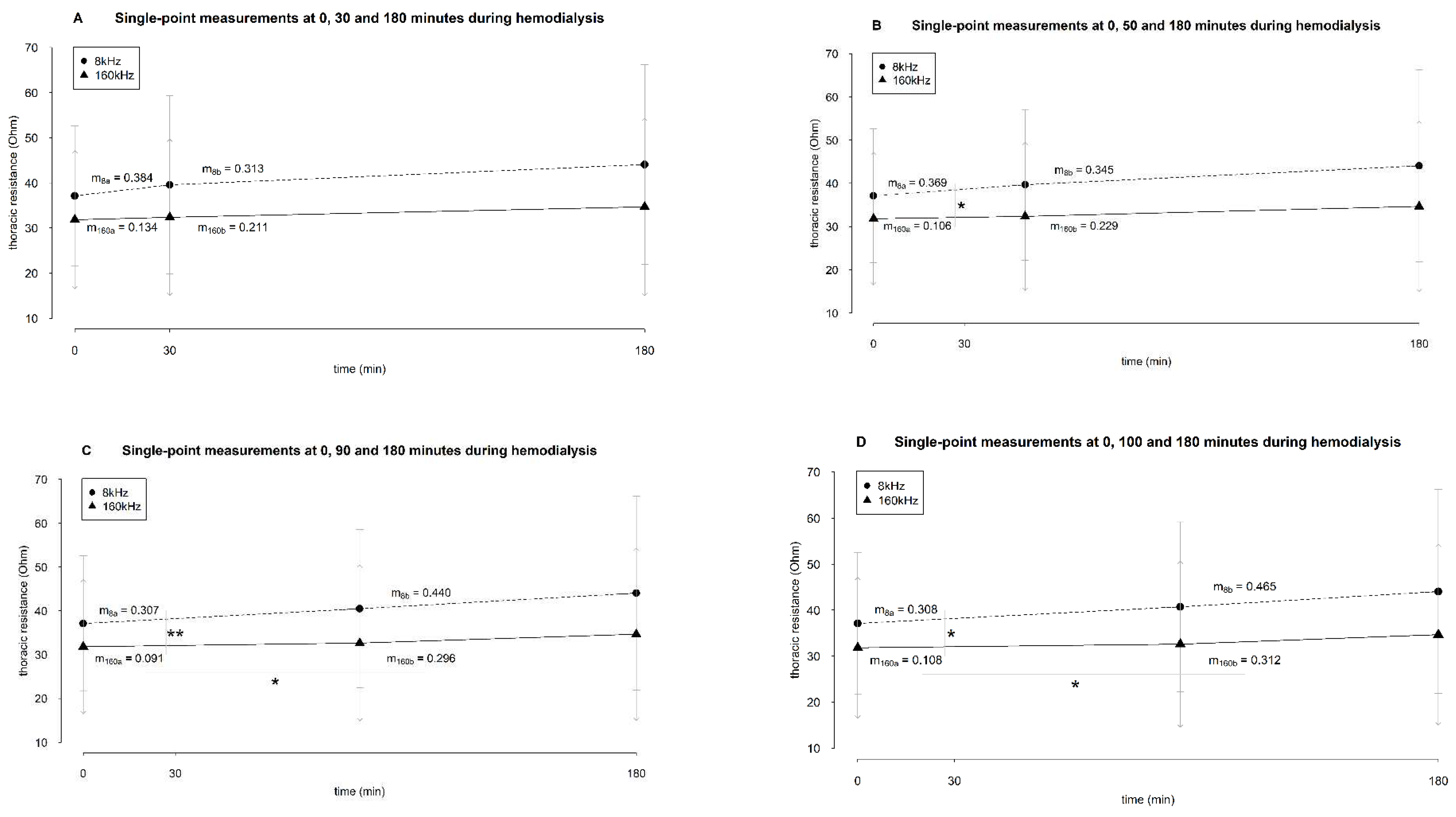
3.4. Selective Analysis of the Slopes
The analysis of the average resistance slopes compiled by a cut-off at 30 minutes revealed no significant difference between the slope
before and
after the cut-off point, nor between frequencies at 8 and 160 kHz (
Figure 5A).
There was no statistical difference between the part
before and
after the cut-off of 50 minutes after the start of dialysis (
Figure 5B). The slope of the resistance at 8 kHz
before this cut-off point was significantly different from the slope at 160 kHz (
p 0.042) (
Figure 5B).
After the cut-off point of 50 minutes, there was no difference between the slopes of both frequencies.
When the cut-off was considered at 90 minutes, no difference in the resistance slopes at 8 kHz between
before and
after the cut-off point was seen (
Figure 5C). However, at 160 kHz, there was a statistical difference (
p 0.013,
Figure 5C). Before the cut-off of 90 minutes, the resistance slope at 8 kHz was significantly different from the one at 160 kHz (
p 0.007,
Figure 5C). After this cut-off point, there was no difference anymore between the slopes of both frequencies.
Setting the cut-off at 100 minutes after the start of dialysis revealed equivalent results as setting the cut-off at 90 minutes (
Figure 5D). Whereas no difference was seen between the resistance slopes
before and
after the cut-off point at 8 kHz, the resistance slopes at 160 kHz showed a significantly different trend (
p 0.015,
Figure 5D). Before this cut-off point, the slope of resistance at 8 kHz was statistically different from the slope at 160 kHz (
p 0.011,
Figure 5D). After 100 minutes, no difference between the slopes of frequency 8 and 160 kHz was seen.
3.5. An Integrated Approach of Analysing Semi-Continuous Measurements
The covariance parameter estimates of the mixed-model are shown in
Table 2. The subject-specific intercepts of both hemodialysis sessions were very variable since both had a high variance [244.98 (standard error (SE) = 52.46) and 241.93 (SE = 52.15), respectively,
Table 2]. In other words, the between-patient variability of the thoracic resistance before the start of dialysis was high.
In addition, a high positive covariance between both random intercepts was noticed (218.98 (SE = 49.58),
Table 2). This means that if a patient has a high Ohm value at the start of the first hemodialysis session, that patient will most likely have a high starting value in the second hemodialysis session. Comparison of the start of both dialysis sessions (same results for 8 kHz and 160 kHz) resulted in an estimated difference of -0.12 Ω (95% CI: -2.29 – 2.05,
p 0.912,
Table 3).
Furthermore, most between-patient variability was captured in the random intercepts [244.98 (SE = 52.46) in the first session and 241.93 (SE = 52.15) in the second session,
Table 2], which were much higher than the random slopes [0.13 (SE = 0.03) and 0.07 (SE = 0.02), respectively,
Table 2]. Hence, the variability in resistance between patients is mainly captured by differences in the starting value of a dialysis session rather than differences during hemodialysis. Even though the variances of the random slopes are small, it still suggests that patients tend to differ with respect to the evolution of their resistance over time.
Next, the positive covariance between both random slopes 0.08 (SE= 0.02) indicates that a higher slope in the first session resulted in a higher slope in the second session (
Table 2). Likewise, a positive covariance between the random intercepts and slopes was seen (
Table 2). This indicates that patients with a higher intercept have a bigger slope compared to those with a lower intercept.
Finally, the within-subject variation 12.66 (SE = 0.29) is clearly less when compared to the between-subject variation 244.98 (SE = 52.47) in the first session and 241.93 (SE = 52.15) in the second session, (
Table 2), which is about 20 times smaller.
The influence of
frequency,
session and
time on resistance is represented in
Table 3 and
Table 4.
Frequency - At 8 kHz, the home-measurement was significantly different compared to the home measurement at 160 kHz (6.212 Ω (SE = 0.289),
p < .0001,
Table 4).
Session - Both sessions started at a significantly different resistance compared to the home-measurement [-5.401 Ω (SE = 2.376),
p 0.023 for the first session and -5.279 Ω (SE = 2.371),
p 0.026 for the second session,
Table 4] at 160 kHz, which resulted to be lower than the home measurement. The evolution of the resistance signal during session 1 was not significantly different than the evolution during session 2 (
p 0.943).
Time - The evolution of the resistance signal at 8 kHz over time was statistically different from the evolution of the resistance signal at 160 kHz (
p < 0.0001). More specifically, to represent the evolution of the resistance over time at 160 kHz, a quadratic term for the first session is needed (
p 0.025,
Table 4), while the evolution in the second dialysis session can be represented with just a straight line (
p 0.089,
Table 4). An individual prediction profile based on the integrated model is shown in
Figure 8.
By including the home measurements into the integrated model, an impression could be made of the interdialytic changes in resistance. At 8 kHz, comparison of the end of session 1 with the home measurement resulted in an average difference in resistance of 2.27 Ω (95% CI: -3.98 – 8.51,
p 0.477,
Table 3). At 160 kHz, comparison of the end of session 1 with the home measurement results in an average difference in resistance of -0.84 Ω (95% CI: -7.09 – 5.41,
p 0.793,
Table 3).
4. Discussion
This paper investigates the feasibility of semi-continuous thoracic bioimpedance measurements by a wearable device with a multifrequency electrical current, both during the course of 4-hour dialysis-sessions, and during the interdialytic interval at home. Longitudinal data demonstrate the different trends over time. Furthermore, a selection of resistance data at 8 and 160 kHz during the first 180 minutes of dialysis suggests different time patterns within and between frequencies. Moreover, an integrated approach to the semi-continuous data reveals the high between-subject variability at the start of hemodialysis, which highlights the importance of a personalized approach for data analysis.
4.1. Technical Feasibility of the Wearable Device
According to the applied outlier detection method, a small percentage of all resistance measurements was labelled as non-reliable. This underlines the capacity of the wearable device to record reliable signals in a semi-continuous way during fluid changes. However, some improvements should be considered. The relatively high number of excluded sessions due to technical problems points out the importance of further optimization of the device. Furthermore, the device that is currently used requests to apply the same electrode configuration for each measurement. Although this was captured by a fixed electrode configuration from the start until the end of this study, the preliminary device as such is not yet suitable for clinical practice. Moreover, its rather large size, the use of cables and multiple electrodes may hinder patients during their daily life activities. Taking the large number of reliable signals into account, together with the aforementioned limitations of the current device, these observations should encourage the further development of a smaller device (i.e. a patch).
4.2. Clinical/Pathophysiological Feasibility during Hemodialysis and beyond
This study reveals several important results. First, by measuring semi-continuously, changes in thoracic bioimpedance signal were detected immediately after the start of hemodialysis. Second, a global increasing resistance towards the end of dialysis was described, for all frequencies. This was confirmed by both the selective and the integrated approach. Third, the resistance at lower frequencies started at a higher Ohm value and showed a steeper increase compared to the resistance at higher frequencies. Fourth, the difference between the slopes of 8 and 160 kHz became clear at 50 minutes after the start of dialysis. Last, the resistance signals at 8 kHz seem to follow a rather linear trend in both sessions, whereas those at 160 kHz suggest a quadratic trend in the first dialysis session.
All these observations can mainly be explained by volume changes
in and
between body compartments. It is known that lower frequencies represent the extracellular volume, whereas higher frequencies additionally take the intracellular volume into account [
1]. As such, changes in the extracellular volume influence both low- and high-frequency signals. Subsequently, an increase in resistance at all frequencies indicates the loss of extracellular volume, as expected according to the principles of fluid extraction during a hemodialysis treatment [
23]. However, a slower increase over time in the higher frequencies compared to the lower frequencies suggests a fluid gain into the intracellular volume compartment. An intracellular fluid gain during hemodialysis has been described earlier on whole-body level and in the limbs, and is mainly due to a decrease in plasma sodium concentration, as is the case in our study population [
24,
25,
26,
27,
28]. Jain et al. focussed on the thoracic segment and found a rather decreasing trend in the intracellular volume of the trunk during hemodialysis [
29]. However, these results were obtained in only five patients, by bioimpedance-based volume predictions, a method which is known to be too imprecise because of intrinsic flaws in the algorithms used to convert the Ohm-values into litres of body water [
30]. Anand et al. described a global increasing thoracic bioimpedance signal during hemodialysis [
16]. As the frequency they used is not reported, no conclusions can be made on changes in volume compartments.
Our observations during hemodialysis revealed a change in resistance at all frequencies, immediately after the start of dialysis. This suggests a change in the central volume compartment directly from the start of dialysis. In addition, changes in blood pressure immediately after the start of dialysis also may play a role in this observed resistance pattern [
15,
31]. Moreover, a significantly different slope in resistance between low versus high frequency was noticed from 50 minutes after the start of dialysis. This difference was emphasized by the differences in slopes further on, suggesting a linear versus non-linear trend of 8 and 160 kHz, respectively. Although the mixed model could not entirely capture the quadratic relation in both dialysis sessions, this feature suggests a certain inertia of the highest frequencies and may be explained by a delayed response of the intracellular volume to the fluid- and osmolar changes induced by dialysis. Indeed, previous research has shown no change in intracellular volume during the first 75 minutes of dialysis [
6], and a change in intracellular volume during the last hour of dialysis [
28].
Next, a significantly higher resistance was measured at home compared to predialysis values of both sessions, and both at low and high frequency. When compared to the measurements taken at the end of the first dialysis session, an average decrease in resistance at all frequencies is observed during the first interdialytic interval. This decrease was more distinct in the lower frequencies compared to the higher frequencies. Indeed, from the integrated approach, an average 2.27 Ω decrease in 8 kHz could be calculated versus a small increase of 0.84 Ω in 160 kHz (
Table 3) on average. As explained above, the larger decrease in resistance at 8 kHz could indicate a fluid gain in extracellular volume and the milder decrease at 160 kHz implies an additional fluid loss in intracellular volume caused by an increase in plasma sodium concentration and plasma volume due to food- and fluid intake from the patient at home [
29]. Additionally, our results showed a larger decrease in resistance during the first interdialytic interval compared to the second interdialytic interval. This could imply that the fluid gain in our study population was larger during the first interdialytic interval. However, some caution has to be taken here. Given the large standard deviation of the measurements during the last hour of dialysis, due to missing values, the mean resistance value may not accurately represent the population mean. In addition, the weight gain during both the interdialytic intervals was the same (0.6 kg). The integrated approach, which takes missing values into account, found on average a larger decrease in resistance during the second interdialytic interval.
Overall, changes in the lower frequencies seem to be more pronounced than the higher frequencies, except during the second interdialytic interval. Again, it seems that a certain inertia is captured within the highest frequencies. This may imply that during the interdialytic interval, the extracellular volume is restored, or overfilled, faster than the intracellular volume.
Lastly, it should be kept in mind that the assumptions of the linear mixed model were violated. As such, some caution is warranted in the interpretation of the results.
4.3. Personalized Feasibility
In addition to the above-discussed findings, the mixed model used in the integrated approach established subject-specific results. First, there is a high between-subject variability in resistance at the start of both dialysis sessions. This can be mainly explained by subject-specific differences in body composition (i.e. fluid volume, muscle- and fat mass), and plasma concentration of electrolytes between patients. Furthermore, the small within-subject variability in resistance at the start of both dialysis sessions suggest that the measurements made by the wearable device are reliable. These results were achieved by a consistent electrode configuration and body position of the patients between both sessions. This confirms the results of our previous work, where thoracic resistance was moderately reproducible between two dialysis sessions [
18]. These results strongly suggest that patients have a subject-specific start-point of thoracic bioimpedance. Consequently, a personalized interpretation of the bioimpedance data instead of an average approach is warranted. Although the variances for the random slopes of both sessions were small, it still suggests that patients do differ with respect to their evolutions over time, as is clearly demonstrated in the individual graphs (
Figure 5 and
Figure 6).
4.4. Clinical Implementation and Future Perspectives
The results obtained by this feasibility study could benefit the population with end-stage renal disease in its broadest sense. Patients treated with hemodialysis, both in-center and at home, could be equipped with a wearable device that semi-continuously measures multifrequency thoracic bioimpedance. The device could function as a remote monitoring tool by interpreting certain trends in the signal, obtained by semi-continuous multifrequency recordings. For example, dedicated clinicians could be warned when a patient reaches a critical maximum thoracic resistance and lower the ultrafiltration rate. From the perspective of the patients, the introduction of a wearable bioimpedance device could modulate their personalized participation in their own hemodialysis treatment. For example, patients could be instructed to schedule a dialysis-session when the thoracic resistance reaches a critical minimum. As such, thoracic bioimpedance may be useful in the determination of dry weight and avoid clinical symptoms of dehydration due to too large fluid extraction. In addition, the home-monitoring function of the device could be extended to patients with end-stage renal disease who are not yet on dialysis. Furthermore, these properties could be applied on the population with chronic heart failure or patients admitted to the intensive care unit, who also often suffer from fluid changes.
This work reaches out to several future perspectives. Future work should focus on the production of a wearable device that is easy-to-use for patients. Hereby, some technical challenges will have to be faced. With respect to the hardware, the introduction of skin-sensors instead of cables and the further miniaturization of the device should be achieved. With respect to the software, a wireless connecting platform from where the research- or nursing staff can detect changes in bioimpedance trends could be created. Furthermore, the extension of the frequency range up to 1000 kHz would lead to the opportunity to create, interpret and model the Cole-Cole plots in order to provide an informative and predictive overview of the bioimpedance signals.
5. Conclusions
In this study, we assessed the feasibility of a wearable device measuring multifrequency thoracic bioimpedance in a dialysis population. We showed that the device is capable of performing semi-continuous measurements during hemodialysis. Semi-continuous and multifrequency measurements provided a broader, and respectively profounder knowledge on the trend of the bioimpedance signal during fluid changes compared to single-point and single-frequency measurements. Hence, measuring bioimpedance of the thoracic region should be performed by a device equipped with a multifrequency electrical current. In the future, this innovative tool should be explored as a remote hemodynamic monitoring application in dialysis patients, which may be a major step forward in the development of personalized dialysis treatment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Research idea and study design: M.K.S., L.L., H.D.C. and P.M.V.; data acquisition: M.K.S.; data analysis/interpretation: M.K.S., L.L., Z.P., H.D.C. and P.M.V..; statistical analysis: M.K.S., Z.P. and L.B.; supervision or mentorship: L.L., H.D.C., A.B., J.K. and P.M.V. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Institutional Review Board Statement
The study was approved by the Ethical Committee (eudract/B-number B371201628917) of Ziekenhuis Oost-Limburg (Genk, Belgium), Jessa Ziekenhuis (Hasselt, Belgium) and UHasselt (Diepenbeek, Belgium). Written informed consent was obtained from each patient prior to study enrollment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are indepted to Seppe Lambeets, Sander Driesen, Hanne Jeurissen, Lennert Snijkers and Michiel De Wever for their invaluable help in the collection of the patient data. We would like to thank the clinical and technical staff at the participating dialysis units for their help and support, and the engineers from imec the Netherlands for their technical support. This research is part of the Limburg Clinical Research Center (LCRC) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk (LSM), province of Limburg, Flemish government, UHasselt, Ziekenhuis Oost-Limburg and Jessa Hospital, Belgium.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Kyle, U.G., et al., Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr, 2004. 23(5): p. 1226-43. [CrossRef]
- Kyle, U.G., et al., Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr, 2004. 23(6): p. 1430-53. [CrossRef]
- Lindeboom, L., et al., On the potential of wearable bioimpedance for longitudinal fluid monitoring in end-stage kidney disease. Nephrol Dial Transplant, 2021. [CrossRef]
- Metry, G., et al., Proportional changes in body fluid with hemodialysis evaluated by dual-energy X-ray absorptiometry and transthoracic bioimpedance with particular emphasis on the thoracic region. Artif Organs, 1997. 21(9): p. 969-76. [CrossRef]
- Schoutteten, M.K., et al., Towards personalized fluid monitoring in haemodialysis patients: thoracic bioimpedance signal shows strong correlation with fluid changes, a cohort study. BMC Nephrol, 2020. 21(1): p. 264. [CrossRef]
- Shulman, T., et al., Preserving central blood volume: changes in body fluid compartments during hemodialysis. Asaio j, 2001. 47(6): p. 615-8. [CrossRef]
- Swatowski, A., et al., Thoracic impedance measurements during orthostatic change test and during hemodialysis in hemodialyzed patients. Asaio j, 2004. 50(6): p. 581-5. [CrossRef]
- Saunders, C.E., The use of transthoracic electrical bioimpedance in assessing thoracic fluid status in emergency department patients. Am J Emerg Med, 1988. 6(4): p. 337-40. [CrossRef]
- Matsushita, K., et al., Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol, 2022. 18(11): p. 696-707. [CrossRef]
- Booth, J., J. Pinney, and A. Davenport, Do changes in relative blood volume monitoring correlate to hemodialysis-associated hypotension? Nephron Clin Pract, 2011. 117(3): p. c179-83. [CrossRef]
- Dasselaar, J.J., F.M. van der Sande, and C.F. Franssen, Critical evaluation of blood volume measurements during hemodialysis. Blood Purif, 2012. 33(1-3): p. 177-82. [CrossRef]
- Keane, D.F., et al., Time to Reconsider the Role of Relative Blood Volume Monitoring for Fluid Management in Hemodialysis. Asaio j, 2018. 64(6): p. 812-818. [CrossRef]
- Reddan, D.N., et al., Intradialytic blood volume monitoring in ambulatory hemodialysis patients: a randomized trial. J Am Soc Nephrol, 2005. 16(7): p. 2162-9. [CrossRef]
- Agarwal, R. and M.J. Andersen, Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int, 2006. 69(2): p. 406-11. [CrossRef]
- Anand, G., et al., Bioimpedance analysis as a tool for hemodynamic monitoring: overview, methods and challenges. Physiol Meas, 2021. 42(3). [CrossRef]
- Anand, I.S., et al., Monitoring changes in fluid status with a wireless multisensor monitor: results from the Fluid Removal During Adherent Renal Monitoring (FARM) study. Congest Heart Fail, 2012. 18(1): p. 32-6. [CrossRef]
- Vonk Noordegraaf, A., et al., Determination of the relation between alterations of total body water and thoracic fluid content during ultrafiltration by bioelectrical impedance analysis. Nephrol Dial Transplant, 1995. 10(3): p. 382-5. [CrossRef]
- Schoutteten, M.K., et al., Comparison of whole body versus thoracic bioimpedance in relation to ultrafiltration volume and systolic blood pressure during hemodialysis. J Appl Physiol (1985), 2023. 135(6): p. 1330-1338. [CrossRef]
- Raimann, J.G., et al., Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int, 2014. 85(4): p. 898-908. [CrossRef]
- Patel, R.V., et al., Estimation of total body and extracellular water using single- and multiple-frequency bioimpedance. Ann Pharmacother, 1994. 28(5): p. 565-9. [CrossRef]
- Van Helleputte N., K.M., Pettine J., A 345 µW Multi-Sensor Biomedical SoC With Bio-Impedance, 3-Channel ECG, Motion Artifact Reduction, and Integrated DSP. IEEE Journal of Solid-State Circuits, 2015. 50(1). [CrossRef]
- Lee, S., et al., Congestive heart failure patient monitoring using wearable Bio-impedance sensor technology. Conf Proc IEEE Eng Med Biol Soc, 2015. 2015: p. 438-41. [CrossRef]
- M., D.P.D.-Y., Ageing and changes in body composition: the importance of valid measurements. In Food for the ageing population. 1st ed. 2009, Abington Hall, Granta Park, Great Abington, Cambridge CB21 6AH, England: Woodhead Publishing Limited.
- Fisch, B.J. and D.M. Spiegel, Assessment of excess fluid distribution in chronic hemodialysis patients using bioimpedance spectroscopy. Kidney Int, 1996. 49(4): p. 1105-9. [CrossRef]
- Jaffrin, M.Y., et al., Continuous monitoring of plasma, interstitial, and intracellular fluid volumes in dialyzed patients by bioimpedance and hematocrit measurements. Asaio j, 2002. 48(3): p. 326-33.
- Kimura, G., et al., A simulation study on transcellular fluid shifts induced by hemodialysis. Kidney Int, 1983. 24(4): p. 542-8. [CrossRef]
- Sarkar, S.R., et al., Fluid dynamics during hemodialysis in relationship to sodium gradient between dialysate and plasma. Asaio j, 2007. 53(3): p. 339-42. [CrossRef]
- Yu, S.J., et al., Assessment of fluid shifts of body compartments using both bioimpedance analysis and blood volume monitoring. J Korean Med Sci, 2006. 21(1): p. 75-80. [CrossRef]
- Jain, A.K. and R.M. Lindsay, Intra and extra cellular fluid shifts during the inter dialytic period in conventional and daily hemodialysis patients. Asaio j, 2008. 54(1): p. 100-3. [CrossRef]
- Schotman, J.M., et al., Towards personalized hydration assessment in patients, based on measurement of total body electrical resistance: Back to basics. Clin Nutr ESPEN, 2020. 35: p. 116-122. [CrossRef]
- Wang, T.W., et al., Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement. Biosensors (Basel), 2022. 12(3). [CrossRef]
Figure 1.
The wearable device (A), schematically presentation of the thoracic electrodes (B), attachment of the electrodes and cables on the thoracic region of a study patient (C). I current, P bias polar, V voltage.
Figure 1.
The wearable device (A), schematically presentation of the thoracic electrodes (B), attachment of the electrodes and cables on the thoracic region of a study patient (C). I current, P bias polar, V voltage.
Figure 2.
Flow chart of the study process indicating technical malfunctioning of the device, outlier detection, and missing measurements.
Figure 2.
Flow chart of the study process indicating technical malfunctioning of the device, outlier detection, and missing measurements.
Figure 3.
The evolution of thoracic resistance (Ohm) with focus on 8 and 160 kHz, and weight (kg) over time (hours) throughout the study (i.e. dialysis session 1 from 0 – 4 hours, home-measurement at 24 hours, and dialysis session 2 from 48 – 52 hours). Every time point represents the mean data from all subjects (n=45), standard deviations are displayed as grey shaded area.
Figure 3.
The evolution of thoracic resistance (Ohm) with focus on 8 and 160 kHz, and weight (kg) over time (hours) throughout the study (i.e. dialysis session 1 from 0 – 4 hours, home-measurement at 24 hours, and dialysis session 2 from 48 – 52 hours). Every time point represents the mean data from all subjects (n=45), standard deviations are displayed as grey shaded area.
Figure 4.
Number of dialysis sessions with increasing or decreasing thoracic resistance, for each frequency, per interval.
Figure 4.
Number of dialysis sessions with increasing or decreasing thoracic resistance, for each frequency, per interval.
Figure 5.
A subject-specific evolution of thoracic resistance (Ohm) at 8 and 160 kHz, and weight (kg) over time, demonstrating an increase in resistance during both dialysis sessions, and a decrease during the interdialytic interval.
Figure 5.
A subject-specific evolution of thoracic resistance (Ohm) at 8 and 160 kHz, and weight (kg) over time, demonstrating an increase in resistance during both dialysis sessions, and a decrease during the interdialytic interval.
Figure 6.
A subject-specific evolution of thoracic resistance (Ohm) at 8 and 160 kHz, and weight (kg) over time, demonstrating a decrease in resistance during dialysis session 1, an increase during the first interdialytic interval, a decrease during the second interdialytic interval, and an increase during dialysis session 2.
Figure 6.
A subject-specific evolution of thoracic resistance (Ohm) at 8 and 160 kHz, and weight (kg) over time, demonstrating a decrease in resistance during dialysis session 1, an increase during the first interdialytic interval, a decrease during the second interdialytic interval, and an increase during dialysis session 2.
Figure 7.
The average slopes of thoracic resistance during hemodialysis based on single-point measurements. Resistance measurements are represented in Ohm as mean ± standard deviation (arrowhead as a straight line for 8kHz and a simple arrowhead for 160 kHz). The cut-off that divided a dialysis session was set at 30 (A), 50 (B), 90 (C), and 100 (D) minutes after the start of dialysis. The slopes were compared using a paired sample T-test. * and ** indicate p values < .05 and < .001, respectively. Abbreviations: m, slope. ma and mb indicate the slope of the part before and after the cut-off point, respectively.
Figure 7.
The average slopes of thoracic resistance during hemodialysis based on single-point measurements. Resistance measurements are represented in Ohm as mean ± standard deviation (arrowhead as a straight line for 8kHz and a simple arrowhead for 160 kHz). The cut-off that divided a dialysis session was set at 30 (A), 50 (B), 90 (C), and 100 (D) minutes after the start of dialysis. The slopes were compared using a paired sample T-test. * and ** indicate p values < .05 and < .001, respectively. Abbreviations: m, slope. ma and mb indicate the slope of the part before and after the cut-off point, respectively.
Figure 8.
Individual predicted evolution over time for a given patient.
Figure 8.
Individual predicted evolution over time for a given patient.
Table 1.
The clinical characteristics of study participants.
Table 1.
The clinical characteristics of study participants.
| |
Total cohort (n = 68) |
| Age (years) |
70.4 ± 13.2 |
| Gender (male) |
46 (67.4%) |
| BMI (kg/m2)a
|
26.3 ± 5.5 |
| Obesityb
|
13 (24.1%) |
| Fistula – Hickmann catheter |
31 (45.6%) – 37 (54.4%) |
| Kt/V |
1.4 ± 0.3 |
| Mean predialysis SBP/DBP (mmHg) |
135.7 ± 20.3 / 66.1 ± 16.4 |
Mean plasma sodium concentration (mmol/l)
- -
Predialytic - -
Postdialytic
|
138.7
138.3 |
| Dialysis vintage (years) |
3.9 ± 3.7 |
| UFV (mL) |
1539.7 ± 897.4 |
| Diabetes mellitus |
31 (45.6%) |
| Heart failurec
|
25 (36.2%) |
| COPD |
6 (11.1%) |
Table 2.
Covariance parameter estimates and standard errors of the statistical model for the integrated approach.
Table 2.
Covariance parameter estimates and standard errors of the statistical model for the integrated approach.
| |
Estimate |
Standard error |
| Variance (Int session 1) |
244.98 |
52.46 |
| Covariance (Int session 1, Int session 2) |
218.98 |
49.58 |
| Variance (Int session 2) |
241.93 |
52.15 |
| Covariance (Int session 1, Slope session 1) |
2.56 |
0.95 |
| Covariance (Int session 2, Slope session 1) |
3.1 |
0.98 |
| Variance (Slope session 1) |
0.13 |
0.03 |
| Covariance (Int session 1, Slope session 2) |
2.06 |
0.74 |
| Covariance (Int session 1, Slope session 2) |
2.23 |
0.74 |
| Covariance (Slope session 1, Slope session 2) |
0.08 |
0.02 |
| Variance (Slope session 2) |
0.07 |
0.02 |
| Residual |
12.66 |
0.29 |
Table 3.
Contrast statistics for the integrated approach.
Table 3.
Contrast statistics for the integrated approach.
| Effect |
Frequency |
Estimate |
Standard error |
t-value |
p value |
95% confidence interval |
| End session 1 versus home measurement |
8 |
2.27 |
3.19 |
0.71 |
0.477 |
-3.98 – 8.51 |
| |
160 |
-0.84 |
3.19 |
-0.26 |
0.793 |
-7.09 – 5.41 |
| Start session 2 versus home measurement |
8 |
-5.28 |
2.37 |
-2.23 |
0.026 |
-9.93 – -0.63 |
| |
160 |
-5.28 |
2.37 |
-2.23 |
0.026 |
-9.93 – -0.63 |
| Start session 1 versus start session 2 |
8 |
-0.12 |
1.11 |
-0.11 |
0.912 |
-2.29 – 2.05 |
| |
160 |
-0.12 |
1.11 |
-0.11 |
0.912 |
-2.29 – 2.05 |
| End session 1 versus end session 2 |
8 |
0.21 |
1.25 |
0.17 |
0.864 |
-2.23 – 2.66 |
| |
160 |
0.21 |
1.25 |
0.17 |
0.864 |
-2.23 – 2.66 |
Table 4.
Solution for fixed effects of the statistical model for the integrated approach.
Table 4.
Solution for fixed effects of the statistical model for the integrated approach.
| Effect |
Frequency |
Session |
Estimate |
Standard error |
t-value |
p value |
| Intercept |
|
|
36.148 |
0.417 |
86.67 |
<.0001 |
| Frequency |
8 |
|
6.212 |
0.289 |
21.47 |
<.0001 |
| Frequency |
160 |
|
0 |
- |
- |
- |
| Session |
|
1 |
-5.401 |
2.376 |
-2.27 |
0.023 |
| Session |
|
2 |
-5.279 |
2.371 |
-2.23 |
0.026 |
| Session |
|
Home |
0 |
- |
- |
- |
| Time*session ( |
|
1 |
0.045 |
0.079 |
0.58 |
0.564 |
| Time*session ( |
|
2 |
0.072 |
0.070 |
1.04 |
0.300 |
| Time*session |
|
Home |
0 |
- |
- |
- |
| Time*time*session |
|
1 |
0.006 |
0.003 |
2.23 |
0.026 |
| Time*time*session |
|
2 |
0.004 |
0.003 |
1.70 |
0.089 |
| Time*time*session |
|
home |
0 |
- |
- |
- |
| Time*frequency |
8 |
|
0.238 |
0.063 |
3.78 |
0.0002 |
| Time*frequency |
160 |
|
0 |
- |
- |
- |
| Time*time*frequency |
8 |
|
-0.005 |
0.003 |
-1.58 |
0.114 |
| Time*time*frequency |
160 |
|
0 |
- |
- |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).