1. INTRODUCTION
RNA helicases, mainly encompassing DEAD- and DEAH-box families, are highly conserved enzymes that participate in all processes of RNA metabolism, from transcription to decay, in an ATP-dependent manner. Each RNA helicase displays a specific function in a diverse number of RNA targets; however, many DEAD/DEAH-box helicases lack target specificity per se. For instance, G-patch proteins act as DEAH-box activators by binding and recruiting them to their action sites (1–4). Upon target recognition they exert its ATPase activity to remodel RNA (5).
We previously described that Dhx15 is a downstream target of Akt and that there is a regulatory crosstalk in the expression of both proteins (6,7). DHX15 participates mainly in mRNA splicing by contributing to the dissociation of the spliceosome subunit U2 upon splicing completion. It is also known to participate in ribosome biogenesis by enhancing small subunit maturation and in viral infections by sensing double RNA strands and stimulating type I IFN and pro-inflammatory cytokines production (8,9).
An emerging interest has aroused in studying the interplay between helicases and cancer (10). Mutations in splicing factors typically occur in many cancers, therefore, recent DHX15 studies focused on elucidating its role in different types of cancer (11). DHX15 acts as a cancer promoter in breast cancer, prostate cancer, acute myeloid leukemia, and hepatocellular carcinoma (HCC), and as an antitumor factor in glioma due to its growth inhibitory function (12–17). We also described a relevant function of Dhx15 in lymphatic and blood vasculature development and functioning in vertebrates. In this context, we demonstrated the role of Dhx15 as a regulator of lung metastasis in a syngeneic mouse model of metastasis (7). Dhx15 gene deficiency resulted in significantly reduced metastasis due to lymphatic vascular defects and impaired endothelial energy metabolism. Further studies analyzing the specificity of DHX15 in cancer and metastasis are necessary, especially since oncologic complications are highly related to metastasis appearance.
Despite the increasing interest in DHX15 and the role played by its upstream regulator Akt in liver function, regeneration, and cytoprotection (18–21), this helicase had not been studied in the liver. Recently, two different studies have evaluated DHX15 expression in hepatocellular carcinoma patients, showing a differential expression of this helicase. Xie C. et al. described significant over-expression of DHX15 in human primary HCC correlated with poor survival (22). Later, Zhao M. et al. described DHX15 as an inhibitor of autophagy that was less expressed in HCC tumor tissues (23). Although both studies show contrasting results, these observations suggest that DHX15 is a protein target for HCC. Thus, it would be necessary to decipher the function of DHX15 in the liver and specifically in the context of HCC. Here, we explore its role in the liver in two different animal models, zebrafish and mouse. We analyze the effects of Dhx15 knockdown in liver organogenesis, liver vasculature and liver regeneration. Furthermore, we evaluate the functions of Dhx15 in HCC and liver metastasis, in Dhx15 heterozygous mice and using the self-delivering AUMsilence ASO technology, providing evidence for its role in the regulation of metastasis and primary tumor growth.
2. RESULTS
2.1. Impaired liver development in Dhx15 deficient zebrafish embryos
As we and others previously described,
Dhx15 deficiency in zebrafish embryos depicts phenotypic abnormalities and ultimately results in lethality at day 8 post-fertilization (dpf). Such mutant embryos are characterized by encephalic and cardiac edema, scoliosis, impaired neural/eye growth and defective pectoral fin and jaw development (7,24). To determine whether
Dhx15 gene deficiency is also associated with liver organogenesis, we generated a
Dhx15 knockout zebrafish model in a red fluorescent protein (RFP) background under the liver-specific promoter fab10. At 5 dpf,
Dhx15-/- embryos presented evident differences from their wild-type (WT) clutch-mates, at this stage mutant embryos lack livers (
Figure 1A). By means of fluorescent microscopy we specifically visualize the hepatic area marked with RFP in zebrafish embryos, we detected residual or no staining tissue in
Dhx15-/- embryos (
Figure 1A, panels c and d). We also determined the percentage of hepatic yolk retention.
Dhx15-/- embryos depicted a 100% of yolk retention, in contrast, WT clutch-mates presented 0-5% of yolk retention. As a consequence of liver absence, in
Dhx15-/- embryos, lipids from the yolk sac were not metabolized and they accumulated in the yolk sac (
Figure 1A, panel b). Heterozygous embryos were also evaluated and displayed similar characteristics as WT embryos, indicating no evident alterations in heterozygosis (Supplementary Figure 1).
To evaluate whether the absence of liver in
Dhx15-/- embryos was caused by genetic alterations induced by
Dhx15-deficiency, we evaluated the gene expression of key mediators of liver development, such as,
Prox1,
Mypt1,
Hdac3,
Foxa1,
Sox17,
Uhrf1 and
Bmp4. We found that
Mypt1,
Prox1 and
Hdac3 were significantly down-regulated in
Dhx15-/- embryos, while only
Gata6 was significantly up-regulated compared to WT embryos. Other genes depicted a non-significant tendency towards a reduced expression (
Figure 1B).
In
Figure 1C, the morphological differences between WT and mutant embryos and the absence of liver in
Dhx15-/- larvae are depicted. Such results urged us to elucidate the role of Dhx15 in the liver.
2.2. Altered liver vasculature in Dhx15 partially deficient mice
In a prior study we described Dhx15-related vascular defects in mutant zebrafish that were also occurring during development (7). In the present study, we wanted to further expand these previous results by studying the vasculature morphology within the liver. Since our
Dhx15-/- zebrafish model presented defects in liver organogenesis, we studied the hepatic angioarchitecture in
Dhx15 gene deficient mice instead. As we previously described,
Dhx15 deficiency in mice causes embryonic lethality (7); however,
Dhx15+/- mice are viable. After CD31 immunostaining, we observed differences in the thickness and expansion of the sinusoids in
Dhx15+/- mice, compared to WT (
Figure 2A), consisting on the presence of thinner sinusoids and lower vascular connectivity.
To study the molecular variations associated with the intrahepatic vascular alterations, we quantified RNA expression of different key factors involved in the generation and maintenance of blood and lymphatic vessels. In heterozygous mice, we observed a reduced RNA expression of
Vegf-c,
Vegfr3,
Podoplanin and
Angiopoietin 1 and a non-significant tendency towards a lower expression of
Vegf-d and
Prox1 (
Figure 2B). Similarly to the results found in hepatic tissue, we detected a reduced expression of
Vegf-c,
Vegf-d and
Angiopoietin 1 in a hepatocyte cell line with the
Dhx15 gene silenced (Hep-siDhx15), compared to WT hepatocytes (
Figure 2C).
2.3. Dhx15 partial gene deficiency decreases the regenerative capacity of the liver in mice
To study the role of Dhx15 during regeneration, we performed two-thirds partial hepatectomy (PHx) in WT and
Dhx15+/- mice. Mice were sacrificed at 2, 3 and 7 days post-PHx; the wet remnant liver weight together with the total body weight was used to calculate the hepatic regenerative rate known as Higgins Index. Seven days after PHx,
Dhx15+/- mice showed decreased regenerative rate associated with a trend of increasing mortality at 72 hours after surgery compared with WT mice (
Figure 3A), although these differences were not significant.
Liver tissue samples obtained at day 2 and 3 after PHx, were used to evaluate hepatocyte proliferation by Ki67 immunostaining. In
Dhx15+/- mice at day 2 post-PHx, we observed a slight but significant decrease in proliferation compared to WT mice (
Figure 3B, panels a and b). At day 3 post-PHx, non-parenchymal cells lead the cellular proliferation in the regenerating liver, at this time point we observed evident and significant reduction of proliferation in
Dhx15+/- mice compared with WT mice (
Figure 3B, panels c and d). Next, we specifically evaluated the presence of endothelial cells in the regenerating liver at 3 days post-PHx by ETS related gene (Erg) immunostaining . We observed a significant reduction of Erg positive cells in the heterozygous livers compared to WT (
Figure 3B panels e and f). The reduced proliferation was accordingly associated with a reduced protein expression of Cyclin d1 but not Pcna (
Figure 3C).
2.4. Dhx15 deficiency alters glucose metabolism.
Previous RNAseq and proteomic analysis results in Dhx15 silenced endothelial cells led us to conclude that Dhx15 participates in carbohydrate metabolism (Ribera J. 2021) (Supplementary Figure 2). To further validate these previous published results, we performed functional metabolic analyses in Dhx15+/- mice.
After partial hepatectomy, glycogen storage is used to feed the highly metabolic demand of liver regeneration; finding its lowest peak at 24h post-PHx, to later recover its normal levels (25,26). To elucidate whether the defects in regeneration were related to metabolic alterations restricting the energetic demands in the liver, we first quantified glycogen levels in the regenerating livers of our
Dhx15 deficient animal experimental model. We observed a significant decrease in glycogen in
Dhx15+/- mice two days after PHx compared to WT (
Figure 4A), indicating that heterozygous mice are unable to normalize glycogen levels post-PHx. We analyzed the expression of glucokinase (
Gck) which converts glucose into glucose 6-phosphate, and the expression of glycogen synthase (
Gs) which converts UDP-glucose into glycogen, before and after hepatectomy. We observed that after hepatectomy,
Dhx15+/- mice presented significantly lower levels of
Gck and significantly higher levels of
Gs (
Figure 4B). This may indicate that the deficiency of the Dhx15 helicase is altering the expression of
Gck and that
Gs expression increases as to compensate such enzymatic deficiency, however, not being able to properly restore the glycogen storage levels. Accordingly, in
Dhx15 silenced hepatocytes (Hep-siDhx15) we evaluated genes participating in glycogenesis and found lower RNA expression of
Phosphoglucomutase 1 and
Udp-glucose pyrophosphorylase that participate in the conversion of glucose into glycogen (
Figure 4C).
In a previous Dhx15 study we observed decreased mitochondrial activity and ATP production in endothelial cells (7). We evaluated now if glucose metabolism might be affected by Dhx15 depletion, possibly reducing hepatocyte proliferation during regeneration.
Dhx15+/- mice presented a lower glucose production after pyruvate injection compared to WT mice, reaching lower levels of maximal glucose production (
Figure 4D). We quantified glucose production in Hep-siDhx15 and observed a significant decreased glucose production caused by Dhx15 deficiency (
Figure 4E). To confirm these results, we evaluated the expression of glycolysis-participating enzymes in Hep-siDhx15, we found decreased expression of
Glucose-6-phosphatase (
G6pc) and
Pyruvate carboxylase (
Pc) which participate in the gluconeogenic conversion of pyruvate into glucose (
Figure 4F), we detected no differences in
Pyruvate kinase (
Pklr) and
Phosphoenolpyruvate carboxykinase (
Pck). These results evidence the metabolic alterations caused by Dhx15 partial deficiency.
2.5. Dhx15-related vascular alterations derive in less hepatic tumor nodule events in an HCC mouse model
Recent studies evaluate the role of DHX15 in different cancer types. In hepatocellular carcinoma (HCC), DHX15 was found over-expressed in human cancerous livers (16). Also, Zhao
et al. described that DHX15 in HCC, has an inhibitor role in HCC proliferation by suppressing autophagy in a hepatoma cell line (23). Since we previously found lymphatic alterations and reduced metastasis in a lung cancer mouse model in
Dhx15 heterozygous mice (7), we now evaluated the role of Dhx15 in HCC and liver metastasis in mice. We benefited from the use of the murine hepatocellular carcinoma cell line named Hepa 1-6 to establish a syngeneic cancer model. Upon subcutaneous injection of Hepa 1-6 cells in the flank of WT and
Dhx15+/- mice, we followed primary tumor formation. Five weeks after injection
Dhx15+/- mice presented similar sized tumors compared to WT mice (
Figure 5A, panels a and b). We evaluated tumor invasion of the liver by Hepa 1-6 and found small nodules within the livers of
Dhx15+/- mice compared to WT mice (65.62±11.77
vs. 111±15.92 µm of nodule size per mouse, respectively; p<0.05). The liver nodules in WT mice were larger and more numerous (
Figure 5A, panels c and d).
Dhx15+/- mice presented less invasive events in the liver compared to WT mice (1.40±0.16
vs. 2.78±0.49 number of nodules per mouse, respectively; p<0.05).
We also studied the lymphatic and blood vasculature within the primary tumor to determine if differences in tumor nodule invasion was related to aberrant vasculature structures. In accordance to reduced tumor nodule formation, we observed clear differences in WT and heterozygous mice, depicting vascular abnormalities in
Dhx15+/- mice. we studied both endothelial and hematopoietic vasculature by Endomucin staining (
Figure 5B, panels a and b). In
Dhx15+/- tumors we observed smaller vases with a reduced lumen. We next studied the organization of lymphatic cells by Lyve-1 immunostaining. Comparing WT and
Dhx15+/- mice, the heterozygous mice presented a significant decrease in % stained area with Lyve-1 (
Figure 5B, panels c and d).
To better analyze lymphatic defects, we quantified the RNA expression of several vascular factors within the primary tumor. We analyzed the expression of
Vegf-d which participates in lymphangiogenesis and in endothelial cell growth, being relevant in the development of new lymphatic vasculature in metastasis (27). We found a significant decrease of
Vegf-d and a significant up-regulation of its receptor
Vegfr3, in the primary tumors of
Dhx15+/- mice. We also found a significant decreased expression of
Vegf-a,
Vegfr1, its receptor, and angiopoietin 1, in the primary tumors of
Dhx15+/- mice (
Figure 5C). The results suggest that the fewer hepatic tumor nodules observed in
Dhx15+/- mice might be due to an impaired growth and development of the vascular network in the primary tumor.
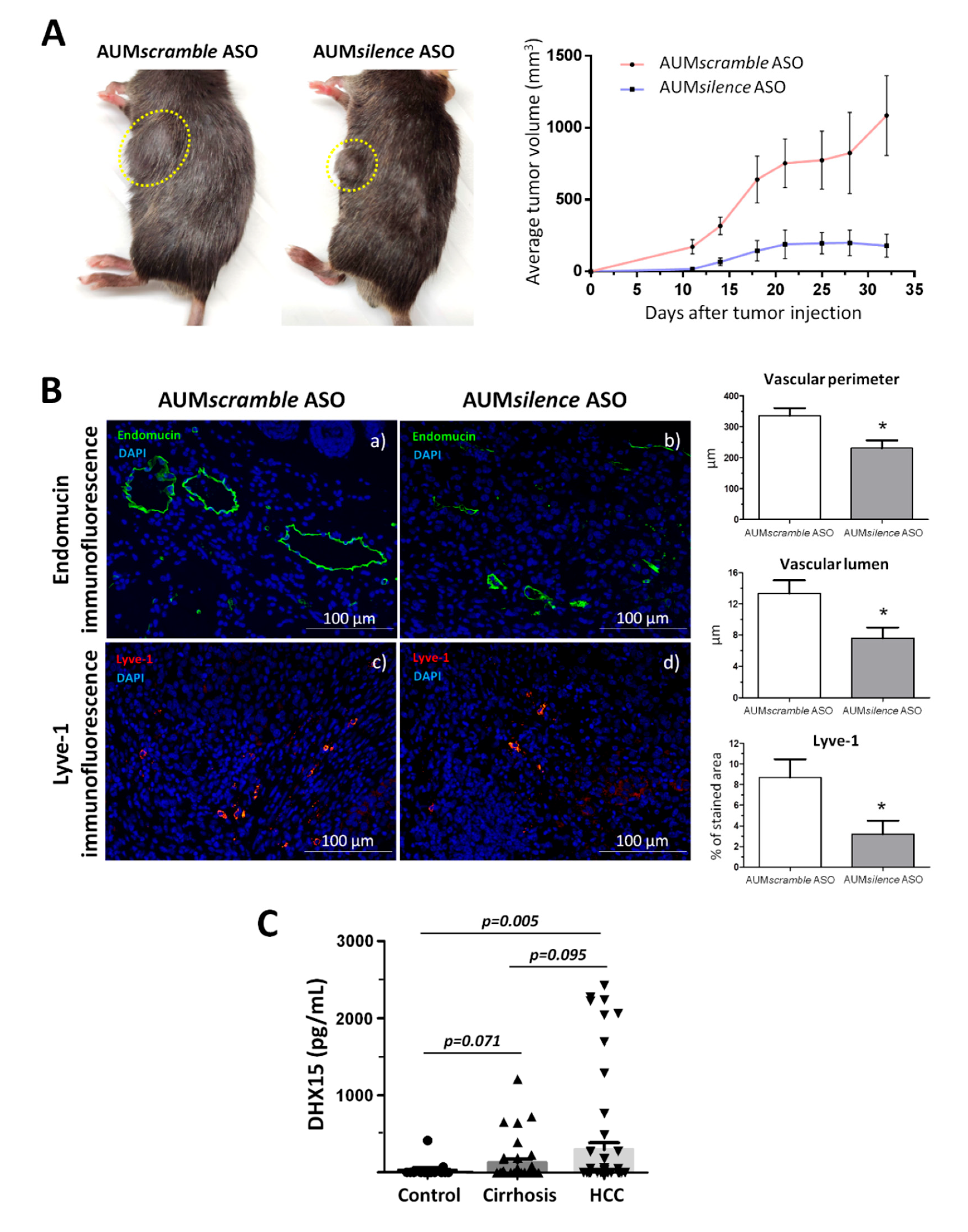
2.6. AUMsilence ASO mediated Dhx15 silencing in mice reduces primary tumor volume in an HCC mouse model
Following previous observations by us and others regarding the function of DHX15 in HCC, we decided to evaluate the potential therapeutic use of Dhx15 deletion in the cancer setting. To do so, we studied the effect of the AUMsilence antisense oligonucleotides (AUMsilenceTM ASO) silencing methodology (28) to silence Dhx15 in vivo. Upon a single dose of AUMsilence Dhx15 intravenous injection, we obtained an 80% of Dhx15 silencing in the liver that was maintained up until 72h post-injection (Supplementary Figure 3A). Dhx15 silencing was also evaluated in the lungs and spleen where we observed a milder Dhx15 deletion.
To evaluate the effects of a strong Dhx15 inhibition in the mouse liver in a cancerous context, we established the Hepa 1-6 HCC model in AUM
silence ASO-injected mice. We followed primary tumor growth and observed a significantly reduced tumor volume in the AUM
silence Dhx15 injected mice compared to AUM
scramble scramble group (AUM
scramble ASO) (179.6±80.06
vs. 1085±277.1 mm
3 primary tumor volume five weeks post-implantation, respectively; p<0.01;
Figure 6A). In agreement with the results found in
Dhx15+/- mice, we also observed a reduction of several vascular genes in the livers of AUM
silence ASO Dhx15 injected mice such as,
Vegf-a,
Vegf-d,
Vegfr1,
Vegfr3 (Supplementary Figure 3B).
We also analyzed the vascular angioarchitecture of the primary tumor and similarly to the HCC model in
Dhx15+/- mouse we observed alterations in the vasculature in terms of a significantly reduced vascular perimeter and lumen of blood vessels detected by Endomucin immunostaining and a significant reduction in lymphatic vessels detected by Lyve-1 immunostaining in the AUM
silence ASO Dhx15 group compared to the AUM
scramble ASO scramble group (
Figure 6B).
Today the biochemical diagnosis of hepatocellular carcinoma continues to be a challenge. Due to the effect of Dhx15 on tumor growth in our experimental model of HCC, we wanted to evaluate the differential diagnostic utility of this marker in patients. With this purpose, we performed serological evaluation of DHX15 levels by ELISA in a cohort of patients with different liver disease etiologies. As shown in
Figure 6C, patients with HCC present significant higher levels of circulating DHX15 compared to healthy subjects (300.3 ± 88.2
vs. 32.4 ± 27.7 pg/mL;
p<0.01; respectively). We also detected a trend of increased circulating values of DHX15 in patients with HCC compared to cirrhotic patients without hepatic tumors, although this trend was not significant (300.3 ± 88.2
vs. 132.0 ± 46.2 pg/mL;
p=0.095; respectively).
3. DISCUSSION
The DEAH-box RNA helicase DHX15 is implicated in diverse biological functions. In this study, we describe a total impairment of liver development caused by the mutation of Dhx15 in our CRISPR/Cas9 zebrafish. To our knowledge, Dhx15 had not previously been described to play a role in liver development; now, we report for the first time a lack of the hepatic organ in zebrafish due to Dhx15 knockout. This implies a redundant and non-compensated function of Dhx15 in liver development. Since DHX15 is a splicing factor, we studied whether Dhx15 knockout was affecting genes classically described to be implicated in liver formation in zebrafish. We found reduced expression of Prox1, which is one of the earliest markers for definitive hepatoblasts, Mypt1 which participates in bud formation and Hdac3 which participates in liver budding and differentiation (29), in Dhx15-/- embryos at 4dpf. Knockout experiments in zebrafish have helped to determine the crucial and non-redundant function of several genes in liver development, such as Gata 4 and 6, Hdac3, Hhex and Mypt1. Single-mutation of these genes results in major hepatic development complications (29–32). For instance, Huang H. et. Al. described a liverless phenotype in Mypt1 mutant zebrafish (33). In such study Mypt1 mutation caused hepatoblast apoptosis that resulted in blockage of liver bud formation. Here, we add Dhx15 to the list of essential genes in liver development in zebrafish. Furthermore, we observed a regulatory crosstalk between Dhx15 and Mypt1, Prox1 and Hdac3, thus supporting the role of Dhx15 in hepatic organogenesis. More studies are needed to evaluate the contribution of each of these factors in the final effect on organogenesis.
In Dhx15-/- zebrafish embryos, liver absence impeded lipid metabolization which depicted lipid accumulation in the yolk sac. At early embryonic stages, zebrafish development energy demands are supplied by the lipidic metabolization that takes place in the liver (34). Our Dhx15-/- zebrafish die at day 8 post fertilization possibly because in the absence of liver and lipid metabolization, their energy demands cannot be met resulting in embryonic lethality.
In mammals, the embryonic liver is an early hematopoietic organ; therefore, mutations affecting liver or blood development may cause early lethality during embryogenesis (35,36). In mouse, hepatogenesis begins with the formation of the liver bud around gestation day 8.25 (37,38). As we previously described, our Dhx15-/- mouse die prior to embryonic stage E8.5 (7). Therefore, we cannot disregard the possibility that embryonic lethality is linked to an impaired liver organogenesis caused by Dhx15 deficiency. Due to early embryonic mortality associated to the loss of Dhx15, this hypothesis could only be tested using Dhx15 conditional KO mouse. In mouse, RNA helicase knockout often results in embryonic lethality, implying their essential role in developmental processes (39–41). For instance, the loss of Ddx3x, which is associated with cell survival and cell cycle control, causes early post-implantation lethality prior to E6.5 (42). So far as we know, no other RNA helicase has been described to play an essential role in liver development.
Knowing that the vasculature is crucial in liver development, we studied the vascular phenotype in the liver of heterozygous mice. We had previously observed vascular defects in Dhx15-/- zebrafish during development (7). Here, we observed differences in thickness and connectivity of liver blood vasculature in Dhx15+/- mice. Additionally, in Dhx15+/- mice we observed a significant reduction of Angiopoietin 1, Vegf-c and Podoplanin which play major roles in the blood and lymphatic vascular growth and maturation. These liver vasculature alterations suggest that Dhx15 might be affecting the development of the hepatic vascular network that is crucial in embryonic stages for the development of the liver and as a result altering liver organogenesis as previously reported by others. (43,44).
Vascular alterations within the liver might alter hepatic functionality. One of the main characteristics of the liver is its capacity to regenerate owing to the proliferative potential of quiescent hepatocytes. It was previously described that loss of Akt hinders hepatic regeneration by reducing cell proliferation, cell hypertrophy, glycogenesis, and lipid droplet formation (19). Since Dhx15 is a downstream target of Akt 1 (6), we evaluated the effects of Dhx15 depletion in liver regeneration. In this context, we observed decreased overall regeneration and cellular proliferation in Dhx15+/- mice. However, the slightly decreased proliferation 48 hours after hepatectomy was not linked to a decreased expression of proliferative genes such as Pcna or Cyclin d1. We also observed a lower mice survival after 72 hours post-PHx. One of the reasons behind heterozygous mouse mortality at 72 hours post-PHx might be correlated with a significantly reduced proliferation that was linked to a decreased expression of Cyclin d1.
We also studied an alternative mechanism to explain impaired function after partial hepatectomy. One of the major functions of the liver is to act as a “glucostat”. It has been demonstrated how glucose supplementation in liver regeneration mouse models increase survival in different gene deficiency models (45). Furthermore, we had previously observed by -omic analysis that endothelial cells with a Dhx15 deficient background showed impaired glucose metabolism (7). Therefore, we analyzed if Dhx15 partial deficiency was promoting metabolic alterations. First, we observed that heterozygous mice were not able to normalize glycogen levels after partial hepatectomy. Such impaired glycogen restorage was linked to alterations in the expression of the enzymes of the glycogenic pathway such as glucokinase and glycogen synthase. During the regenerative process, glycogen is consumed and used as source of energy to meet with the high metabolic demands of regeneration (25,26). This may indicate that the observed liver regeneration alterations may also be the outcome of a decreased energetic availability hindering proper proliferation. In Dhx15+/- mice, we also observed a lower glucose production. Accordingly, we show a significantly decreased glucose production in silenced hepatocytes that may be related to a reduced expression of G6pc, Pklr, Pc which are key enzymes needed for the release of free glucose into blood circulation. The metabolic defects caused by Dhx15 deficiency together with the reduced cellular expression of regulators of the cell cycle progression, such as Cyclin d1, may explain the lower cell proliferation of hepatocytes found in Dhx15+/- livers. These results combined with the endothelial cell defects that surge at 72 hours post PHx result in an impaired liver regeneration derived from Dhx15 partial deficiency.
Next, we evaluated the role of Dhx15 in the context of hepatocellular carcinoma (HCC) and liver metastasis. Recently, several studies have analyzed the role of DHX15 in different cancer types. DHX15 contributes as a cancer promoter in breast cancer, prostate cancer, acute myeloid leukemia and hepatocellular carcinoma, and as an antitumor factor in glioma due to its growth inhibitory function (12–17). In the present study we have evaluated the potential predictive use of DHX15 serologic analysis as a biomarker of HCC. Dhx15 in the serum of the HCC cohort was noticeably higher than that of healthy individuals. However, we did not detect significant differences in the Dhx15 circulating levels when we compared the HCC and the cirrhosis groups, despite detecting a trend of greater concentration of Dhx15 in patients with HCC. One important limitation of our results is the design of this observational clinical study. Therefore, and considering the urgent need of accurate biomarkers for HCC in the clinical laboratory, we guaranteed further prospective validation studies to confirm the diagnostic utility of DHX15 as a non-invasive biomarker for HCC in comparison with other liver tumors.
In a murine HCC xenograft model, we observed the formation of similar-volume primary tumors in WT and Dhx15+/- mice. However, significantly smaller, and lesser tumoral nodules implanted in the liver were linked to Dhx15 depletion. In agreement with the reduced tumoral liver invasion, we observed a dysfunctional lymphatic vasculature in Dhx15+/- mice. In a previous study modelling pulmonary metastasis in mice, we detected a significant metastasis reduction due to Dhx15 depletion (7). Considering the role played by lymphatic vessels in tumor invasion (46) we suggest as a model that the impaired lymphatic growth within the primary tumor associated with Dhx15 deficiency limited cancer cell invasion into other organs, including the liver. These new findings highlight the role of Dhx15 as a potential target in metastasis.
To start evaluating the potential therapeutic benefits of Dhx15 inhibition in cancer and metastasis, we designed specific Dhx15 inhibiting oligonucleotides for in vivo use. We first analyzed the silencing potential of the AUMsilence oligonucleotides in mouse and observed a successful Dhx15 inhibition in the liver and other organs. AUMsilence ASOs are third generation chemically modified oligos which have the capability of self-delivery (gymnosis) without the use of any delivery reagents or formulations. In our experience, AUMsilence oligos are highly sequence specific to their target and show no toxicity. We have shown optimal delivery to the liver using AUMsilence ASOs. Such attributes make AUMsilence ASOs ideal for target discovery and preclinical studies. Then, we established the HCC Hepa 1-6 model in AUMsilence ASO-injected mouse to target Dhx15 expression and we observed significant reduction of average tumor growth. Evaluation of chronic toxicity, routes of administration and dosage are still pending to establish robust conclusions about the biosafety of the AUMsilence ASO Dhx15 treatment.
In summary, our study provided insights into an essential role for Dhx15 in the development of liver in zebrafish. Dhx15 knockout in zebrafish results in a liverless phenotype and early embryonic lethality. An impaired liver development could be the consequence of the observed blood and lymphatic vascular defects together with the reduced expression of hepatogenic enzymes. Also, Dhx15 depletion resulted in hepatic regeneration and metabolic alterations. The observed alterations in liver regeneration may be caused by a reduced proliferation linked to an aberrant glucose metabolism acting together with endothelial defects impeding the de novo formation of hepatic vasculature and impaired liver regeneration. Also, Dhx15 deficiency led to reduced hepatic tumor invasion and tumor growth in a murine HCC model. Regarding the potential use of DHX15 as a diagnostic marker for liver disease, HCC patients showed increased levels of DHX15 in blood samples compared with subjects without hepatic affectation. Therefore, our results support the potential role of DHX15 as a diagnostic and therapeutic target in liver disease, as well as a major regulator of liver regeneration and organogenesis.
4. MATERIALS AND METHODS
4.1. Mouse-induced tumor model
HEPA 1-6 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum, 50 U/ml penicillin and 50 µg/ml streptomycin in humified atmosphere at 37°C and 5% CO2. Syngeneic Hepa 1-6 tumor cells (5x106) were subcutaneously injected into the flank of Dhx15+/- and wild-type mice (n=10). Primary tumor growth was controlled during the first 5 weeks. Tumor growth was monitored by measuring volumes using a digital slide-caliper. Tumor volume was calculated by following formula: V = 4/3 x π x [length x depth x width]. Primary tumors were fixed in 4% PFA and cryopreserved in tissue-tek O.C.T. compound (Sakura, Flemingweg, Netherlands). The Post-surgical metastasis model was performed as follows: five weeks post-injection, Hepa 1-6 injected mice were sacrificed, and the liver was extracted to perform metastasis analyses. Tile scan images of hematoxylin-eosin (H&E) stained paraffin liver sections were visualized using an Olympus BX51 microscope equipped with DP71 camera (Olympus Europa SE & CO.KG. Germany) and the percentage of hepatic metastatic area as percent of total hepatic area was measured with Image J software (ImageJ version 1.52b; National Institutes of Health, Bethesda, MD, USA).
4.2. In vivo knockdown experiments
Knockdown experiments were performed using AUMsilenceTM antisense oligonucleotides (AUMsilenceTM ASOs) were designed and provided by AUM BioTech, LLC, Philadelphia, USA. For general knockdown the oligos were intravenously injected on mouse tail vein at a dose of 10mg/kg/day every third day. In vivo knockdown efficiency was determined by Dhx15 Western Blot determination in different vital organs (Liver, spleen, kidney, lungs, and heart) achieving an 80% of knockdown in the liver. To study the impact of Dhx15 depletion in the Hepa 1-6 HCC model, Hepa 1-6 cells (5x106 cells, subcutaneously injected) were implanted subcutaneously into the flank of the mice (n=10) and allowed to grow for five weeks in previously AUMsilence ASO-injected mouse. An unrelated AUMscramble ASO SCR was used as a control. Tumor growth was monitored by measuring volumes using a digital slide-caliper.
4.3. Patients
A prospective cohort of consecutive patients treated at Hospital Clinic de Barcelona was evaluated. Informed consent was obtained from all patients involved in the study that was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Hospital Clínic de Barcelona.
Included population consisted of patients with 1) HCC diagnosed according to AASL guidelines 2) Cirrhosis associated with hepatitis C virus infection (HCV) without HCC; and healthy volunteers. The demographic and clinical characteristics of the patients included in the study are shown in Supplementary Table 1 and Supplementary Table 2. This study included a total of 24 serum samples from control subjects without neoplastic or liver disease. The samples were collected, anonymized, and stored according to the Ethical rules of the Hospital Clinic.
4.4. Statistical analysis
Quantitative data were analyzed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA), and statistical analysis of the results was performed using unpaired Student’s t-tests and ANOVA models (with Tukey’s post hoc test) with normally distributed data. Partial hepatectomy mortality scores were analyzed by log-rank test and survival curves were generated using the product limit method of Kaplan and Meier. For other type of data, the Mann–Whitney U-test was used. Correlations between variables were evaluated using Spearman’s rho or Pearson’s r, when appropriate. Differences were considered significant at a p-value <0.05. The data are presented as the mean±standard error of the mean.
SUPPLEMENTARY MATERIALS
The following supporting information can be downloaded at the website of this paper posted on Preprints.org: Additional Materials and Methods, Additional Figures and Legends (Figure S1: Heterozygous Dhx15 deficient zebrafish embryos develop liver, Figure S2: Bioinformatic analysis of the proteome and the transcriptome obtained from Dhx15 silenced endothelial cells, and Figure S3: Tissue Dhx15 silencing after AUMsilence ASO treatment), and Additional Tables (Table S1: Demographic characteristics of the patients included in this study, Table S2: Analytical parameters determined in the serum of patients, and Table S3: Experimental and commercial details of the antibodies used in the study).
AUTHOR CONTRIBUTIONS
MM-R conceived the original idea, participated in the statistical analysis and supervised the project. IP and JR planned and carried out all in vivo and in vitro experiments. EL, GC, PM-L, and GF-V helped on in vitro experiments. LB, MS and MR collected patient samples and participated in the analysis of patient-derived data. VA designed and contributed FANA ASO molecules. IP, JR and MM-R wrote the manuscript in consultation with WJ. All authors contributed with critical feedback and approved final version of the article.
FINANCIAL SUPPORT
This study was supported by grants from the Agencia Estatal de Investigación (PDI2019-105502RB-100 and PID2022-138243OB-I00 to MM-R). RedFibro (RED2022-134485-T) of the 2022 call for aid to «RESEARCH NETWORKS», within the framework of the Programa Estatal del Plan Estatal de Investigación Científica, Técnica y de Innovación 2021-2023. Consolidated Research Group of the Generalitat de Catalunya AGAUR (2021 SGR 00881 to MM-R). I.P. was recipient of a predoctoral fellowship supported by MCIN/AEI/10.13039/501100011033 y FSE “El FSE invierte en tu futuro” (BES-2017-080823). PM-L was additionally supported by a fellowship from the Ramon y Cajal Program (RYC2018-0Z23971-I) and a grant (PID2021-123426OB-I00) from the Spanish Ministerio de Ciencia, Innovación y Universidades. M.S. received grant support from Instituto de Salud Carlos III (FI19/00222). L.B. received grant support from Instituto de Salud Carlos III (PI18/00768). M.R. received grant support from Instituto de Salud Carlos III (PI15/00145 and PI18/0358) and the Spanish Health Ministry (National Strategic Plan against Hepatitis C). CIBERehd, is financed by the Instituto de Salud Carlos III.
ETHICS APPROVAL STATEMENT
All animal experiments follow the guidelines of the Investigation and Ethics Committees of Hospital Clinic and the University of Barcelona after study approval. All the individuals were recruited under research protocols approved by the Ethic Committee of their respective institutions. This study was conducted in accordance with the principles of the Declarations of Helsinki and Istanbul.
PATIENT CONSENT STATEMENT
All patients provided written informed consent prior to enrollment.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article and its supplementary information files.
AKNOWLEDGEMENTS
We wish to thank all the patients and family members that participated in the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Studer, M.K.; Ivanovic, L.; Weber, M.E.; Marti, S.; Jonas, S. Structural basis for DEAH-helicase activation by G-patch proteins. Proc Natl Acad Sci USA. 2020, 117, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Silverman E, Edwalds-Gilbert G, Lin RJ. DExD/H-box proteins and their partners: Helping RNA helicases unwind. Gene 2003, 312, 1–16. [Google Scholar] [CrossRef]
- Sloan, K.E.; Bohnsack, M.T. Unravelling the Mechanisms of RNA Helicase Regulation. Trends in Biochemical Sciences [Internet]. 2018, 43, 237–250. [Google Scholar] [CrossRef]
- Robert-Paganin, J.; Réty, S.; Leulliot, N. Regulation of DEAH/RHA helicases by G-patch proteins. BioMed Research International. 2015, 2015. [Google Scholar] [CrossRef]
- Abdelkrim YZ, Banroques J, Tanner NK. RNA Remodeling Proteins: Methods and Protocols. Chapter 3: Known Inhibitors of RNA Helicases and Their Therapeutic Potential. In Methods in Molecular Biology; 2021; pp. 35–52. [Google Scholar]
- Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, et al. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proceedings of the National Academy of Sciences [Internet]. 2014, 111, 12865–12870.
- Ribera J, Portolés I, Córdoba-Jover B; et al. The loss of DHX15 impairs endothelial energy metabolism, lymphatic drainage and tumor metastasis in mice. Communications Biology 2021, 4. [Google Scholar] [CrossRef]
- Lu H, Lu N, Weng L; Zhang, Z. DHX15 Senses Double-Stranded RNA in Myeloid Dendritic Cells. The Journal of Immunology 2014, 193, 1364–1372. [Google Scholar] [CrossRef]
- Wang Y, He K, Sheng B, Lei X, Tao W, Zhu X; et al. The RNA helicase Dhx15 mediates Wnt-induced antimicrobial protein expression in Paneth cells. Proc Natl Acad Sci USA 2021, 118, 1–8. [Google Scholar] [CrossRef]
- Steimer, L.; Klostermeier, D. RNA helicases in infection and disease. RNA Biology. 2012, 9, 751–771. [Google Scholar] [CrossRef]
- Seiler M, Peng S, Agrawal AA, Palacino J, Teng T, Zhu P; et al. Somatic Mutational Landscape of Splicing Factor Genes and Their Functional Consequences across 33 Cancer Types. Cell Reports 2018, 23, 282–296. [Google Scholar] [CrossRef]
- Ito S, Koso H, Sakamoto K, Watanabe S. RNA helicase DHX15 acts as a tumour suppressor in glioma. British Journal of Cancer [Internet]. 2017, 117, 1349–1359. [CrossRef]
- Mosallanejad K, Sekine Y, Ishikura-Kinoshita S, Kumagai K, Nagano T, Matsuzawa A; et al. The DEAH-Box RNA Helicase DHX15 Activates NF- B and MAPK Signaling Downstream of MAVS During Antiviral Responses. Science Signaling 2014, 7, ra40. [Google Scholar] [CrossRef]
- Pan L, Li Y, Zhang HY, Zheng Y, Liu XL, Hu Z; et al. DHX15 is associated with poor prognosis in acute myeloid leukemia (AML) and regulates cell apoptosis via the NF-kB signaling pathway. Oncotarget 2017, 8, 89643–89654. [Google Scholar] [CrossRef]
- Jing Y, Nguyen MM, Wang D, Pascal LE, Guo W, Xu Y. DHX15 promotes prostate cancer progression by stimulating Siah2-mediated ubiquitination of androgen receptor. Oncogene 2018, 37, 638–650. [Google Scholar] [CrossRef]
- Xie, C.; Liao, H.; Zhang, C.; Zhang, S. Overexpression and clinical relevance of the RNA helicase DHX15 in hepatocellular carcinoma. Human Pathology [Internet]. 2019, 84, 213–220. [Google Scholar] [CrossRef]
- Mosallanejad K, Sekine Y, Ishikura-Kinoshita S; et al. The DEAH-Box RNA helicase DHX15 activates NF-κB and MAPK signaling downstream of MAVS during antiviral responses. Science Signaling 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Morales-Ruiz M, Cejudo-Martín P, Fernandez-Varo G, Tugues S, Ros J, Angeli P,; et al. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology 2003, 125, 522–531. [Google Scholar] [CrossRef]
- Pauta M, Rotllan N, Fernández-Hernando A, Langhi C, Ribera J, Lu M,; et al. Akt-mediated foxo1 inhibition is required for liver regeneration. Hepatology. 2016, 63, 1660–1674. [Google Scholar]
- Morales-Ruiz M, Fondevila C, Muñoz-Luque J, Tugues S, Rodríguez-Laiz G, Cejudo-Martín P,;; et al. Gene transduction of an active mutant of Akt exerts cytoprotection and reduces graft injury after liver transplantation. American Journal of Transplantation 2007, 7, 769–778. [Google Scholar] [CrossRef]
- Morales-Ruiz M, Fondevila C, Muñoz-Luque J; et al. Gene transduction of an active mutant of Akt exerts cytoprotection and reduces graft injury after liver transplantation. American Journal of Transplantation 2007, 7, 769–778. [Google Scholar] [CrossRef]
- Xie, C.; Liao, H.; Zhang, C.; Zhang, S. Overexpression and clinical relevance of the RNA helicase DHX15 in hepatocellular carcinoma. Human Pathology [Internet]. 2019, 84, 213–220. [Google Scholar] [CrossRef]
- Zhao M, Ying L, Wang R, Yao J, Zhu L, Zheng M,; et al. DHX15 Inhibits Autophagy and the Proliferation of Hepatoma Cells. Frontiers in Medicine 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McElderry J, Carrington B, Bishop K; et al. Splicing factor DHX15 affects tp53 and mdm2 expression via alternate splicing and promoter usage. Human Molecular Genetics. 2019, 28, 4173–4185. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.B.; Strecker, W.; Silz, S. Ultrastructural changes in rat hepatocytes after partial hepatectomy, and comparison with biochemical results. J Cell Sci. 1981, 50, 433–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Rudnick, D.A. Elucidating the metabolic regulation of liver regeneration. American Journal of Pathology. 2014, 184, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R; et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001, 7, 186–91. [Google Scholar] [CrossRef] [PubMed]
- Kalota A, Karabon L, Swider CR, Viazovkina E, Elzagheid M, Damha MJ,; et al. 2′-Deoxy-2′-fluoro-β-D-arabinonucleic acid (2′F-ANA) modified oligonucleotides (ON) effect highly efficient, and persistent, gene silencing. Nucleic Acids Research. 2006, 34, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Farooq M, Sulochana KN, Pan X, To J, Sheng D, Gong Z; et al. Histone deacetylase 3 (hdac3) is specifically required for liver development in zebrafish. Developmental Biology. 2008, 317, 336–353. [Google Scholar] [CrossRef]
- Wallace, K.N.; Yusuff, S.; Sonntag, J.M.; Chin, A.J.; Pack, M. Zebrafish hhex regulates liver development and digestive organ chirality. Genesis. 2001, 30, 141–143. [Google Scholar] [CrossRef]
- Tao, S.; Witte, M.; Bryson-Richardson, R.J.; Currie, P.D.; Hogan, B.M.; Schulte-Merker, S. Zebrafish prox1b mutants develop a lymphatic vasculature, and prox1b does not specifically mark lymphatic endothelial cells. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Zhao R, Watt AJ, Li J. GATA6 Is Essential for Embryonic Development of the Liver but Dispensable for Early Heart Formation. Molecular and Cellular Biology. 2005, 25, 2622–2631. [Google Scholar] [CrossRef]
- Huang H, Ruan H, Aw MY; et al. Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development. 2008, 135, 3209–3218. [Google Scholar] [CrossRef]
- Anderson, J.L.; Carten, J.D.; Farber, S.A. Zebrafish Lipid Metabolism: From Mediating Early Patterning to the Metabolism of Dietary Fat and Cholesterol. 2011. [Google Scholar]
- Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, et al. An essential role in liver development for transcription factor XBP-1 [Internet]. 2000. Available from: www.genesdev.
- Tao, T.; Peng, J. Liver development in zebrafish (Danio rerio). Journal of Genetics and Genomics. 2009, 36, 325–334. [Google Scholar] [CrossRef]
- Gualdi, R.; Bossard, P.; Zheng, M.; Hamada, Y.; Coleman, J.R.; Zaret^, K.S. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. 1996. [Google Scholar] [CrossRef]
- Duncan SA, Watt AJ. BMPs on the road to hepatogenesis. Genes and Development. 2001, 15, 1879–1884.
- Hildebrandt, M.R.; Germain, D.R.; Monckton, E.A.; Brun, M.; Godbout, R. Ddx1 knockout results in transgenerational wild-type lethality in mice. Scientific Reports. 2015, 5. [Google Scholar] [CrossRef]
- Lee C-G, Da V, Soares C, Newberger C, Manova K, Lacy E, et al. RNA helicase A is essential for normal gastrulation [Internet]. 1998. Available from: www.pnas.org.
- Janknecht, R. Review Article Multi-talented DEAD-box proteins and potential tumor promoters: p68 RNA helicase (DDX5) and its paralog, p72 RNA helicase (DDX17) [Internet]. 2010. Available from: www.ajtr.
- Chen CY, Chan CH, Chen CM, Tsai YS, Tsai TY, Lee YHW, et al. Targeted inactivation of murine Ddx3x: Essential roles of Ddx3x in placentation and embryogenesis. Human Molecular Genetics. 2016, 25, 2905–2922.
- Gouysse G, Couvelard A, Frachon S, Bouvier R, Nejjari M, Dauge M-C, et al. Relationship between vascular development and vascular differentiation during liver organogenesis in humans [Internet]. Available from: www.elsevier.
- DeSesso, J.M. Vascular ontogeny within selected thoracoabdominal organs and the limbs. Reproductive Toxicology. 2017, 70, 3–20. [Google Scholar] [CrossRef]
- Fernández MA, Albor C, Ingelmo-Torres M; et al. Caveolin-1 Is Essential for Liver Regeneration. Science (1979). 2006, 313, 1628–1632. [Google Scholar] [CrossRef]
- Alitalo, A.; Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012, 31, 4499–4508. [Google Scholar] [CrossRef] [PubMed]
- Cermak T, Doyle EL, Christian M; et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Research. 2011, 39, 1–11. [Google Scholar] [CrossRef]
- Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Archives of Pathology. 1931, 12, 186–202. [Google Scholar]
Figure 1.
Dhx15 gene deficiency in zebrafish resulted in a liverless phenotype. (A) In the upper panels, representative images of wild-type (panel a) and Dhx15-/- (panel b) larvae at 5 day post fertilization (dpf) revealing an absence of liver development and lipid retention in the yolk sac. Each area is enclosed with different colors (yellow lines correspond to liver region and green lines to yolk sac). In the lower panels, positive liver red fluorescence in wild-type (panel c) and Dhx15-/- (panel d) larvae. Quantification of liver size is shown in adjacent graph. Bars represent the mean ± SEM, ***p<0.001 vs. wild-type zebrafish. (B) RNA extraction of zebrafish embryos at 4 dpf from either wild-type or Dhx15 knockout larvae was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of the Mypt1, Prox1, Hdac3, Gata6, Foxa1, Sox17, Uhrf1 and Bmp4 genes in the Dhx15+/+ and Dhx15-/- conditions. mRNA levels are shown as fold change relative to Actin mRNA levels. Bars represent the mean ± SEM, *p<0.05 vs. wild-type (n=4). (C) Representative images comparing wild-type and Dhx15-/- larvae at 7 dpf, Dhx15-/- larvae show absence of liver (red fluorescence) and morphological defects including encephalic and cardiac edema, scoliosis, and impaired neural/eye growth.
Figure 1.
Dhx15 gene deficiency in zebrafish resulted in a liverless phenotype. (A) In the upper panels, representative images of wild-type (panel a) and Dhx15-/- (panel b) larvae at 5 day post fertilization (dpf) revealing an absence of liver development and lipid retention in the yolk sac. Each area is enclosed with different colors (yellow lines correspond to liver region and green lines to yolk sac). In the lower panels, positive liver red fluorescence in wild-type (panel c) and Dhx15-/- (panel d) larvae. Quantification of liver size is shown in adjacent graph. Bars represent the mean ± SEM, ***p<0.001 vs. wild-type zebrafish. (B) RNA extraction of zebrafish embryos at 4 dpf from either wild-type or Dhx15 knockout larvae was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of the Mypt1, Prox1, Hdac3, Gata6, Foxa1, Sox17, Uhrf1 and Bmp4 genes in the Dhx15+/+ and Dhx15-/- conditions. mRNA levels are shown as fold change relative to Actin mRNA levels. Bars represent the mean ± SEM, *p<0.05 vs. wild-type (n=4). (C) Representative images comparing wild-type and Dhx15-/- larvae at 7 dpf, Dhx15-/- larvae show absence of liver (red fluorescence) and morphological defects including encephalic and cardiac edema, scoliosis, and impaired neural/eye growth.
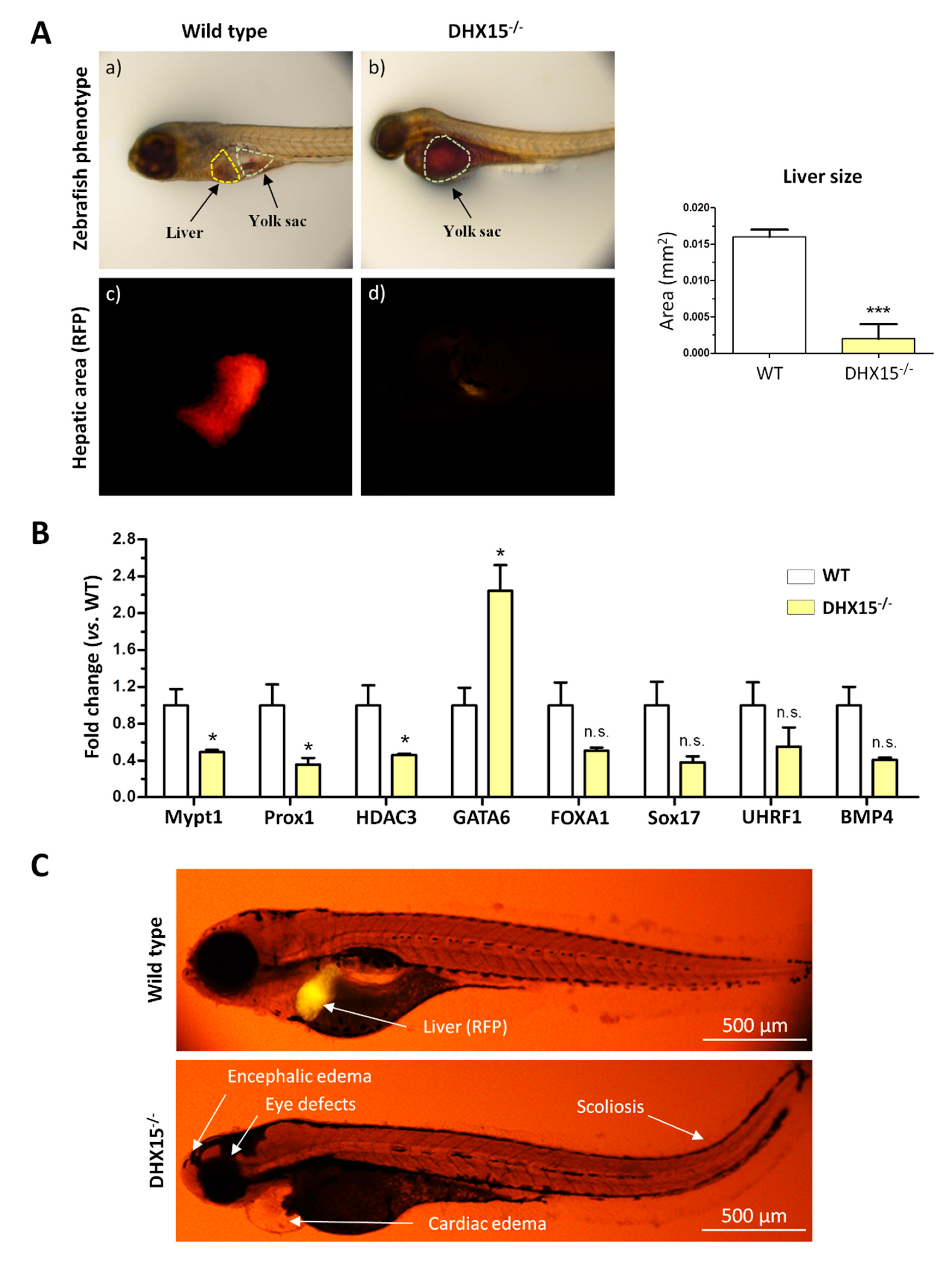
Figure 2.
Intrahepatic liver vasculature was altered in Dhx15+/- mice. (A) Representative Cd31 liver immunostaining (green) for wild-type (panel a) and Dhx15+/- (panel b) mouse. Nuclei counterstaining was performed with DAPI (blue). Confocal microscope, original magnification: 300X. (B) RNA extraction of liver tissue from either wild-type or Dhx15+/- mice was performed. mRNA expression was analyzed by RT-qPCR. Graphs show the expression levels of Vegf-a, Vegf-c, Vegf-d, Vegfr1, Vegfr3, Podoplanin and Angiopoietin 1 genes in the wild-type and Dhx15+/- conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=4). (C) RNA extraction of the hepatocyte cell line without or with silenced Dhx15 gene was performed. mRNA expression was analyzed by RT-qPCR. Graphs show the expression levels of Vegf-a, Vegf-c, Vegf-d and Angiopoietin 1 genes in wild-type and Dhx15 silenced conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=4).
Figure 2.
Intrahepatic liver vasculature was altered in Dhx15+/- mice. (A) Representative Cd31 liver immunostaining (green) for wild-type (panel a) and Dhx15+/- (panel b) mouse. Nuclei counterstaining was performed with DAPI (blue). Confocal microscope, original magnification: 300X. (B) RNA extraction of liver tissue from either wild-type or Dhx15+/- mice was performed. mRNA expression was analyzed by RT-qPCR. Graphs show the expression levels of Vegf-a, Vegf-c, Vegf-d, Vegfr1, Vegfr3, Podoplanin and Angiopoietin 1 genes in the wild-type and Dhx15+/- conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=4). (C) RNA extraction of the hepatocyte cell line without or with silenced Dhx15 gene was performed. mRNA expression was analyzed by RT-qPCR. Graphs show the expression levels of Vegf-a, Vegf-c, Vegf-d and Angiopoietin 1 genes in wild-type and Dhx15 silenced conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=4).
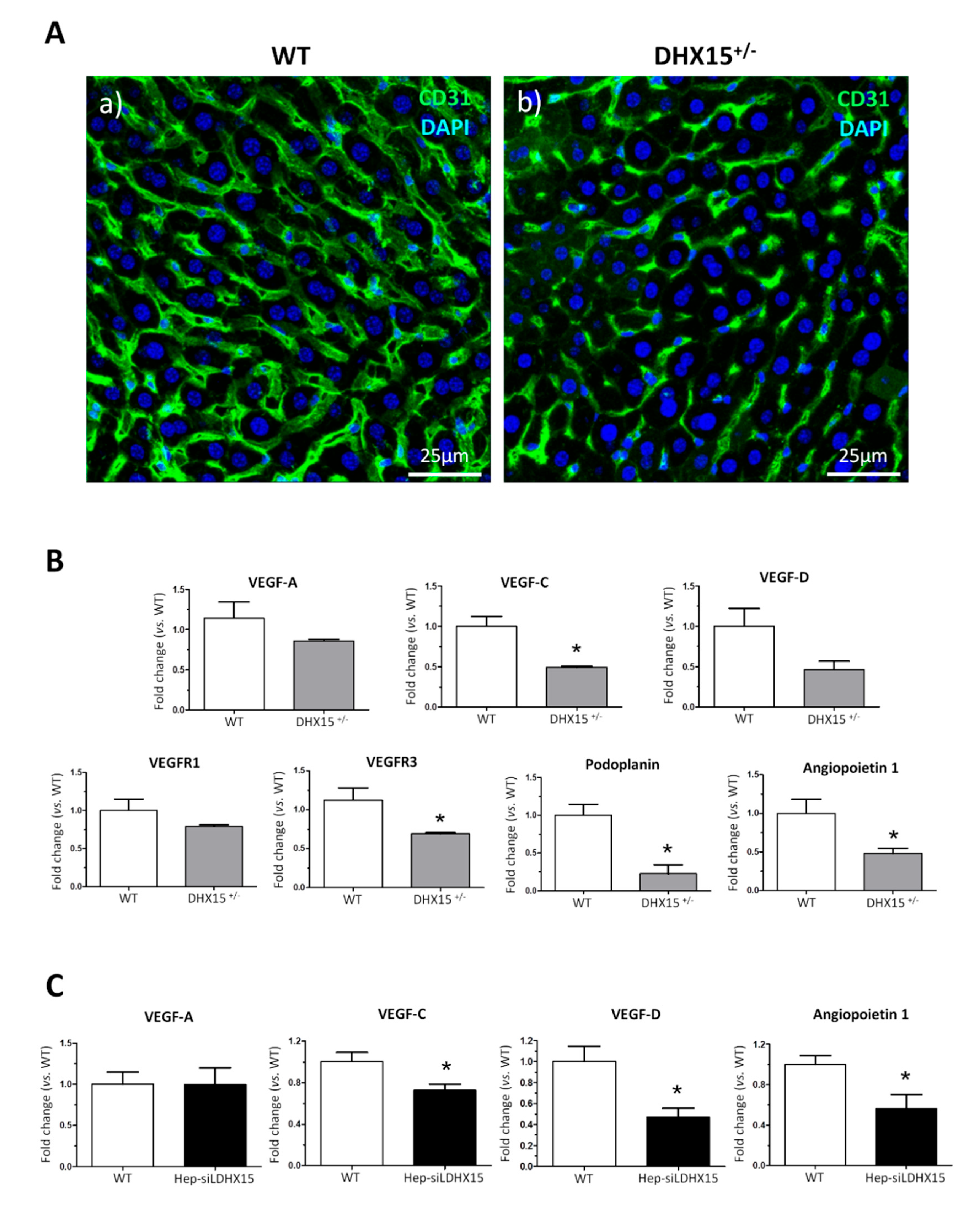
Figure 3.
Dhx15 genetic deficiency impaired liver regeneration in mice after PHx. (A) On the left graph, hepatic regenerative index (Liver weight/Total body weight) obtained in WT and Dhx15+/- mice 7 days after PHx. On the right graph, survival curves from WT and Dhx15+/- mice after PHx generated using the product limit method of Kaplan and Meier. Survival curves were compared using the log-rank test. (B) In the upper panels, representative merged images of immunofluorescence staining of Ki67-positive cells (green) and DAPI (blue) in WT and Dhx15+/- livers at 2 (panels a and b) and 3 (panels c and d) days following PHx. In the lower panels (e and f), representative merged images of immunofluorescence staining of Erg-positive cells (red) and DAPI (blue) in WT and Dhx15+/- livers at 3 days following PHx. Original magnification x200. Graph shows the computer-assisted quantification of Ki67 and Erg-positive cells/total nuclei at different times following PHx. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=4). (C) Expression of Pcna and Cyclin d1 proteins was evaluated by western blot using liver tissue lysates from wild-type and Dhx15+/- mice 3 days after PHx. β-actin was used as a loading control. Densitometric analysis of protein expression is shown on the bar graph. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=3).
Figure 3.
Dhx15 genetic deficiency impaired liver regeneration in mice after PHx. (A) On the left graph, hepatic regenerative index (Liver weight/Total body weight) obtained in WT and Dhx15+/- mice 7 days after PHx. On the right graph, survival curves from WT and Dhx15+/- mice after PHx generated using the product limit method of Kaplan and Meier. Survival curves were compared using the log-rank test. (B) In the upper panels, representative merged images of immunofluorescence staining of Ki67-positive cells (green) and DAPI (blue) in WT and Dhx15+/- livers at 2 (panels a and b) and 3 (panels c and d) days following PHx. In the lower panels (e and f), representative merged images of immunofluorescence staining of Erg-positive cells (red) and DAPI (blue) in WT and Dhx15+/- livers at 3 days following PHx. Original magnification x200. Graph shows the computer-assisted quantification of Ki67 and Erg-positive cells/total nuclei at different times following PHx. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=4). (C) Expression of Pcna and Cyclin d1 proteins was evaluated by western blot using liver tissue lysates from wild-type and Dhx15+/- mice 3 days after PHx. β-actin was used as a loading control. Densitometric analysis of protein expression is shown on the bar graph. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=3).
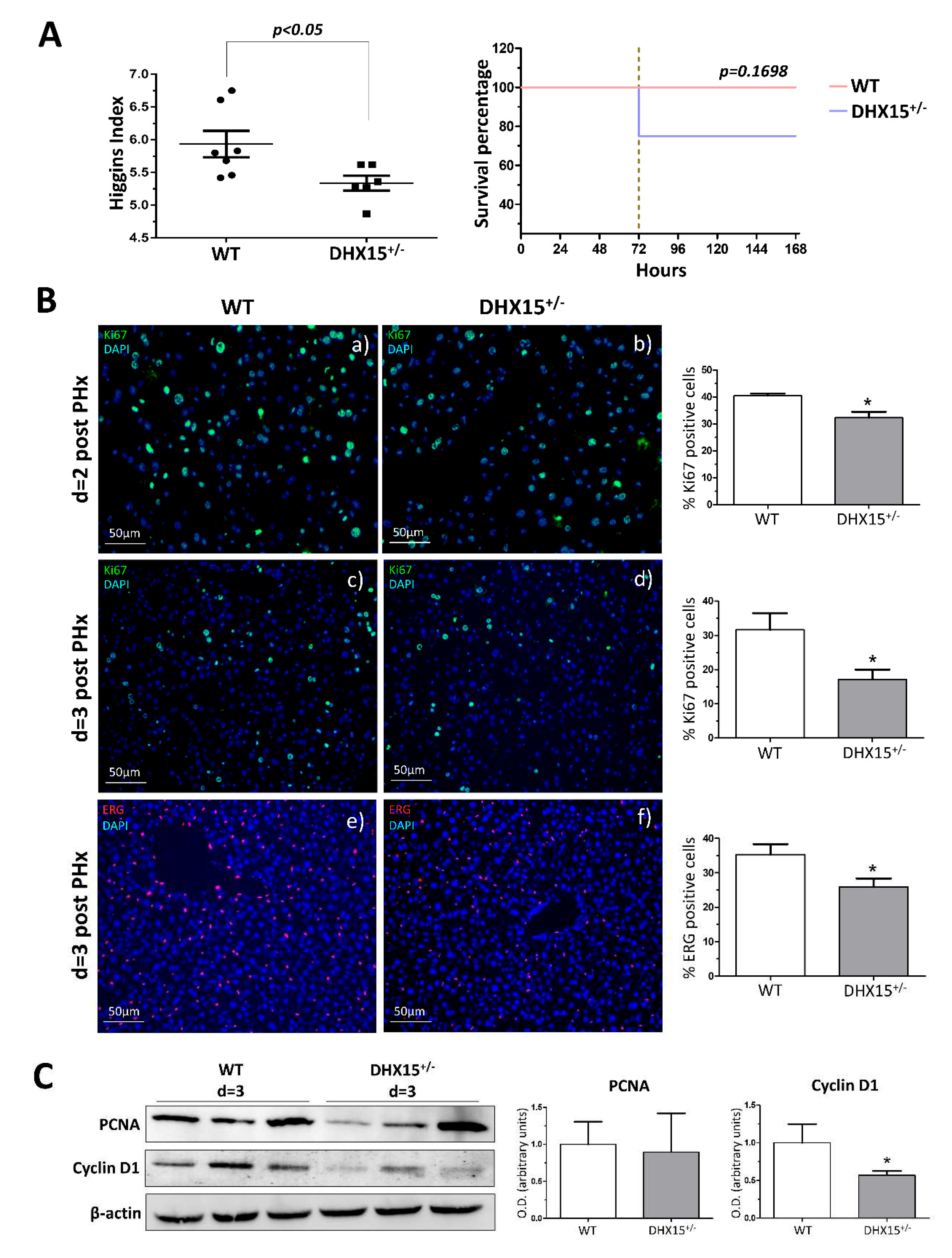
Figure 4.
Impaired glucose metabolism in Dhx15+/- mice. (A) On the left, images from periodic acid Schiff (PAS) staining of wild-type and Dhx15+/- mice in basal condition (upper panels) and 2 days after PHx (lower panels). On the right, glycogen in the hepatic tissue of wild-type and Dhx15+/- mice at 0 and 2 days after PHx measured by colorimetric assay. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=3). (B) RNA extraction of liver tissue from either wild-type or Dhx15+/- mice was performed before and 2 days after PHx. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of glucokinase and glycogen synthase genes in the wild-type and Dhx15+/- mice. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=6). (C) RNA extraction of the hepatocyte cell line without or with silenced Dhx15 gene was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of Ugp2, Pgm1, Gs and Gck genes in wild-type and Dhx15 silenced conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=6). (D) Pyruvate tolerance test performed in wild-type and Dhx15+/- mice with an intraperitoneal injection of sodium pyruvate (2.0 g/kg body weight in 1xPBS) after overnight fasting. Blood glucose levels were measured at 0, 15-, 30-, 60- and 120-min. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=15). (E) Levels of intracellular glucose production in the hepatocyte cell line without or with silenced Dhx15 gene measured by colorimetric assay. Bars represent mean ± SEM, **p<0.01 vs. wild-type (n=3). (F) RNA extraction of the hepatocyte cell line without or with the Dhx15 gene silenced was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of G6pc, Pc, Pklr and Pck1 genes in the wild-type and Dhx15 silenced conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent the mean ± SEM, *p<0.05 vs. wild-type (n=4).
Figure 4.
Impaired glucose metabolism in Dhx15+/- mice. (A) On the left, images from periodic acid Schiff (PAS) staining of wild-type and Dhx15+/- mice in basal condition (upper panels) and 2 days after PHx (lower panels). On the right, glycogen in the hepatic tissue of wild-type and Dhx15+/- mice at 0 and 2 days after PHx measured by colorimetric assay. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=3). (B) RNA extraction of liver tissue from either wild-type or Dhx15+/- mice was performed before and 2 days after PHx. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of glucokinase and glycogen synthase genes in the wild-type and Dhx15+/- mice. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=6). (C) RNA extraction of the hepatocyte cell line without or with silenced Dhx15 gene was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of Ugp2, Pgm1, Gs and Gck genes in wild-type and Dhx15 silenced conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=6). (D) Pyruvate tolerance test performed in wild-type and Dhx15+/- mice with an intraperitoneal injection of sodium pyruvate (2.0 g/kg body weight in 1xPBS) after overnight fasting. Blood glucose levels were measured at 0, 15-, 30-, 60- and 120-min. Bars represent mean ± SEM, *p<0.05 vs. wild-type (n=15). (E) Levels of intracellular glucose production in the hepatocyte cell line without or with silenced Dhx15 gene measured by colorimetric assay. Bars represent mean ± SEM, **p<0.01 vs. wild-type (n=3). (F) RNA extraction of the hepatocyte cell line without or with the Dhx15 gene silenced was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of G6pc, Pc, Pklr and Pck1 genes in the wild-type and Dhx15 silenced conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent the mean ± SEM, *p<0.05 vs. wild-type (n=4).
Figure 5.
Tumor growth and metastases in Dhx15+/- mice following Hepa1-6 tumor induction. (A) On the upper panels, macroscopic images of tumor size in wild-type (panel a) and Dhx15+/- mice (panel b) 5 weeks after mouse Hepa1-6 hepatoma cells implantation. Yellow circles delimitate primary tumor localization. On the lower panels, representative liver sections with metastatic areas after haematoxylin-eosin staining (H&E) in wild-type (panel c) and Dhx15+/- mice (panel d). Original magnification: x10. The lower images of each condition correspond to the enclosed area of the upper images that were taken at higher magnifications (x100 and x200, respectively). Quantifications of tumor volume (cm3), number of metastases and nodule size are shown on the lower graphs. Bars represent mean ± SEM, *p<0.05 vs. wild-type mice (n=10 animals for each condition). (B) Immunostaining of intratumoral vessels in wild-type (blood vessels stained with Endomucin, panel a, and lymphatic vessels stained with Lyve-1, panel c) and Dhx15+/- (blood vessels stained with Endomucin, panel b, and lymphatic vessels stained with Lyve-1, panel d) mice. Quantification of total vascular perimeter and lumen of all intratumoral blood vessels, and percentage of Lyve-1 positive immunostaining are shown on the right graphs. Bars represent mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 vs. wild-type mice (n=10 animals for each condition). Original magnification: 200X. (C) RNA extraction of primary tumors from either wild-type or Dhx15+/- mice was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of Vegf-a, Vegf-d, Vegfr1, Vegfr3 and Angiopoietin 1 genes in wild-type and Dhx15+/- conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05, **p<0.01 vs. wild-type (n=4).
Figure 5.
Tumor growth and metastases in Dhx15+/- mice following Hepa1-6 tumor induction. (A) On the upper panels, macroscopic images of tumor size in wild-type (panel a) and Dhx15+/- mice (panel b) 5 weeks after mouse Hepa1-6 hepatoma cells implantation. Yellow circles delimitate primary tumor localization. On the lower panels, representative liver sections with metastatic areas after haematoxylin-eosin staining (H&E) in wild-type (panel c) and Dhx15+/- mice (panel d). Original magnification: x10. The lower images of each condition correspond to the enclosed area of the upper images that were taken at higher magnifications (x100 and x200, respectively). Quantifications of tumor volume (cm3), number of metastases and nodule size are shown on the lower graphs. Bars represent mean ± SEM, *p<0.05 vs. wild-type mice (n=10 animals for each condition). (B) Immunostaining of intratumoral vessels in wild-type (blood vessels stained with Endomucin, panel a, and lymphatic vessels stained with Lyve-1, panel c) and Dhx15+/- (blood vessels stained with Endomucin, panel b, and lymphatic vessels stained with Lyve-1, panel d) mice. Quantification of total vascular perimeter and lumen of all intratumoral blood vessels, and percentage of Lyve-1 positive immunostaining are shown on the right graphs. Bars represent mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 vs. wild-type mice (n=10 animals for each condition). Original magnification: 200X. (C) RNA extraction of primary tumors from either wild-type or Dhx15+/- mice was performed. mRNA expression was analyzed by RT-qPCR. Graph shows the expression levels of Vegf-a, Vegf-d, Vegfr1, Vegfr3 and Angiopoietin 1 genes in wild-type and Dhx15+/- conditions. mRNA levels are shown as fold change relative to Hprt mRNA levels. Bars represent mean ± SEM, *p<0.05, **p<0.01 vs. wild-type (n=4).
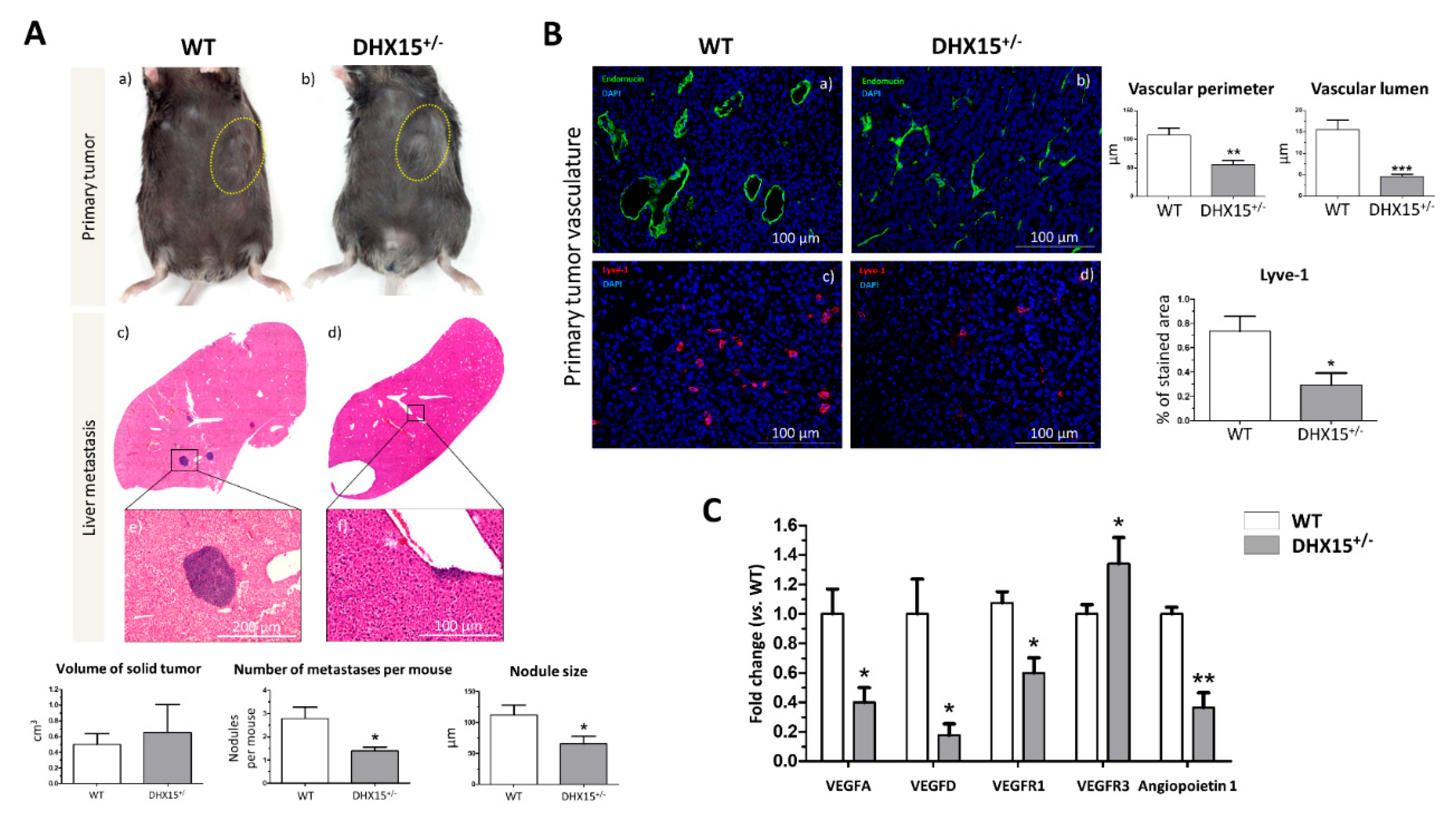
Figure 6.
Tumor growth in wild-type mice following Hepa1-6 tumor induction after Dhx15 inhibition. (A) On the left, macroscopic images of tumor size in wild-type AUMscramble ASO (scramble) and AUMsilence ASO (Dhx15 specific) -injected mice 5 weeks after mouse Hepa1-6 hepatoma cells implantation. Yellow circles delimitate primary tumor localization. On the right, graph shows tumor growth in wild-type AUMsilence ASO (Dhx15 specific) or AUMscramble ASO (scramble) injected mice. Tumor volume was monitored every 3 days until the end of the study. (B) Immunostaining of intratumoral vessels (blood vessels stained with Endomucin, a and b panels, and lymphatic vessels stained with anti-Lyve-1 antibody, c and d panels) in wild-type AUMsilence ASO (Dhx15 specific) or AUMscramble-ASO (scramble) injected mice. Quantifications of total vascular perimeter and lumen of all intratumoral blood vessels, and percentage of Lyve-1 positive immunostaining are shown on the right graph. Bars represent mean ± SEM, *p<0.05 vs. wild-type mice (n=10 animals for each condition). Original magnification: 200X. (C) DHX15 levels in the serum of patients with cirrhosis (n=35), hepatocellular carcinoma (n=62) and healthy controls (n=24).
Figure 6.
Tumor growth in wild-type mice following Hepa1-6 tumor induction after Dhx15 inhibition. (A) On the left, macroscopic images of tumor size in wild-type AUMscramble ASO (scramble) and AUMsilence ASO (Dhx15 specific) -injected mice 5 weeks after mouse Hepa1-6 hepatoma cells implantation. Yellow circles delimitate primary tumor localization. On the right, graph shows tumor growth in wild-type AUMsilence ASO (Dhx15 specific) or AUMscramble ASO (scramble) injected mice. Tumor volume was monitored every 3 days until the end of the study. (B) Immunostaining of intratumoral vessels (blood vessels stained with Endomucin, a and b panels, and lymphatic vessels stained with anti-Lyve-1 antibody, c and d panels) in wild-type AUMsilence ASO (Dhx15 specific) or AUMscramble-ASO (scramble) injected mice. Quantifications of total vascular perimeter and lumen of all intratumoral blood vessels, and percentage of Lyve-1 positive immunostaining are shown on the right graph. Bars represent mean ± SEM, *p<0.05 vs. wild-type mice (n=10 animals for each condition). Original magnification: 200X. (C) DHX15 levels in the serum of patients with cirrhosis (n=35), hepatocellular carcinoma (n=62) and healthy controls (n=24).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).










