Submitted:
08 January 2024
Posted:
10 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
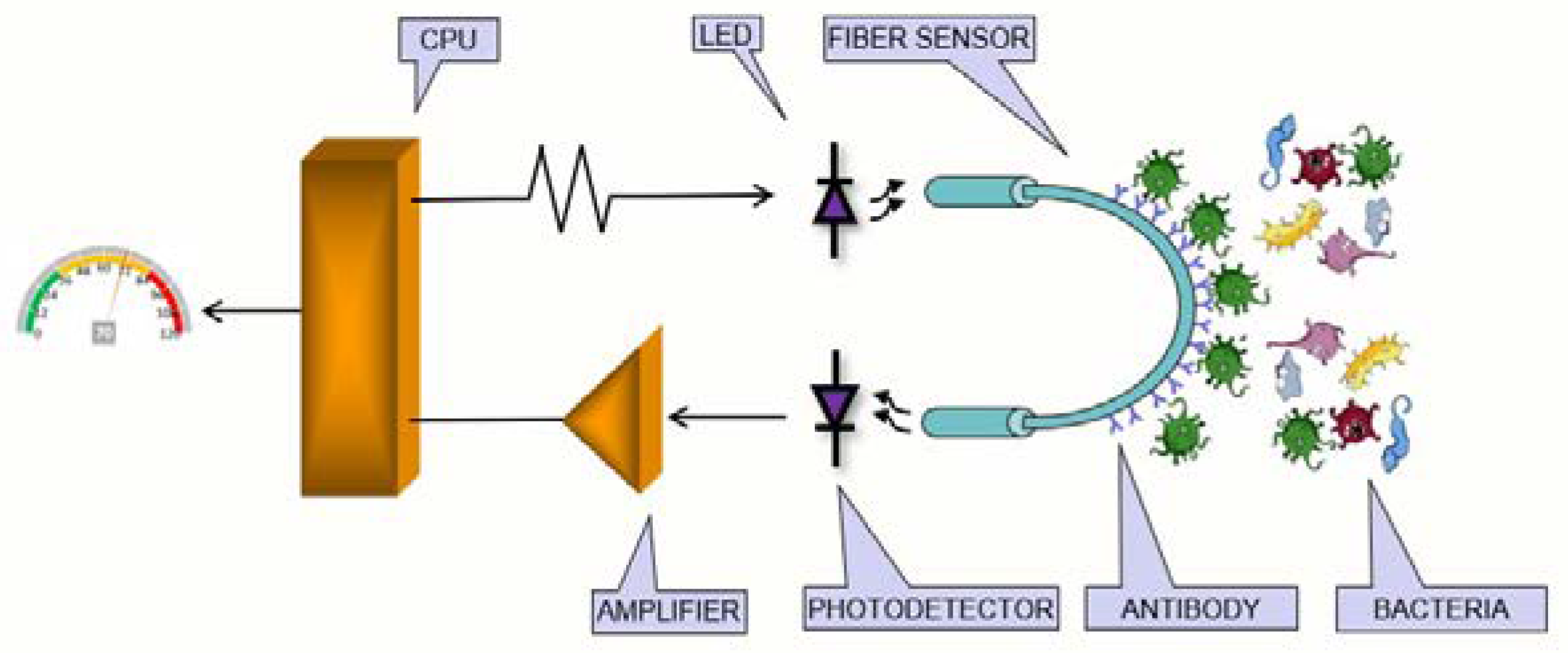
2. Sensing Theory: Physical Principle of a U-Shape POF Sensor
3. Materials e Methods
3.1. Manufacture of U-Shape Sensors
3.2. Sensor Surface Functionalization with Polyethyleneimine (PEI) and Immobilization of Antibodies
3.3. Bacteria Suspension and E. coli Detection Procedures
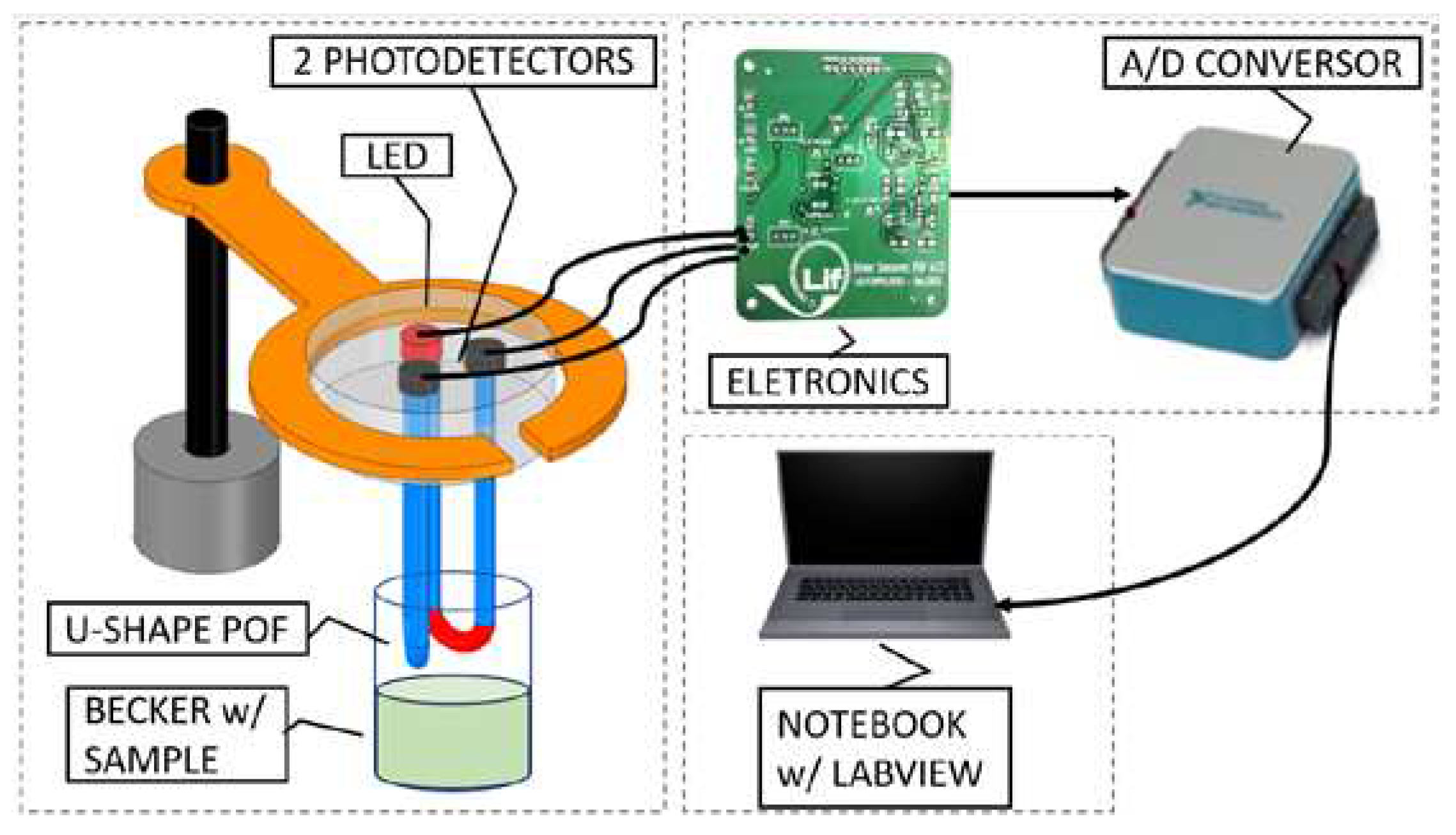
3.4. The Optoelectronic System
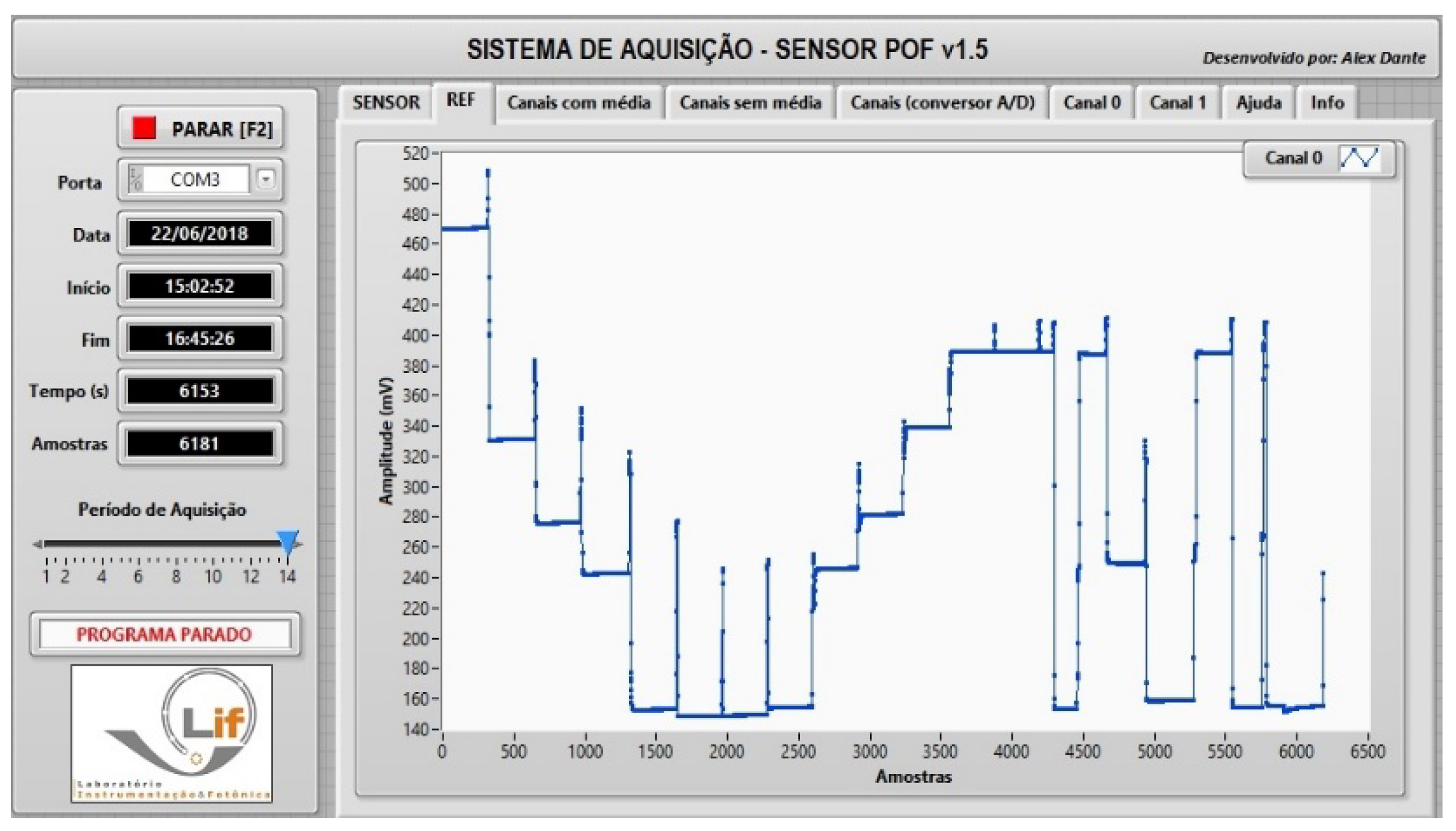
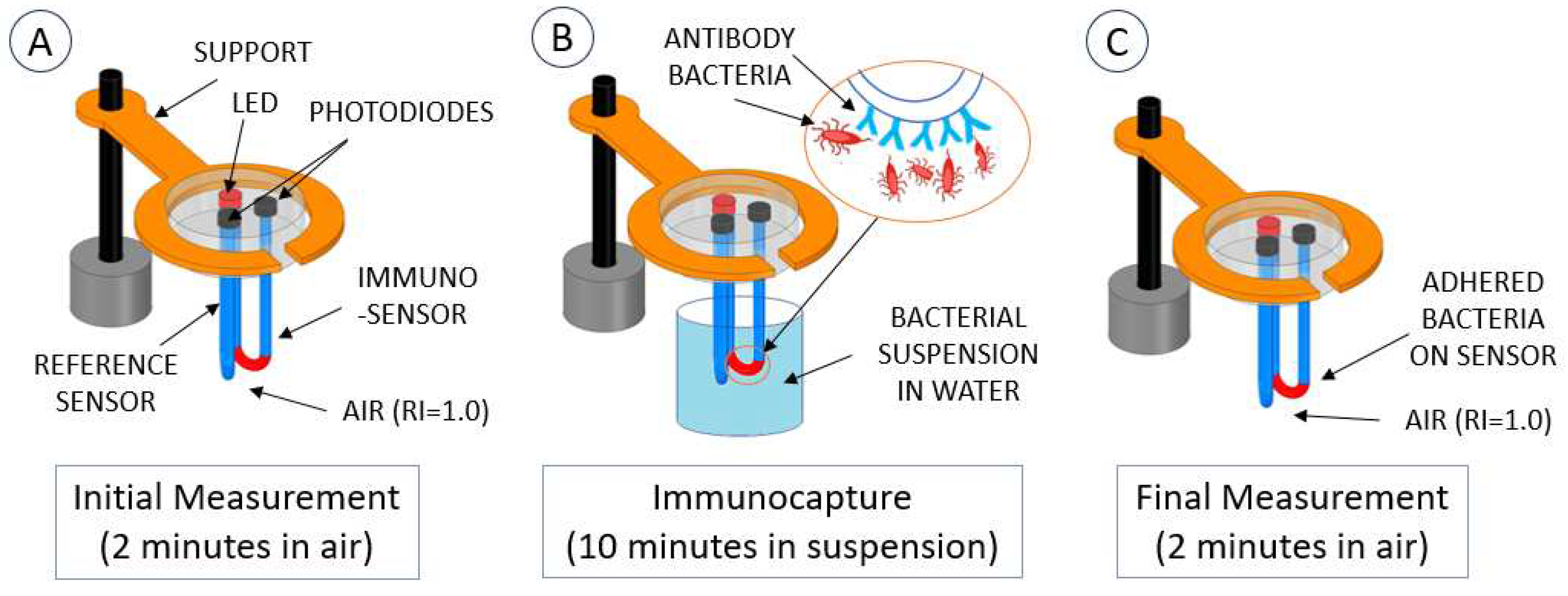
3.5. Measurement Methodology
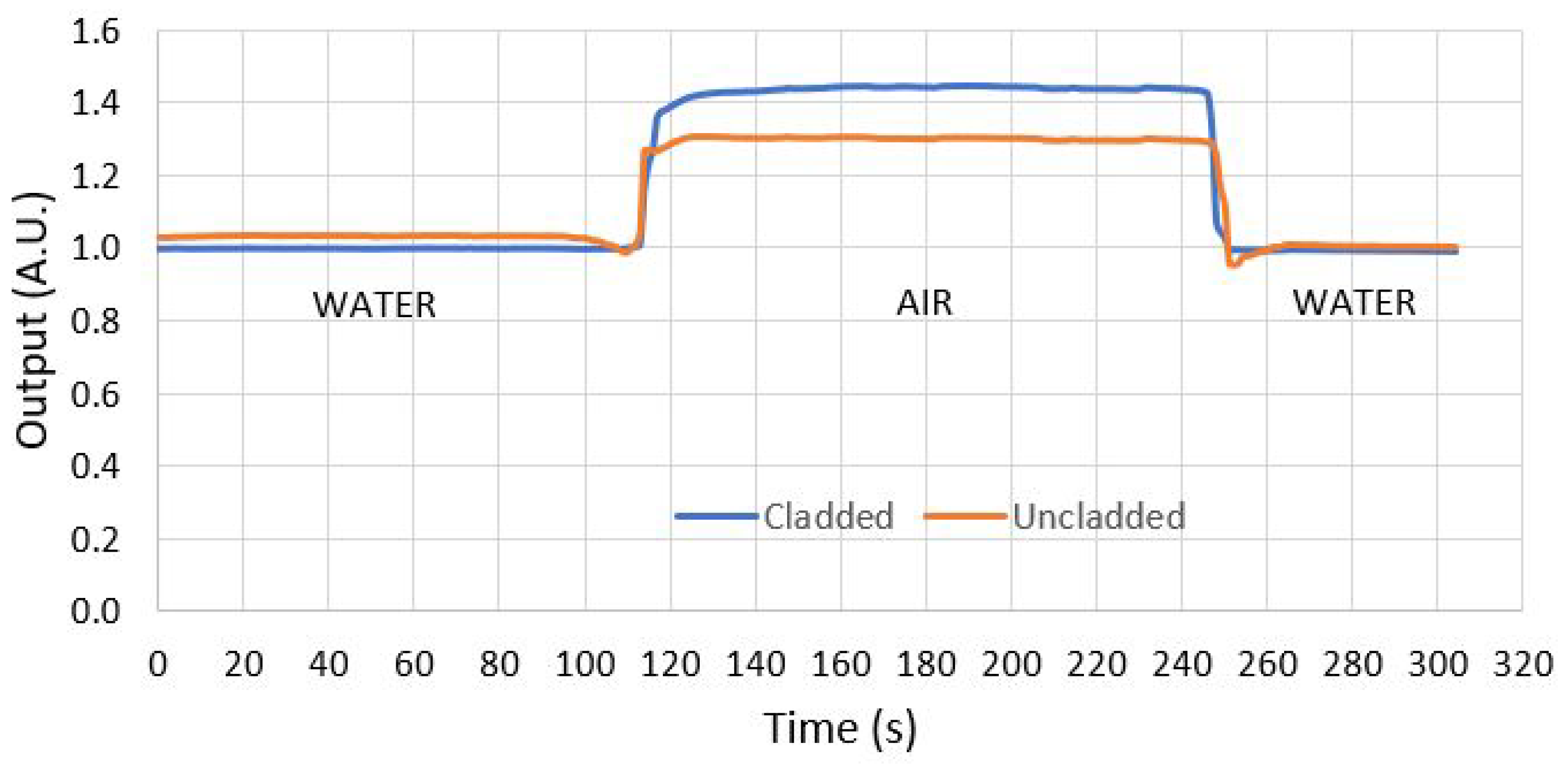
3.6. Test and Simulation of the Methodology
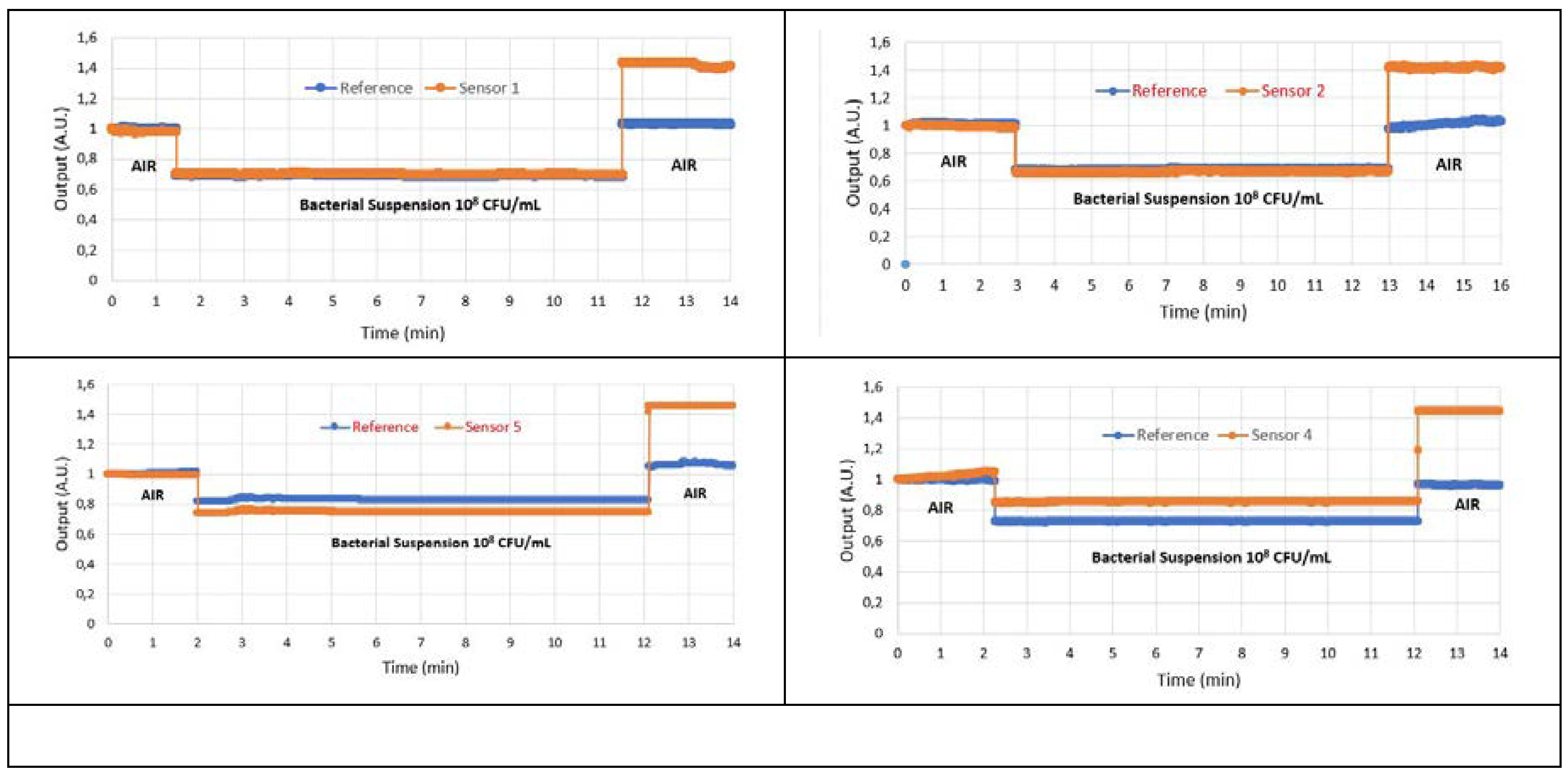
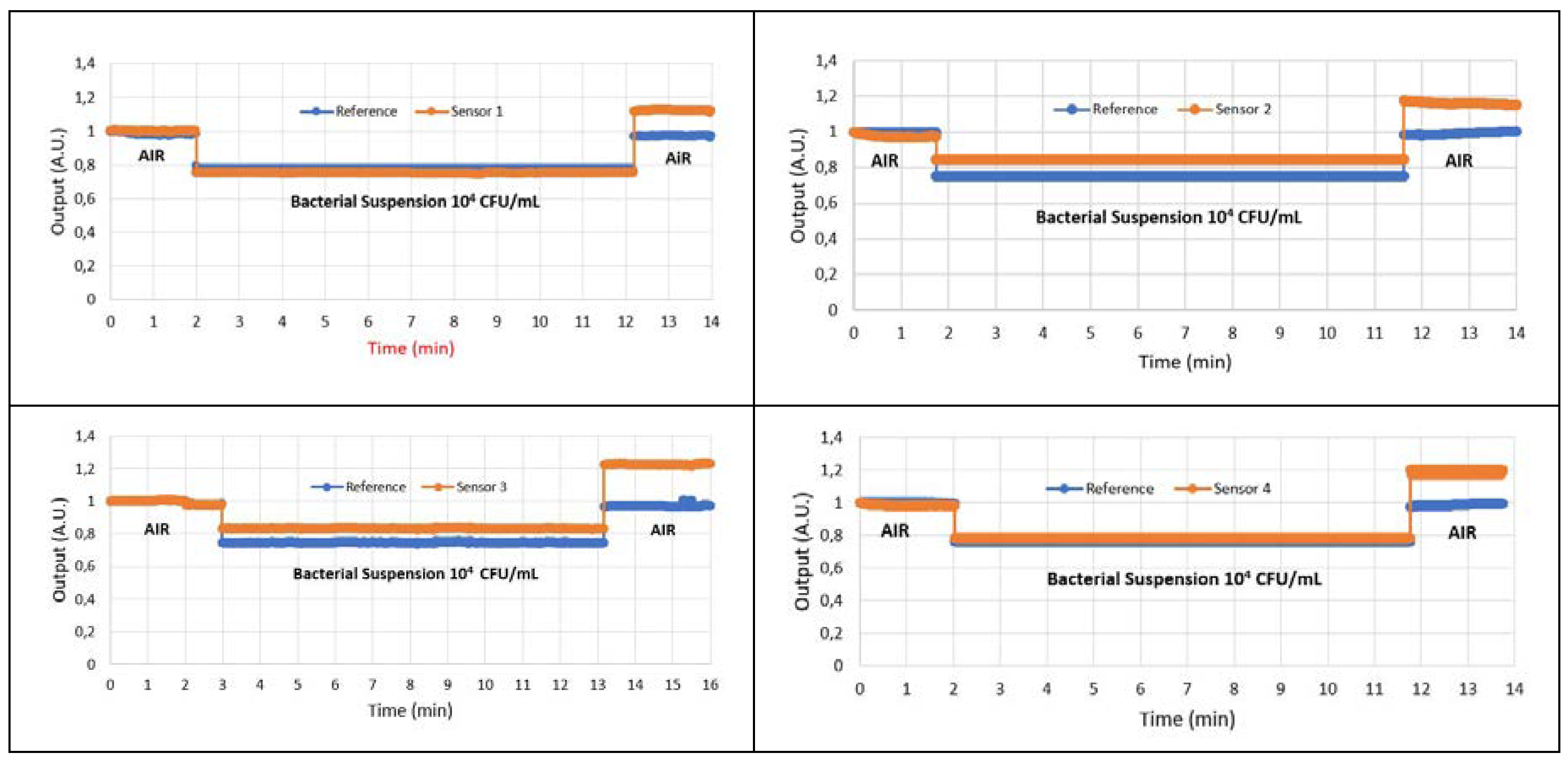
4. Results
4.1. Results of Immunocapture
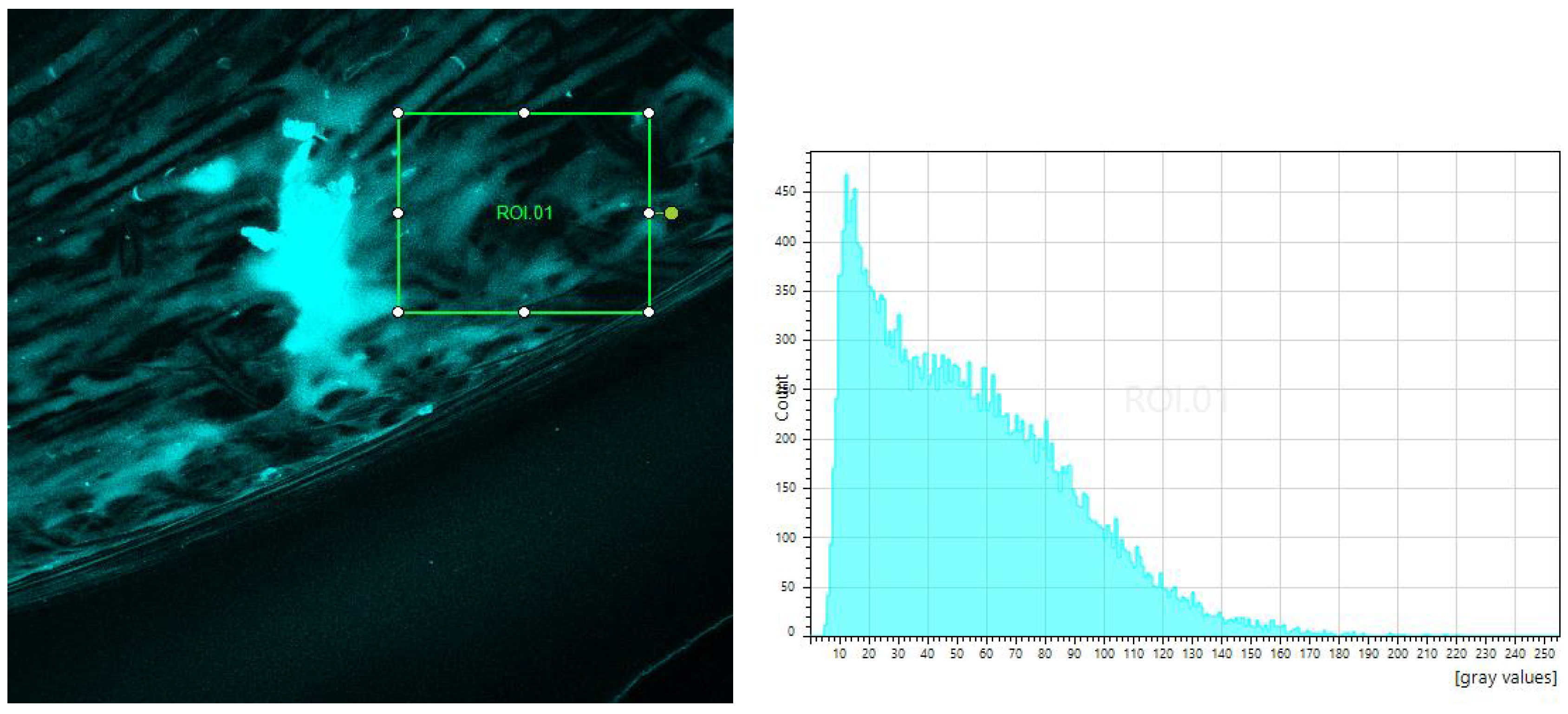
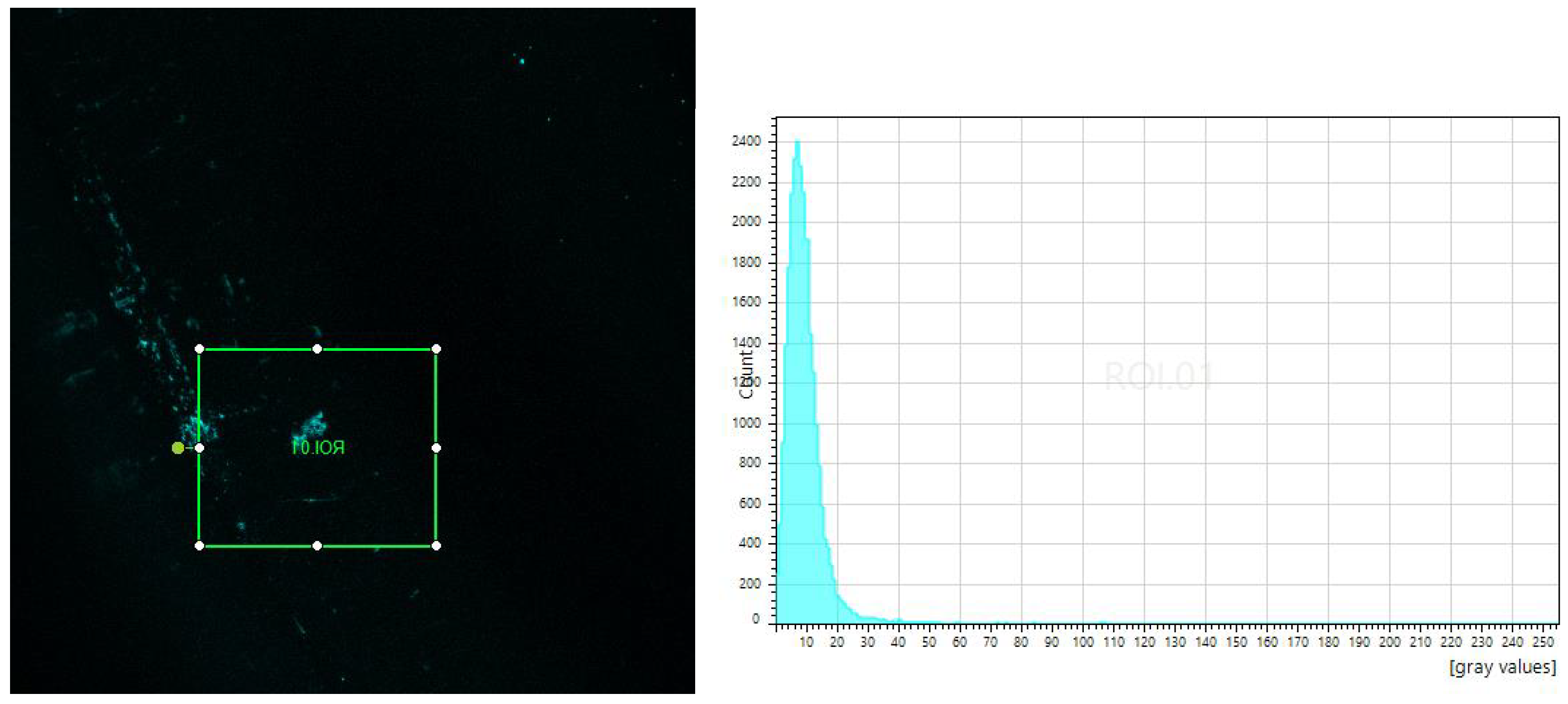
4.3. Tests of Fluorescence in Confocal Microscope
5. Discussion and Conclusions
Author Contributions
Funding
References
- Conelly: J.T.; Baeumner, A.J. Biosensor for the Detection of Waterborne Pathogens. Analytical and Bioanalytical Chemistry, v. 402, p. 117-127, 2012.
- Deshmukh, R.A.; Joshi, K.; Bhand, S.; Roy, U. Recent Developments in Detection and Enumeration of Waterborne Bacteria: a Retrospective Minireview. Microbiology Open. v. 5(6), p. 901-922, 2016.
- N. Kumar, Y. Hu, S. Singh, B. Mizaikoff, “Emerging Biosensor Platforms for the Assessment of Water-borne Pathogens”, Analyst, 143, 359-373, 2018.
- María Dolores Marazuela and María Cruz Moreno-Bondi, “Fiber-optic biosensors – an overview”. Anal Bioanal Chem (2002) 372:664–682. [CrossRef]
- Sobhan, A.; Jia, F.; Kelso, L.C.; Biswas, S.K.; Muthukumarappan, K.; Cao, C.; Wei, L.; Li, Y. A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7. Biosensors 2022, 12, 908. [CrossRef]
- 6. Gisele Wandermur, Domingos Rodrigues, Regina Allil, Vanessa Queiroz, Raquel Peixoto, Marcelo Werneck, Marco Miguel, “Plastic optical fiber-based biosensor platform for rapid cell detection”. Biosensors and Bioelectronics, Volume 54, 2014, Pages 661-666, ISSN 0956-5663. [CrossRef]
- 7. Xu-dong Wang, and Otto S. Wolfbeis, “Fiber-Optic Chemical Sensors and Biosensors (2015−2019)”. Anal. Chem. 2020, 92, 397−430. [CrossRef]
- Razo-Medina, D. A., M. Trejo-Durán, and E. Alvarado-Méndez. "Cholesterol Biosensor Based on a Plastic Optical Fibre with Sol-gel: Structural Analysis and Sensing Properties." Journal of Modern Optics 65.3 (2018): 348-52. [CrossRef]
- 9. Jinze Li, Xin Liu, Hao Sun, Liming Wang, Jianqi Zhang, Xi Huang, Li Deng, Jiawei Xi, and Tianhong Ma. "A New Type of Optical Fiber Glucose Biosensor With Enzyme Immobilized by Electrospinning." IEEE Sensors Journal 21.14 (2021): 16078-6085. [CrossRef]
- 10. Cátia Leitão, Sónia O. Pereira, Carlos Marques, Nunzio Cennamo, Luigi Zeni, Madina Shaimerdenova, Takhmina Ayupova and Daniele Tosi, “Cost-Effective Fiber Optic Solutions for Biosensing”. Biosensors 2022, 12, 575. [CrossRef]
- Jiří Homola, Sinclair S. Yee, Günter Gauglitz, “Surface plasmon resonance sensors: review”. Sensors and Actuators B: Chemical, Volume 54, Issues 1–2, 1999, Pages 3-15, ISSN 0925-4005. [CrossRef]
- Claes Nylander, Bo Liedberg, Tommy Lind, “Gas detection by means of surface plasmon resonance”. Sensors and Actuators, Volume 3, 1982, Pages 79-88, ISSN 0250-6874. [CrossRef]
- Masaru Mitsushio, Kenichiro Miyashita, Morihide Higo, “Sensor properties and surface characterization of the metal-deposited SPR optical fiber sensors with Au, Ag, Cu, and Al”. Sensors and Actuators A: Physical, Volume 125, Issue 2, 2006, Pages 296-303, ISSN 0924-4247. [CrossRef]
- Arcas, A.D.S.; Dutra, F.D.S.; Allil, R.C.S.B.; Werneck, M.M. Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors. Sensors, 2018, 18, 648. [CrossRef]
- Ariadny S. Arcas, Lizeth Jaramillo, Natalia S. Costa, Regina Celia S. B. Allil, and Marcelo M. Werneck, “Localized surface plasmon resonance-based biosensor on gold nanoparticles for Taenia solium detection”. Applied Optics, September, 2021. [CrossRef]
- Nunzio Cennamo, Girolamo D’Agostino, Chiara Perri, Francesco Arcadio, Guido Chiaretti, Eva Maria Parisio, Giulio Camarlinghi, Chiara Vettori, Francesco Di Marzo, Rosario Cennamo, Giovanni Porto and Luigi Zeni, “Proof of concept for a quick and highly sensitive on-site detection of SARS-CoV-2 by plasmonic optical fibers and molecularly imprinted polymers”. Sensors 2021, 21, 1681. [CrossRef]
- Alberti, G.; Pesavento, M.; De Maria, L.; Cennamo, N.; Zeni, L.; Merli, D. An Optical Fiber Sensor for Uranium Detection in Water. Biosensors 2022, 12, 635. [CrossRef]
- Werneck, M.M., & Allil, R.C.S.B. (Eds.). (2019). Plastic Optical Fiber Sensors: Science, Technology and Applications (1st ed.). CRC Press. [CrossRef]
- 19. Carolina Beres, Fábio Vieira Batista de Nazaré, Nathália Correa Chagas de Souza, Marco Antônio Lemos Miguel, Marcelo Martins Werneck. “Tapered plastic optical fiber-based biosensor – Tests and application”. Biosensors and Bioelectronics, Volume 30, Issue 1, 2011, Pages 328-332, ISSN 0956-5663. [CrossRef]
- D.M.C. Rodrigues, R.N. Lopes, M.A.R. Franco, R.C.S.B. Allil, M.M. Werneck, “Sensitivity Analysis of Different Shapes of a Plastic Optical Fiber-Based Immunosensor for Escherichia coli: Simulation and Experimental Results”, Sensors, 17 (2944), 1-16, 2017. [CrossRef]
- R.N. Lopes, D.M.C. Rodrigues, R.C.S. Allil, M.M. Werneck, “Plastic optical fiber immunosensor for fast detection of sulfate-reducing bacteria”, Measurement, Volume 125, September 2018, Pages 377-385. [CrossRef]
- Ashraf, Mainuddin, M. T. Beg, F. Moin, R. Rajesh and G. Singhal, "U-Bent Plastic Optical Fiber Sensor for Iron in Iron Supplements," in IEEE Sensors Journal, vol. 22, no. 15, pp. 14921-14928, 1 Aug.1, 2022. [CrossRef]
- 23. Ashraf, Mohd & Siddique, Mainuddin & Beg, Mirza & Moin, Fiza & Saikia, Ananta & Dwivedi, Sanjai & Kumar, Gagan. (2023). U-shaped plastic optical fiber sensor for phosphate detection in water. Optical and Quantum Electronics. [CrossRef]
- Johari, S.H.; Cheak, T.Z.; Abdul Rahim, H.R.; Jali, M.H.; Mohd Yusof, H.H.; Md Johari, M.A.; Yasin, M.; Harun, S.W. ZnO Nanorods Coated Tapered U-Shape Plastic Optical Fiber for Relative Humidity Detection. Photonics 2022, 9, 796. [CrossRef]
- Hadi, M.U.; Khurshid, M. SARS-CoV-2 Detection Using Optical Fiber Based Sensor Method. Sensors 2022, 22, 751. [CrossRef]
- Ariadny Da Silva Arcas, Regina Célia Da Silva Barros Allil and Marcelo Martins Werneck, “U-Shaped POF sensor coated with gold thin film for E. coli detection”, Paper 56, Proceedings of the 26th International Conference on Plastic Optical Fibres, POF2017, held at Melia Ria Hotel & Spa, Aveiro, Portugal, ISBN: 978-989-97345-2-4, September 13 to 15, 2017.
- 27. Khnouf, R; Karasneh, D; Albiss, B.A. Protein Immobilization on the Surface of Polydimethylsiloxane and Polymethylmethacrylate Microfluidic Devices. Electrophoresis. v. 37, p. 529-535, 2016.
- J. D. Lopez, A. Dante, R. C. da Silva Allil and M. M. Werneck, "The Influence of Geometric Shape on the Performance of Refractive Index Sensors Based on Plastic Optical Fibers: Simulations and Experimental Assessment," in IEEE Sensors Journal, vol. 23, no. 6, pp. 5803-5809, March 15, 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




