1. Introduction
Ever since the first half of the 20th century, we have seen constant shifts on a global scale in the organization of work processes and organizational management. Economic, technological, philosophical, and social changes alter dominant paradigms, introducing different rearrangements from those practiced until then.
These rearrangements bring improvements to the productive process and, at the same time, include new variables and restrictions in managing an organization´s activities.
Management models evolve, and new concepts gain prominence, such as Systemic vision, Risk mindset, and Sustainability.
Continuous improvement should be part of the foundation of management systems. Tracking set objectives and goals allows strategic and operational diagnoses to be more assertive and allows perception of how the system is evolving. At each new cycle, the system should be more robust, undergoing failure review, correcting non-conformities, and making processes interdependent. At each new cycle, the culture matures and makes processes more long-lasting and sustainable.
Possible contributions and gaps in the development of studies about integrated management systems were identified, among them, the need to investigate the impact of certified management systems on sustainable development, as well as the need to develop system integration proposals for the sustainability of corporations to optimize results related to sustainable development. [
1]
Integrating management systems such as quality, environment, health, and occupational safety contributes to Sustainability[
2]. However, to assess the evolution levels of a management system, it is necessary to adopt a maturity model. Maturity models allow for the analysis of incomplete evolution stages (in general, by organizations or processes) using multidimensional criteria [
3].
Another important point regards higher education institutions. Higher Education Institutions (HEIs) are strategic for sustainable development in the dimensions of teaching, research, dissemination, and management. They are also responsible for training professionals who are aware of their role in sustainability and for providing them with the aptitude and competencies necessary for future challenges in this area [
4].
European universities are moving forward in the implementation of Agenda 2030 [
4], and a series of initiatives can be adopted by the HEI to implement sustainability actions like implementing Sustainable Development Goals (SDGs) systematically, starting actions aligned to institutional documents and the strategic mission; integrate the SGDs on the curriculum and in learning; using training to communicate the university community about the topic.
In this context, the adoption of integrated management practices in laboratories aligned with the HEI´s strategic mission and seeking to expand sustainability gradually contributes to the integration of SDGs in the teaching-learning process, and it promotes the awareness of technicians, professors, students, and of other members in the university community.
In view of the above, the following question was formulated: how can a Maturity Model assist chemical analyses laboratories in reaching higher sustainability levels and, with this, add to the Higher Education Institutions (HEI) sustainability policies and actions?
In order to answer the research questions, the following propositions were formulated:
P1: Structuring a Maturity Model based on standardized Management Systems is relevant to managers and positively affects reaching sustainability at the laboratory level.
P2: HEI´s chemical analyses laboratory managers are interested in adopting standardized management systems to organize and improve their processes.
P3: Chemical analyses laboratories at universities and HEI do not have structured management systems; they have isolated tools and/or methodologies to coordinate and control their operations and routines.
P4: It is possible to structure a Maturity Model suited to a chemical analyses laboratory context to achieve sustainable activities.
As main results, it was observed that more than 80% of public and private laboratory managers believe that a Maturity Model would help in organizing internal processes. 86% of public laboratory managers understand that the use of management systems optimized laboratory processes and can contribute to hiring new services, which reveals how relevant this is.
It was also possible to elaborate a maturity model to assess and gradually build an integrated management system for the sustainable development of chemical analyses laboratories.
This paper is divided into four sections.
Section 1 contextualizes and clarifies the intended goals.
Section 2 deals with the methodology used to accomplish this survey.
Section 3 presents the results achieved and the discussions on the data collected to build the model.
Section 4 presents conclusions observed based on the research propositions.
2. Materials and Methods
This is a descriptive exploratory survey in a combined approach (qualitative and quantitative). As to the methods, they were bibliography-based research, field research (survey), and case studies [
5,
6,
7].
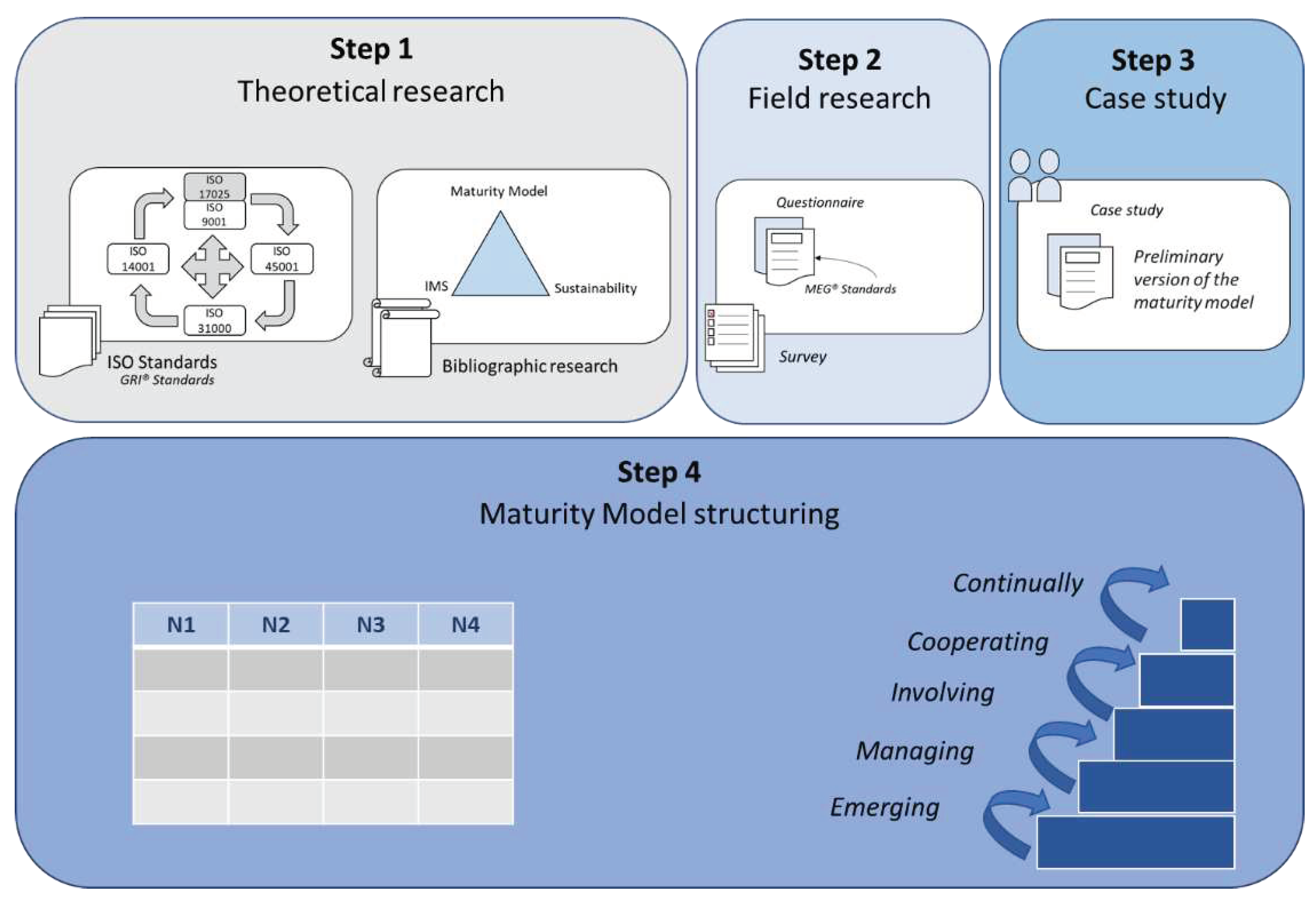
The methodology was divided into four steps: Theoretical research, field research, case study, and Maturity Model structuring (
Figure 1).
In theoretical research, five stages of searches were done to unify three different yet interrelated topics in one single study: Maturity model, sustainability, and integrated management systems applied to the laboratory management setting.
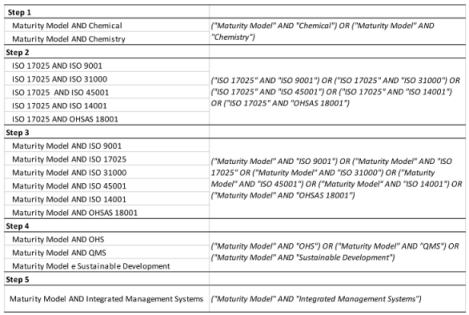
In the first step, we researched papers on Maturity Models associated with Integrated Management Systems. Certifiable Quality standards (ISO 9001), Occupational Health and Safety (ISO 45001 or OHSAS 18001), and Environment (ISO 14001) were used as search parameters, as well as Maturity Models developed and applied to the reality of these systems. The bibliographical research also considered concepts of Sustainability and Risk Management (ISO 31000 standard) associated with constructing these models. The term “ISO 17025” was used as a search parameter for laboratory management, referring to the standard used in the certification of testing laboratories to ensure the quality of operations.
Three scientific databases were used in the research: Emerald Insight, Science Direct, and Scopus. The first survey was carried out between December and January 2019, repeated between December and January 2021, and again in April 2023. Articles were searched between 1995 and 2023 for the words on the
strings anywhere in the article (
Table 1).
In the second stage, field research (Survey) was carried out based on collecting institutional data from the laboratories, their management practices, and the perception of relevance for adopting management systems. Data was collected using a semi-structured questionnaire with open-ended and closed questions, presented in
Appendix A.
The questionnaire was prepared using the Google Forms® platform, in which an explanatory email with an access link was sent to the respective managers. At the end of the questionnaire response period, the collected data were analyzed using statistical software R (version 4.2.2).
To define the target population, we chose laboratories that are in higher education institutions, that do chemical analyses, work with research and development, and provide external services to society (public laboratories) and laboratories that are private legal personalities, certified to the ABNT NBR ISO/IEC 17025 standard, both focusing on chemical analyses aimed at environmental assessments and to the oil, gas, and derivatives industry.
217 laboratories belonging to 147 public higher education institutions were surveyed, based on the Ordinance No. 378, of May 9, 2016, which establishes the list of units that make up the Federal Network of Professional, Scientific, and Technological Education.
545 private laboratories were also selected, using the register of the Brazilian Network of Testing Laboratories (RBLE) of the National Institute of Metrology (INMETRO). To select private laboratories, the following parameters were used: (1) Type of accreditation: “CRL (ABNT NBR ISO/IEC 17025 – TEST LABORATORY)”; Test class: (2) “CHEMICAL TESTS”; (3) Areas of activity: “ENVIRONMENT” and “OIL AND DERIVATIVES, NATURAL GAS, ALCOHOL AND FUEL IN GENERAL.”
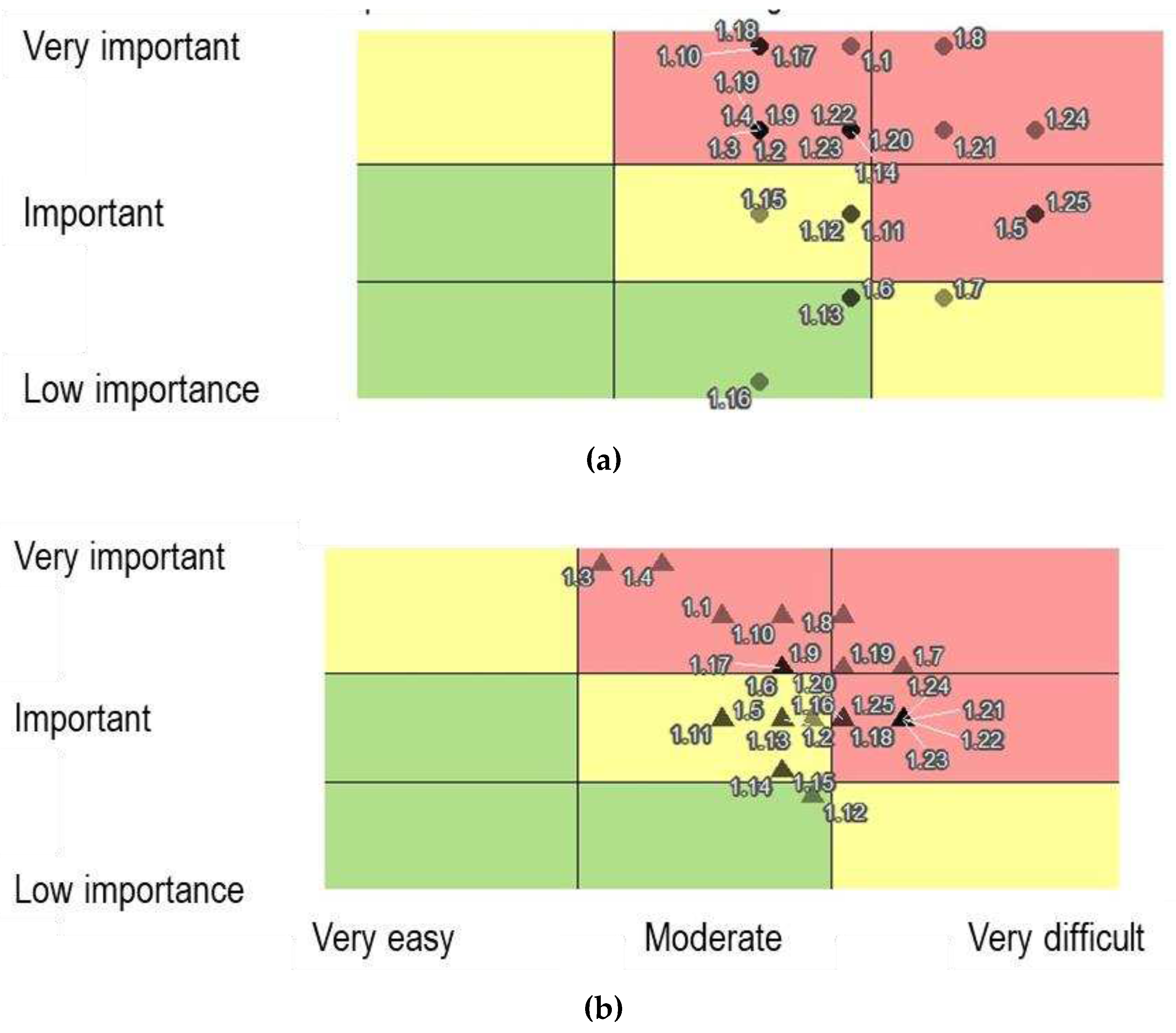
In the third stage, a case study was carried out through a Focus Group to evaluate and validate the management practices formulated for a preliminary maturity model constructed from bibliographical research and questionnaire responses. The focus group included the participation of managers and technicians from a chemical analyses laboratory belonging to a public Higher Education Institution. Participants were asked to assess (1) the levels at which management practices were found in the Maturity Model and (2) the level of importance versus implementation difficulty level.
To evaluate the model, two scales were used:
Level of importance: 1-LOW IMPORTANCE, 2-IMPORTANT, 3-VERY IMPORTANT.
Implementation difficulty level: 1-VERY EASY, 2-EASY, 3-MODERATE, 4-DIFFICULT, 5-VERY DIFFICULT.
The Focus Group's perceptions, suggestions, and changes were analyzed, and they helped adapt the final version of the model.
The fourth and final stage of the work was the development of the maturity model. Based on the guide for developing maturity grids proposed by [
8], the foundations of the maturity model were developed for laboratory application.
Based on the articles researched in the literature review, the answers provided by managers through the questionnaire and the validation of the practices of the preliminary model by the focus group, it was possible to develop a maturity model for the gradual implementation of management systems, considering requirements related to technical skills for calibration and testing laboratories (ISO/IEC 17025), Quality Management (ISO 9001), Occupational Health and Safety Management (ISO 45001), Environmental Management (ISO 14001) and Risk Management (ISO 31000 standard).
The model’s target audience was Managers, Coordinators, and Technical Managers of testing laboratories (chemical analyses) located in Public Higher Education Institutions.
As a framework, its objective was to inform laboratories of which steps can be taken to achieve excellence in Management and Sustainability.
3. Results and Discussion
3.1. Bibliographic Research
Bibliographic research clarified that Higher Education Institutions (HEIs) are essential in disseminating information and in training professionals who will be attentive to sustainability issues. The role of HEIs is preponderant in sustainable development, as it promotes the development of actions to meet SDGs within the institution, raising awareness among professors, students, employees, and other interested parties and contributing to societal changes [
9].
Since the establishment of the SDGs, many universities around the world have adhered to the topic, establishing policies and implementing actions that promote the sustainable development of campus activities [
4,
10]. To do this, they transform their missions, restructure their curricula, modify research programs, promote community engagement, and report their activities to stakeholders [
10].
Linking the Institutional Development Plans (PDI) of Brazilian universities with the SDGs of the 2030 Agenda is a crucial step to be achieved.
Serafini et al. (2022) [
11] point out the following barriers to the implementation of SDGs in universities: the lack of documentation with standardized processes, the lack of training related to the SDGs for the academic community, difficulty in incorporating the SDGs into the institutional systems of HEIs; and cultural resistance to change.
Laboratories that have implemented management systems have their processes standardized and documented; this facilitates incorporation and alignment with the Sustainable Development Goals (SDGs) since it foresees the environmental aspects and impacts caused by their activities in their operations. Furthermore, they help implement the desired culture by minimizing resistance to imposed actions, as their technicians are constantly trained and are conscious of their role.
Teaching and research laboratories play a fundamental role in a country's economic and social development. Scientific and technological advancement and development significantly stem from research and experiments that have been tested, verified, and validated on laboratory benches.
CONMETRO highlights the importance of chemical measurements to decision-making regarding product quality. Once the country is projecting itself as a protagonist in the world trade stage in the food, energy, and environment industries, it points to the need to immediately increase the reliability of the results of the chemical measurements done in Brazil.
The structural adaptation of laboratories inserted in HEIs to meet emerging service demands involves improving the technical skills of their members, which must include aspects related to the quality of operational and management processes, procedures for the safety and health of technicians, students, teachers, and other users; and environmental prevention practices.
Many companies and organizations have implemented management systems emphasizing quality, environmental, and occupational health and safety management to deal with contemporary pressures and complexities [
1,
13]. As a way to achieve the sustainability of their operations, many have opted for standards such as ISO 9001 (Quality), ISO 14001 (environment), and OHSAS 18001 (occupational health and safety) [
13,
14].
A survey carried out with certified Brazilian companies showed that those who achieved better sustainability performances were those who invested in improving the integration of their systems [
2].
Nadae et al. (2020) [
13] analyzed the impact of integrated management systems on sustainability in four Brazilian companies, and concluded that investment in management systems improved the performance of their economic, social and environmental aspects (triple bottom line), despite sustainability not having been the primary motivation for implementing IMS.
Concerning implementing management systems in laboratories, it was possible to verify discussions on the quality of management and operations.
The articles dealt with the benefits of adopting the ISO 9001 and ISO 17025 standards [
12,
15], critical analysis [
17,
18], and even describing implementation steps [
17,
18,
19,
20,
21]. Only 1 (one) article addressed the impact of normative standards [
22,
23,
24,
25,
26] on chemical analyses activities linked to science and technology [
27].
No articles were identified within the laboratories that addressed the use of occupational health and safety standards (ISO 45001) or risk management standards (ISO 31000) independently or integrated with the others.
The popularization of the use of management standards brings with it the need to assess maturity in several areas [
2]. Integration can occur at different levels, and maturity models can help organizations know where and how far they are from achieving best practices.
Domingues (2013) [
28] proposed a maturity model to compare integrated management systems at different levels, evaluating the maturity level of the systems and directing companies to higher maturity levels.
The research was designed using medium-sized companies located in a part of the Portuguese territory as a reference, limiting the sample to aspects related to geographic location, the IMS typology standard, and sectors of activity. The testing or calibration laboratories were not objects of the study.
Furthermore, the model used ISO standards as a conceptual basis before the current versions (2015) and before Annex SL and the High-Level Structure were published. The model also uses the OHSAS 18001 standard as the basis of the Occupational Health and Safety management system, not the ISO 45001:2018 standard. Finally, the model does not include aspects related to the operation of laboratories in its scope [
23].
Regarding the use of Maturity Models applied to laboratories, [
29] proposes a model that aims to evaluate competence, impartiality, and operational consistency for testing and calibration laboratories via self-assessment based on the requirements of Standard ABNT NBR ISO/IEC 17025:2017, employing decision support methods.
The model uses the requirements in the ABNT NBR ISO/IEC 17025:2017 standard and the ABNT NBR ISO 9004:2010 standard to establish the criteria and assessment levels of the maturity model.
Despite its high relevance as an assessment tool, the proposed model only addresses the assessment of the requirements of the ABNT NBR ISO/IEC 17025:2017 standard, and the evaluation of occupational safety and health or environmental aspects is not part of its scope. These are essential aspects of building sustainable institutions.
Gerônimo (2023) [
30] carried out a Systematic Bibliographic Review (RBS) in which he sought to identify maturity models based on Fuzzy logic to assess the degree of maturity of laboratories that had implemented the ISO 17025 standard.
Gerônimo (2023) [
30] proposed a descriptive maturity model to evaluate integrated management systems based on standards ABNT NBR ISO/IEC 17025:2017, ABNT NBR ISO 14001:2015 and ABNT NBR ISO 45001:2018 for testing and calibration laboratories. It proposes using multi-criteria decision support methods and Fuzzy Logic for evaluation. The model, however, is only validated for the requirements of the ABNT NBR ISO/IEC 17025:2017 standard. The author emphasizes that the work was limited to developing a descriptive maturity model without taking improvement actions that could take the laboratory to a higher maturity level.
In this way, the proposed model is limited to providing a situational diagnosis of the IMS's maturity level, and the proposal of effective integration actions that may improve the IMS´s maturity level is not part of its scope.
In summary, the articles found did not demonstrate the existence of a maturity model that would assist chemical analyses laboratories in the gradual implementation of integrated management systems to meet and maintain sustainable levels in their operations. In the development of the theoretical framework, one can observe the role of laboratories in the training and qualification of future professionals and the need to build mechanisms that can handle the demands of quality, risks, safety, and the environment acceptably existing in their activities.
One can also observe that integrated management systems present themselves as a possible solution for this development since they can deal with the complexity of existing variables and because there is a relationship between increased integration and improved sustainability performance. Maturity Models offer the possibility of measuring and following up with the gradual evolution of these systems.
3.2. Field Research
For data collection 6 (six) rounds of emails were sent: the first one between November and December 2022 and the remaining ones between January and March of 2023. Participation in the research was voluntary (by adhesion), ensuring the confidentiality of participants.
701 emails were sent. 42 managers responded to the questionnaire, representing 6.0% of the emails sent. The results described and the observations, analyses, and discussions presented were based only on the laboratories that participated in the survey.
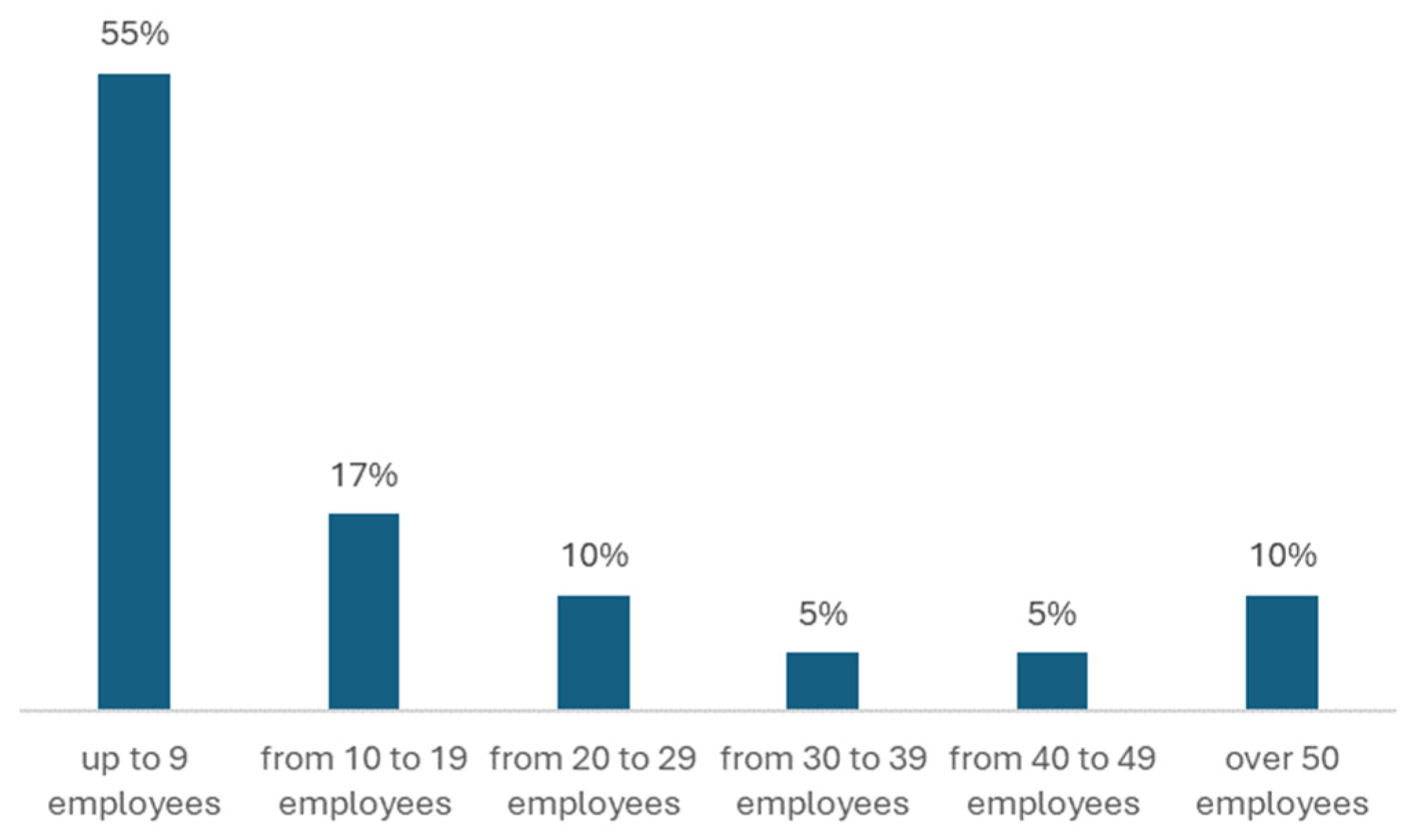
The research showed that the laboratories surveyed have lean structures, mainly operating with up to 20 employees (
Figure 2).
The number of laboratory employees may be considered an impediment to adopting one or more normative systems. Observe [
18] that among the benefits of adopting standard ISO 17025, there is an increase in the number of clients and the resulting increase in the laboratory´s workload, which reflects customer´s demands for accredited calibrations. This could represent a need to expand the workforce, depending on an analysis of the return on the investment.
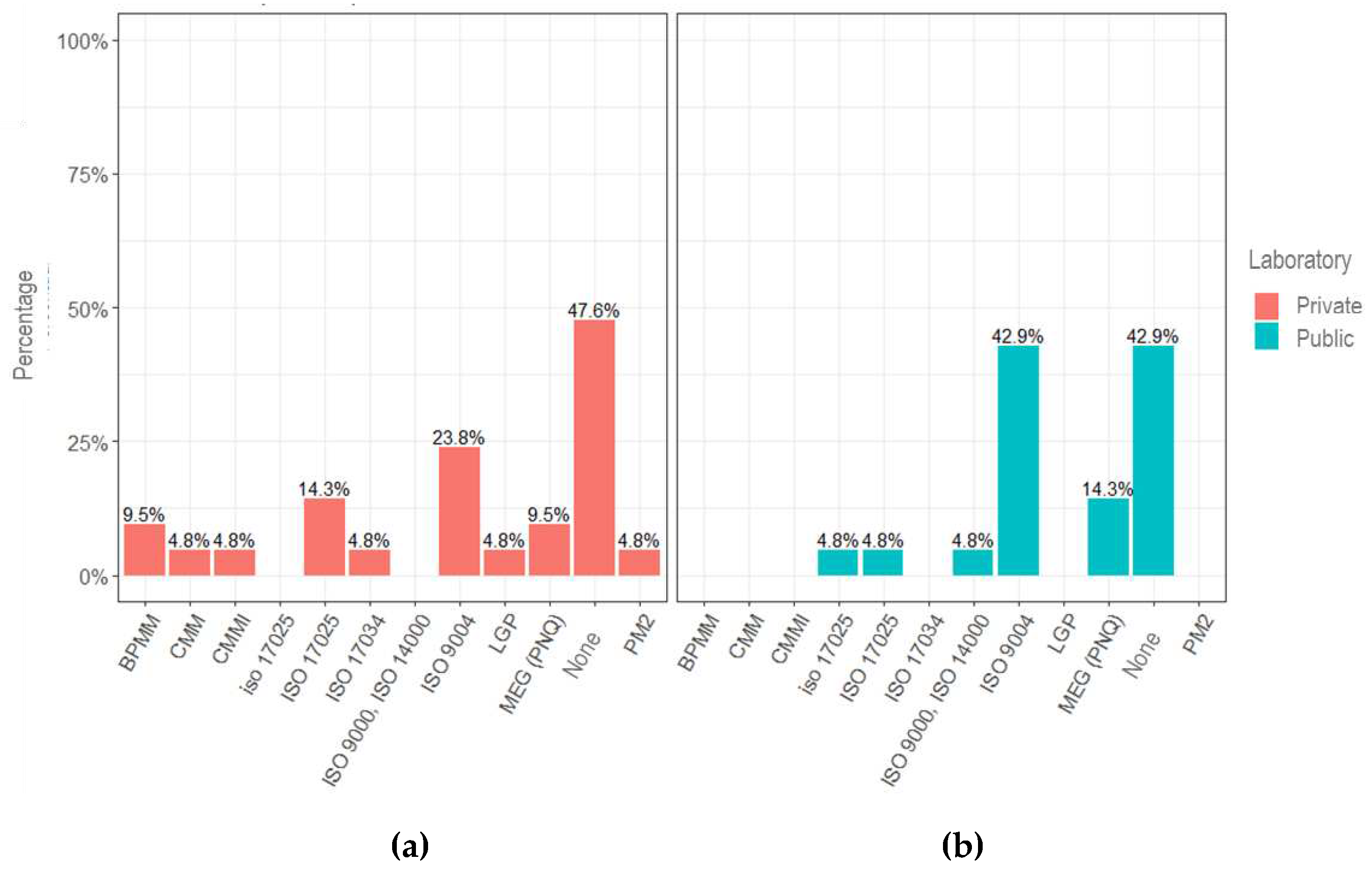
It can also be observed that almost half of the laboratory managers (47.6% of private laboratories and 42.9% of public laboratories) are not aware of the existing maturity models (
Figure 3).
The most cited model is the ABNT NBR ISO 9004 standard – Quality management – Quality of an Organization – Guidance to achieve sustained success.
The ISO 17025 standards regarding laboratory accreditation and ISO 17034 are worth mentioning, which define the requirements for producers of reference material when asked about knowledge of a maturity model applied to the laboratory setting. Both standards provide high standardization of operations and documents but do not establish gradual stages of evolution, allowing the laboratory to see the level of maturity at which it finds itself. The answer may demonstrate a lack of knowledge of the conceptual bases of a maturity model.
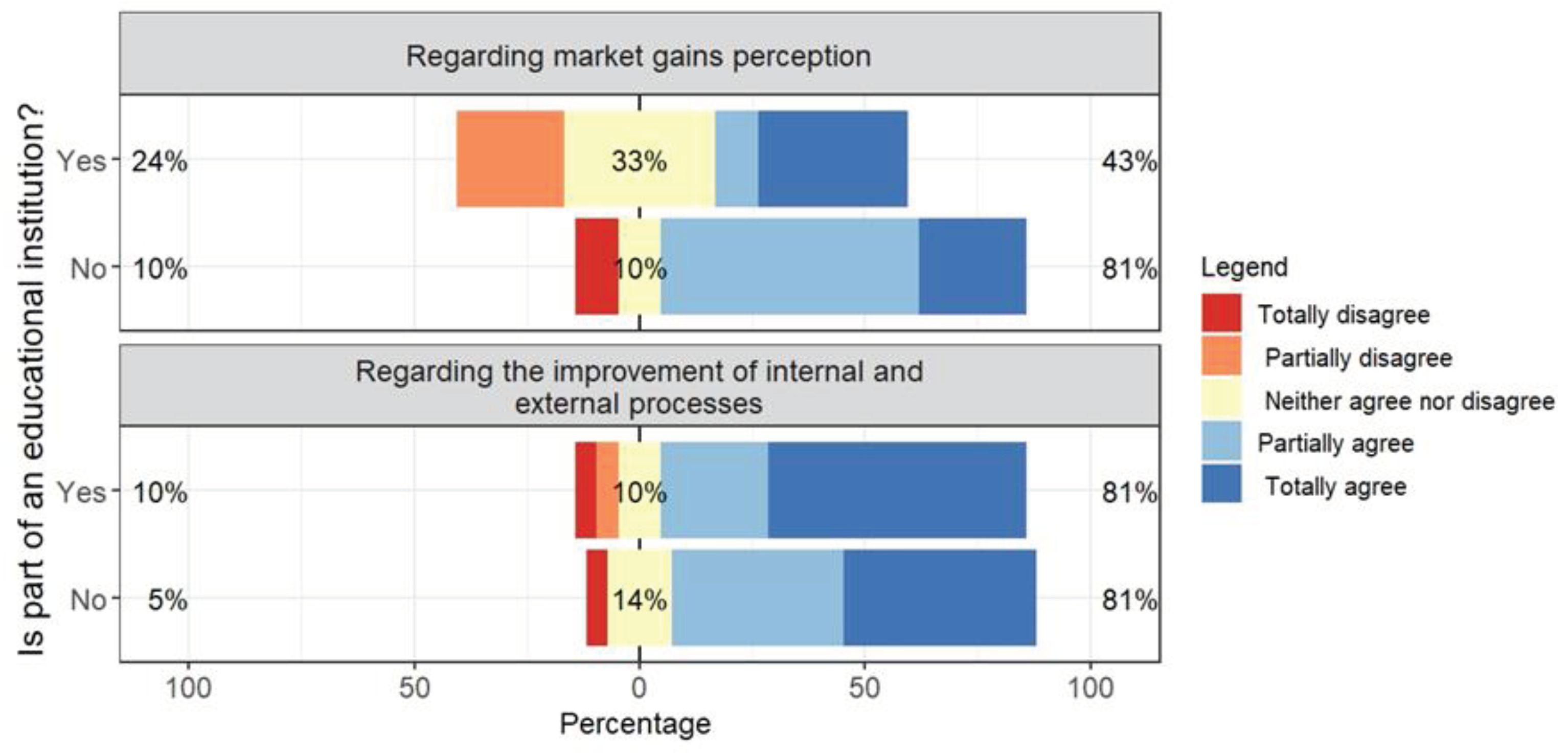
Regarding the relevance of a maturity model, 81% of private and public laboratory managers agree that a model would help improve internal and external processes (
Figure 4).
This vision is consistent with the context in which public managers find themselves, one in which the increasingly growing demand for the provision of quality public services has been the object of constant improvements in the internal processes of universities and continuous training of employees, being an element guiding universities´ Institutional Development Plans (PDI) [
31].
Regarding market gain perception, a much greater variability of opinions was observed for public managers (24% partially disagree, and 33% see it as something indifferent to their reality).
It is essential to highlight, however, that the understanding of market gains for laboratories within universities can translate into a higher success rate in the submission of projects tendered by private entities or even a demand for paid services via foundations, both arising from an acknowledgment of the technical competence of the laboratory.
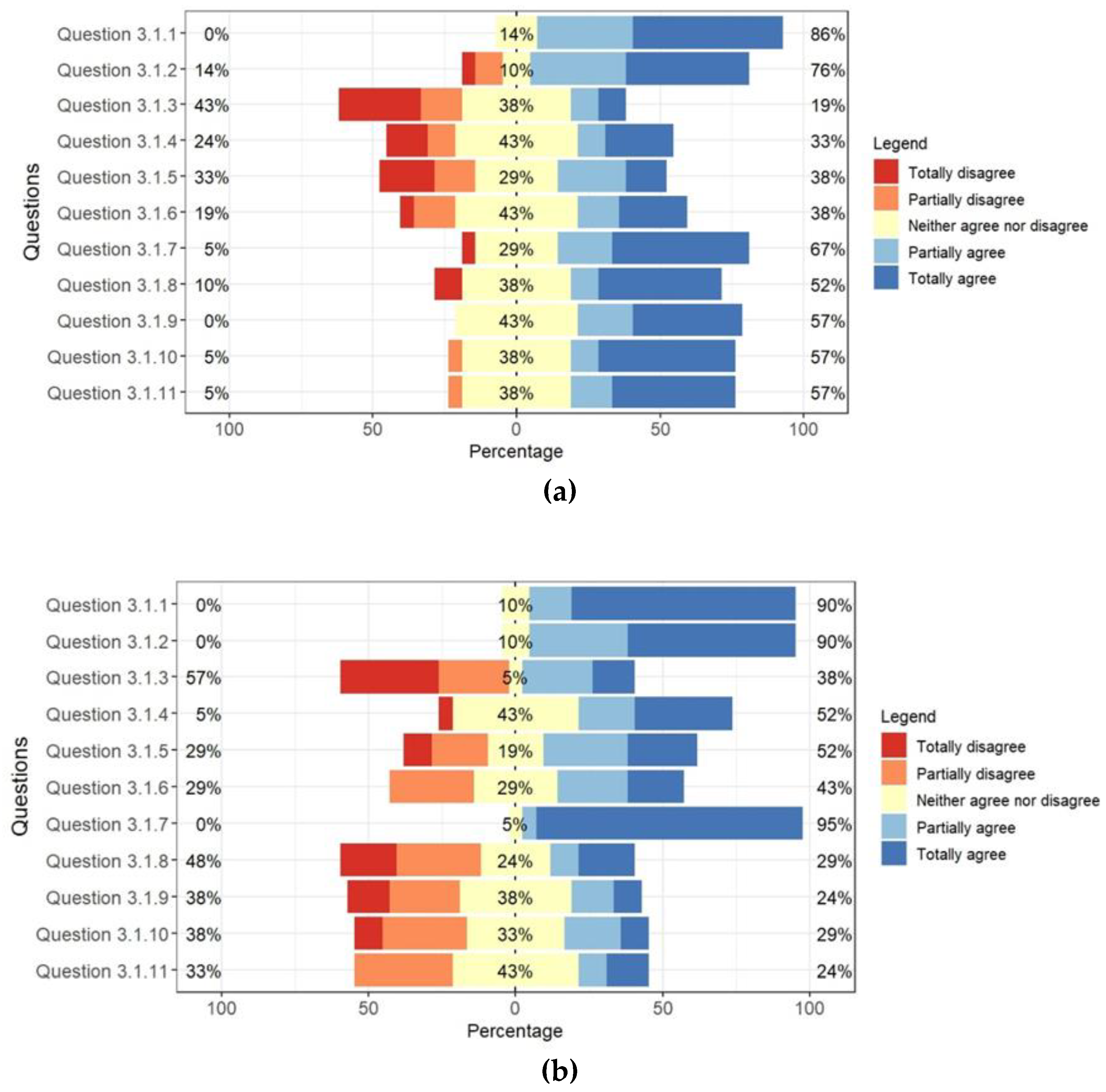
When evaluating the adoption of integrated management systems, research revealed that public managers, for the most part, understand that they optimize laboratory processes (question 3.1.1); they can contribute to the hiring of new services (question 3.1.2), do not negatively impact the time and way in which tasks are performed (question 3.1.3); and understand that accreditation is relevant to improving the quality of operations (question 3.1.7).
Figure 5 presents the commented results.
The responses pointed to greater acceptance of the use of certified management systems by public managers when compared to private laboratory managers.
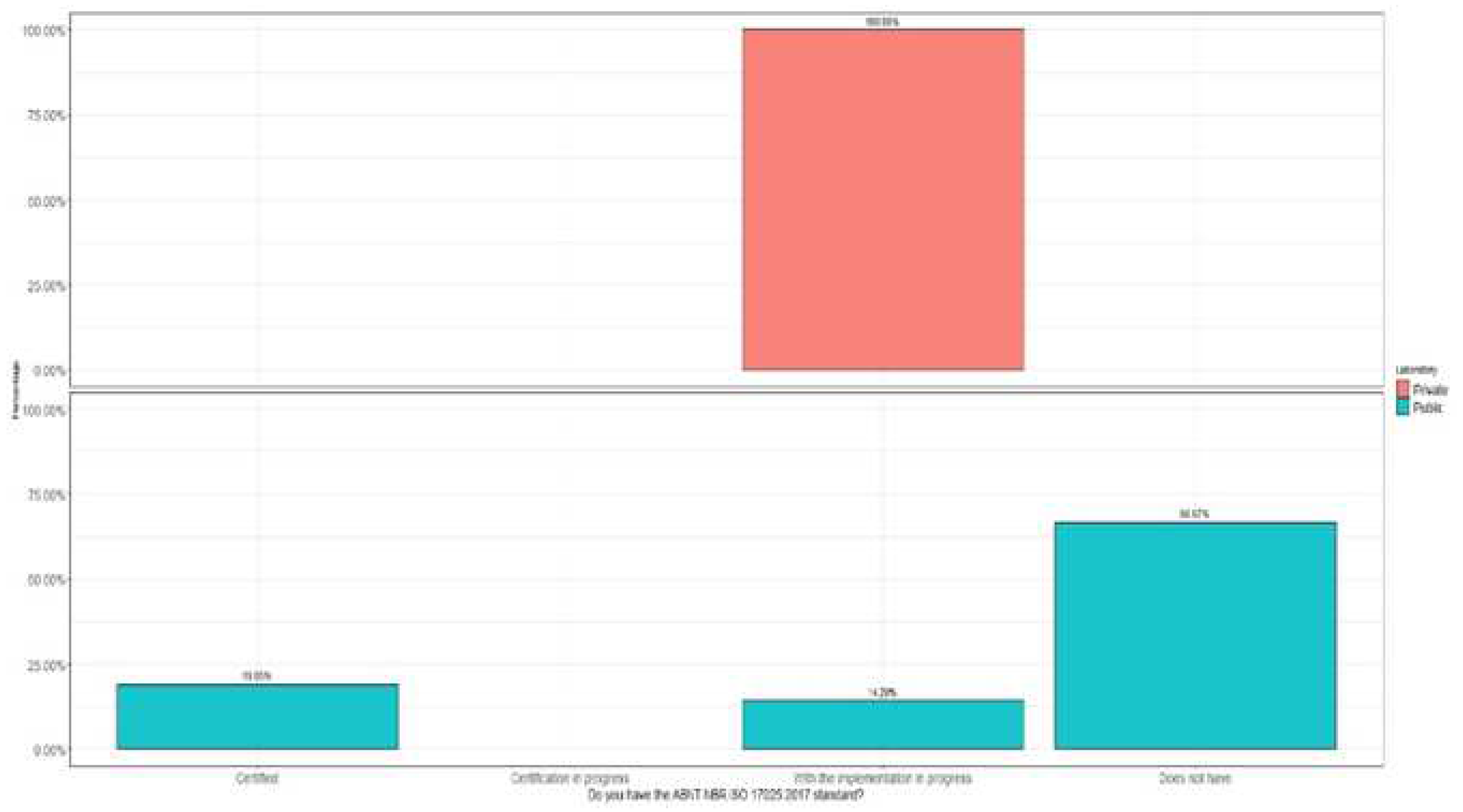
The research indicated that 67% of the laboratories surveyed, belonging to public educational institutions, are not yet accredited in the ISO 17025 standard (
Figure 6).
Choosing accreditation involves carefully assessing internal and external processes and, equally, an economic feasibility study that guarantees the sustainability of operations. The numbers presented, however, highlight the potential to be explored by public higher education institutions.
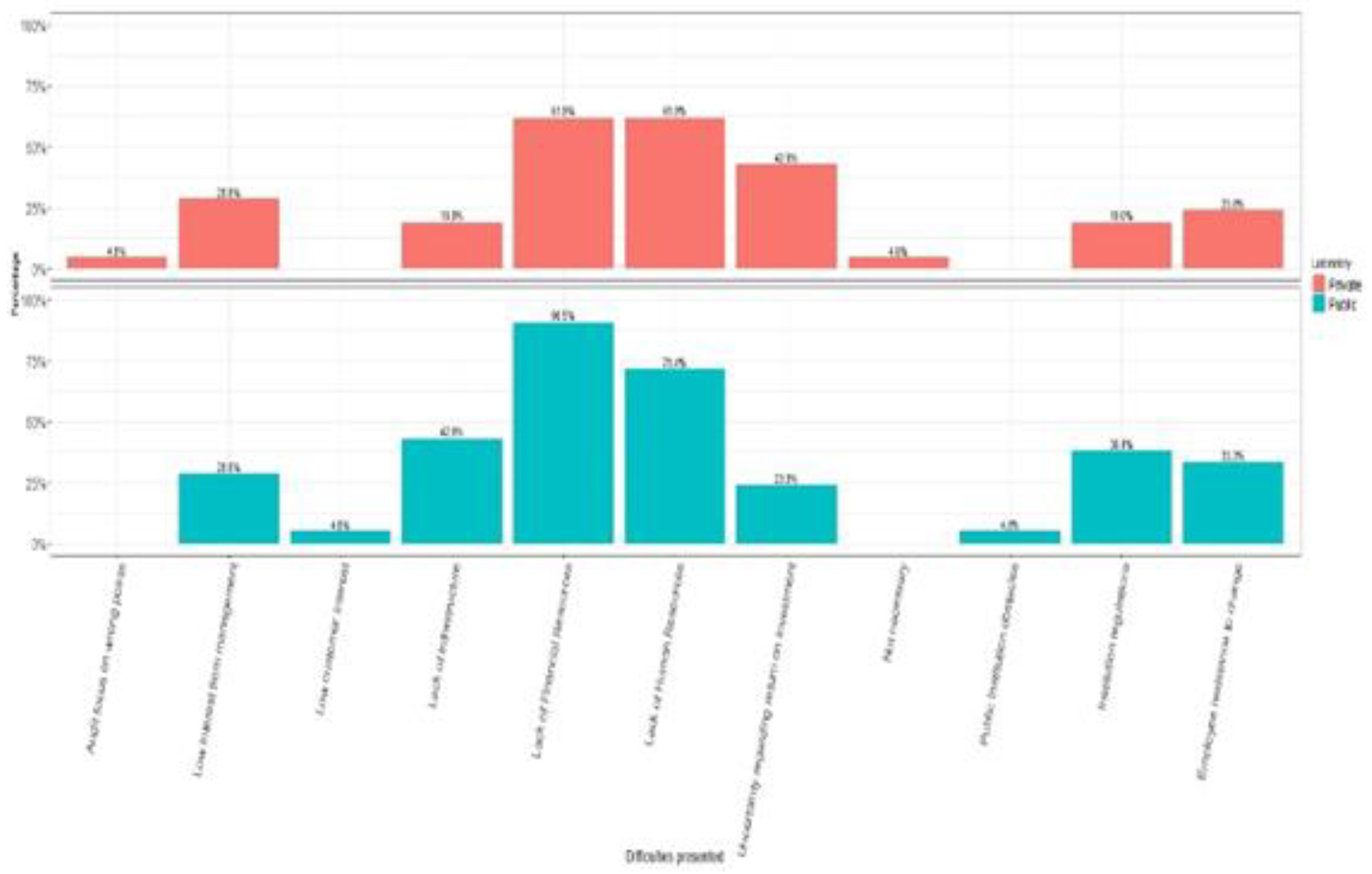
Among the difficulties reported by public managers in implementing the standards, there is a lack of human resources (90.5%), followed by a lack of financial resources (71.4%), and thirdly, a lack of infrastructure (42.9%) (
Figure 7).
Another verified aspect was the use of practices and documents necessary to manage the system well, per the requirements of ISO standards.
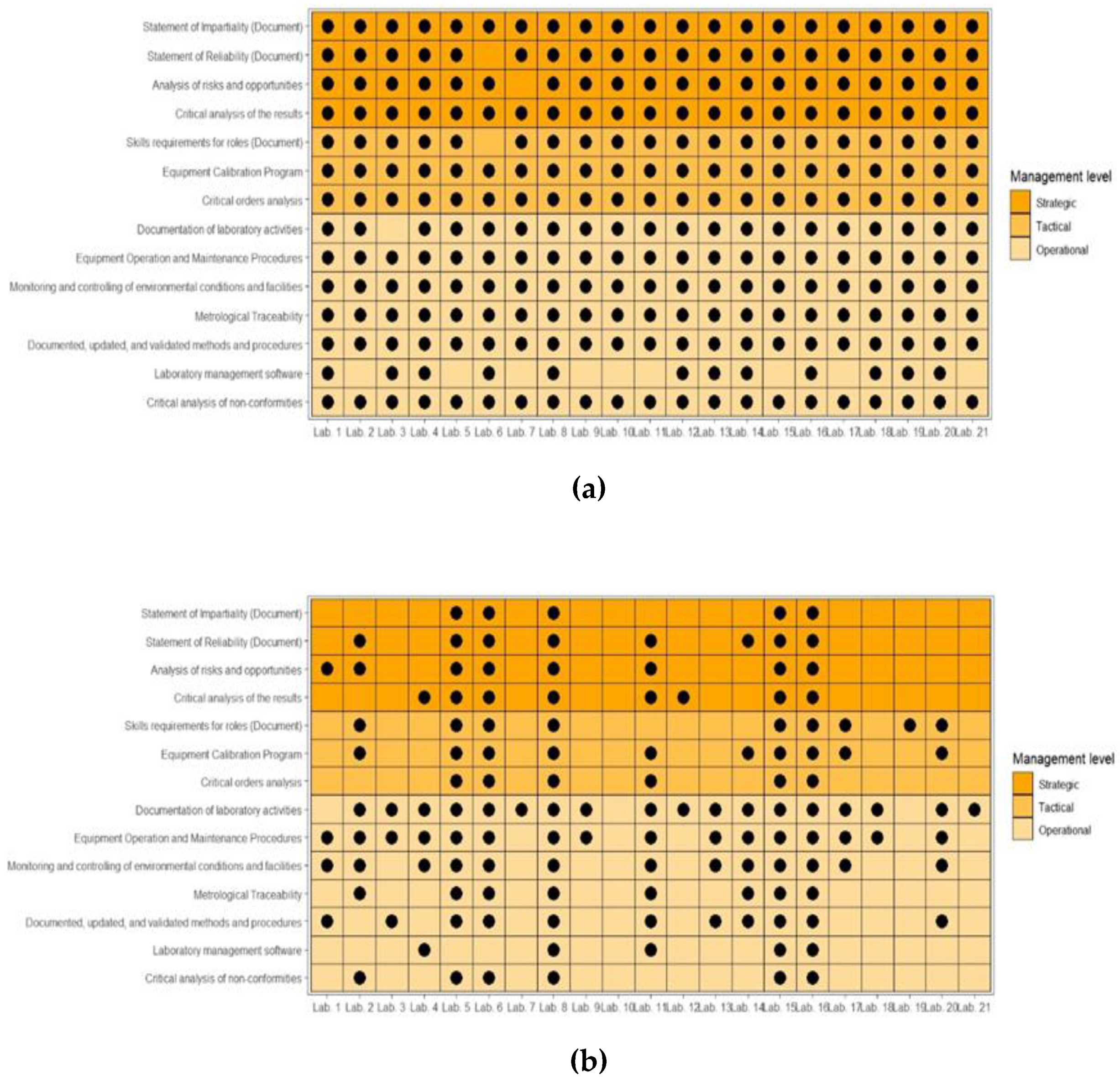
Figure 8 presents the survey carried out for the ISO/IEC 17025 standard for public and private laboratories.
Classified according to a hierarchical perspective of strategic, tactical, and operational management, it is clear that private laboratories have a set of practices and a much more homogeneous documentary structure than public laboratories. Specifically, concerning public laboratories, there is a predominance of more operational management practices and a smaller amount of tactical or strategic practices. Similar situations were observed in the analysis of the other ISO standards that were the subject of this study.
Notably, using these documents allows planning guided by clear objectives and goals, supported by methods and tools that will enable gradual monitoring of activities, with a view to continuous improvement of services. In their structure, they have logically interrelated elements, connecting management mechanisms and providing a feedback cycle, contributing to the sustainability of the laboratory.
3.3. Case study (Focus Group)
The case study revealed the most challenging management practices to implement (in the focus group's view) and the need for more than one level in the maturity model (Level 0) to adapt a laboratory's activities before starting practices leading to accreditation.
Table 2 presents the validation of practices for the “Strategy” dimension. For each management practice, suggested levels of preparation and implementation of the preliminary maturity model were presented. The validated level represents the focus group's view of the positioning of practices within the model.
The focus group also revealed different perceptions between the management and technical teams regarding implementation difficulties and the importance of some practices.
Figure 9 presents the analysis carried out for the “Strategy” dimension.
Among the implementation difficulties pointed out by the focus group in laboratories belonging to HEIs, the following are mentioned:
Fellows´ length of stay in projects: the short length of stay and high turnover affect service performance, as there is a need for recurrent training of new members;
Multipurpose laboratories (teaching, research, and services): in multipurpose laboratories, there is difficulty in implementing access controls necessary to comply with the ISO 17025 standard;
Infrastructure adequacy: Some laboratories within universities need structural adjustments that make it challenging to establish material transport flows, another requirement of standard ISO 17025.
3.4. Maturity Model
Based on the results of the theoretical research and considering the discussions carried out in the field research and the focus group, some assumptions can be established for elaborating the Maturity Model for application in laboratories belonging to public higher education institutions (
Table 3).
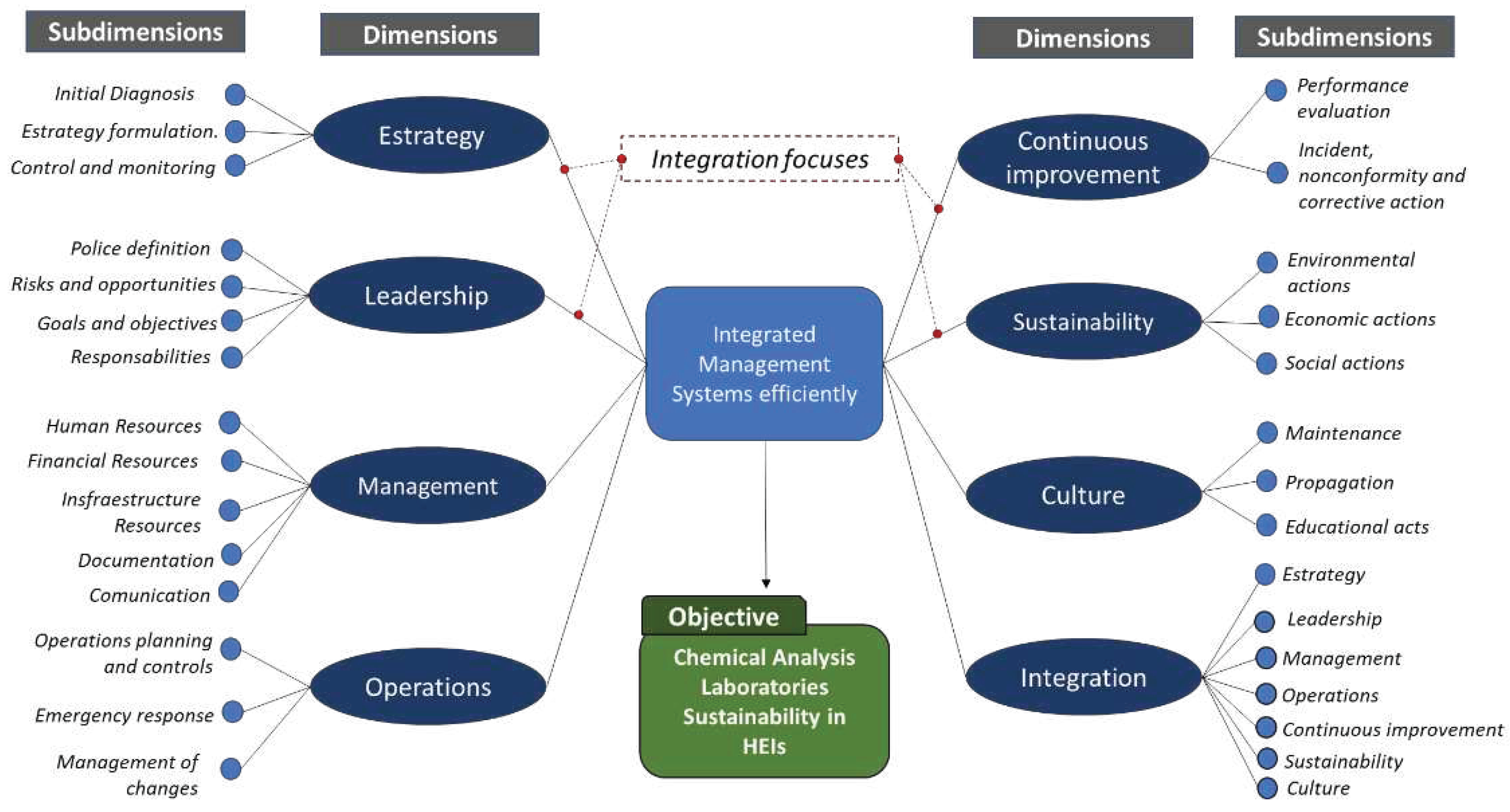
The construction of the Dimensions and Sub-dimensions of the proposed model was carried out through a critical analysis of the process areas used in the maturity models and the integration models researched in the literature review.
The model was built with eight dimensions and 41 sub-dimensions, with an emphasis on systems integration to achieve Sustainability of laboratory operations and based on the following success criteria: Performance
Strategy Clarity,
Leadership Commitment;
Management Excellence,
Operations Reliability;
Continuous Improvement Systems
Integration to promote
Sustainability; Strengthening the
Culture in IMS (
Figure 10).
To build the model's management practices, we observed the requirements of ABNT NBR ISO/IEC 17025:2017 standards; ABNT NBR ISO 9001:2015; ABNT NBR ISO 14001:2015; ABNT NBR ISO 45001:2017; ABNT NBR ISO 31000:2018, in addition to the GRI (Global Report Initiative) standards, all internationally acknowledged as a set of good practices in their respective fields of work.
These practices were classified into the eight dimensions of the conceptual model based on a critical analysis of normative requirements (ISO), grouping them into related categories [
1]. Subdimensions were systematized according to the aggregation of these practices [
32] (p. 128).
Table 4 presents management practices referring to the “Strategy” dimension and the level at which this practice must be fully met. The level of preparation refers to the stage at which the laboratory starts the necessary adjustments for subsequent compliance with management practice.
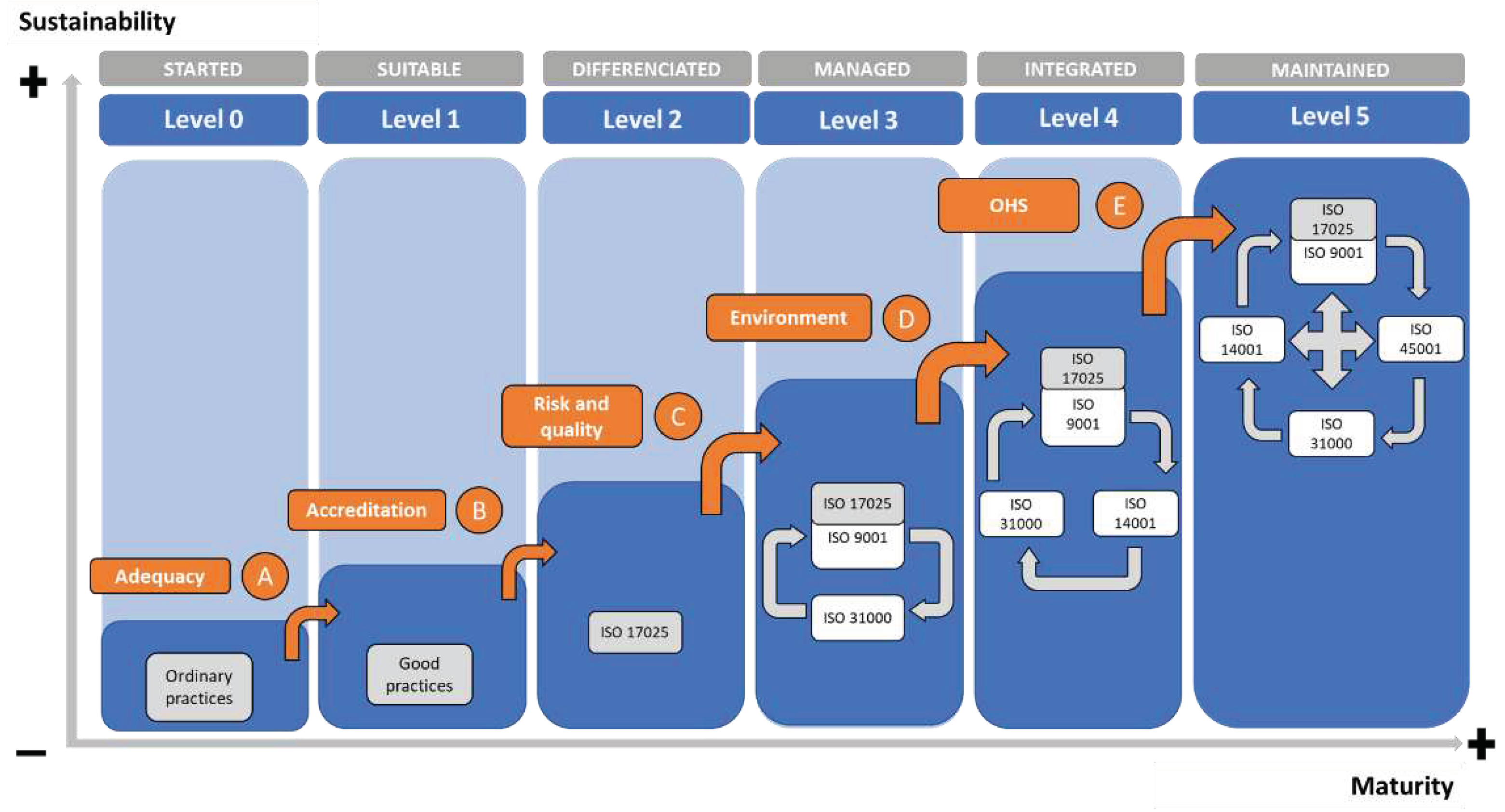
The model has 6 (six) maturity levels. At each level, an objective must be achieved, and a set of management practices must be followed so that the laboratory can evolve progressively (
Figure 11).
Table 5 presents the characteristics expected to be observed in laboratories for the “Strategy” dimension when they reach the desired maturity level.
4. Conclusions
The maturity model (framework) aims to help chemical analyses laboratories located in public higher education institutions achieve higher levels of excellence in management and sustainability.
To achieve this objective, concepts and structures from existing maturity models were linked to Principles, Guidelines, and requirements of standardized standards since they constitute a guide to best management practices. In addition, field research and a case study were carried out to understand whether it was relevant to the target audience.
As reported in the research, maturity models can help laboratories achieve higher levels of excellence by classifying the characteristics that exist at each maturity level and explaining the actions necessary to reach the highest levels.
Based on the propositions presented, it was observed that:
P1: The relevance of a management systems-based model was demonstrated in the theoretical framework and the field research carried out. More than 80% said they believed that a maturity model would help the organization of internal processes, with divergences only regarding market gains.
P2: The interest of managers can be observed in that 86% of public laboratory managers understand that using management systems optimizes laboratory processes and can contribute to hiring new services (
Figure 5).
P3: Chemical analyses laboratories in universities and HEIs do not have structured management systems, relying solely on isolated tools and/or methodologies to coordinate and control their operations and manage routines. This proposition was confirmed when it was verified that the methods and tools were present in laboratory activities without structuring elements that would configure a management system (Policy, Objectives, Goals, Manual, and other documents) (
Figure 8).
P4: It was possible to structure a Maturity Model suited to the context of chemical analyses laboratories to achieve sustainable activities. This proposition was confirmed by structuring the maturity model presented in
Figure 10 and
Figure 11.
The topic is deemed relevant for laboratory managers. It has an immense field to be explored, as many research participants say they do not have fully implemented management systems, observing only isolated tools and methods.
When analyzing the public laboratories researched based on the maturity model developed, it can be observed that the majority of laboratories would be found at Levels 0 or 1 (67% are not accredited in the ISO 17025 standard and do not have a complete documentary structure, which is necessary for sound systems management). The difficulties reported by public managers in implementing the standards to a certain extent point to the reasons for the low level of maturity: lack of human resources (90.5%), financial resources (71.4%), and infrastructure (42.9%).
Comparatively, private laboratories surveyed could be classified in Levels 2 or 3, depending on their compliance with the quality management and risk management criteria present in the IMS (only 14.3% of private laboratories stated that they had ISO 9001 certification or were in the implementation process, and 4.7% for risk management).
It is therefore noted that the Maturity Model could help laboratories achieve higher levels of sustainability, as it indicates the necessary actions to be taken to gradually evolve their operations and management practices.
By conducting a process of gradual implementation of integrated management systems, the model also collaborates with the implementation of sustainability policies and actions within Higher Education Institutions (HEIs), as laboratories with implemented management systems have their processes standardized and documented, which facilitates incorporation and alignment with the Sustainable Development Goals (SDGs), present in the Institutional Development Plans (PDI) of universities. Furthermore, they help to implement the desired culture by minimizing resistance to imposed actions, as their technicians are constantly trained and made aware of quality, safety, and environmental aspects. Based on what was developed, the following are recommended for future research:
Develop a measurement scale for the maturity model based on methods such as Multi-Criteria Decision;
Validate the proposed maturity model based on the evaluation of educational institutions’ laboratories (Benchmarking);
Test and expand the model for laboratories with different characteristics from the research scope.
Evaluate the contribution of the proposed maturity model to the success of project submission (scientific production);
Evaluate the costs of implementing one or more management systems based on the Return on Investment (ROI).
In this way, it is expected that the model will contribute to the improvement of laboratory management, sustainable development of their activities, and quality of services provided to the community by public higher education institutions in the country.
Author Contributions
All the authors contributed to writing and editing the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank the Rio Grande do Norte Federal University (UFRN), Brazil, Department of Petroleum Engineering, Postgraduate Program in Science and Petroleum Engineering (PPGCEP).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Questionnaire Sent to Laboratory Managers
Section 1 – Institutional Data
1.1. Respondent position/role: ______________________________________________
1.2. Year the laboratory was founded: _______________1.3. Number of employees:
1.4. State: __________
1.5. Educational institution: ▯ No ▯ Yes Name: _________________
Section 2 – Maturity Models Perception| A maturity model can be understood as a tool that helps companies understand the quality of their processes and establishes gradual transition stages until a level considered to be excellent is reached. |
2.1. What Maturity Models do you know or have heard of?
- ▯
MEG (PNQ)
- ▯
CMM
- ▯
CMMI
- ▯
QMMG
- ▯
ISO 9004
- ▯
PM2
- ▯
MMGP
- ▯
PMM
- ▯
BPMM
- ▯
OPM-3
- ▯
KMM
- ▯
None
- ▯
Others: ____________
2.2. Do you know of any maturity programs or models applied to the laboratory setting?
- ▯
Yes, which one? ______________
- ▯
No
- ▯
I can´t say
2.3. On a scale of 1 to 5, where 1 (STRONGLY DISAGREE) and (STRONGLY AGREE), classify the following requirements according to their relevance for management, operations, and maintenance of laboratory activities.
| |
|
1 |
2 |
3 |
4 |
5 |
| 2.3.1 |
A Maturity Model applicable to the laboratory setting helps guide the gradual adoption of management practices to improve internal and external processes. |
|
|
|
|
|
| 2.3.2 |
A Maturity Model applicable to the laboratory setting helps define differentiation strategies between competing laboratories, enabling market gains. |
|
|
|
|
|
2.4. On a scale of 1 to 5, where 1 (OF LOW IMPORTANCE) and (VERY IMPORTANT), classify the following requirements according to their relevance for management, operations, and maintenance of laboratory activities.
| |
|
1 |
2 |
3 |
4 |
5 |
| 2.4.1 |
Identify all stakeholders in the laboratory's activities and define their requirements for quality of services. |
|
|
|
|
|
| 2.4.2 |
The laboratory recognizes the external and internal factors that may offer risks and opportunities for its work to continue. |
|
|
|
|
|
| 2.4.3 |
Acknowledging the Value Chain and its respective processes linked to strategic indicators. |
|
|
|
|
|
| 2.4.4 |
Definition of strategic information, processes, and stakeholders, selecting the most important ones for decision-making. |
|
|
|
|
|
| 2.4.5 |
Establishment of relationship channels with stakeholders to handle requests, complaints, and suggestions. |
|
|
|
|
|
| 2.4.6 |
Market analysis and segmentation. Definition of target customers and assessment of satisfaction, loyalty, and dissatisfaction. |
|
|
|
|
|
| 2.4.7 |
Identification, selection, qualification, and performance evaluation of suppliers. Performance communication. |
|
|
|
|
|
| 2.4.8 |
Selection and qualification of workers. Performance evaluation. |
|
|
|
|
|
| 2.4.9 |
Treatment of health and safety hazards and risks. Promotion of improved quality of life, well-being, and satisfaction. |
|
|
|
|
|
| 2.4.10 |
Identification of existing strengths and gaps in management. Definition of current and future competencies. |
|
|
|
|
|
| 2.4.11 |
Identification, development, retention, and protection of knowledge. |
|
|
|
|
|
| 2.4.12 |
Induction, development, and implementation of innovation. |
|
|
|
|
|
| 2.4.13 |
Identification of capacity for change, including assessment of need and capacity for implementation. |
|
|
|
|
|
| 2.4.14 |
Assessment of flexibility for changes, including review of strategies, goals, processes, and products at an appropriate time. |
|
|
|
|
|
| 2.4.15 |
Definition of values, principles, guidelines, and standards of conduct. Ethical relationship with stakeholders. |
|
|
|
|
|
| 2.4.16 |
Risk management, compliance with legal requirements, and transparency with stakeholders. |
|
|
|
|
|
| 2.4.17 |
Mapping of organizational culture to implement strategies and practice values. |
|
|
|
|
|
| 2.4.18 |
Performance analysis of indicators and monitoring of action plans and their resources |
|
|
|
|
|
| 2.4.19 |
Definition of leadership competencies and leader development. |
|
|
|
|
|
| 2.4.20 |
Defining and monitoring economic-financial indicators, cost management, budget, and fiscal control. |
|
|
|
|
|
| 2.4.21 |
Prevention, treatment, and monitoring of environmental impacts. Quick response to emergencies. |
|
|
|
|
|
| 2.4.22 |
Prevention, mitigation, and monitoring of social impacts. |
|
|
|
|
|
| 2.4.23 |
Implementation of information systems with the establishment of security requirements. |
|
|
|
|
|
| 2.4.24 |
Mapping, analysis, and improvement of laboratory processes. |
|
|
|
|
|
| 2.4.25 |
Identification of new product development opportunities |
|
|
|
|
|
Section 3 – Perception of Management Systems| Management Systems can be understood as interrelated and interdependent management practices and methods with pre-defined objectives and goals that help companies continuously improve in managing specific areas, such as Quality, Environment, and others. |
3.1. On a scale of 1 to 5, where 1 (STRONGLY DISAGREE) and 5 (STRONGLY AGREE), classify the following requirements according to their relevance for management, operations, and maintenance of laboratory activities.
| |
|
1 |
2 |
3 |
4 |
5 |
| 3.1.1 |
Adopting a certifiable Management System optimizes the laboratory's internal processes. |
|
|
|
|
|
| 3.1.2 |
Adopting a certifiable Management System enhances the contracting of new services by the laboratory. |
|
|
|
|
|
| 3.1.3 |
Adopting a certifiable Management System leads to rigid laboratory internal processes. |
|
|
|
|
|
| 3.1.4 |
Adopting a certifiable Management System increases laboratory operating costs. |
|
|
|
|
|
| 3.1.5 |
Adopting a certifiable Management System increases the complexity of laboratory management. |
|
|
|
|
|
| 3.1.6 |
Adopting a certifiable Management System requires hiring more professionals to deal with the documents generated by the system. |
|
|
|
|
|
| 3.1.7 |
Adopting the ABNT NBR ISO 17025:2017 standard to improve the quality of operations, compared to what we currently have, is relevant to the laboratory. |
|
|
|
|
|
| 3.1.8 |
Adopting the ABNT NBR ISO 9001:2015 standard for implementing a quality management system for internal processes, compared to what we currently have, is relevant to the laboratory. |
|
|
|
|
|
| 3.1.9 |
Adopting the ABNT NBR ISO 14001:2015 standard for implementing an environmental management system for internal processes, compared to what we currently have, is relevant to the laboratory. |
|
|
|
|
|
| 3.1.10 |
The adoption of the ABNT NBR ISO 45001:2017 standard for implementing an occupational health and safety management system for internal processes, compared to what we currently have, is relevant to the laboratory. |
|
|
|
|
|
| 3.1.11 |
Compared to what we currently have, adopting the ABNT NBR ISO 31000:2018 standard for implementing a risk management system for internal processes is relevant to the laboratory. |
|
|
|
|
|
3.2. Does the laboratory adopt Laboratory Management practices, tools, and methods? Select the option corresponding to the practices adopted (you may select multiple answers).
- ▯
Statement of Impartiality (Document)
- ▯
Statement of Reliability (Document)
- ▯
Documentation of laboratory activities
- ▯
Skills requirements for roles (Document)
- ▯
Equipment Calibration Program
- ▯
Equipment Operation and Maintenance Procedures
- ▯
Monitoring and control of environmental conditions and facilities
- ▯
Metrological Traceability
- ▯
Documented, updated, and validated methods and procedures
- ▯
Laboratory management software
- ▯
Analysis of risks and opportunities
- ▯
Critical orders analysis
- ▯
Critical analysis of the results
- ▯
Critical analysis of non-conformities
- ▯
Others: ____________
3.3. Does the laboratory adopt strategic, tactical, and operational management methods? Select the option corresponding to the practices adopted (you may select multiple answers).
| |
Quality |
Environment |
Occupational Health and Safety |
Risks and opportunities |
Does not adopt |
| Management manual |
|
|
|
|
|
| Policy |
|
|
|
|
|
| Goals and objectives |
|
|
|
|
|
| Written instructions and procedures |
|
|
|
|
|
| Performance indicators |
|
|
|
|
|
| Scheduled inspections |
|
|
|
|
|
| Audits |
|
|
|
|
|
3.4. Select the option that corresponds to the reality of the laboratory (you may select more than one answer). The laboratory has the standard...
| |
Certified |
Certification in progress |
With the implementation in progress |
Does not have |
| ABNT NBR ISO 17025:2017 |
|
|
|
|
| ABNT NBR ISO 9001:2015 |
|
|
|
|
| ABNT NBR ISO 14001:2015 |
|
|
|
|
| ABNT NBR ISO 45001:2018 |
|
|
|
|
| ABNT NBR ISO 31000:2018 |
|
|
|
|
| Others: _______________ |
|
|
|
|
3.5. In your opinion, what reasons make it difficult to implement one or more management systems in the laboratory? (you may select more than one answer)
- ▯
Lack of Human Resources
- ▯
Lack of Financial Resources
- ▯
Lack of infrastructure
- ▯
Institution regulations
- ▯
Uncertainty regarding return on investment
- ▯
Low interest from management
- ▯
Employee resistance to change
- ▯
Others: ____________
Section 4 – Adoption of Management Practices
4.1. Does the laboratory adopt Laboratory Management practices, tools, and methods? Select the option corresponding to the practices adopted (you may select multiple answers).
- ▯
5S Program
- ▯
FMEA
- ▯
Process Mapping
- ▯
Process Quality Control
- ▯
Determining customer requirements
- ▯
Measuring customer satisfaction
- ▯
Others: ____________
4.2. Does the laboratory adopt Laboratory Management practices, tools, and methods? Select the option corresponding to the practices adopted (you may select multiple answers).
- ▯
Effluent treatment
- ▯
Conscious consumption (water/energy)
- ▯
Waste sorting
- ▯
Proper waste disposal
- ▯
3R/5R Program
- ▯
Assessment of Environmental Aspects and Impacts
- ▯
Others: ____________
4.3. Does the laboratory adopt Occupational Health and Safety Management practices, tools, and methods? Select the option that corresponds to the practices adopted (you may select more than one answer).
- ▯
Good Laboratory Practices - GLP
- ▯
Chemical Compatibility Chart
- ▯
Labeling System
- ▯
PPE training
- ▯
Risk Management Program – RMP
- ▯
Environmental Risk Prevention Program – PPRA
- ▯
Occupational Health Medical Control Program – PCMSO
- ▯
Fire prevention and fire fighting measures
- ▯
Hazard and Risk Assessment
- ▯
Others: ____________
4.4. Does the laboratory adopt Laboratory Management practices, tools, and methods? Select the option that corresponds to the practices adopted (you may select more than one answer).
- ▯
Brainstorming
- ▯
Checklists
- ▯
Preliminary Risk Analysis – PRA
- ▯
Hazard Analysis and Critical Control Points – HACCP
- ▯
Failure Modes and Effects Analysis – FMEA
- ▯
Reliability Centered Maintenance – RCM
- ▯
Cause and Effect Analysis
- ▯
Probability and Consequences Matrix
- ▯
Others: ____________
4.5. The space below is intended for additional comments, criticisms, and suggestions regarding the questionnaire and/or about any matters pertinent to management systems and Maturity Models. We thank you in advance
Thank you for taking the time to answer this questionnaire. Thank you. (500 words)
References
- Nunhes, T.V.; Bernardo, M.; Oliveira, O.J. Guiding principles of integrated management systems: towards unifying a starting point for researchers and practitioners. J. of Clean. Prod. 2019, 210, 977–993. [Google Scholar] [CrossRef]
- Poltronieri, C.F.; Ganga, G.M.D.; Gerolamo, M.C. Maturity in management system integration and its relationship with sustainable performance. J. of Clean. Prod. 2019, 207, 236–247. [Google Scholar] [CrossRef]
- Wendler, R. The maturity of maturity model research: a systematic mapping study. Inf. And Soltware Tech. 2012, 54, 12, 13171339. [Google Scholar] [CrossRef]
- Serafini, P.G. et al. Sustainable Development Goals in Higher Education Institutions: a systematic literature review. J. of Clean. Prod. 2022; 370.
- Barbour, R. Grupos Focais. Artmed: Porto Alegre, RS, Brasil, 2009.
- Gil, A. C. Métodos e Técnicas de Pesquisa Social, 6ª ed.; Atlas: São Paulo, SP, Brasil, 2010. [Google Scholar]
- Lakatos, E.M.; Marconi, M.A. Fundamentos de Metodologia Científica, 7ª ed. Atlas: São Paulo, SP, Brasil, 2010.
- Maier, A.; Moultrie, J.; Clarkson, P. Assessing organizational capabilities: Reviewing and guiding the development of maturity grids. IEEE Trans. on Eng. Manag. 2012, 59, 138–159. [Google Scholar] [CrossRef]
- Pedro, E.; Leitão, J.; Alves, H. The intellectual capital of higher education institutions. J. of Intel. Cap. 2019, 20,3, 355–381. [Google Scholar] [CrossRef]
- Alonso-Almeida, M.; Marimon, F.; Casani, F. Diffusion of sustainability reporting in universities: current situation and future perspectives. J. of Clean. Prod. 2015, 106, 144–154. [Google Scholar] [CrossRef]
- Serafini, P. et al. Sustainable Development Goals in Higher Education Institutions: A systematic literature review. J. of Clean. Prod., 2022; 370.
- Brasil, Ministério da Educação, 2017. Resolução no 1, de 26 de julho de 2017. Disponivel: http://portal.mec.gov.br/docman/agosto-2017-pdf/70141-rcp001-17-pdf/file (acessado em 24 de novembro de 2023).
- Nadae, J. de et al. Integrated management systems as a driver of sustainability performance: exploring evidence from multiple-case studies. Inter. J. of Quality & Rel. Manag. 2020; 38, 3, 800–821.
- Zeng, S.X.; Shi, J.J.; Lou, G.X. A synergetic model for implementing an integrated management system: an empirical study in China. J. of Clean. Prod. 2007, 15, 18, 1760–1767. [Google Scholar] [CrossRef]
- Honsa, J.D.; McIntyre, D.A. ISO 17025: Benefícios práticos da implementação de um sistema de qualidade. J. of AOAC Inter. 2003, 86, 1038–1044. [Google Scholar] [CrossRef]
- Kodydková, J.; Vávrová, L.; Zeman, M.; Jirák, R.; Macášek, J.; Staňková, B.; Tvrzická, E. Enzimas antioxidantes e aumento do estresse oxidativo em mulheres depressivas. Rev. Bioquí. Clin 2009, 42, 1368–1374. [Google Scholar]
- Gimeno, C. Systems of quality management in clinical laboratories: certification and accreditation. Enferm. Infec. e Microb. Clínica. 2003, 21, 17-23, 21. [Google Scholar]
- Barradas, J.; Sampaio, P. ISO 9001 and ISO/IEC 17025. Inter. J. of Quality & Rel. Manag. 2017, 34, 406–417. [Google Scholar]
- Guzel, O.; Guner, E.I. Acreditação ISO 15189: Requisitos de qualidade e competência de laboratórios médicos, experiência de um laboratório I. Rev. Clín. Bioquí. 2009, 42, 274–278. [Google Scholar] [CrossRef]
- Peric, M.; Tonković, Z.; Rodić, A.; Surjak, M.; Garašić, I.; Borá, I.; Švaić, S. Análise numérica e investigação experimental de tensões residuais e distorções de soldagem em uma solda de ângulo com junta em T. Rev. Materiais e Design. 2014, 53, 1052–1063. [Google Scholar]
- Biasini, V. Implementation of a quality management system in a public research centre. Accred. and Qual. Assur. 2012, 17, 621–626. [Google Scholar] [CrossRef]
- Associação Brasileira de Normas Técnicas. ABNT NBR ISO 9001: Sistemas de gestão da qualidade - Requisitos. ABNT: Rio de Janeiro, RJ, Brasil, 2015a.
- Associação Brasileira de Normas Técnicas. ABNT NBR ISO 17025: Requisitos gerais para a competência de laboratórios de ensaio e calibração. ABNT: Rio de Janeiro, RJ, Brasil, 2017.
- Associação Brasileira de Normas Técnicas. ABNT NBR ISO 14001: Sistemas da gestão ambiental Requisitos com orientações para uso. ABNT: Rio de Janeiro, RJ, Brasil, 2015b.
- Associação Brasileira de Normas Técnicas. ABNT NBR ISO 26000: Diretrizes sobre responsabilidade social. ABNT: Rio de Janeiro, RJ, Brasil, 2010.
- Associação Brasileira de Normas Técnicas. ABNT NBR OHSAS 18001: Saúde e Segurança Ocupacional. ABNT: Rio de Janeiro, RJ, Brasil, 1999.
- Valcárcel, M.; Lucena, R. Synergistic relationships between analytical chemistry and written standards. A. Chim. Acta. 2013, 788, 1-7; doi.org/10.1016/j. aca.2013.04.008.
- Domingues, J.P.T. Sistemas de Gestão Integrados: desenvolvimento de um modelo para avaliação do nível de maturidade. Tese de Doutorado, Universidade de Minho, Minho, Portugal, 2013.
- Belezia, L.C. Modelo de autoavaliação para laboratórios de ensaio e calibração baseado na Norma ABNT NBR ISO/IEC 17025:2017. Dissertação de Mestrado, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, Brasil, 2019.
- Gerônimo, B.M. Modelo de maturidade de sistema de gestão integrado para laboratórios de ensaio e calibração. Tese de Doutorado, Universidade Tecnológica Federal do Paraná, Ponta Grossa, Brasil, 2023.
- Universidade Federal do Rio Grande do Norte (UFRN). Plano de Desenvolvimento Institucional 2019-2023. UFRN: Natal, RN, Brasil, 2019.
- Xavier, A.F. Proposta de um modelo de maturidade para avaliação das práticas de eco-inovação nas organizações: Eco-mi. UFRJ: Rio de Janeiro, RJ, Brasil, 2017.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).