Submitted:
09 January 2024
Posted:
10 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
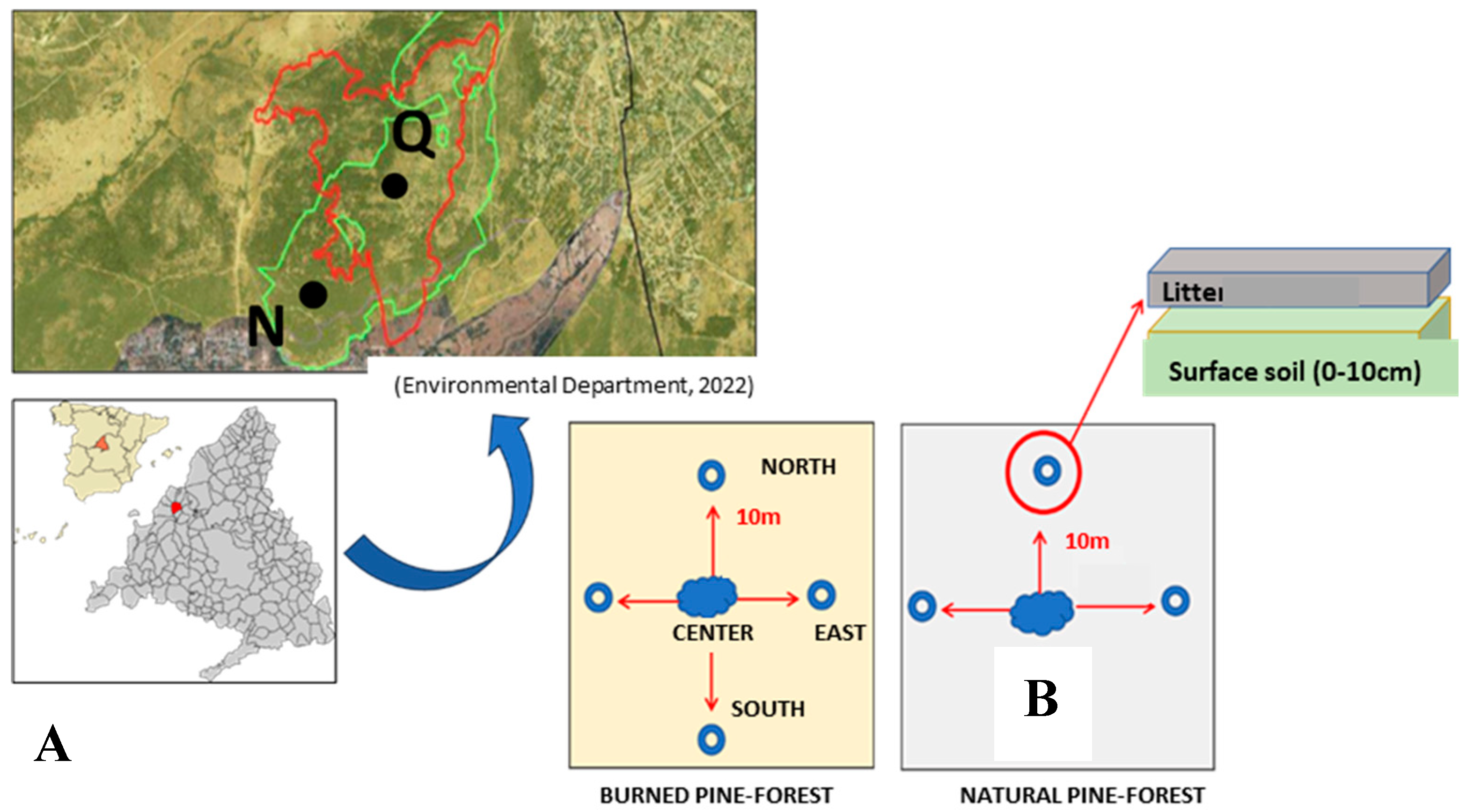
2.1. Study area
2.2. Field work
2.3. Laboratory work
2.3.1. Soil parameters
2.3.2. Faunistic study
2.4. Data analysis
- The Shannon-Wiener Diversity index (H' = - Σpi ln pi).
- The Specific Richness index (Hmax=(LnS)).
- The Pielou's Equity index (J = H/Hmax).
- Simpson's Dominance index (λ =∑pi 2).
3. Results
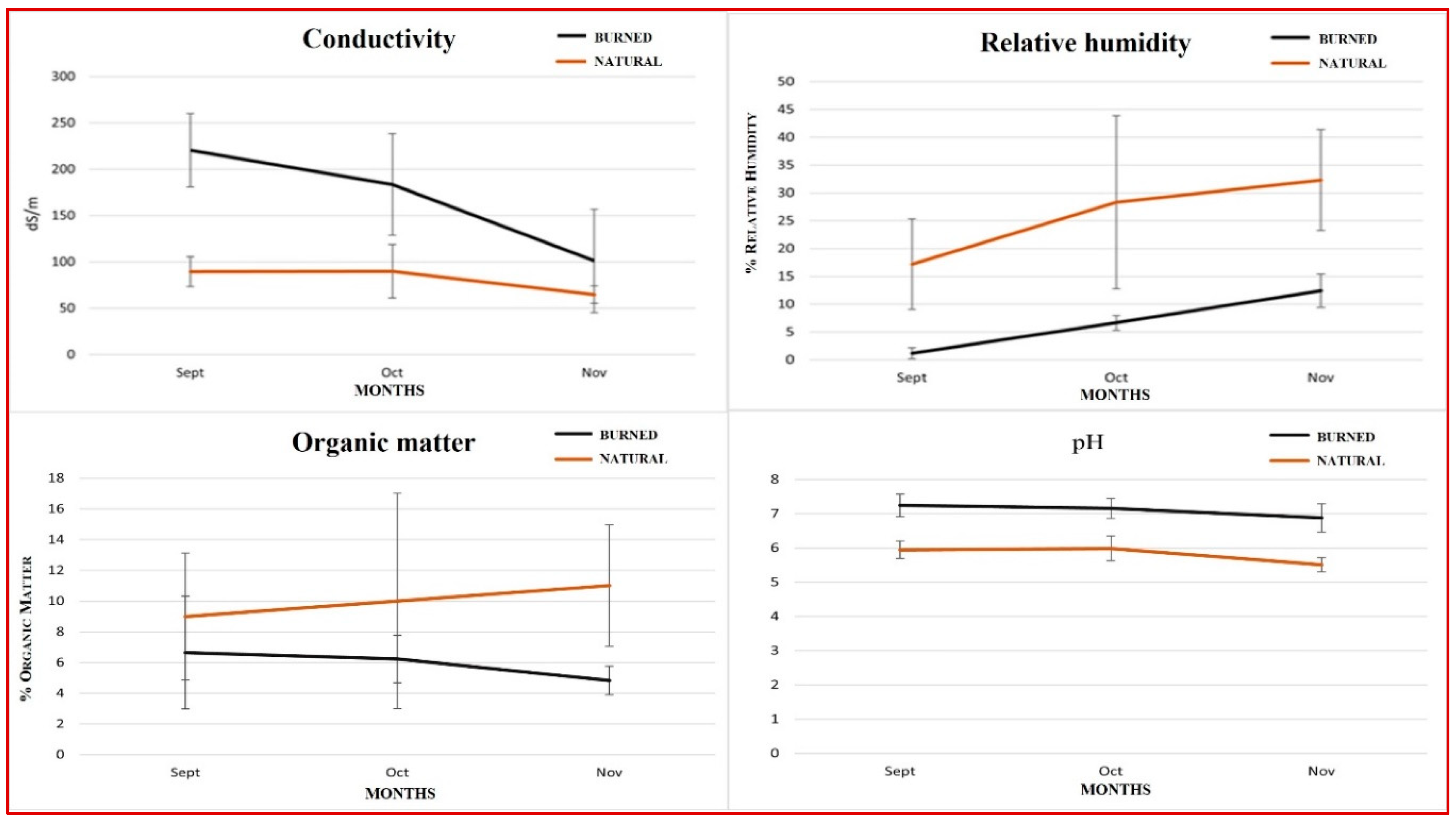
3.1. Edaphic parameters
3.2. Study of springtail communities
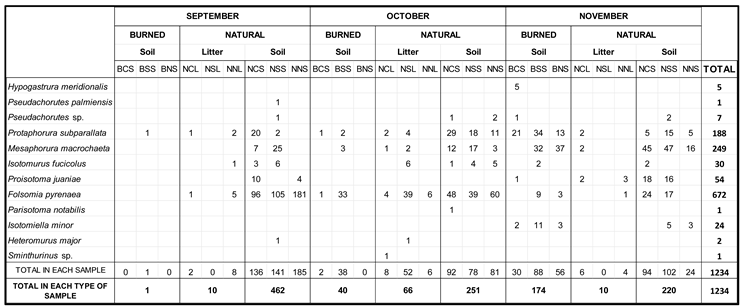
3.2.1. Faunal composition
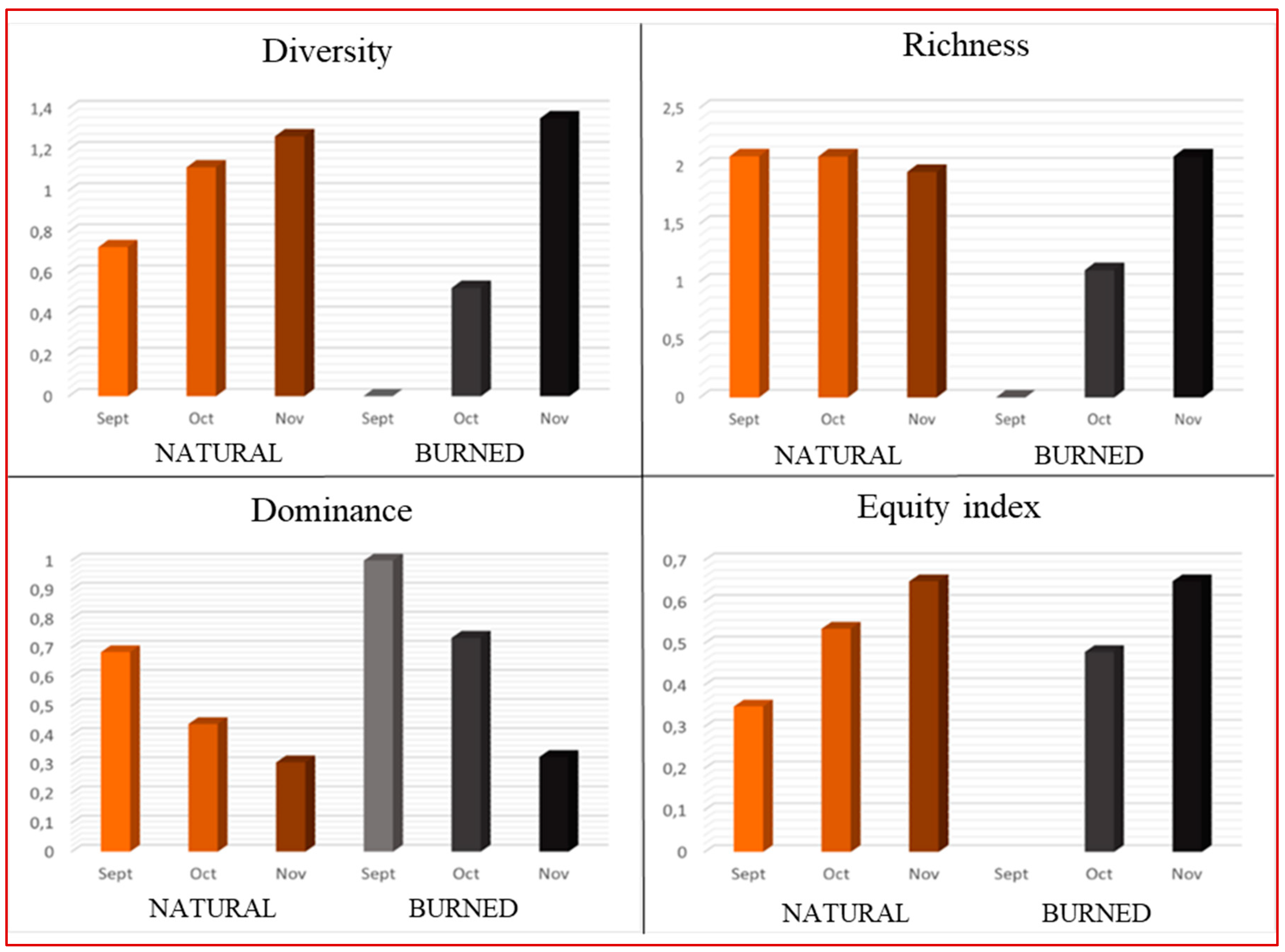
3.2.2. Alpha diversity indexes
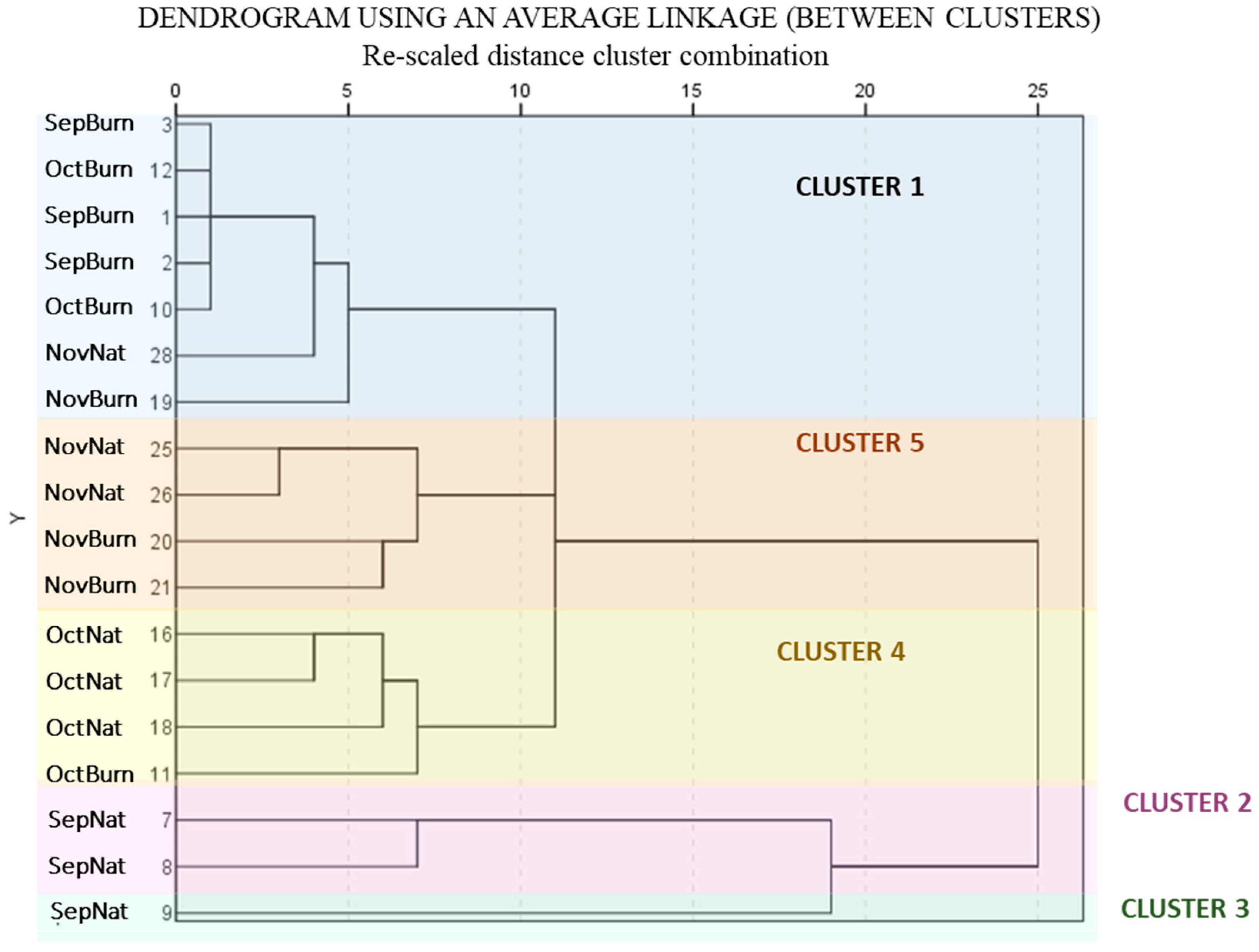
3.2.3. Hierarchical classification
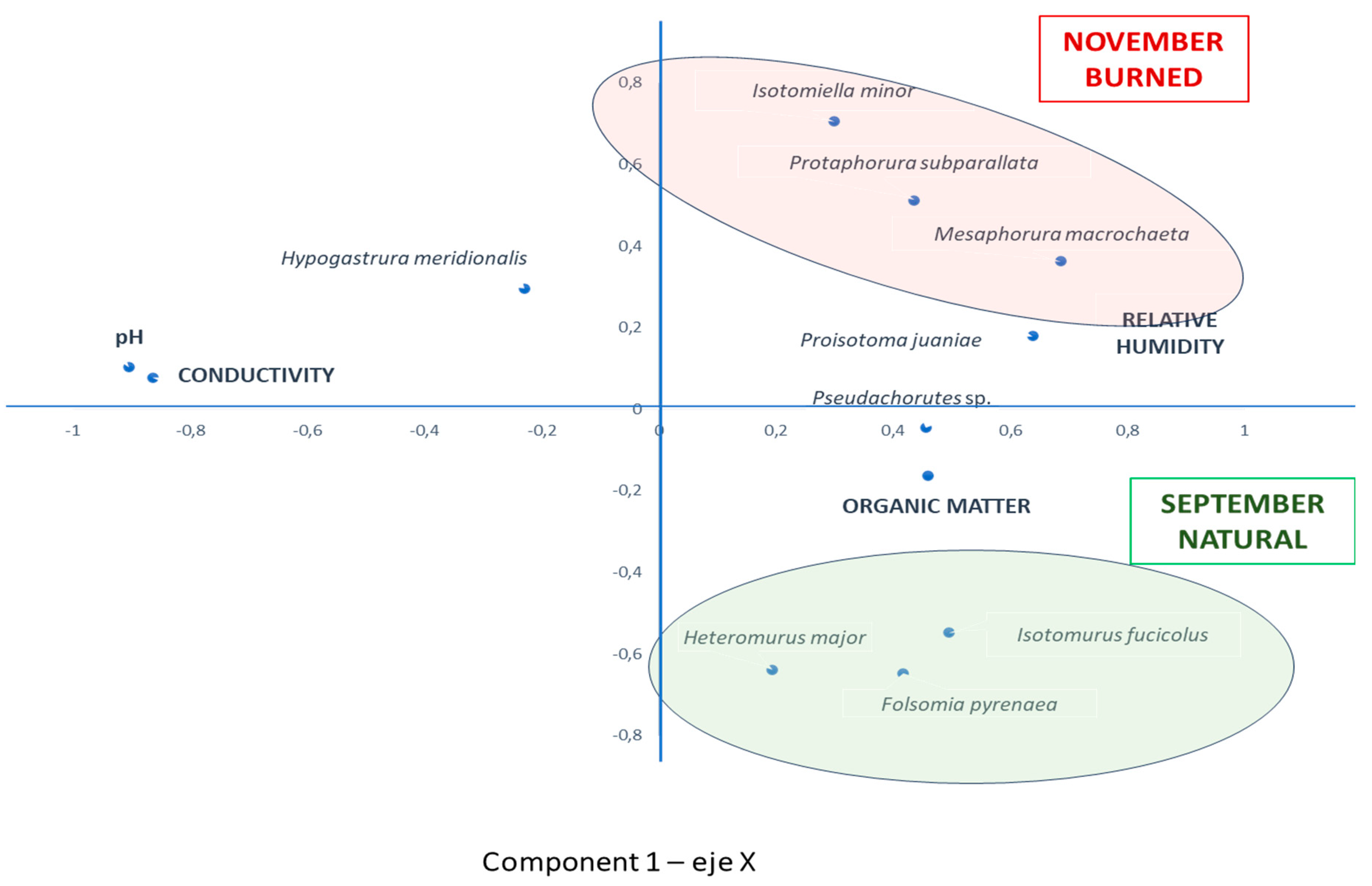
- Mesaphorura macrochaeta: has the smallest mean in cluster 1 (B) and the highest in cluster 5 (Nov).
- Folsomia pyrenaea: shows the lowest mean in cluster 1 (B). The highest means are in clusters 2 and 3 (SepNat), gradually decreasing in clusters 4 (Oct) and 5 (Nov).
- Isotomurus fucicolus: is not present in cluster 1 (B) and its mean decreases consecutively in clusters 2 (SepNat), 4 (Oct) and 5 (Nov).
3.2.4. Principal Component Analysis
4. Discussion
5. Conclusions
- (1)
- The effects of the fire on the physicochemical parameters of the soil have consisted of a lower amount of organic matter and water, and a higher pH and conductivity compared to the natural pine forest. During this study of soil recovery, only two parameters varied in the burned pine forest: relative humidity increased and electrical conductivity decreased. The invariability of the OM percentage in spite of the heavy rains has confirmed the effectiveness of the containment terraces.
- (2)
- The communities of the edaphic fauna of the burned pine forest have increased their diversity and equity with the passage of time, but have not reached the values typical of the natural pine forest. The greater abundance of mites in the burned pine forest is noteworthy.
- (3)
- The populations of springtails have changed considerably during this short-term study, matching the richness and even reaching a higher diversity than that of the natural pine forest communities. In addition, greater similarities have been observed between the springtail populations of the natural and burned pine forest over time, specifically in samples with physicochemical parameters closer to those of the natural pine forest.
Funding
Conflicts of Interest
References
- Alcasena, F.J.; Ager, A.A.; Bailey, J.D.; Pineda, N.; Vega-García, C. Towards a comprehensive wildfire management strategy for Mediterranean areas: Framework development and implementation in Catalonia, Spain. J Environ. Manag. 2019, 231, 303–320. [Google Scholar] [CrossRef]
- Arbea, J.I. Checklist de Fauna Ibérica. Clase Collembola Lubbock, 1870 (Hexapoda) en la Península Ibérica, Islas Baleares y Macaronesia (edición 2021). In Documentos Fauna Ibérica; Ramos, M.A. and Sánchez Ruiz, M., Ed.; Museo Nacional de Ciencias Naturales CSIC: Madrid, 2021; Volume 16, 2 (sn) + 41 pp. [Google Scholar]
- Arbea, J.; Blasco Zumeta, J. Ecología de los Colémbolos (Hexapoda, Collembola) en Los Monegros (Zaragoza, España). Bol. Soc. Entom. Aragonesa (B.S.E.A.) 2001, 28, 35–48. [Google Scholar]
- Arbea, J.I.; Almeida, J. Catálogo de los colémbolos de la Península Ibérica, Baleares y Canarias a partir de fotografías en la naturaleza (Hexapoda, Collembola). Soc Entom Aragonesa, 2022; Monografías electrónicas S E A 10 (30-VI-2022). [Google Scholar]
- Arbea, J.I.; Baquero, E.; Beruete, E.; Pérez Fernández, T.; Jordana, R. Catálogo de los Colémbolos cavernícolas del área Iberobalear e islas Macaronésicas septentrionales (Collembola). Bol Soc Entom Aragonesa (B.S.E.A.) 2021, 68, 1–80. [Google Scholar]
- Arpin, P. : Ponge, J.F.; Dabin, B.; Mori, A. Utilisation des nématodes Mononchida et des collemboles pour caractériser des phénomènes pédobiologiques. Rev Ecol Biol Sol 1984, 21, 243–268. [Google Scholar]
- Ayuntamiento de Collado Mediano. Flora y Fauna. El Cerro del Castillo. 2023. Available online: https://www.aytocolladomediano.es/Tu-Ciudad/Entorno-Natural/Flora-y-Fauna/El-Cerro-Del-Castillo.
- Bardgett, R. Causes and consequences of biological diversity in soil. Zoology 2002, 105, 367–375. [Google Scholar] [CrossRef]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.Th. Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Soil Structure/Soil Biota Interrelationships 1993, 105–118. [Google Scholar] [CrossRef]
- Brevik, E.C.; Cerdà, A.; Mataix-Solera, J.; Pereg, L.; Quinton, J.N.; Six, J.; Van Oost, K. The interdisciplinary nature of soil. SOIL 2015, 1, 117–129. [Google Scholar] [CrossRef]
- Campo Sanchís, A. La restauración arbórea del monte incendiado en Collado Mediano el año 2009. Foresta Especial La Comunidad de Madrid 2011, 52, 158–160. [Google Scholar]
- Castellnou, M.; Guiomar, N.; Rego, F.; Fernandes, P.M. Fire growth patterns in the 2017 mega fire episode of October 15, Central Portugal. In: D X Viegas (Ed.), Advances in forest fire research 2017, ch. 3 Fire management, Universidade de Coimbra: 447-453. [CrossRef]
- Castro, J.; Maranon-Jimenez, S.; Sánchez-Miranda, A.; Lorite, J. Efecto del manejo de la madera quemada sobre la regeneración forestal post-incendio: desarrollo de técnicas blandas de restauración ecológica. Proyectos de Investigación en Parques Nacionales, 2006–2009. 139–157.
- Chauvat, M.; Zaitsev, A.S.; Wolters, V. Successional changes of Collembola and soil microbiota during forest rotation. Oecologia 2003, 137, 269–276. [Google Scholar] [CrossRef]
- Collins, B.M.; Miller, J.D.; Thode, A.E.; Kelly, M.; van Wagtendonk, J.W.; Stephens, S.L. Interactions among wildlandfires in a long-established Sierra Nevada natural fire area. Ecosystems, 2008, 12, 114–128. [Google Scholar] [CrossRef]
- Consejería de Medio Ambiente, Vivienda y Agricultura. Actuaciones urgentes de restauración hidrológico-forestal sobre los terrenos del Mup. Na200 “Cerro del Castillo” afectados por el incendio. 2022; 3–13. [Google Scholar]
- Cowling, R.M.; Rundel, P.W.B.; Lamont, B.B.; Kalin Arroyo, M.; Arianoutsou, M. Plant diversity in mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- . [CrossRef]
- FAO. Soil is a non-renewable resource Its preservation is essential for food security and our sustainable future. Food and Agriculture Organization of the United Nations, 2015; 4p. [Google Scholar]
- García-Orenes, F.; Arcenegui, V.; Chrenková, K.; Mataix-Solera, J.; Moltó, J.; Jara-Navarro, B.; Torres, M.P. Effects of salvage logging on soil properties and vegetation recovery in a fire-affected Mediterranean forest: A two year monitoring research. Sci. Total Environ. 2017, 586, 1057–1065. [Google Scholar] [CrossRef]
- Gómez-Sánchez, M.E.; Lucas-Borja, M.E.; Plaza-Álvarez, P.A.; González-Romero, J.; Sagra, J.; Moya, D.; de las Heras, J. Efecto de los trabajos de restauración forestal post-incendio en ladera sobre la recuperación de la funcionalidad del suelo. Cuadernos de la Sociedad Española de Ciencias Forestales, 2019, 45, 35–44. [Google Scholar]
- Gongalsky, K.B.; Malmström, A.; Zaitsev, A.S.; Shakhab, S.V.; Bengtsson, J.; Persson, T. Do burned areas recover from inside? An experiment with soil fauna in a heterogeneous landscape. Appl. Soil Ecol. 2012, 59, 73–86. [Google Scholar] [CrossRef]
- González Ulibarry, P. Impacto de los incendios forestales en suelo, agua, vegetación y fauna. Departamento de estudios, extensión y publicaciones. Biblioteca del Congreso Nacional de Chile 2017, 1–8. [Google Scholar]
- González-Polo, M.; Agüero, M.; Castán, E. Enmienda con compost y fertilización de suelos afectados por incendios: Respuesta de dos especies nativas de la Patagonia bajo condiciones de invernadero. Ecol. Austr. 2020, 30, 366–379. [Google Scholar] [CrossRef]
- Holden, Z.A.; Morgan, P.; Evans, S. Apredictive model of burn severity based on 20-yearsatellite-inferred burn severity data in a largesouthwestern US wilderness area. Forest Ecol. Manag. 2009, 258, 2399–2406. [Google Scholar] [CrossRef]
- Iglesias, T.; Cala, V.; Gonzalez, J. Mineralogical and chemical modifications in soils affected by a forest fire in the Mediterranean area. Sci. Total Environ. 1997, 204, 89–96. [Google Scholar] [CrossRef]
- Iglesias, T.; Cala, V.; Walter, I.; González, J. Efectos de la temperatura y vegetación en suelos calentados en condiciones controladas de laboratorio. Ecologia 1998, 12, 105–111. [Google Scholar]
- ISO 11465:1993; Determination of dry matter and water content on a mass basis. Gravimetric method (pp. 1–3). Technical Committee: ISO/TC 190/SC 3 Chemical and physical characterization. 1993.
- Jaramillo, D.F. Repelencia al agua en suelos: una síntesis. Rev Acad Colomb Cienc Exactas Fis Nat. 2006, 30, 215–232. [Google Scholar] [CrossRef]
- Jordana, R.; Arbea, J.I. Clave de identificación de los géneros de Colémbolos de España (lnsecta: Collembola). Servicio de Publicaciones de la Universidad de Navarra: Serie zoológica Pamplona. 1989, 19. [Google Scholar]
- Jordana, R.; Arbea, J.I.; Simón, J.C.; Luciáñez, M.J. Collembola, Poduromorpha. In Fauna Ibérica 8. Museo Nacional de Ciencias Naturales; Ramos, M.A., Ed.; CSIC: Madrid, 1997; 807p. [Google Scholar]
- Luciáñez, M.J.; Iniesto, P. Estudio faunístico y ecológico de las comunidades de Colémbolos (Hexapoda, Collembola) de pinares incendiados en la vertiente sur de la Sierra de Gredos. Bol AeE 2006, 30, 75–95. [Google Scholar]
- Luciáñez, M.J.; Simón, J.C. Estudio colembológico de un robledal y un pinar en la vertiente sur de la Sierra de Gredos. Eos 1988, 57–87. [Google Scholar]
- Luciáñez, M.J.; Simón, J.C. Estudio de la variación estacional de la colembofauna en suelos de alta montaña en la Sierra de Guadarrama (Madrid). Miscel Zool 1991, 15, 103–113. [Google Scholar]
- Martín, S.D.; Birk, E.L. The singular role ofthe atmospheric stability in forestfires. Atmósfera 2010, 23, 129–139. [Google Scholar]
- Mataix-Solera, J.; Guerrero, C. Efecto de los incendios forestales en las propiedades edáficas. - In: J Mataix-Solera (ed.): Incendios Forestales, Suelos y Erosión Hídrica. Caja Mediterráneo CEMACAM Font Roja-Alcoi. Alicante, 2007, 1, 7–40. [Google Scholar]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth-Science Reviews, 2011; 109, 44–60. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Moltó, J.; Arcenegui, V.; García-Orenes, F.; Chrenkovà, K.; Torres, P.; Jara-Navarro, A.B.; Díaz, G.; Izquierdo, E. Salvage loggingeffect on soil properties in a fire-affected Mediterranean forest: two years monitoring research. Geophys. Res. Abstr. 2015, 17. EGU General Assembly. [Google Scholar]
- Moritz, M.A.; Batllori, E.; Bradstock, R.A.; Gill, A.M.; Handmer, J.; Hessburg, P.F.; Leonard, L.; McCaffrey, S.; Odion, D.C.; Schoennagel, T.; Syphard, A.D. Learning to coexist with wildfire. Nature, 2014, 515, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Naveh, Z. The evolutionary significance of fire in the mediterranean region. Vegetatio 1975, 29, 199–208. [Google Scholar] [CrossRef]
- Osborne, J. Notes on the use of data transformations. Geophys. Res. Abstr. 2002, 8. [Google Scholar]
- Palacios-Vargas, J.G.; Mejía, B.E. Técnicas de colecta, montaje y preservación de microartrópodos edáficos. Universidad Nacional Autónoma de México, 2007; 72p. [Google Scholar]
- Pausas, J.G. Incendios forestales. Una visión desde la ecología. Catarata y CSIC Madrid, 2012; 128p. [Google Scholar]
- Pedrosa, I.; Juarros-Basterretxea, J.; Robles-Fernández, A.; Basteiro, J.; García-Cueto, E. Pruebas de bondad de ajuste en distribuciones simétricas, ¿qué estadístico utilizar? Univ. Psychol. 2015, 14, 245–254. [Google Scholar] [CrossRef]
- Ponge, J.F.; Gillet, F.; Dubs, E.; Fedoroff, L.; Haese, H.; Sousa, J.P.; Lavelle, P. Collembolan communities as bioindicators of land use intensification. Soil Biol. Biochem. 2003, 35, 813–826. [Google Scholar] [CrossRef]
- Potapov, M.B. Synopses on Palaearctic Collembola: Isotomidae. Staatliches Museum Für Naturkunde Görlitz 2001, 3. [Google Scholar]
- Rivas-Martínez, S. Memoria del mapa de series de vegetación de España ICONA. Ministerio de Agricultura, Pesca y Alimentación Madrid, 1987. [Google Scholar]
- Rothermel, R.C. A mathematical model forpredictingfire spread in wildland fuels; Department of Agriculture, Intermountain Forest and Range Experiment Station: Ogden, Utah, USA, 1972. [Google Scholar]
- Skinner, C.N.; Taylor, A.H.; Agee, J.K. Klamath Mountain bioregion. Pages 170–194 in Sugihara NG, van Wagtendonk JW, Shaffer KE, Fites-Kaufman J and Thode AE (Eds.). Fire in California’s ecosystems. The University of California Press: Berkeley, California, USA, 2006. [Google Scholar]
- Swift, R.S. Sequestration of carbon by soil. Soil Sci. 2001, 166, 858–871. [Google Scholar] [CrossRef]
- Trujillo-González, J.M.; Mahecha, J.D.; Torres-Mora, M. El recurso suelo; un análisis de las funciones, capacidad de uso e indicadores de calidad. Revista de Investigación Agraria y Ambiental (RIAA) 2018, 9, 29–36. [Google Scholar]
- Ubeda Cartañà, X.; Mataix-Solera, J.; Francos, M.; Farguell, J. Grandes incendios forestales en España y alteraciones de su régimen en las últimas décadas. In Geografía, Riscos e Protecao civil. Homenagen ao Professor doutor Luciano Lorenco; RISCOS-Associacao Portuguesa de Riscos, Prevencao e Seguranca, 2021. [Google Scholar] [CrossRef]
- von Saltzwedel, H.; Scheu, S.; Schaeffer, I. Genetic structure and distribution of Parisotoma Jnotabilis (Collembola) in Europe: Cryptic diversity, split of lineages and colonization patterns. PLoS One 2017, 12, e0170909. [Google Scholar] [CrossRef] [PubMed]
| Dependent variable | Burned Mean ± SE | Natural Mean ± SE | F | P-value |
|---|---|---|---|---|
| Conductivity | 168.29 ± 69.7 | 81.36 ± 22.05 | 10.45 | 0.003 |
| Moisture | 6.74 ± 5.07 | 25.93 ± 12.45 | 36.279 | 0.000 |
| O.M. | 5.90 ± 2.33 | 11.53 ± 5.01 | 24.445 | 0.000 |
| pH | 7.09 ± 0.35 | 5.81 ± 0.34 | 56.342 | 0.000 |
| Dependent variable | B-September Mean ± SE | B-Octuber Mean ± SE | B-November Mean ± SE | F | P-value | Multiple comparisons |
|---|---|---|---|---|---|---|
| Conductivity | 220.38 ± 39.82 | 183.48 ± 54.74 | 101.02 ± 55.61 | 7.3 | 0.008 | O = S ≠ N = O |
| Moisture | 1.19 ± 0.99 | 6.65 ± 1.31 | 12.39 ± 2.99 | 40.367 | 0.000 | S ≠ O ≠ N |
| O.M. | 6.65 ± 3.68 | 6.23 ± 1.55 | 4.82 ± 0.93 | 1.256 | 0.32 | S = O = N |
| pH | 7.24 ± 0.33 | 7.16 ± 0.29 | 6.87 ± 0.41 | 1.502 | 0.262 | S = O = N |
 |
| Dependent variable | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | F | P-value |
|---|---|---|---|---|---|---|---|
| Mesaphorura macrochaeta | 2.29± 6.0 | 16±12.72 | 0 | 8.75±6.95 | 40.25±6.99 | 19.451 | 0.000 |
| Isotomurus fucicolus | 0 | 4.5±2.12 | 0 | 2.5±2.38 | 1±1.16 | 5.112 | 0.011 |
| Folsomia pyrenaea | 0.14±0.38 | 100.50±6.37 | 181.00 | 45.00±1.75 | 13.25±9.18 | 186.723 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




