1. Introduction
According to the World Health Organization's (WHO) World Health Statistics 2023, global population health has significantly improved since the 2000s [1]. Notably, over the past two decades, global life expectancy has increased from 67 to 73 years, aligning with the decline in the incidence and mortality of infectious diseases [1]. However, it remains significant that 1 in 5 deaths is still attributable to infections, and six out of the top 10 causes of death in low-income countries are infectious diseases [2]. Meanwhile, global health indicators saw a setback during the COVID-19 pandemic, causing approximately 15 million excess deaths in 2020 and 2021 [3]. This serves as a stark reminder that the emergence and re-emergence of infectious diseases pose significant risks [4]. WHO Director-General Dr. Tedros Adhanom Ghebreyesus emphasizes the importance of learning from the past two decades, including the tragedies of the pandemic era [1].
Although medicinal folklore has been used to treat infections since ancient cultures predating BC, humanity grappled helplessly with various infections and epidemics until the 17th century [5]. During this time, 'little beasties' were observed under a microscope, abiogenesis was refuted, and the question of "What is the nature of infectious diseases?" was addressed [6,7]. The subsequent development of vaccines in the late 18th century and the advent of antibiotics, antivirals, and antifungals in the early to mid-20th century marked progress in understanding and combating infectious diseases, a trend that continues to the present [8-11]. However, the initial euphoria led to the realization that pathogens can develop multiple resistance mechanisms, leading to a decline in new drug discovery and development [12,13]. Pathogens persistently challenge humanity due to the emergence of new and the evolution of known pathogens [14]. Vaccine technology also faces challenges from persistent infections, rapidly evolving pathogens with high sequence variability, complex viral antigens, and new emergences [15]. Thus, the focus on vaccines and antimicrobials, crucial as they are, offers a valuable but insufficient perspective from the fields of immunology and microbiology [16].
In the case of infectious disease outbreaks, clinical solutions traditionally concentrate on efficient pathogen destruction [17]. However, the COVID-19 pandemic emphasized the complexity of infectious diseases as multisystemic conditions, indicating that this perspective needs to be revised to understand survival from infectious diseases [16,17]. This underscores the need to comprehend how we survive infections, which may differ from standard approaches to treating infectious diseases [16]. For instance, antivirals may be effective in patients with "mild" COVID-19 by shortening the infection duration and reducing transmission. However, in severe cases requiring hospitalization and intensive care, antiviral-based strategies may not align with the needs of medical staff and patients fighting for their lives [18]. Effective response to an infectious disease outbreak necessitates a multifaceted and holistic approach [16,17].
There has always been a gap between treating infectious diseases and understanding the mechanisms promoting survival [19]. Moving beyond the traditional view of infectious diseases is imperative to enhance treatment [17]. In addition to the resistance strategies of the host immune response and pathogen destruction, there is a need to develop disease-tolerance drugs that alleviate pathologies or promote physiological functions in the face of these pathologies [16,17]. Against this backdrop, this narrative review interprets the two ways of resistance/tolerance in treating infectious diseases based on the microbiome, recently in the spotlight as a next-generation treatment, and presents the direction of development based on their convergence.
2. Human Microbiome Therapeutics
“Microbiome” is a term derived from the combination of “micro” and “biome,” encompassing the entire habitat, including microorganisms, their genomes, and surrounding environmental conditions [20,21]. According to the Human Microbiome Project (HMP) Consortium report, human feces host 4,180 bacterial species, while the buccal mucosa, anterior nares, supragingival plaque, and posterior fornix harbor 775, 857, 1267, and 255 species, respectively [22]. In numerical terms, the "reference man" carries about 38 trillion bacteria, with a ratio close to 1 compared to approximately 30 trillion human cells [23]. This newfound understanding of scale underscores the significance of the human microbiome. MetaHIT (The European Union Project on Metagenomics of the Human Intestinal Tract) characterized the genetic potential, revealing 3,299,822 microbial genes in human feces, approximately 150 times more than the roughly 23,000 genes constituting the human genome [24]. The shared pool contains around 536,000 unique genes, with 99.1% originating from bacteria, the remaining primarily from archaea, and only 0.1% from eukaryotic and viral sources [24].
These microbial communities profoundly impact host physiology, influencing energy metabolism—converting nutrients into various forms of energy used by the body [25]. Consequently, the human microbiome is referred to as our second genome or other genome [26,27], and it is implicated in various diseases, including infectious diseases, inflammatory bowel disease, multiple sclerosis, diabetes, allergies, asthma, autism, and cancer [28]. This recognition led to Human Microbiome Therapeutics being named one of the Top 10 Emerging Technologies by the World Economic Forum in 2014 [29], catalyzing the rapid development of microbiome-targeting treatments [30]. The U.S. Food and Drug Administration (FDA) issued guidelines for live biotherapeutic products [31]. In 2022, REBYOTA, a microbiome treatment for recurrent Clostridium difficile infection, became the industry's first FDA-approved product [32]. In 2023, Vowst, the inaugural oral microbiota-based product, gained FDA approval for the disease above [33].
3. Probiotics: An Ancestral Approach to Modern Microbiome Therapeutics and Their Role in Treating Infectious Diseases
The term "probiotic" stems from the Latin "pro" (for, in favor of) and Greek "bios" (life) and is defined as "Live microorganisms which when administered in adequate amounts confer a health benefit on the host" [34,35]. Probiotic applications, not confined to a specific definition, encompass single or multiple strains and are utilized in both live and dead forms, sometimes in conjunction with immune stimulants like prebiotics and synbiotics [36]. The roots of probiotics trace back to the dawn of human history, closely intertwined with the advent of agriculture around 10,000 years ago. The journey from folk medicine to modern medicine began with the pioneering work of Russian scientist Elie Metchnikoff in the early 1900s [37]. Consequently, probiotics exhibit various effects, from providing nutritional benefits to impacting various bodily systems, including the intestines, brain, and skin. They are extensively researched in conditions such as irritable bowel disease, allergies, diabetes, cancer, and infectious diseases [38].
Probiotics exert their effects through diverse mechanisms, including modifying intestinal pH, antagonism against pathogens via antimicrobial compound production, competition for pathogen binding and receptor sites, available nutrients and growth factors, and stimulation of immunomodulatory cells [39]. Consequently, probiotics have been a focal point in addressing infectious diseases such as antibiotic-associated diarrhea, pediatric diarrhea, and travelers' diarrhea. Notably, two-thirds of all randomized controlled clinical trials on probiotics have been dedicated to infectious diseases, according to a report [40]. Given the close relationship between the activity of probiotic bacteria and the host's gastrointestinal condition, coupled with changes in the intestinal microbial population, they hold significant potential as alternatives to antibiotics for preventive and therapeutic purposes in gastrointestinal infectious diseases [41].
Nevertheless, an in-depth understanding of the molecular capabilities of probiotic bacteria is expanding the scope beyond gut-microbe interactions. The subsequent paragraphs briefly outline the mechanisms by which probiotics inhibit pathogenic microorganisms.
4. Mechanism of Action of Probiotics in Inhibiting Pathogenic Microorganisms
Antimicrobial compounds produced by probiotic bacteria directly inhibit competing enteropathogens in the gastrointestinal tract, preventing pathogenic colonization [41]. Firstly, bacteriocins such as nisin, plantaricin, and lacticin, within the range of 2 to 10 kDa, play a crucial role [42]. These peptides have various hydrophobic and hydrophilic properties, promoting attachment to microbial cells and penetration of the phospholipid membrane [43]. In Gram-positive bacteria, they primarily inhibit peptidoglycan synthesis, forming pores. In contrast, in Gram-negative bacteria, DNA, RNA, and protein metabolism inhibition occurs by targeting RNA polymerase, DNA gyrase, and aspartyl-tRNA synthetase [44]. Bacteriocins are also inserted into the target cell membrane, inducing depolarization, dissolving the cell wall, and exhibiting enzymatic activities such as DNase, RNase, and phospholipase [44]. Additionally, uncharacterized bacteriocin-like inhibitory substances with similar activity are presented [45]. Probiotics engage in carbohydrate fermentation, producing organic acids like acetic acid, formic acid, succinic acid, and lactic acid [46]. These membrane-soluble protonated acids dissociate upon entering the neutral cytoplasm through simple diffusion [46]. The resulting acidification influences glycolysis, active transport, signal transduction, and metabolic pathway inhibition, increasing cellular osmolarity and turgor pressure [47,48]. Other critical antimicrobial mechanisms involve hydrogen peroxide, causing highly deleterious DNA damage [49], siderophores, iron-chelating ligands sequestering essential iron [50], and biosurfactants, amphipathic molecules inducing permeability through detergent-like effects, causing leakage and dissolution of cell membranes [51,52].
Probiotics exhibit adhesion properties that contribute to a competitive exclusion effect, preventing intestinal pathogens from attaching to intestinal cells [53]. This adhesion relies on the physical interactions of van der Waals forces between surfaces [54]. Bacterial capsular polysaccharides, teichoic/lipoteichoic acids, lipoproteins, and surface proteins like mucin-binding/fibronectin-binding/collagen-binding proteins collaborate to form multi-cellular aggregates [55]. This process involves clumping with pathogens and adhesion to epithelial cells through co-aggregation [56]. Mucin, extracellular matrix, or lectin-like proteins in the digestive tract further promote the colonization of probiotics [55].
Colonized probiotics enhance the integrity of the intestinal epithelial barrier, thereby preventing the invasion of harmful antigens [57]. Probiotics influence the apoptosis and proliferation of intestinal epithelial cells and elevate the levels of transmembrane proteins, occludin and claudin, and junction adhesion molecules at tight junctions, thereby strengthening the intestinal mechanical barrier [57]. Additionally, probiotics increase mucin production by goblet cells [58] and active IgA by IgA plasma cells [59,60]. They also promote the secretion of cationic beta-defensin-2 from intestinal epithelial cells, thereby permeabilizing the lipid bilayer membranes of pathogens [61] and reinforcing both the chemical and immune barriers.
Probiotics play a role in modulating the host’s innate and adaptive immune responses by regulating immune cells such as dendritic cells, macrophages, and B and T lymphocytes [62]. Regarding probiotics, sampling by M cells in Peyer's patches and subsequent engulfment by the innate immune system’s dendritic cells present microbial antigens to naïve T cells in the mesenteric lymph nodes (MLN) [63]. This process is initiated by the recognition of conserved molecular structures known as microbe-associated molecular patterns (MAMPs) by pattern recognition receptors (PRRs) [64]. Subsequently, the activation of naïve T cells occurs through coordinated interactions between antigen-presenting cells, which noncovalently bind infectious agent-derived antigenic peptides to the major histocompatibility complex (MHC) and the T cell receptor (TCR) along with CD4 or CD8 coreceptors [65]. Lactobacillus has been suggested to promote Th1 differentiation and enhance the phagocytic ability of macrophages by secreting IFN-α1, IFN-β, TNF-α, and IL-12, contributing to the prevention of intraintestinal pathogen infections and specific foodborne diseases such as antiviral responses [66]. Additionally, Enterococcus has been shown to maintain intestinal immune homeostasis by reducing the expression of crucial Th2 immune genes such as IL4, IL5, and CCL26, attenuating the typical polarized helminth-mediated Th2 immune response [67]. Th17 cells eliminate fungal and extracellular bacterial infections that are not efficiently removed by Th1- and Th2-type immunity [68]. Interestingly, recent research indicates that segmented filamentous bacteria (SFB) induce the accumulation of Th17 cells in the intestines of various species, including mice [69]. Furthermore, probiotics can generate FoxP3 T cell responses in the small intestine and induce CD4 and CD8 T cell activation in the colon [70]. As illustrated by the provided examples, probiotic bacteria are known to alter the intestinal microbial community, increasing the activity of immune cells such as Th1, Th2, Th17, and Treg cells, as well as B cells [71]. However, literature based on an accurate understanding of immune modulation for pathogen clearance in infectious diseases is insufficient.
5. Expanding Understanding of Infectious Diseases in the Microbiome Era
Since 2005, the traditional Sanger-based approach to DNA sequence analysis using capillary sequencers has undergone a revolutionary transformation with the introduction of next-generation sequencing (NGS) technology, significantly boosting sequencing data output [72,73]. Over the past two decades, the gradual evolution of culture-independent methods has empowered researchers to sequence microbial communities from environmental samples directly [74]. This shift has brought ecological awareness to microbiology, with metagenomics playing a pivotal role in understanding the influence of microbial communities on human health and disease [75]. Consequently, advancements in the study of the human gut microbiome have led to the discovery of novel functional genes, microbial pathways, antibiotic resistance genes, functional dysbiosis, and interactions and coevolution between the microbiota and the host [76].
In addition to genomics, new molecular biochemical analyses, such as transcriptomics, proteomics, and metabolomics, are now applied to evaluate microbial communities’ genome and gene products beyond the gastrointestinal tract. This includes ecosystems with low microbial densities, such as the skin, airway system, and urogenital tract [77]. These analyses have provided insights into the pathogenesis of various human diseases, and the resulting knowledge is expanding into diagnostic, therapeutic, and preventive measures within the realm of personalized precision medicine [77].
According to a report from the U.S. National Institutes of Health, the funding for microbiome projects related to diseases reached $466 million between fiscal years 2012 and 2016, with infectious diseases being the largest category, followed by digestive diseases, neoplasms, respiratory diseases, genitourinary diseases, and endocrine/metabolic diseases [78]. In this microbiome era, the interaction between the gut microbiome and the host is being identified, extending the understanding of microbiome therapeutics for infectious diseases beyond the gastrointestinal tract to encompass regions such as the oral cavity, respiratory system, etc. [79,80].
6. Microbiome-Based Deciphering in Gastrointestinal Infectious Diseases
Through metagenomic analysis, severe imbalances in the composition of the intestinal microbiome for gastrointestinal-related infectious diseases are being deciphered. Infants suffering from acute and persistent diarrhea showed a collapse of indigenous anaerobic microbial communities such as Firmicutes and Bacteroides and aberrant proliferation of facultative anaerobes Proteobacteria. Major pathogenic lineages in diarrheal samples included Klebsiella, Haemophilus, Rothia, Granulicatella, Chelonobacter, and Vibrio [81]. Children with antibiotic-associated diarrhea experience long-term dysbiosis, and in this case, the disease is correlated with the abundance of Bacteroides, as opposed to Lachnospiraceae and amino acid biosynthesis pathways [82]. The β-diversity of the intestinal microbiome in subjects with travelers' diarrhea significantly differed compared to healthy travelers. Although there was no significant difference in α-diversity, travelers with diarrhea exhibited a characteristic gut microbiome profile with a high ratio of Bacteroidetes to Firmicutes [83]. Metagenomic associations between Helicobacter pylori infection status and changes in the gastric microbial community were also identified. After settling in the stomach, H. pylori becomes the dominant species, leading to a decrease in the Shannon index and subsequent reduction in species diversity. Taxa of Stenotrophomonas, Chryseobacterium, Pedobacter, Variovorax, and Pseudomonas, along with pathways of dTDP-L-rhamnose biosynthesis and tetrapyrrole biosynthesis, were more abundant in infected subjects [84].
As our understanding of the gut microbiome deepens, it becomes increasingly clear that it is an essential participant in the classic interpretation of host-pathogen interactions [85]. Accordingly, many studies have shown that the intestinal microbiota affects pathogen infection in various ways, including direct bacterial antagonism, stimulation of host immunity, and bacterial metabolism [85].
Among these, colonization resistance refers to dynamic antagonistic interactions between commensals and pathogenic flora [86] and, through quorum sensing (QS), which regulates bacterial behavior through secreted chemical signals, it possesses competitive mechanisms [87].
In line with Freter's nutrient-niche hypothesis, whereby intestinal bacteria compete for niche space in intestinal niches such as dietary compounds, they simultaneously engage in substrate competition [88]. They release antimicrobial substances such as small short-chain fatty acids, hydrogen peroxide (H2O2), organic acids, bacteriocins, and lipopeptides, causing changes in environmental conditions [89]. The interaction of bacterial components and pattern recognition receptors expressed on various antigen-presenting cells in the gut epithelium mediate communication between microbial communities and the immune system. This mediation leads to the activation of both innate and adaptive immune responses, playing a crucial role in mucosal homeostasis. Various subsets of immune cells, including dendritic cells, macrophages, T cells, B cells, and the secretion of polymeric immunoglobulin A (IgA), generate appropriate immune responses against invading pathogens without inducing overt inflammation [90]. Gut microbial communities metabolize proteins and complex carbohydrates, synthesize vitamins, and mediate crosstalk between gut epithelial and immune cells, producing an immense array of metabolic by-products. Specifically, short-chain fatty acids (SCFAs), the most abundant microbial-derived metabolites in the intestinal lumen, primarily activate G-protein coupled receptors (GPCRs), thereby maintaining barrier integrity and protecting against pathogen invasion [91]. A healthy and balanced composition of microbial communities, known as Eubiosis, contributes to metabolic functions, protects against pathogens, and provides nutrients and energy to the host. This equilibrium significantly impacts health and disease states, including infectious diseases, such as C. difficile infection, induced by an imbalance in microbial diversity, referred to as dysbiosis [92].
Elucidation of the role of the host microbiome in health and infectious diseases has led to the development of live biotherapeutic products (LBPs) [93]. In patients with C. difficile infection, 20 to 40% relapse even after standard treatment with metronidazole or vancomycin, as the indigenous microflora cannot recover after antibiotic use [94]. C. difficile-directed antibiotics are associated with a decrease in α-diversity and differential relative abundance of bacterial and fungal assemblages, potentially enhancing microbial dysbiosis, a crucial determinant of relapse [95]. Against this backdrop, fecal microbiota transplantation (FMT), which restores native intestinal microflora by introducing microorganisms from a healthy donor, has emerged and shown a 90% success rate for recurrent C. difficile infection [96]. In homeostasis after FMT, secondary bile acids, deoxycholic acid (DCA), and lithocholic acid (LCA), produced by 7α-dehydroxylation of primary bile acid deconjugation mediated by microorganisms such as Clostridium scindens, inhibit the spore and vegetative growth of C. difficile [97,98]. Additionally, signals and metabolites such as SCFAs from the microbiota stimulate the mucosa to strengthen epithelial tight junctions and form a thick mucus layer containing antimicrobial peptides and high concentrations of secreted immunoglobulins. They also provide signals such as IL-10, TGFβ, and IL-22 to maintain the mucosal immune system’s non-inflammatory tone and epithelial homeostasis [99]. In this trend, REBYOTA, a fecal microbiota product for rectal administration mentioned above, and Vowst, an oral-fecal microbiota [100].
7. Understanding Nonintestinal Infectious Diseases Based on Microbiome-Host Communication
Microbial communities forming a mutualistic relationship with mammalian hosts can influence various physiological functions by regulating the host's immune system [101]. Recent studies indicate that specific bacteria inhabiting defined niches transmit distinct signals, affecting the functions of both innate and adaptive immune systems. This, in turn, can lead to distal systemic outcomes from the colonization site [101]. Especially in respiratory infections, changes in the composition of the gut microbial community components due to diet, disease, or medical interventions (such as antibiotics) are closely related to alterations in immune responses and homeostasis in the airways through what is termed the 'gut–lung axis' [102].
This phenomenon involves bidirectional communication as part of the shared mucosal immune system [103]. Specifically, a well-developed gut microbiome may enhance resistance to pathogens causing respiratory infections. In contrast, alterations in the gut microbiome and its products during respiratory conditions may influence harmful pathogenic transformations [104]. This concept has been pivotal in microbiome research, notably in tuberculosis. Significant changes in the diversity of intestinal microbes were observed in tuberculosis patients, characterized by a dramatic reduction in microbial diversity, particularly a substantial decrease in bacteria generating SCFAs and related metabolic pathways [105]. SCFAs, potentially involved as chemical messengers in the gut-lung axis, regulate immune responses by binding to GPCRs, including GPR41, GPR43, and GPR109A. Depending on downstream signaling, these receptors can either promote inflammation or exert anti-inflammatory effects [106]. Therefore, the gut microbial community imbalance could precede and contribute to Mycobacterium tuberculosis infection. Concerningly, severe disruptions in the gut microbial community induced by anti-tuberculosis treatment may increase susceptibility to subsequent reinfection or relapse of tuberculosis [107]. In vivo experiments have shown that the bacterial imbalance in the gut microbial community following anti-tuberculosis treatment increased the tuberculosis bacilli burden. Interestingly, this increased susceptibility was reversed by the transplantation of untreated mice's fecal microbial communities [108].
While commensal microbes are predominantly studied in the gut, they also exist on the mucosal surfaces throughout the body. The lungs are no exception, and although considered sterile for decades, recent years have increasingly clarified the presence of a true microbial community in the respiratory tracts of mammals [109]. The pulmonary microbial community is believed to modulate the risk and outcomes of COVID-19 by activating innate and adaptive immune responses. Consequently, an imbalance in the pulmonary microbial community may contribute to acute respiratory distress syndrome (ARDS) in COVID-19 [110]. Indeed, COVID-19 patients showed changes in the bacterial microbial community in the lower respiratory tract during virus infection. Patients exhibited a noticeably high abundance of opportunistic pathogens, especially Acinetobacter baumannii and Candida spp., which correlated positively with inflammatory markers [111]. In essence, SARS-CoV-2 infection, by disrupting lung microbiota eubiosis, may enhance the cytokine storm in the lungs, potentially leading to secondary pathogen infections in COVID-19 patients. Pathogen-associated molecular patterns (PAMPs) released from invading opportunistic pathogens are recognized by host innate lymphocytes, including macrophages and dendritic cells, through PRRs such as Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs). This recognition induces the expression of proinflammatory factors through NF-κB signaling, interferons through IRF3 signaling, and numerous interferon-stimulated genes (ISGs) through JAK/STAT signaling [112]. Such studies may aid in selecting optimal treatments for respiratory infections caused by SARS-CoV-2 [111].
Most Human Immunodeficiency Virus type 1 (HIV-1) infections occur through heterosexual transmission during vaginal–penile sex. The local immune environment at the site of HIV exposure is a crucial factor in determining whether exposure during sex leads to productive infection [113]. The vaginal and penile immune milieus are shaped by local microbiomes, with anaerobic taxa like Prevotella increasing the local density of HIV target cells expressing CCR5 and CD4, thereby increasing the risk of HIV infection. Conversely, the presence of Lactobacillus in the vagina and Corynebacterium in the penile region is associated with reduced inflammation and lower infection risk [113]. Moreover, HIV-1 infection induces changes in diversity and composition, leading to disrupted epithelial barriers, pathogen translocation, increased local inflammation, and the activation of myeloid dendritic cells. This activation drives increased T cell activation and the loss of protective Th17 and Th22 cells, contributing to epithelial barrier breakdown and promoting microbial translocation, potentially fostering bacterial imbalance and inflammation [114]. The potential "crosstalk" between viral and bacterial pathogens in HIV-infected individuals could aid in understanding the role of microbial communities. This insight may facilitate the identification of new antiretroviral factors for use in novel therapeutics [115].
Microbiome research in infectious diseases, including highly resistant and notorious superbugs against antibiotics, is ongoing [116]. As an extension, developing next-generation probiotics is expected to reduce healthcare-associated infections [117]. However, research on the microbiome related to Biosafety level 4 (BSL4) viruses, such as Ebola, one of the deadliest pathogens, is nonexistent. Future pioneering studies on this are imperative.
8. Microbiome Therapeutics in the Management of Infectious Disease
The manipulation of human microbial communities holds significant potential for mitigating the occurrence and severity of various human conditions and diseases. The biomedical research community is actively translating its understanding of microbial communities into beneficial medical therapies [118]. Microbial communities, which share an extensive interface with the host's immune system, emerge as excellent candidates for biomarker development [119]. The Linear Discriminant Analysis Effect Size (LEfSe) is a valuable tool that facilitates the identification of genomic biomarkers characterized by statistical differences between biological groups [120].
Microbiome therapeutics aim to manipulate microbial communities through addition, subtraction, or control strategies to maintain homeostasis, resilience, and resistance to disturbances [121,122]. Rich taxonomic biomarkers in healthy individuals can be approached as potential targets, utilizing technologies such as new organism–media pairings based on culturomics [123,124]. Conversely, a targeted antimicrobial approach is feasible for diseases rich in biomarkers. This involves treating infections by targeting major pathogens while restoring beneficial and healthy microbial flora [125]. For instance, in a microbiome analysis study on pulmonary tuberculosis patients, Bifidobacterium emerged as the genus with the highest Linear Discriminant Analysis (LDA) score in healthy individuals. At the same time, Bacteroides dominated in the patient group [126]. This suggests the potential for an approach involving probiotics for the former and targeted antimicrobials for the latter. Moreover, the analysis of genetically engineered probiotics can be pursued to develop functional biomarkers [127].
Bidirectional communication between humans and their microbial cohabitants is evident, and this dialogue can significantly impact our health in various ways [128]. Bacterial extracellular vesicles (BEVs) from the microbial community of the host's gut can enter the circulatory system, disseminating to distant organs and tissues. Consequently, interest has grown in therapeutic approaches related to BEVs, involving intra and inter-kingdom communication, nutrient delivery within microbial communities, virulence factor transfer, horizontal gene transfer, and modulation of host immunity [129]. BEVs contain numerous MAMPs/PAMPs, including LPS, lipoproteins, peptidoglycan, and bacterial nucleic acids. These engage immune and non-immune cells' PRRs, potentially conferring protective immunity, immunological tolerance, or promoting host pathology [129]. BEVs are presumed to promote maturation and immunological tolerance, protecting against conditions like colitis or sepsis [130]. Simultaneously, the release of exosomes by viruses, parasites, fungi, and bacteria infections is gaining attention for its ability to stimulate or inhibit host immunity by providing antigens to antigen-presenting cells [131].
Comparative mouse studies between germ-free and colonized mice have demonstrated the significant impact of the gut microbial community on the metabolome in distant body sites, including the kidneys, liver, and plasma. This suggests that the microbial community influences the biochemical environment, impacting health and disease [132]. The interaction between microbes in the human body and their metabolites, influencing disease risk, is beginning to be elucidated. Discoveries in this field hold high potential for developing preventive and therapeutic strategies for complex diseases [132]. Strategies are being explored to decode the microbiome-metabolome dialog concerning commensals’ metabolic activity and immunoregulatory properties, particularly in conditions like respiratory fungal diseases. The goal is to develop personalized medical interventions for patients at high risk of infection [133]. The metabolism of dietary fiber by gut microbes influences allergic airway disease and hematopoiesis [134]. This metabolic activity, dependent on G protein–coupled receptor 41 (GPR41), generates bone marrow hematopoiesis characterized by enhanced phagocytic capacity in macrophages and dendritic cell precursors. This leads to lung seeding by impaired dendritic cells that promote T helper type 2 (TH2) cell effector function [134]. Additionally, indole-3-aldehyde, a tryptophan derivative produced by Lactobacillus in the gut microbiome, acts as an aryl hydrocarbon receptor ligand, contributing to the expression of IL-22 and regulating tolerance to Candida albicans in the vaginal microbiome [135]. Therapeutic approaches based on metabolites provide a direct and feasible strategy to address the impact of microbial imbalance on the host. Metabolite-based therapeutic strategies are becoming highly promising [136].
Microbiome therapeutics extend to various interventions, such as intragastric, intravaginal, and intranasal routes, for inhibiting infections in intestinal, urogenital/vaginal, and respiratory infections. Advancements in microbial community analysis tools and technologies are opening up new potential pharmaceutical drugs [137,138].
9. Complex Defense Strategies: Resistance and Tolerance in Microbiome Therapeutics
Traditionally, clinical solutions for infectious diseases have predominantly focused on efficiently eradicating pathogens. However, the COVID-19 pandemic has emphasized infectious diseases’ complexity and multisystemic nature, highlighting the need for a holistic understanding to maximize survival [139]. In the context of COVID-19, the disease's states and potential therapeutic goals vary. For mild initial infections (Phase 1), antiviral treatments like remdesivir aim to reduce symptom duration, minimize infectivity, and prevent progression to severe conditions [140]. In contrast, during Phase 3, characterized by systemic hyperinflammation, immunomodulatory agents, including corticosteroids, IL-6 inhibitors like tocilizumab, and IL-1 receptor antagonists like anakinra, are used to reduce systemic inflammation before causing multiorgan dysfunction [140]. Controversies arise, particularly in severe COVID-19 cases, as observations question the benefits of treatments like lopinavir–ritonavir and suggest delays in virus clearance with steroid use, potentially increasing the duration of hospital stays [141,142]. This underscores the disconnection between methods to treat infections and mechanisms that promote the survival of infections.
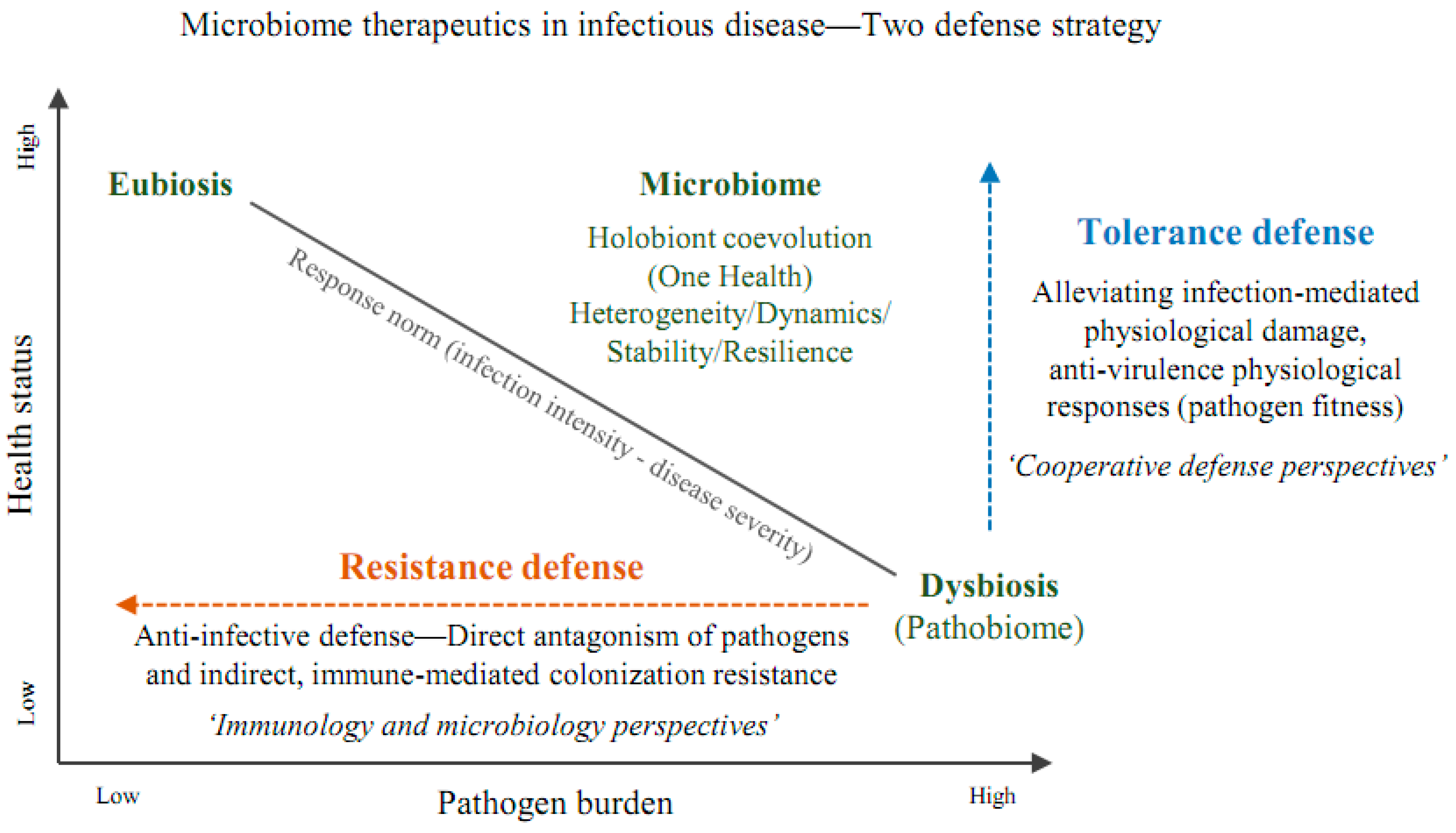
Traditional immunology has focused on mechanisms directly attacking invading microbes to block or remove them. However, recognizing the importance of defense mechanisms limiting infection damage has gained prominence. Consequently, interpreting the host's defense abilities is crucial from the perspectives of resistance and tolerance [143]. Hosts can evolve two types of defense mechanisms, resistance or tolerance, to enhance fitness when challenged by pathogens. Understanding these defenses allows for more efficient treatments for infectious diseases and better explanations of host-pathogen interactions [143]. The microbiome plays a role in evolving a complex mechanism called colonization resistance, including nutrient competition, competitive metabolic interactions, niche exclusion, and induction of host immune responses to inhibit pathogen growth [144]. This is associated with enhancing resistance. Probiotics, for instance, enhance non-immunological defenses, stimulate specific and nonspecific host immune responses, and serve as anti-infective defenses against antibiotic-related conditions like viral gastroenteritis and C. difficile infection [145]. A healthy gut microbiome, rich in Bifidobacterium, Faecalibacterium, Ruminococcus, and Prevotella, is associated with low systemic inflammation. Probiotics, regulating host immune responses, are proposed as an alternative to cope with the 'cytokine storm' during COVID-19 infection, reflecting defense related to tolerance [146]. In a randomized, quadruple-blinded, placebo-controlled trial involving COVID-19 patients, probiotics alleviated symptoms and increased SARS-CoV-2-specific IgM and IgG while reducing nasopharyngeal viral load [147]. These results reflect the composite defense characteristics of resistance and tolerance in microbiome therapeutics. When applied to a reaction norms model concerning disease severity based on infection intensities [148], the interpretation is that the curve of the reaction norm shifts upward due to enhanced resistance, simultaneously with an improvement in tolerance leading to a softened slope of the curve.
As described above, two defense strategies based on resistance and tolerance for microbiome therapeutics are presented in
Figure 1.
10. Understanding the Mechanism of Microbiome Therapeutics Based on a Cooperative Defense System (Future Perspectives)
For the past 50 years, research in immunology and infectious diseases has been rooted in assumptions based on activating immune responses and eliminating pathogens when infected. Efforts have been concentrated on developing vaccines and antibiotics and elucidating immune response mechanisms for infection control [149]. However, a challenge to this mindset arose from cohort studies of SARS-CoV-2 infection, revealing a similarity in virus burden between symptomatic and asymptomatic patients [150]. The discovery of a cooperative defense system, allowing hosts to adapt to pathogens through disease resistance and antiviral defense health mechanisms, has initiated our understanding of this phenomenon [149]. Host-microbe interactions, rarely strictly defined as pathogenic, benign, or beneficial, have begun to be understood through cooperative metabolic strategies that can transition pathogens into a state of symbiosis [151]. As a result, hosts become healthy reservoirs for pathogens, promoting increased transmission to new hosts, such as asymptomatic persistent shedders. This can generate pathogen strains with higher fitness than the parental strain, as experimentally demonstrated [151]. The importance of maintaining a healthy microbiome in overcoming infectious diseases has been extensively documented, possibly interpreted as part of the detoxification of high-threat pathogens through cooperative metabolic strategies. A comprehensive interpretation from the perspective of the pathogen-microbiome-host immuno-metabolic network should follow. We need to learn more about host-encoded tolerance mechanisms based on the microbiome. Integrating the concept of tolerance into host-microbe studies for microbiome therapeutics is an essential challenge for a fundamental understanding of infectious disease treatments.
11. Conclusions
The microbiome encompasses the composition of microbial communities, spatiotemporal heterogeneity/dynamics, network stability/resilience, and the co-evolutionary/holistic characteristics within the framework of the one health concept [152]. On the other hand, defense strategies against microbes are commonly perceived in terms of the immune system's functionality in detecting and eliminating microbes by executing resistance mechanisms [153]. However, this perspective poses challenges in understanding infection response strategies based on host-microbe interactions from a genuine microbiome standpoint. Therefore, a shift in perspective is needed from "how to combat infectious diseases" to "how to survive infections" under the viewpoint of a cooperative defense system [16].
Author Contributions
Conceptualization, H-.Y.S. and H.S.; methodology, H.S.; investigation, H.S.; writing—original draft preparation, H.S.; writing—review and editing, H.S. and H-.Y.S.; supervision, H-.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea's Leading Research Center project (RS-2023-00219563) under the Ministry of Science and ICT. This work was also supported by the MOTIE (Ministry of Trade, Industry, and Energy, Korea)'s Industrial innovation infrastructure construction project (P0021516). This research was also supported by the Soonchunhyang University Research Fund.
Institutional Review Board Statement
Not applicable as this work does not involve human subjects.
Informed Consent Statement
Not applicable as this work does not involve human subjects.
Data Availability Statement
No data sets were generated by the author for this paper. Data presented in the context of this review have been previously published by others and cited within the manuscript.
Acknowledgments
I would like to thank all the researchers of HMMMC at Soonchunhyang University for helping with this study and Uhjin Song, M.D., for providing medical advice and English proofreading for this manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1. WHO. World Health Statistics 2023. Worldwide Health Organization 2023.
- 2. WHO. The top 10 causes of death. Worldwide Health Organization 2020.
- 3. WHO. Global excess deaths associated with COVID-19, January 2020 - December 2021. Worldwide Health Organization 2022.
- 4. Weiss, R.A.; McMichael, A.J. Social and environmental risk factors in the emergence of infectious diseases. Nat Med 2004, 10, S70-76. https://doi.org/10.1038/nm1150. [CrossRef]
- 5. Wainwright, M. Molds in Folk Medicine. Folklore 1989, 100, 162-166. https://doi.org/10.1080/0015587x.1989.9715763. [CrossRef]
- 6. Nuesch, J. Microbiology - an Important Discipline in a Changing World. Fems Microbiol Lett 1992, 100, 5-6. https://doi.org/10.1111/j.1574-6968.1992.tb05671.x. [CrossRef]
- 7. Terry, L.L. The Public Health Service role in medical care administration. Public Health Rep (1896) 1962, 77, 93-96.
- 8. Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: past, present and future. Curr Opin Microbiol 2019, 51, 72-80. https://doi.org/10.1016/j.mib.2019.10.008. [CrossRef]
- 9. Plotkin, S. History of vaccination. Proc Natl Acad Sci U S A 2014, 111, 12283-12287. https://doi.org/10.1073/pnas.1400472111. [CrossRef]
- 10. De Clercq, E. Antivirals: past, present and future. Biochem Pharmacol 2013, 85, 727-744. https://doi.org/10.1016/j.bcp.2012.12.011. [CrossRef]
- 11. Smith, E.B. History of antifungals. J Am Acad Dermatol 1990, 23, 776-778. https://doi.org/10.1016/0190-9622(90)70286-q. [CrossRef]
- 12. Ardal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development - economic, regulatory and societal challenges. Nat Rev Microbiol 2020, 18, 267-274. https://doi.org/10.1038/s41579-019-0293-3. [CrossRef]
- 13. Munita, J.M.; Bayer, A.S.; Arias, C.A. Evolving Resistance Among Gram-positive Pathogens. Clinical Infectious Diseases 2015, 61, S48-S57. https://doi.org/10.1093/cid/civ523. [CrossRef]
- 14. Jackson, R.W.; Johnson, L.J.; Clarke, S.R.; Arnold, D.L. Bacterial pathogen evolution: breaking news. Trends Genet 2011, 27, 32-40. https://doi.org/10.1016/j.tig.2010.10.001. [CrossRef]
- 15. Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589-1603. https://doi.org/10.1016/j.cell.2021.02.030. [CrossRef]
- 16. Ayres, J.S. Surviving COVID-19: A disease tolerance perspective. Science Advances 2020, 6. https://doi.org/10.1126/sciadv.abc1518. [CrossRef]
- 17. Ayres, J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab 2020, 2, 572-585. https://doi.org/10.1038/s42255-020-0237-2. [CrossRef]
- 18. Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical?therapeutic staging proposal. J Heart Lung Transpl 2020, 39, 405-407. https://doi.org/10.1016/j.healun.2020.03.012. [CrossRef]
- 19. Schneider, D.S.; Ayres, J.S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 2008, 8, 889-895. https://doi.org/10.1038/nri2432. [CrossRef]
- 20. Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: a proposal. Microbiome 2015, 3, 31. https://doi.org/10.1186/s40168-015-0094-5. [CrossRef]
- 21. Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 2020, 8, 103. https://doi.org/10.1186/s40168-020-00875-0. [CrossRef]
- 22. Human Microbiome Project, C. A framework for human microbiome research. Nature 2012, 486, 215-221. https://doi.org/10.1038/nature11209. [CrossRef]
- 23. Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 2016, 14, e1002533. https://doi.org/10.1371/journal.pbio.1002533. [CrossRef]
- 24. Qin, J.J.; Li, R.Q.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59-U70. https://doi.org/10.1038/nature08821. [CrossRef]
- 25. Montenegro, J.; Armet, A.M.; Willing, B.P.; Deehan, E.C.; Fassini, P.G.; Mota, J.F.; Walter, J.; Prado, C.M. Exploring the Influence of Gut Microbiome on Energy Metabolism in Humans. Adv Nutr 2023, 14, 840-857. https://doi.org/10.1016/j.advnut.2023.03.015. [CrossRef]
- 26. Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu Rev Genom Hum G 2012, 13, 151-170. https://doi.org/10.1146/annurev-genom-090711-163814. [CrossRef]
- 27. Zhao, L.P. GENOMICS The tale of our other genome. Nature 2010, 465, 879-880. https://doi.org/10.1038/465879a. [CrossRef]
- 28. Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Medicine 2016, 8. [CrossRef]
- 29. WEF. Top 10 Emerging Technologies 2014. World Economic Forum 2014.
- 30. Mimee, M.; Citorik, R.J.; Lu, T.K. Microbiome therapeutics - Advances and challenges. Adv Drug Deliver Rev 2016, 105, 44-54. https://doi.org/10.1016/j.addr.2016.04.032. [CrossRef]
- 31. FDA. FDA Approves First Fecal Microbiota Product Rebyota Approved for the Prevention of Recurrence of Clostridioides difficile Infection in Adults. FDA NEWS RELEASE 2022.
- 32. FitzGerald, M.J.; Spek, E.J. Microbiome therapeutics and patent protection. Nat Biotechnol 2020, 38, 806-810. https://doi.org/10.1038/s41587-020-0579-z. [CrossRef]
- 33. FDA, U.S. FDA Approves First Orally Administered Fecal Microbiota Product for the Prevention of Recurrence of Clostridioides difficile Infection. U.S. Food and Drug Administration 2023.
- 34. Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 2012, 46 Suppl, S1-2. https://doi.org/10.1097/MCG.0b013e318269fdd5. [CrossRef]
- 35. FAO/WHO. Health and Nutrition Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria, Probiotics in food, Health and nutritional properties and guidelines for evaluation. FAO and WHO 2001, 85.
- 36. Reid, G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol 2016, 30, 17-25. https://doi.org/10.1016/j.bpg.2015.12.001. [CrossRef]
- 37. Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J Clin Gastroenterol 2016, 50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015, S116-S119. https://doi.org/10.1097/MCG.0000000000000697. [CrossRef]
- 38. Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann Nutr Metab 2019, 74, 115-124. https://doi.org/10.1159/000496426. [CrossRef]
- 39. Kopp-Hoolihan, L. Prophylactic and therapeutic uses of probiotics: A review. J Am Diet Assoc 2001, 101, 229-241. https://doi.org/10.1016/S0002-8223(01)00060-8. [CrossRef]
- 40. McFarland, L.V. From Yaks to Yogurt: The History, Development, and Current Use of Probiotics. Clinical Infectious Diseases 2015, 60, S85-S90. https://doi.org/10.1093/cid/civ054. [CrossRef]
- 41. van Zyl, W.F.; Deane, S.M.; Dicks, L.M.T. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12. [CrossRef]
- 42. Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Fighting biofilms with lantibiotics and other groups of bacteriocins. Npj Biofilms Microbi 2018, 4. [CrossRef]
- 43. Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. Journal of the American Academy of Dermatology 2005, 52, 381-392. https://doi.org/10.1016/j.jaad.2004.08.026. [CrossRef]
- 44. Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int J Microbiol 2020, 2020. [CrossRef]
- 45. Prasad, S.; Morris, P.C.; Hansen, R.; Meaden, P.G.; Austin, B. A novel bacteriocin-like substance (BLIS) from a pathogenic strain of. Microbiol-Sgm 2005, 151, 3051-3058. https://doi.org/10.1099/mic.0.28011-0. [CrossRef]
- 46. Russell, J.B.; DiezGonzalez, F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol 1998, 39, 205-234.
- 47. Lambert, R.J.; Stratford, M. Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol 1999, 86, 157-164. https://doi.org/10.1046/j.1365-2672.1999.00646.x. [CrossRef]
- 48. Roe, A.J.; O'Byrne, C.; McLaggan, D.; Booth, I.R. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology (Reading) 2002, 148, 2215-2222. https://doi.org/10.1099/00221287-148-7-2215. [CrossRef]
- 49. Zhang, C.; Zhang, S.; Liu, W.; Guo, T.; Gu, R.; Kong, J. Potential Application and Bactericidal Mechanism of Lactic Acid-Hydrogen Peroxide Consortium. Appl Biochem Biotechnol 2019, 189, 822-833. https://doi.org/10.1007/s12010-019-03031-z. [CrossRef]
- 50. Patel, A.K.; Deshattiwar, M.K.; Chaudhari, B.L.; Chincholkar, S.B. Production, purification and chemical characterization of the catecholate siderophore from potent probiotic strains of Bacillus spp. Bioresour Technol 2009, 100, 368-373. https://doi.org/10.1016/j.biortech.2008.05.008. [CrossRef]
- 51. Heerklotz, H.; Seelig, J. Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur Biophys J 2007, 36, 305-314. https://doi.org/10.1007/s00249-006-0091-5. [CrossRef]
- 52. Hajfarajollah, H.; Eslami, P.; Mokhtarani, B.; Akbari Noghabi, K. Biosurfactants from probiotic bacteria: A review. Biotechnol Appl Biochem 2018, 65, 768-783. https://doi.org/10.1002/bab.1686. [CrossRef]
- 53. Van Tassell, M.L.; Miller, M.J. Lactobacillus adhesion to mucus. Nutrients 2011, 3, 613-636. https://doi.org/10.3390/nu3050613. [CrossRef]
- 54. Duary, R.K.; Rajput, Y.S.; Batish, V.K.; Grover, S. Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J Med Res 2011, 134, 664-671. https://doi.org/10.4103/0971-5916.90992. [CrossRef]
- 55. Zawistowska-Rojek, A.; Kosmider, A.; Stepien, K.; Tyski, S. Adhesion and aggregation properties of strains as protection ways against enteropathogenic bacteria. Archives of Microbiology 2022, 204. [CrossRef]
- 56. Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P(6A) and Lactobacillus gasseri P(65). Microb Cell Fact 2017, 16, 155. https://doi.org/10.1186/s12934-017-0769-7. [CrossRef]
- 57. Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Frontiers in Microbiology 2022, 13. [CrossRef]
- 58. Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and Lactic Acid Bacteria Probiotics Influences Intestinal Mucin Gene Expression, Histomorphology and Growth Performance in Broilers. Asian-Australas J Anim Sci 2012, 25, 1285-1293. https://doi.org/10.5713/ajas.2012.12110. [CrossRef]
- 59. Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 1998, 42, 39-44. https://doi.org/10.1016/S0168-1605(98)00056-7. [CrossRef]
- 60. Mei, L.Y.; Chen, Y.; Wang, J.L.; Lu, J.; Zhao, J.X.; Zhang, H.; Wang, G.; Chen, W. Stimulates Intestinal Secretion of Immunoglobulin A in an Individual-Specific Manner. Foods 2022, 11. [CrossRef]
- 61. Wehkamp, J.; Harder, J.; Wehkamp, K.; Wehkamp-von Meissner, B.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-κB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Nissle 1917:: A novel effect of a probiotic bacterium. Infect Immun 2004, 72, 5750-5758. https://doi.org/10.1128/Iai.72.10.5750-5758.2004. [CrossRef]
- 62. Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12. https://doi.org/10.3390/cells12010184. [CrossRef]
- 63. Corthésy, B.; Gaskins, H.R.; Mercenier, A. Cross-Talk between Probiotic Bacteria and the Host Immune System1,2. The Journal of Nutrition 2007, 137, 781S-790S. https://doi.org/10.1093/jn/137.3.781S. [CrossRef]
- 64. Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microbial Cell Factories 2011, 10. [CrossRef]
- 65. Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: naive to memory and everything in between. Adv Physiol Educ 2013, 37, 273-283. https://doi.org/10.1152/advan.00066.2013. [CrossRef]
- 66. Ren, D.Y.; Wang, D.; Liu, H.Y.; Shen, M.H.; Yu, H.S. Two strains of probiotic enhance immune response and promote naive T cell polarization to Th1. Food Agr Immunol 2019, 30, 281-295. https://doi.org/10.1080/09540105.2019.1579785. [CrossRef]
- 67. Myhill, L.J.; Stolzenbach, S.; Mejer, H.; Krych, L.; Jakobsen, S.R.; Kot, W.; Skovgaard, K.; Canibe, N.; Nejsum, P.; Nielsen, D.S.; et al. Parasite-Probiotic Interactions in the Gut: sp. and Regulate Type-2 Inflammatory Responses and Modify the Gut Microbiota of Pigs During Helminth Infection. Frontiers in Immunology 2022, 12. [CrossRef]
- 68. Owaga, E.; Hsieh, R.H.; Mugendi, B.; Masuku, S.; Shih, C.K.; Chang, J.S. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. Int J Mol Sci 2015, 16, 20841-20858. https://doi.org/10.3390/ijms160920841. [CrossRef]
- 69. Tanabe, S. The Effect of Probiotics and Gut Microbiota on Th17 Cells. Int Rev Immunol 2013, 32, 511-525. https://doi.org/10.3109/08830185.2013.839665. [CrossRef]
- 70. Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; de Vos, P. Probiotics Can Generate FoxP3 T-Cell Responses in the Small Intestine and Simultaneously Inducing CD4 and CD8 T Cell Activation in the Large Intestine. Plos One 2013, 8. [CrossRef]
- 71. Guo, N.; Lv, L.L. Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immun Inflamm Dis 2023, 11. [CrossRef]
- 72. Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum Immunol 2021, 82, 801-811. https://doi.org/10.1016/j.humimm.2021.02.012. [CrossRef]
- 73. Mardis, E.R. A decade's perspective on DNA sequencing technology. Nature 2011, 470, 198-203. https://doi.org/10.1038/nature09796. [CrossRef]
- 74. Bragg, L.; Tyson, G.W. Metagenomics Using Next-Generation Sequencing. In Environmental Microbiology: Methods and Protocols, Paulsen, I.T., Holmes, A.J., Eds.; Humana Press: Totowa, NJ, 2014; pp. 183-201.
- 75. Martín, R.; Miquel, S.; Langella, P.; Bermúdez-Humarán, L.G. The role of metagenomics in understanding the human microbiome in health and disease. Virulence 2014, 5, 413-423. https://doi.org/10.4161/viru.27864. [CrossRef]
- 76. Wang, W.L.; Xu, S.Y.; Ren, Z.G.; Tao, L.; Jiang, J.W.; Zheng, S.S. Application of metagenomics in the human gut microbiome. World J Gastroenterol 2015, 21, 803-814. https://doi.org/10.3748/wjg.v21.i3.803. [CrossRef]
- 77. Blum, H.E. The human microbiome. Adv Med Sci 2017, 62, 414-420. https://doi.org/10.1016/j.advms.2017.04.005. [CrossRef]
- 78. Proctor, L.; LoTempio, J.; Marquitz, A.; Daschner, P.; Xi, D.; Flores, R.; Brown, L.; Ranallo, R.; Maruvada, P.; Regan, K.; et al. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007-2016. Microbiome 2019, 7. [CrossRef]
- 79. Lv, L.X.; Jiang, H.Y.; Yan, R.; Li, L.J. Interactions Between Gut Microbiota and Hosts and Their Role in Infectious Diseases. Infect Microbe Dis 2019, 1, 3-9. https://doi.org/10.1097/Im9.0000000000000001. [CrossRef]
- 80. Hatakka, K.; Saxelin, M. Probiotics in intestinal and non-intestinal infectious diseases - Clinical evidence. Curr Pharm Design 2008, 14, 1351-1367. https://doi.org/10.2174/138161208784480162. [CrossRef]
- 81. Thakur, N.; Changotra, H.; Grover, N.; Vashistt, J. Elucidation of bacterial species during childhood diarrhea through 16S rRNA Illumina Miseq approach. Meta Gene 2018, 16, 234-240. https://doi.org/10.1016/j.mgene.2018.03.012. [CrossRef]
- 82. Kwon, J.; Kong, Y.; Wade, M.; Williams, D.J.; Creech, C.B.; Evans, S.; Walter, E.B.; Martin, J.M.; Gerber, J.S.; Newland, J.G.; et al. Gastrointestinal Microbiome Disruption and Antibiotic-Associated Diarrhea in Children Receiving Antibiotic Therapy for Community-Acquired Pneumonia. Journal of Infectious Diseases 2022, 226, 1109-1119. https://doi.org/10.1093/infdis/jiac082. [CrossRef]
- 83. Youmans, B.P.; Ajami, N.J.; Jiang, Z.D.; Campbell, F.; Wadsworth, W.D.; Petrosino, J.F.; DuPont, H.L.; Highlander, S.K. Characterization of the human gut microbiome during travelers' diarrhea. Gut Microbes 2015, 6, 110-119. https://doi.org/10.1080/19490976.2015.1019693. [CrossRef]
- 84. Wang, D.M.; Zhang, T.D.; Lu, Y.Q.; Wang, C.Z.; Wu, Y.M.; Li, J.D.; Tao, Y.; Deng, L.; Zhang, X.Y.; Ma, J.M. infection affects the human gastric microbiome, as revealed by metagenomic sequencing. Febs Open Bio 2022, 12, 1188-1196. https://doi.org/10.1002/2211-5463.13390. [CrossRef]
- 85. Leslie, J.L.; Young, V.B. The rest of the story: the microbiome and gastrointestinal infections. Curr Opin Microbiol 2015, 23, 121-125. https://doi.org/10.1016/j.mib.2014.11.010. [CrossRef]
- 86. Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Frontiers in Microbiology 2019, 9. [CrossRef]
- 87. Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 7, 163-170. https://doi.org/10.1080/19490976.2016.1145374. [CrossRef]
- 88. Stecher, B.; Berry, D.; Loy, A. Colonization resistance and microbial ecophysiology: using gnotobiotic mouse models and single-cell technology to explore the intestinal jungle. Fems Microbiology Reviews 2013, 37, 793-829. https://doi.org/10.1111/1574-6976.12024. [CrossRef]
- 89. Drider, D. Gut Microbiota is an Important Source of Bacteriocins and Their In Situ Expression Can Be Explored for Treatment of Bacterial Infections. Probiotics Antimicro 2021, 13, 1759-1765. https://doi.org/10.1007/s12602-021-09843-y. [CrossRef]
- 90. Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult Sci 2020, 99, 1906-1913. https://doi.org/10.1016/j.psj.2019.12.011. [CrossRef]
- 91. Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8. https://doi.org/10.3390/microorganisms8101587. [CrossRef]
- 92. Gupta, A.; Singh, V.; Mani, I. Dysbiosis of human microbiome and infectious diseases. Prog Mol Biol Transl Sci 2022, 192, 33-51. https://doi.org/10.1016/bs.pmbts.2022.06.016. [CrossRef]
- 93. Ducarmon, Q.R.; Kuijper, E.J.; Olle, B. Opportunities and Challenges in Development of Live Biotherapeutic Products to Fight Infections. J Infect Dis 2021, 223, S283-S289. https://doi.org/10.1093/infdis/jiaa779. [CrossRef]
- 94. Seekatz, A.M.; Aas, J.; Gessert, C.E.; Rubin, T.A.; Saman, D.M.; Bakken, J.S.; Young, V.B. Recovery of the gut microbiome following fecal microbiota transplantation. mBio 2014, 5, e00893-00814. https://doi.org/10.1128/mBio.00893-14. [CrossRef]
- 95. Lamendella, R.; Wright, J.R.; Hackman, J.; McLimans, C.; Toole, D.R.; Bernard Rubio, W.; Drucker, R.; Wong, H.T.; Sabey, K.; Hegarty, J.P.; et al. Antibiotic Treatments for Clostridium difficile Infection Are Associated with Distinct Bacterial and Fungal Community Structures. mSphere 2018, 3. https://doi.org/10.1128/mSphere.00572-17. [CrossRef]
- 96. Austin, M.; Mellow, M.; Tierney, W.M. Fecal microbiota transplantation in the treatment of Clostridium difficile infections. Am J Med 2014, 127, 479-483. https://doi.org/10.1016/j.amjmed.2014.02.017. [CrossRef]
- 97. Studer, N.; Deshamais, L.; Beutler, M.; Brugiroux, S.; Terrazos, M.A.; Menin, L.; Schürch, C.M.; Mccoy, K.D.; Kuehne, S.A.; Minton, N.P.; et al. Functional Intestinal Bile Acid 7α-Dehydroxylation by Associated with Protection from Infection in a Gnotobiotic Mouse Model. Front Cell Infect Mi 2016, 6. [CrossRef]
- 98. Kang, J.D.; Myers, C.J.; Harris, S.C.; Kakiyama, G.; Lee, I.K.; Yun, B.S.; Matsuzaki, K.; Furukawa, M.; Min, H.K.; Bajaj, J.S.; et al. Bile Acid 7α-Dehydroxylating Gut Bacteria Secrete Antibiotics that Inhibit : Role of Secondary Bile Acids. Cell Chemical Biology 2019, 26, 27-+. https://doi.org/10.1016/j.chembiol.2018.10.003. [CrossRef]
- 99. Khoruts, A.; Staley, C.; Sadowsky, M.J. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol 2021, 18, 67-80. https://doi.org/10.1038/s41575-020-0350-4. [CrossRef]
- 100. Jain, N.; Umar, T.P.; Fahner, A.F.; Gibietis, V. Advancing therapeutics for recurrent clostridioides difficile infections: an overview of vowst's FDA approval and implications. Gut Microbes 2023, 15, 2232137. https://doi.org/10.1080/19490976.2023.2232137. [CrossRef]
- 101. Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75-84. https://doi.org/10.1038/nature18848. [CrossRef]
- 102. Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 2019, 12, 843-850. https://doi.org/10.1038/s41385-019-0160-6. [CrossRef]
- 103. Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol 2017, 15, 55-63. https://doi.org/10.1038/nrmicro.2016.142. [CrossRef]
- 104. Zhu, W.; Wu, Y.; Liu, H.; Jiang, C.; Huo, L. Gut-Lung Axis: Microbial Crosstalk in Pediatric Respiratory Tract Infections. Front Immunol 2021, 12, 741233. https://doi.org/10.3389/fimmu.2021.741233. [CrossRef]
- 105. Hu, Y.; Feng, Y.; Wu, J.; Liu, F.; Zhang, Z.; Hao, Y.; Liang, S.; Li, B.; Li, J.; Lv, N.; et al. The Gut Microbiome Signatures Discriminate Healthy From Pulmonary Tuberculosis Patients. Front Cell Infect Microbiol 2019, 9, 90. https://doi.org/10.3389/fcimb.2019.00090. [CrossRef]
- 106. Hung, C.F.; Matute-Bello, G. The Gut-Lung Axis: What's below the Diaphragm Is Also Important. Am J Resp Cell Mol 2022, 67, 617-618. https://doi.org/10.1165/rcmb.2022-0365ED. [CrossRef]
- 107. Eribo, O.A.; du Plessis, N.; Ozturk, M.; Guler, R.; Walzl, G.; Chegou, N.N. The gut microbiome in tuberculosis susceptibility and treatment response: guilty or not guilty? Cell Mol Life Sci 2020, 77, 1497-1509. https://doi.org/10.1007/s00018-019-03370-4. [CrossRef]
- 108. Khan, N.; Mendonca, L.; Dhariwal, A.; Fontes, G.; Menzies, D.; Xia, J.G.; Divangahi, M.; King, I.L. Intestinal dysbiosis compromises alveolar macrophage immunity to. Mucosal Immunology 2019, 12, 772-783. https://doi.org/10.1038/s41385-019-0147-3. [CrossRef]
- 109. Dumas, A.; Corral, D.; Colom, A.; Levillain, F.; Peixoto, A.; Hudrisier, D.; Poquet, Y.; Neyrolles, O. The Host Microbiota Contributes to Early Protection Against Lung Colonization by Mycobacterium tuberculosis. Front Immunol 2018, 9, 2656. https://doi.org/10.3389/fimmu.2018.02656. [CrossRef]
- 110. Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum Microb J 2020, 17, 100073. https://doi.org/10.1016/j.humic.2020.100073. [CrossRef]
- 111. Xie, L.; Chen, L.; Li, X.; Zhou, J.; Tian, H.; Zhao, J.; Li, Z.; Li, Y. Analysis of Lung Microbiome in COVID-19 Patients during Time of Hospitalization. Pathogens 2023, 12. https://doi.org/10.3390/pathogens12070944. [CrossRef]
- 112. Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther 2022, 7, 143. https://doi.org/10.1038/s41392-022-00986-0. [CrossRef]
- 113. Kaul, R.; Liu, C.M.; Park, D.E.; Galiwango, R.M.; Tobian, A.A.R.; Prodger, J.L. The Penis, the Vagina and HIV Risk: Key Differences (Aside from the Obvious). Viruses 2022, 14. https://doi.org/10.3390/v14061164. [CrossRef]
- 114. Dillon, S.M.; Frank, D.N.; Wilson, C.C. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS 2016, 30, 2737-2751. https://doi.org/10.1097/QAD.0000000000001289. [CrossRef]
- 115. Saxena, D.; Li, Y.; Yang, L.; Pei, Z.; Poles, M.; Abrams, W.R.; Malamud, D. Human microbiome and HIV/AIDS. Curr HIV/AIDS Rep 2012, 9, 44-51. https://doi.org/10.1007/s11904-011-0103-7. [CrossRef]
- 116. Baral, B.; Mozafari, M.R. Strategic Moves of "Superbugs" Against Available Chemical Scaffolds: Signaling, Regulation, and Challenges. Acs Pharmacol Transl 2020, 3, 373-400. https://doi.org/10.1021/acsptsci.0c00005. [CrossRef]
- 117. Pamer, E.G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016, 352, 535-538. https://doi.org/10.1126/science.aad9382. [CrossRef]
- 118. Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat Rev Microbiol 2022, 20, 365-380. https://doi.org/10.1038/s41579-021-00667-9. [CrossRef]
- 119. Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D'Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Marcelino, V.R.; Bryant, R.V.; Costello, S.P.; et al. Review article: the future of microbiome-based therapeutics. Aliment Pharm Ther 2022, 56, 192-208. https://doi.org/10.1111/apt.17049. [CrossRef]
- 120. Chang, F.; He, S.S.; Dang, C.Y. Assisted Selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in Microbiome Data. Jove-J Vis Exp 2022. [CrossRef]
- 121. Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J Biosciences 2019, 44. [CrossRef]
- 122. Yadav, M.; Chauhan, N.S. Microbiome therapeutics: exploring the present scenario and challenges. Gastroenterol Rep 2022, 10. [CrossRef]
- 123. Oberhardt, M.A.; Zarecki, R.; Gronow, S.; Lang, E.; Klenk, H.P.; Gophna, U.; Ruppin, E. Harnessing the landscape of microbial culture media to predict new organism-media pairings. Nat Commun 2015, 6, 8493. https://doi.org/10.1038/ncomms9493. [CrossRef]
- 124. Diakite, A.; Dubourg, G.; Dione, N.; Afouda, P.; Bellali, S.; Ngom, II; Valles, C.; Tall, M.L.; Lagier, J.C.; Raoult, D. Optimization and standardization of the culturomics technique for human microbiome exploration. Sci Rep 2020, 10, 9674. https://doi.org/10.1038/s41598-020-66738-8. [CrossRef]
- 125. Stone, V.N.; Xu, P. Targeted antimicrobial therapy in the microbiome era. Mol Oral Microbiol 2017, 32, 446-454. https://doi.org/10.1111/omi.12190. [CrossRef]
- 126. Wang, S.; Yang, L.; Hu, H.; Lv, L.; Ji, Z.; Zhao, Y.; Zhang, H.; Xu, M.; Fang, R.; Zheng, L.; et al. Characteristic gut microbiota and metabolic changes in patients with pulmonary tuberculosis. Microb Biotechnol 2022, 15, 262-275. https://doi.org/10.1111/1751-7915.13761. [CrossRef]
- 127. Sola-Oladokun, B.; Culligan, E.P.; Sleator, R.D. Engineered Probiotics: Applications and Biological Containment. Annu Rev Food Sci Technol 2017, 8, 353-370. https://doi.org/10.1146/annurev-food-030216-030256. [CrossRef]
- 128. Sandrini, S.; Aldriwesh, M.; Alruways, M.; Freestone, P. Microbial endocrinology: host-bacteria communication within the gut microbiome. J Endocrinol 2015, 225, R21-R34. https://doi.org/10.1530/Joe-14-0615. [CrossRef]
- 129. Chronopoulos, A.; Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 2020, 39, 6951-6960. https://doi.org/10.1038/s41388-020-01509-3. [CrossRef]
- 130. Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. Plos One 2013, 8. [CrossRef]
- 131. Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 2015, 16, 24-43. https://doi.org/10.15252/embr.201439363. [CrossRef]
- 132. Lee-Sarwar, K.A.; Lasky-Su, J.; Kelly, R.S.; Litonjua, A.A.; Weiss, S.T. Metabolome-Microbiome Crosstalk and Human Disease. Metabolites 2020, 10. https://doi.org/10.3390/metabo10050181. [CrossRef]
- 133. Goncalves, S.M.; Lagrou, K.; Duarte-Oliveira, C.; Maertens, J.A.; Cunha, C.; Carvalho, A. The microbiome-metabolome crosstalk in the pathogenesis of respiratory fungal diseases. Virulence 2017, 8, 673-684. https://doi.org/10.1080/21505594.2016.1257458. [CrossRef]
- 134. Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014, 20, 159-166. https://doi.org/10.1038/nm.3444. [CrossRef]
- 135. De Luca, A.; Carvalho, A.; Cunha, C.; Iannitti, R.G.; Pitzurra, L.; Giovannini, G.; Mencacci, A.; Bartolommei, L.; Moretti, S.; Massi-Benedetti, C.; et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. Plos Pathog 2013, 9, e1003486. https://doi.org/10.1371/journal.ppat.1003486. [CrossRef]
- 136. Wong, A.C.; Levy, M. New Approaches to Microbiome-Based Therapies. mSystems 2019, 4. https://doi.org/10.1128/mSystems.00122-19. [CrossRef]
- 137. Nataraj, B.H.; Mallappa, R.H. Chapter 19 - Role of probiotics in infections with multidrug-resistant organisms. In Probiotics in the Prevention and Management of Human Diseases, Dwivedi, M.K., Amaresan, N., Sankaranarayanan, A., Kemp, E.H., Eds.; Academic Press: 2022; pp. 265-279.
- 138. Juarez, V.M.; Montalbine, A.N.; Singh, A. Microbiome as an immune regulator in health, disease, and therapeutics. Adv Drug Deliver Rev 2022, 188. [CrossRef]
- 139. Ayres, J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab 2020, 2, 572-585. https://doi.org/10.1038/s42255-020-0237-2. [CrossRef]
- 140. Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant 2020, 39, 405-407. https://doi.org/10.1016/j.healun.2020.03.012. [CrossRef]
- 141. Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020, 382, 1787-1799. https://doi.org/10.1056/NEJMoa2001282. [CrossRef]
- 142. Fernandes, M.; Brabek, J. COVID-19, corticosteroids and public health: a reappraisal. Public Health 2021, 197, 48-55. https://doi.org/10.1016/j.puhe.2021.05.028. [CrossRef]
- 143. Schneider, D.S.; Ayres, J.S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 2008, 8, 889-895. https://doi.org/10.1038/nri2432. [CrossRef]
- 144. Khan, I.; Bai, Y.R.; Zha, L.J.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C.J. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front Cell Infect Mi 2021, 11. [CrossRef]
- 145. Gill, H.S. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol 2003, 17, 755-773. https://doi.org/10.1016/s1521-6918(03)00074-x. [CrossRef]
- 146. Villapol, S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Translational Research 2020, 226, 57-69. https://doi.org/10.1016/j.trsl.2020.08.004. [CrossRef]
- 147. Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, A.T.A.Y.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14. [CrossRef]
- 148. Raberg, L.; Sim, D.; Read, A.F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007, 318, 812-814. https://doi.org/10.1126/science.1148526. [CrossRef]
- 149. Ayres, J.S. The Biology of Physiological Health. Cell 2020, 181, 250-269. https://doi.org/10.1016/j.cell.2020.03.036. [CrossRef]
- 150. Lee, S.; Kim, T.; Lee, E.; Lee, C.; Kim, H.; Rhee, H.; Park, S.Y.; Son, H.J.; Yu, S.; Park, J.W.; et al. Clinical Course and Molecular Viral Shedding Among Asymptomatic and Symptomatic Patients With SARS-CoV-2 Infection in a Community Treatment Center in the Republic of Korea. Jama Intern Med 2020, 180, 1447-1452. https://doi.org/10.1001/jamainternmed.2020.3862. [CrossRef]
- 151. Sanchez, K.K.; Chen, G.Y.; Schieber, A.M.P.; Redford, S.E.; Shokhirev, M.N.; Leblanc, M.; Lee, Y.M.; Ayres, J.S. Cooperative Metabolic Adaptations in the Host Can Favor Asymptomatic Infection and Select for Attenuated Virulence in an Enteric Pathogen. Cell 2018, 175, 146-+. [CrossRef]
- 152. Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.Y.L.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: old concepts and new challenges (vol 8, 103, 2020). Microbiome 2020, 8. [CrossRef]
- 153. Ayres, J.S. Cooperative Microbial Tolerance Behaviors in Host-Microbiota Mutualism. Cell 2016, 165, 1323-1331. https://doi.org/10.1016/j.cell.2016.05.049. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).