1. Introduction
Acid rain has become one of the most serious environmental problems worldwide because of decades of industrial development as well as increases in energy demands and emissions of certain gases, including sulfur dioxide (SO2) and nitrogen oxides (NOX) (Shi et al., 2021). In terms of the total area affected by acid rain, China ranks third behind Europe and North America (Tomlinson et al., 2003). The acid rain-affected regions in China account for 3.8% of the total land area, with Zhejiang province among the regions severely affected by acid rain (average acid rain rate of 47.9%) (Ling et al., 2010). According to Li et al. (2021), because of improvements in the energy supply structure (e.g., use of renewable energy sources) and significant increases in NOX emissions from vehicle exhausts, the main acids associated with acid rain in the subtropical region have recently changed from mainly sulfuric acid to a mixture of sulfuric acid and nitric acid. Despite the active efforts of government departments to control acid rain and mitigate its adverse effects, there are still major environmental problems due to acid rain in the subtropical region.
Simulated acid rain (SAR) initially affects plants by damaging the leaf wax, which leads to the rupture of the epidermis (Du et al., 2017). Additionally, the outer stomatal wall on the abaxial side of leaves also ruptures in response to SAR. The morphology of some epidermal cells is consistent with the effects of lysis. Plants accumulate phenolic compounds in necrotic areas. A subsequent exposure to low-pH stress can damage the leaf mesophyll, ultimately leading to the complete collapse of mesophyll cells (Sant’Anna-Santos et al., 2006). Moreover, SAR can also alter the physical and chemical properties of soil, thereby affecting the absorption of water and nutrients by plant roots, inhibiting plant growth and development, exacerbating plant diseases and pest infestations, accelerating leaf yellowing and shedding, and leading to the severe degradation of terrestrial ecosystems (Wu et al., 2002; Liang et al., 2008). Many studies on SAR that mainly focused on its effects on soil microorganisms in the plant rhizosphere revealed that SAR primarily affects soil respiration and soil physicochemical properties (Lv et al., 2013; Berger et al., 2015; Li et al., 2021; Zheng et al., 2022; Zhang et al., 2023). For evergreen trees in the subtropical region, only low pH acidic conditions have a significant effect, while high pH would not (Shi et al., 2021). Studies have been conducted on the effects of SAR on plant leaves (Francisco et al., 2005; Du et al., 2017) and nutrient elements (Diatta et al., 2020). Recent studies have explored how the frequency of SAR influences soil microorganisms and plants (Liu et al., 2020). There has been considerable research on the effects of SAR on the morphological features and photosynthetic activities of plants. More specifically, the related research on economically important coniferous species, such as Pinus massoniana (PM) and Cunninghamia lanceolata (CL), treated with SAR has mainly focused on biological characteristics, including growth (Zhang et al., 2023), physiological functions (Ren et al., 2013; Fan et al., 2005), wood or fruit quality (Liu et al., 1996), and ecological functions (Wang et al., 2012; Liu et al., 2010).
In terms of plant physiology, subtropical evergreen broad-leaved trees are more tolerant to acidic conditions than coniferous trees (Malla et al.,2023). This is in accordance with the results of an earlier study involving an examination of spectral reflectance, which indicated coniferous tree species are more sensitive to SAR than broad-leaved tree species (Shi et al.,2021). There are few reports describing the changes in the growth characteristics and photosynthetic performance of PM and CL in response to SAR. In addition to being the most representative coniferous tree species in the subtropical region, PM and CL are the most widely cultivated timber species in China (Jiang et al., 2017; Yin et al., 2009; Shan et al., 1988; Sun et al., 2020).
The development of the Multi-Function Plant Efficiency Analyzer (M-PEA) for the simultaneous measurement of prompt chlorophyll fluorescence (PF), delayed chlorophyll fluorescence (DF), and modulated reflection (MR) enabled the examination of the changes in forward and reverse electron transport and/or the redox state of photosystem I (PSI) in many plant species (Strasser et al,2010; Oukarroum et al, 2013). The correlations among the aforementioned signals can provide mutually corroborating and complementary insights into the photosynthetic electron transport chain, including the forward and reverse electron flow and the cyclic electron flow around PSI. A previous study verified the utility of PF, DF, and the MR at 820 nm (MR820) curve for analyzing the degree of plant stress (Zhou et al.,2018). The PF increases in three phases (O–J, J–I, and I–P), which represent three distinct reduction reactions in the electron transport chain. During photosynthesis, PF is detectable after the dark-to-light transition, whereas DF can be detected during the light-to-dark transition (Strasser et al., 2010). In addition, DF is mainly emitted by photosystem II (PSII) rather than PS I (Jursinic et al., 1986). Based on this energy cascade, PF emitted by chlorophyll a reflects the photosynthetic vitality, especially for PSII, and can be measured under saturated actinic light at a wavelength of 627 ± 10 nm (Zushi et al., 2017). The transient MR signals at 820 nm suggest the transport of electrons beyond the plastoquinone (PQ) pool in PSI can be measured under modulated light at a wavelength of 820 ± 25 nm. The DF denoting the charge recombination and re-population of excited PSII antenna chlorophyll can be measured under far-red light at a wavelength of 735 ± 15 nm (Goltsev. et al, 2009; Strasser et al, 2010).
To date, PF, DF, and the MR820 curve have been widely used during analyses of the effects of heat stress (Chen et al.,2015), salt stress (Kan et al.,2017), drought stress (Liu et al.,2018), and heavy metal stress (Poschenrieder et al.,2008). However, there have been relatively few studies that combined PF, DF, the MR820 curve, and JIP-test parameters to investigate the mechanism underlying the response of coniferous tree species to SAR. The objectives of this study were to use the above-mentioned methods to elucidate the mechanism mediating the response of subtropical coniferous species to SAR and to reveal the diversity in the responses of subtropical coniferous species to SAR, thereby providing the theoretical basis for developing effective methods for protecting subtropical coniferous species from acid rain.
2. Results
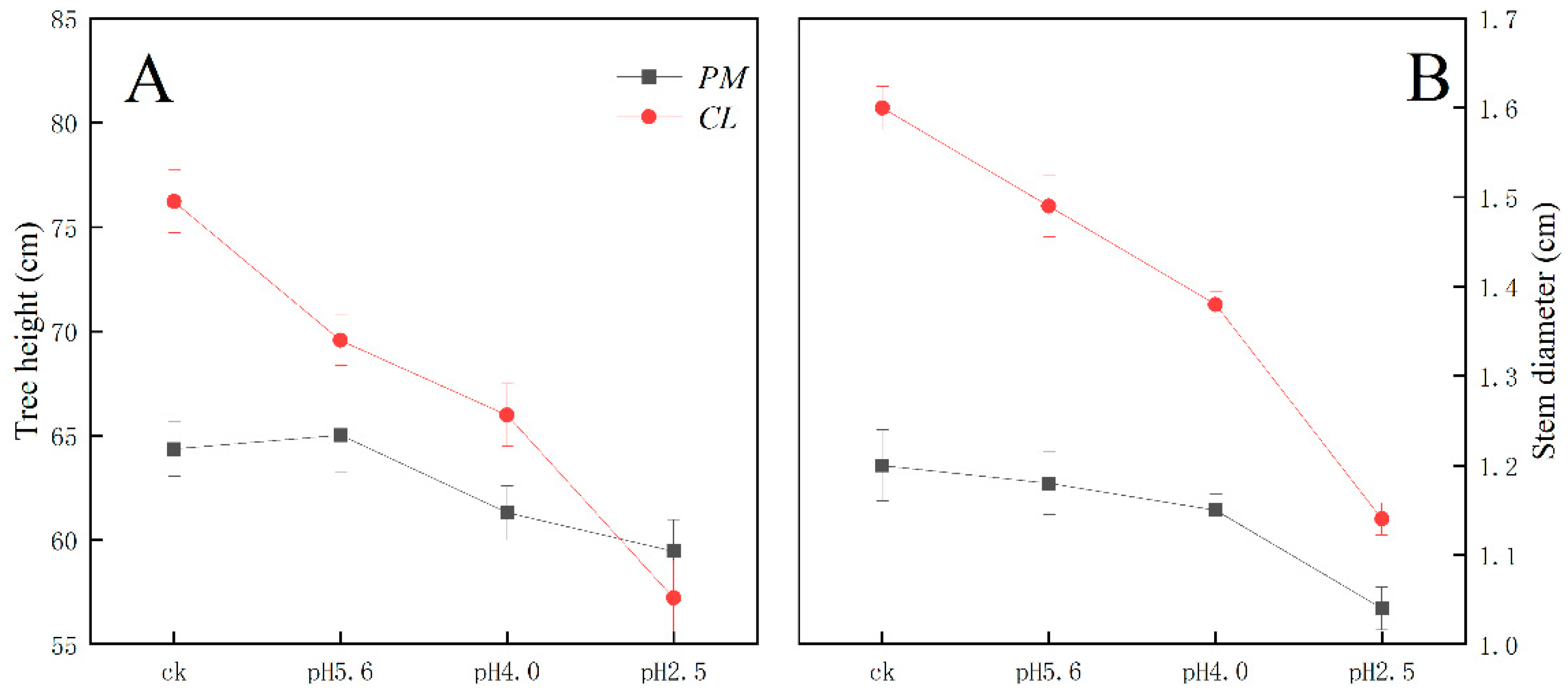
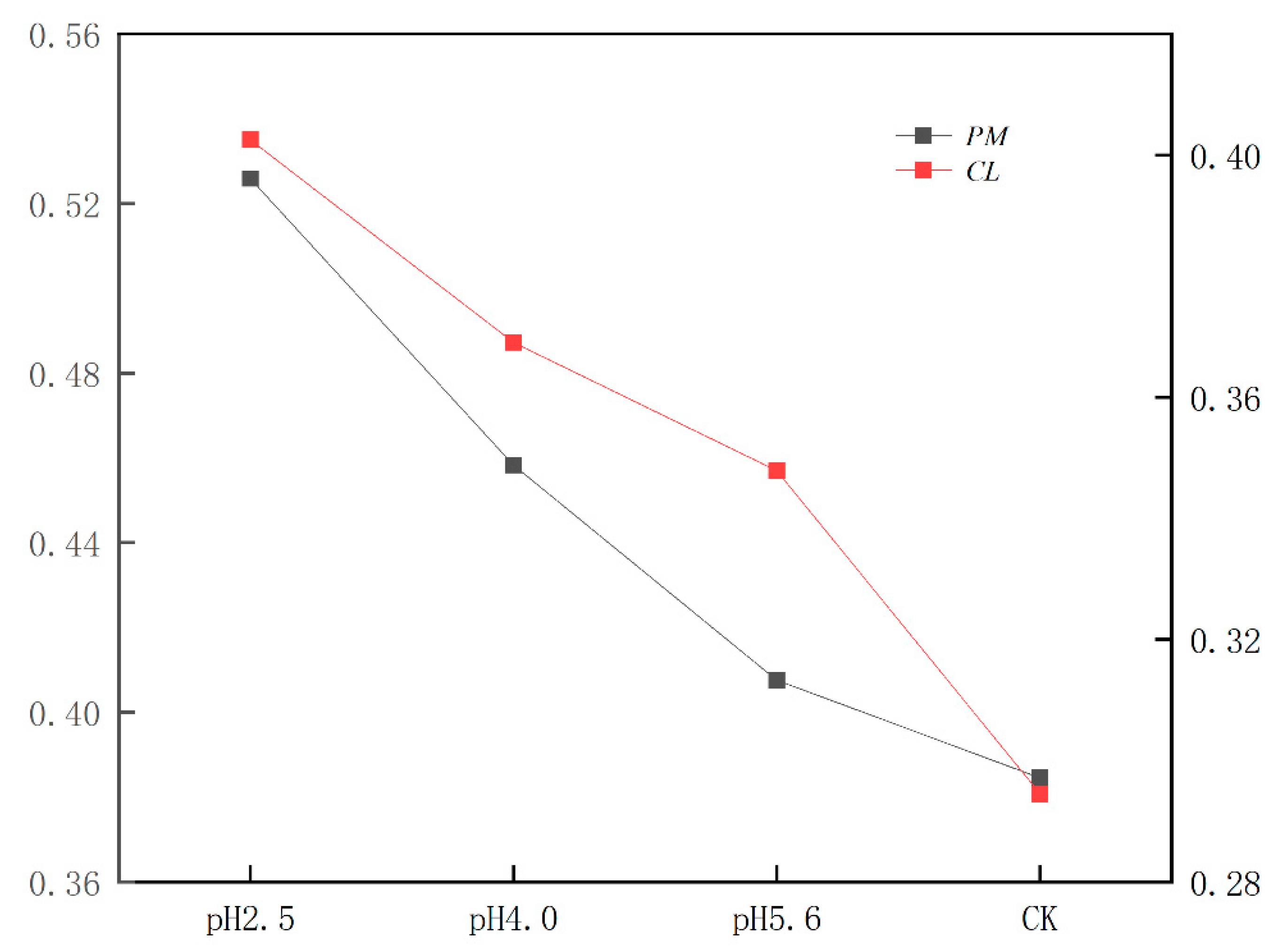
The SAR treatment inhibited PM and CL growth (
Figure 1). Decreases in the pH of the SAR solution resulted in decreases in the tree height and stem diameter of both species. At pH 4.0, the tree height of CL decreased more than that of PM. Additionally, the stem diameter of CL decreased more significantly than that of PM at pH 2.5.
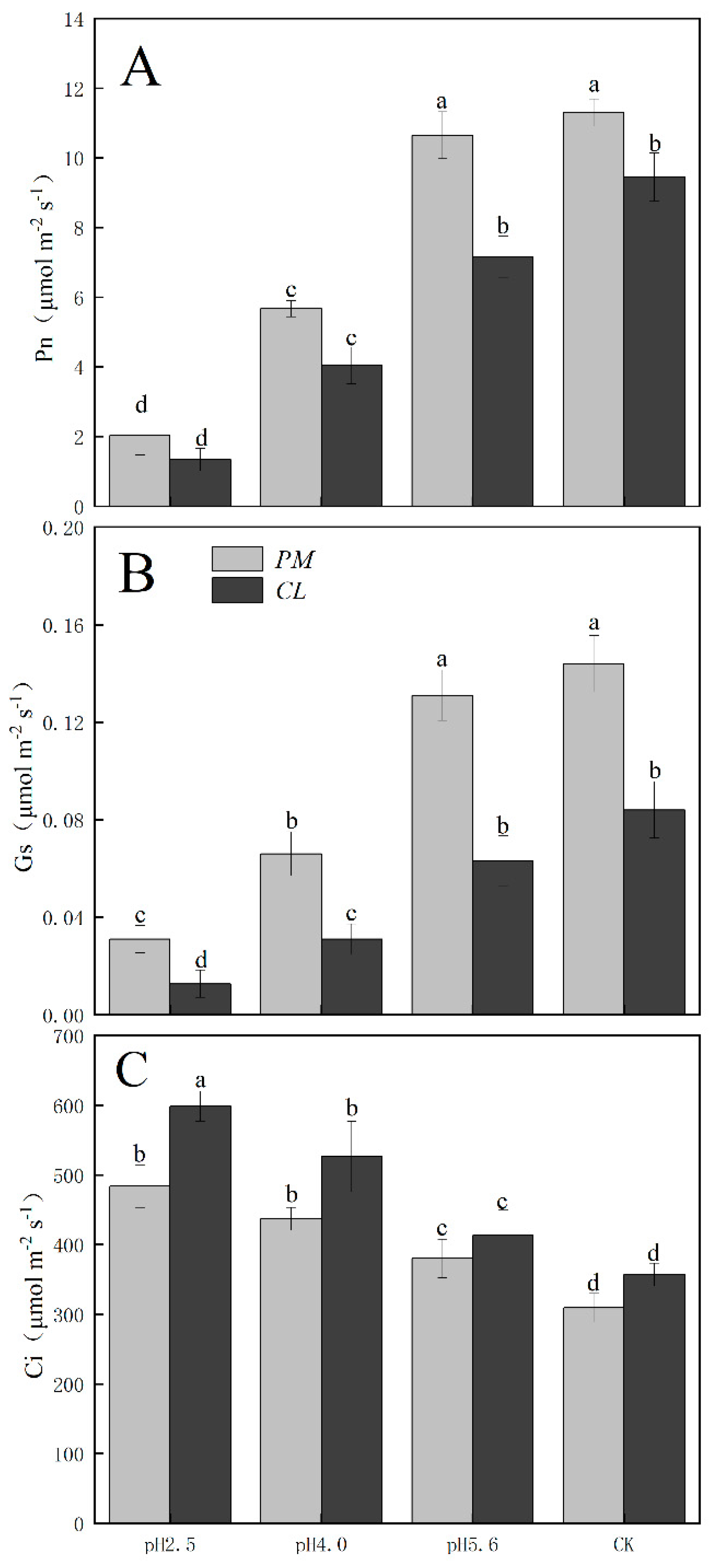
The gas exchange parameters of PM and CL following the SAR treatments are presented in
Figure 2. The application of the SAR solutions significantly decreased Pn and Gs for both PM and CL, whereas Ci increased as the SAR stress increased. After the SAR treatments, Pn and Gs were higher for PM than for CL, whereas the opposite trend was observed for Ci. Compared with the effects of the control treatment, the SAR pH 2.5 treatment of PM and CL resulted in decreases in Pn (by 82.04% and 84.14%, respectively) and Gs (by 81.10% and 84.88%, respectively), but increases in Ci (by 36.02% and 40.37%, respectively).
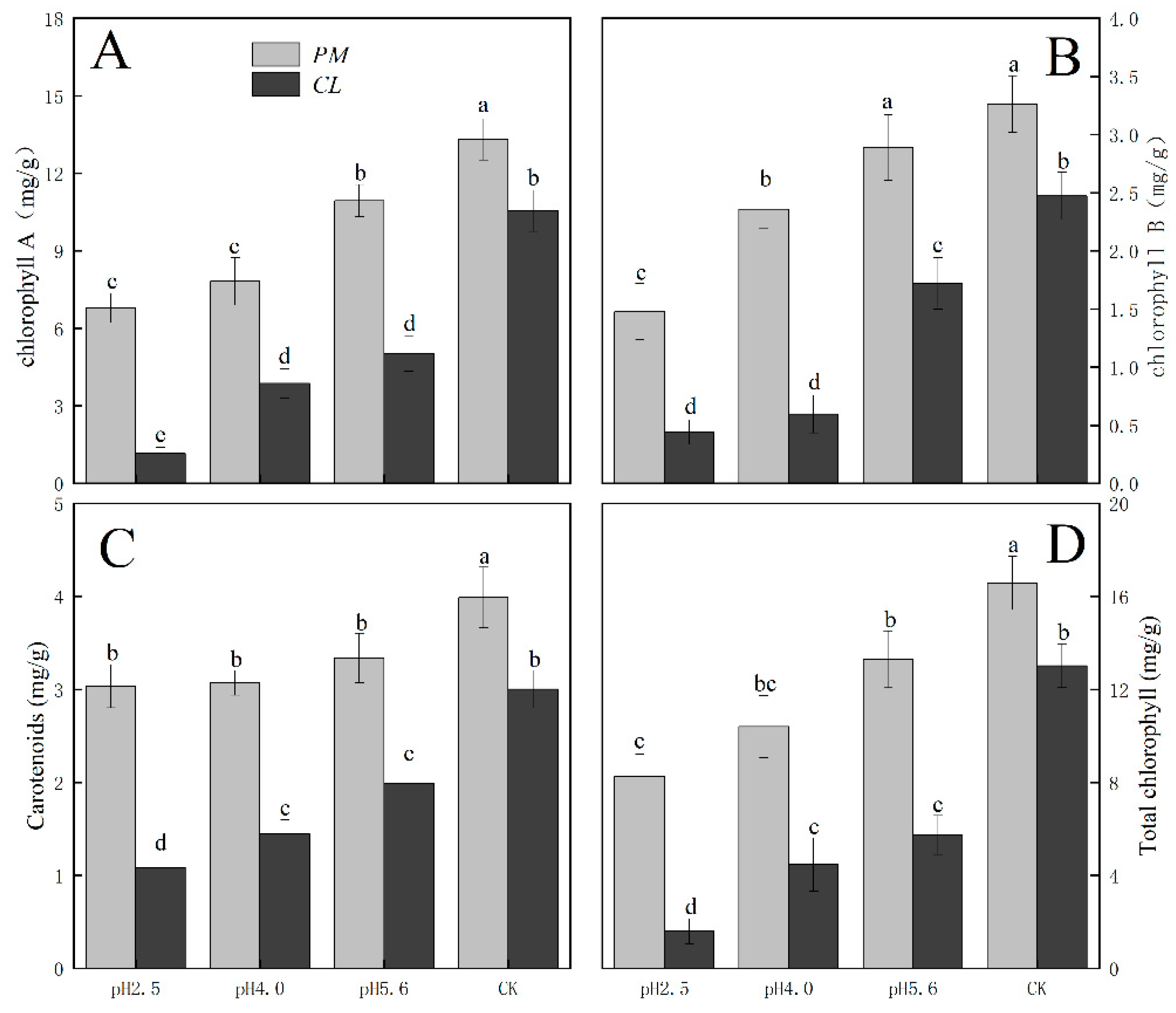
The photosynthetic pigment contents in PM and CL decreased as the pH of the SAR solution decreased. Moreover, there was a negative correlation between the photosynthetic pigment content and the SAR stress level (
Figure 3). The changes in the chlorophyll A, chlorophyll B, and total chlorophyll contents were similar between PM and CL. At pH 2.5, the chlorophyll A contents of PM and CL decreased by 49.00% and 89.04%, respectively. The chlorophyll B contents of PM and CL decreased by 54.74% and 82.20%, respectively. The total chlorophyll contents of PM and CL decreased by 50.14% and 87.75%, respectively. There was no significant change in the carotenoid content of PM as the pH of the SAR solution decreased, whereas the carotenoid content of CL decreased significantly more after the SAR pH 2.5 treatment (by 63.96%) than after the SAR pH 4.0 or 5.6 treatments.
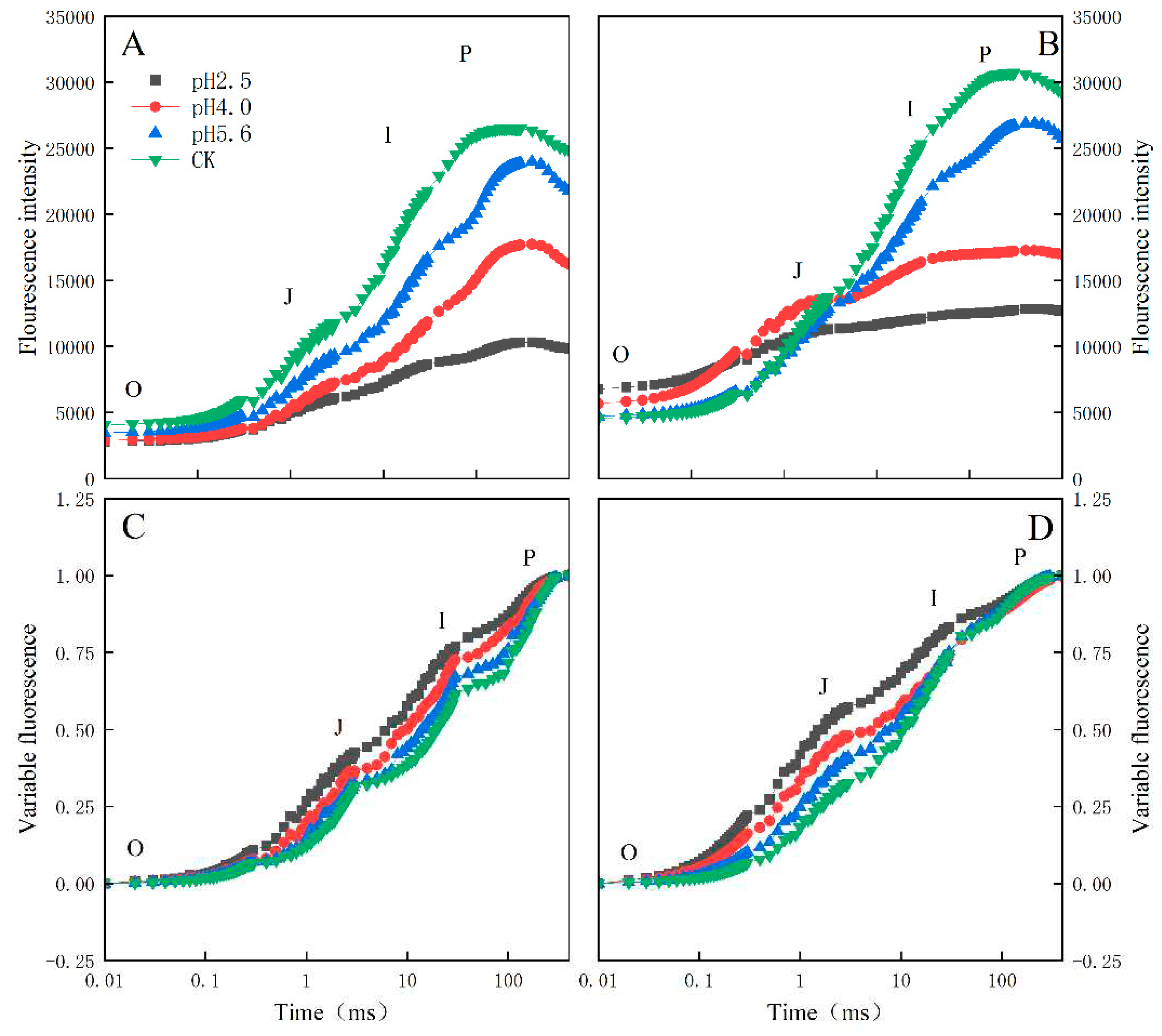
Distinct OJIP curves were generated for both PM and CL treated with different SAR solutions. As the pH of the SAR solution decreased, the I–P phase of the OJIP curve decreased significantly (
Figure 4). However, there was a difference between the two species. More specifically, the overall curve of PM decreased as the pH decreased, but the curve of CL revealed an increase in F
O as the pH decreased. Furthermore, in response to the decrease in pH, the OJIP curve gradually transformed into an OKJIP curve with an obvious K-band. In addition, following the SAR pH 2.5 and 4.0 treatments, the P point was eventually undetectable in the CL curve, whereas it was still detectable in the PM curve.
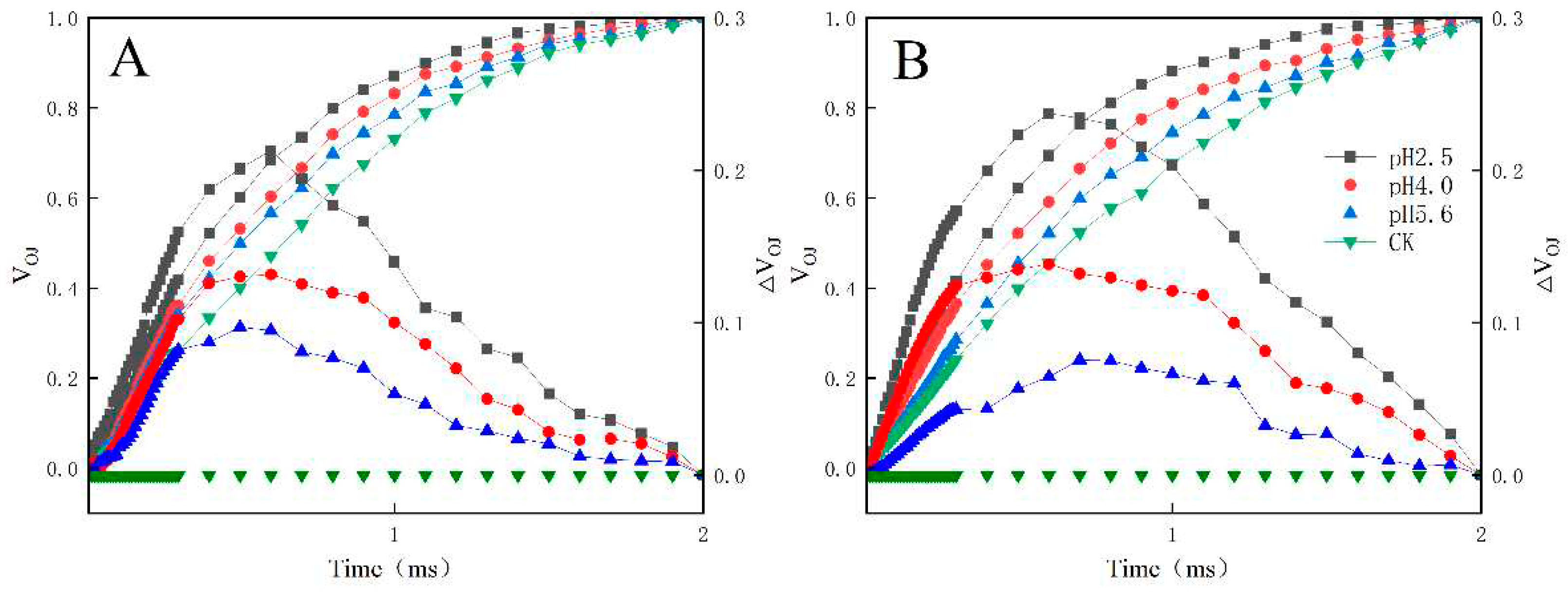
The normalized OJIP curves for both PM and CL indicated that the O–P points increased as the pH of the SAR solution decreased (relative to the control O–P points) (
Figure 4). There were also increasing trends in V
J and V
I for both PM and CL (
Figure 5). For PM, V
J and V
I did not change significantly at pH 5.6, but they increased significantly at pH 4.0. For CL, V
J increased significantly at pH 5.6.
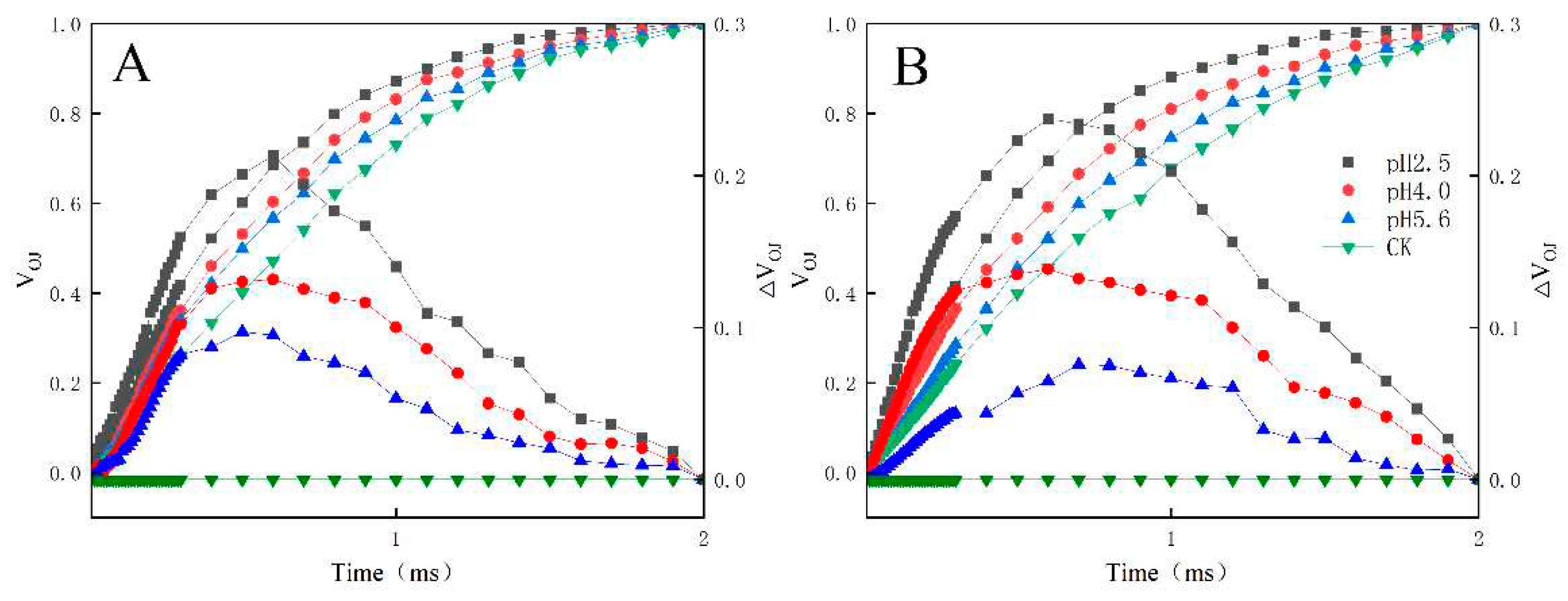
The fluorescence increase kinetics of PM and CL were normalized between the O point (50 μs) and the K point (300 μs) as follows: V
OK = (F
T − F
O)/(F
K − F
O). The K-band of both species was already higher than that of the control at pH 5.6. Further decreases in the pH resulted in significant increases in the K-band (
Figure 6).
For both PM and CL, the L-band was detected at pH 5.6. Further decreases in the pH were accompanied by significant increases in the L-band. Notably, the L-band of CL was higher than that of PM at pH 2.5 (
Figure 7).
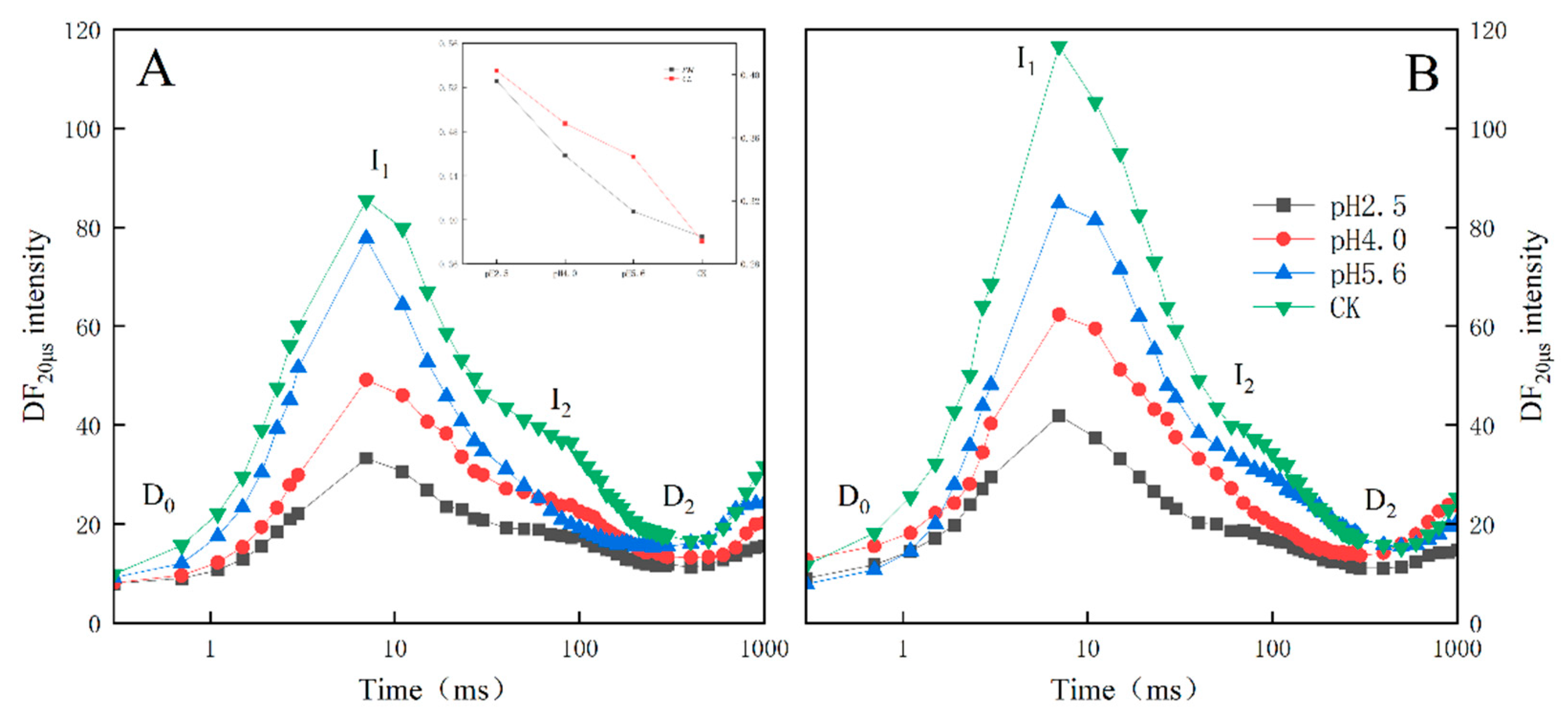
For both PM and CL, the SAR treatments altered the amplitude and shape of the DF induction curves (
Figure 8A,B). The DF curve decreased as the pH of the SAR solution decreased , with the I
1 peak decreasing faster than the I
2 peak. As the pH value decreased, the I
2:I
1 ratio increased significantly, especially at pH 2.5 and 4.0. The I
2:I
1 ratio increased significantly at pH 5.6, but only for CL.
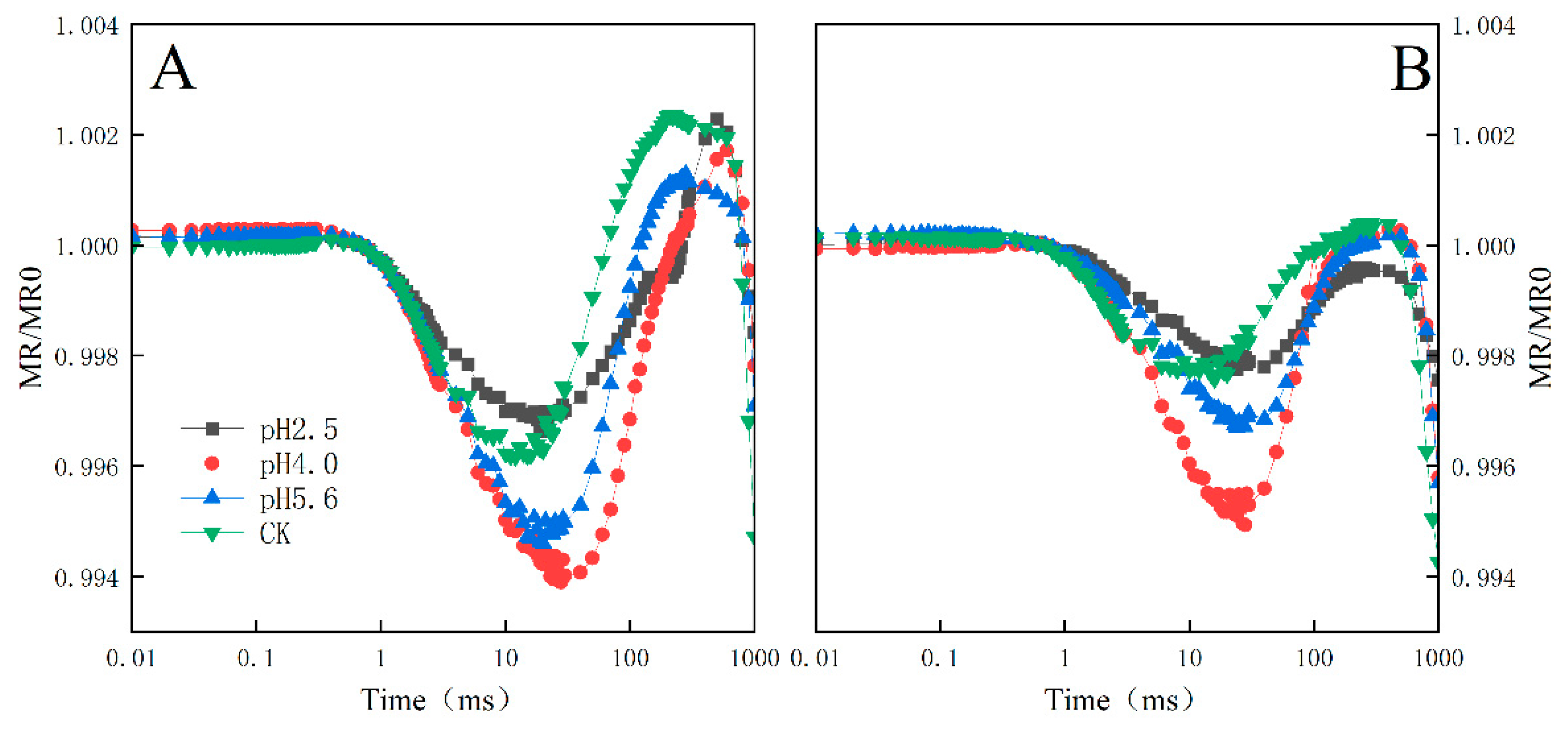
The exposure to various levels of SAR stress led to significant changes to the MR/MR
0 kinetics curves for both PM and CL, although the changes were greater for CL (
Figure 9). Decreases in the pH of the SAR solution shifted the lowest point of the MR/MR
0 kinetics curve to a later time-point. At pH 2.5, both the descending and ascending slopes were lower for CL than for PM (
Figure 10).
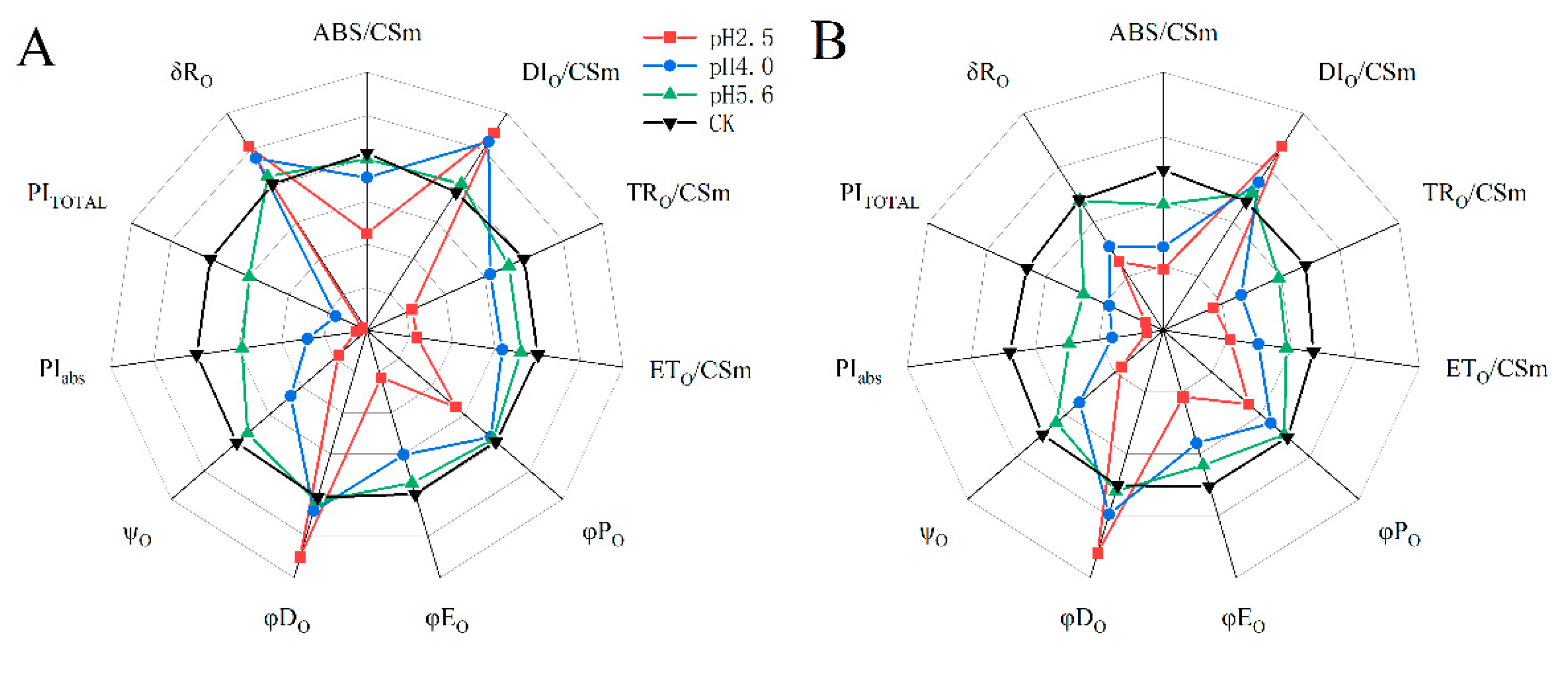
The SAR treatments decreased ABS/CSm, TRO/CSm, ETO/CSm, φPO, φEO, ψO, PIABS, and PITOTAL in both PM and CL, whereas they increased DIO/CSm and φDO. However, there were differences between the two species. Specifically, decreases in pH resulted in increases and decreases in δRO for PM and CL, respectively. Furthermore, significant decreases in various parameters, including ABS/CSm, TRO/CSm, φPO, and φEO, were detected at pH 4.0 and 5.6 for PM and CL, respectively.
3. Discussion
The harmful effects of SAR on plants are reflected by damaged leaf structures and decreased chlorophyll contents, which have inhibitory effects on photosynthesis (Du et al., 2017). Additionally, SAR-induced damages to the stomata and cell membranes of leaves adversely affect plant respiration and other physiological activities (Sant’Anna-Santos et al., 2006). The chlorophyll content, Pn, and Gs decreased significantly in the PM and CL leaves after the SAR pH 4.0 and 2.5 treatments, whereas Ci increased as the pH decreased, implying the decrease in photosynthetic performance in PM and CL was unrelated to stomatal factors.
The main phases of the OJIP curve in response to SAR stress differed between the two analyzed tree species. Specifically, the I–P phase decreased more significantly for CL than for PM. The comparison with CL indicated the fluorescence peak (F
O) increased more in PM as the pH decreased and the I–P phase decreased more under low-pH conditions, eventually resulting in the loss of the P point (
Figure 4). The increase in chlorophyll a fluorescence during the J–I phase reflects the extent of the conversion from QA
− to QA, whereas the decrease in the PQ pool leads to a decrease in the primary electron acceptor QA levels because of the limited induction of PQ reduction (Bhatt et al., 2023). The I point represents the equilibrium between the reduction of the PQ pool by the electrons of PSII and the re-oxidation of the PQ pool, whereas the P point represents the completion of the reduction of all electron acceptors in PSII and PSI (Yang et al., 2023). In the current study, the I–P phase, which is related to the PSI activity, indicated that the low-pH SAR treatment inhibited the electron transfer process in PM and CL. More specifically, blocking the transfer of electrons on the PSII receptor side will lead to an increase in the cyclic electron flow around PSI, thereby affecting the reduction and re-oxidation of the PQ pool. Furthermore, under low-pH SAR stress conditions, the OJIP curve gradually changes to the OKJIP curve, with the K point detected at approximately 300 µs. The appearance of the K point is mainly due to the inhibition of H
2O splitting and the damage to the OEC caused by the partial inhibition of QA, indicative of further damages to PSII.
An increase in V
J is usually indicative of the accelerated restoration of QA, leading to a substantial accumulation of QA in the PS II RC, which then inhibits the transfer of electrons on the receptor side (Goltsev et al., 2016). A previous study showed V
I reflects the capacity of PS I and its receptor quinone to oxidize and reduce (Strasser et al., 2010). Under stress conditions, changes in V
I reportedly represent decreases in the acceptance of electrons by PQ, leading to the inhibited transfer of electrons from QA to QB (Goltsev et al., 2016). In the present study, the SAR treatments of PM and CL had detrimental effects on the ability to accept electrons from PQ, resulting in the accumulation of QA and the inability to transfer electrons from QA to QB (
Figure 5).
A damaged OEC (commonly indicated by V
OK) obstructs the transfer of electrons from the primary electron donor to the secondary electron donor and leads to an imbalanced electron flow between the donor and receptor sides (Salim et al., 2021). The ΔV
OK value represents the standardized V
OK value. The V
OJ value indicates that the rate of electrons captured by the pigments in the PS II RC exceeds the rate of the exchange between QA
− at the QB site and the oxidized PQ pool (Salim et al., 2021). The ΔV
OJ value represents the standardized V
OJ value. In this study, a decrease in the pH of the SAR solution negatively affected both V
OK and V
OJ. As the pH decreased, ΔV
OK and ΔV
OJ increased, but there were significant differences between CL and PM at pH 2.5 (
Figure 6 and
Figure 7).
When electrons are transferred to the PSII RCs, the associated DF is caused by the reverse flow of electrons, leading to a charge recombination and the subsequent re-population of the PSII antenna chlorophylls (Goltsev et al., 2009; Strasser et al., 2010). The intensity of DF directly depends on the rate of the reverse electron transfer reaction in the PSII RCs (Van Gorkom et al., 1973;Jursinic et al., 1986). The shape of the induced DF curve varies among sample types and physiological states (Goltsev et al., 1997;Srivastava et al., 1999; Strasser et al., 2010), but it also depends on DF kinetics (Zaharieva et al., 2003). The induced DF curve was constructed using DF signals recorded at the same decay time-points in multiple DF decay curves (
Figure 8). In the DF curve, the peak value at 3 ms was designated as I
1, the peak value at approximately 100 ms was designated as I
2, the initial minimum value was designated as D
0, and the final plateau was designated as D
2. After the SAR treatment, the DF curve decreased as the pH decreased, with faster decreases in the I
1 peak than in the I
2 peak.
Typical 820 nm kinetics curve include a decreasing phase followed by an increasing phase. The decrease reflects the oxidation of PSI and PC, whereas the subsequent increase is indicative of the reduction of PSI and PC. At the lowest point of the decreasing phase, the PC and PSI reduction rates are equal to their oxidation rates (Strasser et al., 2010). The 820 nm optical reflectance curve is commonly used to represent the effects of different treatments on PSI (Oukarroum et al., 2013). The MR/MR
0 value was calculated, where MR
0 is the value at the onset of the actinic illumination (0.7 ms; the first reliable MR measurement). An increase in MR/MR
0 indicates a decrease in the concentration of the oxidized states of plastocyanin (PC
+) and the PSI RC (P700
+), which is due to the reduction of PC
+ and P700
+ (Strasser et al, 2010). Decreases in the pH of the SAR solution decreased the lowest point of the MR curve and shifted the lowest point to a later time-point (
Figure 9). At pH 4.0, the slope of the curves for PM and CL decreased significantly (
Figure 10), indicative of the severity of the SAR stress. Notably, the decrease in the slope was greater for CL than for PM, implying PM is more tolerant to SAR stress than CL.
According to earlier research, ABS/CS
m, TR
O/CS
m, ET
O/CS
m, and DI
O/CS
m reflect the efficiency of each index per cross-section unit at t = t
FM (Stirbet et al., 2011). In the present study, the exposure to SAR stress significantly decreased ABS/CS
m, TR
O/CS
m, and ET
O/CS
m, but increased DI
O/CS
m (
Figure 11). The decrease in ABS/CS
m was due to the deactivation of the RCs caused by SAR as well as the destruction of the antenna pigment structure, which decreased the captured light energy and the excitation energy and reduction energy of the RC, while also altering the transfer of electrons. The increase in DI
O/CS
m suggests the SAR treatments activated a defense mechanism through which excess excitation energy in the leaves was dissipated in a timely manner to limit damages. Additionally, φP
O, φD
O φE
O, and Ψ
O are important quantum indicators for the electron transport chain (
Figure 11; Wang et al., 2021). Furthermore, δR
O represents the efficiency with which electrons are transferred from the reduction system to the PSI electron acceptor side; it is also a photosynthetic performance index that is based on light absorption (PI
ABS), making it an important parameter for studying the plant photosynthetic status. In this study as well as in earlier studies, PI
TOTAL served as the comprehensive photosynthetic performance index (
Figure 11; Zhou et al., 2019). Under SAR stress conditions, φP
O, φE
O, Ψ
O, PI
ABS, and PI
TOTAL decreased significantly for both PM and CL, which was in contrast to the significant increase in φD
O. For PM, the change in δR
O in response to decreases in pH indicates that PSI was more involved in the cyclic transfer of electrons than in the linear transfer of electrons. Increases in cyclic electron transfer are critical for minimizing the SAR-induced damages to the photosynthetic system. According to the change in δR
O, PSI in PM likely mainly contributed to cyclic electron flow, with the dissipation of excess energy decreasing ROS production.
4. Materials and Methods
4.1. Plant materials and treatments
Two-year-old PM and CL seedlings that were similar in height were used in this study. The seedlings were obtained from Jiujiang Decheng Landscape Greening Co., Ltd. (Jiangxi, China) and then grown in plastic tubes (60 cm tall, 2 cm upper diameter) filled with a 1:1:3 (v/v) mixture of vermiculite, perlite, and peat soil. Plants were grown outdoors at Jiyang College in Zhuji, Zhejiang, China. Approximately 3 months later, 48 PM and CL seedlings were transferred to two growth chambers. The settings for both growth chambers were as follows: 14-h photoperiod with a photosynthetic photon flux density (PPFD) of approximately 600 mol m−2 s−1; relative humidity of approximately 50%; and 25 °C (day)/20 °C night cycle. Fertilizer-containing water along with half-strength Hoagland solution were applied to the plants once per week.
Based on the chemical composition of the acid rain in Zhejiang province in the previous 3 years, H2SO4 and HNO3 (concentration of 98%) were used to prepare the acid rain stock solution (molar ratio of SO42− to NO3− of 2.4:1 and pH 1.0). The stock solution was diluted with deionized water to prepare SAR solutions with varying pH values (2.5, 4.0, and 5.6) that corresponded to different SAR stress levels. Deionized water (pH 6.8) was used for the control treatment. The leaves of PM and CL seedlings (from top to bottom) were sprayed uniformly with the SAR solutions once every 2 weeks.
4.2. Gas exchange measurements
The LI-6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA) was used to measure the gas exchange parameters of PM and CL. Specifically, The third pair of intact functional leaves from the top of the seedling stem were analyzed, with three replicates per leaf. The measurements were conducted using the built-in red–blue light source, with a PPFD of 1,200 µmol m−2 s−1, a CO2 concentration of 400 µmol m−2 s−1, a leaf chamber temperature of 25 °C, and a flow rate of 300 mmol/s. During the measurements, leaves took over the whole chamber room to ensure smooth progress.
4.3. Determination of the chlorophyll content
The third pair of intact functional leaves from the top of the seedling stem were collected for the determination of the chlorophyll content as described by Lichtenthaler (1983).
4.4. Simultaneous measurement of PF, DF, and MR kinetics
The third pair of intact functional leaves from the top of the seedling stem was selected and maintained in darkness for 30 min. The chlorophyll fluorescence parameters were measured using M-PEA (Hannsatech Instruments Ltd., UK). A red-light pulse (650 nm, 3,500 μmol m−2 s−1) resulted in an increase in the fluorescence curve. In terms of the chlorophyll fluorescence curve, O represents the initial fluorescence level; K (300 μs), J (2–3 ms), and I (30 ms) represent the intermediate fluorescence levels; and P (500 ms) represents the peak fluorescence level. We also used the JIP-test to analyze the OJIP fluorescence transients (Strasser et al., 2004; Stirbet and Govindjee, 2011). The JIP-test defines the maximum energy flux of absorption (ABS), trapping (TR), electron transport (ET), and dissipation (DI) in the excitation cross-section (CS) cascade. This test is based on the basic theory of energy flow through the thylakoid membrane and the total energy flow from the collecting complex (i.e., energy flux ratio).
The data were analyzed using the JIP-test (Strasser et al, 2004). Several basic data were collected in this study, including the following: FO (fluorescence intensity at 20 μs), FL (fluorescence intensity at 150 μs), FK (fluorescence intensity at 300 μs), FJ (fluorescence intensity at 2 ms), FI (fluorescence intensity at 30 ms), and FM (maximum fluorescence intensity, which is equal to FP). To analyze the electron transport chain activity, the following relative fluorescence parameters were calculated via the double-normalization of the moment chlorophyll fluorescence values against the end points in different time intervals in the OJIP part of the transient: OP, OK, OJ, OI, and IP; VT, relative variable fluorescence at time T, which is calculated using the formula VT = (FT − FO)/(FM − FO); WOK, ratio of the variable fluorescence (FT − FO) to the amplitude (FK − FO), which is used to represent the L-band; and WOJ, which is calculated using the formula WOJ = (FT– FO)/(FJ – FO) and used to represent the K-band.
The parameters that refer to time 0 include the following: the maximum quantum yield of PSII primary photochemistry (φPO = TRO/ABS = FV/FM); the efficiency with which a trapped exciton moves an electron into the electron transport chain beyond the reduced QA form (QA–) (ψEO = ETO/TRO); the quantum yield of electron transport (φEO = ETO/ABS); the average fraction of open PSII RCs in the time interval between 0 and tFM (Sm/tFM); the fraction of active PSII RCs per CS (RC/CS); absorption flux per CS (ABS/CS); trapped energy flux per CS (TRO/CS); electron transport flux per CS (ETO/CS); absorption flux per RC (ABS/RC); the quantum yield for the reduction of the end electron acceptors on the PSI acceptor side (φRO = REO/ABS); and the probability that an electron is transported from the reduced intersystem electron acceptors to the final electron acceptors of PSI [δRO = REO/ETO= (1 – VI)/(1 – VJ)].
4.5. Data analysis
All experiments were repeated at least three times. All values are expressed herein as the mean ± standard deviation. A one-way ANOVA was performed using SPSS (SPSS Inc.). The Duncan test was used to compare mean values, with P < 0.05 set as the threshold for determining significance.
5. Conclusions
The SAR treatments in this study damaged the cell membranes and stomata of PM and CL, resulting in decreased chlorophyll contents. The I–P phase decrease was due to the inhibited transfer of electrons on the electron acceptor side of PSI, which resulted in an increase in cyclic electron flow around PSI. The increase in chlorophyll a fluorescence during the J–I phase, which was indicated by VJ and VI, reflected the extensive transfer of electrons from PSI to the PQ pool. However, the re-oxidation of the PQ pool was insufficient, leading to the accumulation of QA−. The appearance of the L-band and K-band demonstrates that SAR stress interferes with the transfer of electrons on the donor and acceptor sides, thereby decreasing the transfer of energy to the active RC of PSII. This results in the dissociation of the PSII antenna complex from the PSII core proteins, which adversely affects the OEC and destabilizes the PSII system. In terms of the JIP-test parameters, ABS/CSm, TRO/CSm, ETO/CSm, φPO, φEO, and ΨO decreased significantly after the SAR treatments, resulting in decreases in PIABS and PITOTAL. Furthermore, the SAR-induced increases in both DIO/CSm and φDO protected PSII from membrane photooxidation. Decreases in pH caused δRO to increase, but only for PM. In conclusion, the decrease in chlorophyll contents and the damages to the OEC in PM and CL disrupted the transfer of electrons on the donor and acceptor sides of PSII, ultimately resulting in increased chlorophyll fluorescence and thermal dissipation, which inhibited photosynthesis (Pn) and suppressed the growth of PM and CL. However, PM was more tolerant to SAR than CL, which may be related to the observed difference in δRO between the two tree species. Thus, the physiological functions of CL may already be damaged at pH 5.6, while those of PM are generally unaffected until the pH decreases to 4.0.
Author Contributions
Conceptualization, Pengzhou Shu and Xueqin Li; Data curation, Pengzhou Shu and Xueqin Li; Funding acquisition, Songheng Jin; Investigation, Zhongxu Wang and Penghong Qian; Methodology, Yanlei Du and Yini Han; Resources, Pengzhou Shu and Xueqin Li; Software, Xiaofei Gong; Validation, Pengzhou Shu and Xueqin Li; Writing – original draft, Pengzhou Shu and Xueqin Li; Writing – review & editing, Pengzhou Shu and Xueqin Li.
Funding
This research was funded by the National Key Research and Development Project (2019YFE0118900), the National Natural Science Foundation of China (31971641), and Zhejiang Provincial Team Science and Technology Commissioner Project (Horticulture Team in Wencheng).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Berger, T.W.; Turtscher, S.; Berger, P.; Lindebner, L. A slight recovery of soils from Acid Rain over the last three decades is not reflected in the macro nutrition of beech (Fagus sylvatica) at 97 forest stands of the Vienna Woods. Environment Pollution 2016, 216, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, U.; Sharma, S.; Kalaji, H.M.; Strasser, R.J.; Chomontowski, C.; Soni, V. Sunlight-induced repair of photosystem II in moss Semibarbula orientalis under submergence stress. Functional Plant Biology 2023, 50, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Zhang, M.; et al. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environmental and Experimental Botany 2015, 122, 126–140. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Liu, Y.; Hu, Z.; Shen, X.; Ren, J. Simulated acid rain changed the proportion of heterotrophic respiration in soil respiration in a subtropical secondary forest. Applied Soil Ecology 2015, 86, 148–157. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Sang, L.; Sun, L.; Wu, J. Simulated acid rain offset a warming-induced increase in soil respiration but did not impact the temperature sensitivity of soil respiration in a cropland. Applied Soil Ecology 2021, 164. [Google Scholar] [CrossRef]
- Diatta, J.; Youssef, N.; Tylman, O.; Grzebisz, W.; Markert, B.; Drobek, L.; Lejwoda, P. Acid rain induced leakage of Ca, Mg, Zn, Fe from plant photosynthetic organs – Testing for deciduous and dicotyledons. Ecological Indicators 2021, 121. [Google Scholar] [CrossRef]
- Dou, R.Q.; Xie, Y.L.; Liu Frank, X.; Wang, B.; Xu, F.; Xiao, K.M. In situ mycoremediation of acid rain and heavy metals co-contaminated soil through microbial inoculation with Pleurotus ostreatus, Science of The Total Environment 2023,169020.
- Du, E.Z.; Dong, D.; Zeng, X.T.; Sun, Z.Z.; Jiang, X.F.; Vries, W.D. Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China, Science of The Total Environment. 2017. [Google Scholar]
- Du, E.; Dong, D.; Zeng, X.; Sun, Z.; Jiang, X.; de Vries, W. Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci Total Environ 2017, 605-606, 764-769.
- Goltsev, V.; Yordanov, I. Mathematical model of prompt and delayed chlorophyll fluorescence induction kinetics. Photosynthetica 1997, 33: 571–586.
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russian Journal of Plant Physiology 2016, 63(6), 869–893. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Lu, Y.; Shi, J.; Chen, S.; Strasser, R.J.; Hu, Z. Special issue in honour of Prof Reto, J. Strasser - Effect of AtLFNR1 deficiency on chlorophyll a fluorescence rise kinetics OJIP of Arabidopsis. Photosynthetica 2020, 58(SPECIAL ISSUE), 391-398.
- Jiang, C.W.; Xu, Q.; Zhang, B.B.; Gao, D.Q.; Ma, Y.B. Research Progress on Photosynthetic Physiology and Resource Utilization Efficiency of Pinus massoniana Pinus massoniana Lamb [J]. World Forestry Research 2017, 30, 24–28. [Google Scholar]
- Jursinic, P. Delayed fluorescence: current concepts and status. In: Govindjee, Amesz J, Fork DJ, editors. Light emission by plants and bacteria. Academic Press, Orlando, 1986; 291–328.
- Li, Q.Y.; Liu, X.; Zhang, J.C. Research on the Trend of Acid Rain Transformation in the Yangtze River Delta Region. Journal of Nanjing Forestry University (Natural Sciences Edition) 2021, 45, 168–174. [Google Scholar]
- Li, Y.; Wang, Y.; Zhang, W. Impact of simulated acid rain on the composition of soil microbial communities and soil respiration in typical subtropical forests in Southwest China. Ecotoxicol Environ Saf 2021, 215, 112152. [Google Scholar] [CrossRef]
- Liang, J.; Mai, B.R.; Zheng, Y.F.; et al. Effects of simulated acid rain on the growth, yield, and quality of Brassica napus L. Journal of Ecology 2008, 1, 274–283. [Google Scholar]
- Ling, D.J.; Huang, Q.C.; Ouyang, Y. Impacts of simulated acid rain on soil enzyme activities in a latosol, 45 Ecotoxicology and Environmental Safety. 2010. [Google Scholar]
- Liu, Y.Y.; Jiang, H.; Li, Y.H.; Yuan, H.Y. Short-term Effects of Simulated Acid Rain on Soil Respiration in Cunninghamia lanceolata (Lamb) Hook. Seedling-Soil System. Acta Ecologica Sinica 2010, 30, 2010–2017. [Google Scholar]
- Liu, J.; Guo, Y.Y.; Bai, Y.W.; Camberato, J.J.; Xue, J.Q.; Zhang, R.H. Effects of Drought Stress on the Photosynthesis in Maize. Russian Journal of Plant Physiology 2018, 65(6).
- Liu, Z.; Wei, H.; Zhang, J.; Wang, T.; He, Y.; Zhong, J.; Ma, R. Increasing acid rain frequency promotes the microbial community dissimilarities of forest soil rather than agricultural soil in southern China. Ecotoxicol Environ Saf 2021, 230, 113123. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.; Neupane, P.R.; Köhl, M. Climate change impacts: Vegetation shift of broad-leaved and coniferous forests. Trees, Forests and People 2023, 14.
- Oukarroum, A.; Gharous, M.E.; Goltsev, V.; Strasser, R.J. Delayed fluorescence emission as a probe for the response of photosynthetic organisms to high temperature exposure: A comparative study. Journal of Luminescence 2016, 180, 321–327. [Google Scholar] [CrossRef]
- Oukarroum, A.; Strasser, R.J. Phenotyping of dark light adapted barley plants by the fast chlorophyll a fluorescence rise, O.J.I.P. South African Journal of Botany 2004, 70(2), 277–283. [Google Scholar] [CrossRef]
- Oukarroum, A.; Goltsev, V.; Strasser, R.J. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. PLoS One 2013, 8(3), e59433. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, C.; Benet Gunsu Corrales, I.; et al. A glance into aluminum toxicity and resistance. Science of The Total Environment 2008, 400(1-3):356-368.
- Ren, K.X.; Chen, J.J.; Cui, T.T.; Li, M.; Zhou, C.l.; Zhang, R.H.; et al. Effects of salinity on photosynthesis in maize probed by prompt fluorescence, delayed fluorescence and P700 signals. Trends in Cell Biology 2017, 27(3).
- Salim Akhter, M.; Noreen, S.; Mahmood, S.; Athar, H.-u.-R.; Ashraf, M.; Abdullah Alsahli, A.; Ahmad, P. Influence of salinity stress on PSII in barley (Hordeum vulgare L.) genotypes, probed by chlorophyll-a fluorescence. Journal of King Saud University – Science 2021, 33(1).
- Sant’Anna-Santos, F.B.; Campos da Silva, L.; Alves Azevedo, A.; Marcos de Araújo, J.; Figueiredo Alves, E.; Antônio Monteiro da Silva, E.; Aguiar, R. Effects of simulated acid rain on the foliar micromorphology and anatomy of tree tropical species. Environmental and Experimental Botany 2006, 58(1-3), 158-168.
- Shen, J.; Li, X.; Zhu, X.; Ding, Z.; Huang, X.; Chen, X.; Jin, S. Molecular and photosynthetic performance in the yellow leaf mutant of Torreya grandis according to transcriptome sequencing, chlorophyll a fluorescence, and modulated 820 nm reflection. Cells 2022, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, J.; Xiao, Z.; Lu, T.; Ren, X.; Wei, H. Effects of acid rain on plant growth: A meta-analysis. Journal of Environmental Management 2021, 297. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Strasser, R.J. ; Govindjee. Greening of peas: parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 1999, 37, 365–392. [Google Scholar]
- Stirbet, A. ; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B: Biology 2011, 104(1-2), 236-257.
- Strasser, R.J. Analysis of the Chlorophyll a Fluorescence Transient. Chlorophyll A Fluorescence A Signature of Photosynthesis. 2004.
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Tomlinson, G.H. Acidic deposition, nutrient leaching and forest growth. Biogeochemistry 2003, 65, 51–81. [Google Scholar] [CrossRef]
- Van Gorkom, H.J.; Donze, M. Charge accumulation in the reaction center of, P. S.I.I. Photochem Photobiol 1973, 17, 333–342. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.J.; Li, J.J.; Wang, L.; Wang, H.; Liu, G.D.; Fang, W.P.; Qiang, S.; Strasse, R. J. , Chen S. G. Natural plant inducer 2-Amino-3-Methylhexanoic acid protects physiological activity against high-temperature damage to tea (Camellia sinensis). Scientia Horticulturae 2023, 312, 111836. [Google Scholar]
- Zaharieva, I.; Goltsev, V. Advances on PS II investigation by measurement of delayed chlorophyll fluorescence by a phosporoscopic method. Photochem Photobiol 2003, 77, 292–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, C. Improving yield and quality of rice under acid rain stress by regulating nitrogen assimilation with exogenous Ca2+. Environ Sci Pollut Res 2023, 30, 12085–12097. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zheng, Y.; Li, Y. Effects of Simulated Acid Rain on Soil Enzyme Activity and Related Chemical Indexes in Woodlands. Forests 2022, 13(6).
- Zhou, R.H.; Kan, X.; Chen, J.J.; Hua, H.L.; Li, Y.; Ren, J.J.; et al. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environmental and Experimental Botany 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Using of chlorophyll a fluorescence OJIP transients for sensing salt stress in the leaves and fruits of tomato. Scientia Horticulturae 2017, 219, 216–221. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).