Submitted:
10 January 2024
Posted:
10 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Molecular Structure of the Cerium Compex
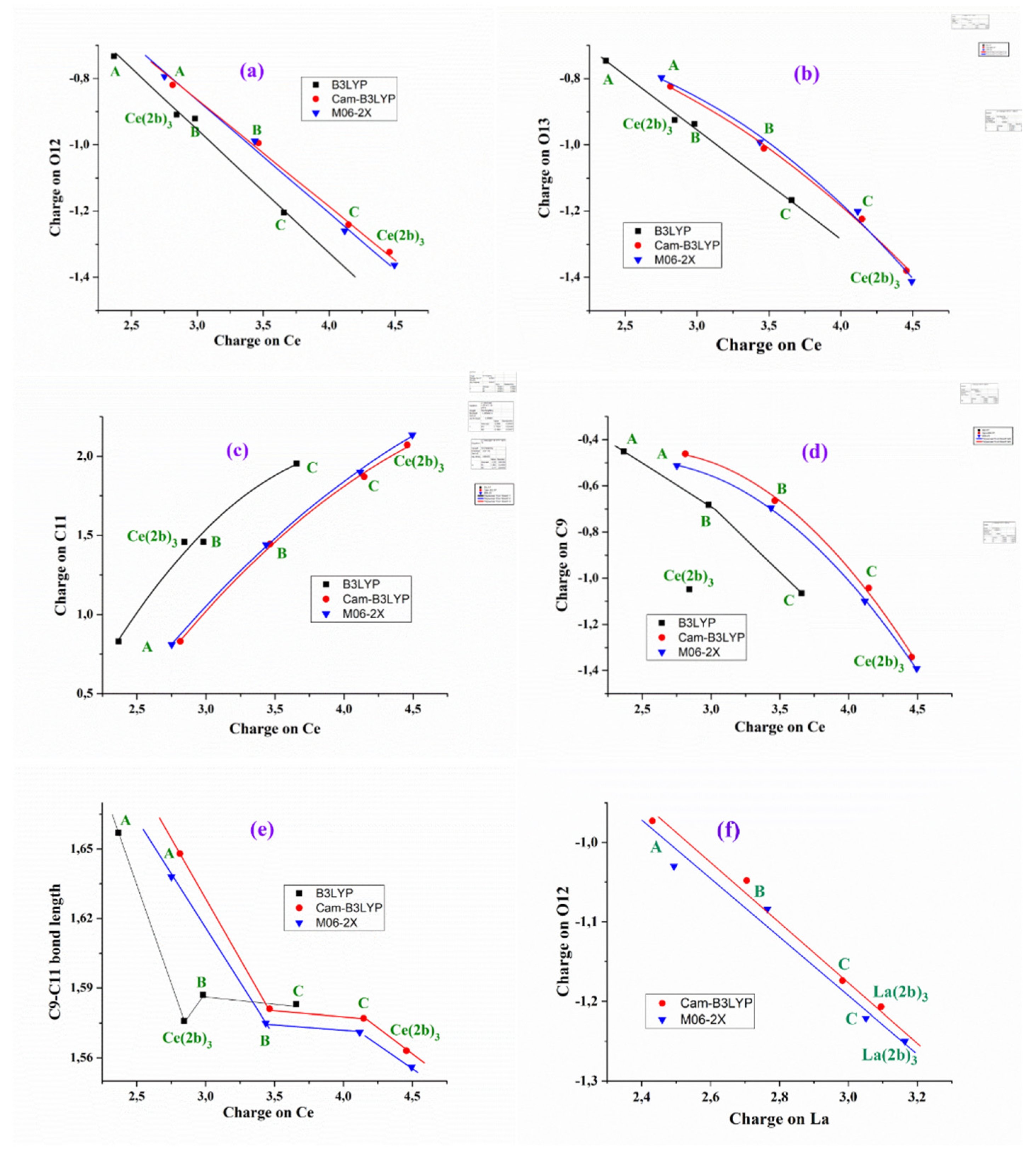
2.2. APT Atomic Charges and Relationships Established
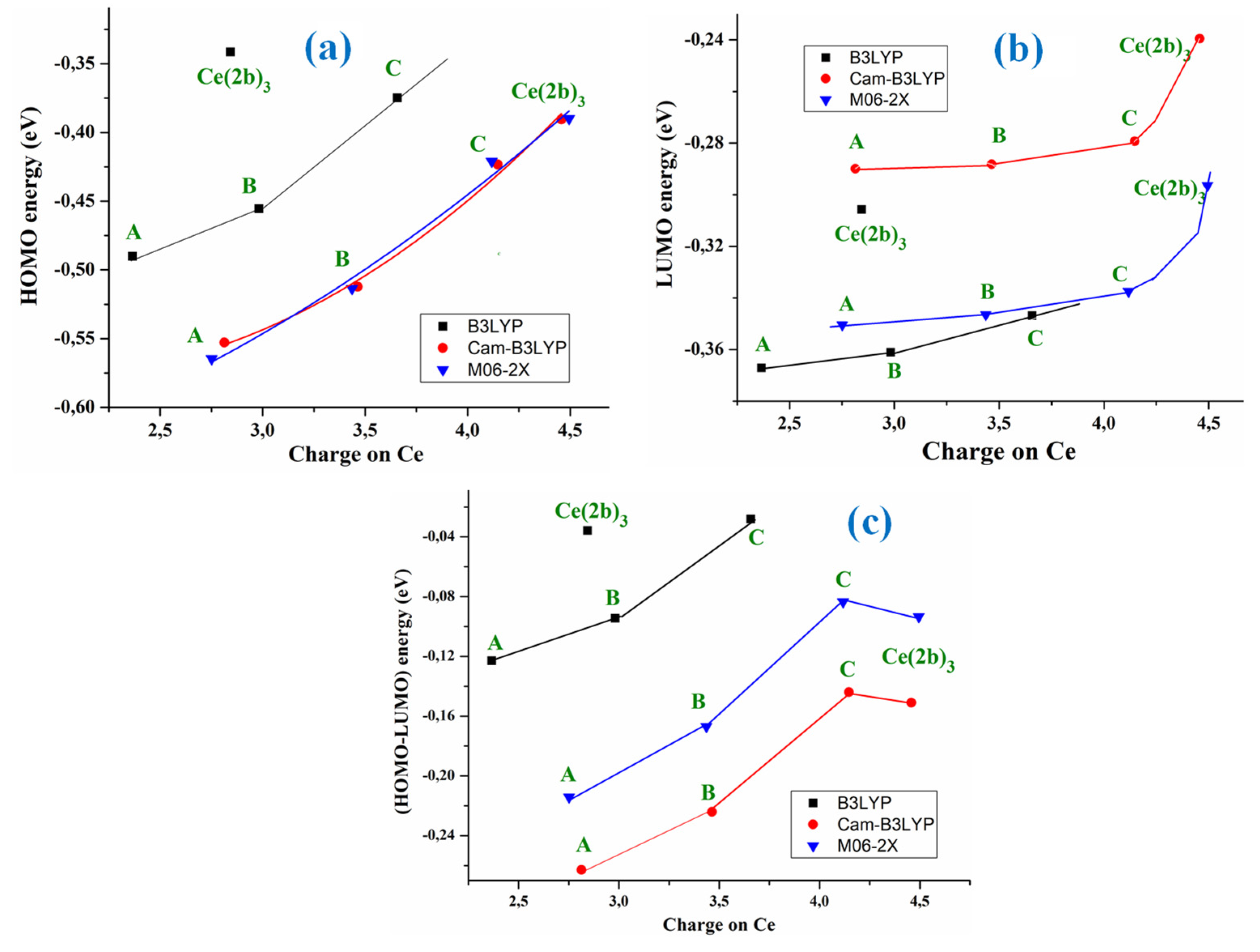
2.3. Molecular Properties
2.4. Vibrational Analysis
| Calculated by CAM-B3LYP | scaled | Experimental | Characterization by CAM-B3LYP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ν | A | S | DP | DU | LSE | PSE | IR | Raman | |
|
3324, 3323, 3323 3312, 3312, 3312 3303, 3303, 3303 3211, 3211, 3211 1664, 1660, 1660 1644, 1644, 1644 1635, 1635, 1635 1558, 1525, 1525 1475, 1464, 1464 1385, 1383, 1383 1362, 1362, 1362 1347, 1339, 1339 1337, 1336, 1336 1306, 1305, 1305 1303, 1301, 1301 1259, 1248, 1248 1238, 1234, 1233 1216, 1215, 1215 1189, 1188, 1188 1168, 1168, 1168 1112, 1109, 1109 1011, 1010, 1010 956, 956, 956 932, 931, 931 887, 887, 887 840, 839, 839 742, 742, 742 713, 709, 709 567, 557, 557 555, 554, 554 474, 474, 473 344, 344, 343 |
47 4 13 5 56 0 0 97 100 7 2 26 7 6 3 40 41 3 1 2 9 4 0 1 0 4 9 4 12 4 16 3 |
1 3 0 3 35 0 92 100 60 7 1 12 16 1 19 3 7 3 0 1 2 1 0 0 4 22 0 11 2 1 2 1 |
0.70 0.64 0.75 0.01 0.09 0.22 0.18 0.75 0.04 0.04 0.75 0.10 0.05 0.75 0.75 0.35 0.75 0.75 0.75 0.75 0.75 0.75 0.73 0.09 0.02 0.04 0.75 0.07 0.75 0.75 0.05 0.75 |
0.82 0.78 0.86 0.02 0.16 0.36 0.31 0.86 0.08 0.07 0.86 0.18 0.09 0.86 0.86 0.52 0.86 0.86 0.86 0.86 0.86 0.86 0.84 0.16 0.04 0.08 0.86 0.13 0.86 0.86 0.10 0.86 |
3057 3048 3040 2960 1623 1605 1598 1503 1450 1380 1362 1342 1339 1312 1309 1263 1250 1234 1211 1194 1143 1057 1011 989 951 909 826 797 666 663 594 481 |
3043 3034 3028 2957 1671 1653 1645 1546 1491 1417 1398 1377 1374 1345 1342 1293 1279 1262 1237 1219 1164 1071 1020 996 954 908 815 783 636 633 554 426 |
2968.1 s 2872.1 m 1577.2 br, vs 1500.1 s 1484.4 vs 1418.0 w 1398.3 m 1372.2 vs 1343.1 s 1301.8 vs 1285.1 s 1246.8 m 1218.1 m 1178.3 m 1091.2 vs 1011.9 m 969.1 vs 914.3 w 829.9 vs 804.8 m 654 m 647.1 m 509.0 m 466.9 m |
1595.0 vs 1504.5 s 1376.8 vs 1171.5 w 1089.9 m 1012.8 w 970.2 s |
20b, ν(C5-H) in aryl (100) 7b, ν(C6-H) in aryl (100) 20a, ν(C2-H) in aryl (100) νs(C-H) in C15H2 in pyrrolidine (100) ν(C8-N14) (45) + νs(CC) (34) 8b, ν(C=C) in aryl (89) 8a, ν(C=C) in aryl (82) ν(C9-C11)+νs(COO)+ν(C4N)+19a,ν(CC,CH) 19a,ν(CC,CH)+ν(C9-C11)+ν(triazol) νas(NNN, CN) +19a,ν(CC)(35)+νs(COO) 19b, ν(CC)(85) ν(triazol)+νas(CO12)+3,δ(CH)+δ(pyrrolidine) νs(COO) (49) + ν(triazol) + δ(pyrrolidine) δ(C-H) in pyrrolidine +νas(COO) νas(CO12) + 3,δ(C-H) aryl + δ(triazol) νas(CO13) + ν(C-N) triazol + Γ(pyrrolidine) νas(CO13) + ν(C-N) triazol + Γ(pyrrolidine) νas(CO12) + ν(C-N) triazol + δ(C-H) in aryl δs(C-H) in pyrrolidine 3, δ(C-H) in aryl νs(COO) + ν(NCCN) + γs(CC,CH) νs(COO) + ν(triazol) + 18a, δ(CC,CH) γas(CC,CH) in pyrrolidine νas(triazol) + νs(COO) + γ(CC,CH) γ(CC) pyrrolidine +νas(NNN) νas(NNN) + 12, δ(CCC) in aryl 17b, γ(C-H) in aryl ν(COO) + ν(triazol) + γ(CC) pyrrolidine Γ(triazol) +δ(COO) + 4,γ(CCC) δ(COO)+ r(triazol) + 4,γCCC) δ(COO) + δ(triazol) + δ(CC) in aryl δ(COO) + δ(triazol) + ν(aryl,C-Cl) |
| Calculated by M06-2X | scaled | Experimental | Characterization by M06-2X | ||||
| ν | A | S | LSE | PSE | IR | Raman | |
| 3326, 3326, 3325 3314, 3314, 3313 3308, 3308, 3307 3223, 3223, 3223 1702, 1700, 1698 1652, 1651, 1651 1646, 1646, 1645 1584, 1550, 1549 1493, 1482, 1481 1407, 1405, 1405 1378, 1378, 1378 1358, 1357, 1352 1345, 1344, 1344 1330, 1325, 1325 1309, 1308, 1308 1283, 1274, 1269 1242, 1242, 1241 1184, 1184, 1183 1168, 1166, 1166 1134, 1132, 1131 1119, 1119, 1118 1027, 1025, 1025 959, 958, 958 945, 944, 944 851, 851, 851 751, 750, 750 725, 724, 723 575, 573, 573 572, 565, 564 478, 476, 475 372, 370, 363 |
37 3 15 6 46 0 1 100 94 5 2 28 8 2 0 81 1 1 5 8 1 4 0 3 2 7 3 0 11 16 4 |
1 1 0 5 20 1 100 73 98 10 0 9 19 14 4 11 4 0 1 1 1 1 1 1 19 0 10 1 2 2 1 |
3050 3039 3034 2961 1649 1607 1603 1520 1461 1395 1372 1349 1343 1326 1312 1278 1255 1205 1189 1159 1149 1068 1010 998 918 831 808 680 671 594 498 |
3036 3027 3022 2957 1699 1655 1651 1565 1503 1434 1409 1386 1378 1361 1345 1309 1285 1231 1214 1182 1170 1083 1019 1006 918 821 795 652 641 554 443 |
2968.1 s 2872.1 m 1577.2 br, vs 1500.1 s 1484.4 vs 1418.0 w 1398.3 m 1372.2 vs 1343.1 s 1301.8 vs 1285.1 s 1246.8 m 1218.1 m 1178.3 m 1091.2 vs 1011.9 m 969.1 vs 914.3 w 829.9 vs 804.8 m 654 m 647.1 m 509.0 m 466.9 m |
1595.0 vs 1504.5 s 1376.8 vs 1171.5 w 1089.9 m 1012.8 w 970.2 s |
7b, ν(C5-H) in aryl (100) 20b, ν(C6-H) in aryl (100) 20a, ν(C2-H) in aryl (100) νs(C-H) in C15H2 pyrrolidine (100) ν(C8-N14) + νs(NNN-NC) in triazol 8b, ν(C=C) in aryl (93) 8a, ν(C=C) in aryl (93) ν(C9-C11)+νs(COO)+νs(CCN) triazol + ν(C4-N4) 19a, ν(CC,CH) + ν(C4-N4) +ν(C9-C11) νas(NNN) in triazol + 19a,ν(CC) in aryl 19b, ν(CC,CH) in aryl νas(COO) + νs(C9-N10) triazol + 19b, ν(CC,CH) in aryl νs(NNN) + νas(COO) +19a,ν(CC) + δ(CC) pyrrolidine νas(COO)+νas(triazol) +νas(CNC) pyrrolidine+ 19a,ν(CC) δs(C-H) in pyrrolidine + νs(NNN) ν(CO13)(45)+ν(CN) triazol (40)+ δs(CC,CH) pyrrolidine (12) νas(CO12) +νs(C9NN) triazol + δs(CC,CH) in pyrrolidine γas(C-H) in pyrrolidine 3, δ(CH) + γas(C-H) in pyrrolidine + νs(NNN) νs(COO)+ ν(triazol)+δas(C-H) in pyrrolidine γas(C-H) in pyrrolidine + νs(triazol) νs(NNN) + 18a, δ(C-H) in aryl δas(CC, CH) in pyrrolidine νas(NC9-C8) + δas(CC, CH) in pyrrolidine νas(NNN)(36) + 12, δ(CCC) in aryl 17b, γ (C-H) in aryl δas(COO) + γ(CC, CH) pyrrolidine +δ(triazol) γ(triazol) + γas(COO) + 8b, δ(CCC) in aryl γas(COO) + γ(triazol) δas(COO) + δ(triazol) + δ(CC) in aryl δas(COO) + δ(triazol) + δ(CC) in aryl |
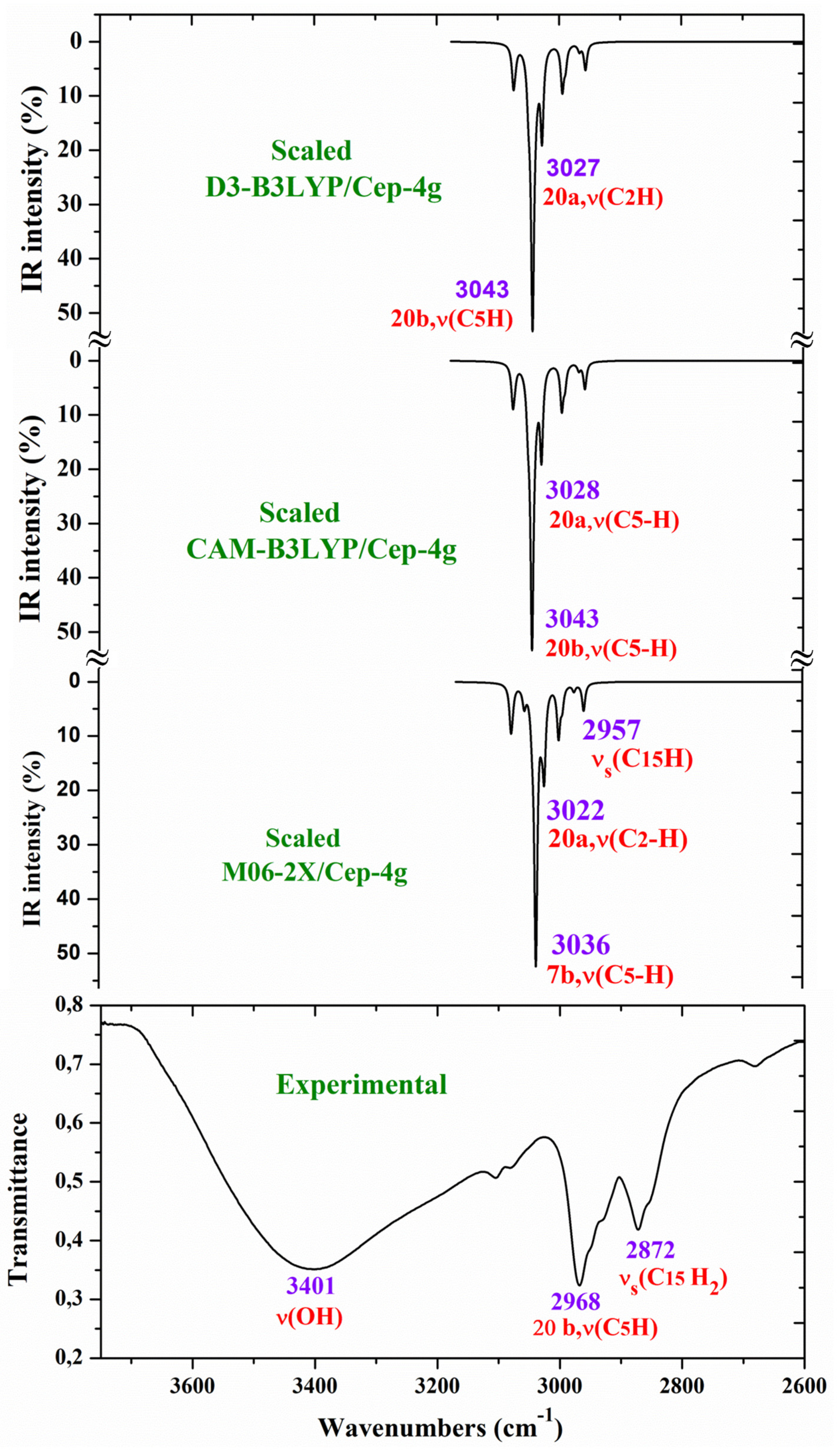
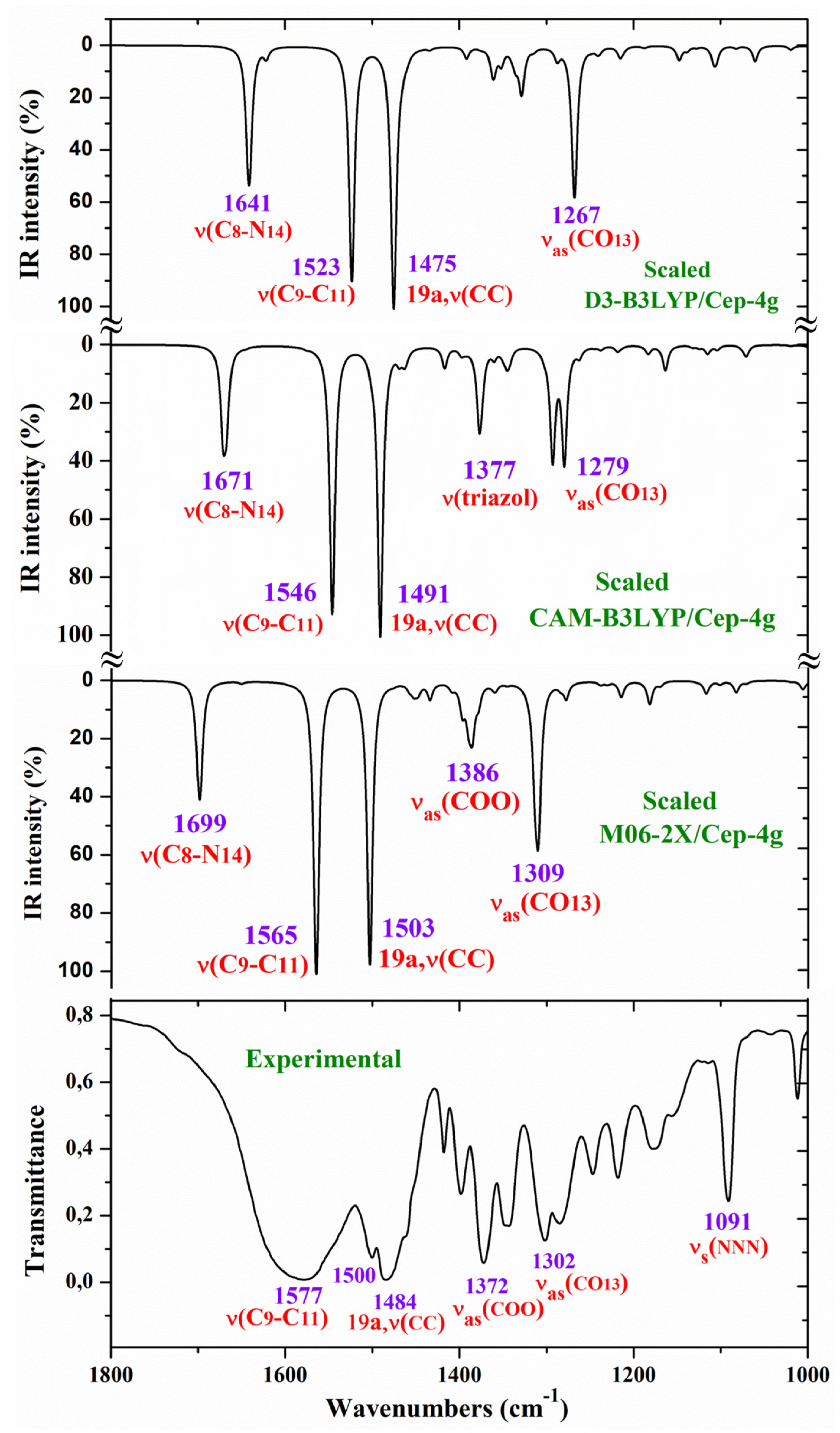
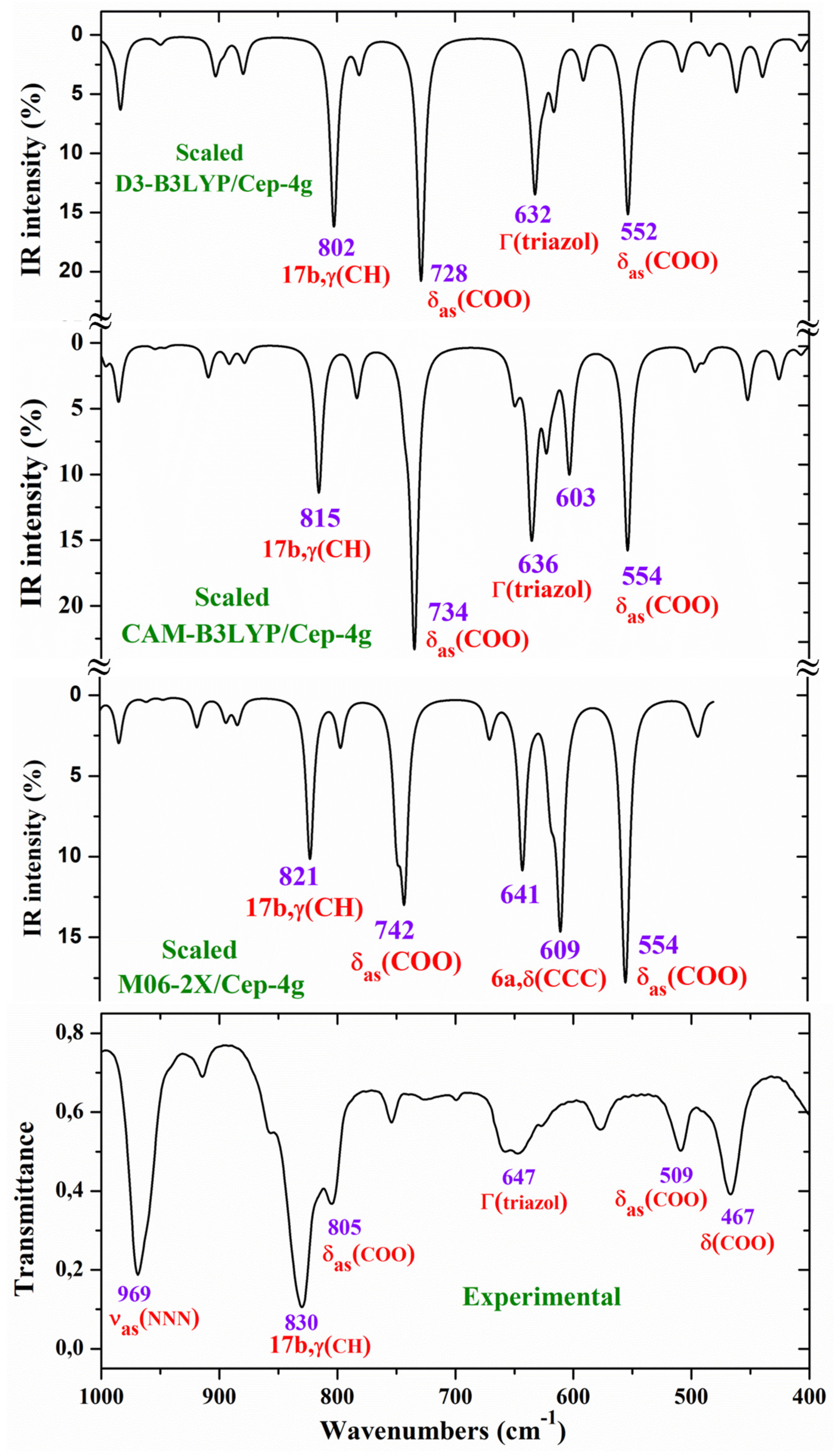
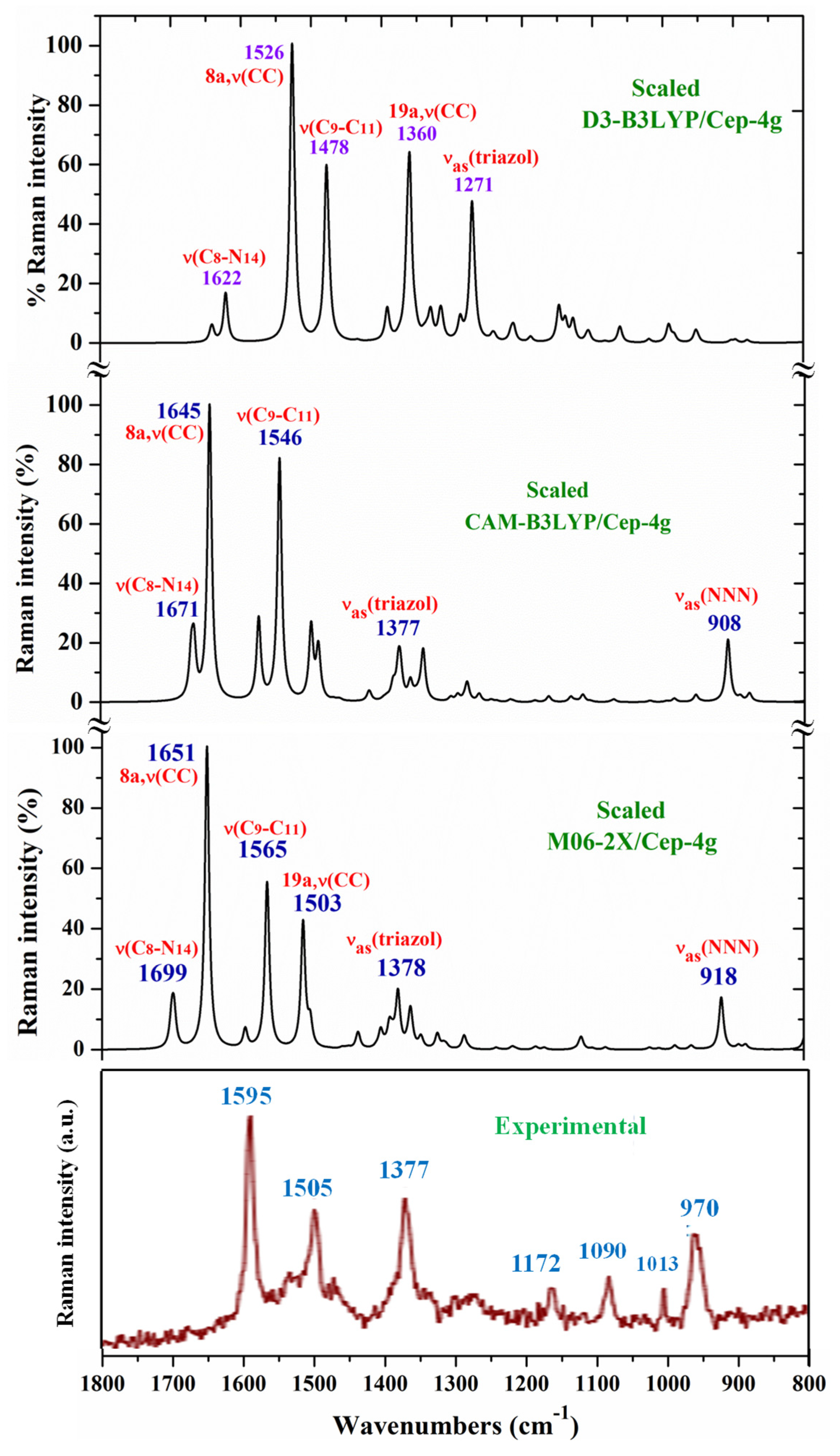
2.4.1. General Comparison of the IR and Raman Spectra
- (i)
- A good accordance between the scaled theoretical spectra with the experimental one has been found, in particular, the scaled strongest vibrations have their correspondence in the experimental spectrum. This feature confirms the scaling carried out on the calculated wavenumbers, therefore the theoretical methods used appear appropriate. Thus, in general, the assignments proposed could be considered true, identifying most of the computed modes in their normal ranges.
- (ii)
- A very broad and strong band centered at 3400.8 cm-1 has been observed in the experimental spectrum that by its position, it can be only assigned to the O-H stretching ν(O-H) mode, corresponding to water molecules strongly H-bonded to the three 2b’ ligands of Ce(2b’)3 complex. These water molecules were not included in our optimized theoretical complex, but in previous studies with lanthanum(III) ion we noted that these water molecules only slightly affect the carboxylate group, the other groups remaining unaffected [3,9]. This hydration appears due to the spatial arrangement of these ligands in the complex, which leaves cavities that can be occupied by water molecules. As it is expected, this band is not observed in the Raman spectrum.
- (iii)
- Another broad but very strong experimental IR band is observed at 1577.2 cm-1. Its large broadening can be interpreted as a result of the additional contribution of the in-plane bending δ(O-H) mode of these hydrated water molecules to the main assignment of this band corresponding to the C8-N14 and C-C stretching, Table 4.
- (iv)
- A noticeable resemblance between the scaled spectra obtained by the D3-B3LYP, CAM-B3LYP and M06-2X methods that include long-range correction has been observed, while that by B3LYP differs noticeably. As compared to the experimental spectrum, the two best methods are CAM-B3LYP and M06-2X and for this reason, their spectra were analyzed in detail and included in Table 4. In this analysis, the scaled wavenumbers by CAM-B3LYP appear slightly better than by M06-2X, that is why it has been mainly utilized in the experimental spectra assignment.
- (v)
- Coordination of the 2b’ ligands to Ce(III) ion remarkably modified the IR and Raman spectra appearing distinct of that found with 2b ligand anion alone [12].
2.4.2. Specific Comparison of the IR and Raman Spectra
2.4.2.1. The Carboxylate COO- Group Modes
2.4.2.2. Triazole Ring Modes
2.4.2.3. Aryl Ring Modes
2.5. Free Radical-Scavenging Activity
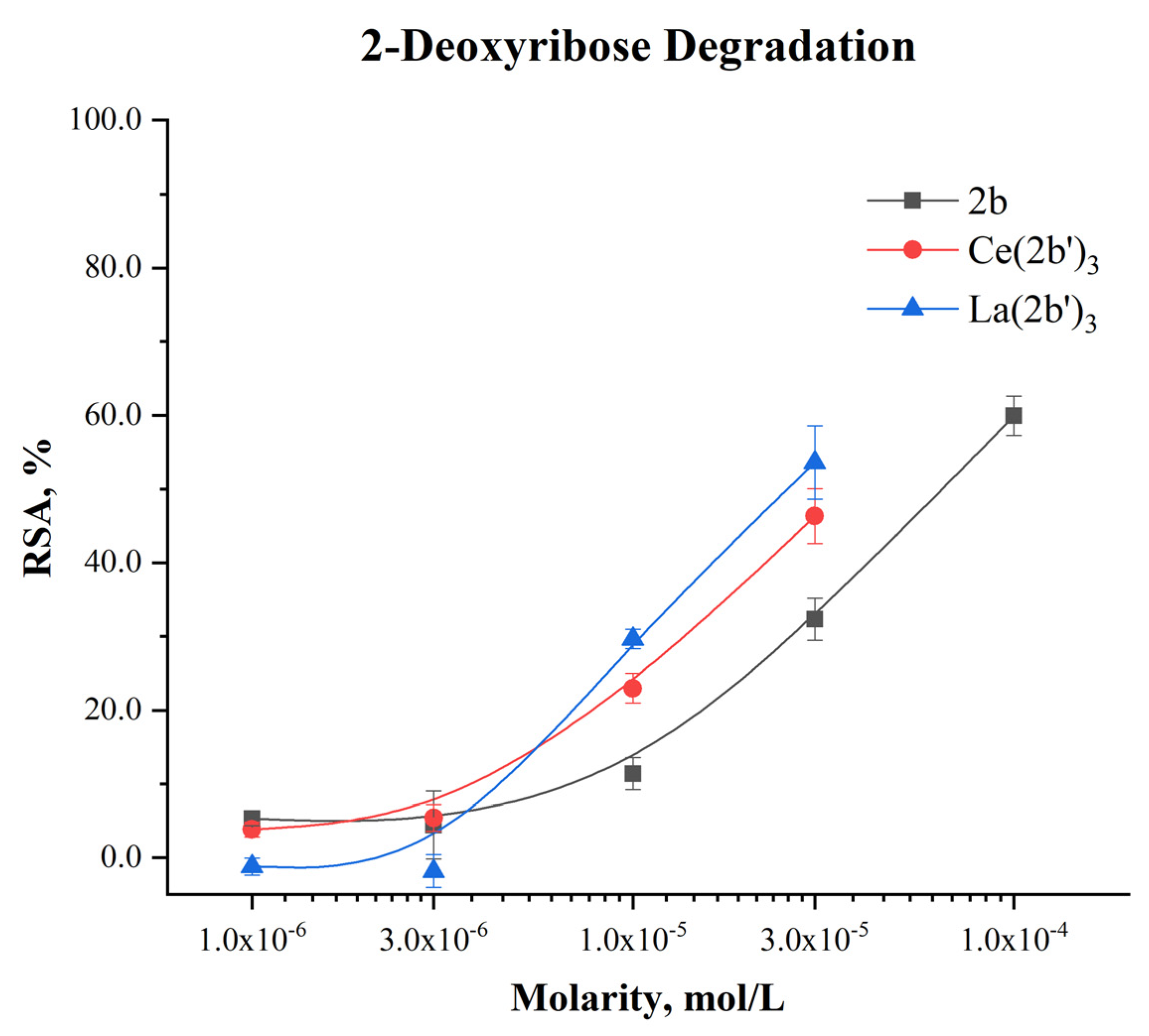
2.5.1. Impact of Ce(2b’)3 on 2-Deoxyribose Degradation
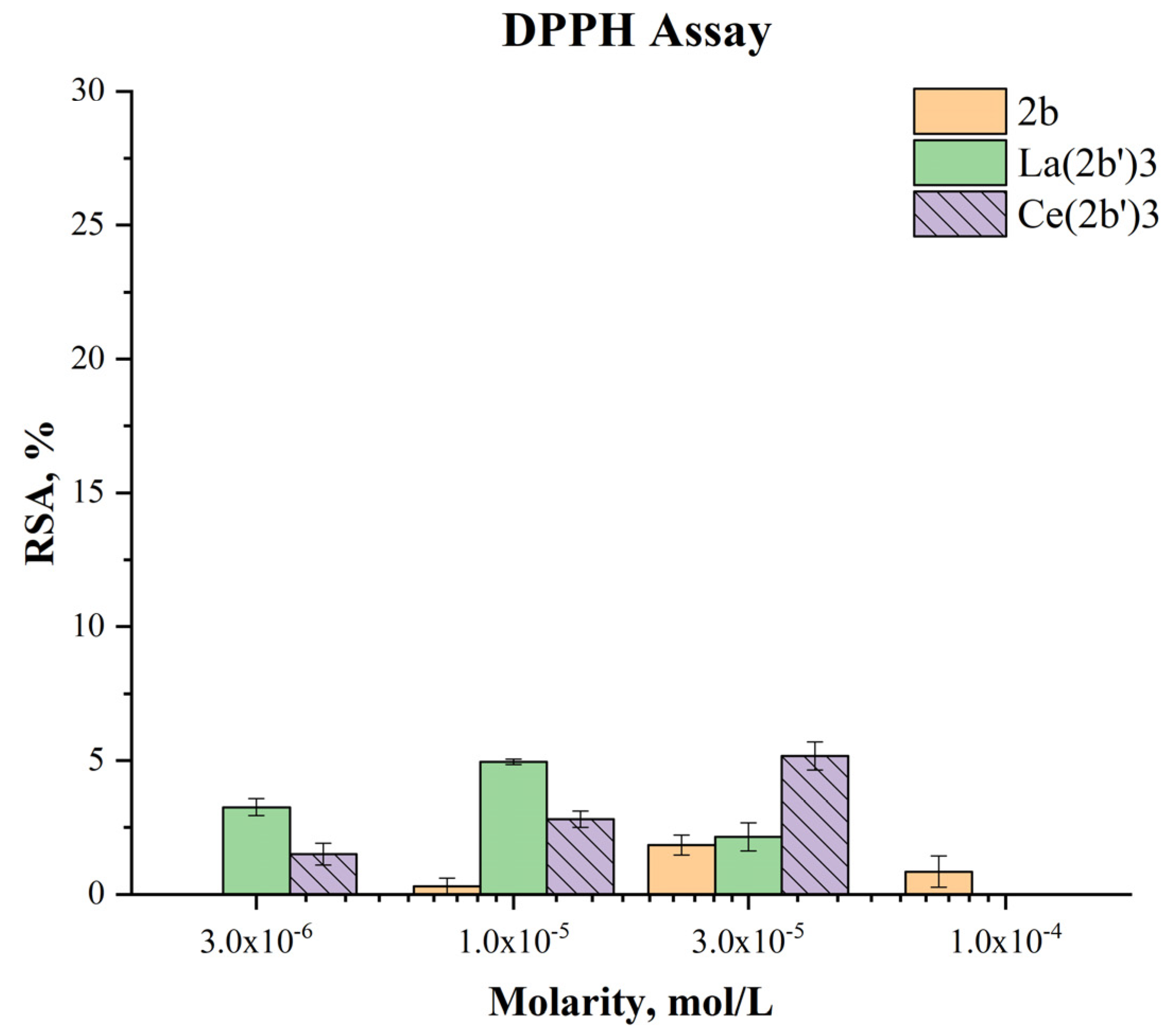
2.5.2. Impact of Ce(2b’)3 on a model system, containing the stable radical DPPH●
2.5.3. Impact of Ce(2b’)3 on a model system, containing the stable radical ABTS●+
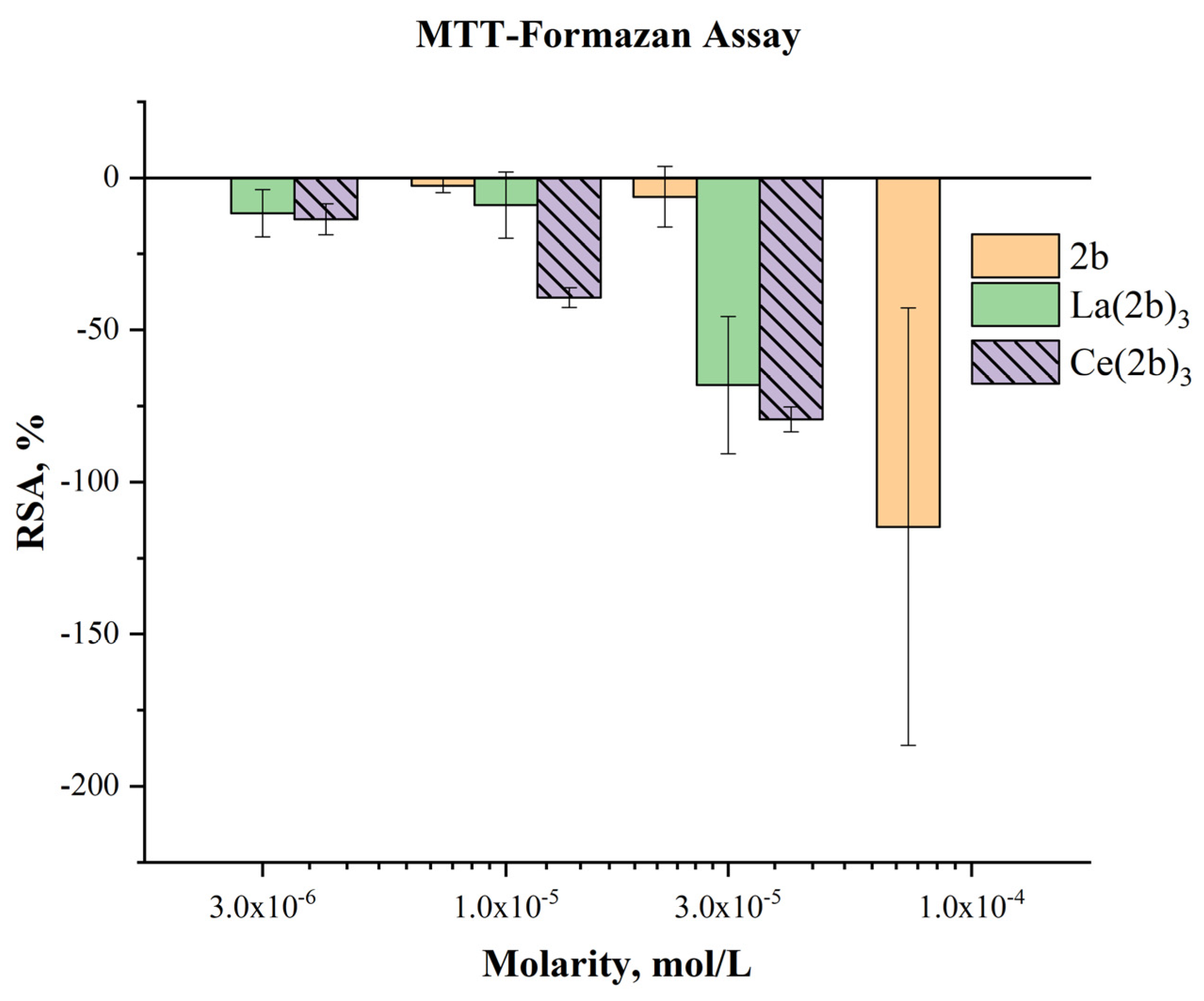
2.5.4. Impact of Ce(2b’)3 on MTT-formazan transformation, triggered by Fenton reaction-derived hydroxyl radicals.
3. Discussion and Conclusions
- 1)
- In the complex formation, the CO bonds of the ligands are lengthened as compared to the free form, which gives rise to a slight shortening of the C9-C11 bond length, to a decrease of the N7=C8 and C9=N10 double bond character, and to more rotated triazole substituents.
- 2)
- The influence of different ligands on the charge and molecular properties of the Ce(III) complex was analyzed. Thus, the cerium charge is increased from the smallest A-complex to the largest Ce(2b’)3 complex due to the better charge distribution on the cerium ion in larger systems. The pyrrolidine ring slightly increments this electron transfer.
- 3)
- New relationships with different ligands were established to find better properties. In particular, the charge on the cerium ion has a large influence in the bond lengths and atomic charges on the ligand atoms in all complexes. An increase in the positive calculated charge of the Ce(III) ion appears well linear associated with an increment in the negative charge of O12 and O13 atoms. The magnitude of the electronic charge lost by the Ce(III) ion is almost the same that was transferred to both O12 and O13 atoms. Large ligands appear to facilitate this negative electron transfer.
- 4)
- Global chemical reactivity descriptors were calculated. The HOMO–LUMO gap value linearly decreases with the system complexity (higher Ce atomic charge), with the exception of Ce(2b’)3 complex which has a similar value to C-complex. Its low energy value indicates a large chemical reactivity and small excitation energies to the manifold of excited states.
- 5)
- The pyrrolidine ligand slightly reduces the reactivity of the Ce(2b’)3 complex, but it gives liposolubility to the complex that would be necessary to cross the cell membrane.
- 6)
- The dipole moment value indicates an almost null water solubility(insolubility) in A- and B-complexes, but remarkable increase when the aryl and pyrrolidine rings are inserted in the ligands (C and Ce(2b’)3 complexes). It is not so significant.
- 7)
- New scaling equations for the D3-B3LYP, CAM-B3LYP and M06-2X DFT methods have been utilized to improve the computed IR and Raman spectra. The noticeable agreement with the experimental ones, raised in both frequencies and intensities, corroborates the spatial orientation of the ligands in the synthesized Ce(2b’)3 complex. The best correlations have been found with CAM-B3LYP method.
- 8)
- The complex Ce(2b’)3 scavenges hydroxyl radicals, generated by UV-infuced water radiolysis. Comparison with the analogous La(2b’)3 shows that in this particular model system the impact of the coordination center, Ce(III) or La(III), on activity is very mild.
- 9)
- In line with previous observations on 2b and La(2b’)3, the ability of Ce(2b’)3 to participate in HAT with DPPH● is very limited. These results, skarkly contrasting with the ones, derived from the 2-deoxyribose degradation assay, suggest that the low DPPH-scavenging activity may be due to steric hindrance, rather than low hydrogen-donating capacity of the complex.
- 10)
- Coordination with Ce(III) seems to improve the ligand’s ability to participate in SET, as observed in the ABTS●+ assay. Ce(2b’)3 seems to scavenge the stable radical much more actively, compared to its La(III) counterpart (almost threefold higher RSA values). In this case we can comfortably deduce that the electron-exchanging capacity is significantly impacted by the type of metal ion, coordinated with 2b.
- 11)
- Ce(2b’)3 reaffirms previous observations that 2b and its lanthanide complexes tend to behave as prooxidants in presence of the clinically significant Fenton reaction. A concentration-dependent increase in MTT-formazan formation was observed with Ce(2b’)3, similar to La(2b’)3 .
4. Materials and Methods
4.1. Experimental Details
4.2. Materials
4.3. Methods
4.3.1. Deoxyribose Degradation Assay
4.3.2. Fenton Reaction MTT Assay
4.3.3. DPPH Assay
4.3.4. ABTS Assay
4.4. Computational details
4.4.1. Scaling the wavenumbers
Author Contributions
Funding
Conflicts of Interest
References
- Lewandowski, W.; Kalinowska, M.; Lewandowska, H. The influence of metals on the electronic system of biologically important ligands. Spectroscopic study of benzoates, salicylates, nicotinates and isoorotates. Review. Journal of inorganic biochemistry 2005, 99, 1407–1423. [Google Scholar] [CrossRef]
- Goswami, A.K.; Kostova, I. Medicinal and Biological Inorganic Chemistry: Walter de Gruyter GmbH & Co KG; 2022; ISBN 1501516116.
- Alcolea Palafox, M.; Belskaya, N.P.; Todorov, L.T.; Kostova, I.P. Structural Study of a La (III) Complex of a 1, 2, 3-Triazole Ligand with Antioxidant Activity. Antioxidants 2023, 12, 1872. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Zhu, W.; Liu, C.; Gu, J. Bacteriostatic effects of cerium-humic acid complex: An experimental study. Biological trace element research 2000, 73, 29–36. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Esfahani, H.S.; Habibi, M.; Hosseinzadeh, G. Preparation of cerium-curcumin and cerium-quercetin complexes and their LEDs irradiation assisted anticancer effects on MDA-MB-231 and A375 cancer cell lines. Photodiagnosis and Photodynamic Therapy 2021, 34, 102326. [Google Scholar] [CrossRef]
- Abd El-Hamid, S.M.; Sadeek, S.A.; Zordok, W.A.; El-Shwiniy, W.H. Synthesis, spectroscopic studies, DFT calculations, cytotoxicity and antimicrobial activity of some metal complexes with ofloxacin and 2, 2′-bipyridine. Journal of Molecular Structure 2019, 1176, 422–433. [Google Scholar] [CrossRef]
- Feng, F.-M.; Cai, S.; Liu, F.-A.; Xie, J.-Q. Studies of DNA-binding and DNA-cutting mechanism of an azamacrocyclic cerium complex with carboxyl branch. Progress in Reaction Kinetics and Mechanism 2013, 38, 283–294. [Google Scholar] [CrossRef]
- Safronov, N.E.; Kostova, I.P.; Palafox, M.A.; Belskaya, N.P. Combined NMR Spectroscopy and Quantum-Chemical Calculations in Fluorescent 1, 2, 3-Triazole-4-carboxylic Acids Fine Structures Analysis. International Journal of Molecular Sciences 2023, 24, 8947. [Google Scholar] [CrossRef]
- Peica, N.; Kostova, I.; Kiefer, W. Theoretical and experimental studies on binding mode of 3, 5-pyrazoledicarboxylic acid in its new La (III) complex. Chemical physics 2006, 325, 411–421. [Google Scholar] [CrossRef]
- Alam, M.M. 1, 2, 3-Triazole hybrids as anticancer agents: A review. Archiv der Pharmazie 2022, 355, 2100158. [Google Scholar] [CrossRef]
- Hrimla, M.; Oubella, A.; Laamari, M.R.; Bahsis, L.; Ghaleb, A.; Auhmani, A.; et al. Click synthesis, anticancer activity, and molecular docking investigation of some functional 1, 2, 3-triazole derivatives. Biointerface Research in Applied Chemistry 2022, 12, 7633–7667. [Google Scholar]
- Palafox, M.A.; Belskaya, N.P.; Kostova, I.P. Study of the Molecular Architectures of 2-(4-Chlorophenyl)-5-(Pyrrolidin-1-Yl)-2H-1, 2, 3-Triazole-4-Carboxylic Acid as the Potential Anticancer Drug by Their Vibrational Spectra and Quantum Chemical Calculations. 2023.
- Mishra, V.R.; Sekar, N. Photostability of coumarin laser dyes-a mechanistic study using global and local reactivity descriptors. Journal of fluorescence 2017, 27, 1101–1108. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical hardness and density functional theory. Journal of Chemical Sciences 2005, 117, 369–377. [Google Scholar] [CrossRef]
- George, S. Infrared and Raman characteristic group frequencies: tables and charts. Wiley: Chichester 2001, 82, 85–87. [Google Scholar]
- Tammer, M.G. Sokrates: Infrared and Raman characteristic group frequencies: tables and charts: Wiley, Chichester, 2004. ISBN 0-470-09307-2, 347 pages, paperback; US $60. Springer; 2004.
- Aziz, S.G.; Elroby, S.A.; Alyoubi, A.; Osman, O.I.; Hilal, R. Experimental and theoretical assignment of the vibrational spectra of triazoles and benzotriazoles. Identification of IR marker bands and electric response properties. Journal of molecular modeling 2014, 20, 1–15. [Google Scholar]
- Törnkvist, C.; Bergman, J.; Liedberg, B. Geometry and vibrations of the 1, 2, 3-triazole anion. A theoretical and experimental study. The Journal of Physical Chemistry 1991, 95, 3119–3123. [Google Scholar] [CrossRef]
- El-Azhary, A.; Suter, H.; Kubelka, J. Experimental and theoretical investigation of the geometry and vibrational frequencies of 1, 2, 3-triazole, 1, 2, 4-triazole, and tetrazole anions. The Journal of Physical Chemistry A 1998, 102, 620–629. [Google Scholar] [CrossRef]
- Palafox, M.A.; Rastogi, V. Spectra and structure of benzonitriles and some of its simple derivatives. Asian Journal of Physics Vol 2013, 22, 1–30. [Google Scholar]
- Varsányi, G.; Láng, L.; Kovner, M.A.E.; Lempert, K. Assignment for vibrational spectra of seven hundred benzene derivatives. (No Title). 1974.
- Galano, A. Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols. Journal of the Mexican Chemical Society 2015, 59, 231–262. [Google Scholar] [CrossRef]
- Kell, D.B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC medical genomics 2009, 2, 1–79. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. Journal of food science and technology 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J sci technol 2004, 26, 211–219. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical biochemistry 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical biochemistry 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical biochemistry 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Burns, W.G.; Sims, H.E. Effect of radiation type in water radiolysis. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 1981, 77, 2803–2813. [Google Scholar] [CrossRef]
- Chrzczanowicz, J.; Gawron, A.; Zwolinska, A.; de Graft-Johnson, J.; Krajewski, W.; Krol, M.; et al. Simple method for determining human serum 2, 2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity–possible application in clinical studies on dietary antioxidants. Clinical Chemical Laboratory Medicine 2008, 46, 342–349. [Google Scholar] [CrossRef]
- Seminario, J.M. Modern density functional theory: a tool for chemistry: Elsevier; 1995. ISBN 008053 6700.
- Palafox, M.A. DFT computations on vibrational spectra: Scaling procedures to improve the wavenumbers. Physical Sciences Reviews 2018, 3, 20170184. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Noncovalent interactions in biochemistry. Wiley Interdisciplinary Reviews: Computational Molecular Science 2011, 1, 3–17. [Google Scholar] [CrossRef]
- Riley, K.E.; Pitonák, M.; Jurecka, P.; Hobza, P. Stabilization and structure calculations for noncovalent interactions in extended molecular systems based on wave function and density functional theories. Chemical Reviews 2010, 110, 5023–5063. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functionals. Chemical Physics Letters 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Alcolea, M.P. Scaling factors for the prediction of vibrational spectra. I. Benzene molecule. International Journal of Quantum Chemistry 2000, 77, 661–684. [Google Scholar] [CrossRef]
- Kullgren, J.; Castleton, C.W.; Müller, C.; Ramo, D.M.; Hermansson, K. B3LYP calculations of cerium oxides. The Journal of chemical physics 2010, 132. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chemical physics letters 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Tawada, Y.; Tsuneda, T.; Yanagisawa, S.; Yanai, T.; Hirao, K. A long-range-corrected time-dependent density functional theory. The Journal of chemical physics 2004, 120, 8425–8433. [Google Scholar] [CrossRef]
- Frisch Me Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; et al. Gaussian 16, revision C. 01. Gaussian, Inc., Wallingford CT; 2016.
- Das, B.; Ghosh, K.; Baruah, J.B. Tris-dipicolinate Cerium Complexes Bearing Dications of Arginine, Histidine, and Ornithine. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 2014, 44, 251–257. [Google Scholar] [CrossRef]
- Levin, J.R.; Dorfner, W.L.; Dai, A.X.; Carroll, P.J.; Schelter, E.J. Density functional theory as a predictive tool for cerium redox properties in nonaqueous solvents. Inorganic Chemistry 2016, 55, 12651–12659. [Google Scholar] [CrossRef]
- Srivastav, G.; Yadav, B.; Yadav, R.K.; Yadav, R. DFT studies of molecular structures conformers and vibrational characteristics of sulfanilamide. Computational and Theoretical Chemistry 2019, 1167, 112588. [Google Scholar] [CrossRef]
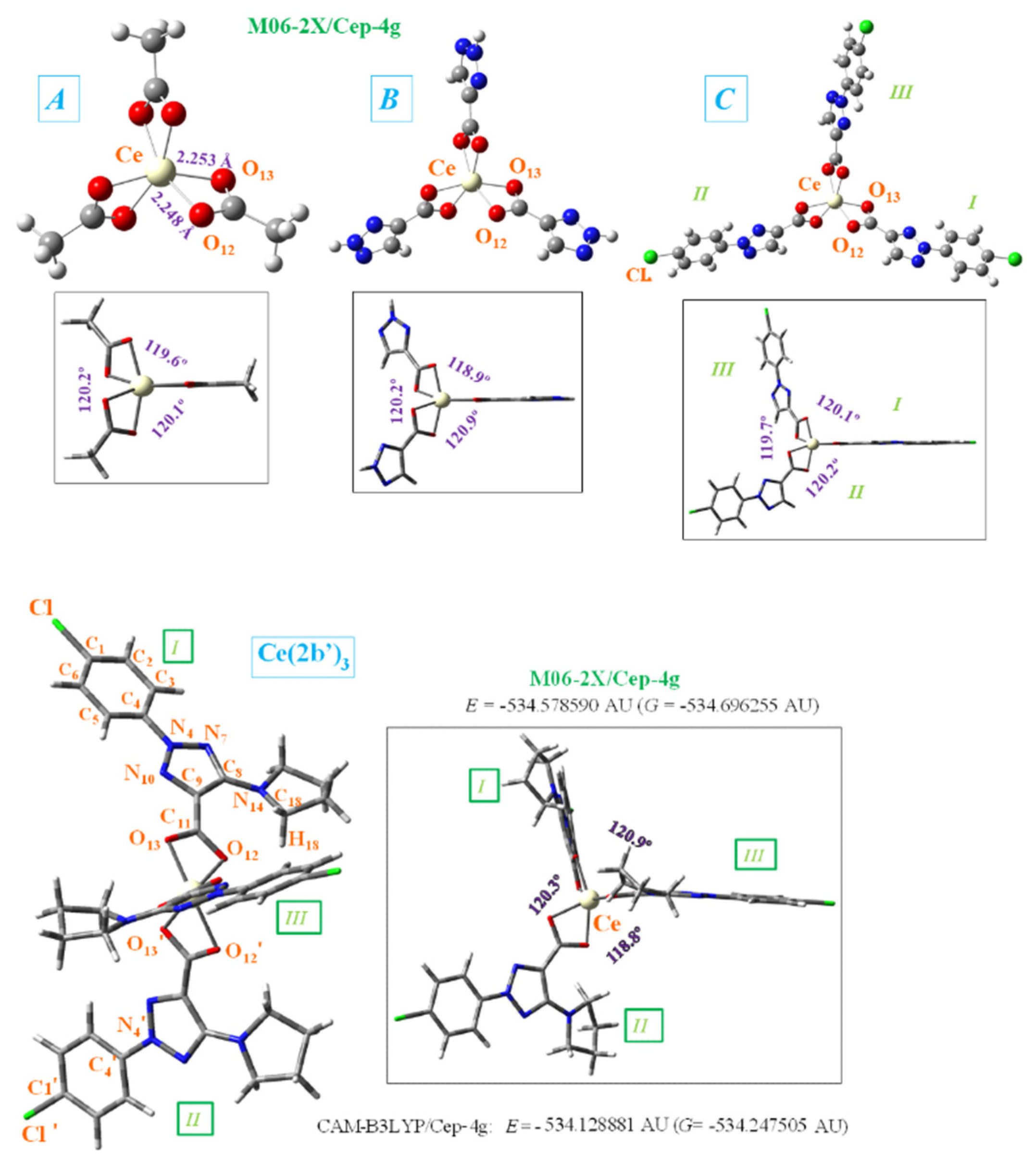
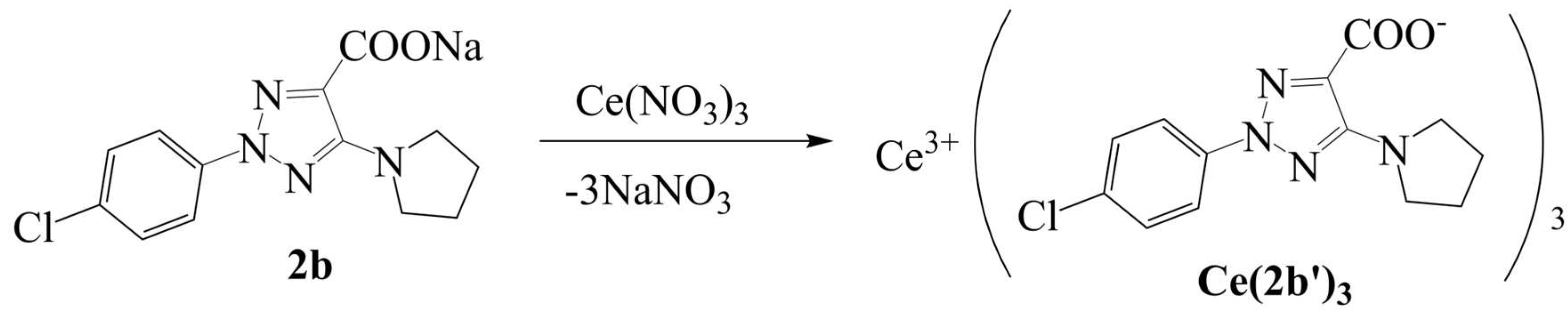

| A-complex | B-complex | C-complex | Ce-(2b’)3 | ||||||||||
| Parameters | B3LYP | CAM-B3LYP | M06-2X/ | B3LYP/ | CAM-B3LYP | M06-2X/ | B3LYP | CAM-B3LYP | M06-2X/ | B3LYP | D3-B3LYP | CAM-B3LYP | M06-2X/ |
| r(C9-C11) r(C=O12) r(C=O13) r(Ce-O12) r(Ce-O13) ∠(C9-C11=O12) ∠(O=C=O) ∠(C=O12-Ce) ∠(C=O13-Ce) ∠(O12-Ce-O′13) ∠(O13-Ce-O′12) ∠(C11-O12···O′12-C′11) ∠(C9-C11···C′11-C′9) ∠(C′9-C′11···C′′11-C′′9) ∠(C11···Ce··· C′11) ∠(C′11···Ce··· C′′11) |
1.657 1.434 1.434 2.287 2.286 123.7 112.5 92.3 92.3 153.9 102.2 57.6 -0.1 0.1 120.1 120.0 |
1.648 1.423 1.423 2.256 2.255 123.9 112.1 92.3 92.4 155.3 100.8 60.6 -0.2 0.3 120.2 119.6 |
1.638 1.413 1.412 2.248 2.253 123.4 113.2 91.9 91.7 100.5 102.1 -98.1 0.5 -0.5 119.6 120.2 |
1.587 1.434 1.435 2.290 2.282 122.2 114.2 90.9 91.2 154.3 102.7 56.9 0.2 2.1 120.2 119.0 |
1.581 1.422 1.423 2.260 2.251 122.4 113.8 91.0 91.4 155.5 101.2 60.5 0.0 2.6 120.2 119.0 |
1.575 1.410 1.414 2.261 2.240 122.0 114.8 90.3 91.0 100.5 100.1 -98.8 1.8 0.2 118.9 120.9 |
1.583 1.436 1.436 2.288 2.281 122.1 114.2 90,9 91.2 153.8 103.1 55.9 0.0 0.0 119,9 120.1 |
1.577 1.423 1.423 2.257 2.251 122.4 113.7 91.1 91.3 155.3 101.3 59.8 0.0 0.1 120.1 120.0 |
1.571 1.413 1.413 2.253 2.245 121.7 114.8 90.5 90.8 101.7 100.4 -99.1 2.7 1.0 120.1 119.7 |
1.576 1.437 1.442 2.304 2.275 125.9 113.7 90.9 91.9 152.4 104.5 56.4 -0.2 -0.1 120.0 119.8 |
1.570 1.437 1.441 2.292 2.272 125.4 113.9 90.8 91.5 153.3 103.4 59.6 -1.1 -1.1 119.9 119.8 |
1.563 1.425 1.433 2.264 2.231 126.6 112.7 91.1 92.2 154.8 101.6 61.7 0.0 -0.1 119.8 119.9 |
1.556 1.415 1.425 2.260 2.224 125.5 113.8 90.4 91.6 99.7 99.9 -103.5 -18.0 16.7 120.3 118.8 |
| A-complex | B-complex | C-complex | Ce-(2b’)3 | ||||||||||
| atom | B3LYP | CAM-B3LYP | M06-2X/ | B3LYP/ | CAM-B3LYP | M06-2X | B3LYP | CAM-B3LYP | M06-2X/ | B3LYP | D3-B3LYP | CAM-B3LYP | M06-2X/ |
|
Ce N4 N7 C8 C9 N10 C11 O12 O13 |
2.366 - - - -0.450 - 0.830 -0.733 -0.747 |
2.814 - - - -0.461 - 0.831 -0.819 -0.823 |
2,752 - - - -0.512 -- 0.810 -0.794 -0.797 |
2.982 -0.596 -0.013 -0.071 -0.682 0.253 1.459 -0.921 -0.937 |
3.464 -0.608 -0.018 -0.090 -0.664 0.262 1.446 -0.995 -1.011 |
3.436 -0.625 -0.031 -0.079 -0.695 0.276 1.441 -0.989 -0.992 |
3.657 -0.210 -0.111 0.021 -1.065 0.335 1.954 -1.205 -1.167 |
4.147 -0.440 -0.076 -0.093 -1.042 0.443 1.871 -1.241 -1.224 |
4.117 -0.383 -0.105 -0.072 -1.100 0.472 1.900 -1.260 -1.201 |
2.844 -0.058 -0.099 0.444 -1.048 0.105 1.458 -0.909 -0.925 |
3.056 -0.109 -0.109 0.453 -1.096 0.157 1.591 -0.974 -1.001 |
4.457 -0.466 -0.124 0.596 -1.342 0.411 2.071 -1.323 -1.380 |
4.494 -0.430 -0.133 0.610 -1.392 0.426 2.134 -1.363 -1.413 |
| Molecular properties | A-complex | B-complex | C-complex | Ce-(2b’)3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAM-B3LYP | M06-2X/ |
CAM-B3LYP | M06-2X | CAM-B3LYP | M06-2X/ | B3LYP | D3-B3LYP | CAM-B3LYP | M06-2X/ | |
| Rotational constants: A (GHz) B C |
0.575 0.572 0.328 |
0.582 0.582 0.331 |
0.133 0.129 0.070 |
0.135 0.131 0.071 |
0.023 0.023 0.013 |
0.028 0.020 0.012 |
0.018 0.018 0.011 |
0.018 0.018 0.011 |
0.018 0.018 0.011 |
0.019 0.017 0.011 |
| Cv (cal/mol·K) S (cal/mol·K) |
51.7 134.3 |
53.6 142.9 |
86.8 188.4 |
83.8 181.6 |
163.8 297.1 |
162.4 296.2 |
231.5 387.8 |
231.0 388.5 |
225.9 383.0 |
224.1 379.4 |
| Dipole moment (Debye) | 0.098 | 0.130 | 0.040 | 0.036 | 5.416 | 2.571 | 11.523 | 10.916 | 10.604 | 5.193 |
| HOMO LUMO Eg IP EA χ η S |
-0.553 -0.290 -0.263 0.553 0.290 0.421 0.131 0.066 |
-0.565 -0.350 -0.214 0.565 0.350 0.457 0.107 0.054 |
-0.512 -0.288 -0.224 0.512 0.288 0.400 0.112 0.056 |
-0.513 -0.346 -0.167 0.513 0.346 0.430 0.083 0.042 |
-0.423 -0.279 -0.144 0.423 0.279 0.351 0.072 0.036 |
-0.421 -0.337 -0.083 0.421 0.337 0.379 0.042 0.021 |
-0.342 -0.306 -0.107 0.342 0.306 0.324 0.018 0.009 |
-0.344 -0.305 -0.039 0.344 0.305 0.324 0.019 0.009 |
-0.390 -0.239 -0.151 0.390 0.239 0.315 0.076 0.038 |
-0.390 -0.296 -0.093 0.390 0.296 0.343 0.047 0.023 |
| Control | Sample | Blank | |
|---|---|---|---|
| Tested compound | no | 200 μL | 200 μL |
| MTT | 200 μL | 200 μL | 200 μL |
| Fe2+/H2O2/Na2-EDTA | 100 μL | 100 μL | no |
| Ascorbic acid | 100 μL | 100 μL | no |
| Bi-destilled water | up to 2.0 mL | дo 2.0 mL | дo 2.0 mL |
| Blank | Control | Sample | |
|---|---|---|---|
| Tested compound | 200 μL | no | 200 μL |
| DPPH | no | 1800 μL | 1800 μL |
| Ethanol | 1800 μL | no | no |
| Bi-distilled water | no | 200 μL | no |
| Blank | Control | Sample | |
|---|---|---|---|
| Tested compound | 100 μL | no | 100 μL |
| R1 | 860 μL | 860 μL | 860 μL |
| R2 | no | 40 μL | 40 μL |
| Bi-destilled water | 40 μL | 100 μL | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





