1. Introduction
Wheat is the largest grain crop in the arid region of Xinjiang, and its production capacity is of great significance for ensuring regional food security (Wang et al., 2018). Rational nitrogen application can significantly improve the yield and quality of arid wheat in Xinjiang, reduce the negative effects of nitrogen loss on its ecological environment, and realize efficient and sustainable wheat production in Xinjiang (Gao et al., 2014; Shibata et al., 2017). However, Xinjiang wheat production has long pursued high nitrogen and high yield, and the soil nitrogen supply capacity has exceeded the crop's absorption capacity and soil fixation capacity, which is the main factor limiting the high yield and efficiency of Xinjiang wheat (Chen et al., 2018). Therefore, exploring suitable nitrogen application rates and achieving synchronization between nitrogen fertilizer supply and demand has become the key to efficient wheat production in arid areas of Xinjiang.
Carbon metabolism is a metabolic pathway closely related to carbohydrate synthesis in crops, including the synthesis and transformation of sucrose and starch (Mahdy et al., 2022). Sucrose is the main form of carbohydrate transport and storage in higher plants and regulates carbon metabolism in plants (Zhang et al., 2019). The balance and coordination of sucrose synthesis and degradation in plants depend on the synergistic effect of sucrose phosphate synthase (SPS) and sucrose synthase (SS), whose activity represents the ability of flag leaves photosynthetic products to convert into sucrose (Kaur et al., 2023). SPS is a key enzyme in the regulation of sucrose synthesis, and the sucrose content in flag leaves is significantly positively correlated with SPS activity (Fan et al., 2022). Different nitrogen fertilizer management methods have a significant regulatory effect on sucrose metabolism. Reducing the amount of nitrogen fertilizer can significantly reduce the SPS activity in flag leaves, slow down the conversion of carbohydrates to sucrose, and lead to a decrease in sucrose content in flag leaves (Wang et al., 2021). Excessive application of nitrogen fertilizer can also inhibit the activity of SPS in flag leaves , SS and soluble starch synthase (SSS) of grains, which is not conducive to sucrose synthesis in leaves and starch synthesis in grains, resulting in a decrease in yield (Jiang et al., 2008). The high activity of SPS in flag leaves and SS in grains indicates a strong ability for sustained supply of assimilates at the source and strong activity at the sink .
Starch is the main component of wheat grains, and the level of sucrose supply and its ability to convert into starch are key factors in starch accumulation. SS, SSS, ADPG - PPase, GBSS, and SBE are key enzymes that control starch synthesis metabolism (Huang et al., 2021). In the process of starch synthesis, there is a significant correlation between the accumulation rate of amylose, amylopectin, and total starch in grains and the activities of SBE, SSS, and GBSS. The SS activity in flag leaves and grains is highly significantly positively correlated with the accumulation rate of starch in grains. Starch content and straight branch ratio significantly affect grain yield and quality (Zheng et al., 2017; Zhao et al., 2008). Yang et al(2020) found in their study of changes in the activity of key enzymes involved in starch synthesis that GBSS, SSS, ADPG - PPase, and SBE activities all increased with increasing nitrogen application at a nitrogen application rate of 0–200 kg·hm-2. Xin et al(2023) believe that compared to non nitrogen treatment, the soluble sugar content, GBSS, SSS, ADPG - PPase, and SBE activities in winter wheat grains significantly increased after nitrogen application, leading to an increase in starch content, thereby increasing starch yield. Further research has found that reducing nitrogen application has a promoting effect on the activities of ADPG - PPase, SSS, and GBSS in carbon metabolism, while excessive nitrogen application inhibits starch formation, thereby affecting grain weight (Yue et al., 2022). However, the research results on the impact of starch content are inconsistent. Studies have shown that increasing nitrogen fertilizer application within the range of 0–240 kg·hm-2 can increase grain starch and component content (Gao et al., 2023; Ran et al., 2020), while in the range of 270–300 kg·hm-2, nitrogen application can reduce starch and amylose content (Zhou et al., 2022), with varying effects on amylopectin content (Wang et al., 2007; Khan and Akma., 2021). This indicates that the efficient utilization of nitrogen fertilizer varies in different wheat regions, and it is necessary to further study the nitrogen response mechanism of starch accumulation and yield formation in drip irrigation wheat in arid areas of Xinjiang.
Drip irrigation technology, which integrates water, fertilizer, and medicine, intelligent field management, and reduces labor and input costs, has become the main cultivation technology for wheat production in Xinjiang. The regulatory mechanism of reducing nitrogen application under drip irrigation conditions on sucrose degradation and starch synthesis pathways in flag leaves and grains is still unclear. The regulatory mechanism of how to not significantly inhibit the activity of wheat carbon metabolism enzymes, but also timely and moderately promote the transport of assimilates to grains and increase yield is not clear. These are all topics that need further exploration. On the basis of existing production technology, this study explores the carbon metabolism characteristics of flag leaves and grains of drip irrigated spring wheat, attempting to solve the problem of i: analyzing the changes in key enzyme activities in the sucrose starch metabolism pathway of flag leaves and grains and their relationship with starch accumulation, revealing the enzymatic mechanism of starch accumulation in drip irrigated spring wheat grains. ii: clarifying the relationship between grain starch and component content, yield formation, and nitrogen application rate. iii: exploring possible ways to synergistically improve yield and nitrogen fertilizer utilization efficiency, proposing suitable nitrogen fertilizer application models for drip irrigation spring wheat in arid areas, and providing scientific basis for nitrogen efficient and green production of drip irrigation spring wheat in Xinjiang.
2. Materials and Methods
2.1. Experimental site
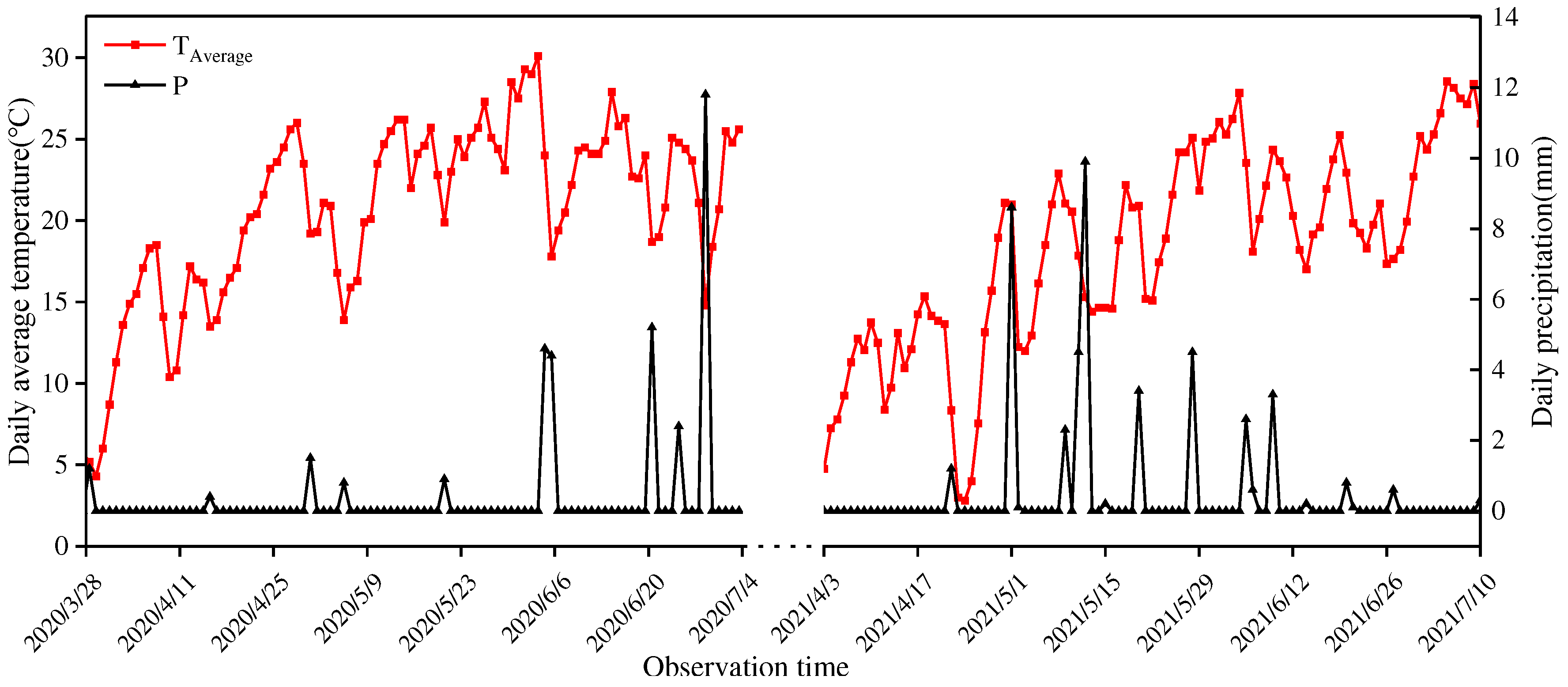
The experiment was carried out at the Experimental Station of Agricultural College of Shihezi University (85°59′ E, 44°18′ N) from April to July in 2020 and 2021.The average temperature in Shihezi ranges from 7.5~8.2 ºC, with a precipitation of 208 mm and evaporation of 1660 mm. It belongs to a typical continental climate, and the changes in meteorological indicators during wheat growth are shown in
Figure 1. The tested soil type is irrigated gray desert soil, and the basic characteristics of the 0-40 cm soil are shown in
Table 1.
Table 1.
The major chemical characteristics of the experimental soil.
Table 1.
The major chemical characteristics of the experimental soil.
| Year |
Total nitrogen content/
(g·kg-1) |
Availine hydrolysis
nitrogen content/
(mg·kg-1) |
Available phosphorus content/
(mg·kg-1) |
Available potassium content/
(mg·kg-1) |
Organic matter content/
(g·kg-1) |
pH |
| 2020 |
1.20 |
58.30 |
14.24 |
137.01 |
13.43 |
7.8 |
| 2021 |
1.27 |
55.71 |
15.96 |
132.02 |
12.84 |
7.7 |
2.2. Experimental design
The experiment adopted a split - plot design, with the variety as the main plot factor. Two spring wheat (Triticum aestivum L.) varieties were used as experimental materials. Xinchun 37 (XC37, protein content 16.3%) is a strong gluten wheat, while Xinchun 6 is a medium gluten wheat (XC6, protein content 13.5%). Root trace nitrogen reduction is a sub plot factor, with four different nitrogen application rates set up, as shown in
Table 2. Each treatment is repeated 3 times, with a planting area of 12 m
2 (3 m×4 m) in the community, a 100 cm depth anti - seepage film is buried between each residential area to prevent fertilizer migration. Except for the non fertilized treatment, 120 kg·hm
-2 of P
2O
5 (diammonium phosphate) was applied as the base fertilizer in each community before sowing, with a basal/topdressing nitrogen fertilzer ratio of 3:7. A total of 9 irrigations were conducted throughout the entire growth period, with a total irrigation amount of 6000 m
3·hm
−2. Each times the amount of irrigation water meter by the precise control.Sowing on April 1, 2020 and April 4, 2021, with a seeding rate of 345 kg·hm
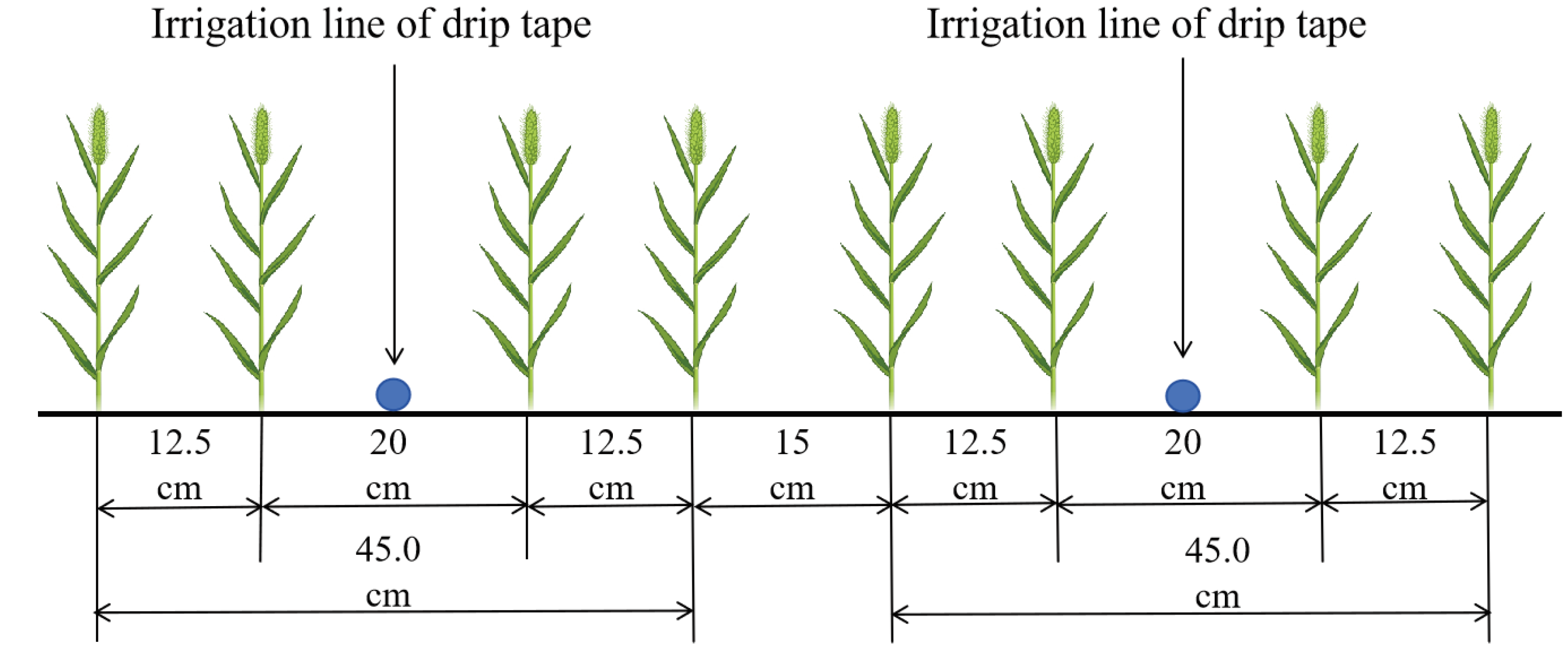
-2. The planting mode is wide and narrow rows, with a "one-tube-four-row" approach, and a row spacing of 12.5+20+12.5+15 cm (
Figure 2). Drip irrigation tape is placed in a 20 cm wide row, with a pipe diameter of 16 mm, a spacing of 30 cm between drip heads, and a flow rate of 2.6 L·h
-1. Wheat was harvested on 4 July 2020 and 7 July 2021. Other field management was similar to local field production.
Table 2.
Nitrogen application rate for each treatment and growth period/(kg·hm-2).
Table 2.
Nitrogen application rate for each treatment and growth period/(kg·hm-2).
| Treatment |
Nitrogen application amount |
Base fertilizer
(20%) |
Top dressing |
Two-leaf one-hearted stage
(8%) |
Tillering stage
(8%) |
Jointing stage
(32%) |
Booting stage
(16%) |
Flowering stage
(12%) |
Milky maturity stage
(4%) |
| CK1 |
300 |
60 |
24.0 |
24.0 |
96.0 |
48.0 |
36.0 |
12.0 |
| A1 |
255 |
51 |
20.4 |
20.4 |
81.6 |
40.8 |
30.6 |
10.2 |
| B1 |
210 |
42 |
16.8 |
16.8 |
67.2 |
33.6 |
25.2 |
8.4 |
| CK2 |
0 |
0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
2.3. Measurement items and methods
2.3.1. Sample collection
Samples of XC37 and XC 6 from each treatment were collected randomly (1 m × 1 m) at 7 - day intervals after the flowering stage. The samples were divided into two parts: one part of the flag leaves and grains were blanced at 105 ℃ and dried at 70 ℃ for dry sample determination, while the other part was stored in −80 ℃ super cold refrigerator to determine the activity of key enzymes in the sucrose-starch metabolism pathway.
2.3.2. Determination of Key Enzyme Activities in Carbon Metabolism of Flag Leaves and Grains
Sucrose synthase (SS) activity and adenosine diphosphate glucose pyrophosphorylase (ADPG - PPase) activity: follow to the method of Douglas et al. (1988). Sucrose phosphate synthase (SPS) activity: according to the method of Keller et al. (1993). Granule-bound starch synthase (GBSS) activity, soluble starch synthase (SSS) activity, and starch branching enzyme (SBE) activity: refer to the method of Nakamura (1989).
2.3.3. Determination of soluble sugar and sucrose content in flag leaves and grains, starch and component content in grains
Soluble sugar content and sucrose content: refer to the method of Vila et al. (2018). Amylose, amylopectin, and total starch content: refer to the method of Jin et al (2009).
2.3.4. Yield measurement
Randomly select 1 square meter of sample for each treatment during the wheat maturity period, harvest all plants and naturally air dry them, weigh the grain weight, and calculate the yield; Simultaneously measure the number of wheat ears in a 1 m2 plot, randomly select 20 plants from them, and use them to measure the number of grains per ear and thousand grain weight. Repeat this process three times.
2.4. Data Analysis
We used SPSS 26.0 to analyze the data. Multiple comparisons were conducted between different treatments using the minimum significant difference test (P<0.05) (n=3). Imageswere plotted using OriginLab 2021 (Northampton, MA, USA).
3. Results
3.1. Effect of Root Layer N Reduction on Carbon Metabolism in Flag Leaves of Spring Wheat
3.1.1. Changes in the activity of key enzymes involved in sucrose metabolism in flag leaves
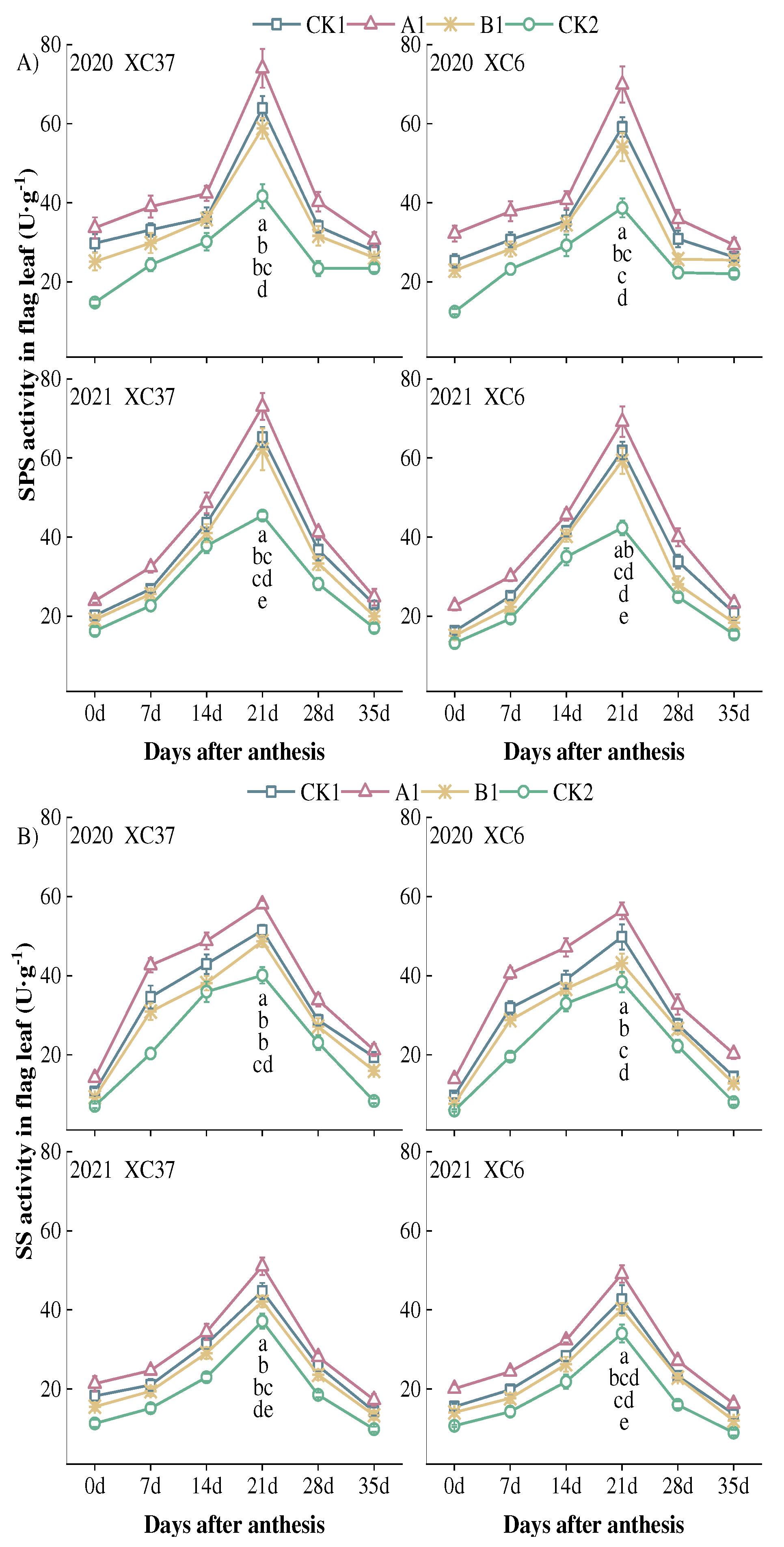
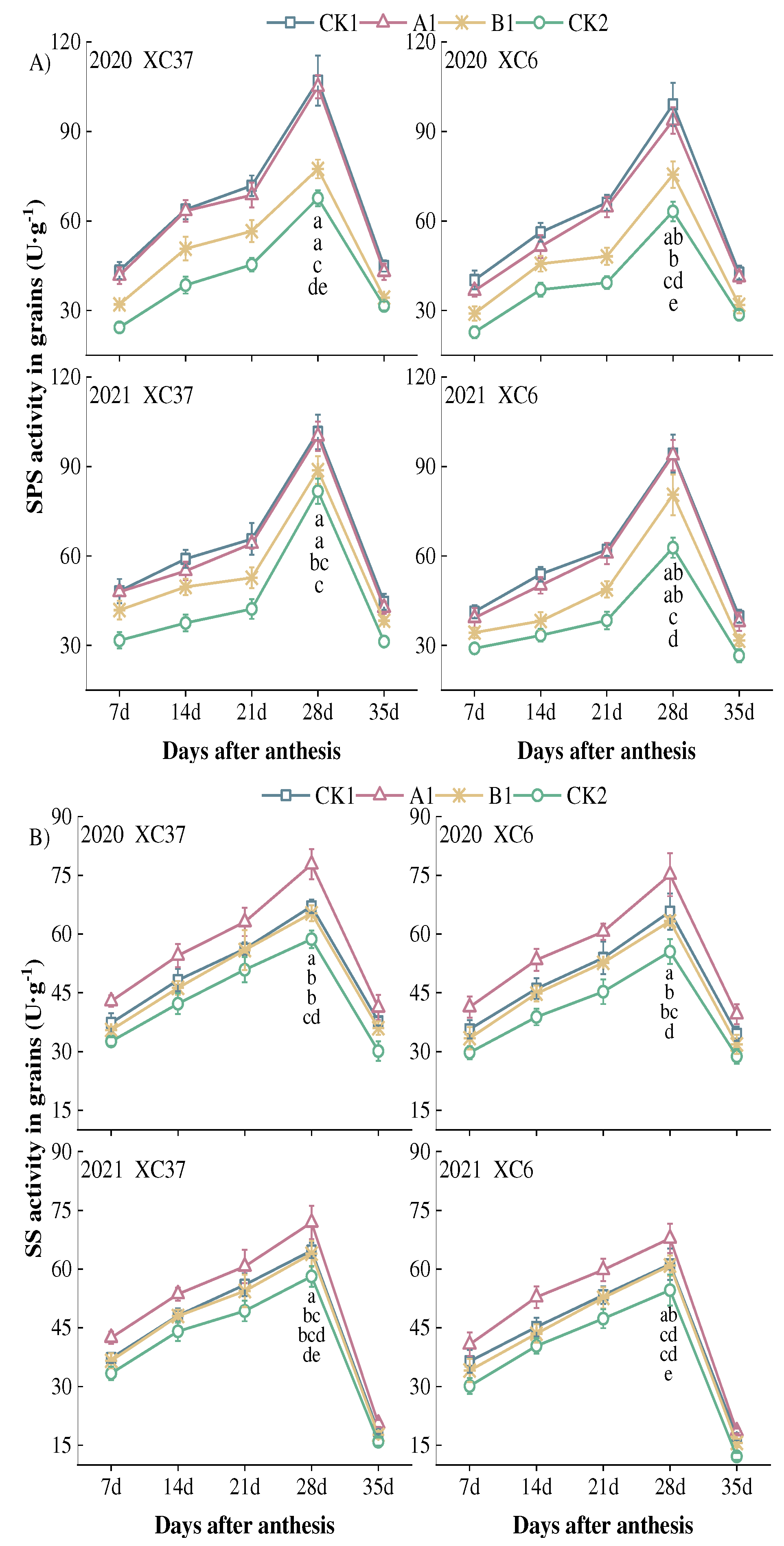
The changes in SPS and SS activities in flag leaves after flowering under nitrogen reduction mode are basically consistent, reaching their peak at 21 days after flowering (
Figure 3). The SPS activity A1 treatment was significantly higher than CK1, B1, and CK2 treatments by 11.76–18.18%, 16.68–29.10%, 60.56–80.60% at 21 days after flowering, and the decrease from 21 days to 28 days after flowering (38.01–52.70%) was greater than the decrease from 28 days to 35 days after flowering (1.27–41.71%). During the entire reproductive period, XC37 was 1.73–26.84% higher than XC 6. The SS activity A1 treatment was significantly higher than CK1, B1, and CK2 treatments by 12.79–14.90%, 19.22–30.81%, 37.43–46.84% at 21 days after flowering, respectively. In the mature stage, the average decrease in SPS activity (52.37–66.33%) is smaller than the average decrease in SS activity (68.06–71.07%). Under A1 treatment, XC37 is 1.06–6.55% higher than XC 6.
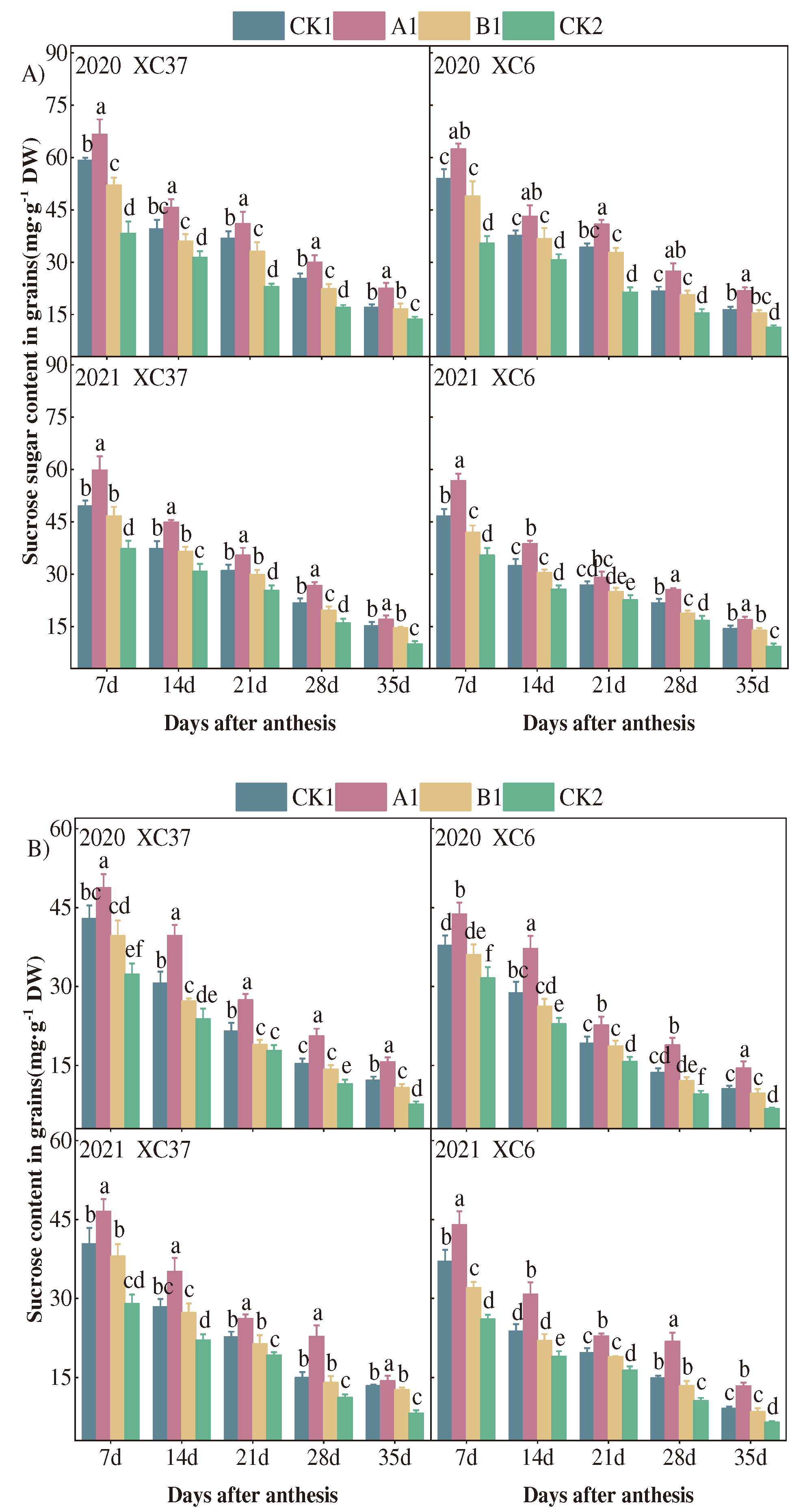
3.1.2. Changes in soluble sugar and sucrose content in flag leaves
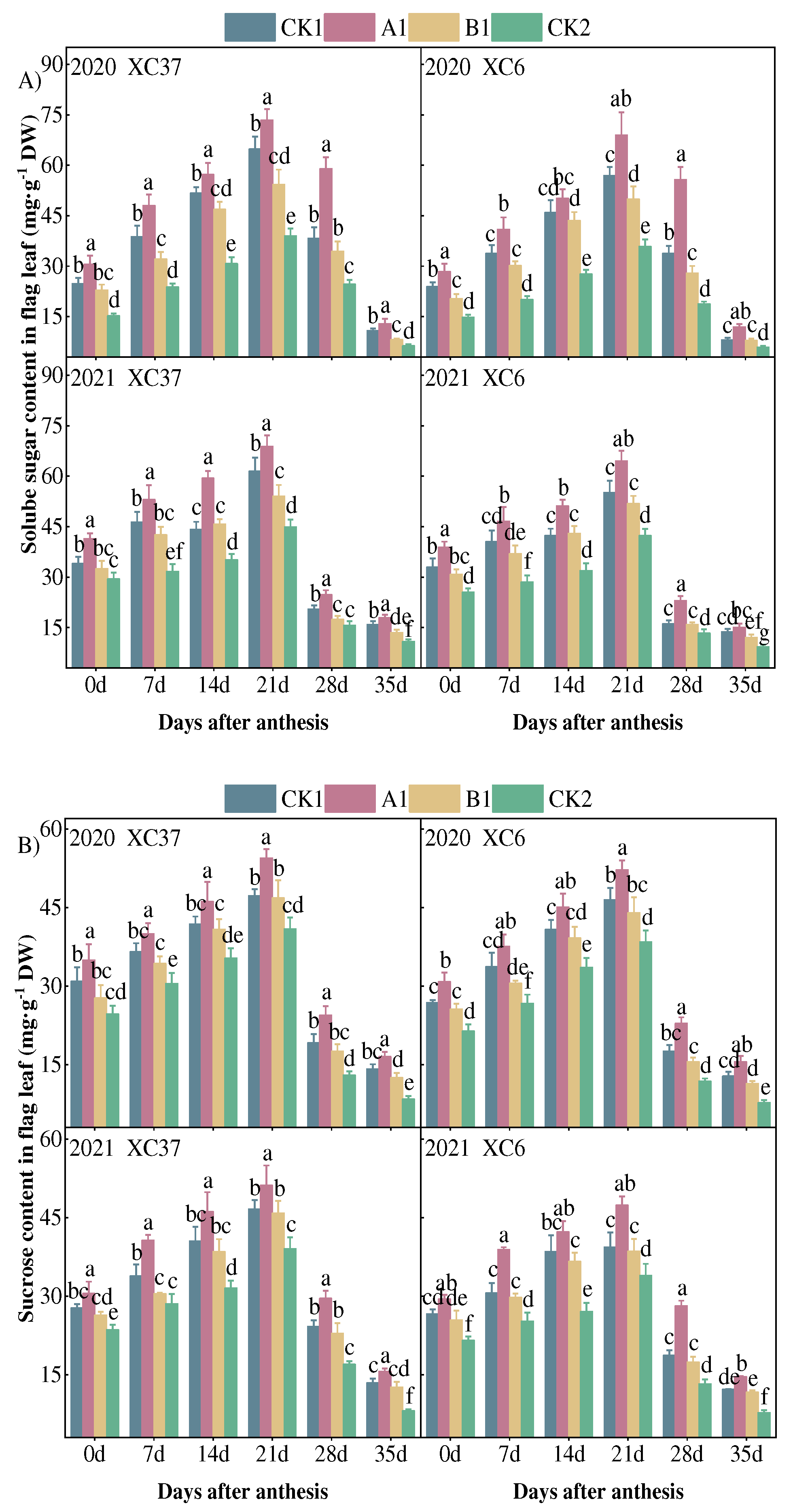
As shown in
Figure 4, the soluble sugar and sucrose content of the flag leaves of both varieties showed an inverted “V-shaped” trend with the number of days after flowering, reaching a peak at 21 days after flowering. From flowering to 21 days after flowering, the soluble sugar content of XC37 and XC 6 increased by 14.44–42.89 mg·g
−1 DW and 16.83–40.61 mg·g
−1 DW, respectively. From 21 days after anthesis to maturity, soluble sugar content decreased by 32.66~60.53 mg·g
−1 DW and 18.61–57.02 mg·g
−1 DW, respectively, indicating that the flag leaves of XC37 accumulated soluble sugar faster and decomposed faster. The content of soluble sugar in the same growth period was as follows: A1 > CK1 > B1 > CK2. A1 differed significantly from the other treatments, with A1 being 11.97–21.05%, 24.50–38.02%, and 52.54–92.33% higher than the other treatments at 21 days after anthesis, and XC37 being 6.45–6.63% higher than XC 6. From flowering to 21 days after flowering, the sucrose content in the flag leaves of XC37 and XC 6 increased by 15.47–20.67 mg·g
−1 DW and 12.43–21.36 mg·g
−1 DW, respectively. From 21 days after flowering to maturity, the sucrose content in the flag leafves decreased by 30.98–37.95 mg·g
−1 DW and 26.21–36.68 mg·g
−1 DW, respectively.This indicates that the decomposition rate of sucrose content in the flag leaves of XC37 is higher than that of XC 6. 21 days after flowering, the sucrose content in A1 treatment was 9.74–20.32%, 11.51–22.71%, 31.08–39.56% higher than other treatments, and XC37 was 4.33–7.98% higher than XC 6.
3.2. Effect of Root Layer Nitrogen Reduction on Grain Carbon Metabolism in Spring Wheat
3.2.1. Changes in the activity of key enzymes involved in sucrose metabolism in grains
The activities of SPS and SS in grains showed a single-peak curve change with the advance of days after anthesis, reaching a maximum at 28 days after flowering (
Figure 5). At the same nitrogen application rate during the same period, the SPS activity showed CK1≥A1>B1>CK2. At 35 days after flowering, the differences between the treatments were small (1.92–13.39 U·g
-1), while at other stages, they were large (0.24–39.38 U·g
-1). Except for A1 treatment, the differences between CK1 and the treatments reached a significant level (
P < 0.05). After 28 days of flowering, CK1 treatment was 0.59–5.78%, 14.48–38.20%, 24.38–58.21% higher than A1, B1, and CK2 treatments, respectively. The whole reproductive period XC37 is 2.53–30.14% higher than XC 6. After 28 days of flowering, A1 was the highest among SS treatments, and the differences between them reached a significant level (
P < 0.05), which was 10.79–15.96%, 11.40–19.13%, and 23.69–35.42% higher than CK1, B1, and CK2. XC37 is 1.46–31.01% higher than XC 6.
3.2.2. Changes in soluble sugar and sucrose content in grains
The trend of changes in soluble sugar and sucrose content in grains under different nitrogen application rates is consistent, both showing a decreasing trend with the number of days after flowering. The maximum value is at 7 days after flowering, showing a trend of A1>CK1>B1>CK2 (
Figure 6). From 7 to 35 days after flowering, the soluble sugar content of XC37 and XC 6 decreased by an average of 34.10–36.57 mg·g
−1 DW and 31.57–33.96 mg·g
−1 DW. A1 was 8.19–33.53%, 16.19–41.38%, 28.63–92.98% higher than the other treatments, and XC37 was 6.75–21.70% higher than XC 6. From 7 days after flowering to maturity, the sucrose content of XC37 and XC 6 decreased by 20.88–33.09 mg·g
−1 DW and 19.59–30.64 mg·g
−1 DW, respectively, indicating that the amplitude of XC37 change was slightly higher than that of XC 6. There were significant differences between A1 and other treatments (P<0.05). A1 was 7.12-52.60%, 13.14-62.92%, and 51.00-114.33% higher than other treatments. XC37 was 4.44–21.04% higher than XC 6.
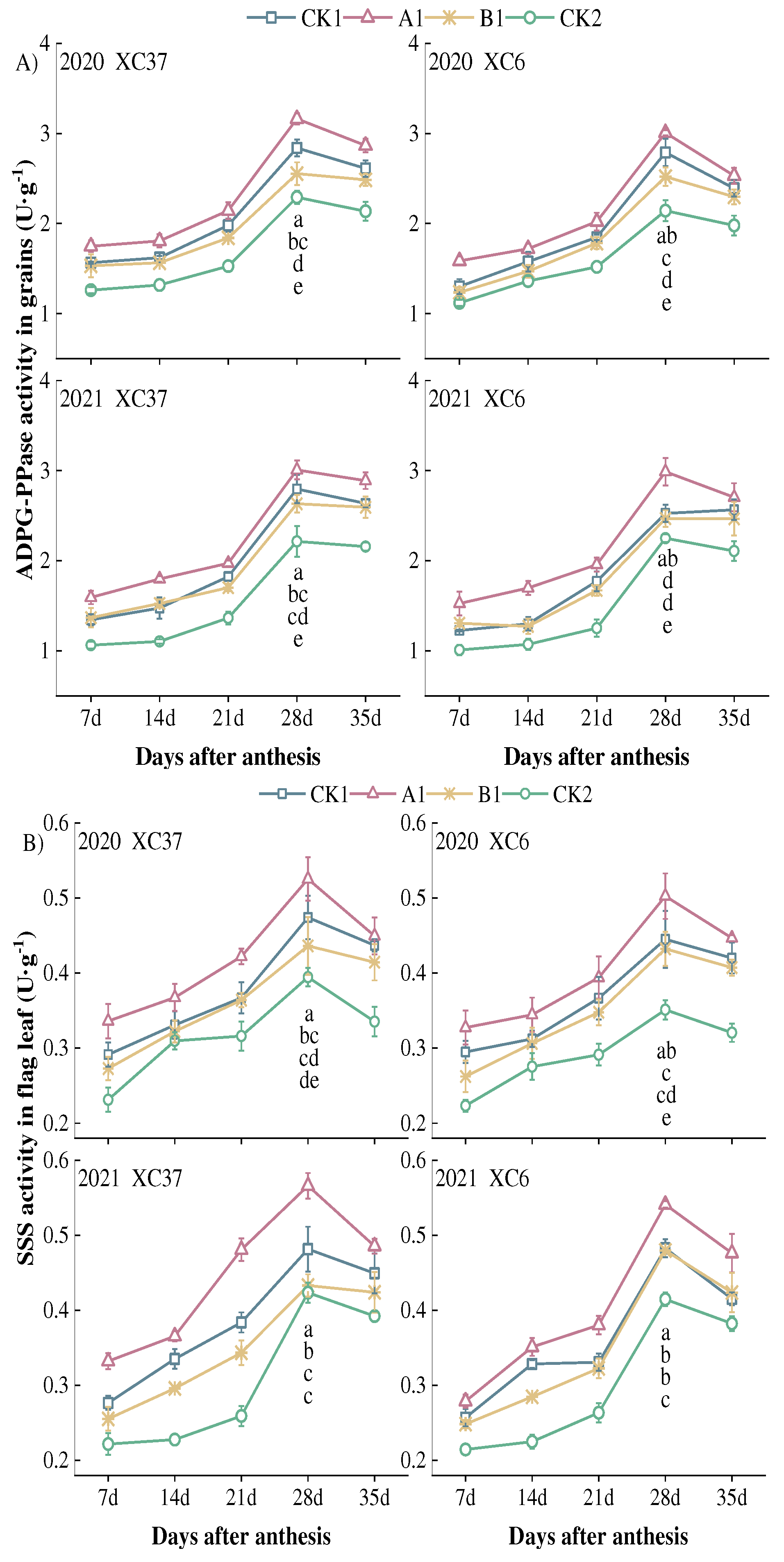
3.2.3. Changes in key enzyme activity of grain starch
As shown in
Figure 7, the ADPG - PPase and SSS activities in grains under reduced nitrogen application showed a single peak curve throughout the entire growth period, reaching their peak at 28 days after flowering. Except for 14 days after flowering, ADPG - PPase showed A1>CK1>B1>CK2 changes. At 28days after flowering, A1 was significantly different from other treatments. A1 was 7.58–18.33%, 14.23–23.87%, 32.76–40.32% higher than the other treatments. XC37 is 0.54–23.88% higher than XC 6. The difference (0.39–0.66 U·g
-1) among different nitrogen reduction treatments after 7 to14 days of flowering is smaller than that after 28 to 35 days of flowering (0.53–0.86 U·g
-1). At 28 days after flowering, the SSS A1 was significantly higher than the other treatments by 10.81–17.51%, 12.78–30.69%, 30.45–43.12%. Under A1 treatment, XC37 is 0.64–26.43% higher than XC 6. After 7 to 14 days of flowering, there was a small difference (0.06–0.13 U·g
-1) among the N reduction treatments; There is a significant difference (0.09–0.23 U·g
-1) between treatments 28 to 35 days after flowering.
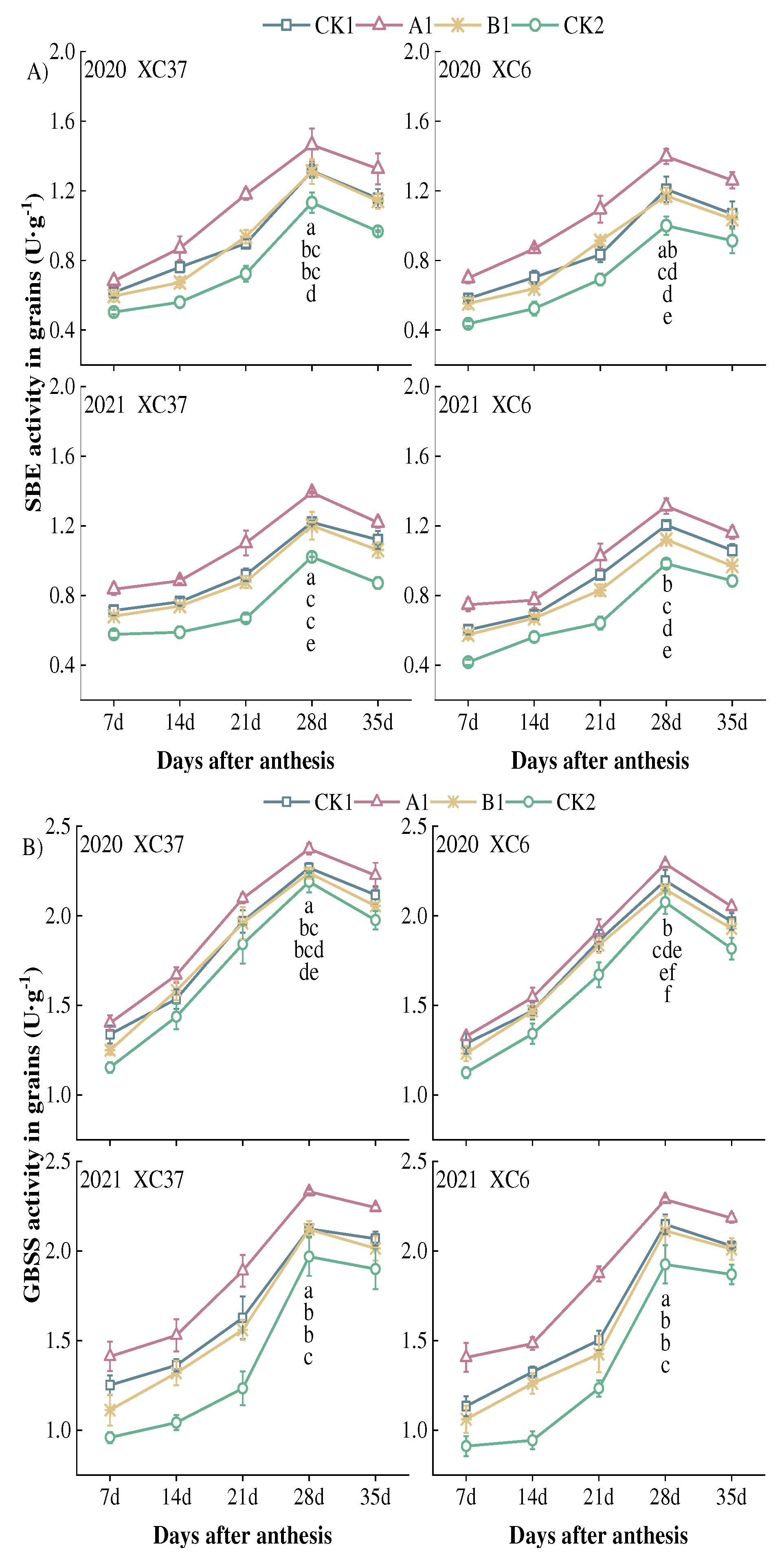
From
Figure 8, it can be seen that the SBE and GBSS activities of grains under reduced nitrogen application showed a single peak curve of first increasing and then decreasing with the filling process, reaching a peak at 28 days after flowering, showing a change of A1>CK1>B1>CK2. At 28 days after flowering, the SBE activity of A1 was significantly higher than other treatments by 9.07–15.65%, 11.57–19.29%, 29.27–39.71%. Under A1 treatment, XC37 is –2.36–14.39% higher than XC 6. SBE activity remained at a high level (0.64–1.46 U·g
-1) from 21 to 35 days after flowering. From 7 to 14 days after flowering, there was a small difference in SBE activity among the treatments, with an average of 0.27 U·g
-1. After 28 days after flowering, there was a significant difference, with an average difference of 0.38 U·g
-1. The GBSS activity of A1 treatment was significantly higher than other treatments at 28 days after flowering, with a range of 4.30–9.81%, 6.02–9.94%, and 8.41–18.80%. GBSS activity remained at a high level (1.23–2.37 U·g
-1) from 21 to 35 days after flowering. Under A1 treatment, XC37 is 0.38–9.29% higher than XC 6. The decrease in XC37 from peak to maturity (2.64–10.82%) is slightly lower than that of XC 6 (2.99–14.25%).
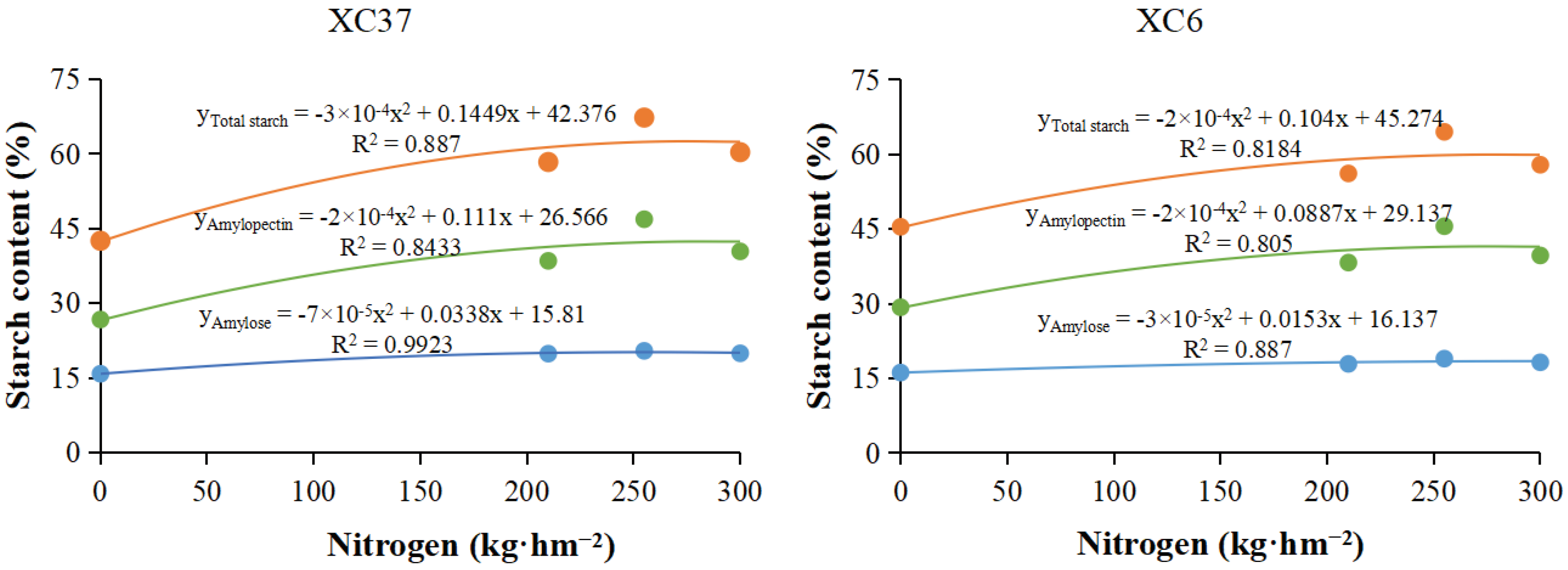
3.3. Effect of root layer nitrogen reduction on starch composition and content in grains
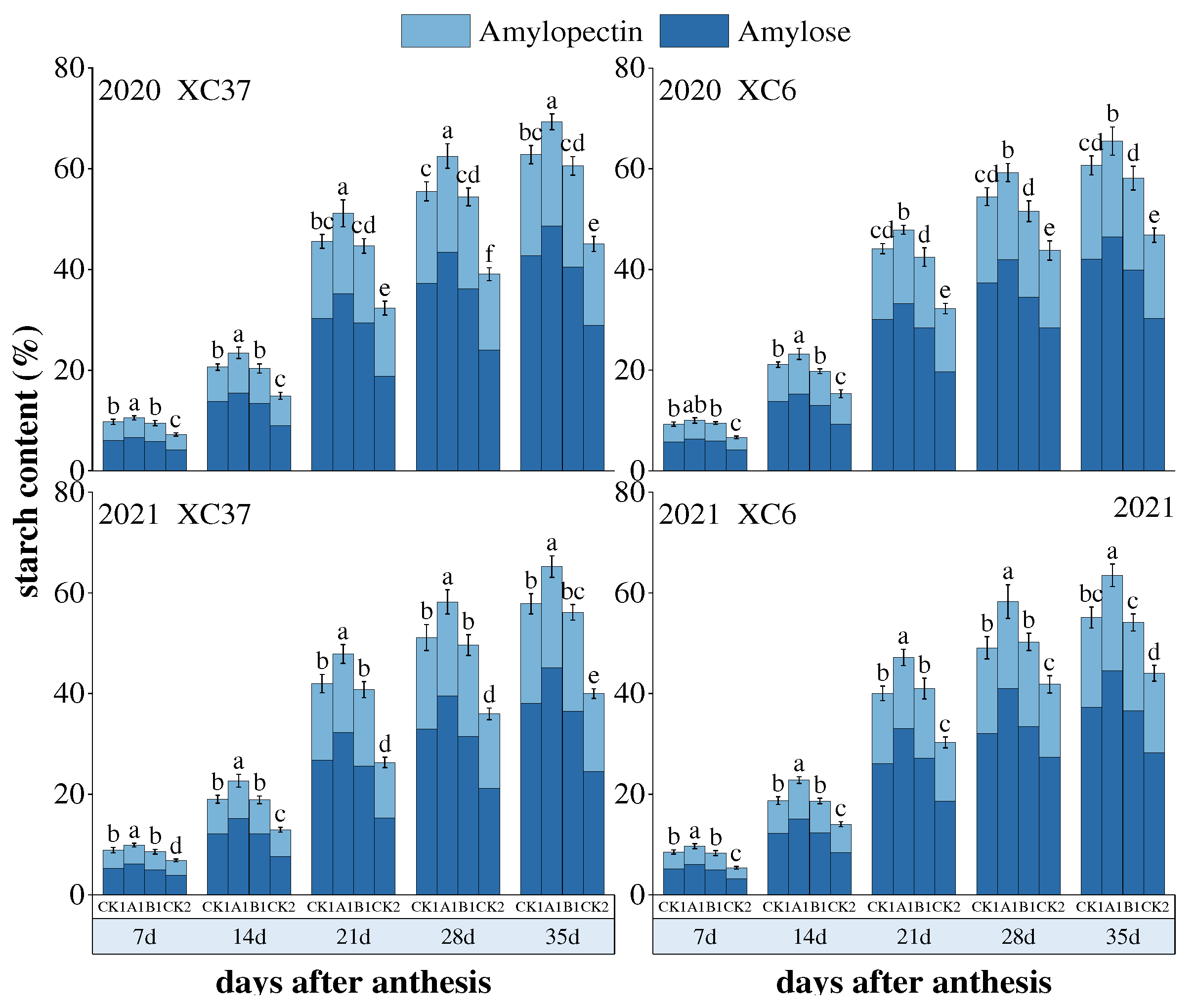
The dynamic changes in amylose and amylopectin content of XC37 and XC 6 are shown in
Figure 9, both of which show a gradual increase with the advancement of the filling period. Throughout the filling period, there is a significant increase in amplitude from 14 to 21days after flowering. During the same period, under different nitrogen fertilizer treatments, the A1 treatment (255 kg·hm
-2) showed the best content of amylose and amylopectin. 35 days after flowering, there were significant differences (
P<0.05) between the two varieties of amylose and amylopectin content A1 and other treatments. After 35 days of flowering, under A1 treatment, the amylose content of XC37 was 20.69% and 20.14%, which increased by 1.86–3.09%, 2.42%~3.28%, and 27.85–30.38% compared to CK1, B1, and CK2 treatments, respectively; The content of amylopectin was 48.63% and 45.08%, which were increased by 13.74–18.49%, 19.97–23.65%, 68.33–83.69%, respectively, compared to CK1, B1, and CK2 treatments. The amylose content of XC 6 was 19.00% and 18.90%, which increased by 2.03–6.09%, 4.08–7.72%, and 15.14–19.52% compared to CK1, B1, and CK2 treatments, respectively; The content of amylopectin content was 46.50% and 44.58%, respectively, which increased by 10.58–19.57%, 16.58–21.85%, and 53.43–57.91% compared to CK1, B1, and CK2 treatments.
3.4. Correlation analysis between yield and carbon metabolism parameters of flag leaves and grains under root layer nitrogen reduction
According to
Table 3, there is a significant relationship between N application rate and wheat yield and various yield factors. The N application rate and variety interaction have a significant impact on the yield and its constituent factors of the two varieties (P<0.05). The trend of changes in 1000-grain weight, spike number, and grain per spike is consistent, with XC37 and XC 6 having the highest yield and composition under A1 treatment.
Through joint yield analysis over the past two years, it was found that as the nitrogen application rate decreased, the yield and composition of both varieties showed an initial increase followed by a decrease. A low nitrogen application rate (0–210 kg·hm
−2) would lead to a decrease in yield and its composition, while an excessive nitrogen application rate (300 kg·hm
−2) had no significant effect on increasing yield. Compared with CK1 treatment, 1000-grain weight, spike number, grain per spike, and yield of XC37 under A1 treatment increased by 2.14–3.11%, 3.96–4.84%, 2.06–7.48%, 3.98–4.68%, respectively; XC 6 increased by 1.33–3.77%, 2.59–4.23%, 1.58–1.93%, 3.06–6.09%. XC37 is 0.54–1.59%, 0.50–3.09%, 0.11–5.93%, 0.75–3.72% higher than XC6, respectively. In
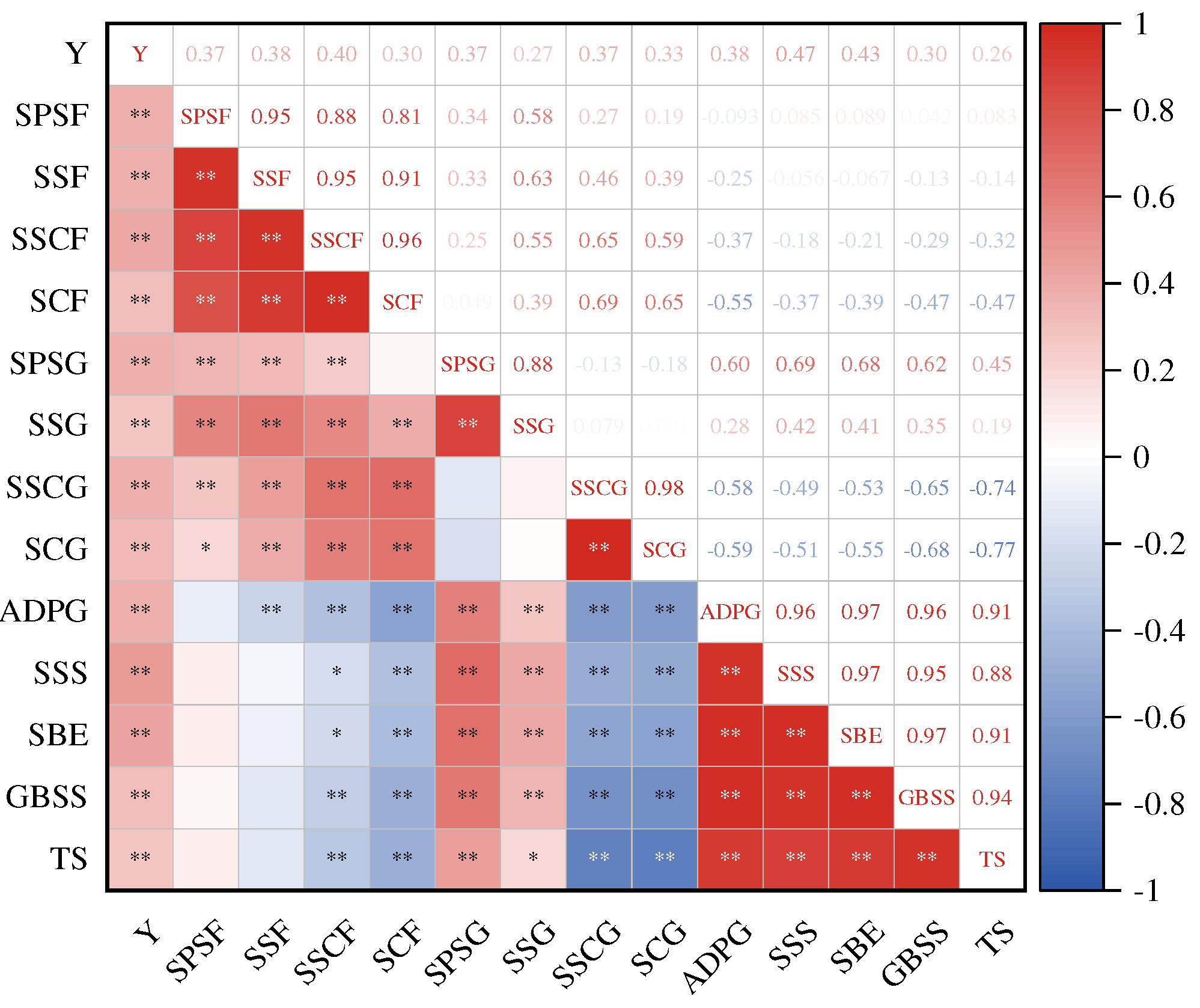
Figure 11, significant correlations were found between yield and various indicators under different nitrogen reduction treatments(
r=0.26*~0.47**). The correlation between soluble sugar and sucrose content in flag leaves and SPS and SS was
r=0.81**~0.95**, while the correlation between starch content in grains and ADPG - PPase, SSS, SBE, and GBSS activities was
r=0.88**~0.94**. Under different N application rates, the activity of sucrose key enzymes in flag leaves can be regulated to regulate sucrose and soluble sugar content, thereby promoting yield improvement. The strength of starch key enzyme activity under nitrogen regulation can also affect the grain quality of drip irrigated spring wheat.
Note: Y, SPSF, SSF, SSCF, SCF, SPSG, SSCG, SCG, ADPG - PPase, SSS, SBE, GBSS TS separate means Yield, Sucrose phosphate synthase in flag leaf, Sucrose synthase in flag leaf, Solube sugar content in flag leaf, Sucrose content in flag leaf, Sucrose phosphate synthase in grain, Sucrose synthase in grain, Solube sugar content in grain, Sucrose content in grain, Adenosine diphosphate glucose ayrophosphorylase, Soluble starch synthetase, Starch branching enzyme, Granule-bound starch synthase, Total starch. * and ** indicate significant difference at 0.05 and 0.01 levels.
Using yield (Y) as the dependent variable, sucrose phosphate synthase in flag leaves SPS (X1), sucrose synthasein flag leaves SS (X2), soluble sugar in flag leaves (X3), sucrose in flag leaves (X4), sucrose phosphate synthase in grains SPS (X5), sucrose synthase in grains (X6), soluble sugar in grains (X7), sucrose in grain (X8), adenosine diphosphate glucose pyrophosphorylase ADPG - PPase (X9), soluble starch synthase SSS (X10), starch branching enzyme SBE (X11), granule-bound starch synthase GBSS (X12), total starch (X13), amylose (X14), and amylopectin (X15) were used as independent variables for stepwise regression analysis. After analysis, the stepwise regression equation was obtained:
(R2=0.845, F=101.714**)
(R2=0.804, F=56.449**)
The regression analysis results showed that soluble sugars in grains, amylopectin, and sucrose in grains had the greatest impact on the yield of XC37, determining 84.5% of the yield. SSS, soluble sugars in grains, amylopectin, and SBE were significant indicators affecting the yield of XC 6, determining 80.4% of its yield.
4. Discussion
4.1. Effect of nitrogen application rate on sucrose metabolism in flag leaves and grains of spring wheat
Sucrose is the substrate for starch synthesis, and SPS and SS are key enzymes for the conversion of photosynthetic products to sucrose (Verma et al., 2011), with SS being a bidirectional catalytic enzyme (Stein and Granot., 2019). The activity of SS in the synthesis direction of flag leaves determines their ability to synthesize sucrose, while the activity of SS in the decomposition direction of grains reflects their ability to degrade sucrose. The higher the SS activity in grains, the faster the degradation rate of sucrose, and the more sufficient the substrate provided for starch synthesis. Appropriate nitrogen fertilizer supply can increase the SPS and SS enzyme activities in wheat flag leaves during the grain filling period, increase the sucrose content in grains, and increase the supply level of starch synthesis substrates, but excessive nitrogen fertilizer is the opposite (Ullah et al., 2023). In this study, we found that the activities of key enzymes of sucrose metabolism (SPS, SS) in flag leaves and grains after flowering showed a tendency to increase and then decrease with the increase in the number of days after flowering under different treatments, which is in agreement with the results of Zhang et al(2023). The activities of flag leaves SPS and SS reached their maximum at 21 days, while the activities of grain SPS and SS reached their maximum at 28 days. Under treatment with A1 (255 kg·hm-2), the SPS and SS activities of flag leaves were significantly higher than those of CK1 (300 kg·hm-2), with increases of 7.95–39.38% and 8.69–43.79%, respectively; The grain SS activity A1 (255 kg·hm-2) was significantly higher than CK1 (300 kg·hm-2) by 7.65–16.87%, indicating that reducing nitrogen fertilizer appropriately can enhance the activity of key enzymes in sucrose metabolism. Soluble sugars and sucrose are substrates for starch synthesis, and their content is related to starch accumulation. After flowering, the soluble sugar and sucrose of flag leaves increased first and then decreased, which was significantly positively correlated with the SPS and SS activities of flag leaves, which was consistent with the results of Yu et al. (2018). The soluble sugars and sucrose in flag leaves continue to increase from 0 to 21 days after flowering, reaching their peak at 21 days. At this time, flag leaves have high SPS and SS activities; After 21 days, the soluble sugar and sucrose content in flag leaves rapidly decreased. This may be due to the aging of flag leaves in the late stage of grain filling, resulting in a decrease in enzyme activity involved in sucrose metabolism, leading to a decrease in sucrose content in flag leaves. On the other hand, the demand for soluble sugar and sucrose in grain starch synthesis increased. The soluble sugars and sucrose in grains show a continuous downward trend after flowering, which may be related to the conversion of sucrose starch in grains. Therefore, application of 255 kg·hm-2 of nitrogen fertiliser could promote sucrose metabolism in flag leaves and grains under drip irrigation in arid areas of Xinjiang. However, further research is needed to investigate the effects of nitrogen fertilizer application period and proportion on sucrose metabolism in flag leaves and grains during wheat growth under drip irrigation.
4.2. Effect of nitrogen application rate on starch metabolism and synthesis in spring wheat grains
The synthesis and accumulation of starch are regulated by the activities of ADPG - PPase, SSS, GBSS, and SBE (Jiang et al., 2003). ADPG - PPase is a rate limiting enzyme for starch synthesis and a determining factor for grain growth rate. Together with SBE, it controls starch synthesis (Wang et al., 2007). SSS and GBSS are the main catalytic enzymes for the synthesis of amylose and amylopectin, respectively (Yong et al., 2018). The above enzyme activities are significantly affected by nitrogen application rate. Insufficient or excessive nitrogen application can lead to varying degrees of decrease in key enzymes involved in starch synthesis, with varying impacts on various stages of starch synthesis (Xin et al., 2023). This study found that under different treatments, the activities of key enzymes in grain starch synthesis (ADPG - PPase, SSS, GBSS, SBE) showed a tendency to increase and then decrease with the increase of days after anthesis, which was in agreement with the findings of Li et al. (2018) At 28 days after flowering, the activity of key enzymes in starch synthesis and starch content showed A1 (255 kg·hm-2)>CK1 (300 kg·hm-2)>B1 (210 kg·hm-2)>CK2 (0 kg·hm-2), and starch content was significantly positively correlated with these enzyme activities. Among them, the ADPG - PPase, SSS, GBSS, and SBE under CK1 (300 kg·hm-2) treatment were significantly lower than those under A1 (255 kg·hm-2) treatment, with decreases of 5.13–23.44%, 2.77–20.17%, 2.99–19.85%, and 8.15–23.91%, respectively, resulting in a decrease of 7.19–17.85% in starch content. Xin et al(2023). believe that wheat can increase ADPG - PPase, SSS, GBSS, SBE activity and yield at a nitrogen application rate of 240 kg·hm-2; However, when the nitrogen application rate is 300 kg·hm-2, both the enzyme activity and yield mentioned above decrease, which may be due to differences in geographical and climatic conditions. The content of starch components in the grains of the two varieties showed a gradual increase as the number of days after anthesis increased, with the highest growth rate from 14 to 21 days. The content of amylose, amylopectin, and total starch increased by 83.51–126.85%, 100.39–127.32%, and 103.09–121.05%, respectively. The content of straight chain, branched chain, and total starch in the grains showed a quadratic parabolic relationship with nitrogen application rate. XC37: , , ; XC 6: , , . It can be seen that drip irrigation of wheat in arid areas of Xinjiang, under A1 (255 kg·hm-2) treatment, is beneficial for improving the key enzyme activity of starch synthesis in strong and medium gluten wheat, and promoting the accumulation of grain starch.
4.3. Effect of nitrogen application rate on spring wheat yield and its composition
Appropriate nitrogen fertilizer supply is the foundation for high yield of wheat (de Lucena Marinho et al., 2022). Yuan et al.(2022) proposed that the main reason for the rational application of nitrogen fertilizer to increase wheat yield is the increase in 1000-grain weight; Wang et al.(2022) believe that the rational application of nitrogen fertilizer is beneficial for improving yield and ensuring harvest spikes. Research has shown that as the nitrogen application rate increases, the 1000 grain weight, spike number, grain per panicle, and yield of both varieties show a trend of first increasing and then decreasing. The yield increased from 6648.67–6832.74 kg·hm-2 under conventional nitrogen application (300 kg·hm-2) to 7053.33–7257.50 kg·hm-2 under reduced nitrogen application (255 kg·hm-2). Further reduction of nitrogen application (210 kg·hm-2) resulted in a decreasing trend in yield and its composition.
Reducing nitrogen fertilizer application in a certain range can promote wheat yield. When too much or too little nitrogen fertilizer is applied, not only can yields not be increased, but yields may even be reduced, as Si et al(2020) have found. The yield of both varieties shows a parabolic relationship with nitrogen application rate, and the grain yield is: (R2=0.9575), (R2=0.9679). When the nitrogen application rate is 255 kg·hm-2, the yield of XC37 is 7004.08 kg·hm-2, and the yield of XC 6 is 6885.69 kg·hm-2. There is a significant interaction effect between nitrogen application rate and variety on 1000-grain weight, spike number, grain per spike, and yield of wheat, indicating that appropriate nitrogen application rate is beneficial for improving 1000-grain weight, spike number, grain per spikem, and yield of wheat. Stepwise regression analysis shows that different varieties have different factors affecting yield. Soluble sugars in grains, amylopectin, and sucrose in grains have the greatest impact on XC37 yield (R2=0.845), while SSS, soluble sugars in grains, amylopectin, and SBE have the greatest impact on XC 6 yield (R2=0.804). Due to differences in land fertility and other factors in different regions, the optimal nitrogen application rate also varies. Therefore, further field evidence is needed to determine the appropriate nitrogen application rate for different regions.
5. Conclusion
Under drip irrigation conditions in arid areas of Xinjiang, compared to 210 kg·hm-2 and 300 kg·hm-2 treatments, drip irrigation significantly increased the activity of key enzymes in flag leaves sucrose metabolism under 255 kg·hm-2 nitrogen application, promoting soluble sugar and sucrose content in spring wheat; The activities of key enzymes involved in grains sucrose metabolism and starch synthesis were significantly increased, leading to a significant increase in seed grains content and grain weight. 255 kg·hm-2 nitrogen application promoted the transformation of sucrose into starch substrate and the synthesis of grain starch, optimized the balance of supply and demand of materials between flag leaves and grain, and finally increased the yield.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We grateful to reviewers for helping us improve our original manuscript. This study was supported by projects of the key project of national natural science foundation of China (Project Nos. 31760346).
References
- Chen, H. , Huang, Z.J., Wang, J.C., Pan, X.J., Zhang, D., & Xu Y.L., (2018). Effect of Water and Nitrogen Coupling on N Absorption, Translocation and Yield of Winter Wheat under Drip Irrigation. Xinjiang Agricultural Sciences. 55(1), 44-56. [CrossRef]
- de Lucena Marinho, J. , Silva, S. R., de Batista Fonseca, I. C., & Zucareli, C. (2022). Nitrogen use efficiency and yield of wheat genotypes affected by nitrogen fertilizing and environmental conditions in Southern Brazil. International Journal of Plant Production, 16(3), 495-510. [CrossRef]
- Douglas, C. D. , Tsung, M. K., Frederick, C. F. 1988. Enzymes of sucrose and hexose metabolism indevelopment kernels of two inbreds of maize. Plant Physiol, 86, 1013-1019. [CrossRef]
- Fan, Y. , Lv, Z., Zhang, Y., Ma, L., Qin, B., Liu, Q., Zhang, W., Ma, S., Ma, C., & Huang, Z. (2022). Pre-anthesis night warming improves post-anthesis physiological activity and plant productivity to post-anthesis heat stress in winter wheat (Triticum aestivum L.). Environmental and Experimental Botany, 197, 104819. [CrossRef]
- Gao, L., Wang, H., Wan, C., Wang, P., Eeckhout, M., & Gao, J. (2023). Suitable nitrogen fertilizer application drives the endosperm development and starch synthesis to improve the physicochemical properties of common buckwheat grain. International Journal of Biological Macromolecules, 235, 123837. [CrossRef]
- Gao, Y., Wu, P., Zhao, X., & Wang, Z. (2014). Growth, yield, and nitrogen use in the wheat/maize intercropping system in an arid region of northwestern China. Field Crops Research, 167, 19-30. [CrossRef]
- Huang, L. , Tan, H., Zhang, C., Li, Q., & Liu, Q. (2021). Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant communications, 2(5). [CrossRef]
- Jiang, D., Cao, W., Dai, T., & Qi, J. (2003). Activities of key enzymes for starch synthesis in relation to growth of superior and inferior grains on winter wheat (Triticum aestivum L.) spike. Plant Growth Regulation, 41, 247-257. [CrossRef]
- Jiang, D. , Fan, X., Dai, T., & Cao, W. (2008). Nitrogen fertiliser rate and post-anthesis waterlogging effects on carbohydrate and nitrogen dynamics in wheat. Plant and Soil, 304, 301-314. [CrossRef]
- Jin, Y. H. , Zhang, K.L., Zhang, X.C., & Du., J.H. (2009). Determination of straight chain and branched chain starch content in wheat and wheat sprouts using dual wavelength method. Journal of the Chinese Cereals and Oils Association, (1),137-140. (in Chinese)
- Kaur, M. , Bhardwaj, R. D., Singla, P., Kaur, S., & Kaur Grewal, S. (2023). Drought Stress-Induced Alterations in Source–Sink Relationships in Barley During Grain Development. Gesunde Pflanzen, 1-12. [CrossRef]
- Keller, F. , & Ludlow, M. M. (1993). Carbohydrate metabolism in drought-stressed leaves of pigeonpea (Cajanus cajan). Journal of Experimental Botany, 44(8), 1351-1359. [CrossRef]
- Khan, G.R. , & Akma, M. (2021). Nitrogen application rate and timing management for improved grain quality parameters of wheat crop. Pakistan Journal of Agricultural Sciences, 58(4). [CrossRef]
- Li, G. , Hu, Q., Shi, Y., Cui, K., Nie, L., Huang, J., & Peng, S. (2018). Low nitrogen application enhances starch-metabolizing enzyme activity and improves accumulation and translocation of non-structural carbohydrates in rice stems. Frontiers in plant science, 9, 1128. [CrossRef]
- Li, Y., Lu, W., Lyu, D., Su, F., Liu, S., Li, H., Wang, X., Liu, Z., & Hu, L. (2018). Effects of different nitrogen application rates on starch accumulation, starch synthase gene expression and enzyme activity in two distinctive potato cultivars. Potato Research, 61, 309-326. [CrossRef]
- Mahdy, R. E., Alghamdi, S. A., Amro, A., & Tammam, S. A. (2022). Changes in Carbon and Nitrogen Metabolites before, at, and after Anthesis for Wheat Cultivars in Response to Reduced Soil Water and Zinc Foliar Application. Plants, 11(9), 1261. [CrossRef]
- Nakamura, Y., Yuki, K., Park, S. Y., & Ohya, T. (1989). Carbohydrate metabolism in the developing endosperm of rice grains. Plant and cell physiology, 30(6), 833-839. [CrossRef]
- Ran, L., Yu, X., Li, Y., Zou, J., Deng, J., Pan, J., & Xiong, F. (2020). Analysis of development, accumulation and structural characteristics of starch granule in wheat grain under nitrogen application. International Journal of Biological Macromolecules, 164, 3739-3750. [CrossRef]
- Shibata, H. , Galloway, J. N., Leach, A. M., Cattaneo, L. R., Cattell Noll, L., Erisman, J. W., Gu, B.J., Liang, X., Hayashi, K., Ma, L., Dalgaard, T., Graversgaard, M., Chen, D., Nansai, K., Shindo, J., Matsubae, K., Oita, A., Su, M.C., Mishima, S.I., & Bleeker, A. (2017). Nitrogen footprints: Regional realities and options to reduce nitrogen loss to the environment. Ambio, 46, 129-142. [CrossRef]
- Si, Z. , Zain, M., Mehmood, F., Wang, G., Gao, Y., & Duan, A. (2020). Effects of nitrogen application rate and irrigation regime on growth, yield, and water-nitrogen use efficiency of drip-irrigated winter wheat in the North China Plain. Agricultural Water Management, 231, 106002. [CrossRef]
- Stein, O. , & Granot, D., (2019). An overview of sucrose synthases in plants. Frontiers in plant science, 10, 95. [CrossRef]
- Ullah, A. , Zhao, C., Zhang, M., Sun, C., Liu, X., Hu, J., Zeeshan, M., Zaid, A., Dai, T., & Tian, Z. (2023). Nitrogen enhances the effect of pre-drought priming against post-anthesis drought stress by regulating starch and protein formation in wheat. Physiologia plantarum, 175(2), e13907. [CrossRef]
- Verma, A.K., Upadhyay, S.K., Verma, P.C., Solomon, S., & Singh, S.B. (2011). Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biology, 13(2), 325-332. [CrossRef]
- Vila, F., & Sanz, A. (2018). A proposal for evaluating laboratory instruction in a plant physiology course. Theoretical and Experimental Plant Physiology, 30, 1-8. [CrossRef]
- Wang, F. , Chen, S., Cheng, F., Liu, Y., & Zhang, G. (2007). The differences in grain weight and quality within a rice (Oryza sativa L.) panicle as affected by panicle type and source-sink relation. Journal of Agronomy and Crop Science, 193(1), 63-73. [CrossRef]
- Wang, J. F., Wang, Z. Z., Gu, F. X., Mou, H. M., Wang, Y., Duan, J. Z., Feng, w., Wang, Y., & Guo, T. (2021). Effects of nitrogen fertilizer and plant density on carbon metabolism, nitrogen metabolism and grain yield of two winter wheat varieties. Sci. Agric. Sin, 54, 4070-4083.
- Wang, L., Sun, J., Zhang, Z., Xu, P., & Shangguan, Z. (2018). Winter wheat grain yield in response to different production practices and soil fertility in northern China. Soil and Tillage Research, 176, 10-17. [CrossRef]
- Wang, W.X., Shen, C.C., Xu, Q.X., Zafar, S., Du, B., Dang, D.Y. (2022). Grain yield, nitrogen use efficiency and antioxidant enzymes of rice under different fertilizer n inputs and planting density. Agronomy, 12(2), 430. [CrossRef]
- Wang, X.Y. , He, M.R., Li, F., Liu, Y.H., Zhang, H.H., & Liu, C.G., (2007). Coupling effects of irrigation and nitrogen fertilizer on grain protein and starch quality of strong-gluten winter wheat. Journal of Plant Nutrition and Fertilizers, 13(3), 361-367. [CrossRef]
- Xin, L. , Fu, Y., Ma, S., L, C., Wang, H., Gao, Y., & Wang, X., (2023). Effects of Post-Anthesis Irrigation on the Activity of Starch Synthesis-Related Enzymes and Wheat Grain Quality under Different Nitrogen Conditions. Plants, 12(24), 4086. [CrossRef]
- Xin, L. , Fu, Y., Ma, S., Li. C., Wang H., Gao, Y., & Wang, X., (2023). Effects of Post-Anthesis Irrigation on the Activity of Starch Synthesis-Related Enzymes and Wheat Grain Quality under Different Nitrogen Conditions. Plants, 12(24), 4086. [CrossRef]
- Yang, G. D. , Hu, Z. Y., Huang, R. D., Hao, Z. Y., Li, J. H., Wang, Q., Ming, X., & Zhou, Y. F. (2020). EFFECT OF NITROGEN ON THE STARCH FORMATION AND YIELD OF HIGH-DENSITY SORGHUM [SORGHUM BICOLOR (L.) MOENCH] IN NORTHERN CHINA. Applied Ecology & Environmental Research, 18(4). [CrossRef]
- YU, H. R. , Guo, Y., ZHU, A. M., LU, F. Y., Wang, L., & ZHANG, Y. X. (2018). Effects of nitrogen fertilizer level on non-structural carbon and nitrogen metabolite levels in oats grown in sandy desert soil. Acta Prataculturae Sinica, 27(5), 61. [CrossRef]
- Yuan, S. , Ling, Y., Xiong, Y., Zhang, C.G., Sha, L.N., You, M.H., Lei, X., Bai, S.Q., & Ma, X., (2022). Effect of nitrogen fertilizer on seed yield and quality of Kengyilia melanthera (Triticeae, Poaceae). PeerJ, 10, e14101. [CrossRef]
- Yue, K., Li, L., Xie, J., Liu, Y., Xie, J., Anwar, S., & Fudjoe, S. K. (2022). Nitrogen supply affects yield and grain filling of maize by regulating starch metabolizing enzyme activities and endogenous hormone contents. Frontiers in Plant Science, 12, 798119. [CrossRef]
- Zhang, W., Wang, J., Huang, Z., Mi, L., Xu, K., Wu, J., Fan, Y., Ma, S., & Jiang, D. (2019). Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Frontiers in plant science, 10, 498. [CrossRef]
- Zhang, Z. Q. , Hu, Y. X., Tung, S. A., Yang, L., Wang, Y., & Zhou, X. B. (2023). Evaluating the Effects of Water-Nitrogen Interactions on Carbon and Nitrogen Accumulation As Well As Related Metabolic Enzymes Activity in Autumn Maize. Journal of Soil Science and Plant Nutrition, 1-12. [CrossRef]
- Zhao, H., Dai, T., Jiang, D., & Cao, W. (2008). Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars. Journal of Agronomy and Crop Science, 194(1), 47-54. [CrossRef]
- Zheng, X. G., Qi, J. C., Hui, H. S., Lin, L. H., & Wang, F. (2017). Starch accumulation in hulless barley during grain filling. Botanical studies, 58(1), 1-12. [CrossRef]
- Zhong, Y. , Chen, Y., Pan, M., Wang, H., Sun, J., Chen, Y., Cai, J., Zhou, Q., Wang, X., & Jiang, D. (2023). Insights into the Functional Components in Wheat Grain: Spatial Pattern, Underlying Mechanism and Cultivation Regulation. Plants, 12(11), 2192. [CrossRef]
- Zhou, T.Y., Li, Z.K., Li, E.P., Wang, W.L., Yuan, L.M., Zhang, H., Liu, L.J., Wang, Z.Q., Gu, J.F., & Yang, J.C. (2022). Optimization of nitrogen fertilization improves rice quality by affecting the structure and physicochemical properties of starch at high yield levels. Journal of Integrative Agriculture, 21(6), 1576-1592. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).