1. Introduction
From December 2020 to March 2022, Thailand experienced five waves of COVID-19 outbreaks, with the initial occurrence in March to May 2020. The subsequent waves transpired from December 2020 to February 2021, April to June 2021, July to December 2021, and January to March 2022, as documented by Puenpa et al. (2022) [
1]. Initially concentrated in Bangkok during the early stage, COVID-19 nationwide dissemination ensued primarily due to a significant migration following the March 2020 Bangkok shutdown [
2]. Examining instances of antropozoonosis, a veterinarian contracted COVID-19 through close contact with an infected cat, highlighting the atypical nature of this transmission mode [
3]. Empirical evidence suggests heightened susceptibility of cats to SARS-CoV-2, with lab experiments showing they can transmit the virus [
4]. Laboratory experiments have further confirmed that cats can transmit the SARS-CoV-2 virus to their feline counterparts [
5,
6]. Consequently, it is plausible for cats residing in households with COVID-19 patients to become integral components of the transmission pathway of the COVID-19 virus, constituting a human-to-animal-to-human transmission cycle.
Surveillance of COVID-19 infections in the feline population is crucial for future epidemic control and prevention planning. There is a moderate likelihood of cats contracting COVID-19 in close contact conditions with their infected owners, as per expert opinion [
7]. The necessity to segregate cats from COVID-19 patients becomes a pivotal measure to avert and mitigate transmission from humans to felines. Continuous scientific inquiry and systematic data collection on COVID-19 infections in cats, especially during human COVID-19 outbreaks, facilitate early detection in the feline demographic and provide essential insights for readiness in preventing and monitoring the spread of COVID-19 among cats [
8,
9,
10]. Notably, during periods of endemic COVID-19 when local testing measures or disease reporting may decrease, utilizing the cat population as sentinels for disease surveillance emerges as a viable alternative for monitoring COVID-19 within both human and animal populations [
11,
12], particularly among companion animals. In this circumstance, serological assay seems to be the most appropriate method for large-scale screening tests and surveillance [
13]. Moreover, the serological assay can diagnose recent SARS-CoV-2 past infection which facilitates determining the actual disease burden [
14]. Recently, Udom et al. (2021) reported the utility of a human anti-N IgG ELISA commercial kit to detect the anti-N antibodies and surrogate virus to detect anti-S neutralizing antibodies in dogs and cats during the Thailand epidemic from April to December 2020 [
15]. Moreover, the seroprevalence against SARS-CoV-2 was investigated. It was found that 1.66 and 0.36% of dogs and cats, respectively, were positive for SARS-CoV-2 antibodies. However, none of them was positive for anti-S neutralizing antibodies by surrogate virus neutralization test (cPass™). After this period, Thailand was attacked by four waves of COVID-19 outbreaks [
1] which predominated by different SARS-CoV-2 clades/variants. Regarding virus adaptation and natural selection, the different SARS-CoV-2 clades/variants have distinct characteristics that could affect transmissibility, disease severity, and serology of hosts [
16]. Hence, it is worth re-assessing the utility of serological tests for diagnosing SARS-CoV-2 infection in domestic animals during the subsequent waves of Thailand’s COVID-19 epidemics.
The ultimate objective of this study was to re-assess the utility of serological tests for diagnosing SARS-CoV-2 infection in cats during five waves of Thailand’s COVID-19 epidemics by using a modified human test kit, namely, anti-S1 RBD IgG ELISA. Additionally, seroprevalence and correlation between SARS-CoV-2 infection in cats and human COVID-19 epidemics in Thailand were investigated. The significance of this research was to gain insights into the feasibility of employing cats as candidate sentinels for future disease surveillance during COVID-19 human outbreaks.
2. Materials and Methods
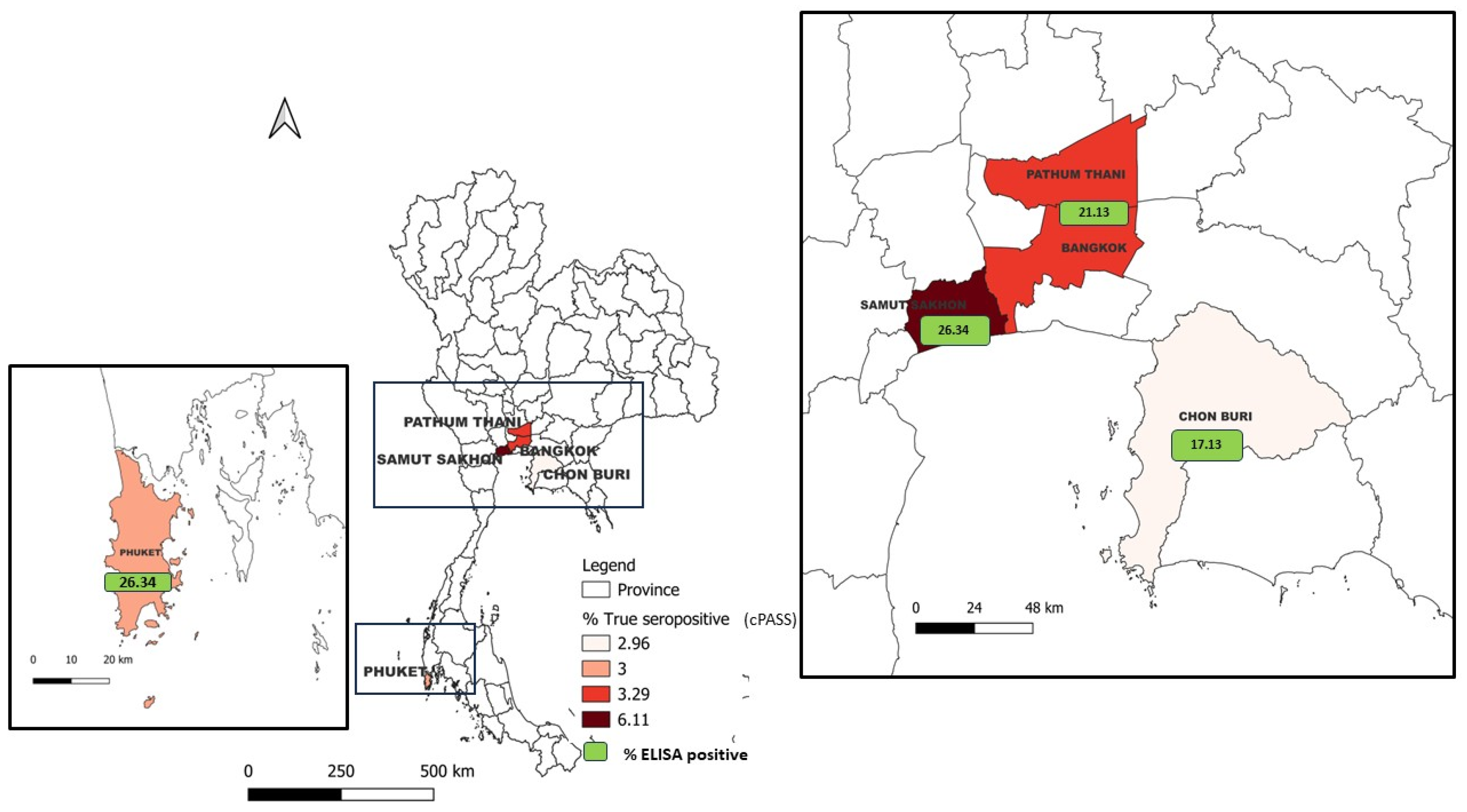
A total of 971 (1,107-136) cat sera samples were collected during between December 2020 and March 2022 in five major provinces according to the high number of confirmed cases in human population, including Bangkok, Pathum Thani, Chon Buri, Samut Sakhon, and Phuket (outbreak areas). Cat sera collected from Nakhon Pathom and Bueng Kan provinces before the report of COVID-19 outbreaks in such areas (non-outbreak areas) were also included in this study. All of samples were shown in map of Thailand (
Figure 1) and separated by wave of infection (
Table 1). Three positive control samples were derived from cats diagnosed with COVID-19, confirmed by PCR and plaque reduction neutralization test. These positive samples were originated from Italy (two samples) and the United States (one sample) and kindly provided by Dr. Nicola Decaro and Dr. Angela Bosco-Lauth. Commercial pooled normal cat sera procured from 200 cats collected prior to the onset of the COVID-19 outbreak (Nordic-MUBio, Inc., Susteren, The Netherlands), were used as a negative control.
Human SARS-CoV-2 S1 RBD IgG antibody ELISA kit (CUSABIO, Hubei, China) was used to perform the indirect ELISA screening test according to the manufacturer’s instructions, except the secondary antibody was substituted with horseradish peroxidase-conjugated goat anti-feline IgG (H+L) (Invitrogen, Camarillo, CA). For optimization, three confirmed SARS-CoV-2 infection-positive sera and commercial pooled normal cat sera (negative control) were diluted 1:100, 1:200, 1:400, and 1:800 in sample diluent. One-hundred microliters of diluted samples were added to each well of commercial SARS-CoV-2 S1 RBD coated assay plates and incubated at 37ºC for 30 minutes at room temperature. Wells were washed three times with 1X wash buffer, and 1:10,000 HRP-conjugated goat anti-feline IgG (H+L) was added. After 30 minutes of incubation, the assay plate was then washed five times, followed by adding TMB substrate and incubated at 37ºC for 20 minutes. The absorbance was measured at the wavelength of 450 and 570 nm using Synergy LX Multi-Mode Microplate Reader (Agilent Biotek, Santa Clara, CA) after adding a stop solution. To evaluate for the potentially cross-reacting cat antibodies, a total of 136 cat sera from 18 categories and 156 cat sera in non-outbreak areas were tested (
supplementary Table S1). All sera were diluted at 1:400 and modified SARS-CoV-2 S1 RBD ELISA was performed as described previously.
Screening of SARS-CoV-2 antibody in cat sera using modified SARS-CoV-2 S1 RBD ELISA was done. In brief, the SARS-CoV-2 positive control, negative control sera (commercial pooled normal cat sera) and tested serum samples were diluted 1:400 in sample diluent. and modified ELISA was performed as described previously. The cut-off value (OD
sample/OD
negative) was determined by ROC curve analysis and computated by Youden’s index [
17,
18] as shown in preliminary data (
supplementary Figure S1). Samples with the value of OD
sample/OD
negative equal to or greater than 2.02 were considered as positive for modified SARS-CoV-2 S1 RBD ELISA.
Cat sera were confirmed for the presence of SARS-CoV-2 antibody by the cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript Biotech, Singapore) following manufacturer’s instructions. In brief, serum samples, positive and negative controls were diluted 1:10 in sample dilution buffer. Subsequently, 60 µl of the diluted samples was incubated with equal volume of 1:3,000 HRP conjugated RBD at 37ºC for 30 minutes. Then, 100 µl of the mixture was transferred to the ACE2-capture plate and incubated for 15 minutes at 37ºC. Following four washes with 1X wash buffer, TMB substrate (100 µl/well) was added, and the plate was then incubated at room temperature for 15 minutes in a dark, humidified box. A stop solution (50 µl/well) was finally added, and the OD was measured at the wavelength of 450 nm using Synergy LX Multi-Mode Microplate Reader. The percentage of inhibition (% inhibition) was calculated according to the manufacturing instruction. The inhibition equal to or greater than 20% was considered as positive for SARS-CoV-2 neutralizing antibody.
Statistical analyses were performed using R Statistical Software (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to determine the proportion of seropositive cats. ANOVA and Kruskal-Wallis H tests were performed to compare the differences between number of positive cat sera and percentage of positive cats by different waves of the epidemic and provinces. The effect of epidemic waves and provinces on the number of positive cat sera and the proportion of seropositive cats was analyzed by two-way ANOVA. Pearson’s and Spearman correlation tests were performed to assess the relationship between reported human cases and the number of positive cat sera. Significance was assessed at the P < 0.05.
3. Results
Cat sera samples collected from the respective provinces were screened for antibody against SARS-CoV-2 by modified SARS-CoV-2 S1 RBD ELISA method, and confirmed by cPass surrogate viral neutralization test. The distribution of seropositive cat sera separated by the waves of epidemic and provinces was shown in
Table 1 and
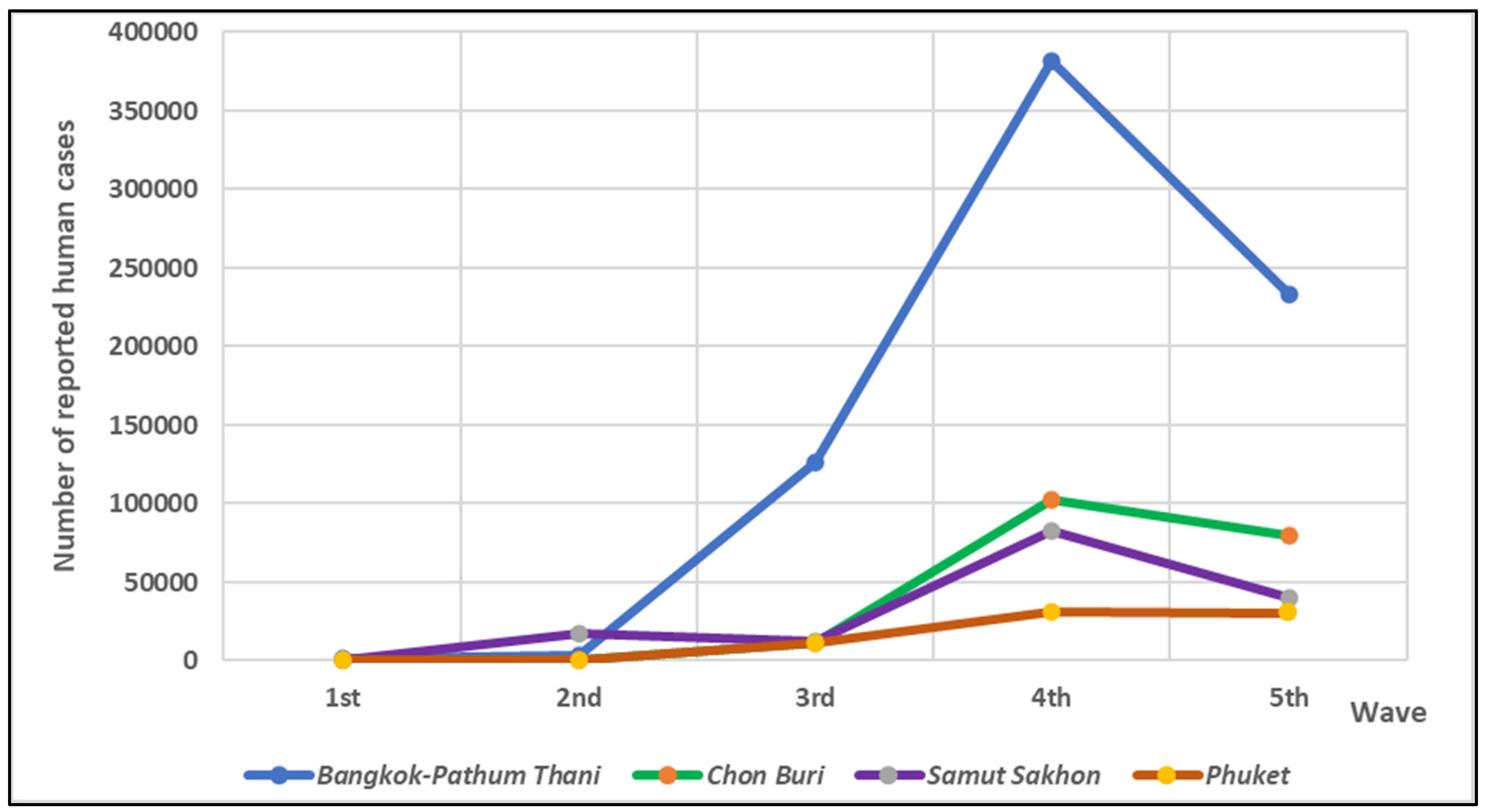
Figure 1. The number of reported human cases across five provinces during five waves of the outbreak in Thailand was shown in
Figure 2 [
19]. Among these five provinces, the number of reported human cases tended to increase over time, the highest number of reported cases was shown at the 4
th wave of epidemic. The highest number of human cases was reported in Bangkok-Pathum Thani area, followed by Chon Buri, Samut Sakhon and Phuket, respectively.
3.1. Prevalence of SAR-CoV-2 antibody in cats by modified SARS-CoV-2 S1 RBD ELISA
Our modified SARS-CoV-2 S1 RBD ELISA had a high accuracy (AUC = 0.903; P <0.0001) and good effectiveness (Youden’s index = 0.788) for detection of SARS-CoV-2 antibodies in cat sera (
Supplementary Figure S1). Of 1,107 feline sera samples, encompassing 878 samples from areas affected by the human COVID-19 outbreaks and 229 from non-outbreak regions. Within the cohort of 878 cat sera from outbreak areas, 22.67% (199/878) demonstrated positivity for SARS-CoV-2 by indirect ELISA during the 2
nd to 5
th waves of the Thailand COVID-19 epidemics. Notably, the trends in the number of positive feline sera and the proportion of positive cats mirrored those observed in human cases, as illustrated in
Figure 3. Surprisingly, samples from non-outbreak areas exhibited a seropositive cat proportion of 17% (39/229).
In the specific context of Bangkok-Pathum Thani, during the 2
nd wave of the epidemic with reported human cases at 2,943, the proportion of ELISA-positive cats was 12.5%, escalating to 15.22% in the 3
rd wave and reaching 24% in the 4
th wave. Only one serum sample was collected during the 5
th wave, yielding a negative result for ELISA. In Chon Buri, no ELISA-positive case was detected during the 2
nd and 3
rd waves, aligning with reported human cases of 662 and 11,254, respectively. However, a substantial proportion of seropositive cats (39.58%) emerged in the 4
th wave, coinciding with a surge in reported human cases to 102,974, followed by a decline to 11.64% in the 5
th wave. In Samut Sakhon, the proportion of seropositive cats was 12.50% in the 3
rd wave, rising twofold to 25.94% in the 4
th wave, concomitant with a surge in reported human cases to 82,921. The highest proportion of seropositivity, reaching 30.65%, was noted in the 5
th wave, despite a 50% reduction in reported human cases. Notably, in Phuket, no seropositive cases were detected during the 2
nd wave. However, the highest proportion of seropositive cats (43.8%) was observed in the 3
rd wave, correlating with 769 reported human cases, followed by proportions of 17.02% and 24.41% in the 4
th and 5
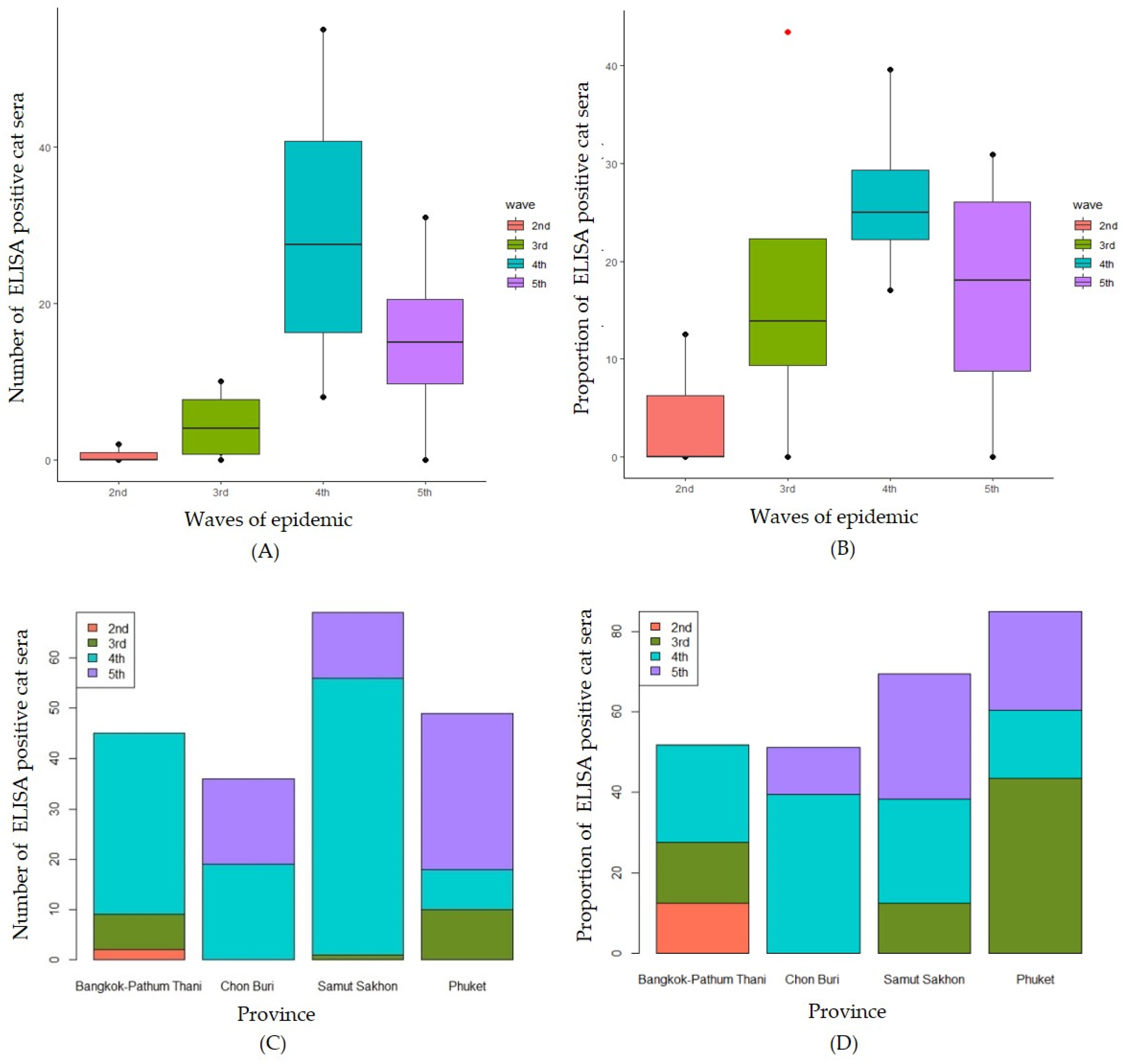
th waves, respectively. The waves of epidemic and provinces did not have a statistically significant effect on the proportion of ELISA positive cats (P = 0.329 and P = 0.802, respectively). The comparison of the numbers of ELISA positive cats by the waves of epidemic was shown in
Figure 3A. There was no statistically significant difference between the numbers of ELISA positive for SAR-CoV-2 by different waves of the epidemic (H (3) = 7.723, P = 0.052). Similarly, the means proportion of seropositive for SAR-CoV-2 cats was not statistically significant difference by difference waves of epidemic (F (3) = 1.632, P = 0.238) (
Figure 3B). Focused on the comparison among provinces, there was no statistically significant difference between the numbers of seropositive for SAR-CoV-2 by different provinces (H (3) = 0.897, P = 0.826) (
Figure 3C). Similarly, no statistically significant difference between the proportion seropositive for SAR-CoV-2 by different provinces (H (3) = 0.471, P = 0.709) was found (
Figure 3D).
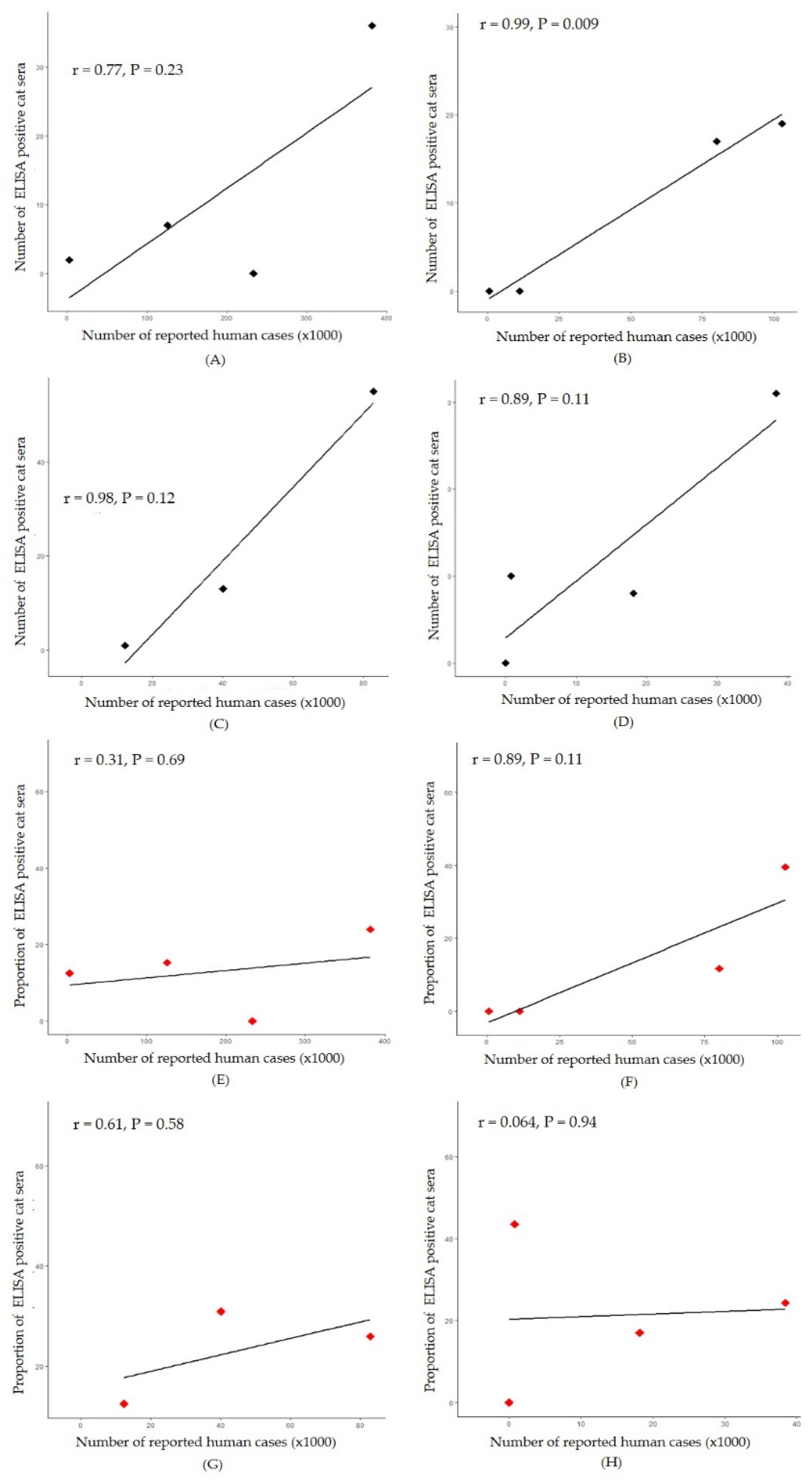
A significant positive correlation was observed between the number of positive cat sera and the number of reported human cases in Chon Buri province (r = 0.99, P = 0.009) (
Figure 4B). Simple linear regression was used to test if the number of reported human cases significantly predicted number of ELISA positive cat sera. The fitted regression model was y = 0.205x - 0.98. The overall regression was statistically significant (R
2 = 0.97, F (1, 2) = 104.3, p < 0.01. It was found that number of reported human cases significantly predicted number of ELISA positive cat sera (β = 0.205, p < 0.01). Conversely, in Bangkok-Pathum Thani, Samut Sakhon, and Phuket, strong correlations (r = 0.77, 0.98, and 0.89, respectively) were observed between the numbers of positive cat sera and reported human cases during the considered period; however, these correlations were not statistically significant (P > 0.05) (
Figure 4A, 4C and 4D).
3.2. Confirmation of SAR-CoV-2 infection in cats by cPass surrogate viral neutralization
In total, the proportion of cat sera which were positive for SARS-CoV-2 by cPass surrogate viral neutralization was 3.99% (35/878). The trends of number of positive cat sera and the proportion of positive cat were the same as occurred in human cases (
Figure 2). Only one cat from Nakhon Pathom province out of 39 seropositive cats (0.44%; 1/229) determined by indirect ELISA from non-outbreak areas were tested positive by cPass surrogate viral neutralization. In Bangkok-Pathum Thani, 213 cat sera were collected during the five waves of epidemic. At the 2
nd wave while reported human case was 2,943, none of cat sera sample found positive by the cPass surrogate viral neutralization. However, at the 3
rd wave of epidemic, while reported human case was 125,788, the proportion of positive cat was 2.17%, then increased to 4.0 % at the 4
th wave. Only one cat serum sample was collected in the 5
th wave which showed negative result. In Chon Buri, with 203 cat sera collected during the five waves, no seropositive cases were detected during the 2
nd and 3
rd waves (662 and 11,254 reported human cases, respectively). However, a notable surge in seropositive cats (39.58%) occurred during the 4
th wave, aligning with a rise in reported human cases to 102,974, followed by a decrease to 11.64% in the 5
th wave. In Samut Sakhon, where 262 cat sera were collected, no positive cats were found during the 3
rd wave (12,407 reported human cases), but the proportion increased to 7.08% (15/212) at the 4
th wave (82,921 reported human cases) and subsequently decreased to 2.38% (1/42) in the 5
th wave, coinciding with a 50% reduction in reported human cases. Finally, in Phuket, with 200 cat sera collected, no positive cases were detected during the 2
nd and 3
rd waves. However, the highest proportion of positives occurred in the 4
th wave (8.51%) with 18,164 reported human cases, followed by a decrease to 1.57% in the 5
th wave.
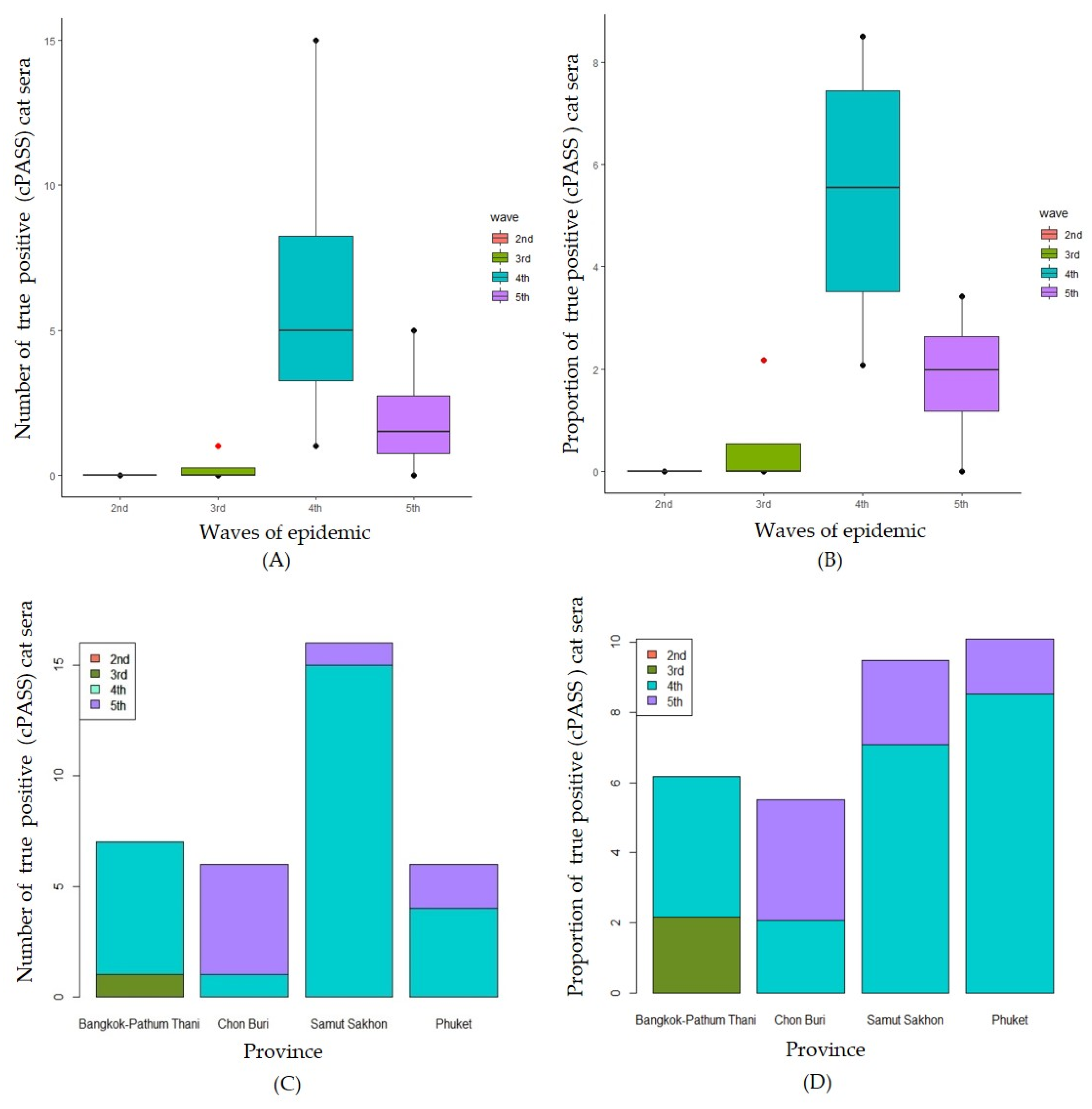
A comparison of the numbers of positive cats by the waves of epidemic is shown in
Figure 5A. There was statistically significant difference between the numbers of cPass surrogate viral neutralization positive for SAR-CoV-2 by different waves of the epidemic (H (3) = 9.268, P = 0.026). The median number of positive viral neutralization cat sera at 2
nd to 5
th wave were 0, 0, 5 and 1.5, respectively. The results show that the median number of positive cats at the 4
th wave was significantly higher than those of the 3
rd wave. Similarly, the medians proportion of positive cPass surrogate viral neutralization for SAR-CoV-2 cats was statistically significant difference by difference waves of epidemic (F (3) = 9.194, P = 0.027) (
Figure 5B). The median proportion of positive viral neutralization cat sera at 2
nd to 5
th wave were 0, 0, 5.54 and 1.98, respectively. The results show that the median number of positive cats at the 4
th wave was significantly higher than those of the 2
nd and the 3
rd waves. Focused on the comparison among provinces, there was no statistically significant difference between the numbers of positive for SAR-CoV-2 by different provinces (H (3) = 0.383, P = 0.944) (
Figure 5C). Similarly, no statistically significant difference between the proportion cPass surrogate viral neutralization positive for SAR-CoV-2 by different provinces (H (3) = 0.616, P = 0.893) was found (
Figure 5D).
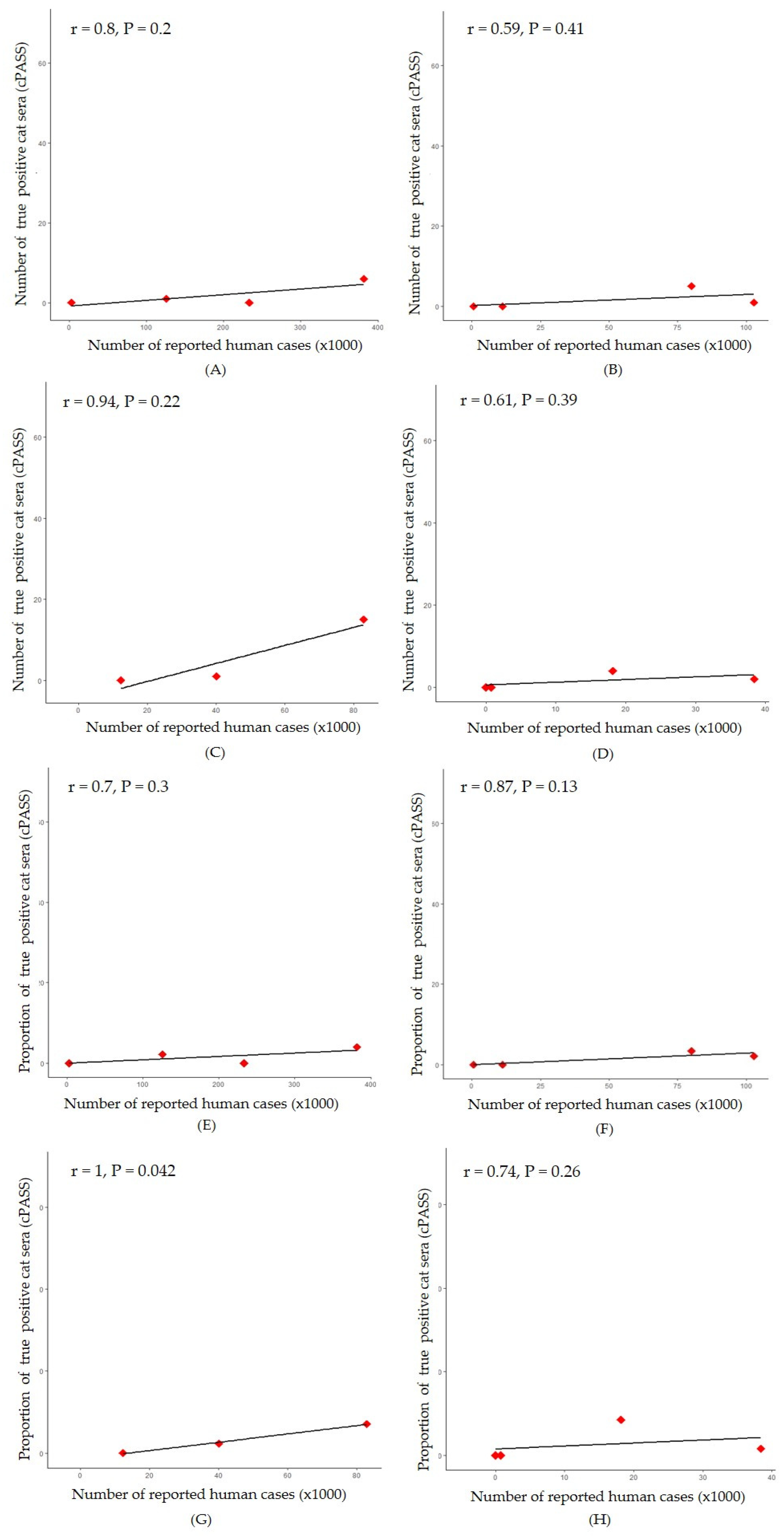
A significant positive correlation was observed between the proportion of positive cat sera and the number of reported human cases in Samut Sakhon province (r = 1, P = 0.042) (
Figure 6G) while, correlation between the number of positive cat sera and the number of reported human cases was not significantly difference in all areas (
Figure 6A-D). Simple linear regression was used to test if the number of reported human cases significantly predicted proportion of cPass surrogate vial neutralization positive cat sera. The fitted regression model was y = 0.1x – 1.42. The overall regression was statistically significant (R
2 = 0.99, F (1, 1) = 234.2, P = 0.04. It was found that the number of reported human cases significantly predicted proportion of positive cat sera (β = 0.1, p = 0.04). Additionally, in Bangkok-Pathum Thani, Chonburi and Phuket, there was a strong correlation (r = 0.70, r = 0.87 and ρ = 0.74, respectively) between the proportion of positive cat sera and number of reported human cases during the considered period, however it was not statistically significant (P > 0.05) (
Figure 6E, 6F and 6H).
Overall, the implement of SAR-CoV-2 ELISA screening test with the cut-off value of 2.02 confirmed by the cPass viral neutralization test gave 100% sensitivity and 78.86% to 89.18% specificity, which was almost comparable to the estimated values (96% sensitivity and 89.89% specificity).
4. Discussion
SARS-CoV-2 infection in companion animals (dogs and cats) appeared to be associated with reported COVID-19-positive status of the human in such household [
20]. In Germany, the seroprevalence in cats was doubled in accordance with the rise of reported human cases, indicating a continuous occurrence of transspecies transmission from infected owners to their cats [
21]. In this study, the human SARS-CoV-2 S1 RBD IgG antibody ELISA kit was employed to screen feline sera followed by the confirmatory test with surrogate virus neutralization assay (cPass™). The investigation into the prevalence of SARS-CoV-2 antibodies in Thai cats during the observed COVID-19 waves yielded similar notable findings with implications for public health and veterinary considerations. The study revealed a 22.67% seropositivity rate in cat serum samples, determined through the indirect ELISA. This percentage closely mirrors trends observed in human cases, reinforcing the interconnectedness of the viral dynamics between the two populations.
Utilizing modified human screening COVID-19 serological tests (anti-S1 RBD IgG ELISA) for SARS-CoV-2 infection surveillance in the cat population presents potential benefits, although the confirmed seropositivity rate via cPass™ surrogate viral neutralization declined to 3.99%. This reduction was attributed to a substantial number of false positive cases identified through the anti-S1 RBD indirect ELISA test. Similarly, the discrepancy results between screening test using anti-N IgG ELISA and cPass™ were reported by Udom et al. (2021) [
15]. Interference from non-specific cross-reactivity in cats with other viral infections, including feline leukemia virus and feline panleukopenia virus, as indicated in preliminary data (
supplementary Table S1), may have influenced the results. Additionally, misdiagnoses from the screening test occurred under unknown circumstances, with cat sera associated with chronic kidney disease and those sampled in non-outbreak areas contributing to false positive results. The reliance on ELISA alone may prove inadequate for assessing relationships during disease outbreaks, as revealed in the results, where differences in analyzing the two datasets with ELISA were predominantly non-significant, except for a significant finding at the median of Chon Buri, attributed to a high false positive rate. In contrast, cPass™ demonstrated significance at the proportion of Samut Sakhon, offering more reliable and true positive results. This underscores the importance of employing multiple testing methods for a comprehensive and accurate assessment, particularly in the context of disease outbreaks.
The true positive cat serum collected in the non-outbreak areas, specifically from Nakhon Pathom province, could suggest an underrepresented transmission between human to cats and could potentially indicate positive human cases in such area before being officially declared as an outbreak area. However, this assumption prompted further investigation. After the 4
th wave, the number of positive cats determined by both tests was decreased or did not show a similar trend as in human epidemics. While the virus strain in human was transitioning to be Omicron during this phase [
22], we speculated that an infection with Omicron strain after the 4
th wave could have been less contagious to cats as the virus evolved [
23].
The study underscores the importance of continuous surveillance for SARS-CoV-2 in both human and feline populations, revealing correlations in specific provinces and highlighting cats as potential sentinels for human infections. While owning a domestic cat appears to pose a low to insignificant risk for additional human infections [
3] based on cat-to-human interactions, separating cats from infected individuals can aid in preventing further transmission [
24,
25]. The decline in cat cases post-fourth wave, amidst rising human cases, suggests a potential association with the emergence of the omicron variant. This temporal alignment with omicron's introduction in Thailand implies viral evolution favoring increased human adaptability [
26,
27,
28], while reducing feline susceptibility [
29,
30]. Molecular and epidemiological investigations may shed light on the intricate interplay between viral evolution and host susceptibility [
31,
32,
33]. Although cPASS-positive cat proportions remained consistent across epidemic waves, the robust correlation in Samut Sakhon underscores the significance of considering regional variations, providing valuable insights into localized factors influencing transmission dynamics between humans and cats across five major provinces.
Despite these significant findings, the study acknowledges certain limitations, including the need for further research to establish causation and the potential influence of confounding variables. Furthermore, the proportion of the positive cat population sampled in this study might not precisely reflect the dynamics seen in human epidemic waves because lockdown measures prevented sample collection at the peak of the epidemic wave [
2,
34]. Additionally, the lack of statistical significance in some correlations, notably in Bangkok-Pathum Thani, and Phuket, highlights the complexity of the relationship between human and feline infections, necessitating continued investigation. Despite the extensive collection of total cases, it is crucial to acknowledge the prolonged outbreak with up to five waves, which may pose challenges in establishing statistical significance across various regions during specific waves.
5. Conclusions
This study investigated SARS-CoV-2 dynamics in human and feline populations during five outbreak waves in Thailand. The 4th wave had the highest human cases, primarily in Bangkok-Pathum Thani. Cat sera were screened using ELISA, reflecting human case trends. No significant effects of epidemic waves or provinces on ELISA-positive cats were found. cPass confirmed a 3.99% cat seropositivity rate. Correlations between positive cat sera and human cases varied in significance. The Omicron variant may explain decreased cat cases post-4th wave. Despite challenges in statistical significance during the prolonged outbreak, the study highlights cats as potential sentinels and emphasizes comprehensive testing methods for accurate assessments in outbreaks.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: ROC curve and ROC curve analysis with Youden index calculation for determining optimal cutoff value by S1-RBD ELISA test (preliminary population, a part of total population in this study).; Table S1: Potential cross-reactivity of cat sera tested by modified indirect ELISA.
Author Contributions
Conceptualization S.T., J.T., P.S., R.Y.; Data curation W.P., R.Y.; Funding acquisition P.S., R.Y.; Resources (Positive control samples) and confirmatory data A.BL., N.D.; Methodology J.T., P.S.; Investigation J.T., P.S., W.P., R.Y. Writing—original draft preparation S.T., J.T., P.S., W.P., P.E., N.T. R.Y.; Formal analysis and software S.T.; Visualization S.T., P.E., R.Y. Writing-review and editing S.T., P.E., N.T., R.Y.; Supervision P.S., P.E., N.T., R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by This work was supported by Program Management Unit for Human Resources & Institutional Development, Research and Innovation (PMU-B), grant number B17F640006.
Institutional Review Board Statement
All animal studies were ethically reviewed and carried out following the guidelines and regulations of the Ethics of Animal Experimentation of the National Research Council of Thailand (NRCT). The procedures in this study were approved by the Kasetsart University Institutional Animal Care and Use Committee (KUIACUC) (ACKU64-VET-021).
Informed Consent Statement
Informed consent was obtained from the owner of the animals.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. The human cases data are available from Department of disease control (DDC), Thailand (
https://covid19.ddc.moph.go.th/).
Acknowledgments
The authors are grateful for the support of the cat’s owner.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Puenpa, J.; Rattanakomol, P.; Saengdao, N.; Chansaenroj, J.; Yorsaeng, R.; Suwannakarn, K.; Thanasitthichai, S.; Vongpunsawad, S.; Poovorawan, Y. Molecular characterisation and tracking of severe acute respiratory syndrome coronavirus 2 in Thailand, 2020–2022. Archives of Virology 2023, 168, 26. [Google Scholar] [CrossRef] [PubMed]
- Triukose, S.; Nitinawarat, S.; Satian, P.; Somboonsavatdee, A.; Chotikarn, P.; Thammasanya, T.; Wanlapakorn, N.; Sudhinaraset, N.; Boonyamalik, P.; Kakhong, B.; et al. Effects of public health interventions on the epidemiological spread during the first wave of the COVID-19 outbreak in Thailand. PLOS ONE 2021, 16, e0246274. [Google Scholar] [CrossRef] [PubMed]
- Sila, T.; Sunghan, J.; Laochareonsuk, W.; Surasombatpattana, S.; Kongkamol, C.; Ingviya, T.; Siripaitoon, P.; Kositpantawong, N.; Kanchanasuwan, S.; Hortiwakul, T.; et al. Suspected Cat-to-Human Transmission of SARS-CoV-2, Thailand, July–September 2021. Emerging Infectious Disease journal 2022, 28, 1485. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, V.; Denzin, N.; Conraths, F.J.; Boden, L.A.; Elvinger, F.; Magouras, I.; Stegeman, A.; Wood, J.L.N.; Urueña, A.C.; Grace, K.E.F.; et al. Does having a cat in your house increase your risk of catching COVID-19? One Health 2022, 14, 100381. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Morozov, I.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerging Microbes & Infections 2020, 9, 2322–2332. [Google Scholar] [CrossRef]
- Doliff, R.; Martens, P. Cats and SARS-CoV-2: A Scoping Review. Animals 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Animals and COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html (accessed on 10 January 2024).
- Dileepan, M.; Di, D.; Huang, Q.; Ahmed, S.; Heinrich, D.; Ly, H.; Liang, Y. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 2021, 12, 1597–1609. [Google Scholar] [CrossRef]
- Jairak, W.; Charoenkul, K.; Chamsai, E.; Udom, K.; Chaiyawong, S.; Bunpapong, N.; Boonyapisitsopa, S.; Tantilertcharoen, R.; Techakriengkrai, N.; Surachetpong, S.; et al. First cases of SARS-CoV-2 infection in dogs and cats in Thailand. Transboundary and Emerging Diseases 2022, 69, e979–e991. [Google Scholar] [CrossRef]
- Panzera, Y.; Mirazo, S.; Baz, M.; Techera, C.; Grecco, S.; Cancela, F.; Fuques, E.; Condon, E.; Calleros, L.; Camilo, N.; et al. Detection and genome characterisation of SARS-CoV-2 P.6 lineage in dogs and cats living with Uruguayan COVID-19 patients. Memórias do Instituto Oswaldo Cruz 2022, 117. [Google Scholar] [CrossRef]
- Antia, R.; Halloran, M.E. Transition to endemicity: Understanding COVID-19. Immunity 2021, 54, 2172–2176. [Google Scholar] [CrossRef]
- Kost, G.J. Diagnostic Strategies for Endemic Coronavirus Disease 2019 (COVID-19): Rapid Antigen Tests, Repeated Testing, and Prevalence Boundaries. Archives of Pathology & Laboratory Medicine 2021, 146, 16–25. [Google Scholar] [CrossRef]
- Sidiq, Z.; Hanif, M.; Dwivedi, K.K.; Chopra, K.K. Benefits and limitations of serological assays in COVID-19 infection. Indian Journal of Tuberculosis 2020, 67, S163–S166. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database of Systematic Reviews 2020. [Google Scholar] [CrossRef]
- Udom, K.; Jairak, W.; Chamsai, E.; Charoenkul, K.; Boonyapisitsopa, S.; Bunpapong, N.; Techakriengkrai, N.; Amonsin, A. Serological survey of antibodies against SARS-CoV-2 in dogs and cats, Thailand. Transboundary and Emerging Diseases 2022, 69, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, L.; Zhu, L.; Zhu, Y.; Yang, S.; Chen, W.; Fan, Y.; Yang, X.; Yang, S.; Zheng, Y.; et al. Viral dynamics during SARS-CoV-2 omicron infection highlight presymptomatic and asymptomatic infectiousness. Journal of Infection 2023, 86, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.E.; Vizard, A.L. Determination of the optimal cutoff value for a serological assay: an example using the Johne's Absorbed EIA. Journal of Clinical Microbiology 1993, 31, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 infected situation reports. Available online: https://covid19.ddc.moph.go.th (accessed on 10 January 2024).
- Kannekens-Jager, M.M.; de Rooij, M.M.T.; de Groot, Y.; Biesbroeck, E.; de Jong, M.K.; Pijnacker, T.; Smit, L.A.M.; Schuurman, N.; Broekhuizen-Stins, M.J.; Zhao, S.; et al. SARS-CoV-2 infection in dogs and cats is associated with contact to COVID-19-positive household members. Transboundary and Emerging Diseases 2022, 69, 4034–4040. [Google Scholar] [CrossRef]
- Michelitsch, A.; Schön, J.; Hoffmann, D.; Beer, M.; Wernike, K. The Second Wave of SARS-CoV-2 Circulation—Antibody Detection in the Domestic Cat Population in Germany. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Suphanchaimat, R.; Teekasap, P.; Nittayasoot, N.; Phaiyarom, M.; Cetthakrikul, N. Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022. Vaccines 2022, 10. [Google Scholar] [CrossRef]
- Sánchez-Morales, L.; Sánchez-Vizcaíno, J.M.; Pérez-Sancho, M.; Domínguez, L.; Barroso-Arévalo, S. The Omicron (B.1.1.529) SARS-CoV-2 variant of concern also affects companion animals. Frontiers in Veterinary Science 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Saied, A.A.; Tiwari, R.; Dhama, K. SARS-CoV-2 infection in domestic and feral cats: current evidence and implications. Veterinary Quarterly 2021, 41, 228–231. [Google Scholar] [CrossRef] [PubMed]
- What You Should Know about COVID-19 and Pets. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/covid-19-pets-prevention.pdf (accessed on 10 January 2024).
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med J 2021, 62, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Torjesen, I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021, 375, n2943. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Michelitsch, A.; Allendorf, V.; Conraths, F.J.; Beer, M.; Denzin, N.; Wernike, K. Dogs and Cats Are Less Susceptible to the Omicron Variant of Concern of SARS-CoV-2: A Field Study in Germany, 2021/2022. Transboundary and Emerging Diseases 2023, 2023, 1868732. [Google Scholar] [CrossRef]
- Saied, A.A.; Metwally, A.A. SARS-CoV-2 variants of concerns in animals: An unmonitored rising health threat. Virus Disease 2022, 33, 466–476. [Google Scholar] [CrossRef]
- Halaji, M.; Heiat, M.; Faraji, N.; Ranjbar, R. Epidemiology of COVID-19: An updated review. J. Res Med Sci 2021. [Google Scholar] [CrossRef]
- Kerner, G.; Quintana-Murci, L. The genetic and evolutionary determinants of COVID-19 susceptibility. European Journal of Human Genetics 2022, 30, 915–921. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfán-Valencia, R. Genetics Insight for COVID-19 Susceptibility and Severity: A Review. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Wongcha-um, P. Thailand expands lockdown areas as COVID-19 cases surge. Available online: https://www.reuters.com/world/asia-pacific/thailand-expands-lockdown-areas-covid-19-cases-surge-2021-07-18/ (accessed on 10 January 2024).
Figure 1.
Map of study highlighted the distribution of SAR-CoV-2 positive cat sera by cPASS (% true positive) and the proportion of SAR-CoV-2 positive cat sera by indirect ELISA (green).
Figure 1.
Map of study highlighted the distribution of SAR-CoV-2 positive cat sera by cPASS (% true positive) and the proportion of SAR-CoV-2 positive cat sera by indirect ELISA (green).
Figure 2.
Number of reported human cases separated by waves of epidemic and provinces.
Figure 2.
Number of reported human cases separated by waves of epidemic and provinces.
Figure 3.
Numbers of ELISA positive cat sera by waves of epidemic (A) and by province (C) and proportions of ELISA positive cat sera separated by waves of epidemic (B) and by provinces (D).
Figure 3.
Numbers of ELISA positive cat sera by waves of epidemic (A) and by province (C) and proportions of ELISA positive cat sera separated by waves of epidemic (B) and by provinces (D).
Figure 4.
Correlation between number of reported human and number of indirect ELISA positive cat sera in four locations: Bangkok-Pathum Thani; r=0.77, P=0.23 (A), Chon Buri; r=0.99, P=0.009 (B), Samut Sakhon; r=0.98, P=0.12 (C) and Phuket; r=0.89, P=0.11 (D). And correlation between number of reported human and proportion of indirect ELISA positive cat sera in four locations: Bangkok-Pathum Thani; r=0.31, P=0.69 (E), Chon Buri; r=0.89, P=0.11 (F), Samut Sakhon; r=0.61, P=0.58 (G) and Phuket; r=0.064, P=0.94(H).
Figure 4.
Correlation between number of reported human and number of indirect ELISA positive cat sera in four locations: Bangkok-Pathum Thani; r=0.77, P=0.23 (A), Chon Buri; r=0.99, P=0.009 (B), Samut Sakhon; r=0.98, P=0.12 (C) and Phuket; r=0.89, P=0.11 (D). And correlation between number of reported human and proportion of indirect ELISA positive cat sera in four locations: Bangkok-Pathum Thani; r=0.31, P=0.69 (E), Chon Buri; r=0.89, P=0.11 (F), Samut Sakhon; r=0.61, P=0.58 (G) and Phuket; r=0.064, P=0.94(H).
Figure 5.
Numbers of true positive cat sera (cPASS) separated by waves of epidemic (A) and by provinces (C) and the proportions of true positive cat sera (cPASS) separated waves of epidemic (B) and by provinces (D).
Figure 5.
Numbers of true positive cat sera (cPASS) separated by waves of epidemic (A) and by provinces (C) and the proportions of true positive cat sera (cPASS) separated waves of epidemic (B) and by provinces (D).
Figure 6.
Correlation between number of reported human cases and number of true positive cat sera (cPASS) in four locations: Bangkok-Pathum Thani; ρ=0.80, P=0.20 (A), Chon Buri; r=0.59, P=0.41 (B), Samut Sakhon; r=0.94, P=0.22 (C) and Phuket; r=0.61, P=0.39 (D). And correlation between number of reported human cases and proportion of true positive cat sera (cPASS) in four locations: Bangkok-Pathum Thani; r=0.70, P=0.30 (E), Chon Buri; r=0.87, P=0.13 (F), Samut Sakhon; r=1, P=0.042 (G) and Phuket; ρ =0.74, P=0.26 (H). .
Figure 6.
Correlation between number of reported human cases and number of true positive cat sera (cPASS) in four locations: Bangkok-Pathum Thani; ρ=0.80, P=0.20 (A), Chon Buri; r=0.59, P=0.41 (B), Samut Sakhon; r=0.94, P=0.22 (C) and Phuket; r=0.61, P=0.39 (D). And correlation between number of reported human cases and proportion of true positive cat sera (cPASS) in four locations: Bangkok-Pathum Thani; r=0.70, P=0.30 (E), Chon Buri; r=0.87, P=0.13 (F), Samut Sakhon; r=1, P=0.042 (G) and Phuket; ρ =0.74, P=0.26 (H). .
Table 1.
Distribution of the indirect ELISA positive cat sera against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) separated by the waves of epidemic in human population in 4 provinces of Thailand from December 2020 to March 2022.
Table 1.
Distribution of the indirect ELISA positive cat sera against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) separated by the waves of epidemic in human population in 4 provinces of Thailand from December 2020 to March 2022.
Location |
Wave |
No. of reported human cases |
No. of cat sera |
No. of positive ELISA |
Proportion of positive sample (%) by ELISA |
No. of positive cPass test |
Proportion of positive sample (%) by cPass test |
| Bangkok-Pathum Thani |
1st
|
1,336 |
|
|
|
|
|
| 2nd
|
2,943 |
16 |
2 |
12.50 |
0 |
0.00 |
| 3rd
|
125,788 |
46 |
7 |
15.22 |
1 |
2.17 |
| 4th
|
381,766 |
150 |
36 |
24.00 |
6 |
4 |
| 5th
|
233,372 |
1 |
0 |
0.00 |
0 |
0.00 |
| Total |
745,205 |
213 |
45 |
21.13 |
7 |
3.29 |
| Chon Buri |
1st
|
71 |
|
|
|
|
|
| 2nd
|
662 |
5 |
0 |
0.00 |
0 |
0.00 |
| 3rd
|
11,254 |
4 |
0 |
0.00 |
0 |
0.00 |
| 4th
|
102,794 |
48 |
19 |
39.58 |
1 |
2.08 |
| 5th
|
80,052 |
146 |
17 |
11.64 |
5 |
3.42 |
| Total |
194,833 |
203 |
36 |
17.73 |
6 |
2.96 |
| Samut Sakhon |
1st
|
14 |
|
|
|
|
|
| 2nd
|
17,109 |
|
|
|
|
|
| 3rd
|
12,407 |
8 |
1 |
12.50 |
0 |
0.00 |
| 4th
|
82,921 |
212 |
55 |
25.94 |
15 |
7.08 |
| 5th
|
40,194 |
42 |
13 |
30.95 |
1 |
2.38 |
| Total |
152,645 |
262 |
69 |
26.34 |
16 |
6.11 |
| Phuket |
1st
|
209 |
|
|
|
|
|
| 2nd
|
3 |
3 |
0 |
0.00 |
0 |
0.00 |
| 3rd
|
769 |
23 |
10 |
43.48 |
0 |
0 |
| 4th
|
18,164 |
47 |
8 |
17.02 |
4 |
8.51 |
| 5th
|
38,414 |
127 |
31 |
24.41 |
2 |
1.57 |
| Total |
57,559 |
200 |
49 |
24.5 |
6 |
3.00 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).