1. Introduction
In 2016, the City of Fairmont, a farming community in southwestern of Minnesota, was forced to issue a public health notice that the city's drinking water supply exceeded Environmental Protection Agency's (EPA) maximum NO
3-N contaminant level of 10 mg/L [
1]. High NO
3-N in drinking water causes harmful effects, one of the most severe beings methemoglobinemia, which can be toxic to infants [
2]. Fairmont uses a groundwater and surface water blending and mechanical treatment to mitigate high NO
3-N spikes, but the capacity of these tools was considered limited because Fairmont’s primary source of drinking water comes from a chain of lakes fed by agricultural watersheds [
1,
3]. Dutch Creek is one of the largest tributaries to the Fairmont chain of lakes with the watershed covering over 4091-Ha and contributing significantly to Fairmont's NO
3-N levels in its drinking water supply [
1]. Data collected since 2000 shows periodic high NO
3-N events in Dutch Creek which peaked three times over the EPA maximum contaminant levels [
3]. Warmer months of the year provide natural NO
3-N removal processes in the lakes and help prevent NO
3-N from reaching the city's drinking water intake. However, in the spring months (March, April and May), when NO
3-N is more readily flushed though the soil profile into tile-drains and though agricultural watersheds, the natural limnologic removal mechanisms are ineffective [
1,
4,
5]. Soil water assessment tool (SWAT) modeling was done and showed seasonal variation in NO
3-N levels. SWAT model results provided more detail suggesting that both spring flushing and summer removal mechanisms were underestimated for the Dutch Creek watershed. This knowledge reinforced the need to adapt NO
3-N removal technologies to help the City of Fairmont achieve safe drinking water.
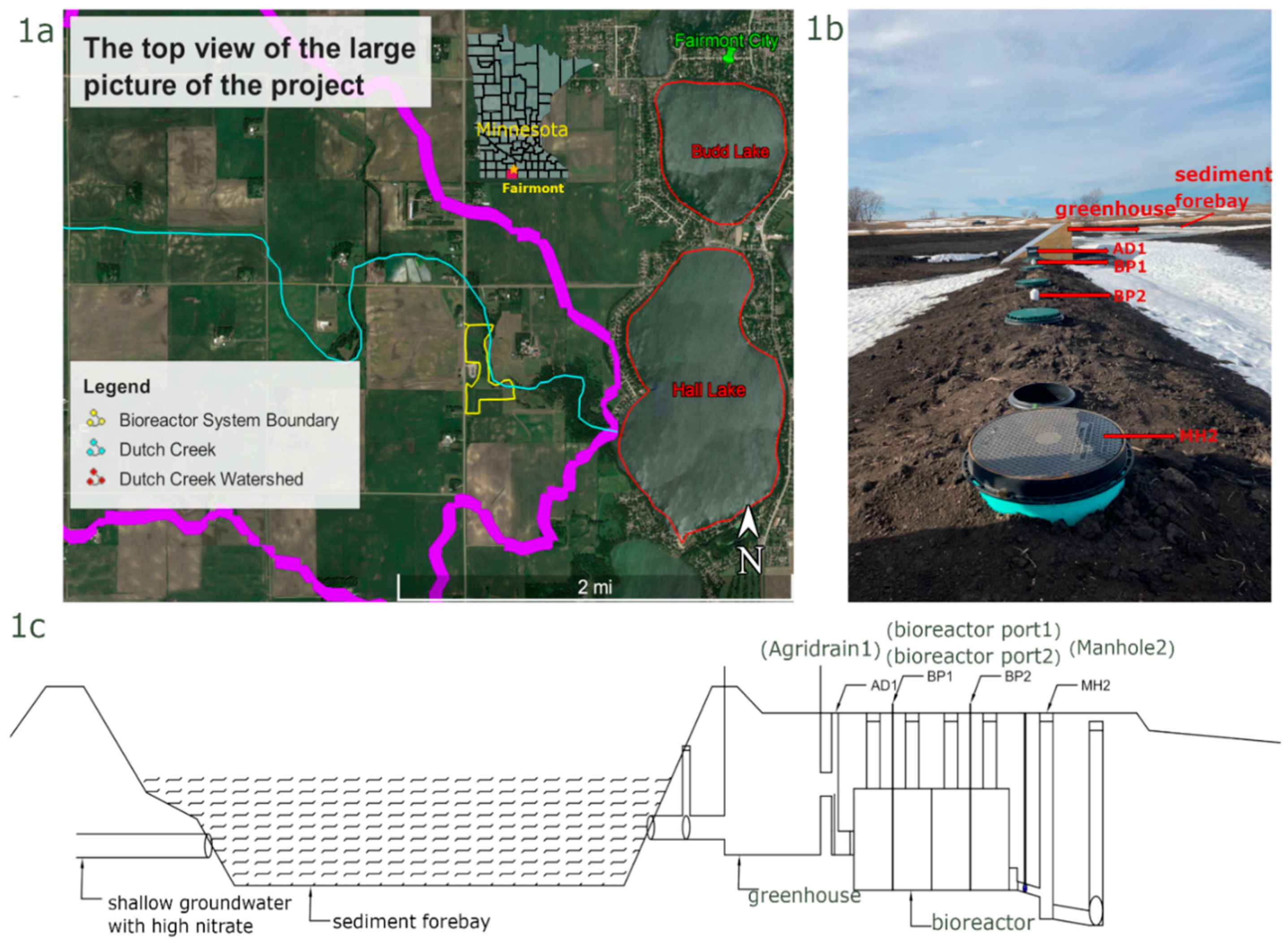
Funding from the Clean Water Act (CWA) Section 319 and the Minnesota Board of Water and Soil Resources (BWSR) was provided to the Martin County Soil and Water Conservation District (SWCD) to implement best management practices (BMP’s) in the Dutch Creek watershed to lower the NO
3-N load to Budd Lake (
Figure 1a). A state BWSR grant of
$86,000 was provided to the SWCD to implement Agricultural BMPs along with
$25,000 of Federal CWA Section 319 Funds. A portion of the funds were used to install two woodchip bioreactors and two saturated buffers in the Fairmont Chain of Lakes/Dutch Creek Watershed to reduce the nitrogen load by 1375 kg/year [
6]. The City of Fairmont was further encouraged to seek Environmental Natural Resources Trust Fund (ENRTF) money via the Legislative Citizens Committee on Minnesota Resources (LCCMR) [
1]. The city was successful in obtaining a grant for
$190,000. The funding was focused on building a novel woodchip bioreactor above the confluence of Budd Lake. Though other NO
3-N BMPS were in the process of being installed in the Dutch Creek watershed the design, construction and performance of the novel greenhouse nitrogen treatment system is the focus of this paper.
A novel denitrifying woodchip bioreactor was built to treat high NO
3-N in Dutch Creek. Denitrifying bioreactors have become common in treating high NO
3-N in a variety of environments including groundwater, agricultural tile drainage, and wastewater treatment [
2,
7,
8]. Solid carbon-rich media is placed in the path of contaminated water to allow facultative anaerobic bacteria to use an electron from carbon and reduce nitrate to nitrogen gas [
8,
9,
10]. Woodchips are commonly used as a carbon source because they decay very slowly, lasting decades, and they provide an ideal growth media for the bacteria [
8,
10]. Fairmont requires denitrification the most during the spring snowmelt when temperatures are near freezing. Denitrification has been shown to occur in bioreactors at temperatures as low as 1-5
oC, but decreased rates are a major concern [
8] for typical woodchip bioreactor performance. Desired denitrification rates begin to occur above 6
oC [
8,
11]. To meet these temperature requirements, a greenhouse pool system was added in the NO
3-N removal system to passively warm water before the denitrification woodchip chamber [
12]. Additionally, a sediment forebay was constructed in front of the greenhouse to prevent sediment from entering the treatment system.
In this study, several water quality parameters were monitored and collected. The desired performance of this designed novel denitrifying woodchip bioreactor would be more biological activity in March and April compared to a standard woodchip bioreactor with typically very cold water from the Spring soil thaw. Intensively managed row-crop land will leach NO
3-N from the soil profile [
2,
3]. NO
3-N treatment is required to obtain sustainable crop growth yet meet the ecosystem service demands of the public. This objective can be exceedingly difficult in a cold climate, therefore new novel ideas must be tested to meet cold water NO
3-N reduction.
2. Materials and Methods
In this bioreactor system, the drainage water from a tributary of Dutch Creek first drains into a forebay to drop sediment, and then drains into a greenhouse and ultimately flows into the bioreactor (
Figure 1b,c).
The bioreactor system was monitored for temperature distribution, water depth, flow, nitrate, sulfate, dissolved organic carbon, and general water quality parameters (TSS, DO, and pH) starting in early March of 2022 until June 29, 2023. Monitoring was stopped in August 2022 when there had been no flow for a month and then began in March of 2023. The first two to three months were primarily dedicated to system (microbial) adjustment, and the test results onNO3-N removal may not be that accurate.
Temperature
Temperature probes (Hach MX Pendant) were placed in 10 locations: Agridrain 2 (AD2, represents inlet water which is challenging to display in the
Figure 1) before the greenhouse, greenhouse air and water, Agridrain 1 (AD1) in the influent of the bioreactor, the bottom of each bioreactor port and 0.91 m off the bottom of each bioreactor port, Manhole 2 (MH2) in the effluent of the bioreactor, and one hung outside of AD1. Temperatures were logged at 30 minutes intervals and downloaded once a month. The AD2 temperature probe stopped recording data on April 17, 2023.
Water Depth
Pressure transducers (Hobo and Solinst) were placed at the bottom of 4 locations in the bioreactor system: the greenhouse, AD1 in the influent, the first bioreactor port (BP1) and MH2 in the effluent. A barologger was also placed outside of AD1. Water pressures were logged at 15 minutes intervals and downloaded once a month. The pressures were compensated with barologger pressures to obtain water depths. Water depths were also measured periodically with a water level meter.
Flow Rates
Flow rates were measured approximately once a month by timing how quickly a container with known volume filled with water at the effluent pipe. The flow rate was controlled in the range of 0.28-L/s-to-1.42-L/s followed by the instruction from the designer to ensure that water had enough time to complete the denitrification process in the bioreactor.
Water Quality
Water quality field parameters were directly measured by a YSI Sonde and Hach Nitratax probe every 15 minutes and indirectly by grab samples. A YSI Sonde with temperature, dissolved oxygen and conductivity probes was deployed first in AD1 to catch the initial water quality of the spring flush and then at BP1 to look at the micro-climate inside the reactor. Periodically, manual measurements were taken at the second bioreactor port (BP2) and for profiles inside the bioreactor at 0.30-m intervals.
A Nitratax nitrate probe was installed in the influent, AD1, in March 2022 and a second probe was installed in the effluent, MH2, at the end of April 2022. Nitrate probes were installed in the influent and effluent in March of 2023. Nitrate readings were recorded every 15 minutes. Two solar panels were installed to power the Nitratax controller and probes. The Nitratax controller, solar panel controller and marine batteries were stored inside a job box at the site. Grab samples were taken twice a week (March through May) and once a week in July of 2022. In 2023 grab samples were taken twice a week March through mid-June. Grab samples were sent to Minnesota Valley Testing Laboratory for nitrate, sulfate, total volatile and suspended solids and dissolved organic carbon.
The measured water quality parameters that were used in this study are summarized in
Table 1. In the following analysis, it is evident that data is missing on certain days due to various factors, such as equipment failures, but it has minimal impact on the overall analysis.
3. Results
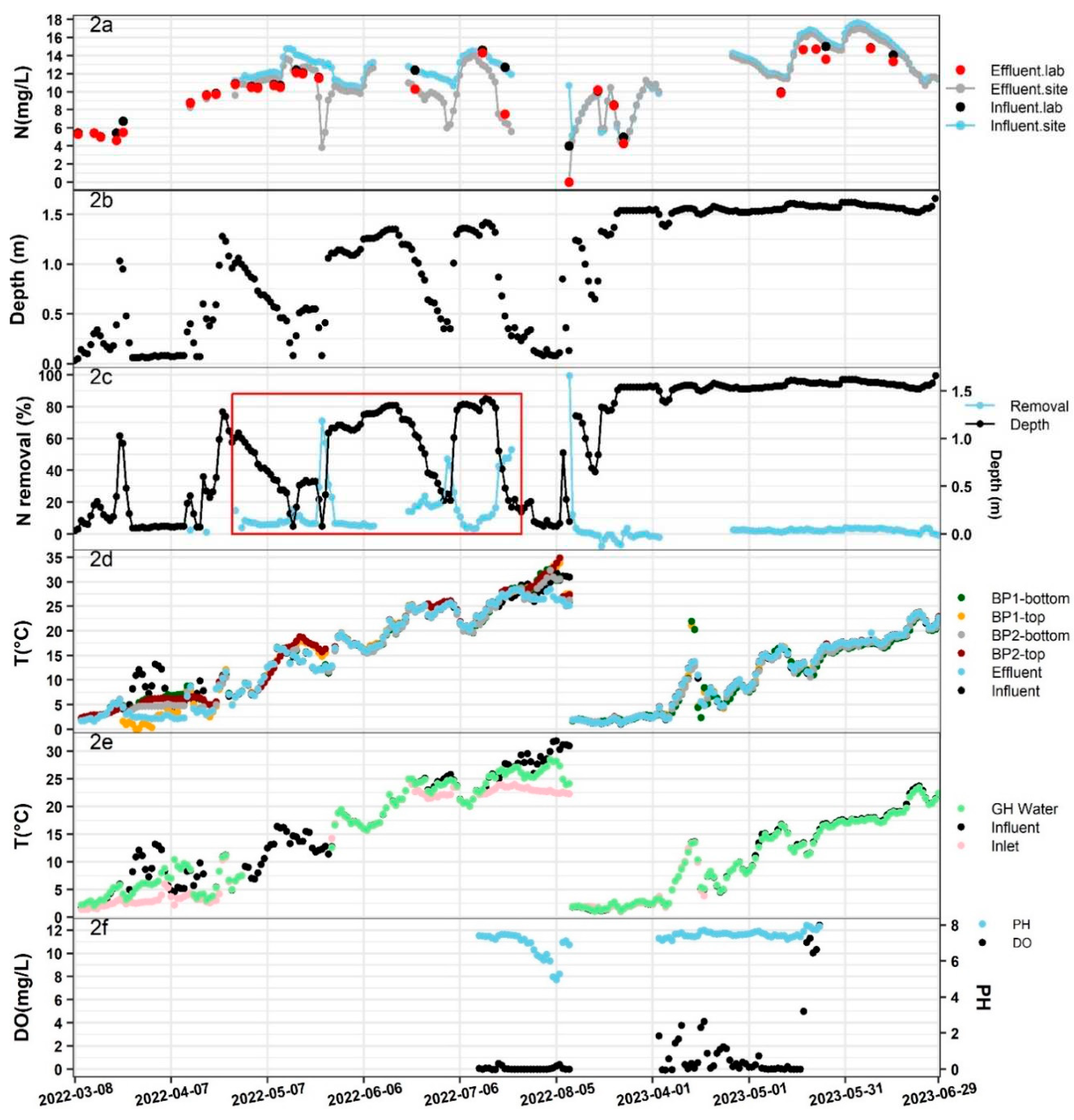
Figure 2a shows the N measurements, with two dash lines representing the N measured at the influent and effluent using the N probe, and two colors of dots representing the N measured at the influent and effluent from the lab. Based on the results, we observed that the N levels increased as the weather got warmer, and the readings from N probe accurately reflected the N removal in the bioreactor during these two years, except for the first two months of adjustment. This was evident as the N difference between influent and effluent remained consistent when using both the probe and lab test (
Figure 2a). Unfortunately, the bioreactor did not function properly in 2023. This could be seen from
Table 2, where the p-value from a single factor ANOVA test indicated no statistical difference in N levels between influent and effluent for the year 2023. The bioreactor only operated properly during the year 2022, as evidenced by a p-value less than 0.05.
Figure 2b shows the water depth in the bioreactor, which is designed as a cylinder with a 1.52 m diameter. The water depth fluctuated in the year 2022 while it remained full for most of the year 2023. During the operational period in the year 2022, the red box in the figure indicates that moments of high denitrification efficiency often corresponded to lower water depth (
Figure 2c). Surprisingly, when the bioreactor exhibited excellent performance with N removal efficiency greater than 40% between May and August, especially from July to August when the system reached a stable state, the corresponding water depth decreased significantly to less than 0.6 m (
Figure 2c).
To investigate the operating performance of the bioreactor, T, pH, and DO, which are the three most important indicators to assess a bioreactor’s performance were studied [
10]. It’s obvious that the T increased as the weather got warmer (
Figure 2d). With the assistance of the greenhouse, the temperature of the inlet water was significantly increased, especially during cold days in 2022. However, in the year 2023, the greenhouse no longer functioned, resulting in no improvement in the inlet temperature (
Figure 2e). This is one of the reasons why N removal was not obvious in the year 2023. From a single-factor ANOVA test on T across the bioreactor, the p-value was greater than 0.05, suggesting there was no significant statistical difference in T across the entire system (
Table 3), as also evidenced in
Figure 2d. Typically, denitrification occurs optimally within the T range between of 15°C-35°C [
13], and given the T increased from 15°C to 35°C between July and August 2022 (
Figure 2d), it is one of the reasons why a higher N removal efficiency was observed during this period.
Regarding pH, it remained relatively constant throughout 2023 with a slight decrease observed from July to August 2022 (
Figure 2f). It is essential to maintain pH being not too high or low, as extremes in pH can negatively impact the bioreactor’s performance [
14]. During bioreactor operation, pH tends to slightly decrease due to fermentation on TOC production [
15]. The DO levels measured at the bottom of the bioreactor were around 0 mg/L from July to August 2022, and they were higher for most of 2023 (
Figure 2f). DO levels above 0.2mg/L was reported to inhibit the denitrification process, as denitrification requires an anaerobic environment [
10]. From
Table 4, we notice a considerable difference in DO levels between the two periods (the only available data we have) in 2022 and 2023. The extremely low DO levels are another significant factor contributing to the substantial improvement in NO
3-N removal observed from July to August 2022.
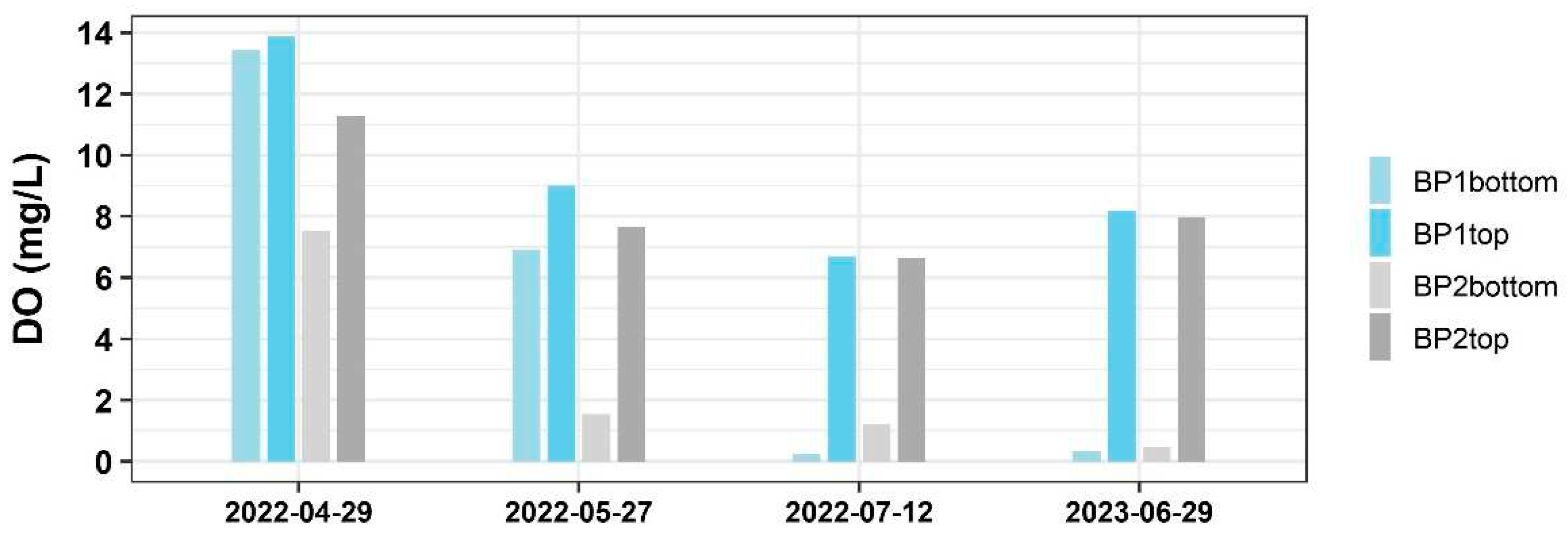
Based on the above findings, the bioreactor showed a good performance between July and August 2022. During this period when the water depth was significantly low, T fell within the optimum range for denitrification to occur and DO levels were low enough to create the anaerobic environment suitable for denitrifying bacteria to thrive. To investigate whether DO exhibits the constant behavior across the bioreactor horizontally and vertically just as T does, several DO measurements were taken at different locations: BP1 top/bottom and BP2 top/bottom. Interestingly, the result shows that unlike other woodchip bioreactors, where DO decreases along the length of the bioreactor [
16], this bioreactor could exhibit a significant vertical stratification of DO, with high DO levels at the top and low DO levels at the bottom (
Figure 3).
This stratification creates an anaerobic zone at the bottom which favors denitrification [
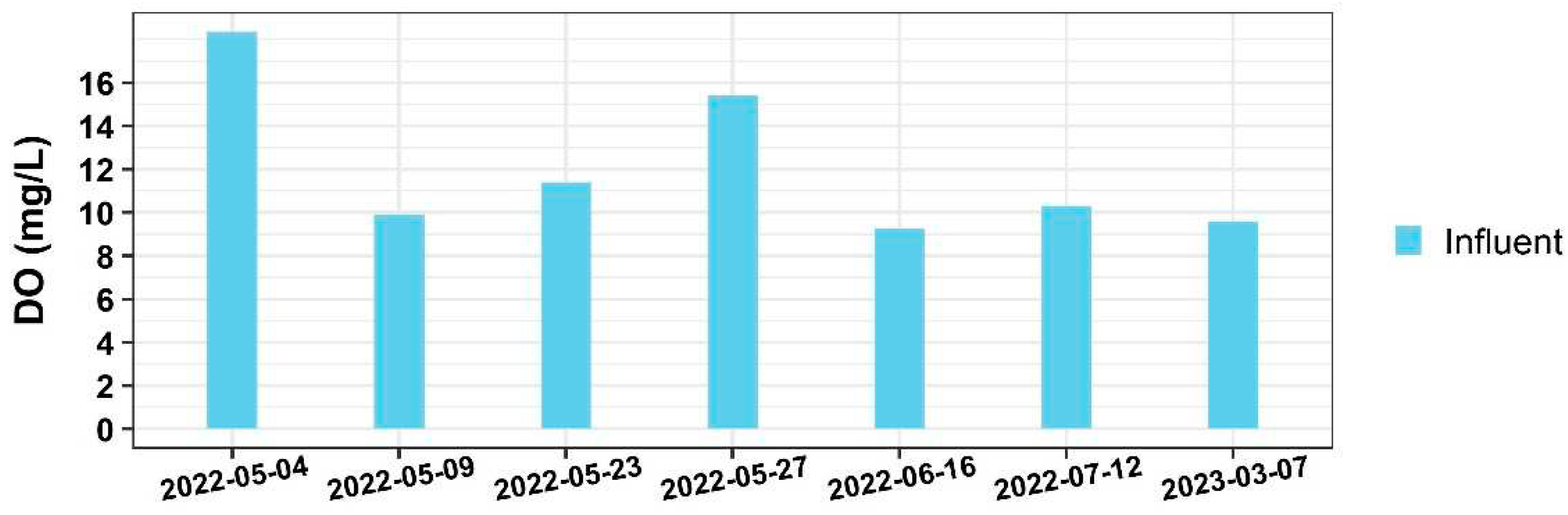
17]. As a result, denitrification does not occur in other parts of the bioreactor besides the bottom zone. The reason for the stratification of DO might be attributed to the presence of algae observed in the bioreactor. With an extra forebay and greenhouse constructed in front of the bioreactor, the incoming water from the shallow groundwater mixed with surface rainfall-runoff, introducing a substantial number of algae in the influent. Typically, the saturated DO levels in a water body ranges between 7mg/l-10mg/l. As depicted in
Figure 4, oversaturated DO caused by algae photosynthesis was observed at the influent.
With the influent entering 0.3-m from the bottom of the bioreactor, algae tend to concentrate at higher levels and physically inhibit oxygen penetration, therefore causing the variable DO distribution vertically. On the other hand, the settling of dead algae tends to deplete DO, causing lower DO levels in the bottom zone [
18]. During July and August 2022, when the water depth was extremely low, less surface water flowed into the bioreactor at a low velocity with low DO and reduced the flux of algae. Additionally, the lower water facilitated faster carbon mineralization of the woodchips by denitrifying bacteria [
19]. Moreover,
Figure 3 reveals variations in DO between BP1 and BP2, indicating that N removal varies along the length of the bioreactor [
20].
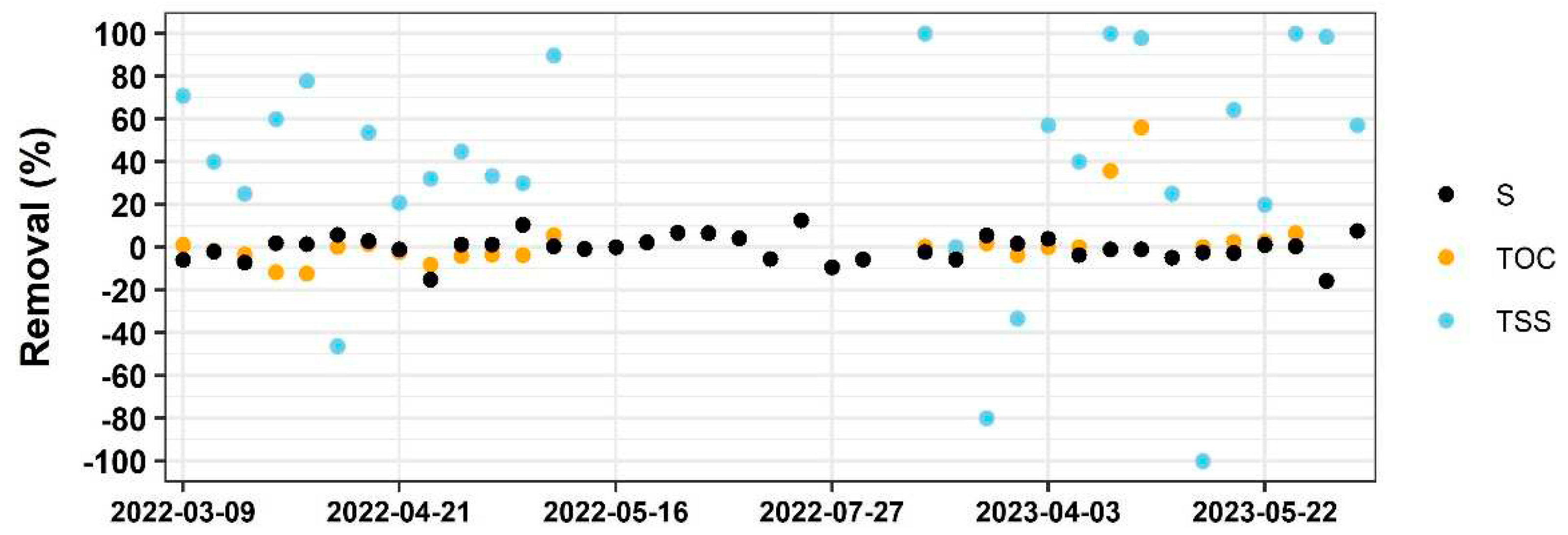
In addition to measuring T, pH, and DO, other chemical properties such as TOC, TSS, and S were also evaluated in both the influent and the effluent to gain a better understanding of the bioreactor’s performance (
Figure 5).
The results show that the removal efficiency of TSS was obvious, which aligns with findings from other studies indicating that the bioreactor is efficient in filtering TSS [
21]. However, unlike other bioreactors, which release a significant amount of TOC from the woodchip [
22], the changes in TOC and S concentration between the influent and effluent were not substantial. Moreover, the dynamic behavior of the bioreactor leads to fluctuation in these three parameters which are challenging to explain.
Author Contributions
Conceptualization, J.M.; methodology, J.M. and K.H.; software, L.M.; validation, J.M., K.H. and L.M.; formal analysis, L.M. and K.H.; investigation, J.M. and K.H.; resources, K.H.; data curation, K.M and L.M.; writing—original draft preparation, L.X. and K.H.; writing—review and editing, J.M.; visualization, L.M. and K.H.; supervision, J.M.; project administration, J.M..; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.