1. Introduction
Sarin (GB, methylphosphonic difluoride) is a representative chemical weapon potent, which is an organophosphorus (OP) neurotoxic agent with high volatility, strong toxicity, and short latency period, this nerve agent can be obtained easily with characteristics of easy synthesis and difficulty in prevention and control [
1,
2]. Through high specificity and affinity of acetylcholinesterase, GB poses a great threat to human health and social public safety [
1,
2,
3]. Therefore, effective detection methods can qualitatively and quantitatively detect GB, improving protection capabilities.
Hitherto, various gas sensing techniques have been developed for GB detection. For instance, field-effect transistors [
4], fluorescence [
5], flame photometry [
6], ion mobility spectrometry [
7], gas chromatography-mass spectrometry [
8], and surface acoustic wave (SAW) [
9], each technique has special advantages and plays unique role in GB detection. Wherein, SAW technique has been systematically and deeply studied in the detection of chemical warfare agents (CWAs), mainly due to its non-destructive nature, compact structure, detectability of nerve agents and blister agents, and ability to perform point or area detection [
10,
11,
12,
13,
14,
15]. SAW sensor has compact structure, small size, high sensitivity, low cost, and fast response, which are in line with current development direction of intelligence in the field of chemical sensors, has becoming a research hotspot in this field [
13,
14,
15,
16]. So far, SAW sensors for detecting various gases such as H
2, SO
2, H
2S, and NO
2 have been developed and have achieved remarkable result [
16,
17], and sensitive film material plays a decisive role in detection effect [
16]. And among various sensitive film materials, polymers are the most commonly used in SAW sensors [
18]. As one of the sensitive film materials, SXFA is an organosilicon compound with special structure and properties [
13,
14,
20]. Its structural unit has a hexafluoroisopropyl (HFIP) functional group, which has strong hydrogen bonding effect on organophosphorus compounds [
14,
19,
20,
21]. Thus, it has superior sensitivity and selectivity in the detection of organophosphorus compounds, and so far, it is one of the most widely studied, and data-rich polymers [
13,
14,
19,
23,
24,
25]. However, after the synthesis of SXFA, SAW-SXFA sensors generally only exists as a type of sensor for detecting hydrogen bonding alkaline gases in sensor arrays, and there are few reports on detection organophosphorus gases individually.
Therefore, in order to individual detect GB with high sensitivity, we synthesized polymer SXFA and construct a SAW-SXFA sensor in this study, discussed its relevant mechanism, analyzed its detection of organophosphorus nerve agents, and evaluated its practical performance.
2. Materials and Methods
2.1. Reagents and Instruments
Allydichloromethylsilane, 95%, Macklin; phenyltrimethylammonium, 20~25% formaldehyde solution, Tokyo Kasel Kogyo Co. LTD; hexafluoroacetone trihydrate (HFA·3H2O), 95%, Macklin; Polyepoxypropyl chloride, average Mw ~700, Macklin; ether, 99.9%, TEDIA; sulfuric acid, AR, Beijing Chemical Plant; magnesium Sulfate, AR, Macklin; toluene, AR, aladdin; ethanol, ≥99.7%, exploration; Dry Ice, Yojanbio; DMMP, AR, Beijing Chemical Plant; GB, 99%, State Key Laboratory of NBC Protection for Civilian.
FTS-185 infrared spectrometer, Bio-Rad, USA; Q100 modulated DSC, Thermal Analysis, USA; Gel Permeation Chromatography (GPC), Waters, USA; Surface acoustic wave oscillator: with central oscillation frequency of 300MHz, delay line surface is quartz layer; Frequency counter, Proteck C3100, South Korea, equipped with RS232 interface for computer connection;Scanning Potentiometer, TSM-6460 series, USA; Dynamic Gas Generator, State Key Laboratory of NBC Protection for Civilian.
2.2. Experimental Methods
2.2.1. Mechanism of Interaction Between Polymer and Gas Molecules
SAW gas sensor utilize sensitive film materials on piezoelectric crystals to generate characteristic responses for gas adsorption, and polymers are commonly used as sensitive film materials on it [
26,
27]. Usually, polymers need to have the following characteristics: (1) non-volatile, which can enable polymers to remain stable on sensors for a long time; (2) Viscoelasticity, which allows gas disperse quickly within the polymer film; (3) Quick response capability, selectivity, recoverability, and has the ability to deploy on sensor surfaces [
28]. Polymer sensitive film materials are mostly composed of polysiloxane as the main chain, which exhibits a viscoelastic state at room temperature and has good adsorption capacity for gas [
29,
30]. Before selecting polymer film materials, it is necessary to analyze principles of interaction between polymer films and gas molecules. Generally, the main interactions between gas molecules and polymer films are van der Waals forces, polarization, and hydrogen bonding [
29,
30].
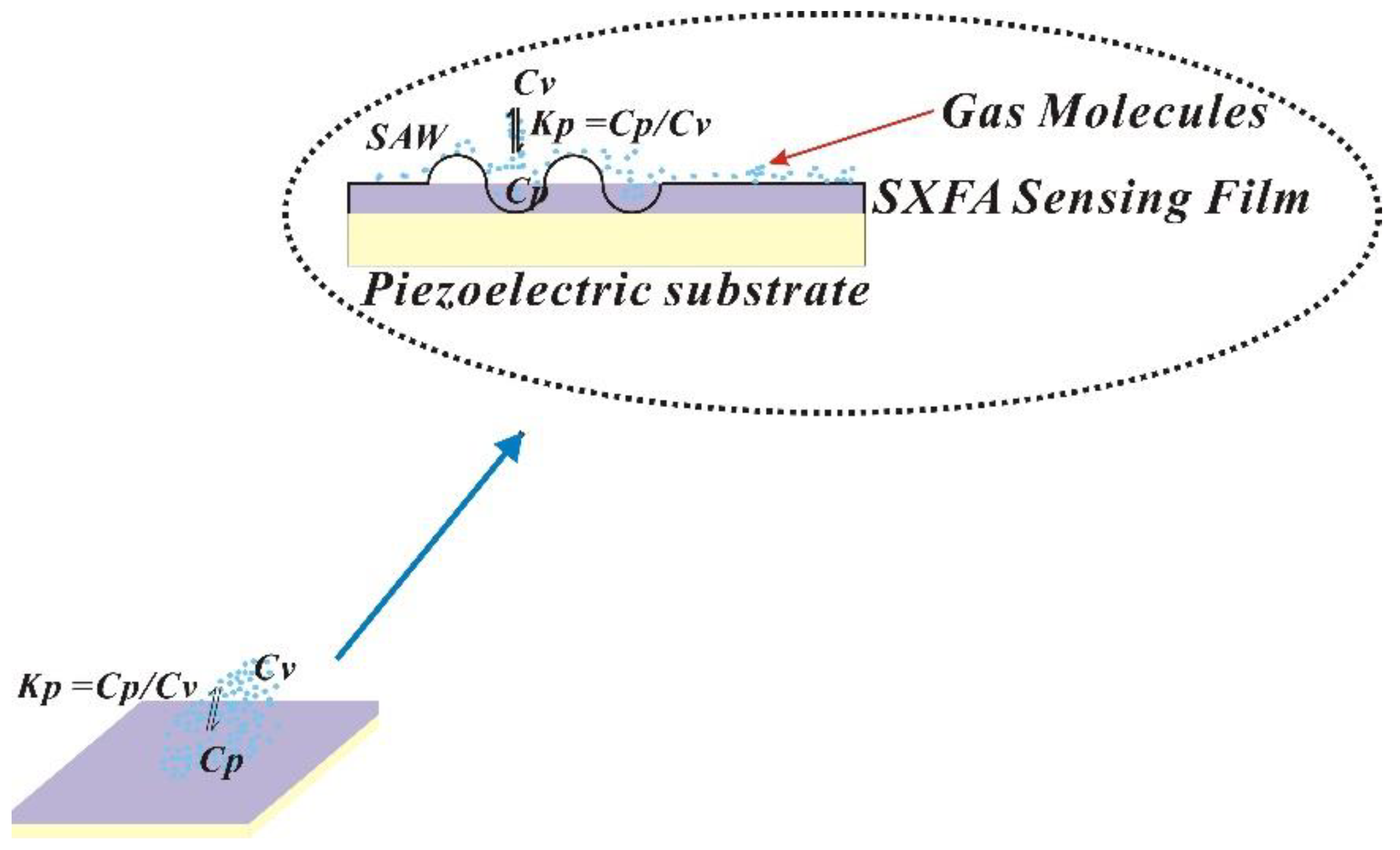
The process of polymer adsorption of gas is similar to that gas dissolution into liquid (
Figure 1). At the interface between polymer film surface and gas phase, target molecules are distributed between gas phase and polymer phase, reaching a thermodynamic equilibrium state. Many previous studies have investigated the distribution equilibrium between adsorbate and stationary phase, and proposed relevant models [
24,
25]. The selective adsorption of gas-phase molecules by polymers and the equilibrium between target molecules in gas phase and polymer phase can be expressed by the following formula:
Where Kp represents the equilibrium of gas entering polymer phase from gas phase, Cv represents the concentration of target molecule in gas phase, and Cp represents the concentration of target molecule in liquid phase. In this model, Kp quantitatively describes the equilibrium of gas entering polymer phase from gas phase, and a larger Kp value represents a stronger gas adsorption capacity.
To determine
Kp value, Grate et al. proposed the linear solvation energy relationship (LSERs) as a model for selective adsorption of gases, this model is established by using gas as solute and polymer as solvent [
31]. The solubility of a gas is expressed by a series of parameters, with LESR representing a linear combination of all forces, and the liner relationship are list as following:
Among them, dissolution parameters
R2, π 2H, α 2H, β 2H and
LogL16 represent various solubility of gases;
R2 refers to gas hyper molar regression parameter, which quantitatively represents
n and
p electrons that play a polarization role;
π 2H represents gas dipole or polarization parameter,
α 2H and
β 2H represents parameters of gas hydrogen bonding acid and hydrogen bonding base, respectively;
LogL16 represents distribution coefficient of solute between gas and liquid phases at 25 ℃ (obtained from gas-liquid chromatography), and represents van der Waals force of gas;
r,
s,
a,
b, and
l are related to the properties of polymer films;
a and
b, as supplements to gas hydrogen bonding acidity and hydrogen bonding alkalinity, represent hydrogen bonding acidity and hydrogen bonding alkalinity of polymer films;
s represents the polarity and dipole effect of polymer films;
l represents dispersion effect of polymer films, and a larger value of
l indicates a significant difference in the distribution coefficients of similar gases;
r represents polarization ability of
n and
π electron pairs between polymer phase and solute molecules;
C is a constant [
32].
According to LSER equation, interaction between a certain gas and polymer film can be calculated [
33]. Polymers should have sensitivity and selectivity towards target gas. Additionally, LSER's polynomial coefficients such as b/a, b/s, s/a, and l/(s+a+b) can also represent selectivity [
34], and
Table 1 presents polymer SXFA dissolution selectivity obtained based on the LSER coefficient.
It is clear that for hydrogen bonded alkaline gases such as GB, the sought polymer should have the largest possible hydrogen bonding acid (b) while having the smallest possible hydrogen bonding alkalinity (a) and dipole polarity (s), represented by b/a and b/s, respectively. As shown in
Table 1, it is clearly that with strong hydrogen bonding acidity, polymer SXFA solubility values of b/a, b/s, s/a, l/(s+a+b) and dispersibility are 6.07, 7.08, 1.17, 0.86, 5.55 and 0.13, making it an ideal hydrogen bonding acidic polymer sensitive membrane material for SAW sensors.
Additionally, SXFA is a polysiloxane film material with low glass transition temperature [
14]. The HFIP functional groups on SXFA have strong hydrogen bonding effects on organic phosphine gases and also exhibit the viscoelastic properties of polysiloxane at room temperature, enabling selective adsorption of organic phosphine compounds [
13,
14], which further proves that SXFA is an ideal organic phosphine adsorption material.
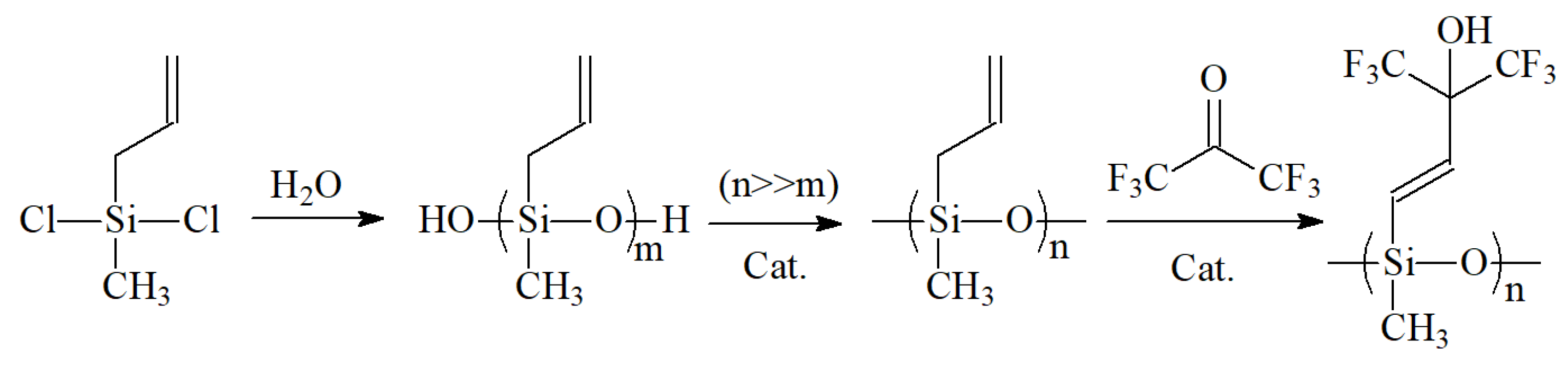
2.2.2. Synthesis Route of Hexafluoro-2-hydroxyisopropyl Polysiloxane
The synthesis of SXFA requires multiple steps and control of reaction conditions, so its synthesis method is relatively complex.
Figure 2 shows the synthesis steps of polymer SXFA. In order to ensure purity and quality of the final product, experimental operations need to be carried out with caution according to designed process. On the on hand, it should be noted that, in synthesis process, it is necessary to control reaction temperature, reaction time and other parameters. On the other hand, it should also pay attention to the synthesis process to avoid generations of impurities and by-products.
The first step in the synthesis of SXFA was to prepare methylvinyl polysiloxane by adding appropriate amount of ether and methylvinyl dichlorosilane, stirred with magnetic stirrer and dripped with distilled water till completely reacted at room temperature. Then, added appropriately amount of ether to extract the upper liquid, dried it overnight with MgSO4, filter it, and evaporated ether, then obtained siloxane. After siloxane was prepared, let it sit for 3 weeks, then added MgSO4 for drying (> 24 h), filtered it, evaporated the ether, and then added a small amount of formaldehyde solution of phenyl trimethylammonium hydroxide. Then, stirred at 400 K until the reaction completed, centrifuged to remove black suspension, and obtained polysiloxane. Finally, transferred the polysiloxane to a high-pressure resistant sealed tube and placed it in a dry ice-cold trap. Dry HFA·3H2O with H2SO4, then obtained HFA gas, and the HFA gas were recovered from the sealed tube in the cold trap. After the reaction was completed, heated the sealed tube at 380K (in a silicone oil bath) for 48 h. After naturally cooling, removed the liquid from the sealed tube, blow with N2 overnight to remove unreacted HFA, and finally, the final product hexafluoro-2-hydroxyisopropyl polysiloxane (SXFA) were obtained.
2.2.3. Preparation of SAW-SXFA Sensor and Its Detection of Organophosphine Agents
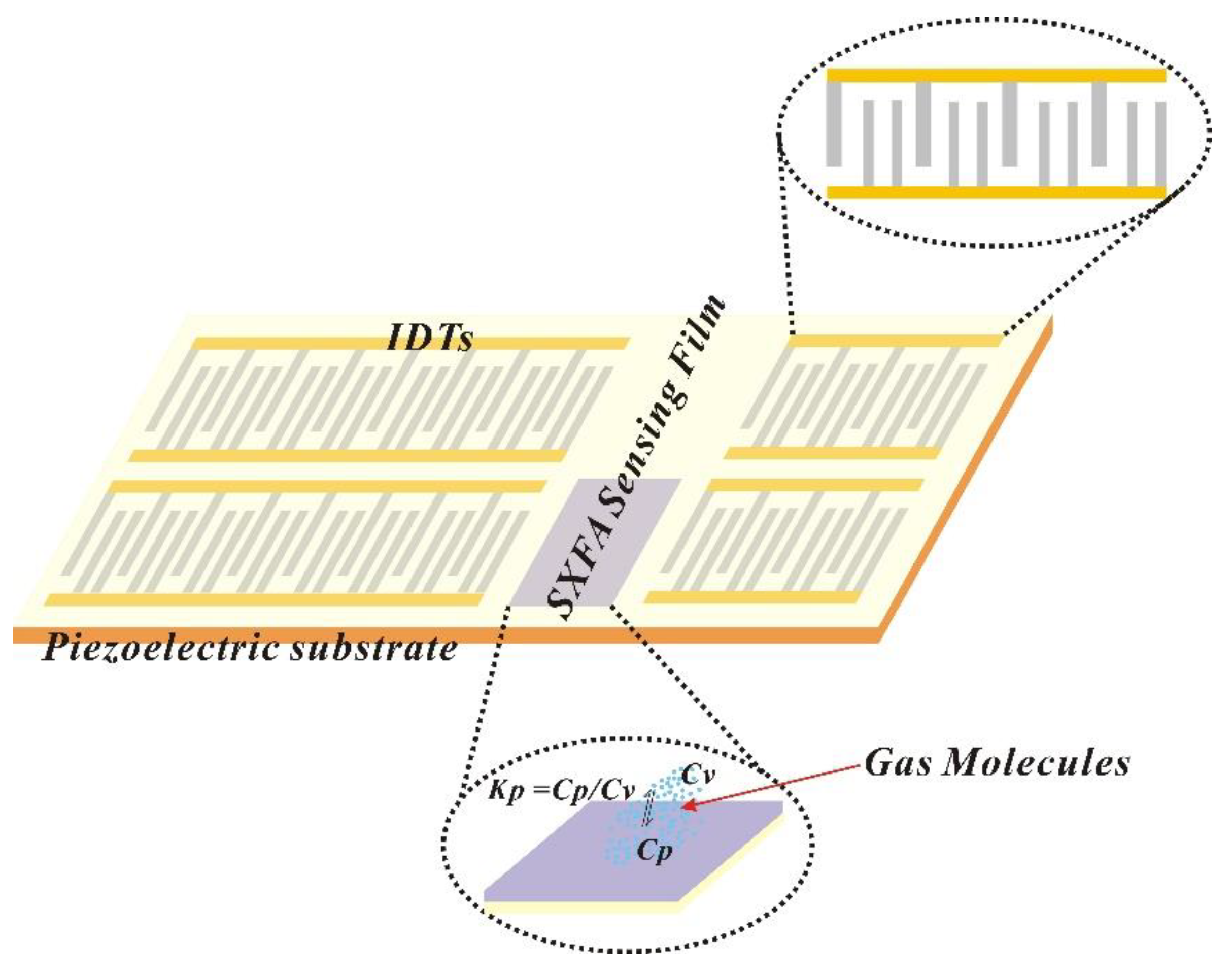
The SAW-SXFA sensor consists of an interdigital transducer (IDT), a piezoelectric substrate, and an SXFA gas-sensitive thin film (
Figure 3). Due to the piezoelectric effect of piezoelectric substrate, the input interdigital transducer converts the input electrical signal into an acoustic signal, while the output interdigital transducer converts the received acoustic signal into an electrical signal output. The SXFA films can adsorbs gas reversibly on the propagation path of surface acoustic waves, and the increase in its mass leads to a change in the propagation speed of surface acoustic waves. The detection of the gas is achieved by measuring its frequency or phase changes. In this research, we used a delayed linear SAW sensor device with a center frequency of 200 MHz, which was based on Y-shaped quartz cutting. And to obtain low loss and single frequency signals, applied unidirectional transducers (SPUDTs) and combed structures. In phase detector circuit, electrical signal emitted by signal source with a frequency of 200 MHz and electrical signal emitted by SAW-SXFA sensor were output through phase detector, and a voltage signal proportional to phase difference of the two signals was then connected to computer through a data transmission module.
The selectivity of film adsorption is not only related to its structure, but also to the morphology of itself. Therefore, in order to improve the separation ability of sensitive polymer film, preparation method of thin films can also be controlled. In this study, polymer SXFA films were prepared on sensor components using drop-coating method to complete the assembly of SXFA-SAW sensor and applied to detect organophosphine agent.
3. Results and Discussions
3.1. Infrared Spectroscopy Characterization and Analysis of SXFA Material
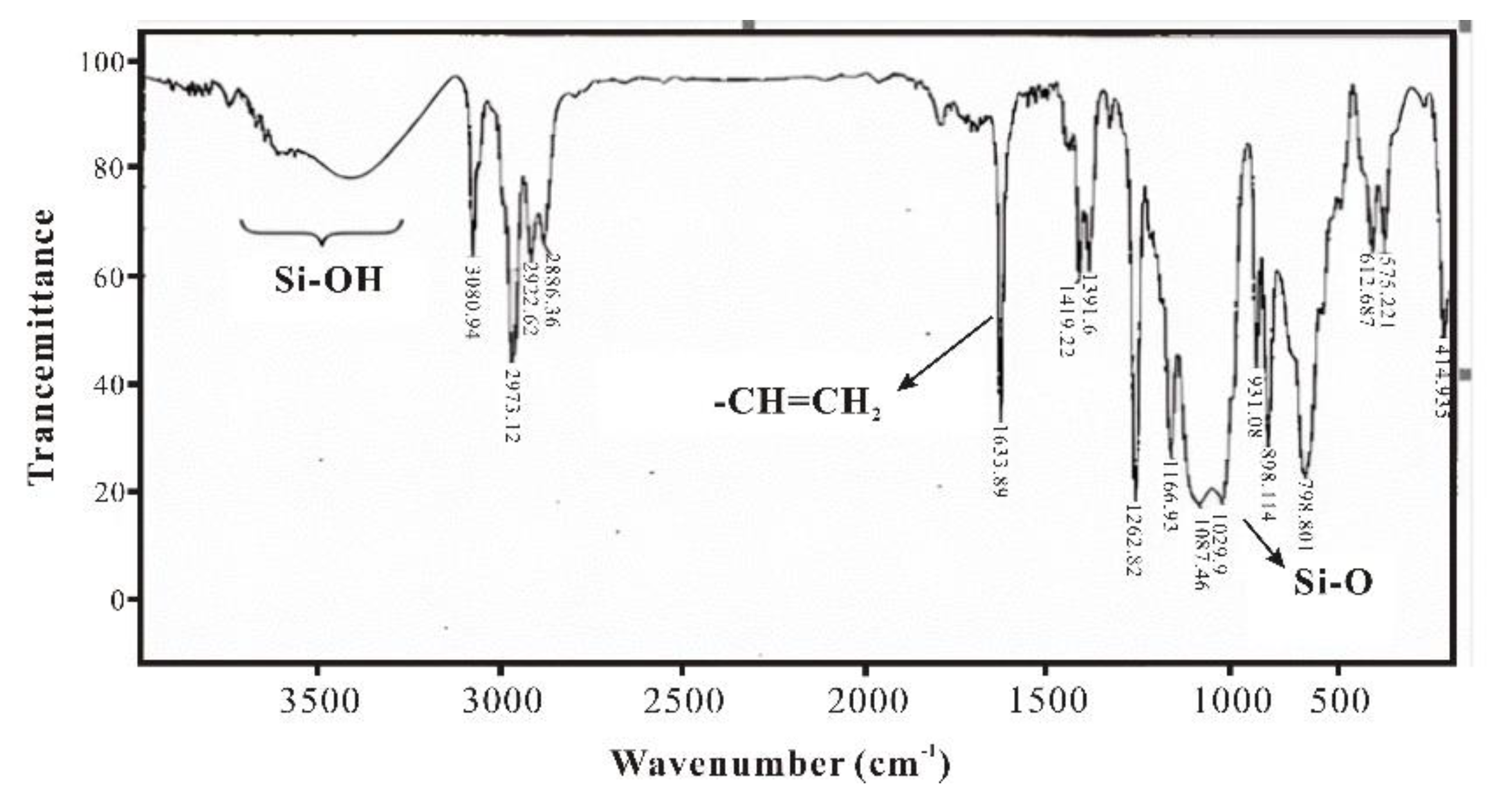
During the synthesis process of SXFA, organosilicon compounds have a strong absorption effect on infrared, and an obvious manifestation is they have strong absorption bands in the infrared absorption spectrum within the range of 1290~800cm-1. In this research, characteristic absorption peaks of the main groups in the synthesis process are Si-Me, Si-CH=CH2, Si-O, Si-O-Si, >SiCl2 and -CF3 (Si-Me located at ~1260 cm-1 and ~765 cm-1; Si-CH=CH2 located at 1613 cm-1, 1410~1390 cm-1, 1020~1000 cm-1, and 980~950 cm-1; Si-OH located at 3390~3200 cm-1 and 910~830 cm-1; Si-O located at 1100~1000 cm-1; Si-O-Si located at 1080 cm-1, 1025 cm-1, ~1020 cm-1, and ~1090 cm-1; >SiCl2 located at 595~535 cm-1; -CF3 located at 1350~1120 cm-1、780~680 cm-1、680~590 cm-1).
Figure 4 shows IR spectrum of methylvinyldichlorosilane, it is obvious that peak 1633 cm
-1 is stretching vibration of vinyl double bond and peak 1263 cm
-1 is symmetric deformation vibration of -CH
3. It can also observe that there is an Si-OH peak near 3500 cm
-1 and an Si-O peak at 1080~1025 cm
-1, which indicating partial methylvinyl dichlorosilane has undergone spontaneous hydrolysis and condensation into siloxane. Therefore, methylvinyl dichlorosilane should be stored in a dry environment, and during hydrolysis reaction, to ensure uniform polymerization of siloxane, it should first be dissolved in organic solvent and then mixed with a suitable amount of water.
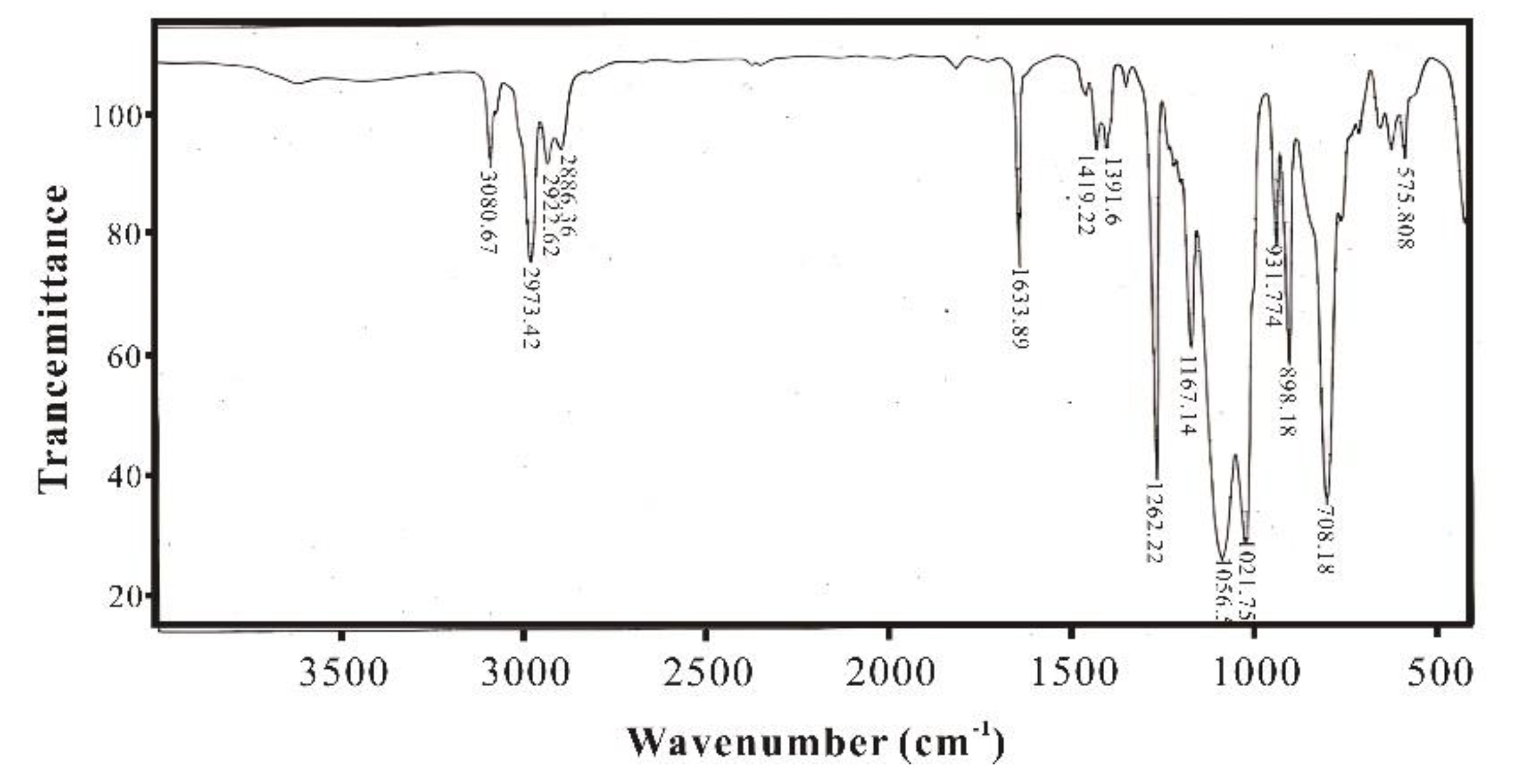
Figure 5 is the IR spectrum of methylvinylpolysiloxane, compared with
Figure 4, it can be found that the Si-OH peak near 3500 cm
-1 disappears, indicating that raw material has been completely hydrolyzed and condensed into siloxane.
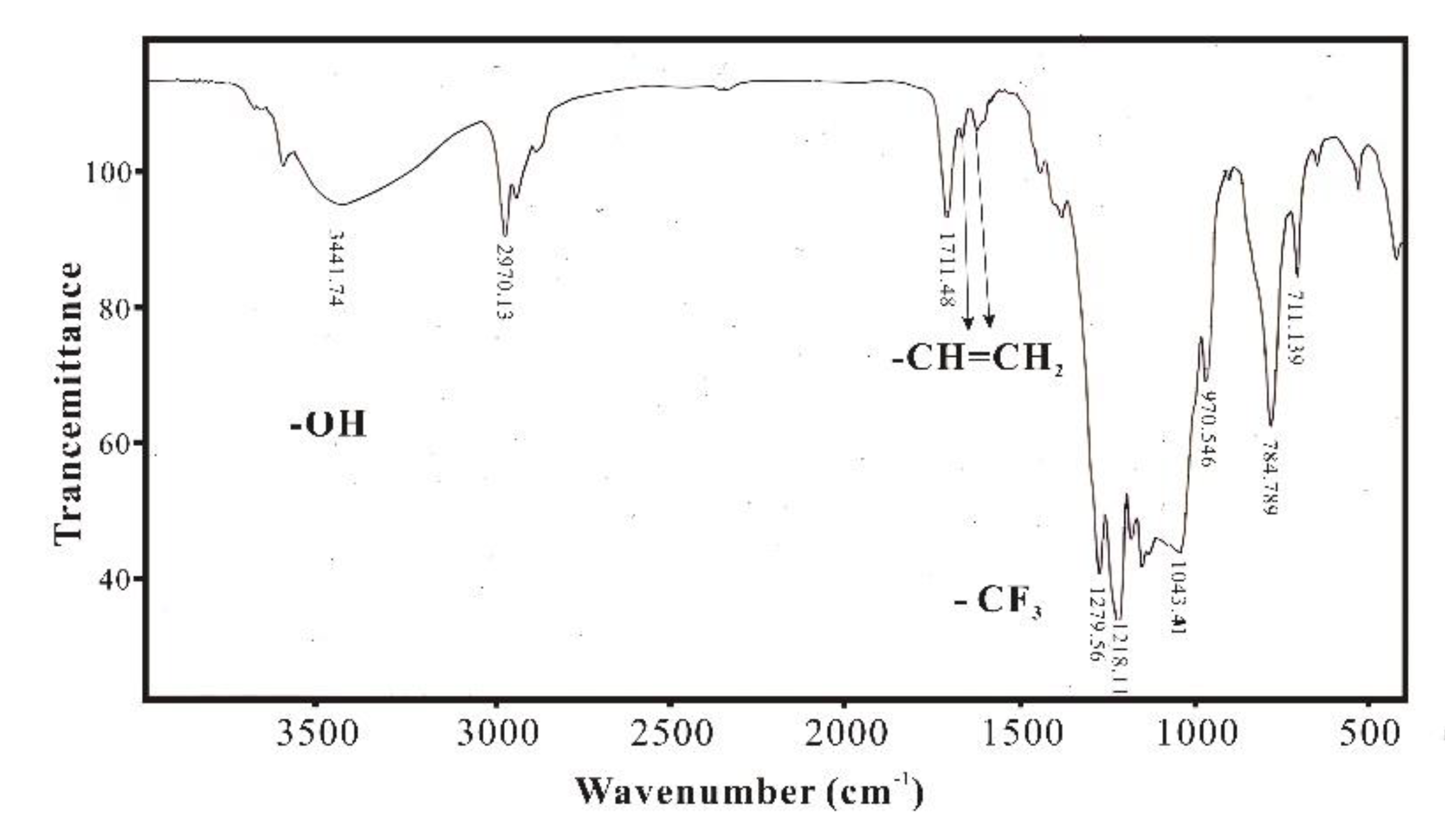
Figure 6 is FTIR spectrum of SXFA, which shows the appearance of an -OH peak near 3500 cm
-1; the original single peak at 1633 cm
-1 for the double bond has become two adjacent double bond peaks; and there is an -CF
3 absorption peak between 1120 cm
-1 and 1350 cm
-1. This phenomenon indicated that some vinyl double bonds undergo addition reaction with HFA, caused double bonds to transfer, and the appearance of hexafluoro-2-hydroxyisopropyl functional group confirms the reaction between methylvinyl polysiloxane and HFA, thus produced the final product SXFA.
3.2. Detection of Organophosphorus Agents
3.2.1. Selective Analysis of SAW-SXFA Sensor
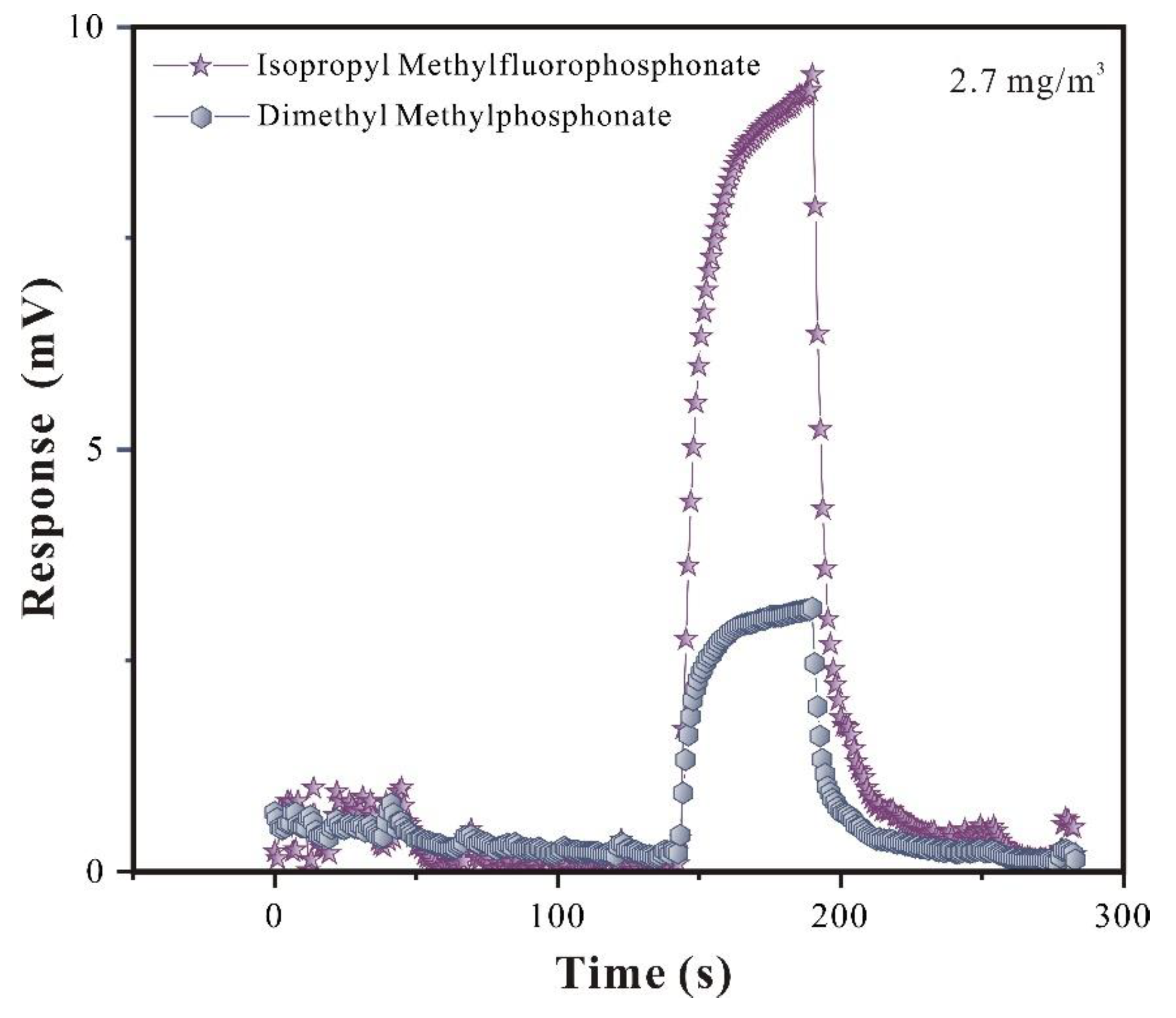
Superior selectivity is extremely important for SAW sensor. So, in this research, a comparative study on the adsorption effect of GB and its analog agent DMMP was conducted (
Figure 7,
Figure 8).
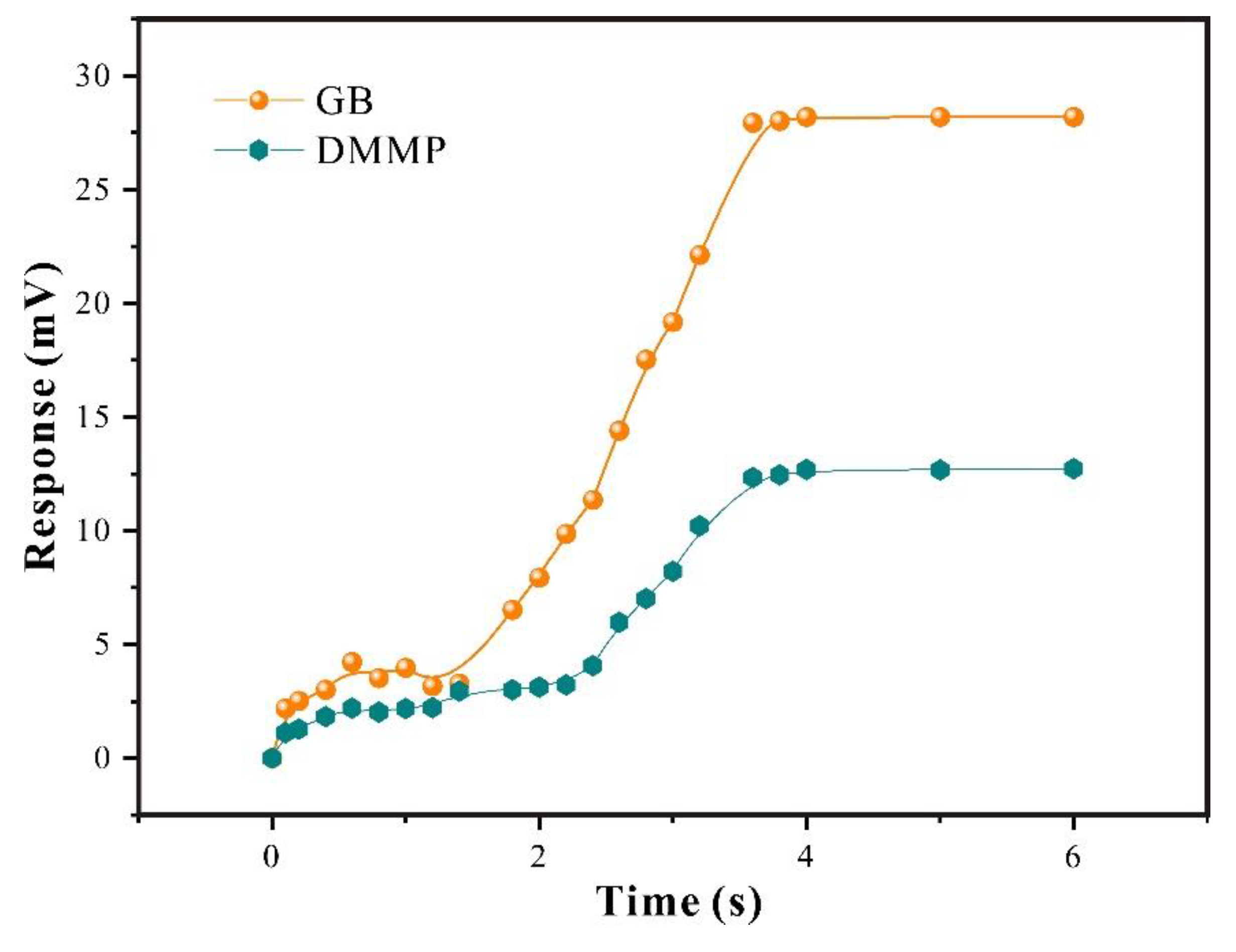
From
Figure 7, it can be seen that at low concentrations, functional group sites on the surface of SXFA were sufficient to adsorb gas molecules in contact with them, which would cause changes in sensor mass load, and thus resulted in significant changes in sensor signal; As concentration gradually increases, with the interaction of low concentrations, functional group sites inside film interacted with gas molecules through stereo adsorption, and jointly caused changes; However, due to the fact that stereo adsorption was based on the diffusion rate of gas within film, time for maximum response was prolonged; When reached high concentration, both surface sites and internal sites of the film tend to saturate, so the increase of GB and DMMP concentrations no longer has a significant impact on sensor response.
By compared the responses of adsorption equilibrium process, it can be seen that the SAW-SXFA sensor has a stronger adsorption response to GB than that to DMMP, differences in response gradually increases with the increase of concentration initially, and gradually stabilized when equilibrium concentration is reached (
Figure 7). Through systematically research, it can be illustrated that the main reason was that SXFA is a linear polysiloxane-based polymer with an HFIP functional group. The hydrogen bond acidity of -OH group on HFIP functional group was enhanced due to the influence of neighboring -CF
3 group, allowed it to selectively adsorb organophosphorus gases with alkaline hydrogen bond interactions, achieved selective adsorption of alkaline organophosphorus compounds [
23]. To visually explored the selectivity of SAW-SXFA gas sensor on GB, the response-recovery curves of GB and DMMP at a concentration of 2 mg/m
3 were compared (
Figure 8). As shown in
Figure 8, the maximum response of GB was 9.236 mV, while the response signal of DMMP was only 3.124 mV. By comparing the responses of the two toxic gases, it was found that at this concentration, the adsorption capacity of SXFA for GB was about three times that of DMMP. Therefore, SAW-SXFA sensor exhibited good selectivity and detection performance for organophosphorus agents.
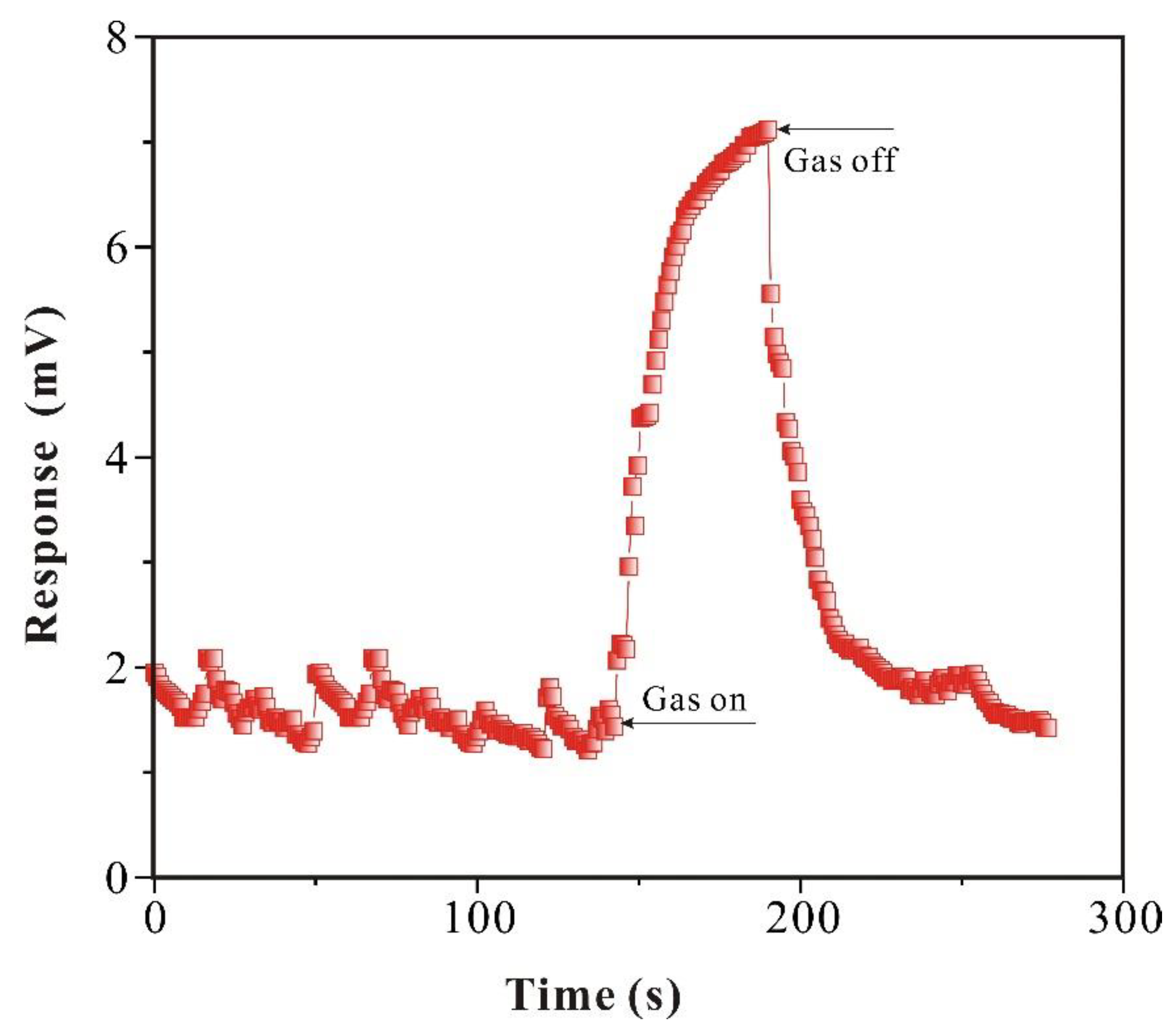
3.2.2. Analysis of Response of SAW-SXFA Sensor
Response of gas is crucial in the research of SAW-SXFA sensor. Therefore, relevant research was conducted in this study. From
Figure 9, it can be seen that the maximum response of SAW-SXFA sensor for GB was 6.118 mV, and noise during sensor equilibration process could be ignored. At the beginning of detection, due to hydrogen bond adsorption between GB and polymer film, the response was 2.475 mV in 10 s, accounting for 40.4% of the maximum response signal, and furthermore, it only costs 50 s to reach 80% of the maximum response. During recovery phase, GB rapidly separated from SXFA polymer film, and the response signal decreased rapidly by 4.283 mV within 10 s, accounting for 70% of the maximum response.
In the initial stage, interactions between GB and polymers mainly involved the adsorption of surface hydrogen bonding sites, which indicated a high adsorption efficiency, and therefore caused significant changes in sensor mass loading and response signals. After 1 min, some GB molecules penetrated into interior of polymer through steric adsorption effects, but the adsorption efficiency on internal sites of the polymer was lower than that on surface, so mass loading of the sensor increased slowly, resulted in a slower response. During the recovery period, GB molecules on the surface of SXFA polymer quickly escaped, resulted in a significant change in sensor signal. Immediately after, with the passage of time, the rate of sensor recovery slowed down, as GB molecules inside the polymer film had to overcome the obstruction of polymer chains to escape.
3.2.3. Detection Limit of SAW-SXFA Sensor
Table 2.
Relationship between concentration of GB and response of SAW-SXFA sensor (17.3℃, RH = 27%).
Table 2.
Relationship between concentration of GB and response of SAW-SXFA sensor (17.3℃, RH = 27%).
| Intensity (mg/m3) |
Response (mV) |
Recovery (mV) |
Recovery Rate (%) |
| 0.1 |
2.168 |
0.02 |
98 |
| 0.2 |
2.509 |
0.05 |
95 |
| 0.4 |
3.002 |
0.09 |
91 |
| 0.6 |
4.216 |
0.08 |
92 |
| 0.8 |
3.523 |
0.12 |
88 |
| 1.0 |
3.972 |
0.12 |
88 |
| 1.4 |
3.283 |
0.14 |
86 |
| 1.8 |
6.515 |
0.20 |
80 |
| 2.2 |
9.859 |
0.23 |
77 |
| 2.6 |
14.394 |
0.27 |
73 |
| 3.0 |
19.172 |
0.30 |
70 |
| 3. 4 |
27.605 |
0.35 |
65 |
Figure 10.
SAW-SXFA Sensor for Minimum Detection Concentration of GB (18.6℃, RH = 29%).
Figure 10.
SAW-SXFA Sensor for Minimum Detection Concentration of GB (18.6℃, RH = 29%).
Determination of sensor detection limits plays an important role in ensuring normal operation of sensors, improve usage efficiency, and enhancing safety. In this research, variations of SAW-SXFA sensor response with GB concentration was shown in
Table 2. When GB at a relatively high concentration (≥ 1.0 mg/m
3), response signals of SAW-SXFA sensor showed an increasing trend with the increase of GB concentration within the same time period, which conformed to the law of solid adsorption isotherms [
25], and the SAW-SXFA sensor could recover more than 65% of the response signal. However, when at a relatively low concentration (< 1.0 mg/m
3), the response of SAW-SXFA sensor increased first and then decreased, and reached a maximum response at 0.6 mg/m
3 in this period, which were similar to liquid adsorption isotherm curve [
25].
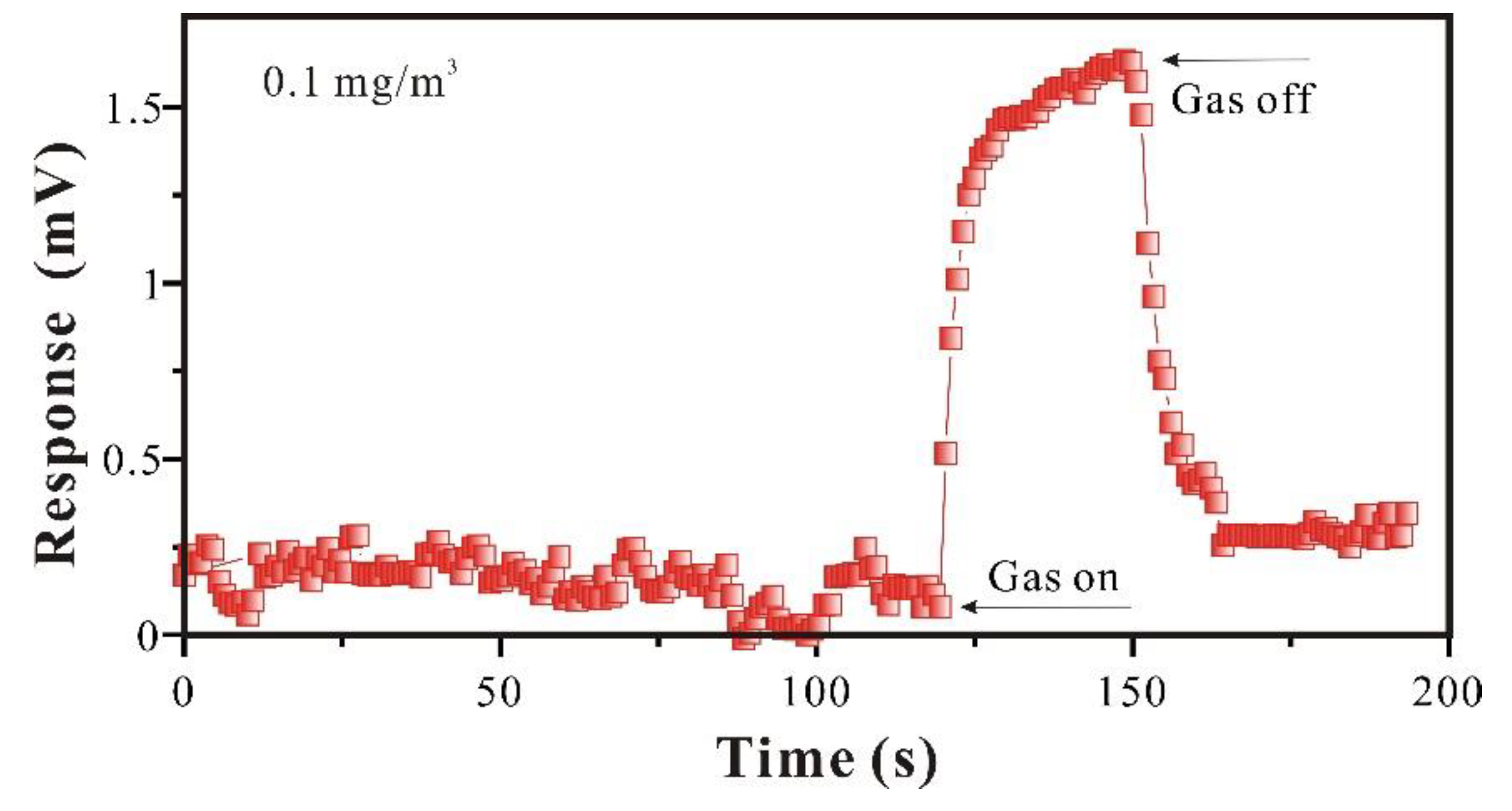
To study the sensitivity of SAW-SXFA sensor at low concentration, further analysis was conducted for GB at the concentration of 0.1 mg/m
3. As shown in
Figure 10 and
Table 2, SAW-SXFA sensor response was 1.563 mV in 140 s, and it continues to increase with time. Initial signal changes of response in first 10s was 0.326 mV, which was much larger than the SAW-SXFA sensor's noise, and can be inferred that the sensor has potential to detect lower concentrations. In addition, it was found that SAW-SXFA sensor has an "accumulation" function when detecting low concentrations of GB. Under the conditions of lower sample concentration and higher alarm response, the SAW-SXFA sensor can achieve a pre-alarm purpose by accumulating adsorption over a long period of time, and finally exceeded the alarm limit. Therefore, in the detection of low concentration GB, the SAW-SXFA sensor has high sensitivity and practical performance in rapid alarm.
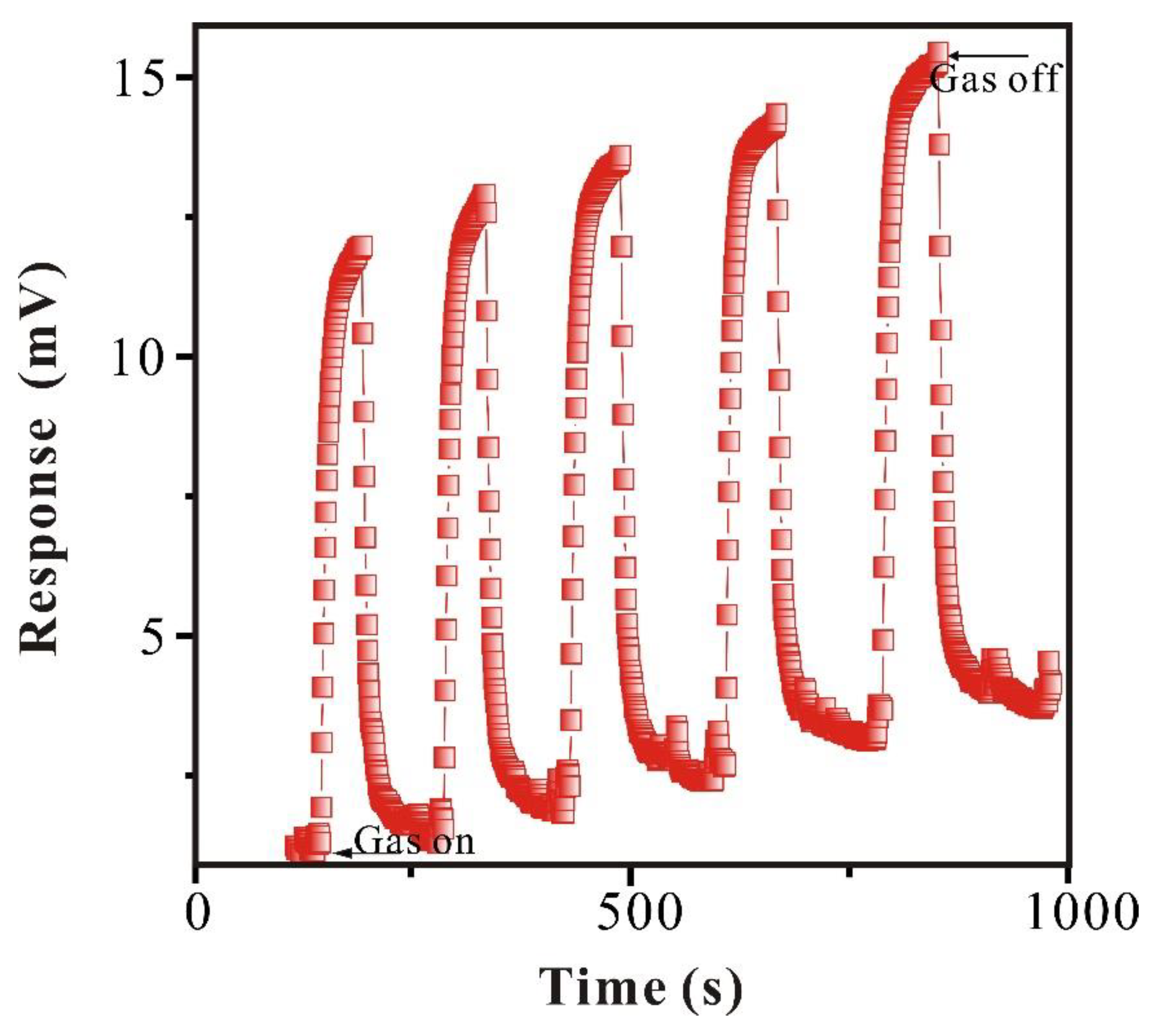
3.2.3. Reproducibility Study of SAW-SXFA Sensor
To research the stability of the SAW-SXFA sensor, GB with the same concentration was detected continuously for 5 times at the same conditions, responses and recovery time were both set to be 120 s, and the results are shown in
Figure 11 and
Table 3.
It can be seen from
Figure 11 that changes in SAW-SXFA sensor response and recovery have a periodic nature about 120 s. However, in this periodic nature of changes, due to the limited recovery time, GB molecules cannot completely desorb from the sensitive film, leaded to continuous accumulation of molecules within sensitive film, and thus resulted in a longer recovery time. Although the SAW-SXFA sensor cannot fully recovered to initial value, the impact on response within the time set in the experiment was very small (as shown in
Table 3). The standard deviation of maximum response for 5 times was only 0.11 mV, with a coefficient of variation of 0.01; and the standard deviation of maximum recovery signal was 0.22 mV, with a coefficient of variation of 0.02. Therefore, the SAW-SXFA sensor has good reproducibility for detecting GB, which is of great significance for future quantitative detection of SAW-SXFA sensor.
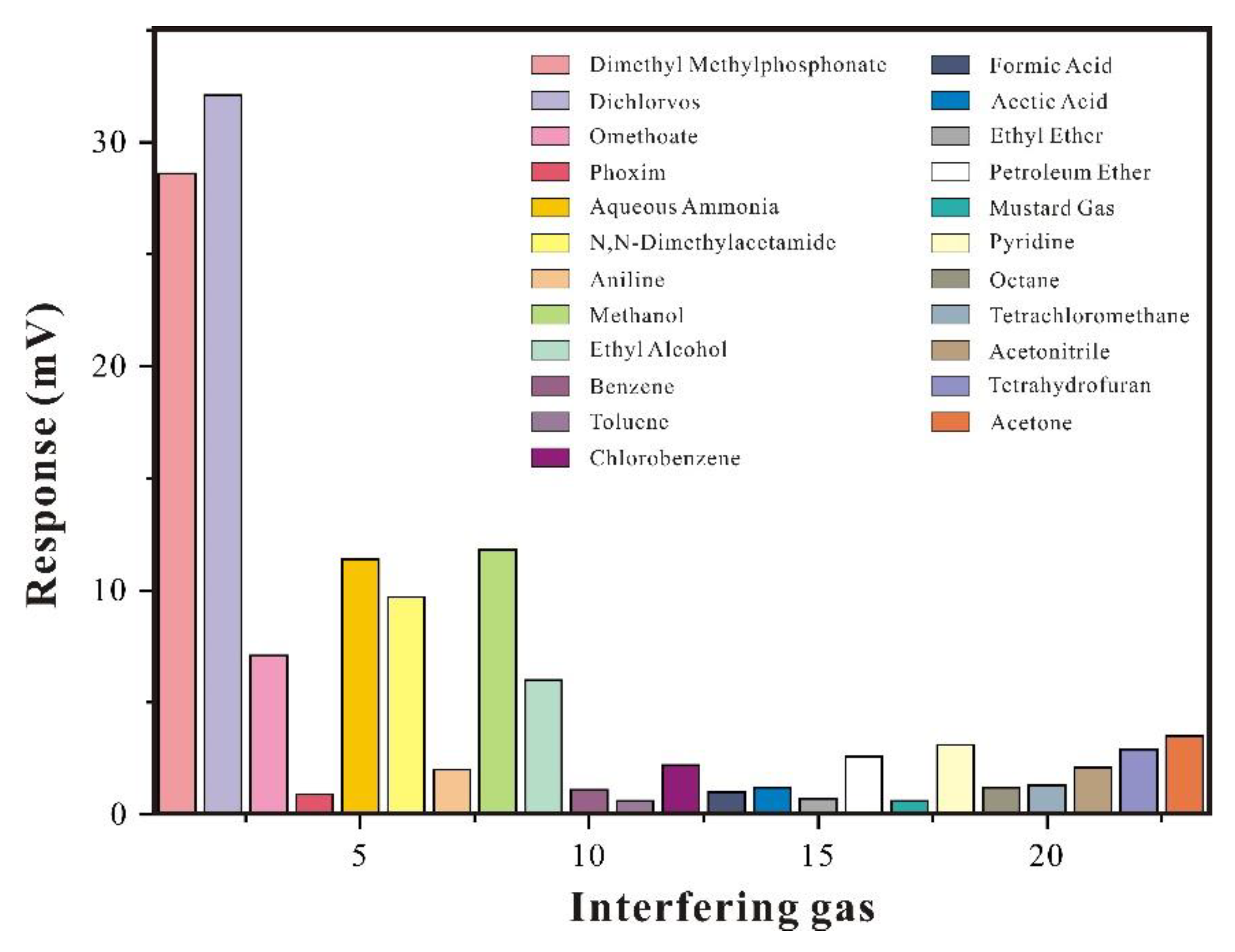
3.2.4. Interference Gas Research
The composition of air is quite complex, and for gas-sensitive sensor, its ability to resist interference is particularly important. Therefore, in order to verify the selectivity of film material and evaluate the sensor performance, it is important to test interference gases before detecting the target gas. The SAW-SXFA sensor was subjected to comparative experiments with various high-concentration interference gases, with each gas concentration set at 500 mg/m
3, and results are shown in
Figure 12. From
Figure 12, it can be seen that SAW-SXFA sensor has a strong response to organophosphorus gases, especially for DMMP and DFP. In addition, due to the hydrogen bond alkalinity of organophosphorus and amine gases, they can adsorb on the surface of SXFA polymer through hydrogen bond interaction, resulting in a noticeable response to high concentrations of ammonia gas and N, N-dimethylacetamide. Due to strong polarity of the HFIP group, made this group adsorb various polar gases, and causing certain interference of polar gases (such as alcohols) on the SAW-SXFA sensor, and comprehensive comparison revealed that this effect is much weaker than the hydrogen bond adsorption effect.
4. Conclusions
Synthesis of SXFA and the detection of organic phosphorus gas by SAW-SXFA sensor was researched in this study, and several conclusions are drawing. Firstly, through analyzed reaction mechanism and synthesis route of SXFA, it was found that ether plays a dispersing role in solve dichlorosilane, resulted in the formation of low degree cyclic siloxane after hydrolysis and condensation; phenyltrimethylamine hydroxide has superior catalytic activity, allowed cyclic siloxanes to undergo ring opening and generate chain like polysiloxanes with high degree of polymerization; HFA has amphiphilic properties and undergoes an addition reaction with methylallyl dichlorosilane, generated HFIP functional groups and transferring the position of allyl double bonds. Secondly, characterization of SXFA and its relevant materials confirmed methylallyl polysiloxane reacts with HFA, and finally generate SXFA. Then, detection of GB and its relevant agents indicated that SAW-SXFA sensor has strong sensitivity ( detection limit < 0.1mg/m3) , fast response and recovery speed, strong reproducibility and periodicity; and due to its viscoelastic state, response increase first and then decrease with the increase of GB concentration at low concentrations, and maximum response increase with the increase of GB concentration at high concentrations. And finally, the interfering gases experiment suggested that SAW-SXFA sensor has good detection performance and anti-interference ability against organophosphine agents, although high concentrations of organic phosphines, amines, and alcohol compounds may interfere with sensors, those effect is much weaker than hydrogen bonding adsorption.
Comprehensive research suggests that SAW-SXFA sensor has good detection effect and interference resistance against organic phosphorus agents and has characteristics such as short response time, good selectivity, high sensitivity, and strong reproducibility in GB detecting, which proves that the SAW-SXFA sensor has superior detection performance for GB.
Author Contributions
Conceptualization, C.C.Y. and Y.P.; methodology, C.C.Y. and T.X.G.; software, C.C.Y. and Y.P.; validation, C.C.Y., Y.P. and L.Z.; formal analysis, C.C.Y. and Y.P.; investigation, C.C.Y..; resources, C.C.Y. and Y.P.; data curation, C.C.Y.; writing—original draft preparation, C.C.Y.; writing—review and editing, C.C.Y. and Y.P.; visualization, C.C.Y., Y.P., M.L. Q. and J.C.Y.; supervision, Y.P.; project administration, Y.P.; funding acquisition, Y.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abu-Qare, A.W.; Abou-Donia, M.B. Sarin: health effects, metabolism, and methods of analysis. Food Chem. Toxicol. 2002, 40, 1327–1333. [Google Scholar] [CrossRef]
- Lee, E.C. Clinical manifestations of sarin nerve gas exposure. Jama-J. Am. Med. Assoc. 2003, 290, 659–662. [Google Scholar] [CrossRef]
- Tokuda, Y.; Kikuchi, M.; Takahashi, O.; Stein, G.H. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. 2006 , 68, 2, 193–202.
- Yoo, J.; Kim, D.; Yang, H.; Lee, M.; Kim, S.O.; Ko, H.J.; Hong, S.; Park, T.H. Olfactory receptor-based CNT-FET sensor for the detection of DMMP as a simulant of sarin.Sensors and Actuators B-Chemical, 2022, 354.
- Hamel, M; Hamoniaux, J; Rocha, L; Normand, S. Ppb detection of Sarin surrogate in liquid solutions. Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XIV. 2013, 8710.
- O’Neill, H.J.; Brubaker, K.L.; Schneider, J.F.; Sytsma, L.F.; Kimmell, T.A. Development of an analytical methodology for sarin (GB) and soman (GD) in various military-related-wastes. J. Chromatogr. A 2002, 962, 183–195. [Google Scholar] [CrossRef]
- Maziejuk, M.; Ceremuga, M.; Szyposzynska, M.; Sikora, T.; Zalewska, A. Identification of organophosphate nerve agents by the DMS detector. Sens. Actuators B-Chem. 2015, 213, 368–374. [Google Scholar] [CrossRef]
- Black, R.M.; Clarke, R.J.; Read, R.W.; Reid, M.T.J. Application of gas-chromatography mass-spectrometry and Gas-chromatography tandem mass-spectrometry to the analysis of chemical warfare samples, found to contain residues of the nerve agent sarin, sulfur mustard and their degradation products. J. Chromatogr. A 1994, 662, 301–321. [Google Scholar] [CrossRef]
- William, H.K. Pizoelectric Sorption Detector. Anal. Chem. 1964, 36, 1735–1739. [Google Scholar]
- Stevenson, A.C.; Mehta, H.M.; Sethi, R.S.; Cheran, L.E.; Thompson, M.; Davies, I.; Lowe, C.R. Gigahertz surface acoustic wave probe for chemical analysis. Analyst 2001, 126, 1619–1624. [Google Scholar] [CrossRef]
- Fahim, F.; Mainuddin, M.; Mittal, U.; Kumar, J.; Nimal, A.T. Novel SAW CWA Detector Using Temperature Programmed Desorption. IEEE Sens. J. 2021, 21, 2915–5922. [Google Scholar] [CrossRef]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Sharma, M.U.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sens. Actuators B-Chem. 2013, 178, 647. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, G.; Guo, T.; Liu, X.; Liu, X.; Zhang, C.; Yang, J.; Cao, B.; Zhang, C.; Wang, W. Environmental characteristics of surface acoustic wave devices for sensing organophosphorus vapor. Sens. Actuators B Chem. 2020, 315, 127986. [Google Scholar] [CrossRef]
- Pan, Y.; Qin, M.; Wang, P.; Yang, L.; Zhang, L.; Yan, C.; Zhang, C.; Wang, W. Interface and Sensitive Characteristics of the Viscoelastic Film Used in a Surface Acoustic Wave Gas Sensor. ACS Sens. 2022, 7, 612–621. [Google Scholar] [CrossRef]
- Matatagui, D.; Martí, J.; Fernández, M.J.; Fontecha, J.L.; Gutiérrez, J.; Gràcia, I.; Cané, C.; Horrillo, M.C. Chemical warfare agents simulants detection with an optimized SAW sensor array. Sens. Actuators B-Chem. 2011, 154, 199–205. [Google Scholar] [CrossRef]
- Lama, S.; Kim, J.; Ramesh, S.; Lee, Y.J.; Kim, J.; Kim, J.H. Highly Sensitive Hybrid Nanostructures for Dimethyl Methyl Phosphonate Detection. Micromachines 2021, 12, 6. [Google Scholar] [CrossRef]
- Lurz, F.; Ostertag, T.; Scheiner, B.; Weigel, R.; Koelpin, A. Reader Architectures for Wireless Surface Acoustic Wave Sensors. Sensors 2018, 18, 6. [Google Scholar] [CrossRef]
- Palla-Papavlu, A.; Voicu, S.I.; Dinescu, M. Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors. Chemosensors 2021, 9, 5. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Zhang, Y.; Pan, Y.; Liang, Y.; Li, J. Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature. Sensors 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; He, S.; Li, S.; Liu, M.; Pan, Y. Advances in SXFA-Coated SAW Chemical Sensors for Organophosphorous Compound Detection. Sensors 2011, 11, 1526–1541. [Google Scholar]
- Grate, J.W. Hydrogen Bond Acidic Polymers for Surface Acoustic Wave Vapor Sensors and Arrays. Anal. Chem. 1999, 71, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Andonian-Haftvan, J.; Du, C.M.; Diart, V.; Whiting, G.S.; Grate, J.W.; Andrew, M.R. Hydrogen bonding. Part 29. Characterization of 14 sorbent coatings for chemical microsensors using a new solvation equation. J. Chem. Soc. Perkin Trans. 1995, 2, 369–378. [Google Scholar] [CrossRef]
- Freudenberg, J.; Schickfus, M.V.; Hunklinger, S. A SAW immunosensor for operation in liquid using a SiO2 protective layer. Sens. Actuators B Chem. 2001, 76, 147–151. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Wood, B.; Ramisetty, K.A.; Stewart, A.A.; Howard, C.A.; Brett, D.J.L.; Rodriguez-Reinoso, F. Characterization of the adsorption site energies and heterogeneous surfaces of porous materials. J. Mater. Chem. A 2019, 7, 17. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A; Da'ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393. [Google Scholar] [CrossRef] [PubMed]
- Kindlund, A.; Sundgren, H.; Lundstrm, I. Quartz crystal gas monitor with a gas concentrating stage. Sens. Actuators 1984, 6, 1–17. [Google Scholar] [CrossRef]
- Finklea, H.O.; Phillippi, M.A.; Lompert, E.; Grate, J.W. Highly sorbent films derived from ni(scn)2(4-picoline)4 for the detection of chlorinated and aromatic hydrocarbons with quartz crystal microbalance sensors. Anal. Chem. 1998, 70, 1268–76. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.F.; Stephen, J.M. Materials characterization using surface acoustic wave devices. Appl. Spectrosc. Rev. 1991, 26, 73–149. [Google Scholar]
- Grate, J.W.; Wenzel, S.W.; White, R.M. Flexural plate wave device for chemical analysis. Anal. Chem. 1991, 63, 1552–1561. [Google Scholar] [CrossRef]
- Grate, J.W.; Klusty, M.; Mcgill, R.A.; Abraham, M.H.; Whiting, G.; Andonian-Haftvan, J. The predominant role of swelling-induced modulus changes of the sorbent phase in determining the responses of polymer-coated surface acoustic wave vapor sensors. Anal. Chem. 1992, 64, 610–624. [Google Scholar] [CrossRef]
- Tascon, M.; Romero, L.M.; Acquaviva, A.; Keunchkarian, S.; Castells, C. Determinations of gas-liquid partition coefficients using capillary chromatographic columns. alkanols in squalane. J. Chromatogr. A 2013, 1294, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; RosÉS, M.; Poole, C.F.; Poole, S.K. Hydrogen bonding. 42. characterization of reversed-phase high-performance liquid chromatographic c18 stationary phases. J. Phys. Org. Chem. 2015, 10, 358–368. [Google Scholar] [CrossRef]
- Grate, J.W.; Patrash, S.J.; Abraham, M.H. Method for estimating polymer-coated acoustic wave vapor sensor responses. Anal. Chem. 1995, 67, 2162–2169. [Google Scholar] [CrossRef]
- Grate, J.W.; Kaganove, S.N.; Bhethanabotla, V.R. Comparisons of polymer/gas partition coefficients calculated from responses of thickness shear mode and surface acoustic wave vapor sensors. Anal. Chem. 1998, 70, 199–203. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).