1. Introduction
Stroke is a typical target disease in rehabilitation. The factors that cause stroke patients to require some support in their daily lives include the appearance of symptoms such as motor paralysis, sensory disturbance, and higher brain dysfunction. Among these, motor paralysis significantly impacts daily life and quality of life, and improvement through rehabilitation is strongly required.
In this context, Mental Practice (MP) is one intervention to rehabilitate gait, balance, and upper limb function after a stroke. MP is the continuous repetition of Motor Imagery (MI) to improve performance on motor tasks, and its usefulness has been reported in systematic reviews of stroke patients [
1,
2]. Based on the results of many such studies, MP is also classified as Grade A in guidelines published by the American Heart Association [
3].
However, it has been pointed out that there is no standardized intervention method in the implementation of MP for gait, balance, and upper limb function after stroke because of the wide variety of intervention methods, such as MP intervention time, intervention frequency, and intervention duration [
4,
5]. In other words, the clinical use of MP to improve gait, balance, and upper limb function after stroke is left to the subjective judgment of the practitioner, and the development of intervention methods is required for future development.
This scoping review focuses on MP for paralytic upper limb function. It aims to understand the current status and identify problems for more effective MP for paralytic upper limb function and its further application in clinical practice. The scoping review systematically maps studies of MP for post-stroke paralytic upper limb function and comprehensively clarifies the methodology of MP that has been used to date.
2. Materials and Methods
Our scoping review methodology was originally conceived by Arksey and O’Malley [
6], developed in detail by Levac et al. [
7], and was implemented based on “Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Review (PRISMA-ScR)” as compiled by Triccol et al. [
8]. We structured our protocol by applying a four-step process: identifying the research question, identifying the studies, selecting the studies, and extracting and analyzing the data.
The purpose of this scoping review was to comprehensively clarify the methodology of MP to date by systematically mapping studies that have performed MP on post-stroke paralytic upper extremity function. Specifically, (1) When is the most common timing of MP intervention after stroke onset? (2) What is the MP load (intervention time, number of intervention days, and intervention period)? (3) What are the most common methods of MI recall and MI tasks during MP? (4) Is MP often used in conjunction with individual rehabilitative therapies? (5) What is the paralyzed side's upper limb and cognitive function level at the start of MP intervention?
We searched for articles that included “stroke” and “mental practice (motor imagery training).” The databases used were PubMed, Scopus, Medline, and the Cochrane Library; the last search date was July 19, 2022. The PubMed search prompts are shown below as an example.
“cerebrovascular disorder” OR stroke OR “Brain infarction” OR “Brain Stem Infarctions” OR “Cerebral Infarction” OR Lacunar OR “Brain injury”
AND
“mental practice” OR “motor imagery training” OR “motor image”
Duplicate papers were removed after extracting papers from each database.
Papers meeting our criteria were selected from among English language publications, and all study designs were included, including studies that performed MP on the paralyzed upper limb function after stroke. Five authors selected eligible articles using the Rayyan literature screening software (
https://www.rayyan.ai/). For each article, the first author (Akira Nakashima) and two other authors (from among Takefumi Moriuchi, Kengo Fujiwara, Ryohei Okamura, or Toshio Higashi) checked whether it met the eligibility criteria. In case of disagreement, the five authors reviewed the manuscript until 100% agreement was reached.
The following information was extracted from the articles for the eligible articles. Author, year of publication, study design, country of study, age of participants with MP, type of stroke, timing of MP intervention, cognitive function at the start of MP, paralytic upper limb function at the start of MP, duration of MP intervention, daily MP intervention time, MP intervention days per week, how MI was performed during MP, and whether MP was combined with individual rehabilitation therapy. Akira Nakashima and Takefumi Moriuchi subsequently identified the level of evidence and study design of 62 articles using the American Journal of Occupational Therapy’s systematic review guidelines [
9].
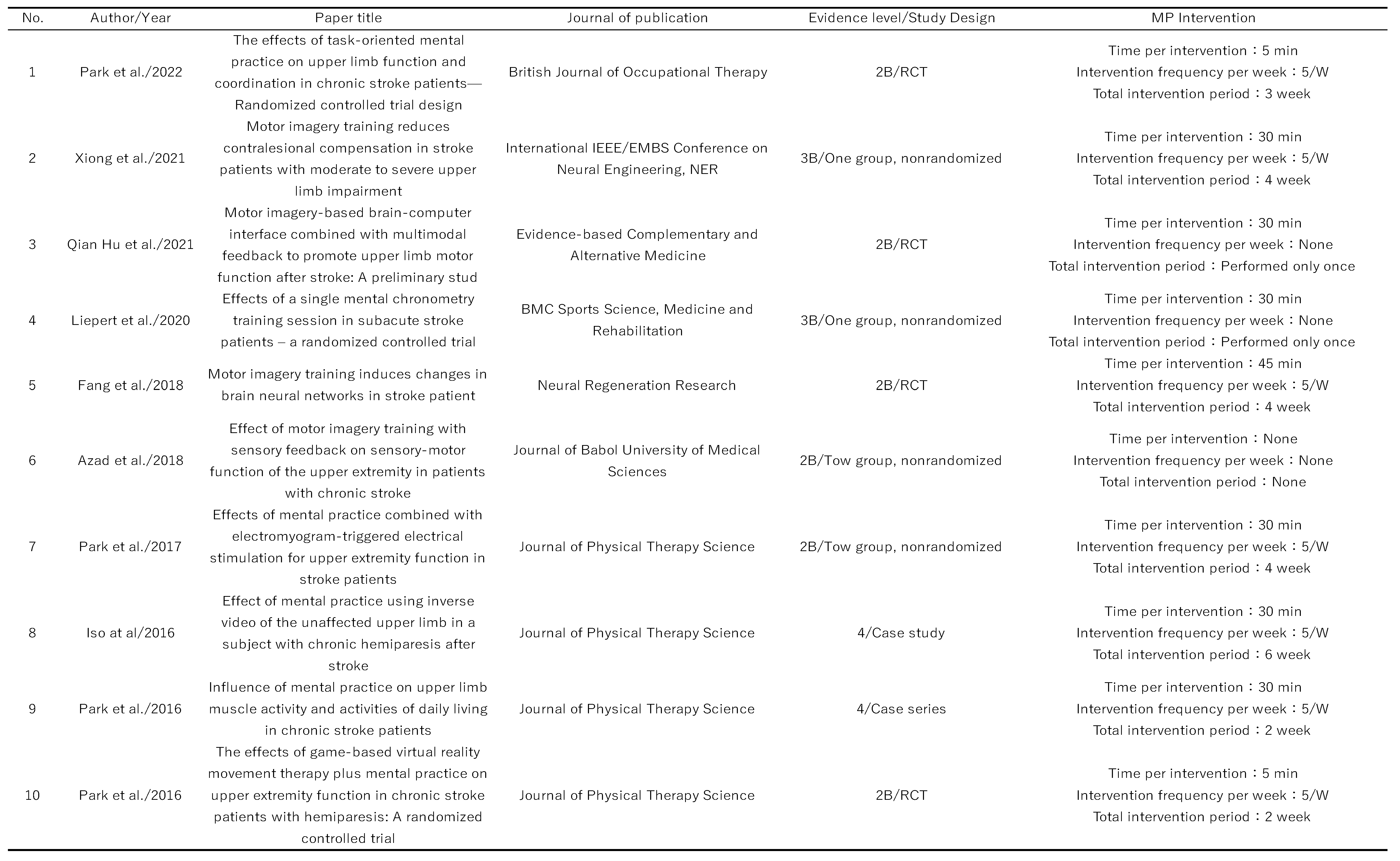
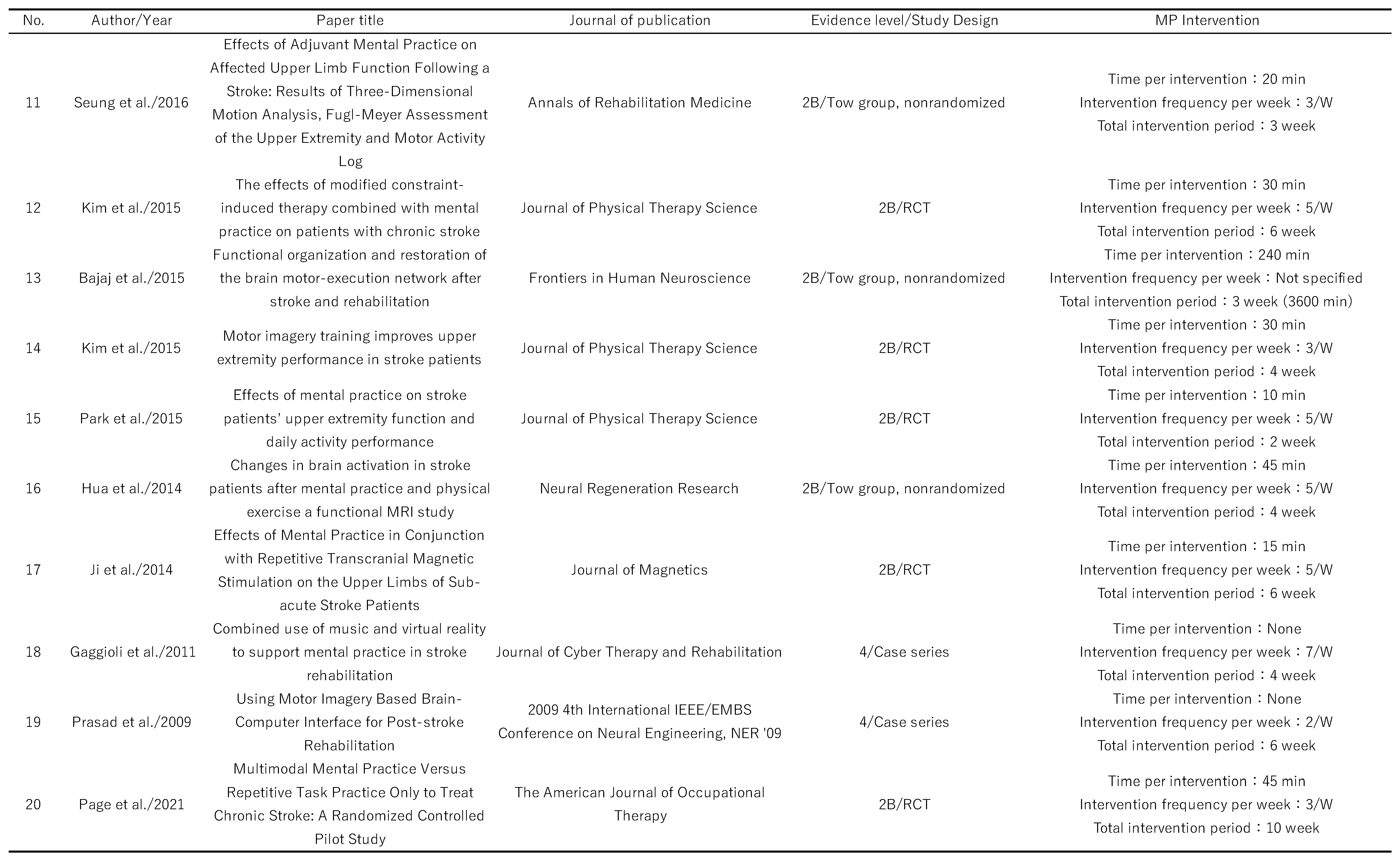
Table 1.
Levels of evidence and forms of intervention for the articles included in this scoping review.
Table 1.
Levels of evidence and forms of intervention for the articles included in this scoping review.
3. Results
English-language articles identified were 694 for this study, of which 62 were selected for inclusion (
Figure 1). The 62 articles selected are listed in
Table 1. The study designs included 26 randomized controlled trials, 16 pre/post comparisons, 11 case series, 4 quasi-randomized controlled trials, 4 single case studies, and 1 crossover comparison study. The majority of the randomized controlled trials had several methodological problems, and the quality of the studies was poor. The largest number of participants in each study was between 11 and 20 (18 studies), and 12 included more than 30 participants, of which the largest number was 121 [
10]. The participants in each study were between 51 and 70 years old in the majority of 48 studies. Only a single study included participants aged 70 years or older [
11]. The most common stroke type in each study was not described in 29 papers, and among those that did, 20 papers included both cerebral hemorrhage and cerebral infarction.
- (1)
When is the most common time to start MP intervention for post-stroke paralytic side upper limb function after stroke?
The timing of MP intervention was within 6 months after stroke onset in 20 studies, after 6 months in 37 studies, and 5 studies did not provide information confirming the start of MP intervention. The study that started MP the earliest was 27.8 ± 19.2 days after stroke onset [
12], and the study that started MP the latest was 72.2 ± 20.3 months [
13]. The timing of the intervention in all studies is described in
Figure 2.
- (2)
What is the MP load (intervention time, number of intervention days, and intervention period)?
The intervention times for MP varied across the studies: 13 studies had MP intervention times of 20 minutes or less, 26 studies had MP intervention times of 30 minutes or less, 14 studies had MP intervention times of 60 minutes or less, 1 study had MP intervention times of 60 minutes or longer, and 8 studies lacked information concerning MP intervention time. The study with the longest MP intervention time was by Butler et al. [
14], at 180 minutes. Regarding the intervention frequency, 23 studies reported 5 weekly interventions, followed by 15 with 3 weekly interventions, 11 with 2 weekly interventions, and 7 with 7 weekly interventions. There were no studies in which MP was performed once per week or 6 times a week. Five studies did not mention any MP. Next, regarding the duration of the MP intervention in each study, 4 weeks was the most common (18 studies), followed by 6 weeks (14 studies), 2 weeks and 10 weeks (7 studies). The most common combination of intervention time per intervention, intervention frequency per week, and overall intervention period were 30 minutes per intervention, 5 times per week for 4 weeks (5 studies [
15,
16,
17,
18,
19], followed by 4 papers with 45 minutes per intervention, 5 times per week, and four weeks [
10,
20,
21,
22].
- (3)
What are the most common methods of MI recall and MI tasks during MP?
The most common method of recalling MI during MP was using an audio guide to prompt MI while giving verbal instructions (23 studies), MI alone (18 studies), and combined with motor observation (7 studies).
Other methods included using BCI in 5 papers using BCI [
23,
24,
25,
26,
27] and VR in 3 papers [
28,
29,
30].
The most common MI tasks used in MP were daily activities such as “drinking water from a glass” and “buttoning a shirt” (28 papers). Twenty-two studies used joint movements such as hand flexion and wrist dorsiflexion. Seven studies used both daily activities and joint exercises.
- (4)
Is MP often used in conjunction with individual rehabilitative therapies?
Forty-nine studies examined the effects of MP in combination with individual rehabilitative therapies, and 10 studies used MP alone.
- (5)
What is the upper limb and cognitive function level on the paralyzed side at the start of MP intervention?
In this scoping review, FMA, ARAT, MAL, and BBT data were extracted from each article to confirm the status of paralyzed side upper limb function at the start of MP. As a result, FMA, ARAT, MAL, and BBT were implemented in 38, 21, 9, and 6 studies.
Twelve studies measured cognitive function at the start of MP using the MMSE. Nineteen studies used MMSE>24 or 25 or 27 as the inclusion criterion. 11 studies used the Modified Mini-Mental State Examination>69 or 70 as the inclusion criterion. Nineteen studies did not test cognitive function. Among the 12 studies that measured cognitive function using the MMSE at the beginning of the MP intervention, the minimum MMSE score was 25±2 points [
12].
4. Discussion
This scoping review aimed to systematically map studies in which MP was performed for post-stroke paralytic lateral upper extremity function to provide a comprehensive picture of the MP methodologies used to date. Among these, our investigation considered (1) When is the most common timing of MP intervention after stroke onset? (2) What is the MP load (intervention time, number of intervention days, and intervention period)? (3) What are the most common methods of MI recall and MI tasks during MP? (4) Is MP often used in conjunction with individual rehabilitative therapies? (5) What is the upper limb and cognitive function level on the paralyzed side at the start of MP intervention?
- (1)
When is the most common timing of MP intervention after stroke onset?
Most studies were conducted in the chronic phase after stroke onset, and this scoping review suggests that MP is an effective intervention strategy for upper limb function on the paralyzed side 3 months after stroke onset. However, very few studies have examined the effect of intervention in the acute phase of a stroke. The need for such intervention can be inferred from how cognitive aspects have a significant impact on how MP is conducted and that the participant is unable to perform adequate MI during MP in the acute phase of stroke onset because of impaired consciousness. Further, from the viewpoint of research design, it is difficult to derive the effects of specific approaches for participants in the acute and subacute phases of stroke because there are many factors (cerebral edema, diaschisis, improvement of penumbra) [
31] that may improve physical function, and researchers are reluctant to publish negative data. However, studies in the acute and subacute phases of stroke onset are essential to determine the appropriate time to start MP intervention, and future research should focus on the effectiveness of MP in the acute and subacute phases.
- (2)
What is the MP load (intervention time, number of intervention days, and intervention period)?
In all studies, there were no clear criteria for the amount of MP loading, and the intervention time, days of intervention, and duration of intervention varied in a wide variety of situations. The only study that has investigated MP loading was Page et al.’s study of the MP intervention period [
32]. In addition, in recent years, when considering MP load, it has become clear that MI can cause muscle and mental fatigue with sustained repetition, which can also affect performance improvement [
33,
34,
35,
36,
37]. Against this backdrop, systematic reviews on MP have pointed out the importance of formulating interventions that account for fatigue associated with sustained repetition of MI [
38]. In the future, it will be important to cooperate with basic researchers to establish standardized intervention criteria for MP and to investigate what level of load is most effective from a neurophysiological perspective.
- (3)
What are the most common methods of MI recall and MI tasks during MP?
The most common method of conducting MP was using an audio guide to facilitate MI while giving verbal instructions, and it is important to know how MI can be performed to maximize the effectiveness of MP [
39]. Several recent studies have begun to use VR and BCI to enhance MI clarity [
23,
24,
25,
26,
27,
28,
29,
30], and we believe that it will be important to apply MI clarifying techniques to optimize MP efficacy in the future.
The most common tasks used during MP were tasks for daily activities, such as “drinking water from a glass” and “buttoning a shirt.” This may be because of the combination of task-oriented training, the ease of generalization to daily activities, and the use of familiar activities to ensure MI clarity.
- (4)
Is MP often used in conjunction with individual rehabilitative therapies?
The majority of the studies used MP in conjunction with individual rehabilitative therapies. MP is ultimately a complementary intervention to physical training, which is believed to be more effective than MP as a stand-alone training method. In this context, Page et al. reported that combining exercise observation, physical exercise [
40], and MP improved paralyzed upper limb function after more than 45 minutes of physical exercise and 15 minutes each of motor observation, physical exercise, and MP. These findings suggests that it may be necessary not only to combine these exercises in the future, but also to consider the order of the exercises and the allocation of time for each exercise within the overall practice time.
- (5)
What is the upper limb and cognitive function level on the paralyzed side at the start of MP intervention?
Depending on the FMA score, MP tends to be performed on participants with mild-to-moderate paralysis, and according to MMSE, MP tends to be performed on participants with relatively preserved cognitive function. There is no indication criteria for MP concerning paralytic upper limb function or cognitive status. For example, in CI therapy, the criteria for indication include the ability to perform a 10° extension of the MP and IP joints and a 20° dorsiflexion of the wrist in the paralyzed upper extremity, and an MMSE score of 24 or higher in cognitive function [
41]. In the case of MP, it is important to perform clear MI tasks to realize their effects fully, and the participant must understand the practitioner’s explanations. From this point of view, participants with relatively preserved cognitive functions are likely to benefit from MP. However, it is important to combine AO and VR to ensure motor imagery ability and to prepare the environment so that even participants with diminished cognitive function can benefit from MP. Future accumulation of research data is needed to accumulate studies on people with severe paralytic upper limb dysfunction and cognitive decline to investigate the extent to which people with paralytic upper limb function and cognitive function can benefit from MP.
One limitation of this study was that this was a scoping review, so we did not evaluate the advantages and disadvantages given by MP in each study. For this reason, it is not possible to describe the effectiveness of MP in rehabilitation interventions in this study. In addition, although five experienced occupational therapists reviewed each study in this study, we cannot deny the possibility that another occupational therapist or a different team of occupational therapists would have had a different opinion.
5. Conclusions
In this study, we comprehensively reviewed the MP methodologies used to date for the rehabilitation of paralyzed upper extremity function. We found that the duration of MP interventions varied widely and that many studies differed in their methods of MI recall. In the future, conducting accumulation of research in cooperation with basic and clinical researchers will be important to unify the varied widely MP methodologies identified in this study.
Author Contributions
A.N., R.O., T.M., K.F., and T.H. contributed to study selection and data extraction; A.N., T.M., contributed to methodological quality assessment; A.N., T.H., and K.T. contributed to data and outcome synthesis; and A.N., T.H., and K.T. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (grant number 21K17513).
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 2016, 10, 422. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; Lang, C.E.; MacKay-Lyons, M.; Ottenbacher, K.J.; Pugh, S.; Reeves, M.J.; Richards, L.G.; Stiers, W.; Zorowitz, R.D.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Malouin, F.; Jackson, P.L.; Richards, C.L. Towards the integration of mental practice in rehabilitation programs. A critical review. Front Hum Neurosci. 2013, 7, 576. [Google Scholar] [CrossRef] [PubMed]
- Guerra, Z.F.; Lucchetti, A.L.G.; Lucchetti, G. Motor imagery training after stroke: a systematic review and meta-analysis of randomized controlled trials. J Neurol Phys Ther 2017, 41, 205–214. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: advancing the methodology. Implement Sci 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; Hempel, S.; Akl, E.A.; Chang, C.; McGowan, J.; Stewart, L.; Hartling, L.; Aldcroft, A.; Wilson, M.G.; Garritty, C.; Lewin, S.; Godfrey, C.M.; Macdonald, M.T.; Langlois, E.V.; Soares-Weiser, K.; Moriarty, J.; Clifford, T.; Tunçalp, Ö.; Straus, S.E. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018, 169, 467–473. [Google Scholar] [CrossRef]
- American Occupational Therapy Association: Guidelines for systematic reviews, 2020.
- Ietswaart, M.; Johnston, M.; Dijkerman, H.C.; Joice, S.; Scott, C.L.; MacWalter, R.S.; Hamilton, S.J. Mental practice with motor imagery in stroke recovery: randomized controlled trial of efficacy. Brain 2011, 134, 1373–1386. [Google Scholar] [CrossRef]
- Braun, S.M.; Beurskens, A.J.; Kleynen, M.; Oudelaar, B.; Schols, J.M.; Wade, D.T. A multicenter randomized controlled trial to compare subacute ‘treatment as usual’ with and without mental practice among persons with stroke in Dutch nursing homes. J Am Med Dir Assoc 2012, 13, 85.e1–85.e857. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, M.W.; Park, K.H.; Choi, Y.A. The effects of additional electrical stimulation combined with repetitive transcranial magnetic stimulation and motor imagery on upper extremity motor recovery in the subacute period after stroke. Medicine 2021, 100, e27170. [Google Scholar] [CrossRef]
- Park, J. The effects of task-oriented mental practice on upper limb function and coordination in chronic stroke patients—Randomized controlled trial design. Br J Occup Ther 2022, 85, 164–171. [Google Scholar] [CrossRef]
- Butler, A.J.; Page, S.J. Mental practice with motor imagery: evidence for motor recovery and cortical reorganization after stroke. Arch Phys Med Rehabil 2006, 87, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, H.; Wang, X.; Sun, L.; Guo, X. Motor imagery training reduces contralesional compensation in stroke patients with moderate to severe upper limb impairment. 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Italy; 2021; pp. 876–879. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, J.B.; An, D.H.; Chang, M.Y. Effects of mental practice combined with electromyogram-triggered electrical stimulation for upper extremity function in stroke patients. J Phys Ther Sci 2017, 29, 1819 –1820. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Xiong, X.; Sun, C.; Zhu, B.; Xu, Y.; Fan, M.; Tong, S.; Sun, L.; Guo, X. Motor imagery training after stroke increases slow-5 oscillations and functional connectivity in the ipsilesional inferior parietal lobule. Neurorehabil Neural Repair 2020, 34, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yin, D.; Zhu, Y.; Fan, M.; Zang, L.; Wu, Y.; Jia, J.; Bai, Y.; Zhu, B.; Hu, Y. Cortical reorganization after motor imagery training in chronic stroke patients with severe motor impairment: a longitudinal fMRI study. Neuroradiology 2013, 55, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Bütefisch, C.M.; Seitz, R.J.; Hömberg, V. Mental practice improves hand function after hemiparetic stroke. Restor Neurol Neurosci 2007, 25, 501–511. [Google Scholar] [PubMed]
- Li, F.; Zhang, T.; Li, B.J.; Zhang, W.; Zhao, J.; Song, L.P. Motor imagery training induces changes in brain neural networks in stroke patients. Neural Regen Res 2018, 13, 1771–1781. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; Zhang, T. Changes in brain activation in stroke patients after mental practice and physical exercise: a functional MRI study. Neural Regen Res 2014, 9, 1474–1484. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; Zhang, T. Mental practice combined with physical practice to enhance hand recovery in stroke patients. Behav Neurol 2014, 2014, 876416. [Google Scholar] [CrossRef]
- Prasad, G.; Herman, P.; Coyle, D.; McDonough, S.; Crosbie, J. Using motor imagery based brain-computer interface for post-stroke rehabilitation. 2009 4th International IEEE/EMBS Conference on Neural Engineering; 2009; pp. 258–262. [Google Scholar] [CrossRef]
- Chowdhury, A.; Meena, Y.K.; Raza, H.; Bhushan, B.; Uttam, A.K.; Pandey, N.; Hashmi, A.A.; Bajpai, A.; Dutta, A.; Prasad, G. Active physical practice followed by mental practice using BCI-driven hand exoskeleton: a pilot trial for clinical effectiveness and usability. IEEE J Biomed Health Inform 2018, 22, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Okuyama, K.; Takahashi, Y.; Hiramoto, M.; Nishimura, A.; Ushiba, J.; Fujiwara, T.; Liu, M. Change in reciprocal inhibition of the forearm with motor imagery among patients with chronic stroke. Neural Plast 2018, 2018, 3946367. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Pisotta, I.; Pichiorri, F.; Kleih, S.; Paolucci, S.; Molinari, M.; Cincotti, F.; Kübler, A.; Mattia, D. Proof of principle of a brain-computer interface approach to support poststroke arm rehabilitation in hospitalized patients: design, acceptability, and usability. Arch Phys Med Rehabil 2015, 96, S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Gao, T.H.; Li, J.; Tao, J.C.; Bai, Y.L.; Lu, R.R. Motor imagery-based brain-computer interface combined with multimodal feedback to promote upper limb motor function after stroke: a preliminary study. Evid Based Complement Alternat Med 2021, 2021, 1116126. [Google Scholar] [CrossRef] [PubMed]
- Trobia, J.; Gaggioli, A.; Antonietti, A. Combined use of music and virtual reality to support mental practice in stroke rehabilitation. J Cyber Ther Rehabil 2011, 4, 57–61. [Google Scholar]
- Gaggioli, A.; Meneghini, A.; Morganti, F.; Alcaniz, M.; Riva, G. A strategy for computer-assisted mental practice in stroke rehabilitation. Neurorehab Neural Repair 2006, 20, 503–507. [Google Scholar] [CrossRef]
- Gaggioli, A.; Morganti, F.; Meneghini, A.; Pozzato, I.; Greggio, G.; Pigatto, M.; Riva, G. Computer-guided mental practice in neurorehabilitation. Stud Health Technol Inform 2009, 145, 195–208. [Google Scholar]
- Teasell, R.; Bayona, N.A.; Bitensky, J. Plasticity and reoeganization of the brain post stroke. Top Stroke Rehabil 2005, 12, 11–26. [Google Scholar] [CrossRef]
- Page, S.J.; Dunning, K.; Hermann, V.; Leonard, A.; Levine, P. Longer versus shorter mental practice sessions for affected upper extremity movement after stroke: a randomized controlled trial. Clin Rehabil 2011, 25, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.D.; Sonne, M.W.; Bray, S.R. It wears me out just imagining it! Mental imagery leads to muscle fatigue and diminished performance of isometric exercise. Biol Psychol 2014, 103, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rozand, V.; Lebon, F.; Papaxanthis, C.; Lepers, R. Does a mental training session induce neuromuscular fatigue? Med Sci Sports Exerc 2014, 46, 1981–1989. [Google Scholar] [CrossRef]
- Rozand, V.; Lebon, F.; Stapley, P.J.; Papaxanthis, C.; Lepers, R. A prolonged motor imagery session alter imagined and actual movement durations: potential implications for neurorehabilitation. Behav Brain Res 2016, 297, 67 –75. [Google Scholar] [CrossRef]
- Nakashima, A.; Moriuchi, T.; Matsuda, D.; Hasegawa, T.; Nakamura, J.; Anan, K.; Satoh, K.; Suzuki, T.; Higashi, T.; Sugawara, K. Corticospinal excitability during motor imagery is diminished by continuous repetition-induced fatigue. Neural Regen Res 2021, 16, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Moriuchi, T.; Matsuda, D.; Nakamura, J.; Fujiwara, K.; Ikio, Y.; Hasegawa, T.; Mitunaga, W.; Higashi, T. Continuous repetition motor imagery training and physical practice training exert the growth of fatigue and its effect on performance. Brain Sci 2022, 12, 1087. [Google Scholar] [CrossRef]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. Neural plasticity during motor learning with motor imagery practice: review and perspectives. Neuroscience 2017, 341, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. The influence of imagery capacity in motor performance improvement. Exp Brain Res 2017, 235, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Page, S.J.; Levine, P. Multimodal mental practice versus repetitive task practice only to treat chronic stroke: a randomized controlled pilot study. Am J Occup Ther 2021, 75, 7506205020. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, D.; Sirtori, V.; Castellini, G.; Moja, L.; Gatti, R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev 2015, 2015, CD004433. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).