Submitted:
10 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Synergistic Hydration of SGM
3. Fresh Properties of SGM
3.1. Fluidity
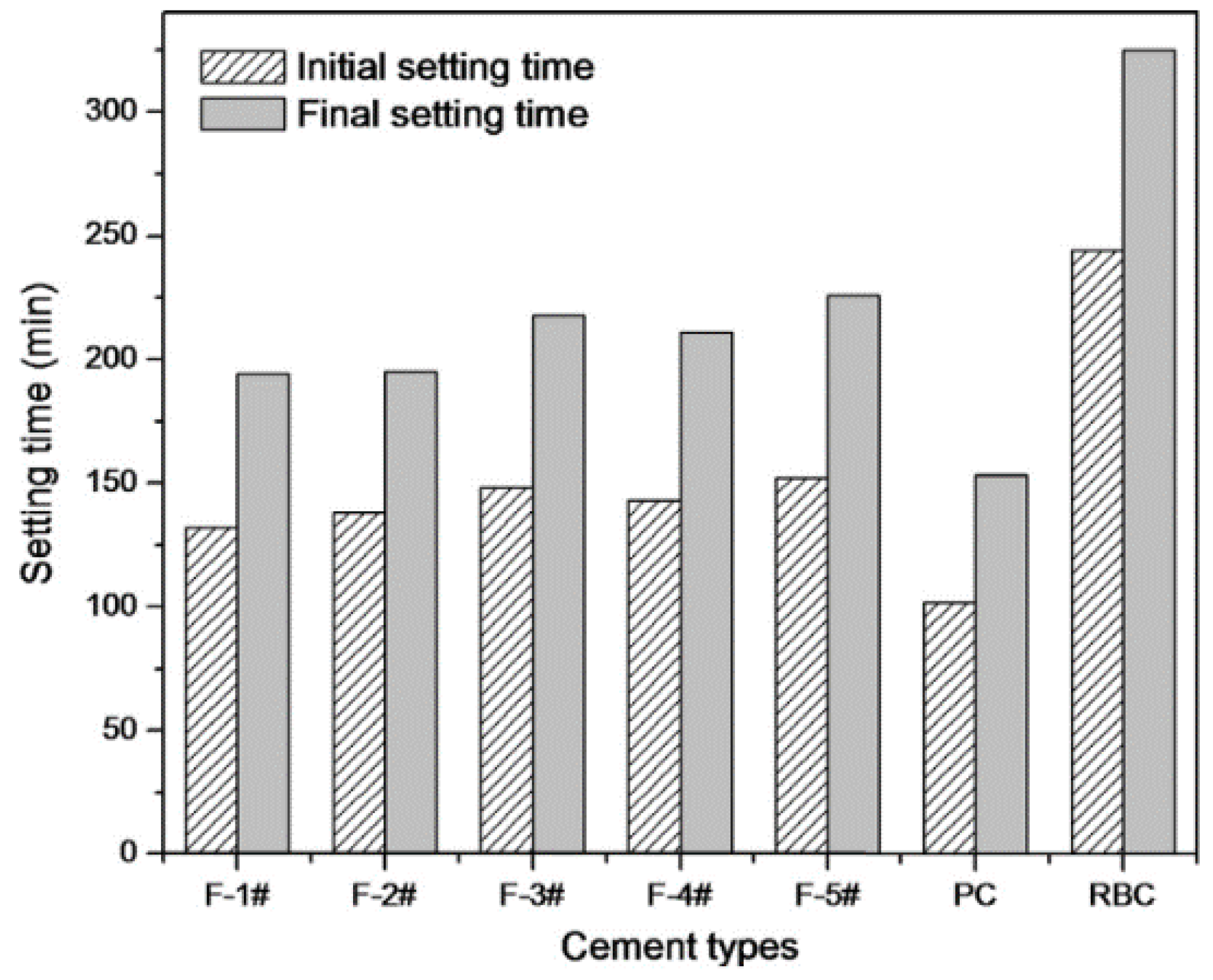
3.2. Setting Time
4. Hardened Properties of SGM
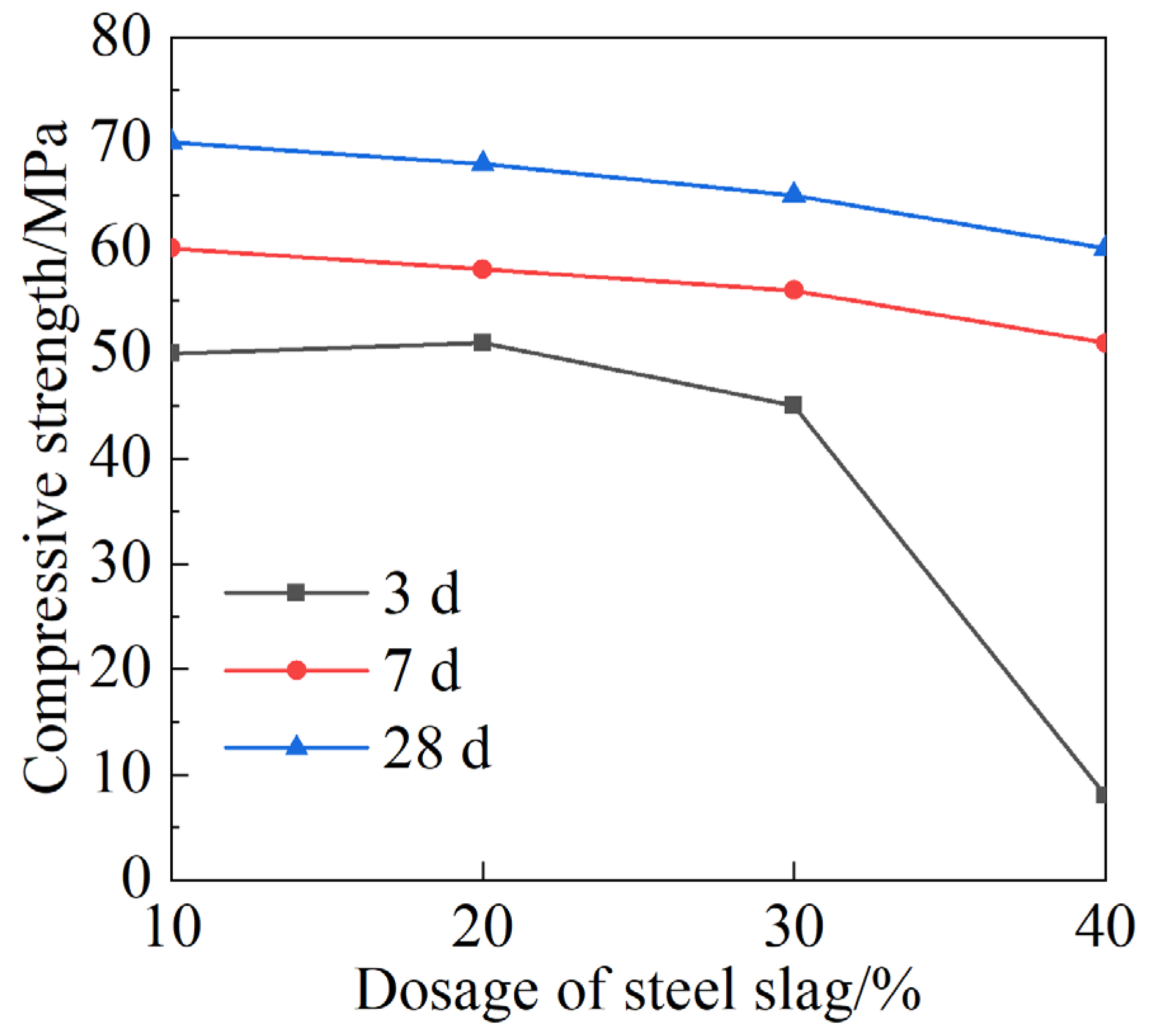
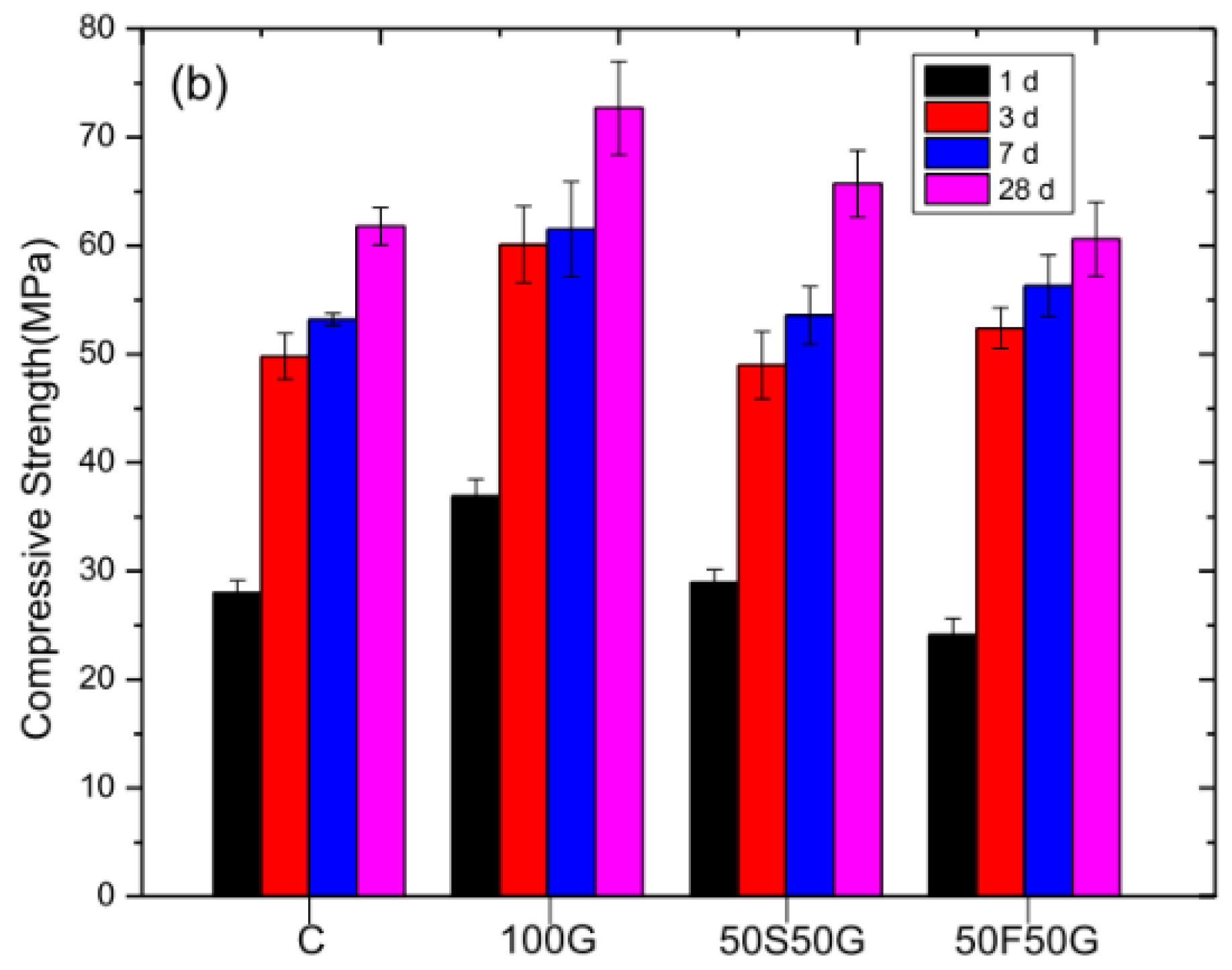
4.1. Mechanical Strength
4.2. Durability
4.3. Shrinkage
5. Conclusion and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li J J, Ni W, Wang X, et al. Mechanical activation of medium basicity steel slag under dry condition for carbonation curing. J. Build. Eng. 2022, 50, 104123. [Google Scholar] [CrossRef]
- Gaow H, Zhou W T, Lyu X J, et al. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Guo J L, Bao Y P, Wang M. Steel slag in China, Treatment, recycling, and management. Waste Manage. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Yang M Q, Yang J Y. Vanadium extraction from steel slag: Generation, recycling and management. Environ. Pollut. 2024, 343, 123126. [Google Scholar] [CrossRef]
- Aliyah F, Kambali I, Setiawan A F, et al. Utilization of steel slag from industrial waste for ionizing radiation shielding concrete: A systematic review. Constr. Build. Mater. 2023, 382, 131360. [Google Scholar] [CrossRef]
- Kurniati E O, Pederson F, Kim H. Application of steel slags, ferronickel slags, and copper mining waste as construction materials: A review. Resour. Conserv. Recy. 2023, 198, 107175. [CrossRef]
- Shu K, Sasaki K. Occurrence of steel converter slag and its high value-added conversion for environmental restoration in China: A review. J. Clean. Prod. 2022, 373, 133876. [Google Scholar] [CrossRef]
- Connor J O, Nguyen T B T, Honeyands T, et al. Production, characterisation, utilisation, and beneficial soil application of steel slag, a review. J. Hazard Mater. 2021, 419, 126478. [CrossRef]
- Borat, J.P. Sustainability footprint of steelmaking byproducts. Ironmak. Steelmak. 2012, 39, 270–277. [Google Scholar] [CrossRef]
- Zhuang S Y, Wang Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Shi C J, Qian J S. High performance cementing materials from industrial slags-a review. Resour. Conserv. Recy. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Luo T, Wang X, Zhuang S. Value-added utilization of steel slag as a hydration heat controlling material to prepare sustainable and green mass concrete. Case Stud. Constr. Mater. 2023, 19, e02619. [Google Scholar]
- Zhuang S Y, Wang Q, Luo T. Effect of C12A7 in steel slag on the early-age hydration of cement. Cem. Concr. Res. 2022, 162, 107010. [Google Scholar] [CrossRef]
- Wang Q, Yan P Y. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Sun J W, Hou S Y, Guo Y H. Effects of high-temperature curing on hydration and microstructure of alkali-activated typical steel slag cementitious material. Developments in the Built Environment, 2024, 17, 100314. [Google Scholar] [CrossRef]
- Li Y, Wu B H, Ni W, et al. Synergies in early hydration reaction of slag-steel slag-gypsum system. J Northeastern Uni. 2020, 41, 581–586. [Google Scholar]
- Cui X W, Ni W, Reng C. Chemical activation of cementitious materials with all solid waste based of steel slag and blast furnace slag. Chinese Journal of Materials Research, 2017, 9, 687–694. [Google Scholar]
- Wang Q, Yan P Y, Feng J W. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J. Hazard Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef]
- Guo X L, Shi H S. Effects of steel slag admixture with GGBFS on performances of cement paste and mortar. Adv. Cem. Res. 2015, 26, 93–100. [Google Scholar]
- Wang Q, Yan P Y, Mi G D. Effect of blended steel slag-GBFS mineral admixture on hydration and strength of cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Singh S P, Tripathy D P, Ranjith P G. Performance evaluation of cement stabilized fly ash-GBFS mixes as a highway construction material. Waste Manage. 2008, 28, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Chen P, Ma B G, Tan H B, et al. Improving the mechanical property and water resistance of β-hemihydrate phosphogypsum by incorporating ground blast-furnace slag and steel slag. Constr. Build. Mater. 2022, 344, 128265. [Google Scholar] [CrossRef]
- Zeng Q S, Liu X M, Zhang Z Q, et al. Synergistic utilization of blast furnace slag with other industrial solid wastes in cement and concrete industry, Synergistic mechanisms, applications, and challenges. Green Energy and Resources, 2023, 1, 100012. [Google Scholar] [CrossRef]
- Zhang M G, Li K Q, Ni W, et al. Preparation of mine backfilling from steel slag-based non-clinker combined with ultra-fine tailing. Constr. Build. Mater. 2022, 320, 126248. [Google Scholar] [CrossRef]
- Wang Q, Miao M, Yan P Y. The influence of high-temperature curing on the hydration characteristics of a cement-GGBS binder. Adv. Cem. Res. 2012, 24, 33–40. [Google Scholar] [CrossRef]
- Xiao B, Wen Z, Envelope S M P, et al. Utilization of steel slag for cemented tailings backfill, Hydration, strength, pore structure, and cost analysis. Case Stud. Constr. Mater. 2021, 15, e00621. [Google Scholar]
- Huang X, Wang Z J, Liu Y, et al. On the use of blast furnace slag and steel slag in the preparation of green artificial reef concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Wang Q, Yang J W, Yan P Y. Cementitious properties of super-fine steel slag. Powder Technol. 2013, 245, 35–39. [Google Scholar] [CrossRef]
- Song S J, Hamlin M J. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar]
- Hamdan A, Song H, Yao Z B, et al. Modifications to reaction mechanisms, phase assemblages and mechanical properties of alkali-activated slags induced by gypsum addition. Cem. Concr. Res. 2023, 174, 107311. [Google Scholar] [CrossRef]
- Li Z, Lu T, Liang X, et al. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Fu Q, Bu M X, Zhang Z, et al. Hydration characteristics and microstructure of alkali-activated slag concrete, A review. Engineering, 2021, 20, 162–179. [Google Scholar]
- Lee K M, Park P J. Estimation of the environmental credit for the recycling of granulated blast furnace slag based on LCA. Resour. Conserv. Recy. 2005, 44, 139–151. [Google Scholar] [CrossRef]
- Zhao J H, Li Z H, Wang D M, et al. Hydration superposition effect and mechanism of steel slag powder and granulated blast furnace slag powder. Constr. Build. Mater. 2023, 336, 130101. [Google Scholar]
- Chia-Jung T, Ran H, Wei-Ting LIN, et al. Using GGBOS as the alkali activators in GGBS and GGBOS blended cements. Constr. Build. Mater. 2014, 70, 501–507. [Google Scholar] [CrossRef]
- Sangita M, Raut S P, Ansari K, et al. Waste slags as sustainable construction materials, a compressive review on physico mechanical properties. J. Mate. Res. Technol. 2023, 23, 5821–5845. [Google Scholar] [CrossRef]
- Zhao, J. Grinding and hydration characteristics of steel slag and composition and properties of composite cementitious materials containing steel. Beijing, Doctoral thesis, China University of Mining and Technology, China, 2015.
- Xiong X L, Yang Z X, Yan X Y et al. Mechanical properties and microstructure of engineered cementitious composites with high volume steel slag and GGBFS. Constr. Build. Mater. 2014, 70, 501–507. [Google Scholar]
- Ghorbani S, Stefanini L, Sun Y B, et al. Characterisation of alkali-activated stainless steel slag and blast-furnace slag cements. Cement Concre. Comp. 2023, 143, 105230. [Google Scholar] [CrossRef]
- Siddique R, Bennacer R. Use of iron and steel industry by-product (GGBS) in cement paste and mortar. Resour. Conserv. Recy. 2012, 69, 29–34. [Google Scholar] [CrossRef]
- You N, Li B, Cao R, et al. The influence of steel slag and ferronickel slag on the properties of alkali-activated slag mortar. Constr. Build. Mater. 2019, 227, 116614. [Google Scholar] [CrossRef]
- Li J S, Li J M, Ge X, et al. Preparation and characterization of early strengthened of binding materials steel slag and blast furnace slag. Journal of Anhui University of Technology (Natural Science), 2020, 37, 321–326. [Google Scholar]
- Zhou Y Q, Sun J, Liao Y W. Influence of ground granulated blast furnace slag on the early hydration and microstructure of alkali-activated converter steel slag binder. J. Therm. Anal. Calorim. 2020, 182, 1–10. [Google Scholar]
- Sun J W, Chen Z H. Effect of silicate modulus of water glass on the hydration of alkali-activated converter steel slag. J. Therm. Anal. Calorim. 2019, 138, 1–10. [Google Scholar]
- Atis C D, Bilim C, Celik O, et al. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- A T L, A H S, A Z A, et al. Influence of sodium silicate powder silica modulus for mechanical and chemical properties of dry-mix alkali-activated slag mortar. Constr. Build. Mater. 2020, 233, 117354. [Google Scholar] [CrossRef]
- Liu Z Y, Ni W, Li Y, et al. The mechanism of hydration reaction of granulated blast furnace slag-steel slag-refining slag-desulfurization gypsum-based clinker-free cementitious materials. J. Build. Eng. 2021, 40, 103289. [Google Scholar]
- Li Y Y, Liang W T, Ni W, et al. Characteristics of hydration and hardening of steel slag mud-blast furnace slag-desulphurization gypsum system. Bulletin of The Chinese Ceramic Society, 2022, 41, 536–544. [Google Scholar]
- Xu C W, Ni W, Li K Q, et al. Activation mechanisms of three types of industrial by-product gypsums on steel slag-granulated blast furnace slag-based binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Zhao J H, Wang D M, Yan P Y. Design and experimental study of a ternary blended cement containing high volume steel slag and blast-furnace slag based on Fuller distribution model. Constr. Build. Mater. 2017, 140, 248–256. [Google Scholar] [CrossRef]
- You N Q, Li B L, Cao R L, et al. The influence of steel slag and ferronickel slag on the properties of alkali activated slag mortar. Constr. Build. Mater. 2019, 227, 116614. [Google Scholar] [CrossRef]
- Feng J J, Sun J W. A comparison of the 10-year properties of converter steel slag activated by high temperature and an alkaline activator. Constr. Build. Mater. 2020, 234, 116948. [Google Scholar] [CrossRef]
- Maria C, Willian A, Isabel S. Microstructural and Mechanical Properties of Alkali Activated Colombian Raw Materials. Materials, 2016, 9, 158. [Google Scholar] [CrossRef]
- Sun J W, Zhang Z Q, Zhuang S Y, et al. Hydration properties and microstructure characteristics of alkali-activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Cao R L, Li B L, Nan Q Y, et al. Properties of alkali-activated ground granulated blast furnace slag blended with ferronickel slag. Constr. Build. Mater. 2018, 192, 123–132. [Google Scholar] [CrossRef]
- Zhang G Q, Wu P C, Gao S J. Properties and microstructure of low-carbon whole-tailings cemented paste backfill material containing steel slag, granulated blast furnace slag and flue gas desulphurization gypsum. Acta Microsc. 2019, 28, 770–780. [Google Scholar]
- Brakat A, Zhang Y. Shrinkage mitigation of alkali activated slag with natural cellulose fibers. Adv. Cem. Res. 2017, 31, 47–57. [Google Scholar]
- Furlani E, Maschiom S, Magnane M, et al. Synthesis and characterization of geopolymers containing blends of unprocessed steel slag and metakaolin, The role of slag particle size. Ceram. Int. 2018, 44, 5226–5232. [Google Scholar] [CrossRef]
- Hu S G, He Y J, Lu L N, et al. Effect of fine steel slag powder on the early hydration process of Portland cement. Journal of Wuhan University of Technology (Materials Science Edition), 2006, 21, 147–149. [Google Scholar] [CrossRef]
- İ. A A, Ismail Y. Study on steel furnace slags with high MgO as additive in Portland cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Liu K S, Zhang Z Q, Sun J W. Advances in understanding the alkali-activated metallurgical slag. Adv. Civ. Eng. 2021, 2021, 8795588.
- Xu C W, Ni W, Li K Q, et al. Hydration mechanism and orthogonal optimisation of mix proportion for steel slag-slag-based clinker-free prefabricated concrete. Constr. Build. Mater. 2019, 228, 117036. [Google Scholar] [CrossRef]
- Xiao, B. Steel slag binder and its application in the cemented tailings backfill. Doctoral thesis, University of Science and Technology, Beijing, China, 2020. [Google Scholar]
- Adesanya E, Ohenoja K, Maria A D, et al. Alternative alkali-activator from steel-making waste for one-part alkali-activated slag. J. Clean. Prod. 2020, 274, 123020. [Google Scholar] [CrossRef]
- Han F H, Yan P Y. Hydration characteristics of slag-blended cement at different temperatures. J. Sustain. Cem. -Based. 2015, 4, 34–43. [Google Scholar]
- B. A C, P. W B. Formation of calcium sulfoaluminate hydrate compounds. Cem. Concr. Res. 2000, 30, 233–240. [Google Scholar] [CrossRef]
- E. G, P.W. B, J.V. B J. The formation of ettringite at elevated temperature. Cem. Concr. Res. 1993, 23, 981–987. [Google Scholar] [CrossRef]
- Wang M Q, Qian B B, Jiang J, et al. The reaction between Ca2+ from steel slag and granulated blast-furnace slag system, a unique perspective. Chem. Pap. 2020, 74, 4401–4410. [Google Scholar] [CrossRef]
- Xiang X D, Xi J C, Li C H, et al. Preparation and application of the cement-free steel slag cementitious material. Constr. Build. Mater. 2016, 114, 874–879. [Google Scholar] [CrossRef]
- Li Q, Zhao F Q, Li H, et al. Durability of slag and steel slag-based cementitious materials. China Concrete and Cement Products, 2011, 1, 23–27. [Google Scholar]
- Wang X, Ni W, Li J J, Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [CrossRef]
- Nan Q Y, Shi J J, Zhang Y M. Corrosion behaviour of low-carbon steel reinforcement in alkali-activated slag-steel slag and Portland cement-based mortars under simulated marine environment. Corros. Sci. 2020, 175, 108874. [Google Scholar] [CrossRef]
- Sun J W, Chen Z H. Effect of silicate modulus of water glass on the hydration of alkali-activated converter steel slag. J. Therm. Anal. Calorim. 2019, 138, 47–56. [Google Scholar] [CrossRef]
- Shi C J, Qu B, Provis J L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Zhang B, Zhu H, Feng P, et al. , A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems, effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Liu S H, Li Q L, Han W W. Effect of various alkalis on hydration properties of alkali-activated slag cements. J. Therm. Anal. Calorim. 2018, 131, 3093–3104. [Google Scholar] [CrossRef]
- Hou D H, Li T, Wang P. Molecular dynamics study on the structure and dynamics of NaCl solution transport in the nanometer channel of CASH gel. Acs Sustain. Chem. Eng. 2018, 7, 9498–9509. [Google Scholar]
- Sun X F, Peng X Q, Zhang G Z, et al. Effect of steel slag content on properties of alkali-activated steel slag and slag based grouting material. New Building Materials, 2017, 44, 10–13. [Google Scholar]
- Darko K, Branislav Z. Effects of dosage and modulus of water glass on early hydration of alkali-slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [CrossRef]
- Bernal S A, Gutierrez R M, Provis J L, et al. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



