Submitted:
10 January 2024
Posted:
11 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
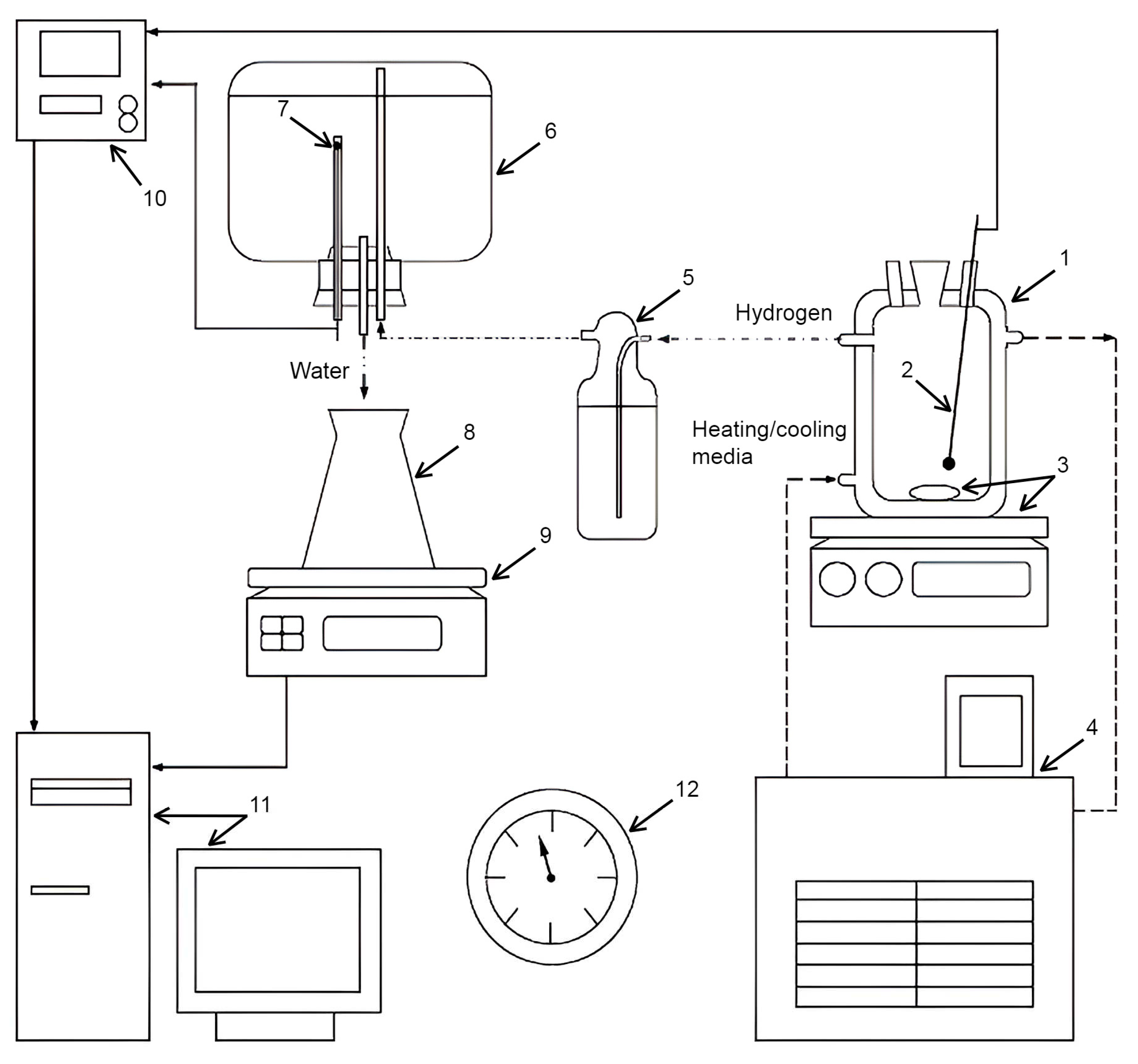
2. Materials and Methods
3. Results and Discussion
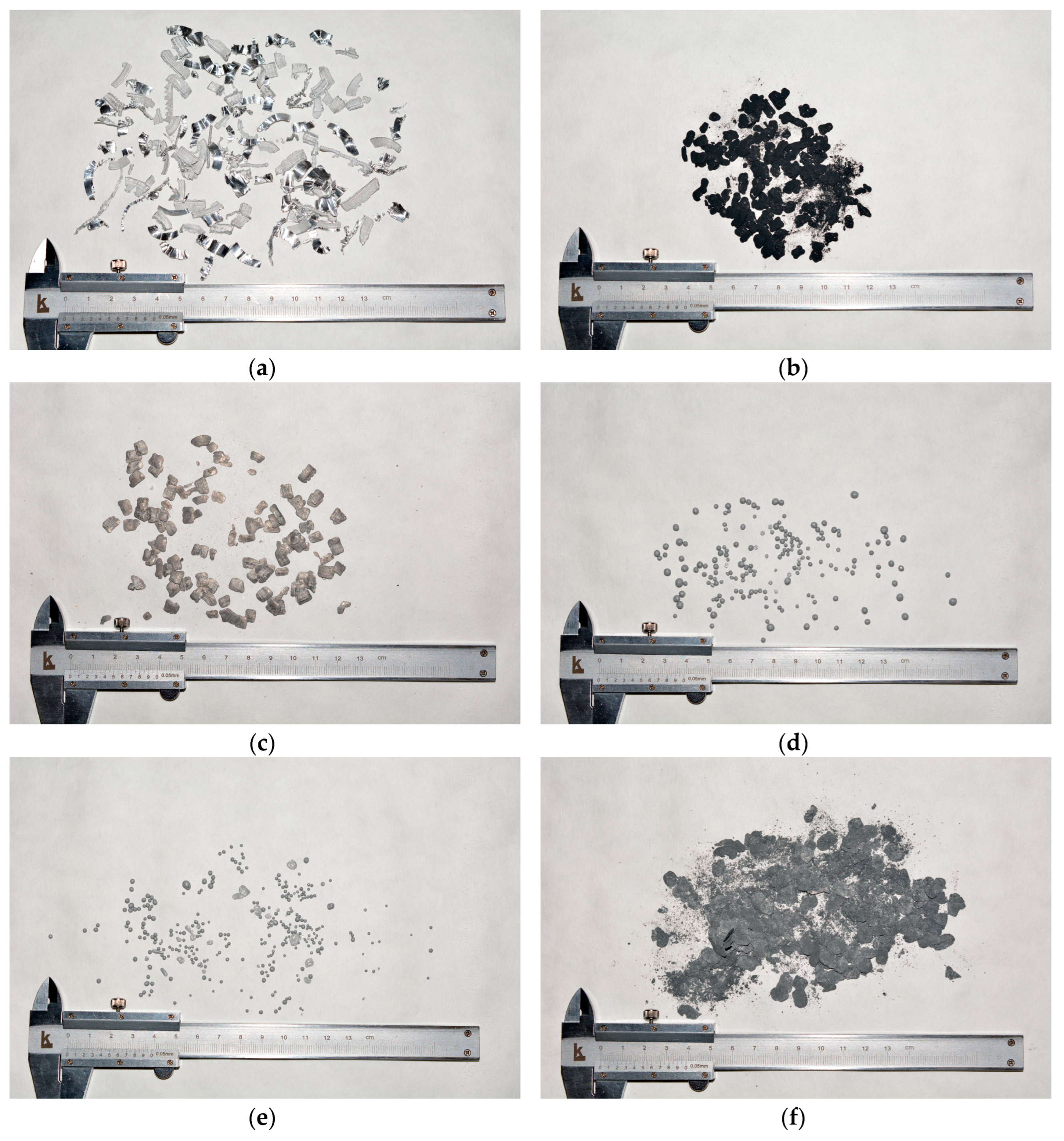
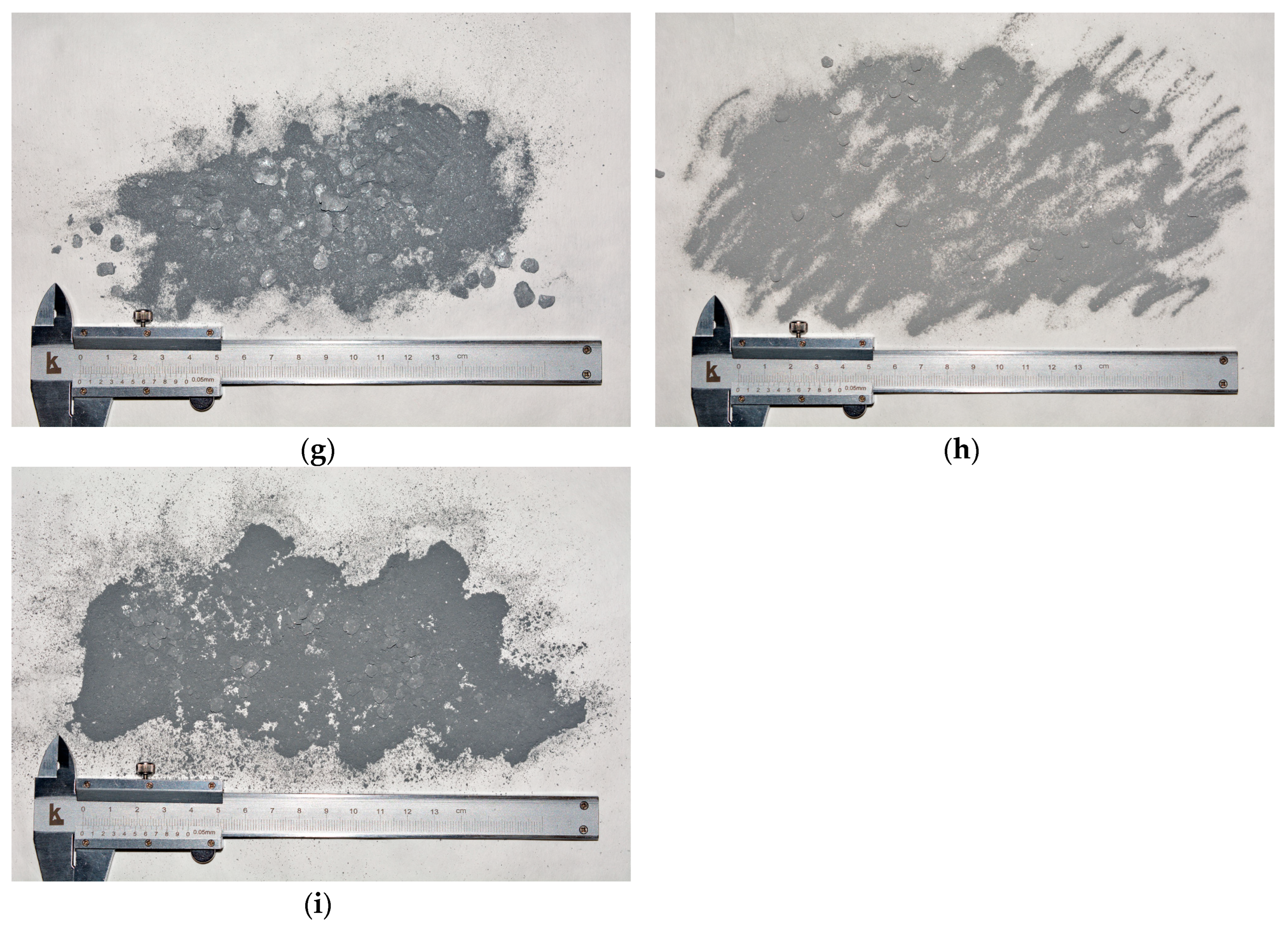
3.1. Trials for Powder Manufacture
3.2. Phase Composition
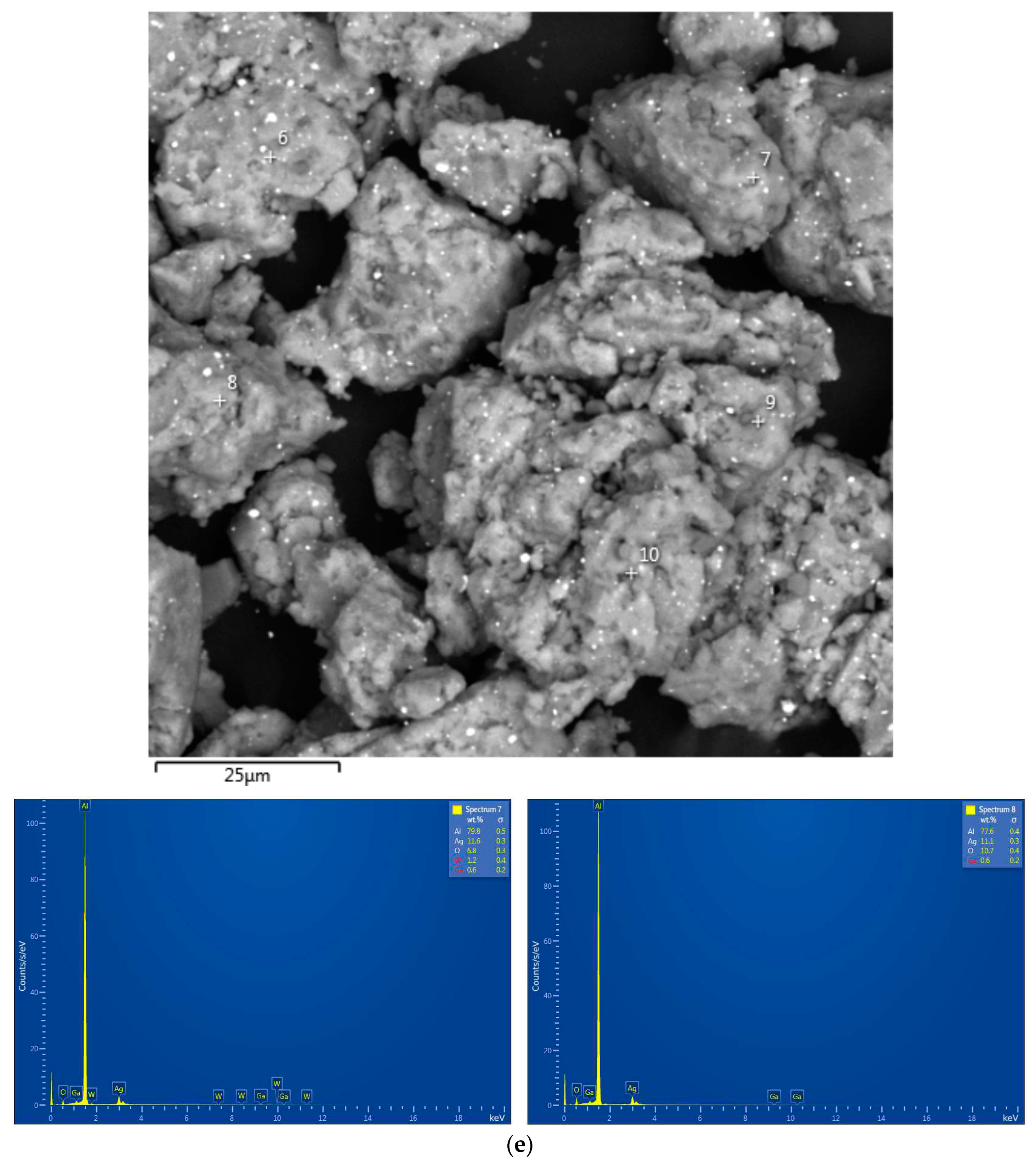
3.3. Microstructure, Specific Surface Area, and Elemental Composition
3.4. Reaction Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wang, J.; Su, Z.; Li, H.; Ding, L.; Zhu, H.; Gaidai, O. Imposing a wake effect to improve clean marine energy harvesting by flow-induced vibrations. Ocean Engineering 2020, 208, 107455. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, X. Synchronous steam generation and photodegradation for clean water generation based on localized solar energy harvesting. Energy Conversion and Management 2018, 173, 158–166. [Google Scholar] [CrossRef]
- Al-Qadami, E.H.H.; Mustaffa, Z.; Al-Atroush, M.E. Evaluation of the Pavement Geothermal Energy Harvesting Technologies towards Sustainability and Renewable Energy. Energies 2022, 15, 1201. [Google Scholar] [CrossRef]
- Perera, S.M.H.D.; Putrus, G.; Conlon, M.; Narayana, M.; Sunderland, K. Wind Energy Harvesting and Conversion Systems: A Technical Review. Energies 2022, 15, 9299. [Google Scholar] [CrossRef]
- Kharel, S.; Shabani, B. Hydrogen as a Long-Term Large-Scale Energy Storage Solution to Support Renewables. Energies 2018, 11, 2825. [Google Scholar] [CrossRef]
- Amirante, R.; Cassone, E.; Distaso, E.; Tamburrano, P. Overview on recent developments in energy storage: Mechanical, electrochemical and hydrogen technologies. Energy Conversion and Management 2017, 132, 372–387. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, Q.; Tapia-Bastidas, C.; Gray, E.; Irwanto, B.; Venezuela, J.; Liu, Q. Influence of Hydrogen on Steel Components for Clean Energy. Corrosion and Materials Degradation 2020, 1, 3–26. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Deterioration of Property of Aluminum Alloys (EN AW-1050A, EN AW-5754 and EN AW-6060) by Absorbed Hydrogen. Applied Sciences 2022, 12, 1392. [Google Scholar] [CrossRef]
- Cui, S.; Zhu, G.; He, L.; Wang, X.; Zhang, X. Analysis of the fire hazard and leakage explosion simulation of hydrogen fuel cell vehicles. Thermal Science and Engineering Progress 2023, 41, 101754. [Google Scholar] [CrossRef]
- Lin, H.; Luan, H.; Yang, L.; Han, C.; Zhang, S.; Zhu, H.; Chen, G. Numerical simulation and consequence analysis of accidental hydrogen fires in a conceptual offshore hydrogen production platform. International Journal of Hydrogen Energy 2023, 48, 10250–10263. [Google Scholar] [CrossRef]
- Li, J.-Q.; Li, J.-C.L.; Park, K.; Kwon, J.-T. Investigation on the changes of pressure and temperature in high pressure filling of hydrogen storage tank. Case Studies in Thermal Engineering 2022, 37, 102143. [Google Scholar] [CrossRef]
- Li, J.-Q.; Li, J.-C.; Park, K.; Jang, S.-J.; Kwon, J.-T. An Analysis on the Compressed Hydrogen Storage System for the Fast-Filling Process of Hydrogen Gas at the Pressure of 82 MPa. Energies 2021, 14, 2635. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. International Journal of Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, T.; Son, Y.; Park, J.; Kim, M.; Eun, H.; Park, J.-W.; Kim, Y. Metallic Material Evaluation of Liquid Hydrogen Storage Tank for Marine Application Using a Tensile Cryostat for 20 K and Electrochemical Cell. Processes 2022, 10, 2401. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. Journal of Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage – past, recent progress and future outlook. Journal of Alloys and Compounds 2020, 827, 153548. [Google Scholar] [CrossRef]
- Bunker, B.C.; Nelson, G.C.; Zavadil, K.R.; Barbour, J.C.; Wall, F.D.; Sullivan, J.P.; Windisch, C.F.; Engelhardt, M.H.; Baer, D.R. Hydration of Passive Oxide Films on Aluminum. The Journal of Physical Chemistry B 2002, 106, 4705–4713. [Google Scholar] [CrossRef]
- Grigorenko, A.V.; Ambaryan, G.N.; Valyano, G.E.; Vlaskin, M.S.; Gromov, A.A.; Zmanovsky, S.V. Kinetics of Aluminum Micron Powder Oxidation in Hot Distilled Water and Product Microstructure Investigation. IOP Conference Series: Materials Science and Engineering 2018, 381, 012028. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S.; Grigorenko, A.V. Effect of Thermal Treatment of Aluminum Core-Shell Particles on Their Oxidation Kinetics in Water for Hydrogen Production. Materials 2021, 14, 6493. [Google Scholar] [CrossRef]
- Kader, M.S.; Zeng, W.; Johnston, E.; Buckner, S.W.; Jelliss, P.A. A Novel Method for Generating H2 by Activation of the μAl-Water System Using Aluminum Nanoparticles. Applied Sciences 2022, 12, 5378. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Dudoladov, A.O.; Buryakovskaya, O.A.; Ambaryan, G.N. Modelling of aluminum-fuelled power plant with steam-hydrogen enthalpy utilization. International Journal of Hydrogen Energy 2018, 43, 4623–4631. [Google Scholar] [CrossRef]
- Trowell, K.; Goroshin, S.; Frost, D.; Bergthorson, J. Hydrogen production rates of aluminum reacting with varying densities of supercritical water. RSC Advances 2022, 12, 12335–12343. [Google Scholar] [CrossRef] [PubMed]
- Trowell, K.A.; Goroshin, S.; Frost, D.L.; Bergthorson, J.M. The use of supercritical water for the catalyst-free oxidation of coarse aluminum for hydrogen production. Sustainable Energy & Fuels 2020, 4, 5628–5635. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L.; Stowe, R.; Ringuette, S. Enhanced hydrogen generation from aluminum-water reactions. International Journal of Hydrogen Energy 2013, 38, 14992–15002. [Google Scholar] [CrossRef]
- Godart, P.; Fischman, J.; Seto, K.; Hart, D. Hydrogen production from aluminum-water reactions subject to varied pressures and temperatures. International Journal of Hydrogen Energy 2019, 44, 11448–11458. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Valyano, G.E.; Zhuk, A.Z.; Shkolnikov, E.I. Oxidation of coarse aluminum in pressured water steam for energy applications. International Journal of Energy Research 2020, 44, 8689–8715. [Google Scholar] [CrossRef]
- Kwon, J.; Eom, K.; Kim, M.; Toor, I.; Oh, S.; Kwon, H. Fabrication of Al-Ni Alloys for Fast Hydrogen Production from Hydrolysis in Alkaline Water. Materials 2023, 16, 7425. [Google Scholar] [CrossRef] [PubMed]
- Mezulis, A.; Richter, C.; Lesnicenoks, P.; Knoks, A.; Varnagiris, S.; Urbonavicius, M.; Milcius, D.; Kleperis, J. Studies on Water–Aluminum Scrap Reaction Kinetics in Two Steps and the Efficiency of Green Hydrogen Production. Energies 2023, 16, 5554. [Google Scholar] [CrossRef]
- Kale, M.; Yılmaz, İ.H.; Kaya, A.; Çetin, A.E.; Söylemez, M.S. Pilot-scale hydrogen generation from the hydrolysis of black aluminum dross without any catalyst. Journal of the Energy Institute 2022, 100, 99–108. [Google Scholar] [CrossRef]
- Tekade, S.P.; Shende, D.Z.; Wasewar, K.L. Hydrogen Generation in an Annular Micro-Reactor: An Experimental Investigation and Reaction Modelling by Shrinking Core Model (SCM). International Journal of Chemical Reactor Engineering 2018, 16. [Google Scholar] [CrossRef]
- Tekade, S.P.; Pednekar, A.S.; Jadhav, G.R.; Kalekar, S.E.; Shende, D.Z.; Wasewar, K.L. Hydrogen generation through water splitting reaction using waste aluminum in presence of gallium. International Journal of Hydrogen Energy 2020, 45, 23954–23965. [Google Scholar] [CrossRef]
- Tekade, S.P.; Shende, D.Z.; Wasewar, K.L. Hydrogen Generation in an Annular Micro-Reactor: an Experimental Investigation of Water Splitting Reaction Using Aluminum in Presence of Potassium Hydroxide. International Journal of Chemical Reactor Engineering 2019, 17. [Google Scholar] [CrossRef]
- Ambaryan, G.N.; Vlaskin, M.S.; Dudoladov, A.O.; Meshkov, E.A.; Zhuk, A.Z.; Shkolnikov, E.I. Hydrogen generation by oxidation of coarse aluminum in low content alkali aqueous solution under intensive mixing. International Journal of Hydrogen Energy 2016, 41, 17216–17224. [Google Scholar] [CrossRef]
- Liu, H.; Yang, F.; Yang, B.; Zhang, Q.; Chai, Y.; Wang, N. Rapid hydrogen generation through aluminum-water reaction in alkali solution. Catalysis Today 2018, 318, 52–58. [Google Scholar] [CrossRef]
- Yang, B.C.; Chai, Y.J.; Yang, F.L.; Zhang, Q.; Liu, H.; Wang, N. Hydrogen generation by aluminum-water reaction in acidic and alkaline media and its reaction dynamics. International Journal of Energy Research 2018, 42, 1594–1602. [Google Scholar] [CrossRef]
- Kanehira, S.; Kanamori, S.; Nagashima, K.; Saeki, T.; Visbal, H.; Fukui, T.; Hirao, K. Controllable hydrogen release via aluminum powder corrosion in calcium hydroxide solutions. Journal of Asian Ceramic Societies 2013, 1, 296–303. [Google Scholar] [CrossRef]
- Alviani, V.N.; Hirano, N.; Watanabe, N.; Oba, M.; Uno, M.; Tsuchiya, N. Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water. Applied Energy 2021, 293, 116909. [Google Scholar] [CrossRef]
- Martínez-Salazar, A.L.; Melo-Banda, J.A.; Coronel-García, M.A.; González-Barbosa, J.J.; Domínguez-Esquivel, J.M. Hydrogen generation by aluminum alloy corrosion in aqueous acid solutions promoted by nanometal: Kinetics study. Renewable Energy 2020, 146, 2517–2523. [Google Scholar] [CrossRef]
- Alviani, V.N.; Setiani, P.; Uno, M.; Oba, M.; Hirano, N.; Watanabe, N.; Tsuchiya, N.; Saishu, H. Mechanisms and possible applications of the Al–H2O reaction under extreme pH and low hydrothermal temperatures. International Journal of Hydrogen Energy 2019, 44, 29903–29921. [Google Scholar] [CrossRef]
- Hiraki, T.; Takeuchi, M.; Hisa, M.; Akiyama, T. Hydrogen Production from Waste Aluminum at Different Temperatures, with LCA. MATERIALS TRANSACTIONS 2005, 46, 1052–1057. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Shi, X.; Wang, N.; Chai, Y. Synergistic effect of the fresh Co, Ni, and anion ions on aluminum or magnesium with water reactions. International Journal of Energy Research 2019, 43, 430–438. [Google Scholar] [CrossRef]
- Cuzacq, L.; Polido, C.; Silvain, J.-F.; Bobet, J.-L. Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2. Metals 2023, 13, 1868. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Kurbatova, A.I.; Vlaskin, M.S.; Valyano, G.E.; Grigorenko, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Waste to Hydrogen: Elaboration of Hydroreactive Materials from Magnesium-Aluminum Scrap. Sustainability 2022, 14, 4496. [Google Scholar] [CrossRef]
- Eom, K.; Cho, E.; Kwon, H. Feasibility of on-board hydrogen production from hydrolysis of Al-Fe alloy for PEMFCs. International Journal of Hydrogen Energy 2011, 36, 12338–12342. [Google Scholar] [CrossRef]
- Kim, M.; Eom, K.; Kwon, J.; Cho, E.; Kwon, H. On-board hydrogen production by hydrolysis from designed Al-Cu alloys and the application of this technology to polymer electrolyte membrane fuel cells. Journal of Power Sources 2012, 217, 345–350. [Google Scholar] [CrossRef]
- Eom, K.; Kim, M.; Oh, S.; Cho, E.; Kwon, H. Design of ternary Al-Sn-Fe alloy for fast on-board hydrogen production, and its application to PEM fuel cell. International Journal of Hydrogen Energy 2011, 36, 11825–11831. [Google Scholar] [CrossRef]
- Kahveci, O.; Kaya, M.F. Hydrogen production from Al–Cu alloy using electric vehicle's waste DC motor coils. International Journal of Hydrogen Energy 2022, 47, 12179–12188. [Google Scholar] [CrossRef]
- Eom, K.S.; Kwon, J.Y.; Kim, M.J.; Kwon, H.S. Design of Al-Fe alloys for fast on-board hydrogen production from hydrolysis. Journal of Materials Chemistry 2011, 21, 13047–13051. [Google Scholar] [CrossRef]
- He, T.; Xiong, Y.; Du, S.; Yuan, Z.; Liang, X.; Huttula, M.; Cao, W. Impact of Li Addition in Al-Rich Alloys on Hydrogen Production in Water. Journal of Materials Engineering and Performance 2019, 28, 2459–2464. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, H.; Shi, J.; Wang, H.; Gao, X.; Gao, Q.; Sun, X. Unveiling the role of indium and tin in Al–Ga based alloys for on-demand hydrogen supply from simulation to validation. Journal of Power Sources 2023, 554, 232268. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, Y.; Tang, Z.; Fan, X.; Wang, T.; Li, T.; Liaw, P.K. The superior hydrogen-generation performance of multi-component Al alloys by the hydrolysis reaction. International Journal of Hydrogen Energy 2019, 44, 3527–3537. [Google Scholar] [CrossRef]
- Alinejad, B.; Mahmoodi, K. A novel method for generating hydrogen by hydrolysis of highly activated aluminum nanoparticles in pure water. International Journal of Hydrogen Energy 2009, 34, 7934–7938. [Google Scholar] [CrossRef]
- Mahmoodi, K.; Alinejad, B. Enhancement of hydrogen generation rate in reaction of aluminum with water. International Journal of Hydrogen Energy 2010, 35, 5227–5232. [Google Scholar] [CrossRef]
- Skrovan, J.; Alfantazi, A.; Troczynski, T. Enhancing aluminum corrosion in water. Journal of Applied Electrochemistry 2009, 39, 1695. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of addition of water-soluble salts on the hydrogen generation of aluminum in reaction with hot water. Journal of Alloys and Compounds 2016, 679, 364–374. [Google Scholar] [CrossRef]
- Zhu, L.; Zou, M.; Zhang, X.; Zhang, L.; Wang, X.; Song, T.; Wang, S.; Li, X. Enhanced Hydrogen Generation Performance of Al-Rich Alloys by a Melting-Mechanical Crushing-Ball Milling Method. Materials 2021, 14, 7889. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guan, X.; Wang, H.; Dong, S.; Luo, P. Hydrogen generation from splitting water with Al–Bi(OH)3 composite promoted by NaCl. International Journal of Hydrogen Energy 2020, 45, 13139–13148. [Google Scholar] [CrossRef]
- Wang, H.-W.; Chung, H.-W.; Teng, H.-T.; Cao, G. Generation of hydrogen from aluminum and water – Effect of metal oxide nanocrystals and water quality. International Journal of Hydrogen Energy 2011, 36, 15136–15144. [Google Scholar] [CrossRef]
- Dupiano, P.; Stamatis, D.; Dreizin, E.L. Hydrogen production by reacting water with mechanically milled composite aluminum-metal oxide powders. International Journal of Hydrogen Energy 2011, 36, 4781–4791. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Tang, Y.-B.; Zhu, L.-L.; Sakka, Y.; Ye, J. Effect of different modification agents on hydrogen-generation by the reaction of Al with water. International Journal of Hydrogen Energy 2010, 35, 9561–9568. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum–water reaction. International Journal of Hydrogen Energy 2013, 38, 795–806. [Google Scholar] [CrossRef]
- Amrani, M.A.; Haddad, Y.; Obeidat, F.; Ghaleb, A.M.; Mejjaouli, S.; Rahoma, I.; Galil, M.S.A.; Shameeri, M.; Alsofi, A.A.; Saif, A. Productive and Sustainable H2 Production from Waste Aluminum Using Copper Oxides-Based Graphene Nanocatalysts: A Techno-Economic Analysis. Sustainability 2022, 14, 15256. [Google Scholar] [CrossRef]
- Amrani, M.A.; Alrafai, H.A.; Al-nami, S.Y.; Obeidat, F.; Alwahbani, F.; Alhammadi, M.A.; Qasem, A. Green synthesis of Size-Controlled copper oxide nanoparticles as catalysts for H2 production from industrial waste aluminum. International Journal of Energy Research 2022, 46, 14023–14035. [Google Scholar] [CrossRef]
- Wei, H.; Wang, C.; Yang, S.; Yin, B.; Huang, Y.; Yu, F.; Han, J.; Lu, Y.; Liu, X. Integrated design of hydrogen production and thermal energy storage functions of Al-Bi-Cu composite powders. International Journal of Hydrogen Energy 2023, 48, 14931–14940. [Google Scholar] [CrossRef]
- Wang, C.; Yin, B.; Lin, K.; Wang, M.; Deng, R.; Guo, Y.; Zhang, J.; Yang, S.; Liu, X. Effect of Fe on the Hydrogen Production Properties of Al-Bi-Sn Composite Powders. Materials 2022, 15, 6702. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Du Preez, S.P.; Bessarabov, D.G. The Hydrolysis of Ball-Milled Aluminum–Bismuth–Nickel Composites for On-Demand Hydrogen Generation. Energies 2022, 15, 2356. [Google Scholar] [CrossRef]
- Davies, J.; du Preez, S.P.; Bessarabov, D.G. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites. Materials 2022, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ji, F.; Cheng, D.; Yin, C.; Niu, J.; Brnic, J. Hydrolysis-Based Hydrogen Generation Investigation of Aluminum System Adding Low-Melting Metals. Energies 2021, 14, 1433. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Ambaryan, G.N.; Suleimanov, M.Z.; Tarasenko, A.B.; Vlaskin, M.S. Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy. Materials 2023, 16, 4450. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Liu, Y.; Chen, X.; Zou, H.; Qiu, C.; Yang, S.; Liu, X. Design and Fabrication of High Activity Retention Al-Based Composite Powders for Mild Hydrogen Generation. Materials 2019, 12, 3328. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Ambaryan, G.N.; Tarasenko, A.B.; Suleimanov, M.Z.; Vlaskin, M.S. Effects of Bi–Sn–Pb Alloy and Ball-Milling Duration on the Reactivity of Magnesium–Aluminum Waste-Based Materials for Hydrogen Production. Materials 2023, 16, 4745. [Google Scholar] [CrossRef] [PubMed]
- Ambaryan, G.N.; Buryakovskaya, O.A.; Kumar, V.; Valyano, G.E.; Kiseleva, E.A.; Grigorenko, A.V.; Vlaskin, M.S. Hydrothermal Oxidation of Coarse Aluminum Granules with Hydrogen and Aluminum Hydroxide Production: The Influence of Aluminum Purity. Applied Sciences 2023, 13, 7793. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Ren, F.; Stock, H.-R. Effect of different Cu contents on the microstructure and hydrogen production of Al–Cu-Ga-In-Sn alloys for dissolvable materials. Journal of Alloys and Compounds 2020, 821, 153489. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Du, S.; Deng, S. Microstructure and hydrogen production of the rapidly solidified Al–Mg-Ga-In-Sn alloy. Journal of Alloys and Compounds 2020, 827, 154290. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Chen, X.; Yang, S.; Wang, C. Hydrogen generation from hydrolysis of activated Al-Bi, Al-Sn powders prepared by gas atomization method. International Journal of Hydrogen Energy 2017, 42, 10943–10951. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Zou, J.; Shen, X.; Qin, K.; Ban, C.; Cui, J.; Nagaumi, H. Enhancement of the discharge performance of Al-0.5Mg-0.1Sn-0.05Ga (wt.%) anode for Al-air battery by directional solidification technique and subsequent rolling process. Journal of Alloys and Compounds 2020, 827, 154272. [Google Scholar] [CrossRef]
- Han, Z.; Xu, Y.; Zhou, S.; Xu, L.; Zhu, P. The Effect of Annealing on Electrochemical Performances of an Al–Sn–Ga–Mg Alloy as an Anode for Al–air Batteries in Alkaline Electrolytes. Journal of The Electrochemical Society 2020, 167, 100541. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Zheng, Y.; Zou, J.; Yang, D.; Guo, C.; Qin, K.; Ban, C.; Cui, J.; Nagaumi, H. Electrochemical behaviors and discharge properties of Al–Mg–Sn–Ca alloys as anodes for Al-air batteries. Journal of Power Sources 2021, 493, 229724. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Ryu, H.-Y.; Cho, S.-S.; Han, M.-H.; Bae, K.-S.; Lee, J.-H. Effect of Si content on H2 production using Al–Si alloy powders. International Journal of Hydrogen Energy 2011, 36, 15111–15118. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Yun, K.-S.; Kalubarme, R.S.; Park, C.-N.; Park, C.-J. Hydrogen generation using the corrosion of Al-Sn and Al-Si alloys in an alkaline solution. Metals and Materials International 2014, 20, 619–627. [Google Scholar] [CrossRef]
- Eom, K.S.; Kwon, J.Y.; Kim, M.J.; Kwon, H.S. Design of Al–Fe alloys for fast on-board hydrogen production from hydrolysis. Journal of Materials Chemistry 2011, 21, 13047–13051. [Google Scholar] [CrossRef]
- Kim, M.; Eom, K.; Kwon, J.; Cho, E.; Kwon, H. On-board hydrogen production by hydrolysis from designed Al–Cu alloys and the application of this technology to polymer electrolyte membrane fuel cells. Journal of Power Sources 2012, 217, 345–350. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Iturbe-García, J.L.; González-Zavala, F.; Solis-Casados, D.A.; Pérez-Hernández, R.; Haro-Poniatowski, E. Hydrogen production by ultrasound assisted liquid laser ablation of Al, Mg and Al-Mg alloys in water. Applied Surface Science 2019, 478, 189–196. [Google Scholar] [CrossRef]
- Soler, L.; Macanás, J.; Muñoz, M.; Casado, J. Aluminum and aluminum alloys as sources of hydrogen for fuel cell applications. Journal of Power Sources 2007, 169, 144–149. [Google Scholar] [CrossRef]
- Rajulwar, V.V.; Shyrokykh, T.; Stirling, R.; Jarnerud, T.; Korobeinikov, Y.; Bose, S.; Bhattacharya, B.; Bhattacharjee, D.; Sridhar, S. Steel, Aluminum, and FRP-Composites: The Race to Zero Carbon Emissions. Energies 2023, 16, 6904. [Google Scholar] [CrossRef]
- Yolcular, S.; Karaoglu, S.; Karasoglu, M. Hydrogen generation performance of waste aluminum alloy chips and powders. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 2022, 44, 1529–1540. [Google Scholar] [CrossRef]
- Singh, K.; Meshram, A.; Gautam, D.; Jain, A. Hydrogen production using waste aluminium dross: from industrial waste to next-generation fuel. 2019.
- Zhang, B.; Xu, K.; Zheng, X.; Yao, X.; Wang, Y.; Ge, J. Study of a Hydrogen Inhibition Method with Sodium Tungstate for Wet Aluminum Dust Removal Systems. Coatings 2020, 10, 431. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S. Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution. Materials 2022, 15, 8699. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, H.; Hao, T.; Xu, K.; Wang, Y. Hydrogen Inhibition as Explosion Prevention in Wet Metal Dust Removal Systems. Coatings 2022, 12, 349. [Google Scholar] [CrossRef]
- Liu, B.; Yin, W.; Xu, K.; Zhang, Y. Inerting Waste Al Alloy Dust with Natural High Polymers: Sustainability of Industrial Waste. Materials 2022, 15, 5540. [Google Scholar] [CrossRef]
- Michalik, M.; Kasina, M.; Kajdas, B.; Kowalski, P. Form of the Occurrence of Aluminium in Municipal Solid Waste Incineration Residue–Even Hydrogen Is Lost. Energies 2022, 15, 8186. [Google Scholar] [CrossRef]
- Avila, Y.; Silva, R.V.; de Brito, J. Alkali-Activated Materials with Pre-Treated Municipal Solid Waste Incinerator Bottom Ash. Applied Sciences 2022, 12, 3535. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Marín-Genescà, M.; Massagués Vidal, L.; Dagà-Monmany, J.M. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials 2021, 14, 7323. [Google Scholar] [CrossRef] [PubMed]
- Buryakovskaya, O.A.; Meshkov, E.A.; Vlaskin, M.S.; Shkolnokov, E.I.; Zhuk, A.Z. Utilization of Aluminum Waste with Hydrogen and Heat Generation. IOP Conference Series: Materials Science and Engineering 2017, 250, 012007. [Google Scholar] [CrossRef]
- Wei, L.K.; Abd Rahim, S.Z.; Al Bakri Abdullah, M.M.; Yin, A.T.M.; Ghazali, M.F.; Omar, M.F.; Nemeș, O.; Sandu, A.V.; Vizureanu, P.; Abdellah, A.E.-h. Producing Metal Powder from Machining Chips Using Ball Milling Process: A Review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef] [PubMed]
- Salueña Berna, X.; Marín-Genescà, M.; Dagà-Monmany, J.M. Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen. Metals 2021, 11, 598. [Google Scholar] [CrossRef]
- Salueña Berna, X.; Marín-Genescà, M.; Dagà-Monmany, J.M. Analysis of the Use of Recycled Aluminum to Generate Green Hydrogen in an Electric Bicycle. Metals 2023, 13, 357. [Google Scholar] [CrossRef]
- Acevedo-Hurtado, P.O.; Sundaram, P.A.; Caceres-Valencia, P.G.; Fachini, E.R.; Miller, C.E.; Placzankis, B.E. Characterization of atmospheric corrosion in Al/Ag lap joints. Corrosion Science 2008, 50, 3123–3131. [Google Scholar] [CrossRef]
- Afzali, P.; Yousefpour, M.; Borhani, E. Evaluation of the effect of ageing heat treatment on corrosion resistance of Al–Ag alloy using electrochemical methods. Journal of Materials Research 2016, 31, 2457–2464. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z. On the electrochemical dealloying of Al-based alloys in a NaCl aqueous solution. Physical Chemistry Chemical Physics 2010, 12, 1453–1472. [Google Scholar] [CrossRef]
- Afzali, P.; Yousefpour, M.; Borhani, E. Effect of Deformation-Induced Defects on the Microstructure and Pitting Corrosion Behavior of Al-Ag Alloy. International Journal of Engineering 2018, 31, 2092–2101. [Google Scholar]
- Riveros, V.; Gulppi, M.; Páez, M.; Zagal, J.H.; Rangel, C.M.; Huerta, D.; Skeldon, P.; Thompson, G.E. Influence of surface treatments in the initial stages of anodizing Al–Ag alloys in neutral electrolytes. Journal of Solid State Electrochemistry 2006, 10, 83–90. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Suleimanov, M.Z.; Vlaskin, M.S.; Kumar, V.; Ambaryan, G.N. Aluminum Scrap to Hydrogen: Complex Effects of Oxidation Medium, Ball Milling Parameters, and Copper Additive Dispersity. Metals 2023, 13, 185. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of ball size on steady state of aluminum powder and efficiency of impacts during milling. Powder Technology 2015, 284, 149–158. [Google Scholar] [CrossRef]
- Shelekhov, E.; Tcherdyntsev, V.; Pustov, L. Computer simulation of mechanoactivation process in the planetary ball mill: Determination of the energy parameters of milling/Metastable, mechanically alloyed and nanocrystalline materials, pts 1 and 2 Book Series: Materials science forum Volume: 343-3, P: 603-608, Part 1, 2. 2000.
- Buryakovskaya, O.A.; Vlaskin, M.S. Microstructural Transformation and Hydrogen Generation Performance of Magnesium Scrap Ball Milled with Devarda's Alloy. Materials 2022, 15, 8058. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R. Aluminum and aluminum alloys; ASM international: 1993.
- Siddesh Kumar, N.M.; Dhruthi; Pramod, G.K.; Samrat, P.; Sadashiva, M. A Critical Review on Heat Treatment of Aluminium Alloys. Materials Today: Proceedings 2022, 58, 71–79. [CrossRef]
- Ozdemir, F.; Witharamage, C.S.; Darwish, A.A.; Okuyucu, H.; Gupta, R.K. Corrosion behavior of age hardening aluminum alloys produced by high-energy ball milling. Journal of Alloys and Compounds 2022, 900, 163488. [Google Scholar] [CrossRef]
- Kaufman, J.G. Introduction to aluminum alloys and tempers; ASM international: 2000.
- Zhang, Y.; Li, J.B.; Liang, J.K.; Liu, Q.L.; Xiao, Y.G.; Zhang, Q.; Rao, G.H.; Li, C.R. Thermodynamic assessment of the Ag–Ga system. Calphad 2006, 30, 316–322. [Google Scholar] [CrossRef]
- Hovington, P.; Timoshevskii, V.; Burgess, S.; Demers, H.; Statham, P.; Gauvin, R.; Zaghib, K. Can we detect Li K X-ray in lithium compounds using energy dispersive spectroscopy? Scanning 2016, 38, 571–578. [Google Scholar] [CrossRef]
- Durdziński, P.T.; Dunant, C.F.; Haha, M.B.; Scrivener, K.L. A new quantification method based on SEM-EDS to assess fly ash composition and study the reaction of its individual components in hydrating cement paste. Cement and Concrete Research 2015, 73, 111–122. [Google Scholar] [CrossRef]
- Ohfuji, H.; Yamamoto, M. EDS quantification of light elements using osmium surface coating. Journal of Mineralogical and Petrological Sciences 2015, 110, 189–195. [Google Scholar] [CrossRef]
- Seah, M.P.; Gilmore, I.S.; Spencer, S.J. Measurement of data for and the development of an ISO standard for the energy calibration of X-ray photoelectron spectrometers. Applied Surface Science 1999, 144–145, 178–182. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Droubay, T.; Chambers, S.A.; Bagus, P.S. Spectroscopic Evidence for Ag(III) in Highly Oxidized Silver Films by X-ray Photoelectron Spectroscopy. The Journal of Physical Chemistry C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; ULVAC-PHI, Inc.: Chigasaki, 1995; p. 261. [Google Scholar]
- Acharya, P.V.; Kar, A.; Shahriari, A.; Bhati, A.; Mhadeshwar, A.; Bahadur, V. Aluminum-based promotion of nucleation of carbon dioxide hydrates. The Journal of Physical Chemistry Letters 2020, 11, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; He, M.-F.; Zhu, L.-F.; Lyu, Y.-J.; Yang, H.-Q.; Hu, C.-W. Cooperative catalytic performance of Lewis and Brønsted acids from AlCl3 salt in aqueous solution toward glucose-to-fructose isomerization. The Journal of Physical Chemistry C 2019, 123, 4879–4891. [Google Scholar] [CrossRef]
- Natishan, P.M.; O’Grady, W.E. Chloride Ion Interactions with Oxide-Covered Aluminum Leading to Pitting Corrosion: A Review. Journal of The Electrochemical Society 2014, 161, C421. [Google Scholar] [CrossRef]
- Fu, S.-W.; Lee, C.C. A corrosion study of Ag–Al intermetallic compounds in chlorine-containing epoxy molding compounds. Journal of Materials Science: Materials in Electronics 2017, 28, 15739–15747. [Google Scholar] [CrossRef]
- Liu, W.-p.; Xu, H.; Yang, X.-y.; Shi, X.-c.; Chen, Y. Thermodynamic equilibrium diagram of CaCl2-Ca(OH)2-H2O system. Journal of Central South University 2012, 19, 2751–2754. [Google Scholar] [CrossRef]
- Pathak, A.D.; Nedea, S.; Zondag, H.; Rindt, C.; Smeulders, D. A DFT-based comparative equilibrium study of thermal dehydration and hydrolysis of CaCl 2 hydrates and MgCl 2 hydrates for seasonal heat storage. Physical Chemistry Chemical Physics 2016, 18, 10059–10069. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corrosion science 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Role of Ball Milling of Aluminum Powders in Promotion of Aluminum-Water Reaction to Generate Hydrogen. Metallurgical and Materials Transactions E 2014, 1, 247–256. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Liu, Z. Active aluminum composites and their hydrogen generation via hydrolysis reaction: A review. International Journal of Hydrogen Energy 2022, 47, 365–386. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Li, J. Hydrogen generation from hydrolysis of activated aluminum/organic fluoride/bismuth composites with high hydrogen generation rate and good aging resistance in air. Energy 2019, 170, 159–169. [Google Scholar] [CrossRef]
- Grosjean, M.H.; Zidoune, M.; Roué, L.; Huot, J.Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials. International Journal of Hydrogen Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. International Journal of Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Poirier, D.; Drew, R.A.L.; Trudeau, M.L.; Gauvin, R. Fabrication and properties of mechanically milled alumina/aluminum nanocomposites. Materials Science and Engineering: A 2010, 527, 7605–7614. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Farhad, S. Heavy liquids for rapid separation of cathode and anode active materials from recycled lithium-ion batteries. Resources, Conservation and Recycling 2021, 174, 105749. [Google Scholar] [CrossRef]
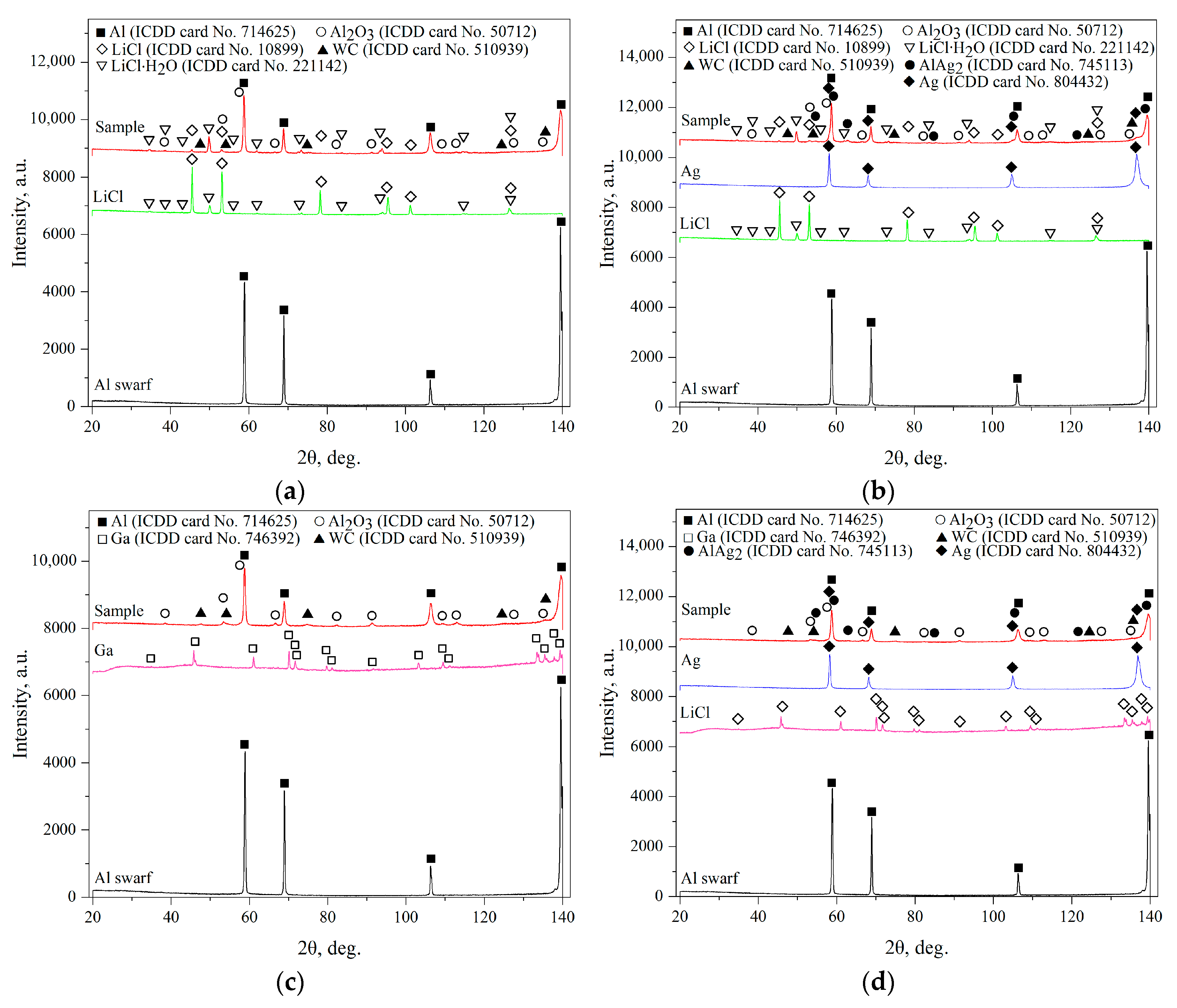
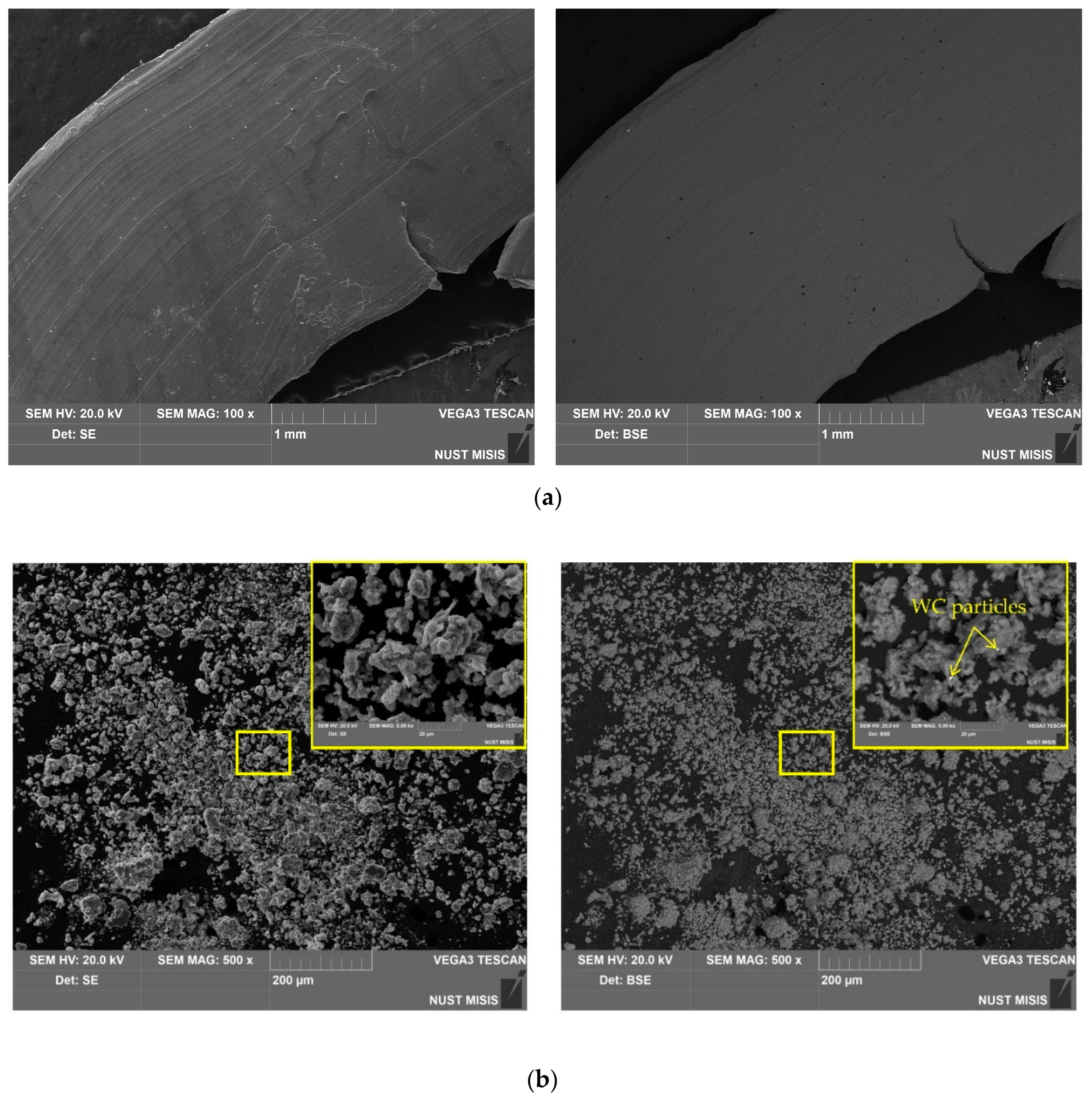
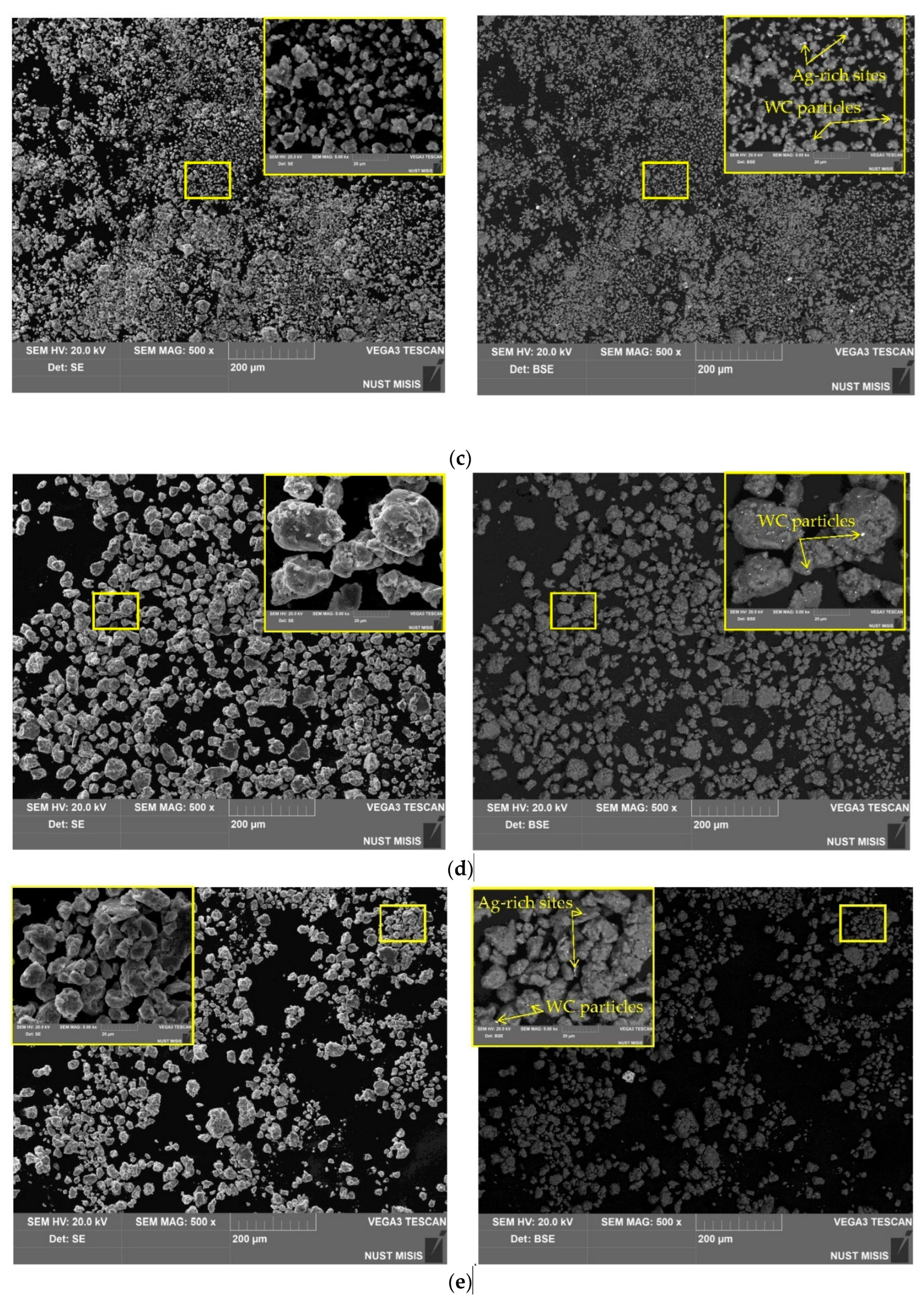
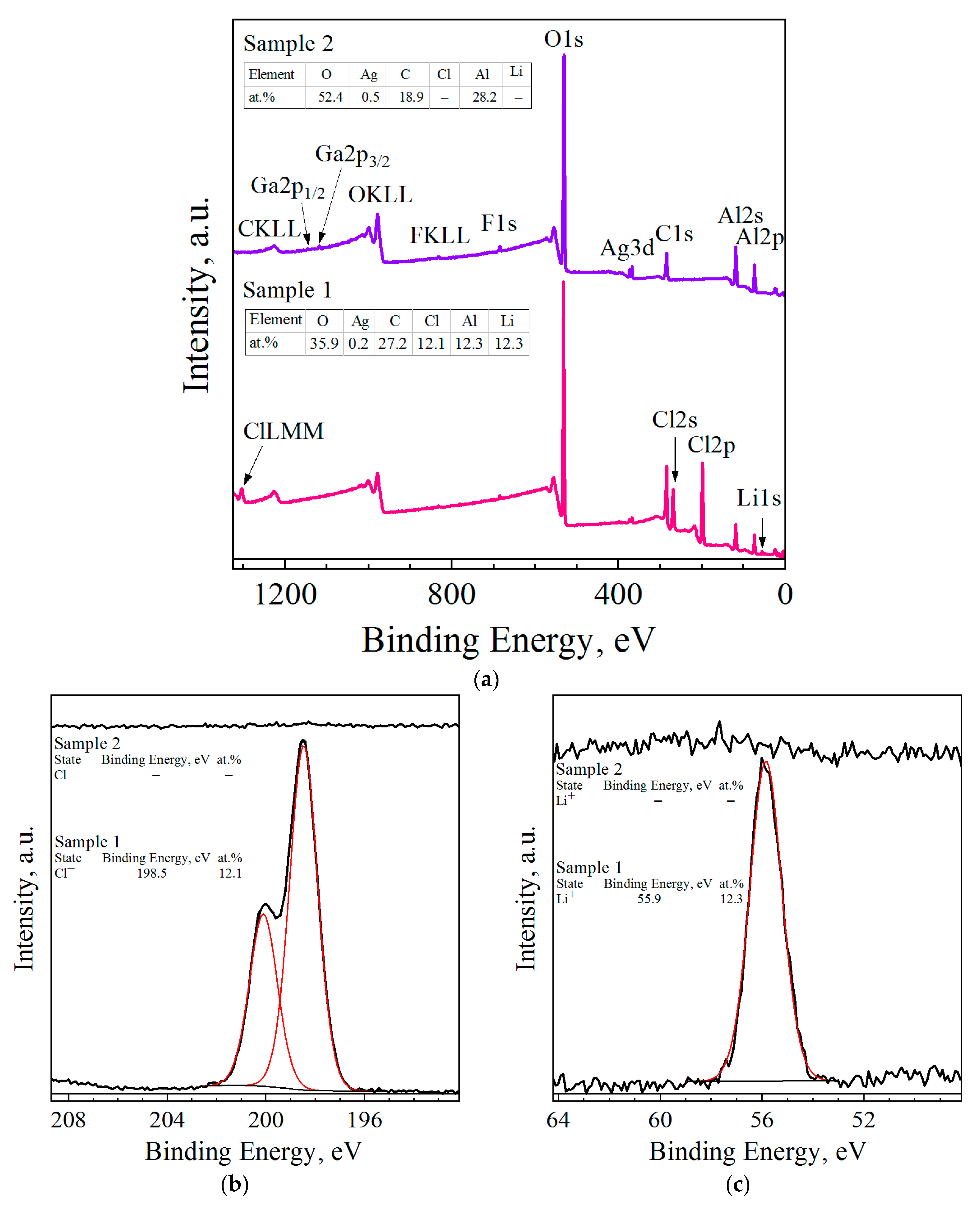
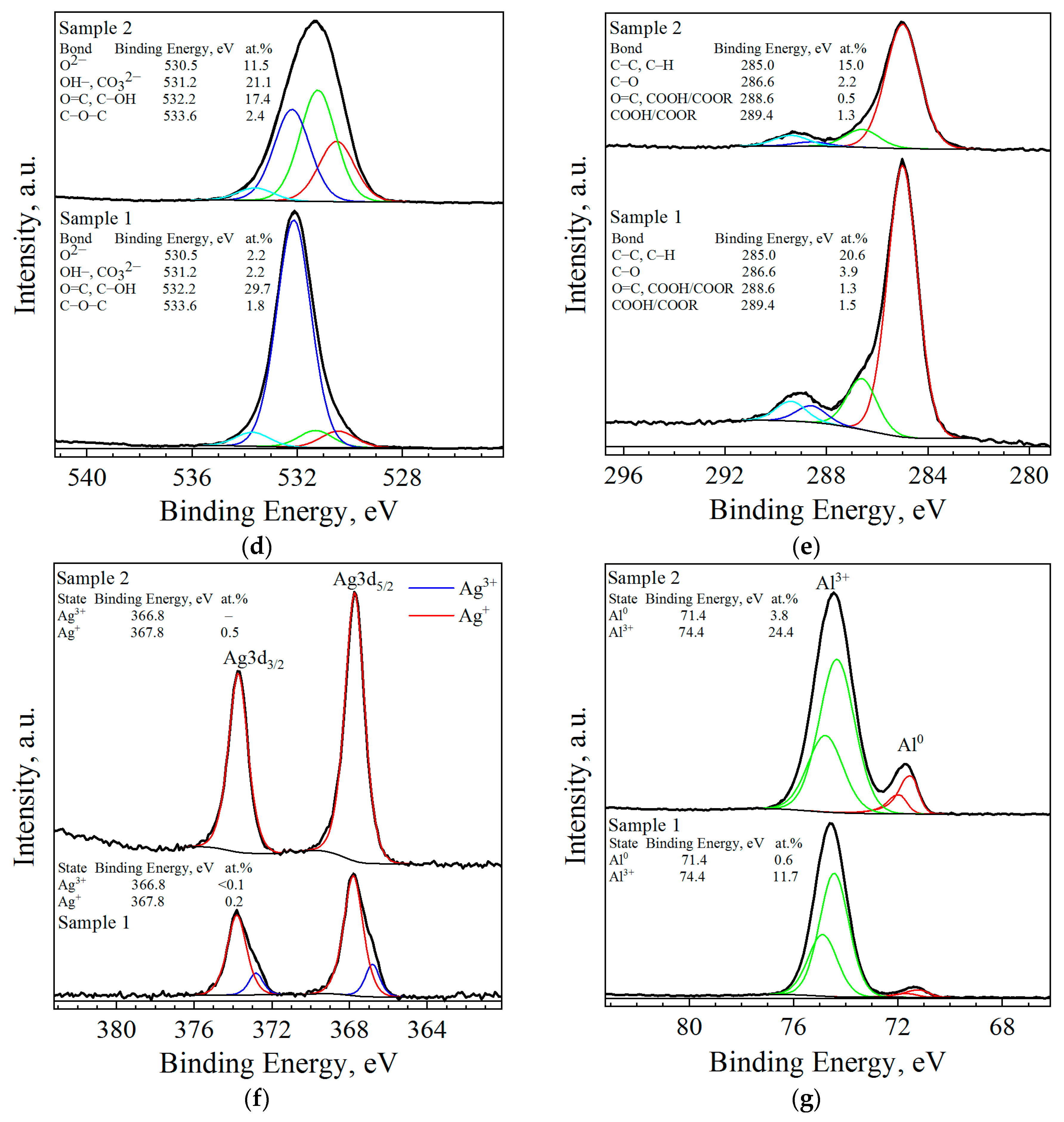

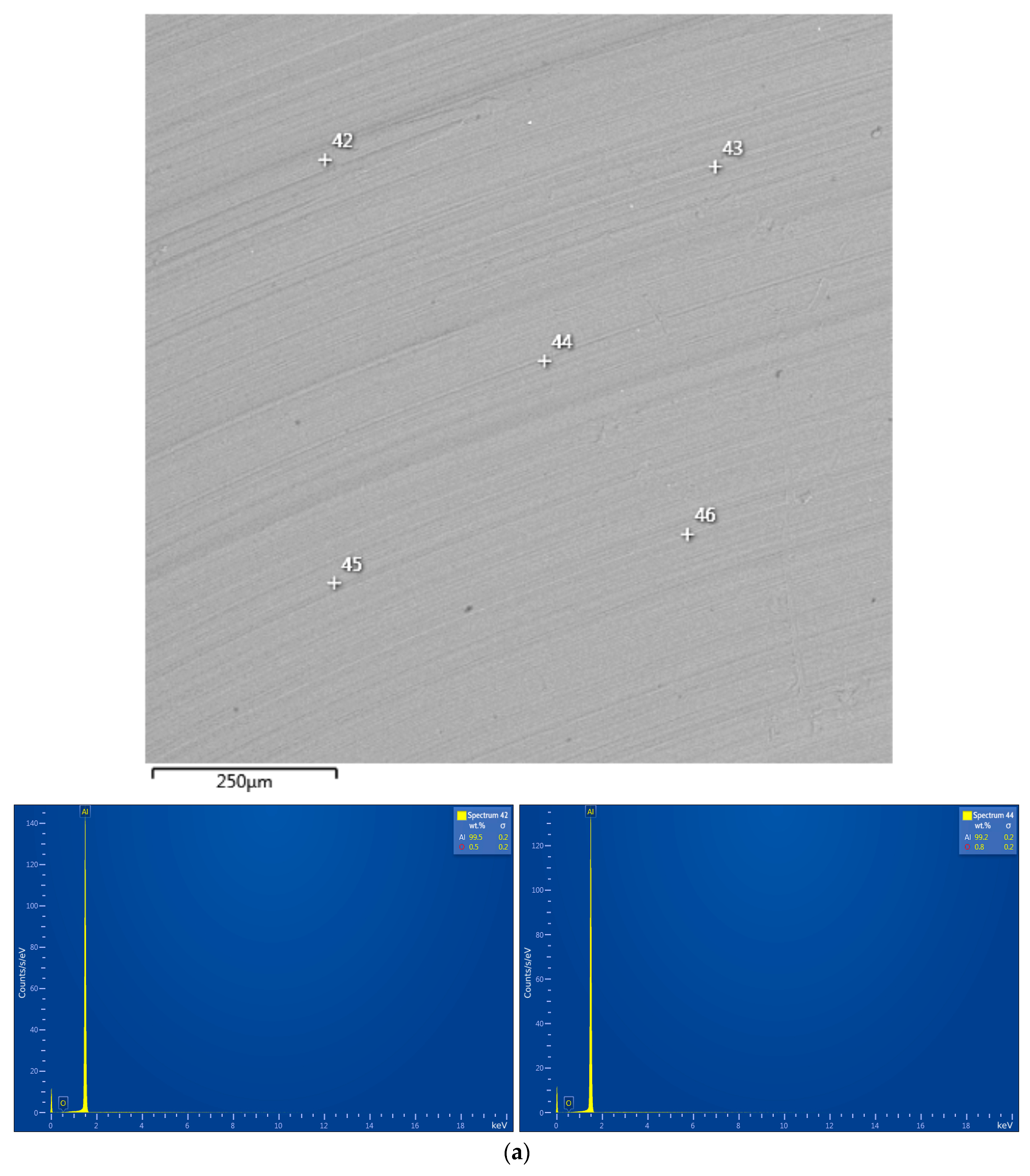
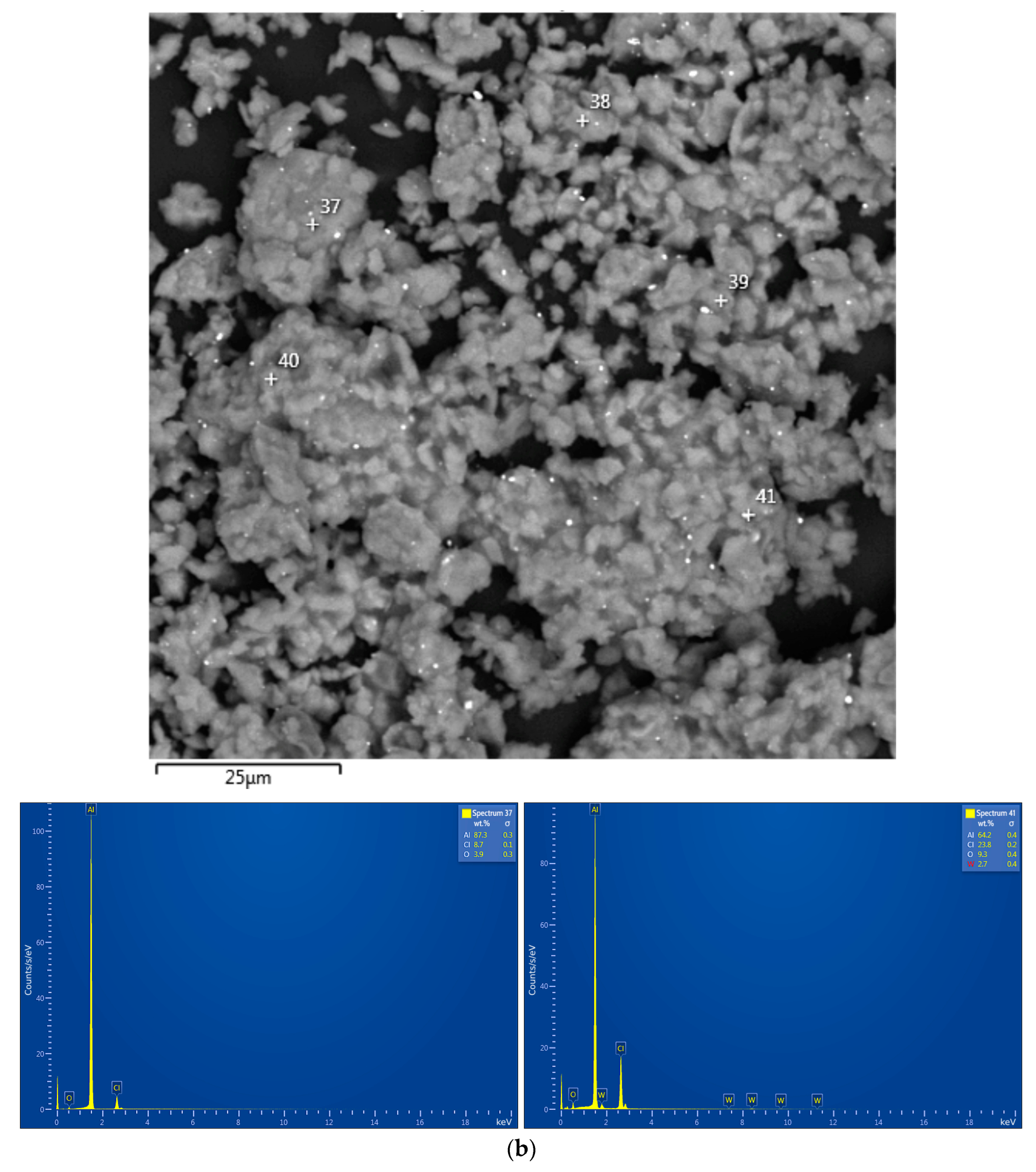
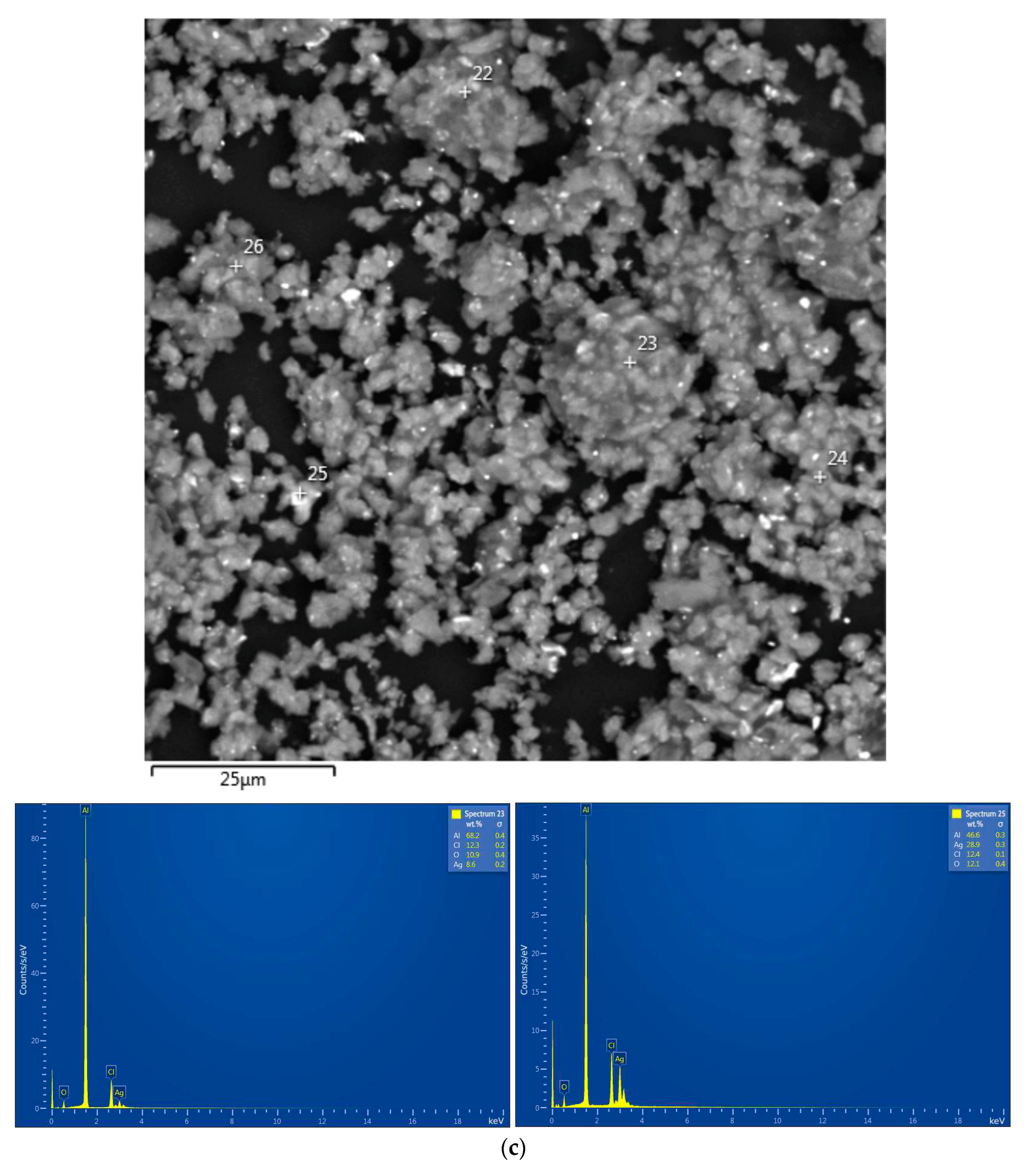
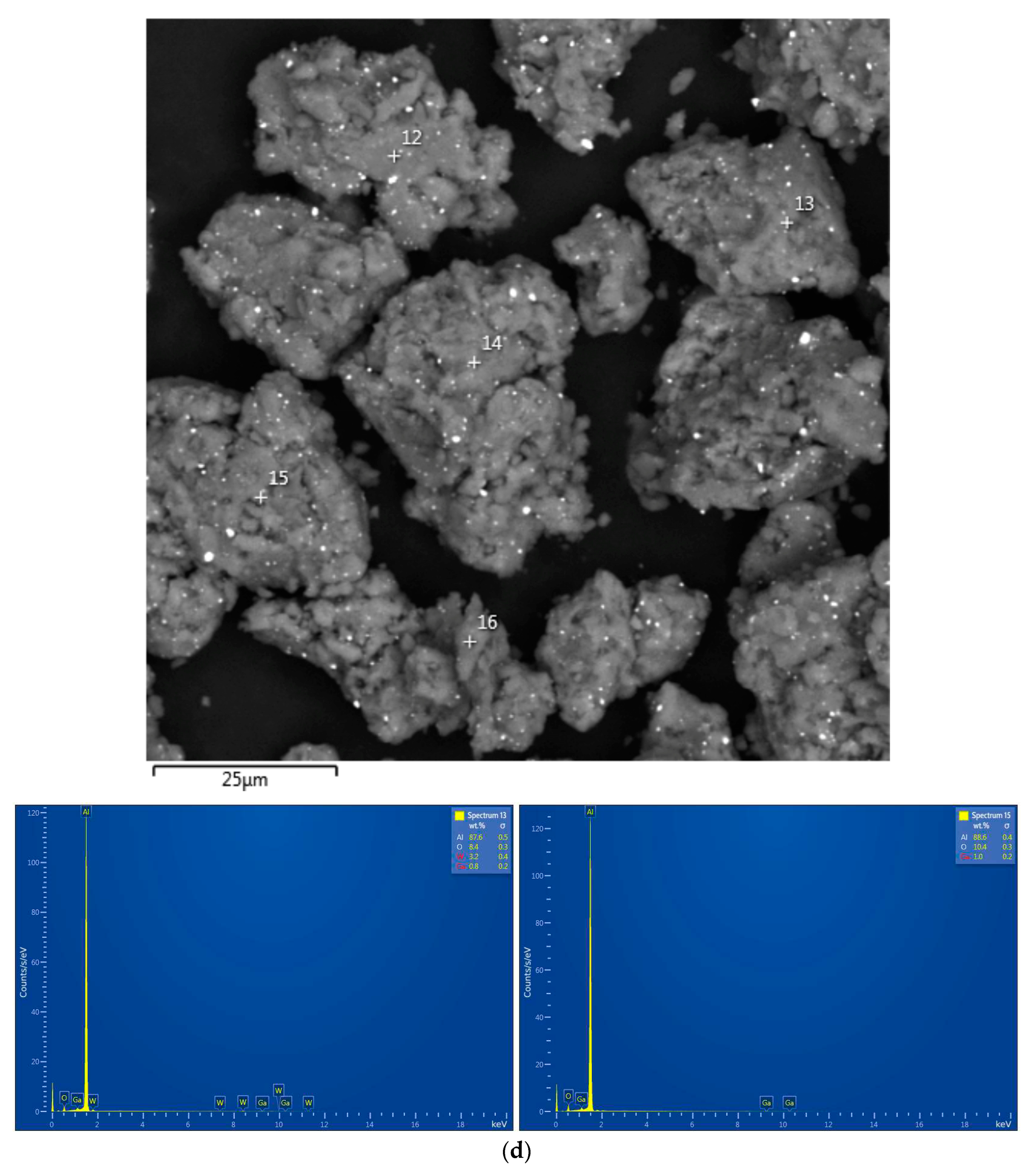
| Sample | Spectra No. | Al | O | Cl | Ga | Ag | W |
|---|---|---|---|---|---|---|---|
| Al swarf | 42 | 99.5±0.2 | 0.5±0.2 | - | - | - | - |
| 44 | 99.2±0.2 | 0.8±0.2 | - | - | - | - | |
| Al–Li | 37 | 87.3±0.3 | 3.9±0.3 | 8.7±0.1 | - | - | - |
| 41 | 64.2±0.4 | 9.3±0.4 | 23.8±0.2 | - | - | 2.7±0.4 | |
| Al–Li–Ag | 23 | 68.2±0.4 | 10.9±0.4 | 12.3±0.2 | - | 8.6±0.2 | - |
| 25 | 46.6±0.3 | 12.1±0.4 | 12.4±0.1 | - | 28.9±0.3 | - | |
| Al–Ga | 13 | 87.6±0.5 | 8.4±0.3 | - | 0.8±0.2 | - | 3.2±0.4 |
| 15 | 88.6±0.4 | 10.4±0.3 | - | 1.0±0.2 | - | - | |
| Al–Ga–Ag | 7 | 79.8±0.5 | 6.8±0.3 | - | 0.6±0.2 | 11.6±0.3 | 1.2±0.4 |
| 8 | 77.6±0.4 | 10.7±0.4 | - | 0.6±0.2 | 11.1±0.3 | - |
| Sample | Solution | Temperature, °C | Hydrogen Yield, % | Maximum Hydrogen Evolution Rate, mL/g/min. |
|---|---|---|---|---|
| Al–Ga–Ag | AlCl3 | 60 | 80.2±0.5 | 792 |
| Al–Ga | 76.7±0.7 | 588 | ||
| Al–LiCl–Ag | 84.6±0.2 | 586 | ||
| Al–LiCl | 86.8±1.4 | 183 |
| Sample | Solution | Temperature, °C | Hydrogen Yield, % | Maximum Hydrogen Evolution Rate, mL/g/min. |
|---|---|---|---|---|
| Al–Ga–Ag | CaCl2 | 80 | 31.8±1.9 | 72 |
| Al–LiCl–Ag | 46.7±2.1 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





