Submitted:
09 January 2024
Posted:
11 January 2024
You are already at the latest version
Abstract
Keywords:
CHAPTER 1: INTRODUCTION
background of the Study
Problem Statement
SIGNIFICANCE OF THE STUDY
- Enhanced Soil Remediation: The study’s findings will provide valuable insights into optimizing the use of bitter-leaf and other bio-remediators in effectively restoring hydrocarbon-contaminated soils. This knowledge can contribute to the development of more efficient and sustainable soil remediation techniques, benefiting not only Ogoni land but also other regions grappling with similar soil pollution challenges.
- Restoration of Hope: By offering promising solutions for remediating polluted soils, the study brings hope to the Ogoni people and other affected areas. It provides a glimmer of optimism for communities that have experienced the devastating consequences of hydrocarbon contamination, assuring them that there are potential ways to reclaim their lands and restore environmental balance.
- Stimulating Further Research and Development: The study’s outcomes will serve as a catalyst for additional research and development efforts in the field of soil remediation. By contributing to knowledge and highlighting the effectiveness of bitter-leaf and other bio-remediators, it will inspire researchers to delve deeper into this area, leading to advancements in bio-remediation techniques and expanding the range of available options.
- Environmental Sustainability: The successful utilization of bitter-leaf and other bio-remediators can contribute to environment socioeconomic benefits for affected communities. As the study provides insights into effective soil remediation techniques, it opens up opportunities for improved agricultural productivity, land utilization, and economic development in regions struggling with the consequences of hydrocarbon contamination. Overall, the significance of the study lies in its potential to optimize soil remediation practices, restore hope to affected communities, stimulate further research, promote environmental sustainability, and generate socioeconomic benefits in areas impacted by soil pollution.
AIM OF THE STUDY
THE OBJECTIVES OF THE RESEARCH STUDY
SCOPES OF STUDY
- Focus on Bitter Leaf Extracts: The study primarily focuses on evaluating the performance of bitter leaf extracts, specifically Vernonia Galamensis and Vernonia Amygdalina, in remediating hydrocarbon-contaminated soil. It explores the potential of these extracts as bio-remediators.
- Laboratory Testing: The research involves conducting small-scale laboratory tests to assess the effectiveness of bitter leaf in remediating oil-contaminated soil. The testing will be carried out under controlled conditions to obtain empirical data.
- Evaluation of Different Soil Types: The study considers the remediation effectiveness of bitter leaf in different soil types, including sandy-loamy, clay, and swamp soil. By examining multiple soil types, the research aims to understand how bitter leaf performs in diverse soil compositions.
- Concentration-Effect Relationship: The research aims to establish a relationship between the concentration of bitter leaf and its remediation effect. It seeks to identify the optimal dosage of bitter leaf extract required to achieve efficient soil remediation.
- Statistical Modeling: The study aims to develop a statistical model that describes the bio-remediation process using bitter leaf. This model may incorporate variables such as bitter leaf concentration, treatment duration, and soil pH to enhance the understanding and prediction of remediation outcomes. It’s important to note that the scope of the study may also include any specific limitations or constraints, such as time, resources, or geographical boundaries, which should be acknowledged and considered during the research process.
- Research Report: The primary deliverable would be a comprehensive research report outlining the findings, methodologies, and recommendations. This aligns with SDG 9 (Industry, Innovation, and Infrastructure) as it contributes to the advancement of knowledge and innovation in the field of soil remediation.
- Recommendations for Sustainable Soil Remediation: The research study can provide practical recommendation for the use of bitter leaf and other bio-remediators in soil remediation practices. These recommendations can contribute to SDG 12 (Responsible Consumption and Production) by promoting sustainable and environmentally friendly approaches to address soil pollution.
- Knowledge Sharing and Awareness: Disseminating the research findings through publications, conferences, and workshops can help raise awareness about the potential of bitter leaf and bio-remediation techniques. This aligns with SDG 4 (Quality Education) and SDG 13 (Climate Action), as it promotes scientific knowledge sharing and sustainability practices.
- Policy Recommendations: Based on the research outcomes, the study can provide policy recommendations to relevant stakeholders, such as governmental agencies and environmental organizations. These recommendations can contribute to SDG 15 (Life on Land) by supporting efforts to restore and protect ecosystems affected by soil pollution.
- Capacity Building: The research study can also contribute to capacity building initiatives by providing guidance and training materials for professionals in the field of soil remediation. This aligns with SDG 8 (Decent Work and Economic Growth) and SDG 17 (Partnerships for the Goals) by promoting skill development and collaboration to address environmental challenges. By considering the SDGs in the selection and implementation of deliverables, the research study can have a broader impact on sustainable development, environmental conservation, and socio-economic well-being.
CHAPTER 2: LITERATURE REVIEW
2.1. Review of Previous Works in Bio-Remediation
2.2. Limitations and Environmental Issues of Bioremediation in the Niger Delta
2.3. Current Bioremediation Technologies for Crude Oil Contaminated Sites
2.3.1. In Situ Land Treatment
2.3.2. Composting Technology
2.3.3. Bioreactors

2.3.4. Biodegradation
2.4. Sources and Effects of polluted soils in the Niger Delta
2.4.1. Sources
2.4.2. Effects of Oil Spillage on the Environment
- a. Human Health Risk
- b. Damage to The Ecosystem
- c. Health Hazard to The Aquatic Animals
- d. Risk to Food Security
- e. Loss of Aquatic Plants
- f. Depletion of Fish Population
- g. A Danger to The Wildlife in General
- h. Pollutes The Air Leading to Other Illness.
- i. Acid Rain
- j. Poverty
2.5. Mechanisms for Phytoremediation of Hydrocarbons
2.5.1. Degradation
2.5.2. Stabilization
2.1.3. Volatilization
CHAPTER 3: MATERIALS AND METHODS
STUDY SITE
FOR SOIL SAMPLING PURPOSES,
FOR THE VERNONIA LEAF SAMPLE COLLECTION AND EXTRACT PREPARATION
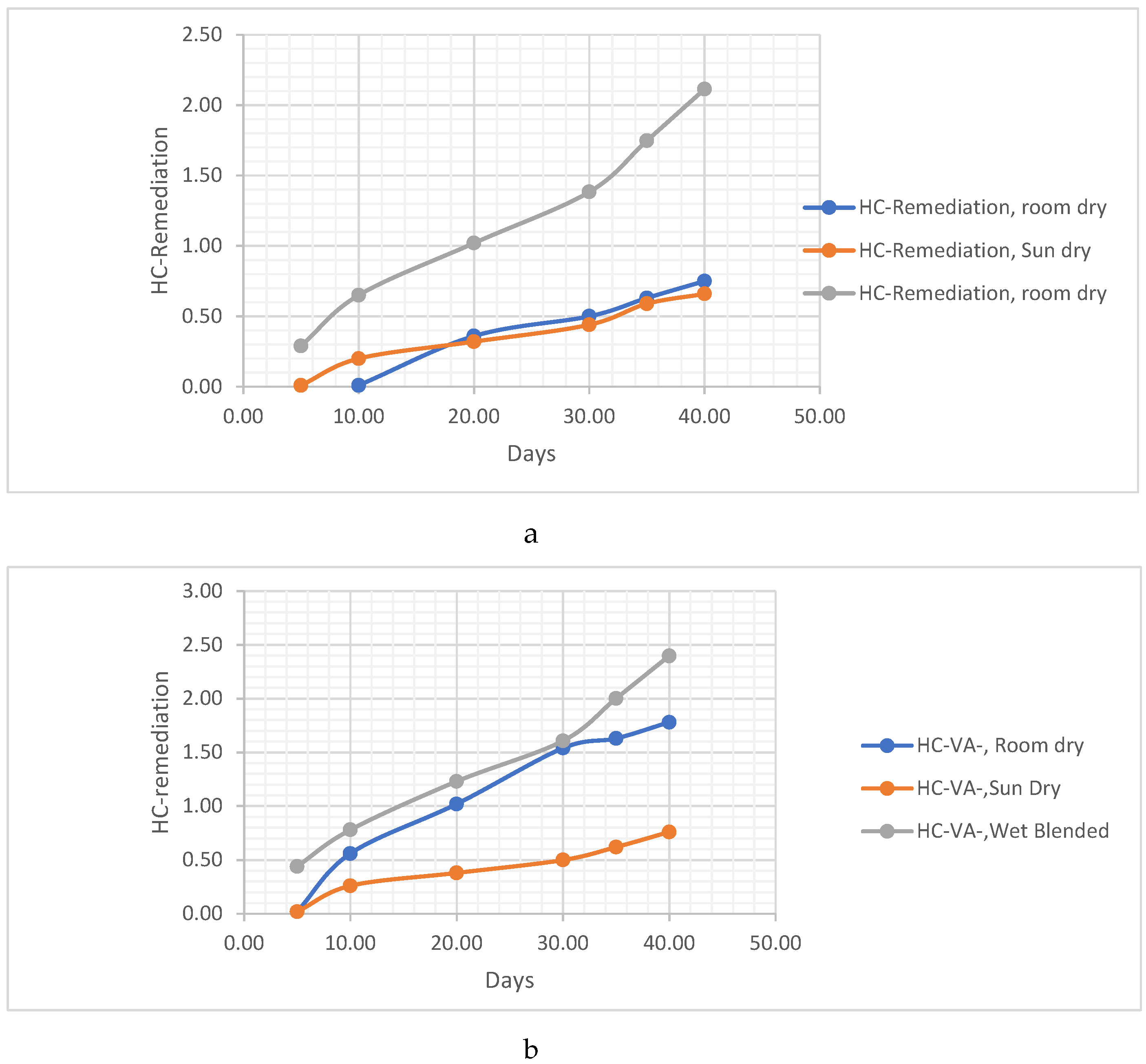
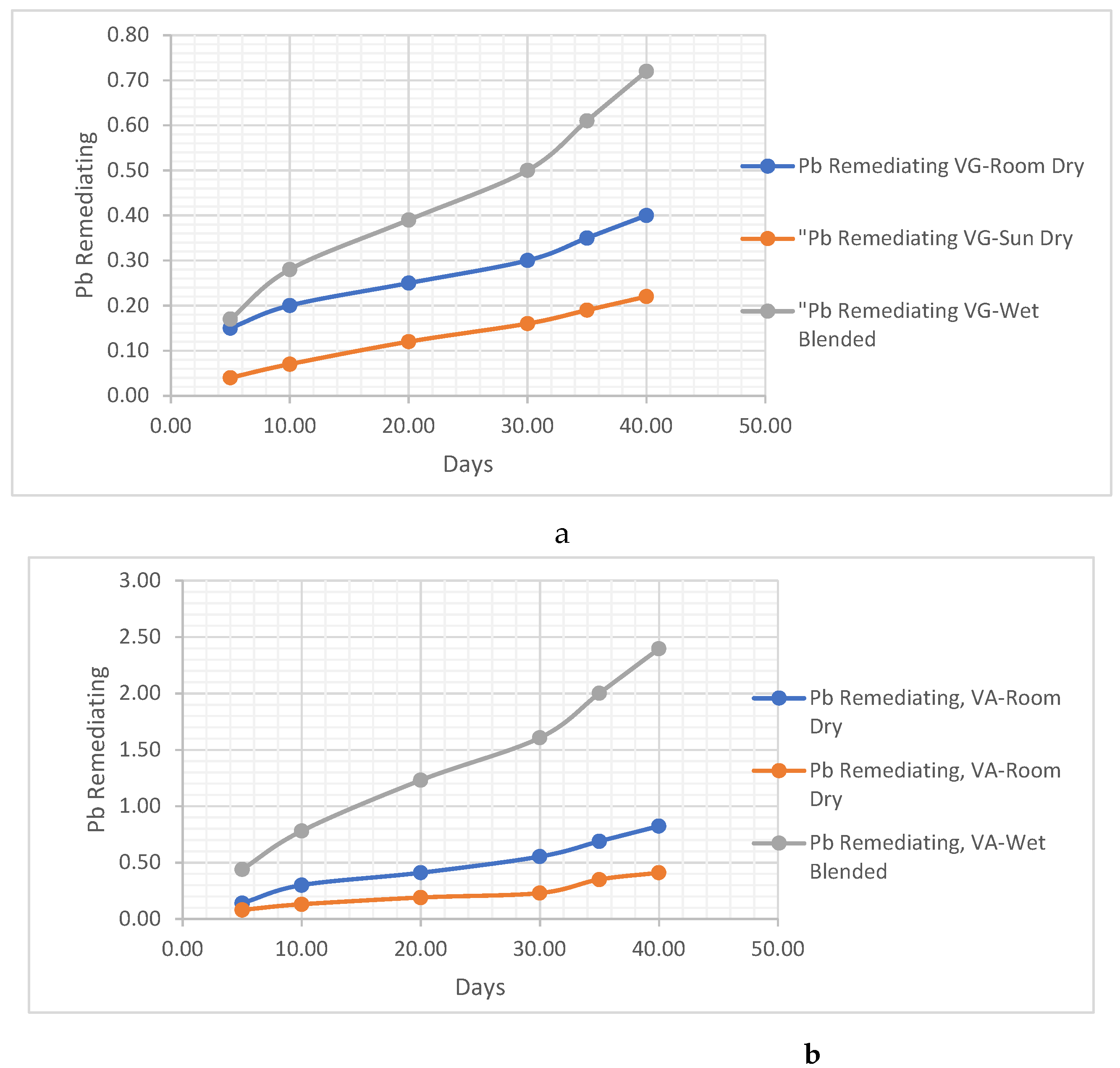
THE POST-TREATMENT METHODS FOR THE VERNONIA CHAFF/REMAINS
- The Vernonia chaff/remains were placed in the sun to dry. This method involves exposing the chaff to sunlight, allowing the moisture to evaporate naturally and facilitating the drying process.
- The Vernonia chaff/remains were placed to dry in room conditions. In this method, the chaff is left in a room with normal environmental conditions, allowing air circulation to aid in the drying process.
- The Vernonia chaff/remains were left wet. This indicates that no specific drying method was employed, and the chaff was kept in its wet condition for further analysis. These different post-treatment techniques provide variations in the moisture content and physical properties of the Vernonia chaff, which may impact subsequent analysis or experimentation.
- Physio-chemical analysis: This analysis aims to determine the composition of specific chemical species present in the Vernonia extract. It includes assessing factors such as pH, presence of certain compounds, elemental composition, or other physicochemical properties of the extract.
- Microbial analysis: This analysis is performed to investigate whether the Vernonia extract can support the presence and stability of microorganisms. It helps determine if the extract provides a favorable environment for microbial growth, colonization, or inhibition. By conducting these analyses, valuable insights can be gained regarding the chemical composition and microbial properties of the Vernonia extract, contributing to a better understanding of its potential applications and effects.
3.3.2. TO CONDUCT THE MICROBIAL ANALYSIS, THE FOLLOWING MATERIALS ARE REQUIRED:
- Conical flask: Used for holding and mixing liquids during the analysis.
- One 5 ml syringe: Used for precise measurement and transfer of liquids.
- One 1 ml syringe: Used for more accurate and smaller volume measurements.
- Distilled water: Used as a solvent or diluent for various solutions.
- Nutrient agar: A solid growth medium used for culturing and isolating microorganisms.
- Vernonia leaf extract from the four species of bitter leaf (Vernonia amygdalina and Vernonia galamensis bitter leaf): The extract obtained from the Vernonia leaves, which will be used for microbial analysis.
- 30 Petri dishes: Used as containers for the agar medium and microbial cultures.
- Masking tape: Used for labeling and identification purposes.
- Cotton wool: Used for sterilization and plugging of test tubes
- Aluminium foil: Used for covering the Petri dishes to protect the cultures.
- Test tubes: Used for preparation and storage of samples or media.
- Nutrient broth: A liquid growth medium used for cultivating microorganisms.
- Autoclave: A device used for sterilizing equipment and media through high-pressure steam.
- Weighing balance: Used for accurately measuring the weight of substances. These materials are essential for conducting the microbial analysis and ensuring the proper cultivation, identification, and characterization of microorganisms present in the Vernonia leaf extracts.
- Wash the conical flask, test tubes, and petri dishes with distilled water. This step ensures cleanliness and reduces the risk of contamination during the analysis.
- Prepare the nutrient agar culture: a. Dissolve 28 grams of nutrient agar in 1 liter of distilled water to prepare the standard culture. This forms the base for the nutrient agar medium. b. Using a spatula, carefully place the powdered nutrient agar on the foil. c. In a conical flask, dissolve 3 grams of the weighed nutrient agar powder in 100 ml of distilled water. d. Cover the conical flask with cotton wool to prevent contamination. After completing these steps, you can proceed with further analysis and experimentation. It is important to maintain a sterile environment throughout the process to ensure accurate results and prevent unwanted microbial growth.
- Place a small piece of aluminum foil on the digital weighing balance and zero the scale to ensure accurate measurements.
- Using a spatula, carefully place the pellets of nutrient broth on the foil until the weighing balance reads 5 grams. This ensures the correct amount of nutrient broth for the culture.
- In a conical flask, dissolve the weighed 5 grams of nutrient broth in 500 ml of distilled water. Stir or swirl the flask gently to aid in the dissolution process. By following these steps, you will have prepared the nutrient broth culture. This liquid medium is commonly used for cultivating and growing a wide range of microorganisms. It provides necessary nutrients to support microbial growth and can be used for various purposes in microbial analysis, such as culturing and studying specific bacteria or fungi strains.
- Using the 1 ml syringe, collect 20 milliliters of the nutrient broth water
- Carefully transfer the collected nutrient broth water into 6 test tubes, distributing approximately 20 milliliters in each test tube.
- Cover each test tube with cotton wool. This acts as a barrier to prevent contamination from external microorganisms.
- 4 Wrap aluminum foil around each test tube to further protect them and maintain their sterility.
- Once the test tubes are properly covered and wrapped, they are ready for sterilization using an autoclave. Autoclaving is a process that effectively kills microorganisms and ensures the sterility of the samples. Follow the manufacturer’s instructions or standard laboratory protocols for autoclaving to properly sterilize the test tubes. By following these steps, you will have prepared the nutrient broth culture in the test tubes, ensuring their sterility and readiness for further analysis or experimentation.
- 1 ml syringe: Used for precise measurement and transfer of liquids during the dilution process.
- 6 sterilized test tubes with nutrient broth: These test tubes containing nutrient broth are pre-sterilized and ready for use in the dilution process. They provide a growth medium for the microorganisms present in the Vernonia leaf extract.
- Vernonia leaf extract: The Vernonia leaf extract obtained previously, which will be diluted for further analysis. 4.
- Bunsen burner: A gas burner used for sterilization purposes. It provides a high-temperature flame to sterilize the syringe and other equipment by passing them through the flame. These materials are essential for performing a serial dilution, a technique used to decrease the concentration of a substance, in this case, the Vernonia leaf extract, to a level suitable for microbial analysis. The dilution process allows for the isolation and enumeration of microorganisms present in the extract by reducing their initial concentration.
- Collect 1 ml of Vernonia leaf extract from each different species and place it in a separate test tube containing nutrient broth. This step ensures that each species extract is diluted individually.
- Shake each test tube vigorously to ensure thorough mixing of the Vernonia leaf extract with the nutrient broth.
- From the first test tube, take 5 ml of the mixture and transfer it to a new test tube.
- Shake the second test tube vigorously to mix the 5 ml of the mixture with the nutrient broth.
- From the second test tube, take 1 ml of the mixture and transfer it to another new test tube.
- Repeat this process by taking 1 ml from the previous test tube and adding it to a new test tube until you obtain about 20 test tubes that have been serially diluted.
- Pour approximately 20 ml of nutrient agar into each sterilized petri dish. Make sure to distribute the agar evenly across the dish.
- Invert the petri dishes so that the nutrient agar hangs from the top of each dish. This is done to allow the agar to solidify and adhere to the bottom surface of the dish.
- Wait until the nutrient agar has completely solidified and sticks to the top of the petri dishes. This ensures a stable surface for the subsequent steps.
- Take 1 ml from each of the serially diluted broth cultures and place it in a separate petri dish. This step involves transferring the diluted broth culture onto the solidified nutrient agar.
- Carefully spread the 1 ml of diluted broth culture across the surface of each petri dish using a sterile spreader or an appropriate technique, ensuring an even distribution.
- Once the diluted broth culture has been spread, cover the petri dishes and place them into an incubator or a temperature-controlled environment where the temperature is maintained at 37°C (not 370°C, as that would be extremely high). By following these steps, you will have prepared the culture by inoculating the serially diluted broth cultures onto nutrient agar plates. The incubation at 37°C promotes the growth of microorganisms, allowing for their visual observation and further analysis.
FOR THE COLD ETHANOIC AND COLD AQUEOUS EXTRACTION METHODS,
TO TEST FOR THE PRESENCE OF ALKALOIDS,
TO TEST FOR THE PRESENCE OF PHENOLICS.
TO TEST FOR THE PRESENCE OF TANNINS.
TEST FOR STEROIDS
BIOREMEDIATION EXPERIMENT
PRE-ANALYSIS TEST
PROCEDURES
INTERMEDIATE TESTING.
CHAPTER 4: RESULTS AND DISCUSSIONS
BIO REMEDIAL ANALYSIS
4.1.1. Swampy Soil Bio Remedial Analysis
| pH and HC readings for samples before contaminant | |||||
| Initial Content sample | pH | HC | Pb (ug/ml) | Zn (ug/ml) | Cr(ug/ml) |
| Sandy Loam Soil, SLSi | 6.76 | 2.59 | 0.018 | 0.022 | 0.015 |
| pH and HC readings for samples after contaminant | |||||
| Final Content sample | pH | HC | Pb (ug/ml) | Zn (ug/ml) | Cr(ug/ml) |
| swamp soil, SSf | 6.81 | 4.7 | 1.24 | 0.921 | 1.107 |
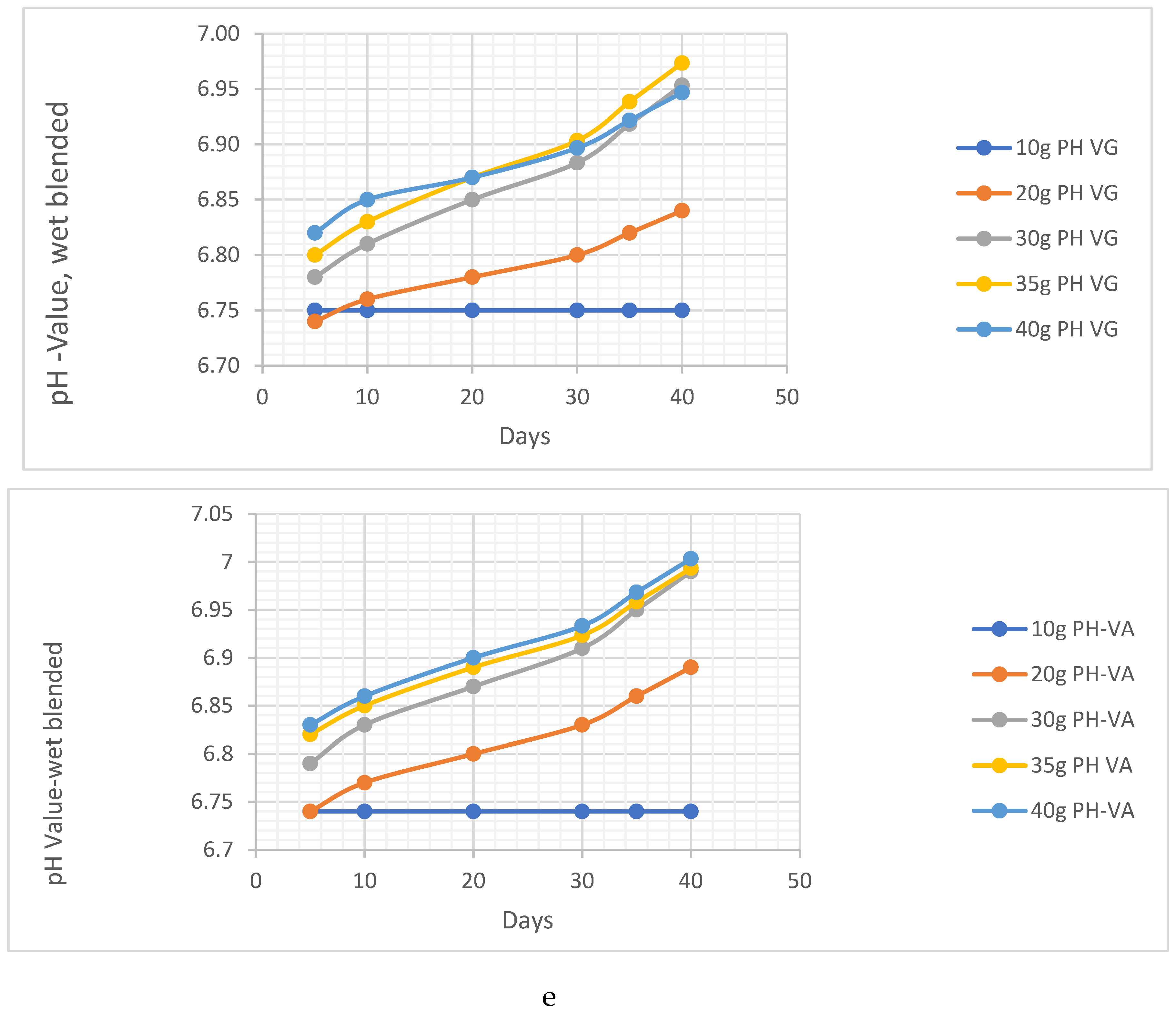
pH Analysis
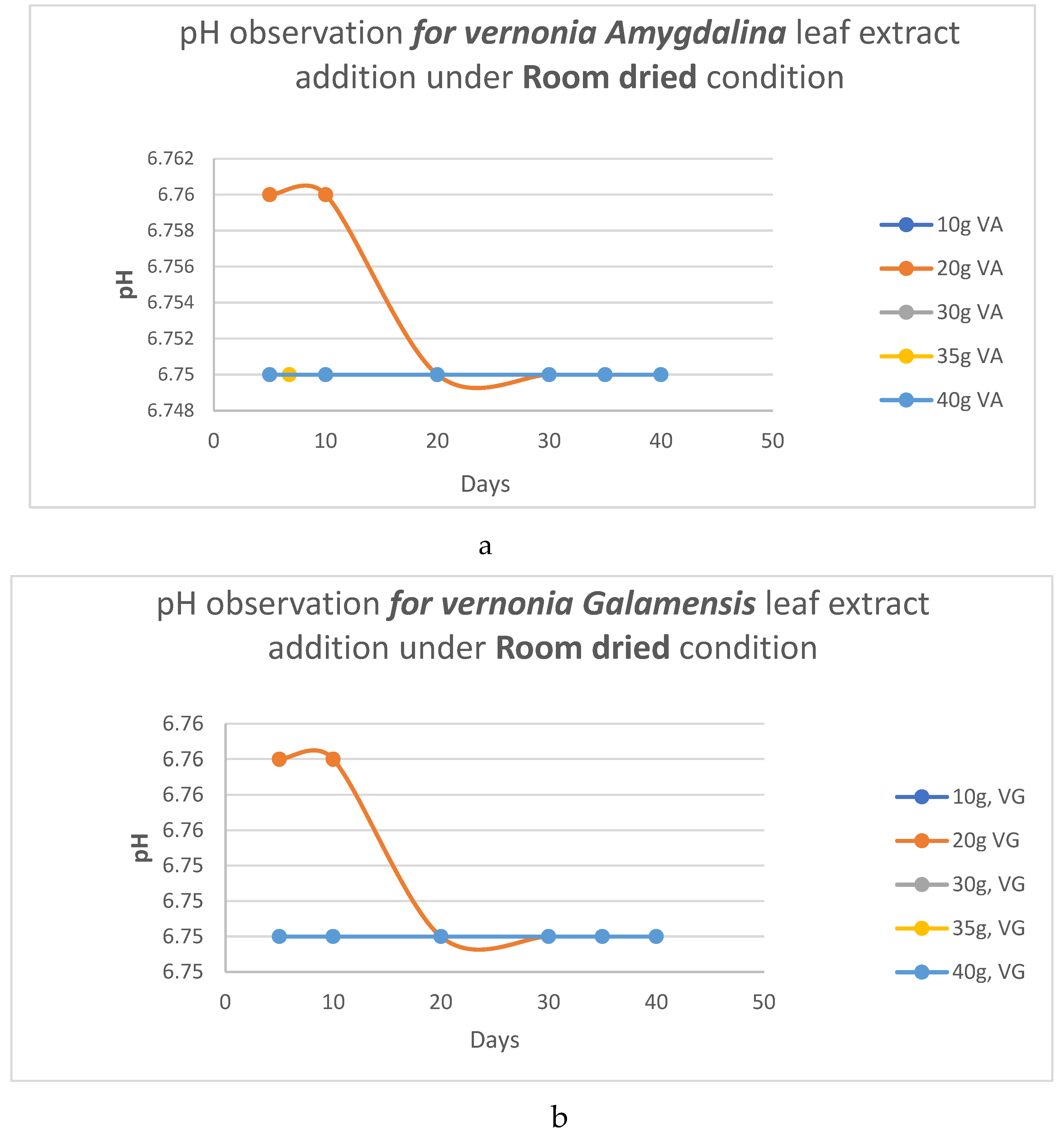
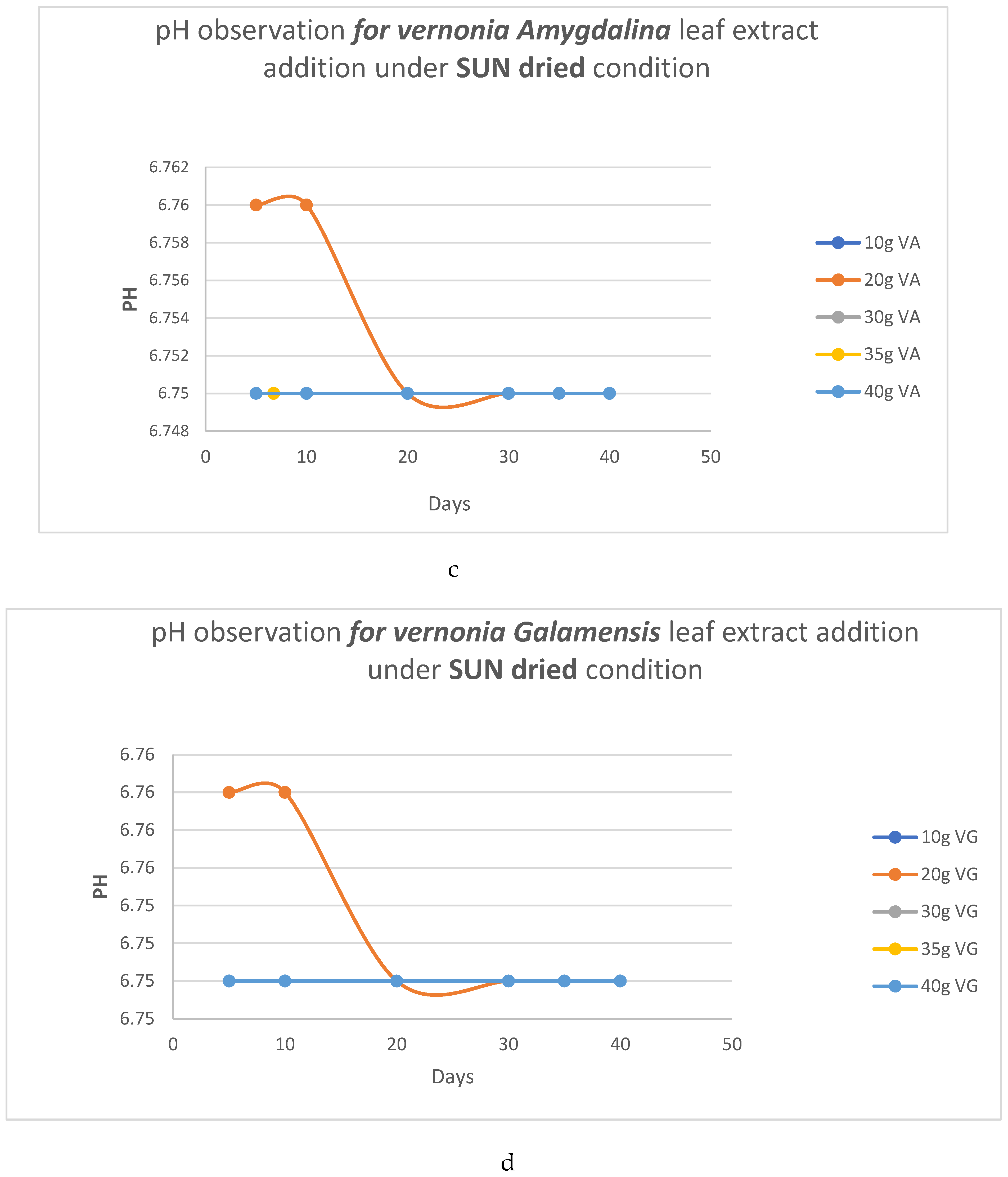
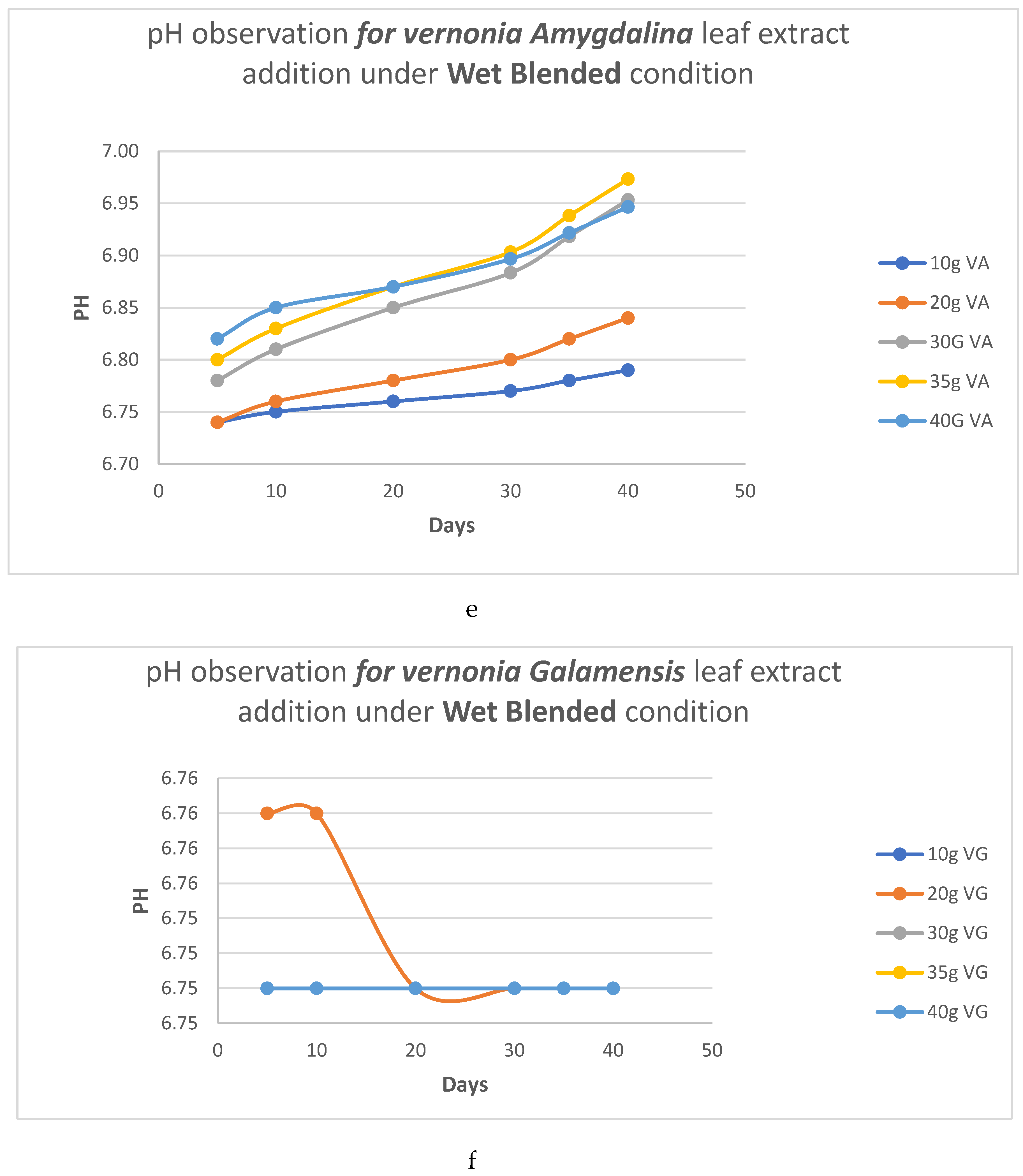
- b. HC analysis.
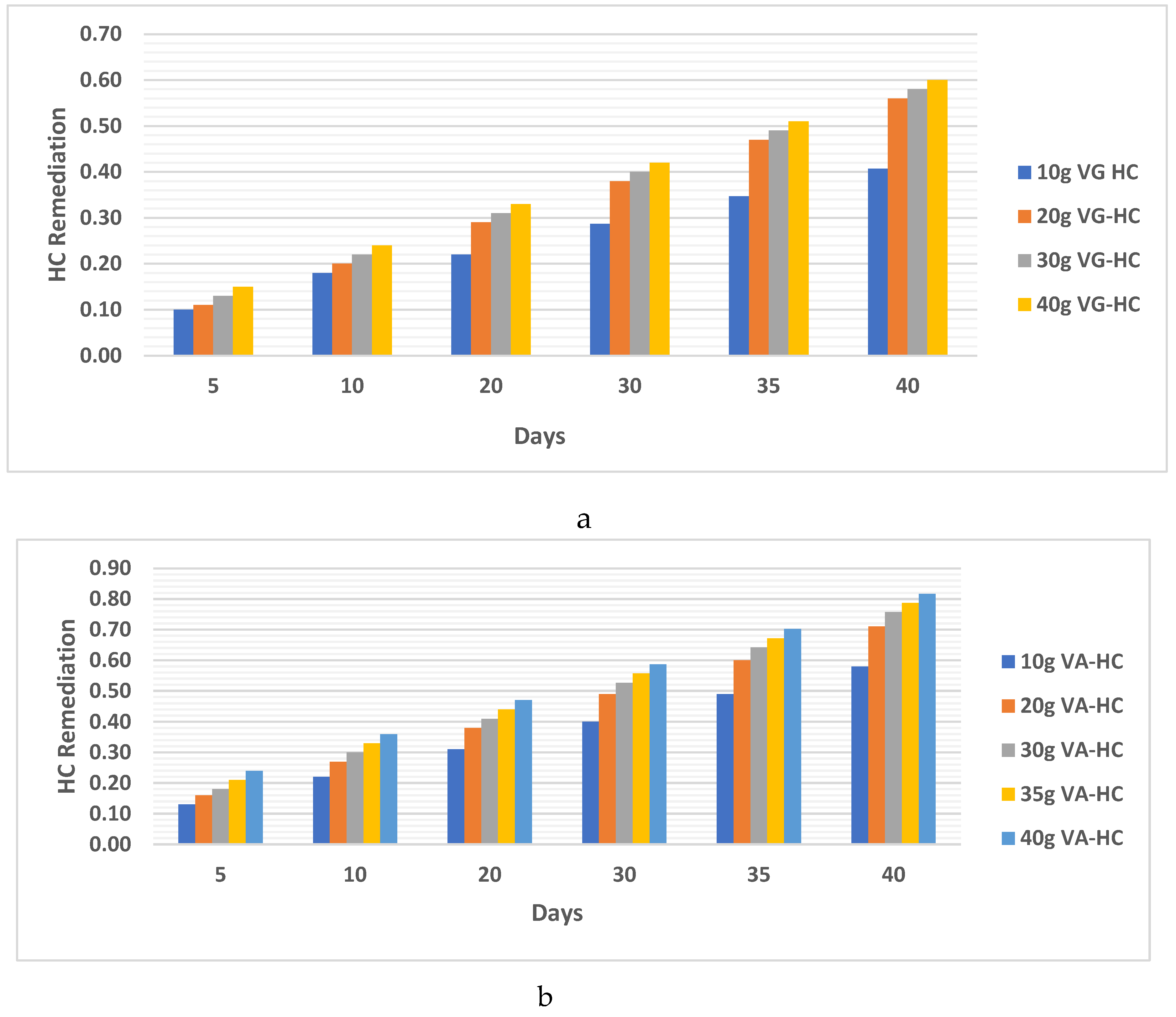
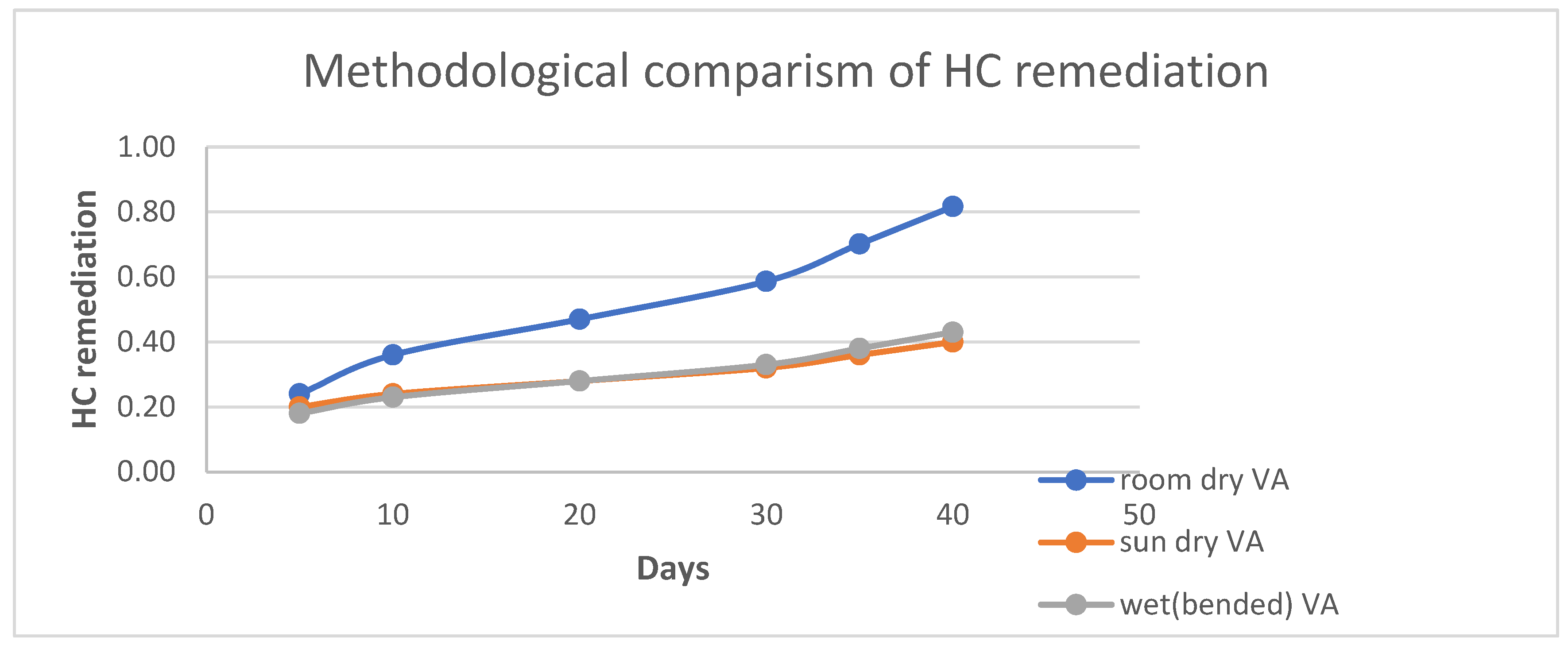
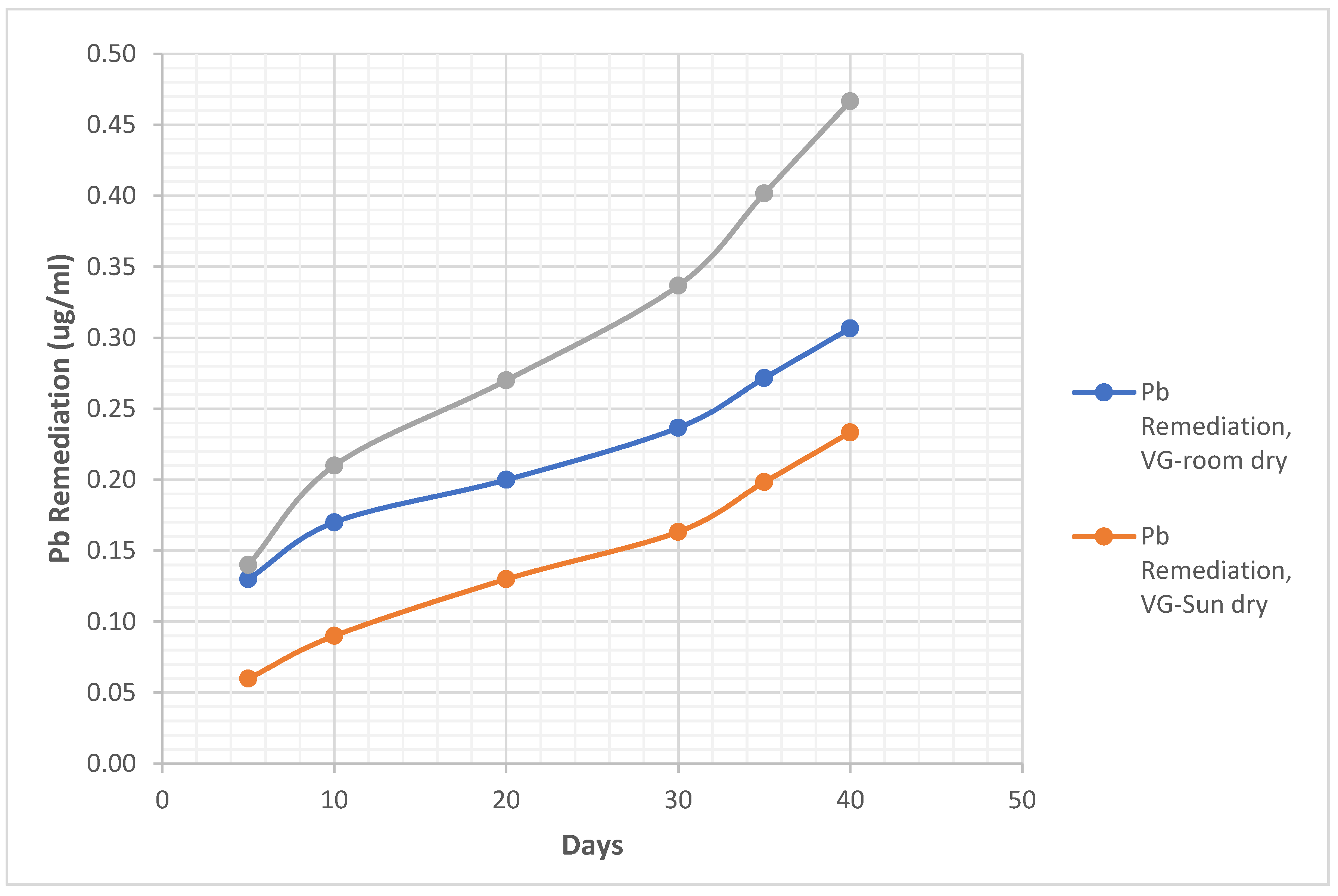
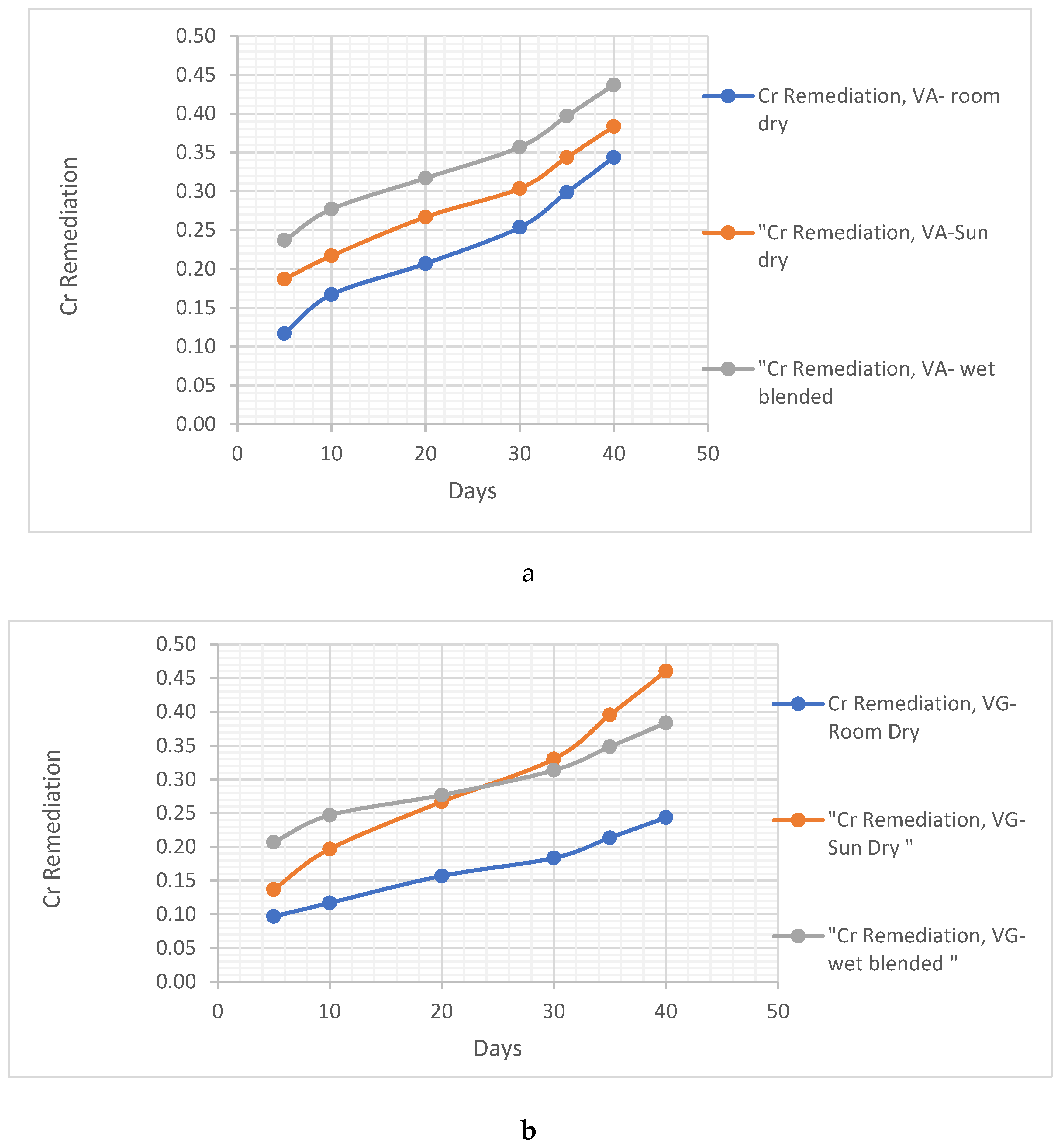
4.1.1. Clay Soil Bio Remedial Analysis
| pH and HC readings for samples before contaminant | |||||
| Initial Content sample | pH | HC | Pb (ug/ml) | Zn (ug/ml) | Cr(ug/ml) |
| Clay soil, Csi | 6.47 | 1.3 | 0.005 | 0.000 | 0.002 |
| pH and HC readings for samples after contaminant | |||||
| Final Content sample | pH | HC | Pb (ug/ml) | Zn (ug/ml) | Cr(ug/ml) |
| Clay soil, Csf | 6.64 | 4.69 | 1.21 | 0.924 | 1.105 |
- a. pH Analysis
- a. HC analysis
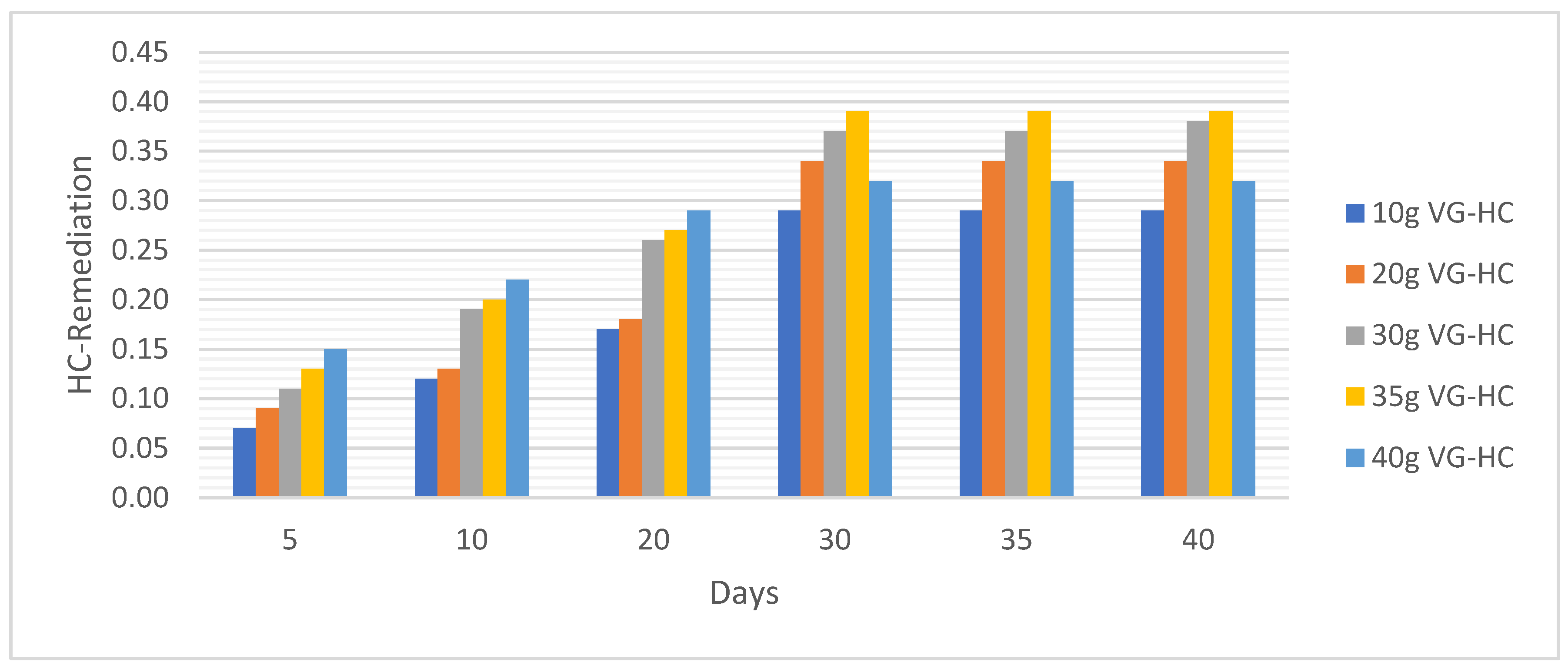
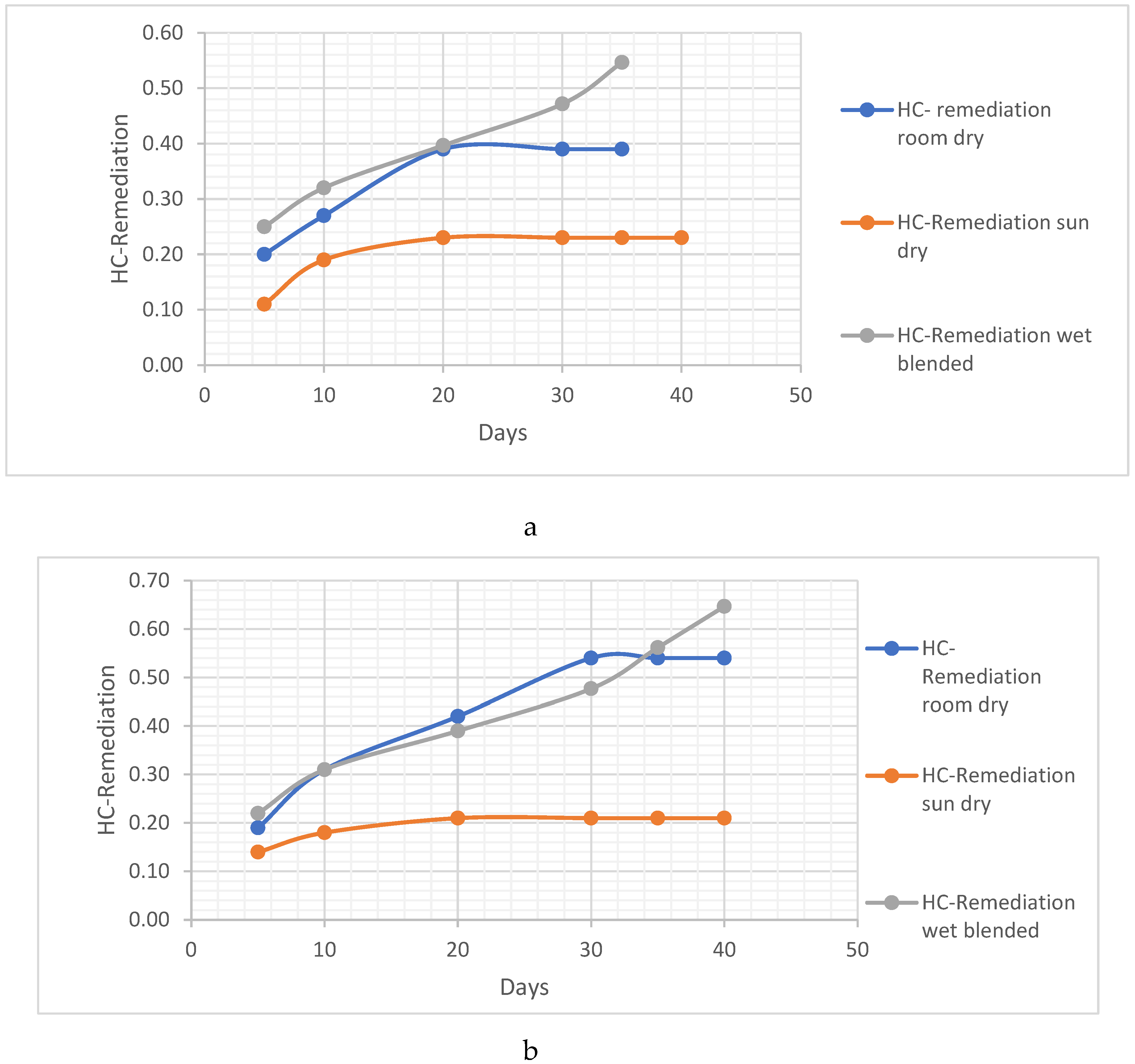
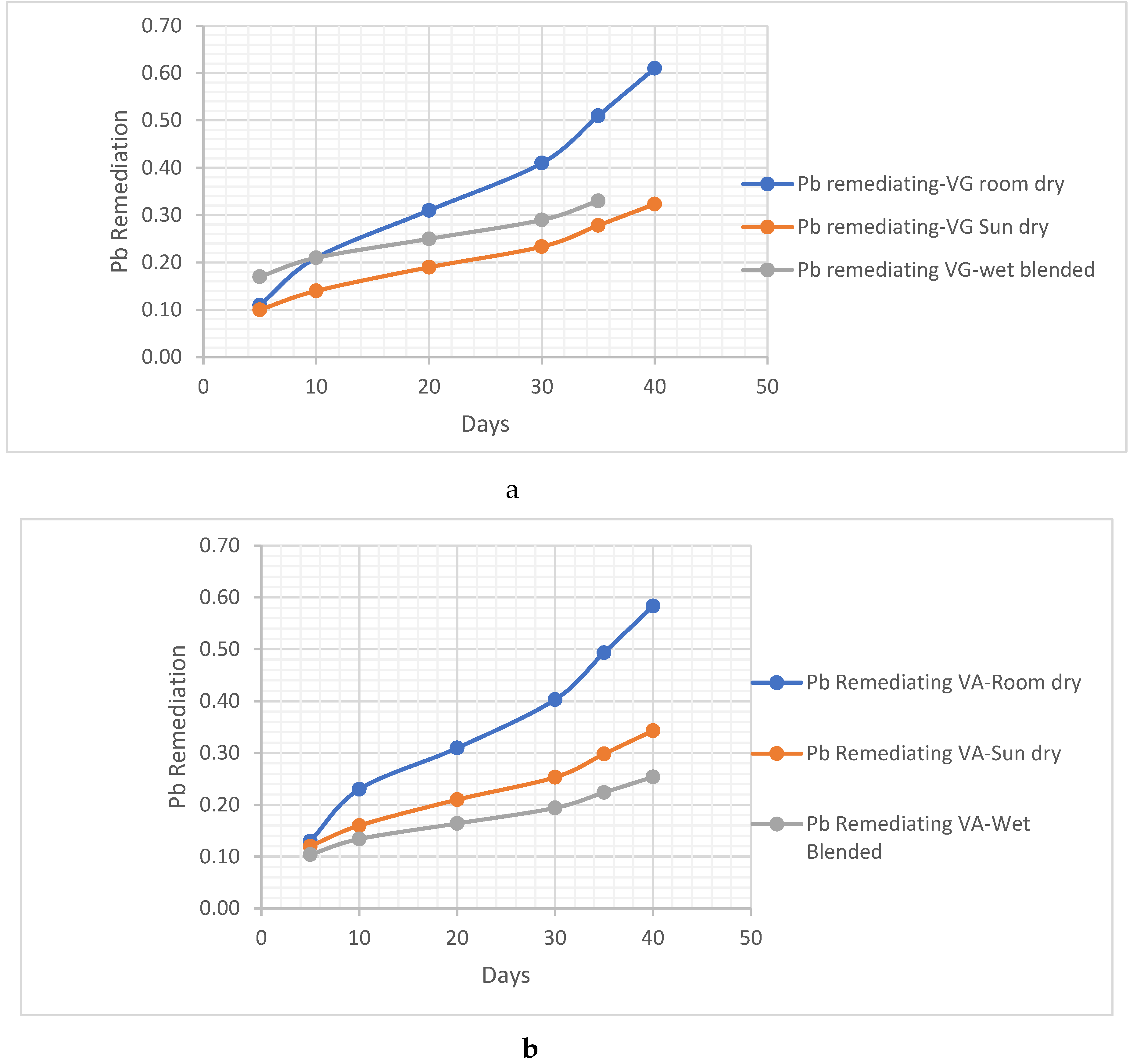
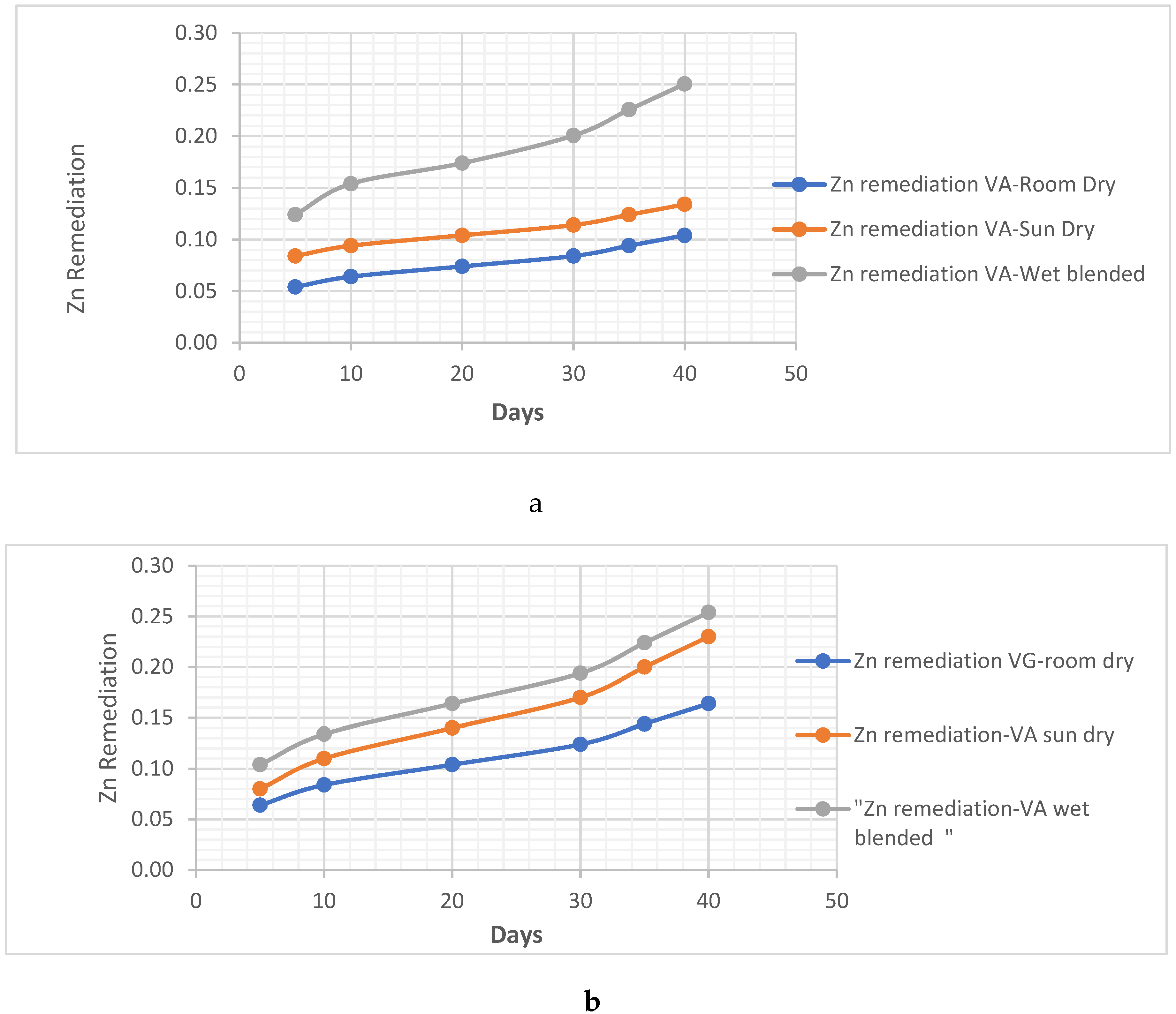
Metal Analysis
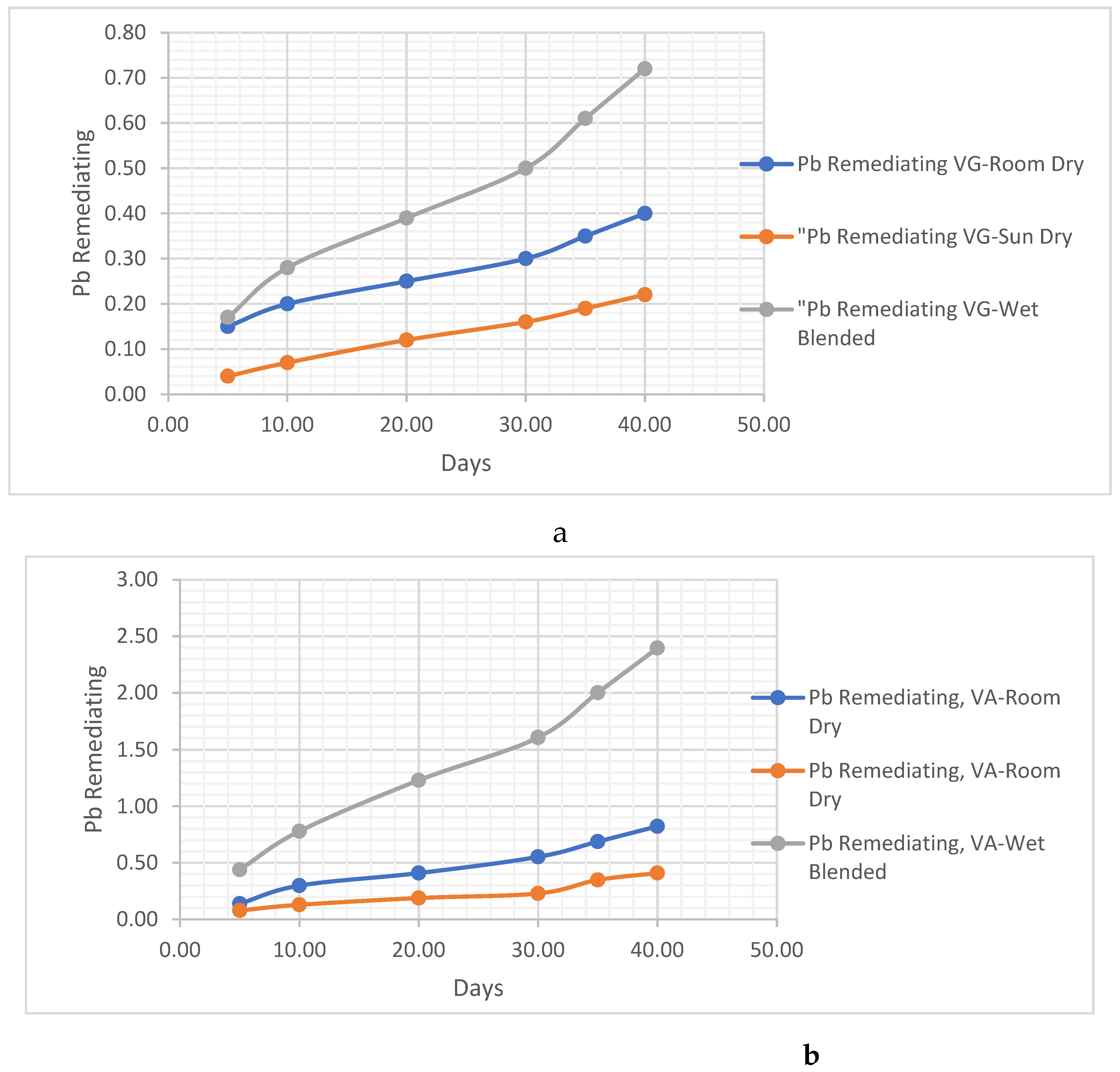
- i. Pb Remediating Response
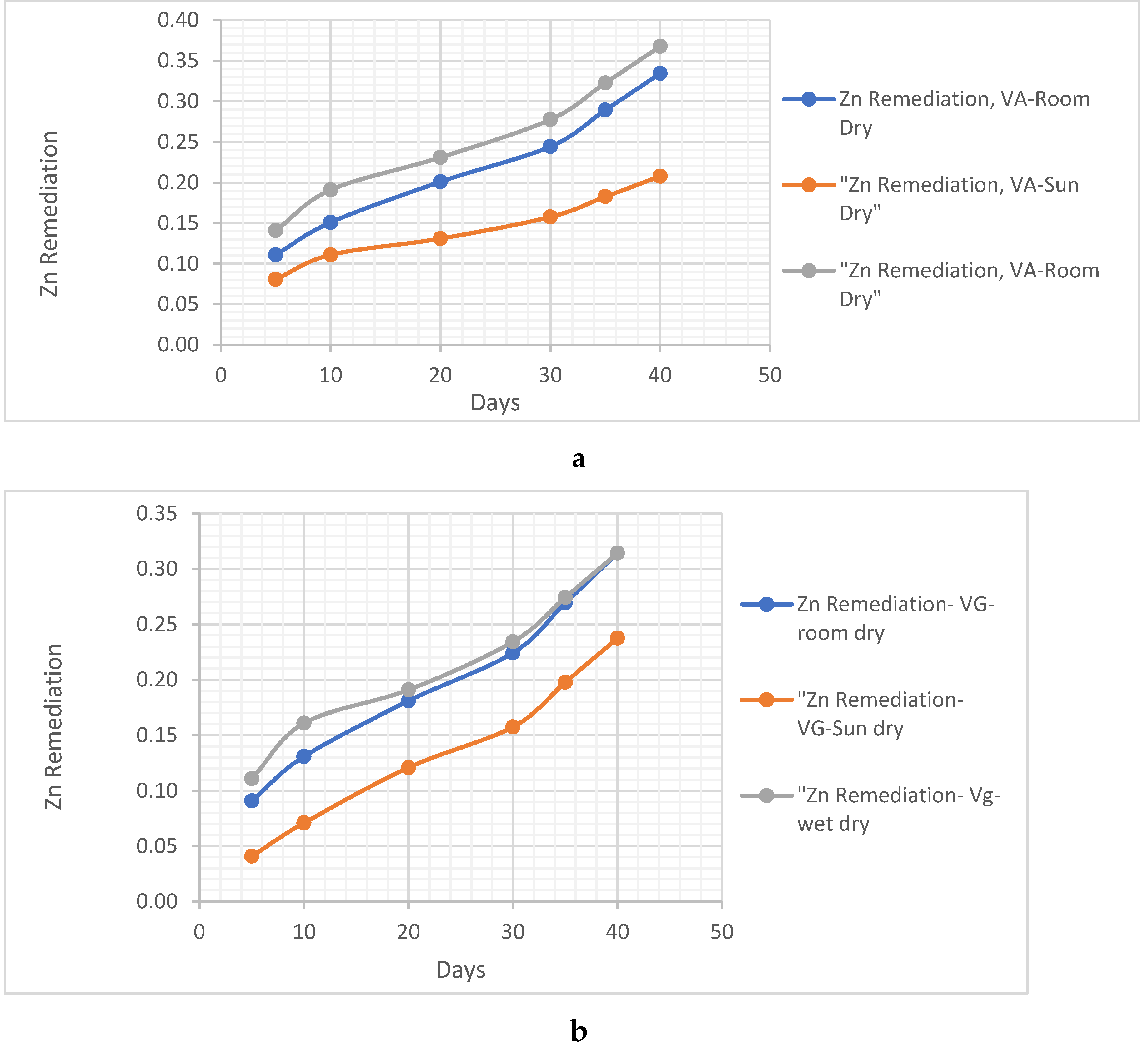
Zn Remediating Response
Cr remediating Response
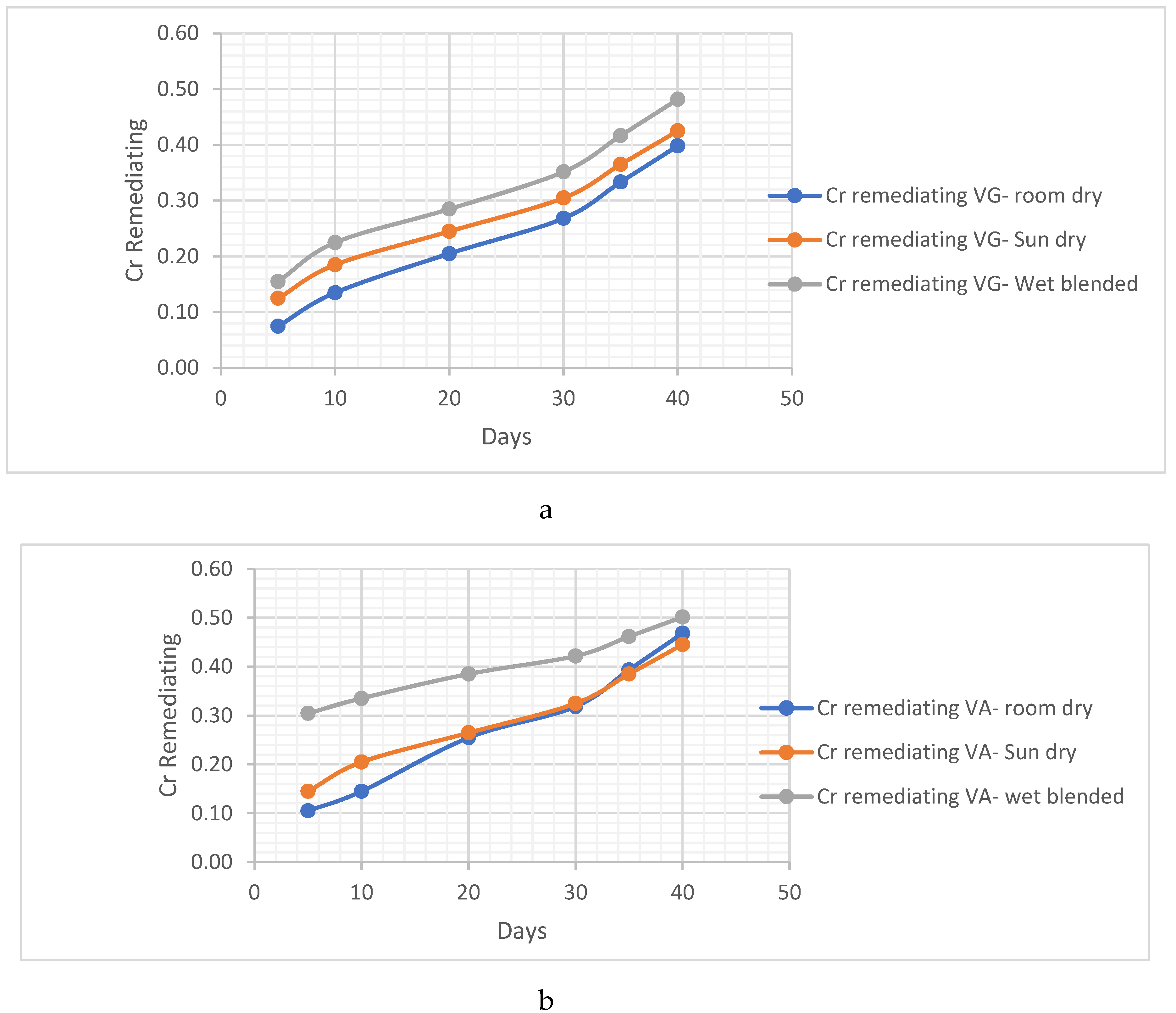
4.1.3. Sandy-Loamy Soil Bio Remedial Analysis
| pH and HC readings for samples before contaminant | |||||
| Initial Content sample | pH | HC | Pb (ug/ml) | Zn (ug/ml) | Cr(ug/ml) |
| Sandy Loam Soil, SLSi | 6.76 | 2.59 | 0.018 | 0.022 | 0.015 |
| pH and HC readings for samples after contaminant | |||||
| Final Content sample | pH | HC | Pb (ug/ml) | Zn (ug/ml) | Cr(ug/ml) |
| Sandy Loam Soil, SLSf | 6.75 | 4.67 | 1.22 | 0.923 | 1.103 |
PH Analysis
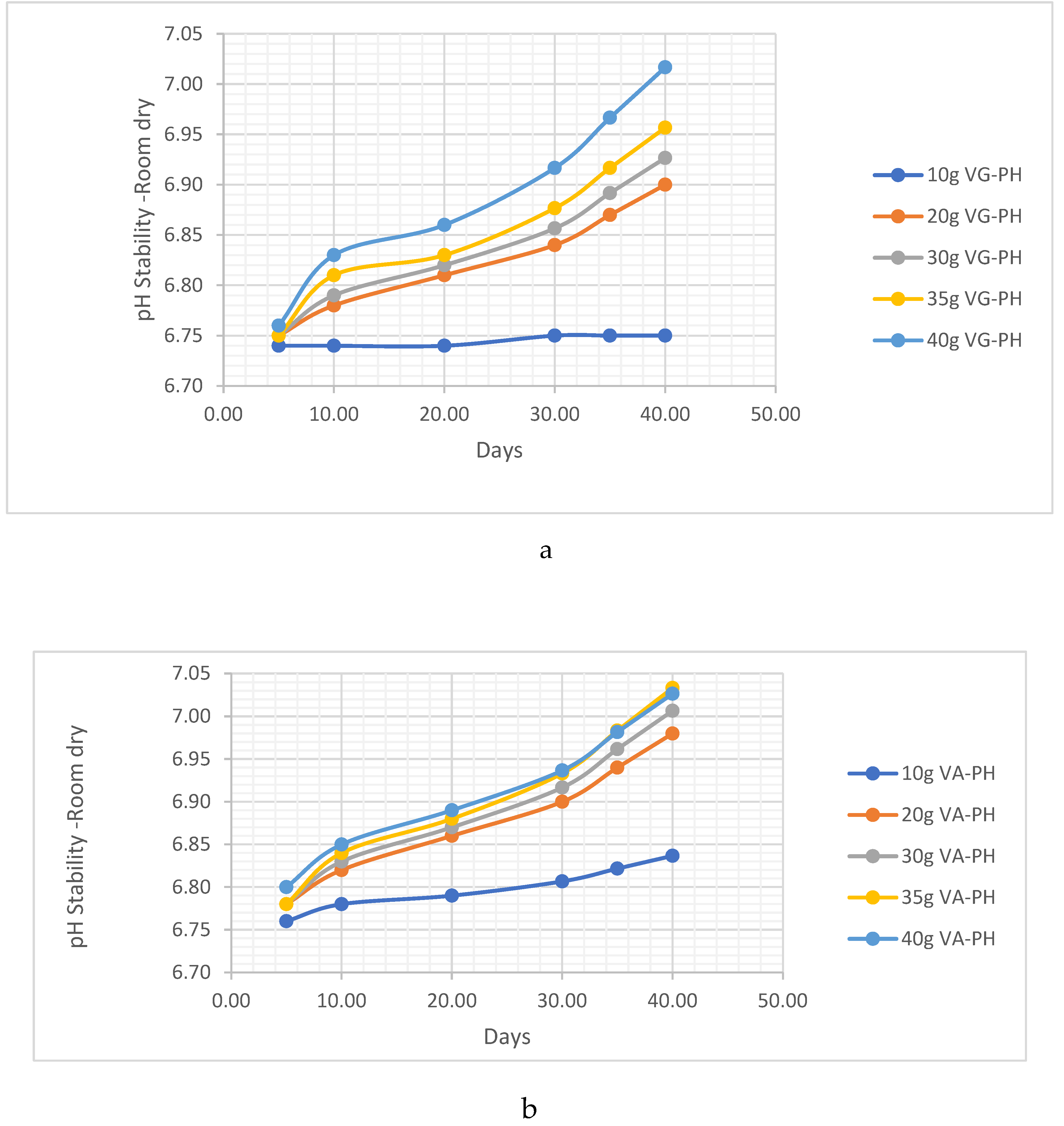
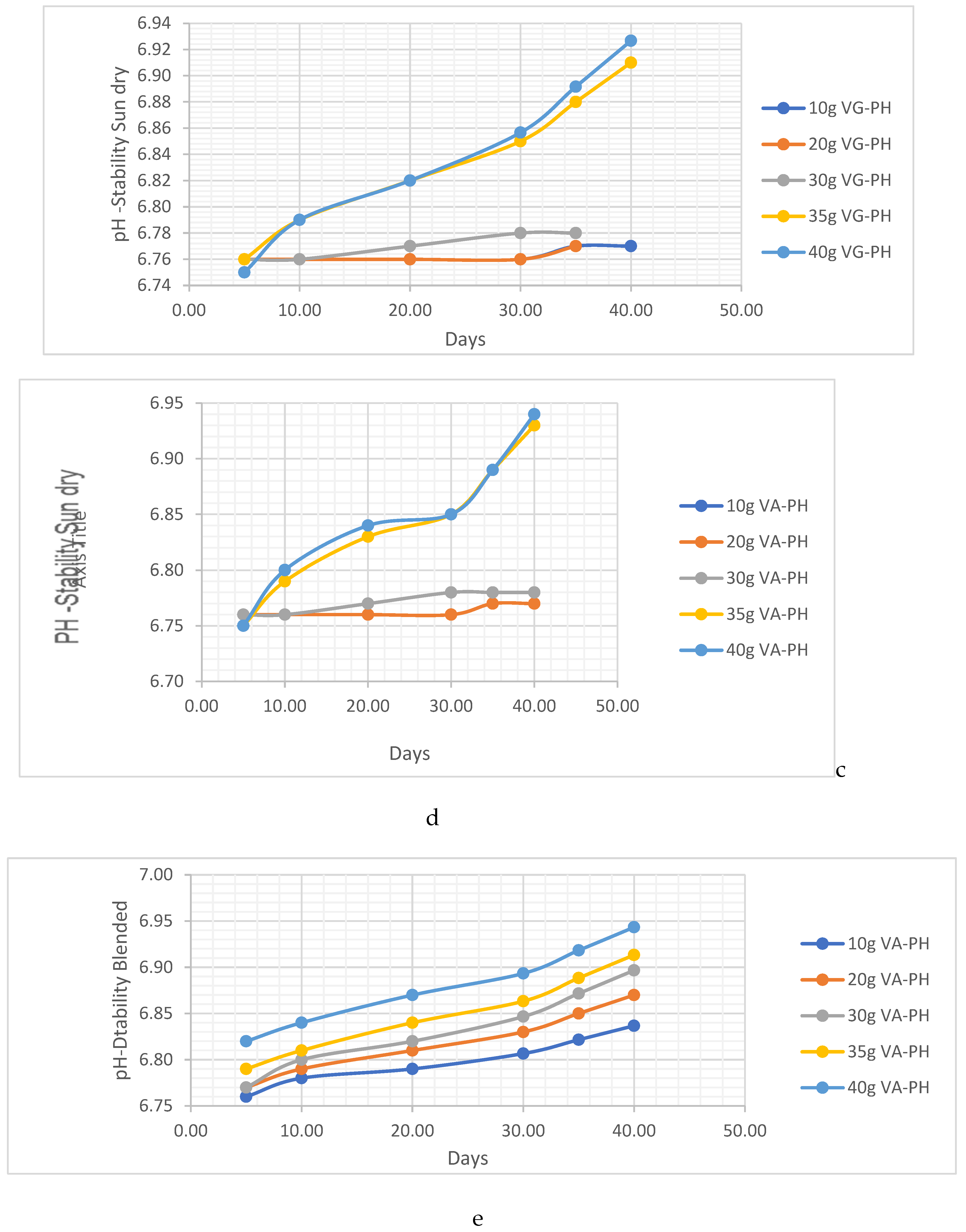
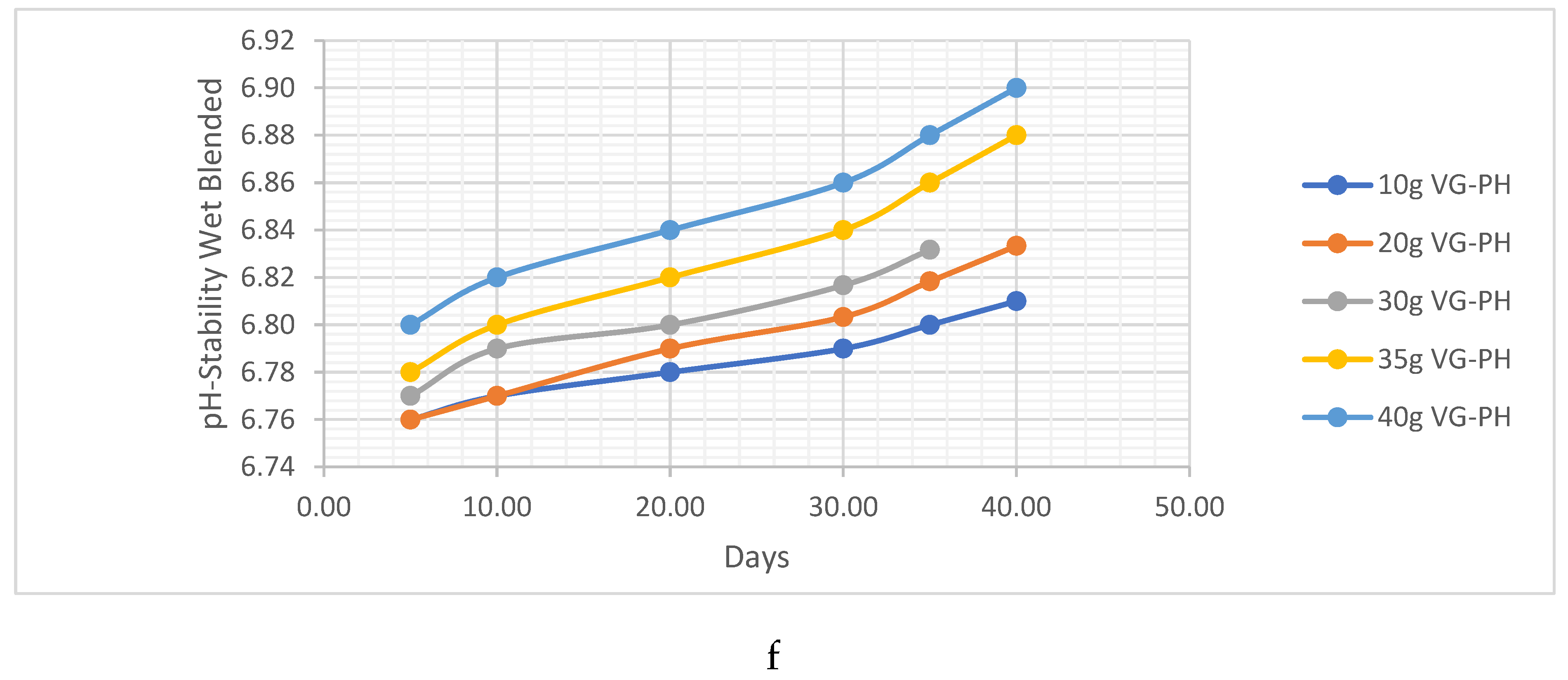
HC Analysis .
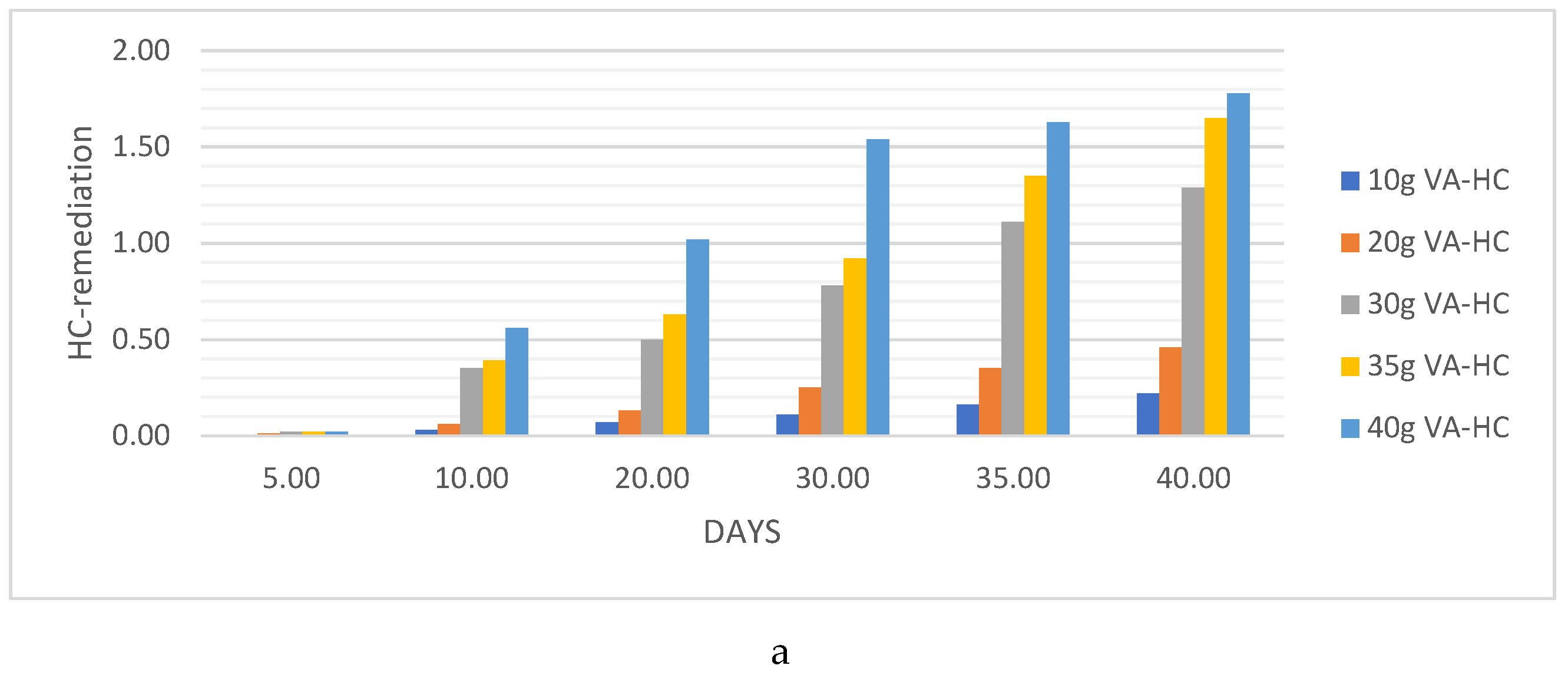
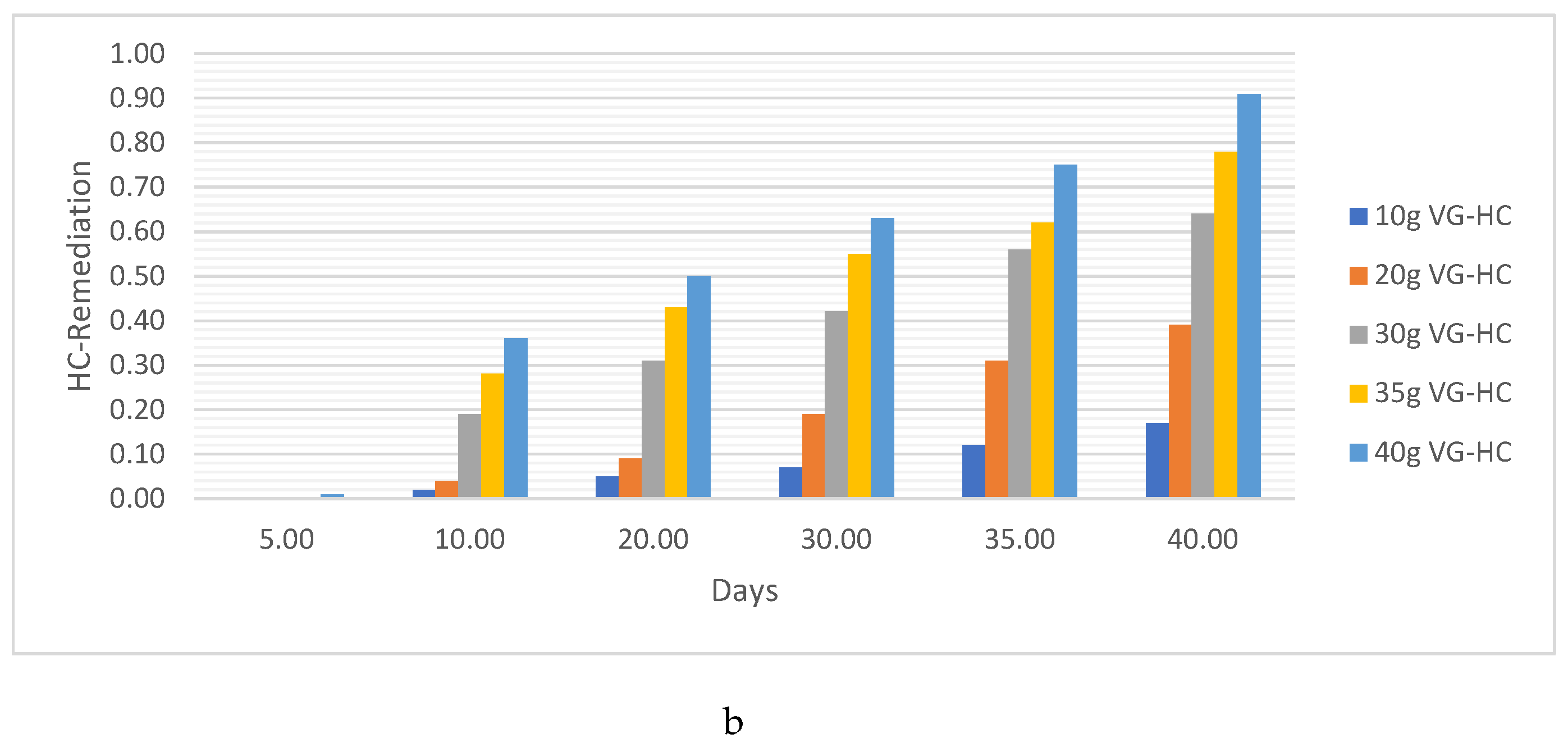
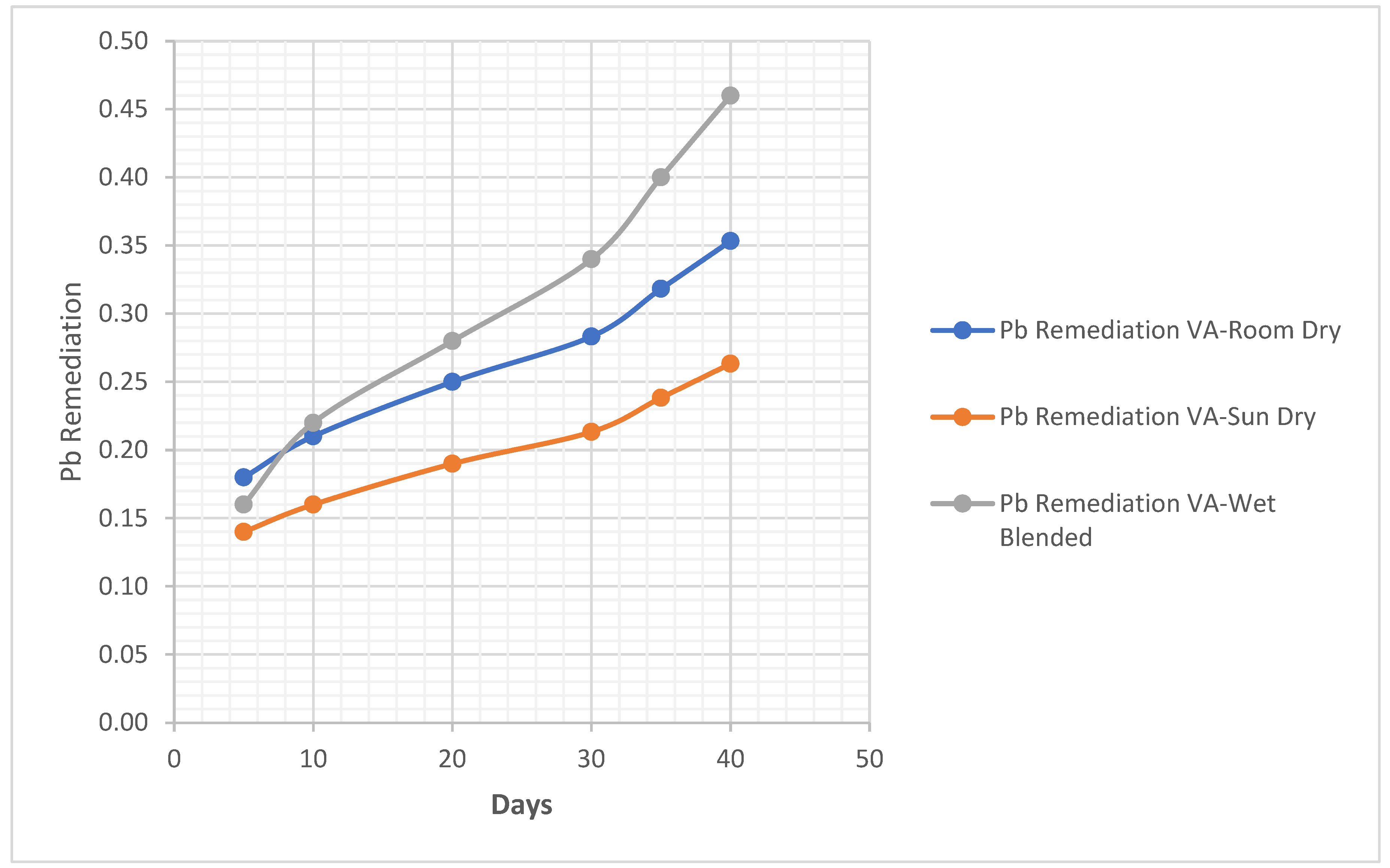
Pb. Metal Analysis
Pb Remediating Response For Sandy-Loamy Soil.
- i. Zn Remediating Response
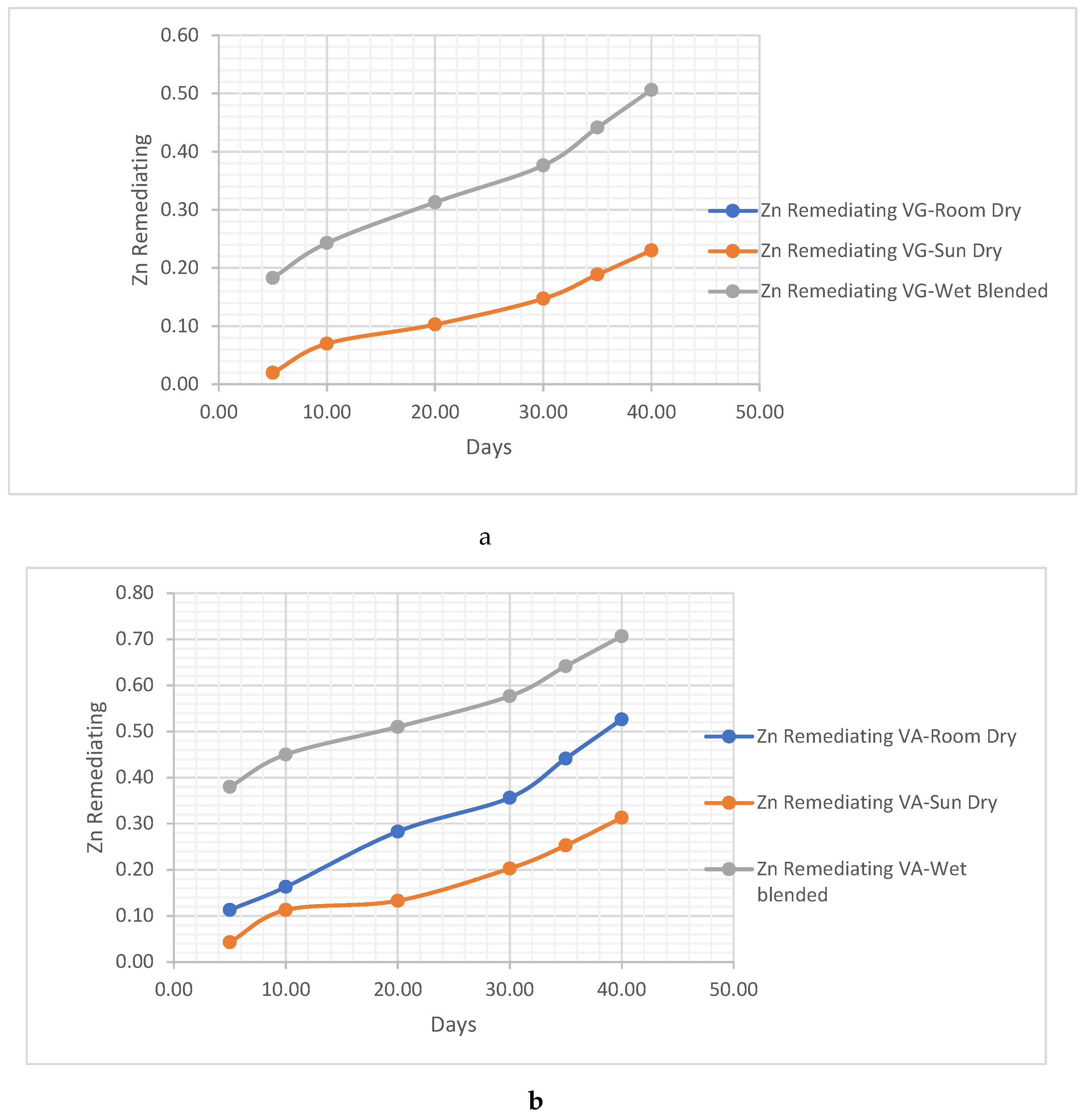
- ii. Cr remediating Response
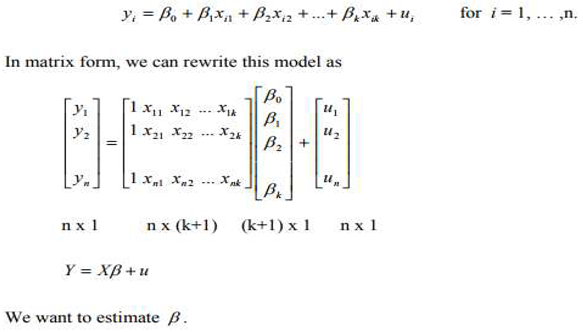
4.2-. Modal- Prediction Analysis
4.2.1. Model for Swamp Soil
- Constant: 11.040
- Time: 0.0041616 with a standard error (SE) of 0.0003246
- Mass: 0.0010702 with a standard error (SE) of 0.0003751
- pH: -1.627 with a standard error (SE) of 1.359.
- Time: T = 12.82, P < 0.000 (highly significant)
- Mass: T = 2.85, P = 0.008 (significant)
- pH: T = -1.20, P = 0.242 (not significant
ADDITIONAL STATISTICS:
- Constant: 3.45
- Time: 0.0077294 with a standard error (SE) of 0.0009772
- Mass: 0.001892 with a standard error (SE) of 0.001129
- pH: -0.509 with a standard error (SE) of 4.091.
- Time: T = 7.91, P < 0.000 (highly significant)
- Mass: T = 1.68, P = 0.106 (not significant)
- pH: T = -0.12, P = 0.902 (not significant).
- S: The standard error of the regression (S) is 0.0576976.
- R-Squared (R-Sq): The R-squared value is 78.3%, indicating that 78.3% of the variation in the Pb concentration can be explained by the regression model.
- Adjusted R-Squared (R-Sq(adj)): The adjusted R-squared value is 75.8%, which takes into account the number of predictor variables in the model.
- Constant: 10.40
- Time: 0.0058693 with a standard error (SE) of 0.0004501
- Mass: 0.0015317 with a standard error (SE) of 0.0005201
- pH: -1.541 with a standard error (SE) of 1.885
- Time: T = 13.04, P < 0.000 (highly significant)
- Mass: T = 2.94, P = 0.007 (significant)
- pH: T = -0.82, P = 0.421 (not significant)
- S: The standard error of the regression (S) is 0.0265765.
- R-Squared (R-Sq): The R-squared value is 91.3%, indicating that 91.3% of the variation in the Zn concentration can be explained by the regression model.
- Adjusted R-Squared (R-Sq(adj)): The adjusted R-squared value is 90.3%, which takes into account the number of predictor variables in the model.
- Constant: -7.31
- Time: 0.0051582 with a standard error (SE) of 0.0003631
- Mass: 0.0069393 with a standard error (SE) of 0.0004196
- pH: 1.070 with a standard error (SE) of 1.520
- Time: T = 14.21, P < 0.000 (highly significant)
- Mass: T = 16.54, P < 0.000 (highly significant)
- pH: T = 0.70, P = 0.488 (not significant
Additional Statistics:
- S: The standard error of the regression (S) is 0.0214408.
- R-Squared (R-Sq): The R-squared value is 96.0%, indicating that 96.0% of the variation in the Cr concentration can be explained by the regression model.
- Adjusted R-Squared (R-Sq(adj)): The adjusted R-squared value is 95.5%, which takes into account the number of predictor variables in the model.
- Constant: 3.0969
- Time_1: 0.0058911 with a standard error (SE) of 0.0005299
- Mass_1: 0.0033893 with a standard error (SE) of 0.0007112
- pH_1: -0.4596 with a standard error (SE) of 0.1432
- Time_1: T = 11.12, P < 0.000 (highly significant)
- Mass_1: T = 4.77, P < 0.000 (highly significant)
- pH_1: T = -3.21, P = 0.004 (significant)
- S: The standard error of the regression (S) is 0.0166601.
- R-Squared (R-Sq): The R-squared value is 93.4%, indicating that 93.4% of the variation in the HC concentration can be explained by the regression model.
- Adjusted R-Squared (R-Sq(adj)): The adjusted R-squared value is 92.7%, which takes into account the number of predictor variables in the model.
- Constant: 0.726
- Time_1: 0.008814 with a standard error (SE) of 0.001413
- Mass_1: 0.002037 with a standard error (SE) of 0.001897
- pH_1: -0.1037 with a standard error (SE) of 0.3820
- Time_1: T = 6.24, P < 0.000 (highly significant)
- Mass_1: T = 1.07, P = 0.293 (not significant)
- pH_1: T = -0.27, P = 0.788 (not significant)
- S: The standard error of the regression (S) is 0.0444367.
- R-Squared (R-Sq): The R-squared value is 87.6%, indicating that 87.6% of the variation in the Pb_1 concentration can be explained by the regression model.
- Adjusted R-Squared (R-Sq(adj)): The adjusted R-squared value is 86.1%, which takes into account the number of predictor variables in the model.
- Constant: 1.713, SE Coef: 1.689, T: 1.01, P: 0.320
- Time: 0.0082985, SE Coef: 0.0009276, T: 8.95, P: 0.000
- Mass: 0.006083, SE Coef: 0.001410, T: 4.31, P: 0.000
- pH: -0.2708, SE Coef: 0.2548, T: -1.06, P: 0.298
- ● Degrees of Freedom (DF): 3
- ● Sum of Squares (SS): 0.35398
- ● Mean Square (MS): 0.11799
- ● F-value: 92.43
- ● p-value: 0.000
- ● Degrees of Freedom (DF): 26
- ● SS: 0.03319
- ● MS: 0.00128
- ● Degrees of Freedom (DF): 29
- ● SS: 0.38717
- Constant: -13.772, SE Coef: 1.484, T: -9.28, P: 0.000
- Time_1: 0.0024258, SE Coef: 0.0009678, T: 2.51, P: 0.019
- Mass_1: -0.002502, SE Coef: 0.001491, T: -1.68, P: 0.105
- pH_1: 2.0617, SE Coef: 0.2248, T: 9.17, P: 0.000
- Standard Deviation (S): 0.0345595
- Coefficient of Determination (R-Sq): 96.8%
- Adjusted R-Square (R-Sq(adj)): 96.5%
- ● Degrees of Freedom (DF): 3
- ● Sum of Squares (SS): 0.95150
- ● Mean Square (MS): 0.31717
- ● F-value: 265.55
- ● p-value: 0.000
- ● Degrees of Freedom (DF): 26
- ● SS: 0.03105
- ● MS: 0.00119
- ● Degrees of Freedom (DF): 29
- ● SS: 0.98255
- Constant: 3.962, SE Coef: 1.697, T: 2.33, P: 0.028
- Time_1: 0.008439, SE Coef: 0.001107, T: 7.62, P: 0.000
- Mass_1: 0.005892, SE Coef: 0.001705, T: 3.46, P: 0.002
- pH_1: -0.5983, SE Coef: 0.2571, T: -2.33, P: 0.028
- Standard Deviation (S): 0.0395213
- Coefficient of Determination (R-Sq): 84.2%
- Adjusted R-Square (R-Sq(adj)): 82.4%
- ● Degrees of Freedom (DF): 3
- ● Sum of Squares (SS): 0.216307
- ● Mean Square (MS): 0.072102
- ● F-value: 46.16
- ● p-value: 0.000
- ● Degrees of Freedom (DF): 26
- ● SS: 0.040610
- ● MS: 0.001562
- ● Degrees of Freedom (DF): 29
- ● SS: 0.256917
- Time_1: 0.0025315
- Mass_1: 0.0018940
- pH_1: 0.24446
- Standard Error (SE) represents the precision of the coefficient estimates.
- T-value indicates the significance of the coefficient, where larger absolute values suggest more significant relationships.
- P-value shows the probability of observing a coefficient as extreme as the one obtained, assuming the null hypothesis (no relationship) is true.
- The constant term in the regression equation is -1.6346, indicating the estimated Zn_1 value when all predictors are zero.
- The Standard Error (S) of the regression is 0.00870020, which represents the average distance between the observed and predicted values.
- R-squared (R-Sq) is 98.1%, indicating that the predictor variables explain 98.1% of the variability in Zn_1.
- R-squared (adjusted) (R-Sq(adj)) is 97.8%, which considers the number of predictor variables and adjusts R-squared accordingly.
- Degrees of Freedom (DF): 3
- um of Squares (SS): 0.099379
- Mean Square (MS): 0.033126
- F-value: 437.64
- P-value: 0.000
- Degrees of Freedom (DF): 26
- Sum of Squares (SS): 0.001968
- Mean Square (MS): 0.000076
- Degrees of Freedom (DF): 29
- Sum of Squares (SS): 0.101347
- Time_1: 0.0068464
- Mass_1: 0.009396
- pH_1: -0.2788
- Standard Error (SE) represents the precision of the coefficient estimates.
- T-value indicates the significance of the coefficient, where larger absolute values suggest more significant relationships.
- P-value shows the probability of observing a coefficient as extreme as the one obtained, assuming the null hypothesis (no relationship) is true.
- The constant term in the regression equation is 1.801, indicating the estimated Cr_1 value when all predictors are zero.
- The Standard Error (S) of the regression is 0.0278546, which represents the average distance between the observed and predicted values.
- R-squared (R-Sq) is 94.9%, indicating that the predictor variables explain 94.9% of the variability in Cr_1.
- R-squared (adjusted) (R-Sq(adj)) is 94.3%, which considers the number of predictor variables and adjusts R-squared accordingly.
- Degrees of Freedom (DF): 3
- Sum of Squares (SS): 0.37409
- Mean Square (MS): 0.12470
- F-value: 160.72
- P-value: 0.000
- Degrees of Freedom (DF): 26
- Sum of Squares (SS): 0.02017
- Mean Square (MS): 0.00078
- Degrees of Freedom (DF): 29
- Sum of Squares (SS): 0.39426
- Constant: -125.51 (P = 0.003)
- Time: -0.02073 (P = 0.127)
- Mass: -0.01570 (P = 0.247)
- pH: 18.619 (P = 0.004)
- Standard Error (S): 0.328763
- R-squared (R-Sq): 62.3%
- Adjusted R-squared (R-Sq(adj)): 57.9%
- ● Degrees of Freedom (DF): 3
- ● Sum of Squares (SS): 4.6396
- ● Mean Square (MS): 1.5465
- ● F-value: 14.31 (P = 0.000)
- ● Degrees of Freedom (DF): 26
- ● Sum of Squares (SS): 2.8102
- ● Mean Square (MS): 0.1081
- ● Degrees of Freedom (DF): 29
- ● Sum of Squares (SS): 7.4498
- SS (Sum of Squares): This column indicates the sum of squares for each source of variation.
- F (F-value): This column shows the F-value, which is a ratio of the mean squares between regression and residual error. It measures the significance of the regression model.
- P (p-value): This column provides the p-value associated with the F-value, indicating the significance level of the regression model.
- Source: This column indicates the source of the variation in the analysis.
- DF (Degrees of Freedom): This column represents the degrees of freedom associated with each source of variation.
- SS (Sum of Squares): This column indicates the sum of squares for each source of variation.
- MS (Mean Square): This column represents the mean square, which is the sum of squares divided by the degrees of freedom.
- F (F-value): This column shows the F-value, which is a ratio of the mean squares between regression and residual error. It measures the significance of the regression model.
- P (p-value): This column provides the p-value associated with the F-value, indicating the significance level of the regression model.
References
- Aldrett, S., Bonner, J.S., McDonalds, T.J., Mills, M.A., Autenrieth, R.L. (1997) Degradation of crude oil enhanced by commercial microbial cultures. Proceedings of 1997 International Oil Spill Conference. American Petroleum Institute, Washington DC, 995-996. [CrossRef]
- Anderson, I: (2005). Niger River basin: A Vision for Sustainable Development, The World Bank Pp. 1-131.
- Atlas, R. M., (1988). Biodegradation of hydrocarbons in the environment, In: Environmental Biotechnology Omenn, GS EdPlenum Press, NewYork. [CrossRef]
- Atlas, R. M., (1991). Microbial hydrocarbon degradation: bioremediation of oil spills. J. Chem. Technol. Biotechnol., 52, 149-156. [CrossRef]
- Azarowicz, R.M. (1973) Microbial degradation of petroleum. US Patent 3,769,164.
- Baird, J.,(2010). “Oil’s Shame in Africa”. Newsweek: 27. [CrossRef]
- Banerjee, D. K., Fedora, P. M., Hashimoto, A., Masliyah, J. H., Pickard, M.A. andGray, M. R., (1995). Monitoring the biological treatment of anthracite-contaminated soil in a rotating –drum bioreactor. Appl. Microbial. Biotechnol., 43, 521-528.
- Barbee, G. C., Brown, K. W., Thomas, J. C., Donelley, K. C., Murray, H. E., (1996). Mutagenic activity (Ames test) of wood-preserving waste sludge applied to soil Bull. Environ Contam Toxicol., 57, 54-62. [CrossRef]
- Bogumil T, (2014). Oil-Induced Displacement and Resettlement: Social Problem and Human Rights Issue,http://www.conflictrecovery.org/bin/Bogumil_Terminski-Oil.
- Bonaventura, C., Boneventura, J., Bodishbaugh, D. F., (1995). Environmental Bioremediation: Approaches and processes. In: Ecotoxicity and Human Health (Eds) de serres, F.J and Bloom, AD CRS Lewis Publ. Boca Raton, New York. 199-200.
- Bronwen M.M (1999). The Price of Oil Human Rights Watch. 1999. Retrieved November 9, 2007.
- Bronwen, M., (1999). The Price of Oil Human Rights Watch. Retrieved November 9, 2007.
- Cheesbrough, M., 2002. District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press, UK., pp: 135-162.
- CNN, (2006). Pipeline explosion kills at least 200. Retrieved May 29, 2007.
- Compeau, G. C., Mahaffey, W. D., Patras, L., (1991). Full scale bioremediation of contaminated soil and water. In : Environmental biotechnology for waste treatment. Eds. GS Sayler, R. Fox, JW Blackbourn. Plenum Press, NewYork and London. Environ. Sci. Res., 25, 91-109.
- Environmental Inquiry - Bioremediation., retrieved 2017 (http://ei.cornell.edu/biodeg/bioremed/).
- Forsyth, J.V., Tsao, Y.M., Blem, R.D. (1995) Bioremediation: when is augmentation needed? In Hinchee, R.E. et al. (eds) Bioaugmentation for Site Remediation. Battelle Press, Columbus, OH, pp1 14.
- Goldstein, R.M., Mallory, L.M., Alexander, M. (1985) Reasons for possible failure of inoculation to enhance biodegradation. Applied and Environmental Microbiology, 50, 977-983. [CrossRef]
- Gray, M. R., Banerjee, D. K., Fedora, K. P. M., Habhimoto,A., Maslryah,J. H., Pickard, M.A., (1994). Biological remediation of anthracene-contaminated soil in rotating bioreactors. Appl. Microbial Biotechnol., 40, 933-944.
- Hozumi, T., Tsutsumi, H. and Kono, M. (2000) Bioremediation on the shore after an oil spill from the Nakhodka in the Sea of Japan. I. Chemistry and characteristics of the heavy oil loaded on the Nakhodka and biodegradation tests on oil by a bioremediation agent with microbial cultures in the laboratory. Marine Pollution Bulletin, 40, 308-314. [CrossRef]
- Huan Jing Ke Xue (February 2007). “Chemical fixation of metals in soil using bone char and assessment of the soil genotoxicity”. Huan Jing Ke Xue. 28: 232–7. PMID 17489175.
- Hupe, K., Luth, J. C., Heerenklage, J., Stegmann, R., (1995). Blade-milking reactors in the biological treatment of contaminated soils, in Biological unit processes for HazardousWasteTreatment. Ed by HuncheeRE, Skeen RS, and Sayles GD, Battele Press, Columbus. 153-159.
- Jenkins, K. D., Sanders, B. M., (1992). Biomonitoring with biomarkers:A multi-tiered framework for conducting the ecological impact of contaminants. In: Ecological indicators. Mekenzie, D., Hyatt, E., McDonald, J. Eds. Elsever, NewYork.
- Kris S. Freeman (January 2012). Remediating Soil Lead with Fishbones. Environmental Health Perspectives. 120: A20–1.
- Le Floch, S., Merlin, F.X., Guillerme, M., Dalmazzone, C., and Le Corre, P. (1999) A field experimentation on bioremediation: Bioren. Environmental Technology, 20, 897-907. [CrossRef]
- Le Floch, S., Merlin, F.X., Guillerme, M., Tozzolino, P., Ballerini, D., Dalmazzone, C., and Lundh, T. (1997) Bioren: recent experiment on oil polluted shoreline in temperate climate. In: In-Situ and On-Site Bioremediation: Volume 4, Battelle Press, Columbus, OH, pp. 411-417.
- Leahy, J. A., Colwell, R. R., (1990). Microbial degradation of hydrocarbons in the environment. Microbiological Reviews, 54, 305-315. [CrossRef]
- Lovley, D. R., (2003). Cleaning up with genomics: applying molecular biology to bioremediation. Nature Reviews/Microbiology, 1, 35-44. Available at: (www.nature.com/reviews/micro). [CrossRef]
- Mauro, G. and Wynne, III, B.J. (1990) Mega Borg oil spill: an open water bioremediation test. Texas General Land Office, Austin, Texas.
- Meagher, RB (2000). Phytoremediation of toxic elemental and organic pollutants. Current Opinion in Plant Biology. 3 (2): 153–162. [CrossRef]
- Microbiological cultures for the treatment of an oil spill. Marine Pollution Bulletin, 40, 320 324.
- Moffat, F and Linden, K., (2009). Perception and Reality: Assessing Priorities for Sustainable Development in the Niger River Delta.
- Mohan, R.R., Byrd, G.H., Nixon, J., and Bucker, E.R. (1975) Use of microorganisms to disperse and degrade oil spills. US Patent 3,871,957.
- National Research Council (NRC), (1985). Oil in the sea; inputs, fates and effects. National Academy Press, Washington, DC. [CrossRef]
- Nwilo, P.C. & Badejo, O. T.:(2001). Impacts of Oil spills along the Nigerian coast ,The Association for Environmental Health and Sciences, 2001.
- Olapade, OA; Ronk, AJ (2014). Isolation, Characterization and Community Diversity of Indigenous Putative Toluene-Degrading Bacterial Populations with Catechol-2,3-Dioxygenase Genes in Contaminated Soils. Microbial Ecology. 69: 59–65. [CrossRef]
- O’Loughlin, E. J; Traina, S. J.; Sims, G. K. (2000). Effects of sorption on the biodegradation of 2 methylpyridine in aqueous suspensions of reference clay minerals. Environ. Toxicol. And Chem. 19: 2168–2174. [CrossRef]
- Olson, B. H., Tsai, Y., (1992). Molecular approaches to environmental management. In: Environmental Microbiology ed Ralph Mitchell. John Wiley and sons Inc. Pub. New York,239-263.
- Onwurah, I. N. E., (1999a). Restoring the crop sustaining potential of crude oil polluted soil by means of Azotobacter inoculation. Plant Prod. Res, J., 4, 6-16.
- Onwurah, I. N. E., (2000). A Perspective of Industrial and Environmental Biotechnology. Snaap Press / Publishers Enugu, Nigeria, 148.
- Paerl, H., Piehler, M., Swistak, J., (1996). Coastal diesel fuel pollution effects on the native microbial community. Poster presentedat the Meeting of the American Society of Microbiology, New Orleans May 19-23.
- Prince, P. C., (1992). Bioremediation of oil spills with particular reference to the spill from Exxon Valdez. Microbial Control of Pollution. (Eds. Fry Jc, Gadd GM Herbert RA, Jones CW and Watson-Craik 1A.) Society for General Microbiology symposium 48.
- Ritter, W. F., Scarborough, R. W., (1995). A review of bioremediation of contaminated soils and ground water. J. Environ Sci Health. A., 30, 323-330. [CrossRef]
- Robson,B.D (2003) : ‘Phytoremediation of hydrocarbon-contaminated soil using plants adapted to the western Canadian climate’, Department of Soil Science, University of Saskatchewan, Saskatoon.
- Rosenberg, E. and Ron, E.Z (1996) Bioremediation of petroleum contamination, In R.L. Crawford and D.L. Crawford (Eds.), Bioremediation: principles and Applications, Cambridge University Press, UK, 100-124. [CrossRef]
- Shell International Petroleum Company, Developments in Nigeria (London: March 1995).
- Simon, M., Autenrieth, R.L., McDonald, T.J., Bonner, J.S.(1999) Evaluation of bioaugmentation for remediation of petroleum in a wetland. Proceedings of 1999 International Oil Spill Conference. American Petroleum Institute, Washington DC.
- Sims, G.K. (2006). “Nitrogen Starvation Promotes Biodegradation of N-Heterocyclic Compounds in Soil”. Soil Biology & Biochemistry. 38: 2478–2480. [CrossRef]
- Stroo, H. F., (1992). Biotechnology and hazardous waste treatment .J Environ. Qual., 21, 167. [CrossRef]
- Swannell, R.P.J., Lee, K., and McDonagh, M. (1996) Field evaluations of marine oil spill bioremediation. Microbiological Reviews, 60(2), 342-365. [CrossRef]
- The Daily Independent Lagos (2010).“Shell And The N15bn Oil Spill Judgement Debt”. . 2010-07 19. Retrieved 27 July 2010.
- Tsutsumi, H., Kono, M., Takai, K., Manabe, T. (2000) Bioremediation on the shore after an oil spill from the Nakhodka in the Sea of Japan. III. Field test of a bioremediation agent with.
- UNDP.( 2006 )“Niger Delta Human Development Report”. . p. 76. Retrieved 19 June 2011.
- Venosa, A. D., Lee, K., Suidan, M. T., Garcia-Blanco, S., Cobanli, S., Moteleb, M., Haines, J.R., Tremblay, G., and Hazelwood, M. (2002) Bioremediation and biorestoration of a crude oilcontaminated freshwater wetland on the St. Lawrence River. Bioremediation Journal, 6.
- Vidal, J., (2010). “Nigeria’s agony dwarfs the Gulf oil spill. The US and Europe ignore it”. The Observer. Retrieved 27 July 2010. government’s national oil spill detection and response agency (Nosdra) says that between 1976 and 1996 alone, more than 2.4m barrels.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





