1. Introduction
Probiotics have played a pivotal role in advancing human health, from their initial discovery to their contemporary integration into healthcare practices. The foundational understanding of probiotics can be attributed to Élie Metchnikoff, recognized as the progenitor of
Lactobacillus, and a Nobel laureate for his pioneering work on phagocytes [
1]. In 2001, the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) formally defined probiotics as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.” [
2]. This definition underscores the transformative potential of probiotics in promoting host well-being by modulating microbial balance, particularly within the gastrointestinal tract.
In recent years, the utilization of probiotics in disease treatment has developed into a dynamic and multidimensional field. Probiotics exhibit considerable promise in addressing disorders associated with dysbiosis, encompassing allergic, digestive, and metabolic conditions, as well as cancers and cardiovascular diseases [
3]. The mechanisms underlying these functions are diverse and heterogeneous and have not yet been fully elucidated. Moreover, delivery of adequate probiotics into the gastrointestinal tract poses several challenges, stemming from the harsh conditions of the digestive system. Advancements in chemistry, biology, and research on membrane vesicles in the last decade have significantly enhanced the efficiency and targeted delivery of engineered probiotics, thereby extending their applicability to the treatment of a broader spectrum of diseases [
4,
5,
6].
This review comprehensively explores the intrinsic mechanisms through which probiotics influence overall host health. Subsequently, we discuss probiotic-based delivery systems, offering an overview of the predominant current delivery methods. These methods encompass not only chemical engineering modifications, but also genetic engineering strategies designed to augment the efficiency of probiotic delivery to crucial anatomical sites. The innovative application of membrane vesicles derived from probiotics was also discussed. Finally, this review outlines the applications of functional probiotics in disease treatment, discuss the outcomes of probiotic interventions in preclinical experiments, and offer insights into the prospective role of probiotics therapy in future clinical applications.
2. Probiotic mechanisms of action for disease treatment
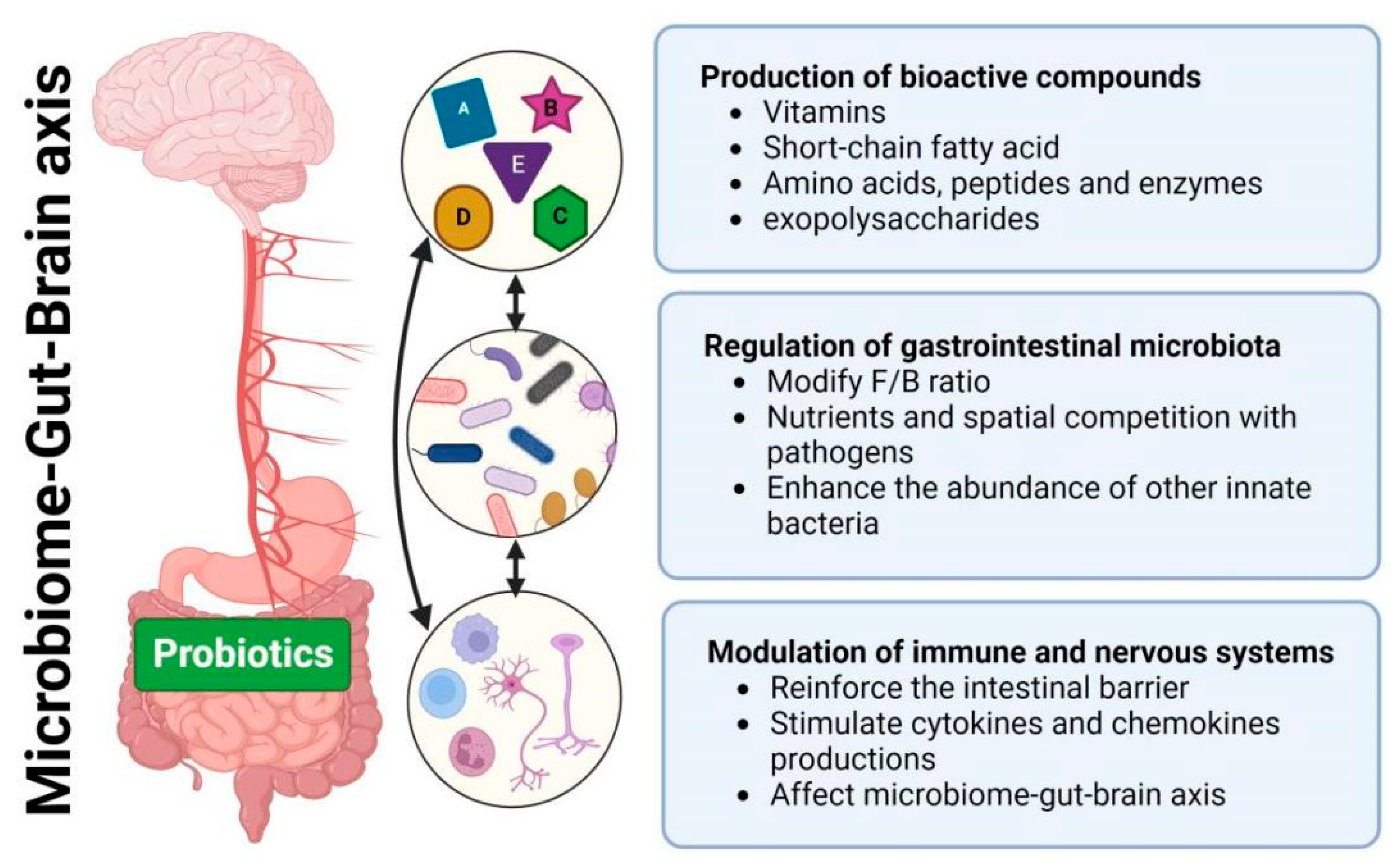
Although the positive impact of probiotic consumption on human health is widely acknowledged, they are often recommended as dietary supplements rather than pharmaceutical interventions for disease treatment [
7]. The accessibility and non-prescriptive nature of dietary supplements contribute to the widespread adoption of probiotics. However, our limited understanding of probiotic action mechanisms constrains their effective use in disease treatment. Recognizing the imperative to comprehend these mechanisms becomes essential, as it has the potential to enhance the engineering of probiotics for more effective disease treatment, optimizing their impact on human health through informed and targeted applications. This encourages us to initially explore and outline the primary mechanisms of probiotic action (
Figure 1): (1) production of bioactive compounds; (2) regulation of the gastrointestinal microbiome; (3) modulation of the immune and nervous systems.
2.1. Production of bioactive compounds
Probiotics generate a diverse range of bioactive compounds, including vitamins, short-chain fatty acids (SCFAs), amino acids, peptides, enzymes, and exopolysaccharides (EPS), thereby exhibiting beneficial health effects on the host [
8]. For examples, probiotic bacteria are able to produce B-group vitamins (B
6, B
12, folate, and thiamine) and K-group vitamins (K
1 and K
2), effectively addressing vitamin deficiencies and supporting the treatment of inflammatory bowel disease (IBD) and carcinogenesis [
9]. SCFAs (mainly lactate, acetate and butyrate) are essential metabolic products of probiotics. Zhang et al. discovered that
Lactobacillus plantarum-derived indole-3-lactic acid enhanced the anti-tumor immunity of CD8+ T cells through epigenetic regulation, thereby ameliorates colorectal tumorigenesis [
10]. SCFAs play a crucial role in various physiological functions, including energy expenditure, mucosal barrier maintenance, and immune system modulation. Administering probiotics producing SCFAs holds promise as an adjunctive therapy for conditions like obesity, diabetes, Alzheimer’s, tumors, and cancer [
11,
12]. Probiotics also secrete specific amino acids, small peptides or proteins to fight pathogens and to activate host immunity. Recently, Zhou et al. genetically engineered
E. coli Nissle 1917 (ECN) to express antioxidant enzymes (catalase and superoxide dismutase) for the treatment of IBD [
13]. The EPS produced by probiotics was reported with anti-tumor and anti-inflammatory activities on melanoma mice [
14]. These bioactive compounds can exert their effects by directly interacting with host cells. However, the majority of these compounds influence the host by interacting with other gastrointestinal bacteria and modulating the composition of microbiota.
2.2. Regulation of gastrointestinal microbiota
The human gastrointestinal (GIT) system, crucial for health, accommodates more than 10
14 microorganisms of over 1000 species. Changes in the GIT microbial composition have been associated with a wide spectrum of human diseases, including cancer, obesity, inflammatory disorder, and even metal illness and neurological disorder [
15,
16].
Probiotics contribute to the regulation of the gastrointestinal microbiota through diverse mechanisms. For examples, probiotics of
Lactobacillus,
Bacillus and
Saccharomyces show potential to reduce the Firmicutes/Bacteroidetes (F/B) ratio that indicate therapy application in obesity and IBD [
17]. Osbelt et al. observed nutrient competition, where commensal
Klebsiella oxytoca strains inhibited the growth of multidrug-resistant
Klebsiella pneumoniae by outcompeting it through beta-glucoside utilization, thereby reducing the risk of bloodstream infections [
18]. Zou et al. found that sortase A-anchored SDPs in
Lactobacillus gasseri Kx110A1 competitively exclude
Helicobacter pylori through steric hindrance, reducing the initial colonization of
H. pylori in the mouse stomach, a key risk factor for stomach cancer development [
19]. Furthermore, Zhou et al. discovered that administration of engineered ECN enhances the abundance of beneficial innate probiotics,
Lachnospiraceae_NK4A136 and
Odoribacter, thereby promoting intestinal homeostasis and mitigating IBD [
13]. These studies highlight the profound impact of probiotics on gut microbiota composition, prompting a deeper exploration of their effects on modulating the immune and nervous systems given their close interactions [
20].
2.3. Modulation of immune and nervous systems
Probiotics exhibit significant effects in modulating both immune and nervous systems, offering promising avenues for therapy of related diseases.
Probiotics exhibit the capability to bolster both innate and adaptive immune responses by interacting with intestinal epithelial cells (IECs), immune cells or other gastrointestinal microbiota. They reinforce the intestinal barrier by increasing mucins, tight junction proteins, supporting the function of Goblet and Paneth cells. Probiotics also stimulate the production of various cytokines and chemokines through Toll-like receptors, initiating a cascade of signaling events. These cytokines, in turn, facilitate the stimulation of adaptive immune responses and establish a network of communication among different immune cells. La Fata et al [
21] and Galdeano et al [
22] systematically reviewed these.
In recent years, scientific studies have increasingly centered on investigating the impact of probiotics on various neurological conditions in humans. This exploration has given rise to a new category of probiotics termed ‘psychobiotics’, which have the ability to generate or stimulate the production of SCFAs, neurotransmitters, enteroendocrine hormones and anti-inflammatory cytokines. As a result, they exhibit potential benefits for a spectrum of neurological diseases like anxiety, depression, Parkinson’s disease, autism spectrum disorders, and Alzheimer’s disease [
23,
24]. In addition, probiotics show therapeutic potential for aging-related cognitive decline. For instance, Yang et al. found that ProBiotic-4 administration improve cognitive function in aging that associated with inhibition of both TLR4-and RIG-I-mediated NF-κB signaling pathway and inflammatory responses [
25].
Probiotics exert a significant and far-reaching influence on human health through the production of functional compounds and modulation of the microbiome-gut-brain axis. However, their effects vary extensively based on factors such as strain species, dosage, and individual differences. Therefore, conducting in-depth molecular mechanism studies, animal experiments, and clinical trials is crucial to refine their applications and target specific diseases more effectively.
3. Probiotics engineering strategies
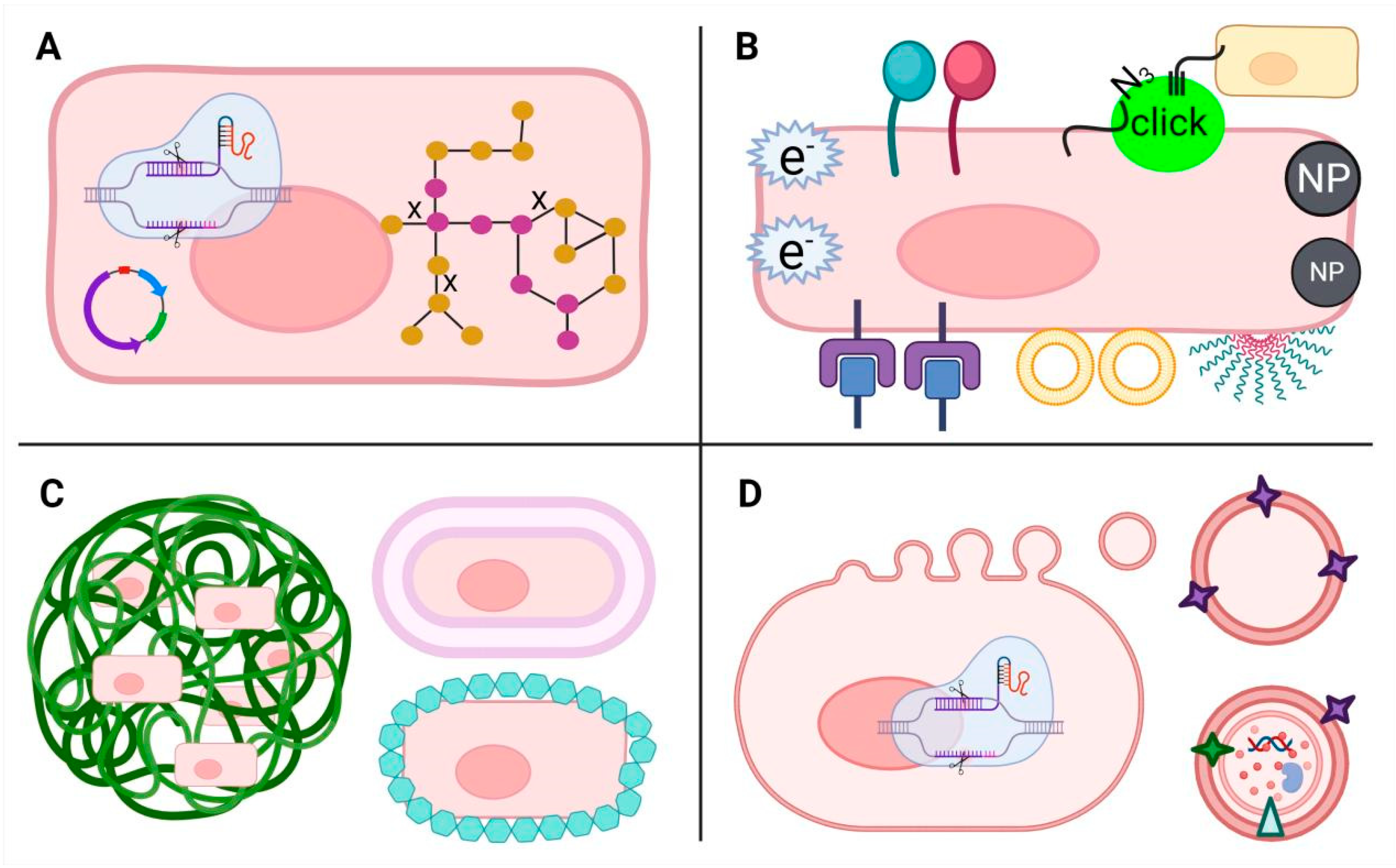
Probiotics hold promising clinical potential for treating various diseases. However, to achieve these effects, probiotics must endure numerous environmental challenges, such as low gastric pH, enzymatic breakdown, the antimicrobial impact of bile salts, and competition with other bacteria. Ensuring their efficacy involves the ability to withstand these hurdles and reach specific locations within the body in adequate quantities. Therefore, different strategies (
Figure 2), including genetic and metabolic engineering, surface modification and encapsulation, have been utilized to engineer probiotics, enhancing their capabilities for efficient and target delivery, as well as precision therapy. Moreover, the evolution of nanotechnology has sparked considerable interest in membrane vesicles derived from probiotics.
3.1. Genetic and Metabolic Engineering
Progress in synthetic biology offers expanded avenues for genetic and metabolic engineering of probiotics, including exogenous gene expression, genome editing, and metabolic regulation. These advancements fortify probiotics against digestive challenges, enhance the production of beneficial substances, and diminish their immunogenicity, thereby bolstering their potential in disease treatment.
Many researchers have suggested that engineered probiotics harboring specific genes or pathways show programmable functions of disease treatment. For example, Yan et al. inserted a 3-hydroxybutyrate (3HB) pathway into the ECN genome and deleted the competitive branch pathways, which significantly increased the production of bioactive compounds 3HB and SCFAs and thus ameliorated colitis in mice [
26]. Praveschotinunt et al. genetically modified ECN to generate a curly fiber matrix composed of curli nanofibers displaying trefoil factors (TFFs), promoting intestinal barrier function and epithelial repair, leading to enhanced gut epithelial integrity and alleviated colitis in a mouse model [
27]. Isabella et al. engineered ECN to express Phe-metabolizing enzymes that achieved live bacterial therapeutic for metabolic disease phenylketonuria in mice and primates [
28]. Developed genetic and metabolic engineering technologies, together with chemical methods have also facilitated the development of surface modification and encapsulation techniques of probiotics.
3.2. Surface Modification
Altering the surface properties of probiotics can enhance their adherence to specific target areas in the body or improve their interaction with other substances. Surface engineering techniques include modifying surface charge, roughness, or adding functional groups to facilitate specific interactions.
Surface modification of probiotics encompasses a range of techniques, including chemical, physical, and biological approaches. For instances, Song et al. pioneered a click chemistry-driven approach to fortify
Clostridium butyricum’s intestinal presence. By introducing azido-modified D-alanine (N3-DAA) to generate azide groups on gut microbiota surfaces and subsequently surface-modifying probiotics with dibenzocyclooctyne (DBCO), they enabled engineered probiotics to engage in precise click chemistry reactions upon oral administration. This innovative strategy ensured prolonged intestinal residency and notably alleviated disease in a colitis mouse model, underscoring the potential of click chemistry in enhancing probiotic colonization efficacy [
29]. Pan et al. utilized a dual approach: they genetically enhanced
E. coli MG1655 by overexpressing cytolysin A (ClyA) within cells and chemically adorned Bi
2S
3 nanoparticles (BNPs) onto the cell surface. This modification resulted in remarkable tumor-targeting capabilities and improved sensitivity to radiotherapy for efficient tumor ablation, while mitigating damage to the surrounding normal tissues [
30]. Recently, Wang et al. and Wu et al. have systematically reviewed diverse physicochemical and biological technologies for cell surface decoration, such as covalent conjugation with existing functional groups like amine, thiol, carboxyl, diol, and artificially introduced groups, electrostatic and/or hydrophobic interactions, receptor-ligand interactions, avidin-biotin interactions, and others [
31,
32].
3.3. Encapsulation
Unlike surface modification, probiotic encapsulation envelops either bulk or individual cells within protective wall materials, shielding them from harsh environments, enabling controlled release, and enhancing their bioavailability.
Conventional probiotic encapsulation techniques include extrusion, emulsion, and spray-drying that used polysaccharides, lipids and proteins as protective matrix materials [
33]. Advancements in nanotechnology have led to the creation of nanofibers and nanoparticles for bulk encapsulation, alongside the innovation of biofilm, biomembrane, and nanocoating techniques for individual probiotic nanoencapsulation. These developments aim to ensure high cell viability, resistance to gastric conditions and temperature, and extended shelf lives [
34]. For example, Harimoto et al. utilized inducible synthetic gene circuits to generate surface capsular polysaccharides, enabling self-encapsulation of engineered ECN. This increased the fraction of microbial translocation among mouse tumors, resulting in therapeutic efficacy in distal tumors [
35]. Cao et al. employed red blood cell membranes to encapsulate engineered probiotics, accomplished through a straightforward process of extruding erythrocyte membranes with bacteria. This innovative method effectively shields bacteria from triggering a high inflammatory response or being eliminated by macrophages, preserving their biological activity [
36].
3.4. Membrane Vesicles (MVs)
In recent years, the therapeutic potential of membrane vesicles (MVs) derived from probiotics has garnered significant attention alongside the engineered probiotics. These MVs, ranging from 20 to 400 nm in diameter, encapsulate a variety of macromolecules and play pivotal roles in microbial activities, facilitating inter-microbial and host-microbe communication, metabolite efflux, and gene transfer [
37]. Their cost-effective production and facile molecular manipulation for antigens display make bacterial MVs promising for therapeutic development. Their nanoparticle size enables broad tissue dissemination, their innate immunogenicity aids vaccines design, and their potential targeted delivery via surface receptors hints at precise therapeutic applications [
38].
A type of nanoprobiotics was created by Li et al., encapsulating manganese dioxide (MnO
2) with MVs from ECN, and combining with the anti-inflammatory drug metformin (Met) for treating IBD. The MnO
2 reduced oxidative stress induced by IBD, while the ECN-derived MVs regulated the gut microbiome, and Met further improved the inflammatory environment. In a colitis model, this approach reduced inflammatory markers, improving gut health without disrupting the healthy microbial balance [
39]. Wang et al. explored the therapeutic potential of MVs from
Akkermansia muciniphila in addressing IBD and colorectal cancer. They found that these MVs aided in the growth of beneficial bacteria, helping to rebalance gut microbes linked with these diseases. Additionally, the MVs could interact with immune cells, triggering increased production of immunoglobulin A (IgA) and improving intestinal cell adhesion and mucus secretion, crucial for maintaining gut barrier integrity [
40]. Moreover, since MVs can penetrate the central nervous system, it has the potential to provide novel noninvasive therapies against blood-brain barrier infections [
41].
Despite numerous articles highlighting the benefits of MVs in disease treatment, such as their non-self-replicating nature that enhances safety in contrast to probiotics, the broader utilization of MVs encounters specific challenges. Firstly, one of the most critical issues is that MVs, like other biological membranes, have very low yields, resulting in relatively high production costs for large-scale applications. Liu et al. reported a method using synthetic biology to induce bacterial peptidoglycan hydrolase to increase MV production, but its industrial application requires more assessment [
42]. Secondly, the mechanism of MV formation remains to be elucidated in detail and necessitates further research. Lastly, as a biological product, it is essential to explore the relationship between the suitable storage conditions for MVs and their effective functionality.
4. Engineered probiotics for living therapeutics
Using the various engineering methods mentioned earlier opens an exciting path for creating probiotics designed specifically for living therapeutics. By harnessing probiotics’ natural abilities or introducing novel traits, engineered probiotics hold immense potential for targeted disease treatment. Their precise manipulation allows for customization, enabling them to address diverse health challenges, combating metabolic, immune and nervous-related diseases. Apart from validating the therapeutic efficacy of probiotics in mice, numerous clinical trials associated with probiotics have achieved initial success (
Table 1). In recent preclinical studies, engineered probiotics have made significant advancements in treating IBD, cancer, neurologic disorders and other diseases.
4.1. IBD
IBD is a non-infectious chronic gastrointestinal inflammatory disorder, primarily encompassing two main conditions, Crohn’s Disease (CD) and Ulcerative Colitis (UC) [
43]. Since 1990, the global number of IBD patients has steadily increased. It is estimated that more than 3 million people in the United States and Europe suffer from IBD. In many countries across North America, Oceania, and Europe, the prevalence of IBD is estimated to exceed 0.3%. Furthermore, the incidence of IBD is on the rise in several emerging industrialized nations [
44]. In clinical practice, traditional treatment methods involve the use of 5-aminosalicylic acid, tofacitinib, or biologics such as anti-tumor necrosis factor (anti-TNF), anti-integrins, and anti-interleukins [
5,
45,
46]. It is worth noting that IBD patients often exhibit disruptions in their gut microbiota, accompanied by a high level of reactive oxygen species (ROS) in the affected areas [
47,
48,
49]. Therefore, the development of engineered probiotics with the ability to scavenge ROS is a feasible therapeutic approach.
The primary challenge that needs to be overcome when using probiotics to treat IBD is the high sensitivity of probiotics to the harsh environment of the GIT. Therefore, it is essential to employ suitable methods to protect probiotics, enabling them to overcome the challenges during delivery and establish colonization at the affected sites [
50]. Liu et al. reported an engineered probiotic system [
51]. In this system, poly(propylene sulfide) (PPS) and hyaluronic acid (HA) self-assemble to form HA-PPS nanoparticles (HPN). They then coated the probiotic’s surface with norepinephrine (NE) to create HPN-NE-EcN for treating IBD. PPS can be oxidized into sulfone by ROS, thereby mitigating the high ROS environment in the gut. HA, as a highly biocompatible material, resolves the challenge of applying PPS in the body due to its high hydrophobicity. Norepinephrine assists ECN in withstanding the harsh gastrointestinal conditions and enhances its adhesion properties, prolonging ECN’s retention in the gut, and ultimately facilitating its modulation of gut microbiota.
Cao et al. developed an engineered
Bifidobacterium longum probiotic that also possesses the capability to scavenge ROS and modulate the gut microbiota [
52]. Notably, their work not only demonstrated superior therapeutic effects in a murine IBD model but also exhibited promising IBD alleviation capabilities in large animals, such as beagle dogs. This bodes well for potential clinical applications in the future.
4.2. Cancer
Cancer is a prominent global cause of mortality, with data from the WHO indicating that it accounted for nearly 10 million deaths in 2020. The primary clinical modalities for treatment include surgical intervention and the application of radiotherapy and chemotherapy. Radiotherapy operates by either activating signaling pathways that lead to cell death or inducing cellular damage, thereby triggering cellular defense mechanisms, ultimately culminating in the demise of cancer cells [
53]. Nonetheless, radiotherapy is often accompanied by challenges, including a scarcity of highly specialized medical professionals, elevated costs associated with radiopharmaceuticals, and the potential harm to non-tumor tissues caused by radiation [
54]. Chemotherapy involves the use of chemical agents to eradicate cancer cells. However, there are currently no chemotherapeutic drugs specifically targeting cancer cells, and as a result, drug toxicity remains one of the limiting factors for the widespread application of chemotherapy. Furthermore, chemotherapy drugs are primarily administered via intravenous injection, which may lead to patient resistance and diminish treatment efficacy upon repeated injections [
55]. Some studies have reported the oral delivery of chemotherapy drugs; however, oral administration entails the passage of chemotherapy drugs through the harsh GIT environment, which raises the possibility of drug inactivation [
56]. As the body of literature related to engineered bacteria continues to expand, the utilization of bacteria for drug delivery has garnered widespread research attention.
Wang et al. created an engineered probiotic hybrid material, designated as EcN@HPB, by combining anticancer drug paclitaxel and BAY-876 bound human serum albumin nanodrugs with ECN [
57]. Due to the hypoxic conditions within tumor tissues as compared to normal tissues, [
58] ECN actively targets tumor tissues, thereby purposefully delivering loaded drugs to the desired locations. At the tumor site, ECN competes with tumor cells for glucose uptake, while BAY-876 further reduces tumor cell glucose uptake via inhibiting glucose transporter 1. Through their synergistic actions, they activate the AMPK signaling pathway in tumor cells, enhancing macropinocytosis in tumor cells. This leads to increased internalization of HPB, raising the concentration of paclitaxel within tumor cells and consequently achieving the goal of antitumor therapy.
With the advancements in immunology, immunotherapy for cancer is gradually emerging as one of the treatment modalities for cancer. The primary goal of immunotherapy is to harness the patient’s immune system to combat malignant tumors [
59]. Immunotherapy encompasses various approaches, including immune checkpoint blockade therapy, [
60] adoptive T cell therapies, [
61] and cancer vaccines [
62]. Similar to chemotherapy, immunotherapy still faces the challenge of non-specifically affecting tumor tissues, resulting in systemic toxicity. Therefore, leveraging specific probiotics for tumor hypoxia targeting can effectively address the targeting issue in immunotherapy, further expanding the application of immunotherapy in cancer treatment.
Savage et al. utilized synthetic biology techniques to engineer ECN, enabling it to express human chemokine CXCL16 [
63]. The primary function of this cytokine is to promote the migration of lymphocytes, particularly T cells, to inflammatory sites, thereby activating the immune response. Furthermore, researchers designed strains expressing CCL20 to target the presentation of tumor-derived antigens by dendritic cells. The synergistic action of these two engineered probiotics effectively restrained tumor progression in the MC38 tumor model mice. Chimeric antigen receptor (CAR)–T cell therapy has shown promising results in the treatment of hematologic malignancies, but its effectiveness in solid tumors has been disappointing [
64,
65]. Vincent et al. developed engineered ECN cells that express specific targets to induce CAR–T cell enrichment and destruction of tumor cells [
66]. ECN can target the hypoxic tumor microenvironment, and due to the clever design of a synchronized lysis circuit, when the number of ECN cells reaches a critical threshold, they actively lyse to release the target. Subsequently, CAR–T cells recognize these antigen targets released by these probiotic bacteria, effectively killing these tumor cells in situ. To enhance T cell enrichment, the researchers combined this system with engineered ECN that were reported earlier in their research to express CXCL16, and the results showed that it effectively suppressed tumor growth in mouse models of human and mouse cancer. This provides a new approach for the application of CAR-T cell therapy in solid tumors.
4.3. Others
Engineered probiotics, in addition to achieving promising results in the treatment of IBD and cancer, have also been reported in the literature for the treatment of various other diseases. Ventilator-associated pneumonia (VAP) is an acute pulmonary infection, typically caused by pathogens such as
Pseudomonas aeruginosa or
Staphylococcus aureus [
67]. Zhang et al. developed a micro-robot for VAP treatment by modifying
Chlamydomonas reinhardtii with antibiotic nanoparticles encapsulated within neutrophil membranes through click chemistry reactions [
68]. Due to
Chlamydomonas reinhardtii’s advantageous mobility, it can, to some extent, evade phagocytosis by macrophages. Furthermore, with the assistance of the neutrophil membrane, this micro-robot can reduce the chances of clearance. In a mouse model of pneumonia induced by Pseudomonas aeruginosa infection, the authors observed that this system exhibited substantial antibacterial capabilities, leading to increased mouse survival rates.
Candida vaginitis, a commonly encountered inflammatory fungal infection of the female genital tract, is typically caused by
Candida albicans [
69]. Statistics indicate that approximately 75% of women worldwide suffer from this disease, which significantly impairs their quality of life [
70]. Currently employed clinical treatments often result in damage to normal vaginal cells and tissues, exacerbating the situation [
71]. Wei et al. developed a novel approach for treating
Candida by combining a nanozyme capable of catalyzing hydrogen peroxide into
•OH radicals with
lactobacilli [
72]. These
lactobacilli were encapsulated within a hyaluronic acid hydrogel. Hyaluronidase secreted by
Candida albicans degrades the hydrogel, releasing the composite probiotics.
Lactobacilli produce lactic acid, restoring the vaginal environment to its normal acidic state. Simultaneously, the nanozyme breaks down the hydrogen peroxide produced by
lactobacilli, generating harmful free radicals detrimental to pathogenic microorganisms. Remarkably, researchers observed satisfying therapeutic effects in a murine disease model. Furthermore, this system exhibited a superior treatment capacity compared to clinical medications, with no significant impact on the vaginal microbiota.
Probiotics also play an important role in neurodevelopment and various brain functions by affecting the gut-brain axis through producing bioactive compounds and modulating gut microbiota. Guo et al. utilized a simple bio-interface supramolecular self-assembly approach, using a methacrylic acid and ethyl acrylate-based pH-responsive anionic copolymer to encapsulate
Lactobacillus plantarum, enhancing the probiotic’s survival capabilities under extreme conditions [
73]. Approximately 2 hours after reaching the intestines, these probiotics began synthesizing biologically active substances. The researchers observed significant symptom relief in a mouse model of Parkinson’s disease. This study provides a novel approach to the clinical treatment of neurological disorders.
Engineered probiotics can be endowed with multiple functions, such as resistance to extreme pH environments, drug delivery for therapeutic purposes, and the recruitment of immune cells to activate the host’s immune response. As living entities capable of adapting to their environment, engineered probiotics stand at the forefront of innovative medical interventions, heralding a new era of personalized and precise therapeutics.
5. Conclusion and outlook
Certainly, probiotics have been demonstrated to exert positive effects on host health. However, commercial probiotics still face various challenges. For instance, probiotics, while exhibiting lower toxicity compared to other microorganisms, still possess immunogenicity, and adverse outcomes like inflammatory reactions can be anticipated during treatment. Moreover, there remains a risk of sepsis when probiotics are administered intravenously. While oral ingestion significantly reduces the risk of sepsis, the extreme pH conditions in the GIT and the presence of various enzymes pose significant barriers to whether probiotics can reach specific sites.
The engineering of probiotics using chemical and biological approaches offers a promising avenue to overcome the limitations mentioned above and expand their clinical applications. Several engineered probiotics have seen successful commercialization. For instance, the FDA has sanctioned a genetically engineered probiotic,
Bacillus subtilis ZB183, developed and commercialized by ZBiotics (
https://zbiotics.com/). This engineered probiotic, hosting an acetaldehyde dehydrogenase enzyme, transforms acetaldehyde into acetic acid, effectively reducing post-alcohol consumption acetaldehyde accumulation and thus relieve the alcohol hangover [
74]. Despite the known safety of the parent strain, the modified variant underwent thorough safety assessments before gaining recognition as safe for general use [
75]. This marks the world’s pioneering commercialized engineered probiotic, heralding a promising future for engineered probiotics in various commercial applications. In addition, Synlogic is an established biotechnology company specializing in the development of engineered probiotics with therapeutic applications. Among their products are the strains SYNB1618 and SYNB1934, which have been engineered to combat phenylketonuria (PKU). These strains employ ECN as the genetic backbone. However, it is important to acknowledge that, despite the advancements in this domain, the project is currently in phase 2 of clinical trials, as outlined by Vockley et al [
76].
Engineered probiotics currently face stringent regulatory challenges, requiring extensive safety and efficacy testing that is time-consuming and costly. Ensuring consistent effectiveness among diverse individuals, long-term safety, and monitoring for potential side effects adds complexity. Public acceptance and competition with natural probiotics further complicate their adoption. Ethical considerations around genetic modification are also pivotal. For engineered probiotics to advance in clinical applications, advancements in science must align with adjustments in regulatory frameworks to facilitate their utilization.
Author Contributions
Conceptualization, M.L. and H.L.; validation, Y.L.; Supervision, Y.L. and H.L.; visualization, J.C. and I.P.W.D.; writing—original draft, M.L.; writing—review and editing, M.L., J.C., I.P.W.D. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Shenzhen Science and Technology Innovation Commission, China (grant number JCYJ20220530114409020 and 20231115134555001).
References
- Shortt, C. The probiotic century: historical and current perspectives. Trends in Food Science & Technology 1999, 10 (12), 411-417. [CrossRef]
- Who, F. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria – Joint FAO/WHO Expert Consultation. 2001.
- Li, Z.; Wang, Y.; Liu, J.; Rawding, P.; Bu, J.; Hong, S.; Hu, Q. Chemically and biologically engineered bacteria-based delivery systems for emerging diagnosis and advanced therapy. Advanced Materials 2021, 33 (38), 2102580. [CrossRef]
- Singh, T. P.; Natraj, B. H. Next-generation probiotics: a promising approach towards designing personalized medicine. Critical Reviews in Microbiology 2021, 47 (4), 479-498. [CrossRef]
- Lin, S.; Wu, F.; Zhang, Y.; Chen, H.; Guo, H.; Chen, Y.; Liu, J. Surface-modified bacteria: synthesis, functionalization and biomedical applications. Chemical Society Reviews 2023, 52 (19), 6617-6643. [CrossRef]
- Hahn, J.; Ding, S.; Im, J.; Harimoto, T.; Leong, K. W.; Danino, T. Bacterial therapies at the interface of synthetic biology and nanomedicine. Nature Reviews Bioengineering 2023. [CrossRef]
- Feng, Q.; Chen, W. D.; Wang, Y. D. Gut microbiota: an integral moderator in health and disease. Frontiers in Microbiology 2018, 9, 151. [CrossRef]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Current Opinion in Food Science 2020, 32, 76-82. [CrossRef]
- Zhai, Z.; Dong, W.; Sun, Y.; Gu, Y.; Ma, J.; Wang, B.; Cao, H. Vitamin—;ndash;microbiota crosstalk in intestinal inflammation and carcinogenesis. Nutrients, 2022; Vol. 14. [CrossRef]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8+ T cell immunity. Cell Metabolism 2023, 35 (6), 943-960.e949. [CrossRef]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the treatment of overweight and obesity in humans—a review of clinical trials. Microorganisms, 2020; Vol. 8. [CrossRef]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H. F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s disease: a systematic review. Nutrients, 2022; Vol. 14. [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nature Communications 2022, 13 (1), 3432. [CrossRef]
- Wang, Q. a.; Jiang, B.; Wei, M.; He, Y.; Wang, Y.; Zhang, Q.; Wei, H.; Tao, X. Antitumor effect of exopolysaccharide from Lactiplantibacillus plantarum WLPL09 on melanoma mice via regulating immunity and gut microbiota. International Journal of Biological Macromolecules 2024, 254, 127624. [CrossRef]
- Lee, J.-Y.; Tsolis, R. M.; Bäumler, A. J. The microbiome and gut homeostasis. Science 2022 377 (6601), eabp9960. [CrossRef]
- Chin Fatt, C. R.; Asbury, S.; Jha, M. K.; Minhajuddin, A.; Sethuram, S.; Mayes, T.; Kennedy, S. H.; Foster, J. A.; Trivedi, M. H. Leveraging the microbiome to understand clinical heterogeneity in depression: findings from the T-RAD study. Translational Psychiatry 2023, 13 (1), 139. [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms, 2020; Vol. 8. [CrossRef]
- Osbelt, L.; Wende, M.; Almási, É.; Derksen, E.; Muthukumarasamy, U.; Lesker, T. R.; Galvez, E. J. C.; Pils, M. C.; Schalk, E.; Chhatwal, P.; et al. Klebsiella oxytoca causes colonization resistance against multidrug-resistant K. pneumoniae in the gut via cooperative carbohydrate competition. Cell Host & Microbe 2021, 29 (11), 1663-1679.e1667. [CrossRef]
- Zuo, F.; Appaswamy, A.; Gebremariam, H. G.; Jonsson, A.-B. Role of sortase a in Lactobacillus gasseri Kx110A1 adhesion to gastric epithelial cells and competitive exclusion of helicobacter pylori. Frontiers in Microbiology 2019, 10. [CrossRef]
- Fung, T. C.; Olson, C. A.; Hsiao, E. Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nature Neuroscience 2017, 20 (2), 145-155. [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M. H. Probiotics and the gut immune system: indirect regulation. Probiotics and Antimicrobial Proteins 2018, 10 (1), 11-21. [CrossRef]
- Maldonado Galdeano, C.; Cazorla, Silvia I.; Lemme Dumit, José M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Annals of Nutrition & Metabolism 2019, 74 (2), 115-124. [CrossRef]
- Dinan, T. G.; Stanton, C.; Cryan, J. F. Psychobiotics: A novel class of psychotropic. Biological Psychiatry 2013, 74 (10), 720-726. [CrossRef]
- Ross, K. Psychobiotics: Are they the future intervention for managing depression and anxiety? A literature review. EXPLORE 2023, 19 (5), 669-680. [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharmaceutica Sinica B 2020, 10 (3), 475-487. [CrossRef]
- Yan, X.; Liu, X.-Y.; Zhang, D.; Zhang, Y.-D.; Li, Z.-H.; Liu, X.; Wu, F.; Chen, G.-Q. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cellular & Molecular Immunology 2021, 18 (10), 2344-2357. [CrossRef]
- Praveschotinunt, P.; Duraj-Thatte, A. M.; Gelfat, I.; Bahl, F.; Chou, D. B.; Joshi, N. S. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nature Communications 2019, 10 (1), 5580. [CrossRef]
- Isabella, V. M.; Ha, B. N.; Castillo, M. J.; Lubkowicz, D. J.; Rowe, S. E.; Millet, Y. A.; Anderson, C. L.; Li, N.; Fisher, A. B.; West, K. A.; et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nature Biotechnology 2018, 36 (9), 857-864. [CrossRef]
- Song, W.-F.; Yao, W.-Q.; Chen, Q.-W.; Zheng, D.; Han, Z.-Y.; Zhang, X.-Z. In situ bioorthogonal conjugation of delivered bacteria with gut inhabitants for enhancing probiotics colonization. ACS Central Science 2022, 8 (9), 1306-1317. [CrossRef]
- Pan, P.; Dong, X.; Chen, Y.; Zeng, X.; Zhang, X.-Z. Engineered bacteria for enhanced radiotherapy against breast carcinoma. ACS Nano 2022, 16 (1), 801-812. [CrossRef]
- Wang, Y.; Li, Z.; Mo, F.; Chen-Mayfield, T.-J.; Saini, A.; LaMere, A. M.; Hu, Q. Chemically engineering cells for precision medicine. Chemical Society Reviews 2023, 52 (3), 1068-1102. [CrossRef]
- Wu, F.; Liu, J. Decorated bacteria and the application in drug delivery. Advanced Drug Delivery Reviews 2022, 188, 114443. [CrossRef]
- Reque, P. M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: applications in functional food industry. Trends in Food Science & Technology 2021, 114, 1-10. [CrossRef]
- Centurion, F.; Basit, A. W.; Liu, J.; Gaisford, S.; Rahim, M. A.; Kalantar-Zadeh, K. Nanoencapsulation for probiotic delivery. ACS Nano 2021, 15 (12), 18653-18660. [CrossRef]
- Harimoto, T.; Hahn, J.; Chen, Y.-Y.; Im, J.; Zhang, J.; Hou, N.; Li, F.; Coker, C.; Gray, K.; Harr, N.; et al. A programmable encapsulation system improves delivery of therapeutic bacteria in mice. Nature Biotechnology 2022, 40 (8), 1259-1269. [CrossRef]
- Cao, Z.; Cheng, S.; Wang, X.; Pang, Y.; Liu, J. Camouflaging bacteria by wrapping with cell membranes. Nature Communications 2019, 10 (1), 3452. [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nature Reviews Microbiology 2019, 17 (1), 13-24. [CrossRef]
- Bitto, N. J.; Kaparakis-Liaskos, M. The therapeutic benefit of bacterial membrane vesicles. International Journal of Molecular Sciences, 2017; Vol. 18. [CrossRef]
- Li, J.; Sun, M.; Liu, L.; Yang, W.; Sun, A.; Yu, J.; Liu, D.; Zhao, W.; Cheng, M.; He, Z.; et al. Nanoprobiotics for remolding the pro-inflammatory microenvironment and microbiome in the treatment of colitis. Nano Lett. 2023, 23 (18), 8593-8601. [CrossRef]
- Wang, X.; Lin, S.; Wang, L.; Cao, Z.; Zhang, M.; Zhang, Y.; Liu, R.; Liu, J. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Science Advances 2023, 9 (11). [CrossRef]
- Srivastava, P.; Kim, K.-s. Membrane vesicles derived from gut microbiota and probiotics: cutting-edge therapeutic approaches for multidrug-resistant superbugs linked to neurological anomalies. Pharmaceutics, 2022; Vol. 14. [CrossRef]
- Liu, Y.; Chen, J.; Raj, K.; Baerg, L.; Nathan, N.; Philpott, D. J.; Mahadevan, R. A universal strategy to promote secretion of G+/G– bacterial extracellular vesicles and its application in host innate immune responses. ACS Synthetic Biology 2023, 12 (1), 319-328. [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R. J. Microbial genes and pathways in inflammatory bowel disease. Nature Reviews Microbiology 2019, 17 (8), 497-511. [CrossRef]
- Alatab, S.; Sepanlou, S. G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M. R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet Gastroenterology & Hepatology 2020, 5 (1), 17-30. [CrossRef]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D. D. Novel and emerging therapies for inflammatory bowel disease. Frontiers in Pharmacology 2021, 12, 651415. [CrossRef]
- Baumgart, D. C.; Le Berre, C. Newer biologic and small-molecule therapies for inflammatory bowel disease. The New England Journal of Medicine 2021, 385 (14), 1302-1315. [CrossRef]
- (IIBDGC), T. I. I. G. C.; Jostins, L.; Ripke, S.; Weersma, R. K.; Duerr, R. H.; McGovern, D. P.; Hui, K. Y.; Lee, J. C.; Philip Schumm, L.; Sharma, Y.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491 (7422), 119-124. [CrossRef]
- Investigators, I.; Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A. N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T. W.; Andrews, E.; Ajami, N. J.; Bonham, K. S.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569 (7758), 655-662. [CrossRef]
- Plichta, D. R.; Graham, D. B.; Subramanian, S.; Xavier, R. J. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell 2019, 178 (5), 1041-1056. [CrossRef]
- Anselmo, A. C.; McHugh, K. J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Advanced Materials 2016, 28 (43), 9486-9490. [CrossRef]
- Liu, J.; Wang, Y.; Heelan, W. J.; Chen, Y.; Li, Z.; Hu, Q. Mucoadhesive probiotic backpacks with ROS nanoscavengers enhance the bacteriotherapy for inflammatory bowel diseases. Science Advances 2022, 8 (45), eabp8798. [CrossRef]
- Cao, F.; Jin, L.; Gao, Y.; Ding, Y.; Wen, H.; Qian, Z.; Zhang, C.; Hong, L.; Yang, H.; Zhang, J.; et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nature Nanotechnology 2023. [CrossRef]
- Petroni, G.; Cantley, L. C.; Santambrogio, L.; Formenti, S. C.; Galluzzi, L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nature Reviews Clinical Oncology 2022, 19 (2), 114-131. [CrossRef]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A. M.; Lewis, J. S. Radiotheranostics in oncology: current challenges and emerging opportunities. Nature Reviews Clinical Oncology 2022, 19 (8), 534-550. [CrossRef]
- Chi, Y. H.; Lee, J. H.; Kim, J. H.; Tan, H. K.; Kim, S. L.; Lee, J. Y.; Rim, H.-K.; Paik, S. H.; Lee, K.-T. Pharmacological characterization of BR-A-657, a highly potent nonpeptide angiotensin II receptor antagonist. Biological & Pharmaceutical Bulletin 2013, 36 (7), 1208-1215. [CrossRef]
- Pridgen, E. M.; Alexis, F.; Farokhzad, O. C. Polymeric nanoparticle technologies for oral drug delivery. Clinical Gastroenterology and Hepatology 2014, 12 (10), 1605-1610. [CrossRef]
- Wang, J. W.; Chen, Q. W.; Luo, G. F.; Ji, P.; Han, Z. Y.; Song, W. F.; Chen, W. H.; Zhang, X. Z. Interference of glucose bioavailability of tumor by engineered biohybrids for potentiating targeting and uptake of antitumor nanodrugs. Nano Lett 2022, 22 (21), 8735-8743. [CrossRef]
- Wang, J. J.; Lei, K. F.; Han, F. Tumor microenvironment: recent advances in various cancer treatments. European Review for Medical and Pharmacological Sciences 2018, 22 (12), 3855-3864. [CrossRef]
- Waldman, A. D.; Fritz, J. M.; Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunology 2020, 20 (11), 651-668. [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B. A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S. K.; Poon, C.; Gant, K. L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186 (8), 1652-1669. [CrossRef]
- Cascone, T.; McKenzie, J. A.; Mbofung, R. M.; Punt, S.; Wang, Z.; Xu, C.; Williams, L. J.; Wang, Z.; Bristow, C. A.; Carugo, A.; et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metabolism 2018, 27 (5), 977-987.e974. [CrossRef]
- Goldman, B.; DeFrancesco, L. The cancer vaccine roller coaster. Nature Biotechnology 2009, 27 (2), 129-139. [CrossRef]
- Savage, T. M.; Vincent, R. L.; Rae, S. S.; Huang, L. H.; Ahn, A.; Pu, K.; Li, F.; De Los Santos-Alexis, K.; Coker, C.; Danino, T.; et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity. Science Advances 2023, 9 (10), eadc9436. [CrossRef]
- Chen, N.; Li, X.; Chintala, N. K.; Tano, Z. E.; Adusumilli, P. S. Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Current Opinion in Immunology 2018, 51, 103-110. [CrossRef]
- Hyrenius-Wittsten, A.; Roybal, K. T. Paving new roads for CARs. Trends in Cancer 2019, 5 (10), 583-592. [CrossRef]
- Vincent, R. L.; Gurbatri, C. R.; Li, F.; Vardoshvili, A.; Coker, C.; Im, J.; Ballister, E. R.; Rouanne, M.; Savage, T.; De Los Santos-Alexis, K.; et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science 2023, 382 (6667), 211-218. [CrossRef]
- Lisboa, T.; Diaz, E.; Sa-Borges, M.; Socias, A.; Sole-Violan, J.; Rodríguez, A.; Rello, J. The ventilator-associated pneumonia PIRO score. Chest 2008, 134 (6), 1208-1216. [CrossRef]
- Zhang, F.; Zhuang, J.; Li, Z.; Gong, H.; de Ávila, B. E.-F.; Duan, Y.; Zhang, Q.; Zhou, J.; Yin, L.; Karshalev, E.; et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nature Materials 2022, 21 (11), 1324-1332. [CrossRef]
- Ilkit, M.; Guzel, A. B. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: A mycological perspective. Critical Reviews in Microbiology 2011, 37 (3), 250-261. [CrossRef]
- Watson, M. C.; Grimshaw, J. M.; Bond, C. M.; Mollison, J.; Ludbrook, A. Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 2002, 109 (1), 85-95. [CrossRef]
- Denning, D. W.; Kneale, M.; Sobel, J. D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. The Lancet Infectious Diseases 2018, 18 (11), e339-e347. [CrossRef]
- Wei, G.; Liu, Q.; Wang, X.; Zhou, Z.; Zhao, X.; Zhou, W.; Liu, W.; Zhang, Y.; Liu, S.; Zhu, C.; et al. A probiotic nanozyme hydrogel regulates vaginal microenvironment for Candida vaginitis therapy. Science Advances 2023, 9 (20), eadg0949. [CrossRef]
- Guo, M.; Yang, C.; Li, B.; Cheng, S.-X.; Guo, Q.; Ming, D.; Zheng, B. Bionic dormant body of timed wake-up for bacteriotherapy in Vivo. ACS Nano 2022, 16 (1), 823-836. [CrossRef]
- Merlo, A.; Abbott, Z.; Alford, C.; Balikji, S.; Bruce, G.; Gunn, C.; Iversen, J.; Iversen, J.; Johnson, S. J.; Kruisselbrink, L. D.; et al. Proceedings of the 10th alcohol hangover research group meeting in utrecht, the netherlands. Proceedings, 2020; Vol. 43. [CrossRef]
- Appala Naidu, B.; Kannan, K.; Santhosh Kumar, D. P.; Oliver, J. W. K.; Abbott, Z. D. Lyophilized B. subtilis ZB183 spores: 90-day repeat dose oral (Gavage) toxicity study in wistar rats. Journal of Toxicology 2019, 2019, 3042108. [CrossRef]
- Vockley, J.; Sondheimer, N.; Puurunen, M.; Diaz, G. A.; Ginevic, I.; Grange, D. K.; Harding, C.; Northrup, H.; Phillips, J. A., 3rd; Searle, S.; et al. Efficacy and safety of a synthetic biotic for treatment of phenylketonuria: a phase 2 clinical trial. Nature Metabolism 2023, 5 (10), 1685-1690. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).